Abstract
Hypoxia increases the release of neurotransmitters from chemoreceptor cells of the carotid body (CB) and the activity in the carotid sinus nerve (CSN) sensory fibers, elevating ventilatory drive. According to previous reports, perinatal hyperoxia causes CSN hypotrophy and varied diminishment of CB function and the hypoxic ventilatory response. The present study aimed to characterize the presumptive hyperoxic damage. Hyperoxic rats were born and reared for 28 days in 55%–60% O2; subsequent growth (to 3.5–4.5 months) was in a normal atmosphere. Hyperoxic and control rats (born and reared in a normal atmosphere) responded with a similar increase in ventilatory frequency to hypoxia and hypercapnia. In comparison with the controls, hyperoxic CBs showed (1) half the size, but comparable percentage area positive to tyrosine hydroxylase (chemoreceptor cells) in histological sections; (2) a twofold increase in dopamine (DA) concentration, but a 50% reduction in DA synthesis rate; (3) a 75% reduction in hypoxia-evoked DA release, but normal high [K+]0-evoked release; (4) a 75% reduction in the number of hypoxia-sensitive CSN fibers (although responding units displayed a nearly normal hypoxic response); and (5) a smaller percentage of chemoreceptor cells that increased [Ca2+]1 in hypoxia, although responses were within the normal range. We conclude that perinatal hyperoxia causes atrophy of the CB–CSN complex, resulting in a smaller number of chemoreceptor cells and fibers. Additionally, hyperoxia damages O2-sensing, but not exocytotic, machinery in most surviving chemoreceptor cells. Although hyperoxic CBs contain substantially smaller numbers of chemoreceptor cells/sensory fibers responsive to hypoxia they appear sufficient to evoke normal increases in ventilatory frequency.
The parenchyma of the carotid body (CB) is formed by chemoreceptor and sustentacular cells organized in clusters surrounded by a dense network of capillaries. Sensory nerve terminals of the carotid sinus nerve (CSN) penetrate the clusters to synapse with chemoreceptor cells (Verna, 1997). Functionally, chemoreceptor cells are activated by hypoxia and hypercapnia, and they respond with an increased release of neurotransmitters that activate the sensory nerve endings of the CSN and produce an increase in the action potential frequency in the CSN. Central projections of the CSN produce a ventilatory response, which facilitates homeostasis of blood O2 and CO2 levels. Catecholamines (CA) are the most abundant neurotransmitters present in chemoreceptor cells. Many groups have demonstrated that CA metabolism, including rate of synthesis and release, parallel the level of CB stimulation and action potential frequency in the CSN (Gonzalez et al. 1992, 1994 and references therein).
In adult mammals the CB is responsible for the entire hyperventilation evoked by hypoxia and for about 30–50% of the hyperventilation triggered by hypercapnia and acidosis (Cherniack & Altose 1997; Gonzalez et al. 2002a). In the intact animal a decrease in arterial PO2from 100 to about 75 mmHg produces only minor changes in the basal level of CSN activity or ventilation. At PO2 below the apparent threshold of 75 mmHg there is an almost exponential increase in either response. CSN activity and ventilation double at about 50–55 mmHg, and increase by a factor of four near 40 mmHg. In the case of CO2 the activity in the CSN and the ventilation mediated by the CB increase linearly with the PCO2, doubling every 15–20 mmHg (Gonzalez et al. 1994).
In neonatal animals, the apparent threshold for the hypoxic response is set at a much lower PO2, in the range of 20–25 mmHg, i.e. at PO2 comparable to that found in utero. At lower PO2, the hypoxic response increases with a lower slope than in adults. In response to elevated CO2 there is a comparable hyposensitivity in newborn animals (Hanson & Kumar 1994; Donnelly, 1997). Functional maturation of the CB during postnatal life occurs in the first few weeks after birth, with some differences among species. In the rat, at four weeks of age the responses are fully developed (Eden & Hanson 1987a; Donnelly & Doyle 1994; Rigual et al. 2000; Donnelly, 1997). Maturation of CB function is greatly affected by the ambient PO2 in the perinatal period. Thus, if animals are born and reared during the first few weeks in a hypoxic environment with an arterial PO2 mimicking that found in utero, maturational changes are delayed or fail to develop fully. For example, rats reared in a 13% O2 atmosphere even at 10 weeks of age still present a biphasic (activation followed by depression) ventilatory response to hypoxia, similar to that seen in newborn animals; however, their CSN response to hypoxia was identical to control rats (Eden & Hanson 1987b). In kittens, however, both ventilation and CSN response remain flattened after perinatal hypoxia. Thus, perinatal hypoxia seems to produce a long-lasting alteration of the ventilatory response to hypoxia in all species, including humans (Gauda & Lawson 2000), but the maturational alteration of the CB appears to be species-specific (Hanson & Kumar 1994).
Similarly, perinatal hyperoxia produces profound and permanent alterations in the CSN response to hypoxia or asphyxia, which was found to be totally absent (Eden & Hanson 1986) or greatly attenuated (Ling et al. 1997a; Prieto-Lloret et al. 2003). In the experiments of Eden & Hanson (1986) the ventilatory response to hypoxia was normal and developed earlier following perinatal hyperoxia, leading the authors to conclude that the response originated outside the CB. Ling et al. (1996, 1997a,b) demonstrated in adult rats that were previously exposed to perinatal hyperoxia that the hypoxic ventilatory responses in awake animals, as well as phrenic nerve responses to isocapnic hypoxia in anaesthetized animals, were significantly reduced at moderate intensities of hypoxic stimulation; they also observed that hyperoxic animals needed more intense hypoxic stimulation than controls to generate comparable maximal or near maximal responses. However, suprathreshold electrical stimulation of the central stump of the sectioned CSN elicited an identical phrenic activity in hyperoxic and control animals within the entire range (0–20Hz) of stimulus frequencies (Ling et al. 1997c). In subsequent articles (Erickson et al. 1998; Fuller et al. 2002), the same group found a marked hypoplasia of the CB and a clear degeneration of the CSN chemosensory fibers, which correlated with the attenuated responses to hypoxia. However, there is no such clear relationship between degeneration of the chemosensory fibers and the normal effects of the CSN stimulation on phrenic activity (Ling et al. 1997c).
The present study was designed to identify the cellular process(es) in the CB that are ‘damaged’ by perinatal hyperoxic treatment, in order to determine the cause of the apparent permanent hypofunctionality observed in adult animals. We have also attempted to clarify some of the inconsistencies previously reported with respect to effects of perinatal hyperoxia on hypoxia-evoked responses monitored via CSN activity versus ventilation.
Material and methods
Experimental animals and perinatal exposure to hyperoxia
Experiments were performed in Sprague–Dawley rats. Control rats were born from mothers maintained in a normal atmosphere (barometric pressure in Valladolid ∼710 mmHg) for the entire pregnancy and reared in the same atmosphere until the age of 3.5–4.5 months, when the postexposure experiments were performed. Experimental animals were born from mothers exposed in a hyperoxic atmosphere (55–60% O2, balance N2) for the last 6–7 days of pregnancy and maintained in the same atmosphere for the first month of postnatal life; this protocol of hyperoxic treatment is the same used by Ling et al. (1996) except that the period of hyperoxia before birth in their experiments lasted 2–5 days. Two groups of seven pregnant rats (control and experimental) were caged individually at day one of pregnancy. Fifteen days later, the cages with the experimental animals were placed in a glass chamber (125 × 50 × 50 cm) that was continuously flushed with the specified gas mixture. Except for the periods of measurement of the percentage O2, the outlet of the chamber flowed to the building exterior. The accumulation of CO2 and water vapor (and the increase of temperature; 22–25°C) inside the chamber was prevented by the gas flow and by the presence in the floor of the chamber of a layer of soda lime. Every 4 days the case was opened for cleaning and feeding purposes, and the entire cleaning procedure required approximately 30 min. After cleaning, the glass cage was flushed at high flow so that in 15–20 min the percentage O2 measured at the outlet of the chamber was 55–60%. The control animals were maintained in the same room for the entire period. When the pups were one month old, both control and experimental mothers and litters were transferred to the general vivarium until the experiments were conducted. The experimental observations reported in this study were obtained in animals from four different series of experiments performed over a period of two years. On the day of the experiment the animals were weighed and a blood sample was taken for analysis (Advia 120 Cell Counter, Bayer).
Whole-body plethysmography
A multichannel pump (air supply unit Oxilet 4 LE400-4; Letica Scientific Instruments, Barcelona, Spain) delivered the selected gas mixture to cylindrical chambers, each containing a rat (chamber volume approximately 2.5 l; gas flow 2 l min−1). The outflow of each chamber was directed to a flow head (MLT10L; ADInstruments, Castle Hill, Australia) suitable for rats. Part of the flow was deflected to the spirometer and the remainder released to the room; in each experiment, the flow delivered to the spirometer was adjusted to obtain an adequate signal (oscillations related to the respiratory movements) in the recording computer. A small gas-sample outlet was used to monitor O2 percentage. In the present experimental conditions it took around 3.0 min to reach the delivered O2 percentage. The animals breathed a desired gas mixture for periods of at least 10 min, and evaluated the change in breathing parameters during the last five minutes. Prior to recording, each animal was in the chamber for 30 min to allow acclimation and to acquire a standard resting behaviour. Signals were fed to a computer for visualization and storage for later analysis with Chart 4 software (ADInstruments, Castle Hill, Australia). In the present experiments we evaluated only the respiratory frequency. As shown by several authors, the hyperventilation produced by hypoxia in the normal rat is due mainly to a change in respiratory frequency (Ling et al. 1996; Roux et al. 2000; Fernandez et al. 2003). Although this pattern of hypoxic response can be altered by experimental treatments (Roux et al. 2000), this does not appear to be case of perinatal hyperoxia, because the increase in minute ventilation produced by hypoxia in control and perinatally hyperoxic rats was due mostly to an increase in respiratory frequency in both groups of animals (Ling et al. 1996, 1997a). The protocols for hypoxic stimulation while recording ventilatory frequency shall be described with the results.
Surgical procedures
Rats were anaesthetized with Na-pentobarbital (60 mg kg−1, i.p.) and the carotid arteries were dissected past the carotid bifurcation. In selected animals the CSN was identified under a dissecting microscope and a block of tissue, including the carotid bifurcation and the glossopharyngeal nerve, was removed and placed in a lucite chamber filled with ice-cold/100% O2-equilibrated Tyrode (140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm Hepes and 5 mm glucose) for further dissection of tissue surrounding the CB and the CSN. In other experiments the CBs were cleaned free of the CSN and nearby connective tissue. Animals were killed by an intracardiac overdose of Na-pentobarbital. The Institutional Committee of the University of Valladolid for Animal Care and Use approved the protocols.
Tyrosine hydroxylase immunostaining and morphometry of the CB sections
Four control and four hyperoxic rats were perfused by gravity (1 m column) through the left ventricle. Perfusion consisted of 150 ml of phosphate-buffered saline (10 mm; pH = 7.40; PBS) at 37°C, followed by 250 ml of 4% (v/v) paraformaldehyde in 0.1 m phosphate buffer (PB; pH = 7.40) at 4°C. The carotid bifurcations were removed and the CB cleaned of surrounding tissues. CBs were postfixed for 1 h in 4% paraformaldehyde in PB and transferred to 30% (w/v) sucrose in PB for cryoprotection. After embedding in Tissue-® (Sakura Finetek, Zoeterwoude, The Netherlands) the CBs were frozen at –20°C. Serial sections 10 μm thick were obtained in a Leitz Cryostat (1720) and collected in glass slides coated with 3-aminopropyltriethoxy-silane (Sigma, Spain). The sections were washed in PBS at room temperature (five minutes), and incubated in PBS containing 0.1% (v/v) Triton X-100 and 2% (v/v) nonimmunized goat serum (permeabilizing-blocking solution) for 30 min. Incubation with the primary antibody (mouse anti-tyrosine hydroxylase; Abcam, Cambridge, UK) at a dilution of 1: 1000 in permeabilizing-blocking solution was carried out for one hour at room temperature. After extensive washing with PBS (three times 10 min), the sections were incubated with secondary antibody (goat anti-mouse–FITC; Sigma, Spain) at 1: 1000 in blocking solution for one hour at room temperature. Finally, the sections were washed with PBS (four times five minutes) and distilled water, and mounted in an aqueous-base mounting medium (Vectashil, Vector Laboratories). Negative controls were similarly incubated but in the absence of primary antibody.
Sections were examined with a fluorescence microscope (Axioscop 50, Zeiss) equipped with excitation and emission filters for FITC. Images (10×; Phan-Neofluor) were captured with a CoolSnap camera and analysed using Spot 3.4.2. Tyrosine hydroxylase-positive areas and the entire area of the CB tissue in each section were measured and used to calculate the percentage area immunopositive to the enzyme.
Recording of the activity in the CSN and voltammetric measurement of the release of CA
The CB-CSN preparation was transferred to a perfusion chamber mounted on the stage of a dissecting microscope (Leitz) and superfused (35°C) with bicarbonate/CO2-buffered saline (120 mm NaCl, 3 mm KCl, 2 mm CaCl2, 1 mm Na2HPO4, 1 mm MgSO4, 24 mm NaHCO3 and 10 mm glucose). Single fibre to whole CSN recordings were made with a suction electrode. The pipette potential was amplified (Neurolog Digitimer, Hertfordshire, England), displayed on an oscilloscope and stored on computer (2 kHz acquisition rate; Datasponge, WPI, Berlin). Chemoreceptor activity was identified (spontaneous generation of action potentials at irregular intervals) and confirmed by its increase to hypoxia (normoxia, perfusate equilibrated with 20% O2/5% CO2 (v/v); hypoxia, 0% or 5% O2/5% CO2 (v/v); balance N2). Chamber PO2 was recorded with an electrode polarized to –0.8 V against an Ag/AgCl ground electrode and stored in the computer with the raw nerve signal. CSN activity was converted to logic pulses, summed every second and converted to a voltage proportional to the sum.
Free tissue CA concentrations were measured using a carbon fibre electrode inserted into the CB tissue as described by Rigual et al. (2000). The electrode was a 5 μm carbon fibre insulated (apart from the tip) with a polyethylene tube (ProCFE, Dagan Instruments, Minneapolis, MN, USA). The electrode was attached to an EI-400 potentiostat (Ensman, Bloomington, IN, USA) with a potential at +200 mV. Calibration was made with DA concentrations in the recording chamber between 0 and 5 μm. The activity of the CSN and the levels of free tissue CA were measured in normoxia and in hypoxia. The hypoxic response in the CSN is expressed as Δ frequency (peak – basal normoxic frequency in Hz) and the release of CA is expressed as free tissue CA concentration.
Labeling of CA stores: synthesis and release of 3H-CA
The CA stores of the CBs were labelled by incubating the organs in small glass vials (4–8 CBs per vial for two hours) containing 0.5 ml of 100% O2-pre-equilibrated Tyrode solution and placed in a shaker bath at 37°C. The incubating solution contained 3H-tyrosine (30 μm; specific activities of 6 and 48 Ci mmol−1 for the synthesis and release experiments, respectively). The incubating solution also contained 100 μm 6-methyl-tetrahydropterine and 1 mm ascorbic acid, cofactors for tyrosine hydroxylase and dopamine-β-hydroxylase, respectively (Nagatsu 1973; Fidone & Gonzalez 1982).
In the synthesis experiments, at the end of the incubation the CBs were transferred (five minutes) to vials containing 10 ml of ice-cold precursor-free Tyrode solution to wash out the 3H-tyrosine present in the extracellular space. Afterwards the CBs were transferred to cold eppendorf tubes containing 75 μl of 0.4 m perchloric acid. After weighing the CBs in an electrobalance (Supermicro, Sartorius, Pacisa, Madrid), they were glass-to-glass homogenized at 0–4°C. The homogenates plus an aliquot of 50 μl of 0.4 m perchloric acid (used to collect quantitatively the tissue homogenate) were centrifuged for 10 min, 0–4°C at 12.000 g, in a microfuge (Beckmann, Madrid). 3H-CA synthesized and free 3H-tyrosine present in the tissues were measured in the supernatants.
In the release experiments, following incubation to label 3H-CA stores, each CB was transferred to a scintillation glass vial containing 4 ml of precursor-free Tyrode bicarbonate solution (composition as above except for the substitution of 24 mm NaCl by 24 mm NaHCO3). Vials were kept in a shaker bath at 37°C for the entire experiment. Solutions were continuously bubbled with 20% O2/5% CO2/75% N2 (v/v/v) saturated with water vapor, except during hypoxic stimulation (see Results). During the first hour, the incubating solutions were renewed every 20 min and discarded. Thereafter, solutions were collected every 10 min for analysis of 3H-CA content. Hypoxic and depolarizing stimuli consisted of 10 min incubations with low PO2-equilibrated and high K+-containing solutions. Collected solutions were acidified with glacial acetic acid to pH = 3 and maintained at 4°C to prevent any degradation of 3H-CA until analysis. At the end of the experiments, CB tissues were prepared for analysis of 3H-CA content.
Analytical procedures
Free 3H-tyrosine accumulated by the CBs during the period of synthesis was calculated from the difference between total radioactivity present in an aliquot of the tissue supernatants and radioactivity in the form of 3H-CA. For the analysis of endogenous unlabelled and 3H-labelled CA in the tissue extracts, aliquots (10–50 μl) of the supernatants were injected directly into the HPLC system. The HPLC system was composed of a Milton Roy CM 400 pump, a Waters C18 column (particle size 4 μm), a Waters U6K injector, and a Bioanalytical Systems LC-4 A electrochemical detector (set at a holding potential of 0.75 mV and a sensitivity of 1–5 nA). The signal was fed to an analogue-to-digital converter controlled by Peak Sample Chromatography System software (Buck Scientific, East Northwalk, CT, USA). Identification and quantification of endogenous CA in tissue samples were verified with external standards. Identification of 3H-catechols in CBs labelled with 3H-tyrosine was also verified with external standards; quantification was made by minute-to-minute collection of the HPLC column effluent and scintillation counting. The analysis of 3H-catechols present in the collected incubating solutions included adsorption to alumina (100 mg) at pH = 8.6, the washing of alumina with distilled water, bulk elution of all catechols with 1 ml of 1 m HCl and scintillation counting.
Chemoreceptor cell culture and intracellular Ca2+ measurements
Cleaned CBs were enzymatically dispersed, and dissociated cells were plated on poly l-lysine-coated coverslips maintained in culture for up to 48 h as described previously (Perez-Garcia et al. 1992). Coverslips were incubated with 10 μm fura-2 AM (Molecular Probes) diluted in Tyrode-Hepes solution with 0.1% (v/v) Pluronic F-127 (Molecular Probes) at 20°C for 60 min. After fura-2 loading the culture coverslips were mounted in a perfusion chamber, placed on the stage of a Nikon Diaphot 300 inverted microscope and the cells superfused with a solution containing 116 mm NaCl, 5 mm KCl, 1.1 mm MgCl2, 2 mm CaCl2, 25 mm NaCO3H, 10 mm glucose, 10 mm Hepes (pH 7.4 bubbling with 5% CO2/20% O2/75% N2, v/v/v). The temperature was maintained at 37°C. Dual-wavelength measurements of fura-2 fluorescence were performed, using the two-way wavelength illumination system DX-1000 (Solamere Technology Group, Salt Lake City, Utah). A 100 W Hg lamp was used as the light source (Optiquip, New York, NY). Light was focused and collected through a Nikon Fluor 40/1.30 objective. The wavelength for dye excitation was alternated between 340 and 380 nm, and fluorescence emission at 540 nm was collected with a SensiCam digital camera (PCO CCD imaging, Kelheim, Germany). Four-by-four binning was applied to obtain ratio images of 320 × 256 pixels (12 bits per pixel) at 0.5 Hz. The illumination system and the camera were driven by an Axon Imaging Workbench 4.0 (Axon Instruments, CA, USA), using a Pentium computer. Calcium was computed offline through the ratio images obtained from the background, subtracted f340 and f380 images and calibration parameters measured in selected experiments (after perfusing the cells for 30 min with zero Ca2+ plus 10 mm EGTA, or with 2 mm Ca2+-containing solutions in the presence of 50 μm ionomycin). In all the experiments, hypoxia and 40 mm KCl were used as stimuli. At the end of the experiment, cells were fixed with 2% (v/v) paraformaldehyde for 15 min at 20°C, washed in permeabilizing solution and blocked with permeabilizing-blocking solution for 10 min. Antibodies to tyrosine hydroxylase (TH) were diluted in blocking solution and incubated with the cells for 30 min. After several washes in permeabilizing solution, cells were incubated with FITC-conjugated goat anti-mouse secondary antibodies for 30 min. After washing with PBS, TH-labelling was examined with the appropriate set of filters.
Statistics
Data are expressed as mean ±s.e.m. and compared for statistical significance using a two tails Student t-test for unpaired data. Significance level was established at P < 0.05.
Results
Animals and their basic blood parameters
Even though at delivery the body size appeared comparable in baby rats of both control and hyperoxic litters, at the time of weaning hyperoxic animals were smaller, with a size about half of that of the control animals. However, by the time of the experiments (3.5–4.5 months of age), hyperoxic animals were closer in size to controls, but still significantly smaller. Mean ±s.e.m. weight of 41 control male rats at the time of the experiments was 328 ± 9 g and that of 36 hyperoxic age-matched males was 292 ± 10 g (P < 0.01); the mean weight of 41 control female was 227 ± 5 g and that of hyperoxic age-matched females was 200 ± 5 g (P < 0.001). Blood samples were withdrawn from a group of 24 control and 16 hyperoxic rats of both sexes to determine basic blood parameters including counts of red and white cells, platelets, haemoglobin concentration, and haematocrit. As shown in Table 1, no differences were observed between the two groups.
Table 1.
Basic blood parameters in control and hyperoxic animals
| Blood parameter | Control (n= 24) | Hyperoxic (n= 16) | Units |
|---|---|---|---|
| Red blood cells | 7.76 ± 0.15 | 7.64 ± 0.20 | ×106μl−1 |
| Haemoglobin | 14.56 ± 0.19 | 14.52 ± 0.25 | g dl−1 |
| Haematocrit | 43.35 ± 0.56 | 44.02 ± 0.97 | percentage |
| White blood cells | 3.32 ± 0.39 | 3.08 ± 0.37 | ×103μl−1 |
| Platelets | 845.0 ± 51.6 | 854.7 ± 63.4 | ×103μl−1 |
Respiratory frequency responses to hypoxia in freely moving animals
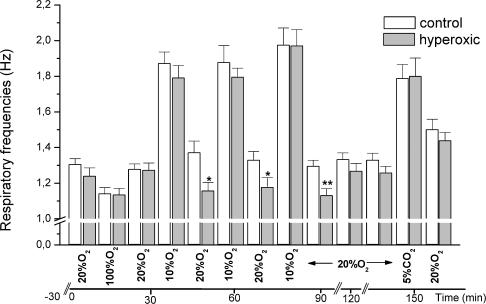
To assess the ventilatory response to hypoxia we made a continuous recording of the respiratory frequency while switching from air to the desired gas mixture (Fig. 1). Sixteen control and 16 hyperoxic animals (eight males and eight females) were used in these experiments. Basal (room air) respiratory frequency is not statistically different in control and hyperoxic animals. The depression of the respiratory frequency produced while breathing 100% (v/v) O2 was comparable in control (14%) and hyperoxic animals (9%). The evoked increase in ventilatory frequency was identical (44%) in control and hyperoxic animals during the first exposure to 10% (v/v) O2. In two additional exposures to hypoxia there was a small tendency towards sensitization of the response so that by the third hypoxic exposure the increase in ventilatory frequency had reached 52% in control and 60% in hyperoxic animals. After 60 min of recovery a hypercapnic test (breathing 20% O2/5% CO2/75% N2, v/v/v) was applied and again there was no difference in the response between the two groups. The only difference seen between groups was that following each hypoxic test there was a statistically significant depression of breathing in hyperoxic animals, representing a decrease in ventilatory frequency between 16 and 19% (P < 0.05 for the first and second posthypoxic period and P < 0.01 in the third posthypoxic period). It should be emphasized that the 10 min duration of the hypoxic tests used in our study is sufficient to fully activate the response of the CB chemoreceptors to hypoxia. CB-mediated hypoxic ventilation develops promptly, in less than five minutes. Limiting the observations to 10 min of hypoxia should avoid or minimize the unwanted complicating effects of longer hypoxic exposures such as hypothermia and hypometabolism (Nakano et al. 2001; Barros & Branco 2002).
Figure 1. Modification of the respiratory frequencies induced by hyperoxia, hypoxia and hypercapnia in control and perinatally hyperoxic animals.
The animals were individually caged in the chambers of the plethysmograph that were flushed sequentially with the gas mixtures shown at the bottom of the graph while recording respiratory frequencies. The columns represent mean ±s.e.m. of 16 individual data. *P < 0.05; **P < 0.01.
The normal increase in respiratory frequency in response to hypoxia in hyperoxic animals prompted the experiments that follow. Their purpose was to search for mechanisms capable of explaining how an apparently hypotrophic CB–CSN complex (Erickson et al. 1998) is able to produce an increase in respiratory frequency to hypoxia similar to that of control animals. We started with a basic morphological study of the CB.
The carotid body: basic morphometric data
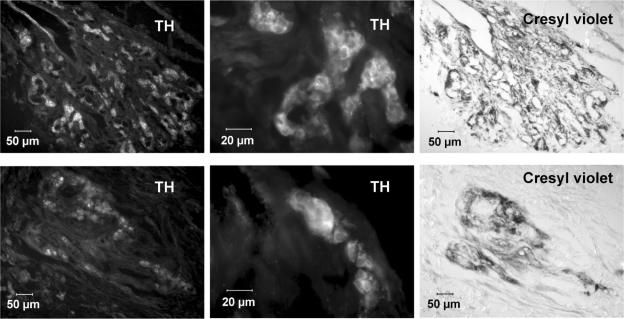
CBs in hyperoxic versus control animals were difficult to locate and dissect due to their small size (hyperoxic: 19.90 ± 1.49 μg, n= 48; normal: 48.97 ± 2.48 μg, n= 69). The size of control CBs in this series of experiments is comparable to that found in previous studies, which ranged between 40 and 60 μg (Gonzalez et al. 1979; Fidone & Gonzalez 1986; Vicario et al. 2000). In contrast, the superior cervical ganglion of hyperoxic animals was only 12% smaller than the organs of control animals, a difference that is comparable to the percent decrease in total body weight. Consistent with this marked difference in the weight of CBs, sections of control and hyperoxic organs close to their equatorial plane showed marked differences in size (Fig. 2). The number of sections as well as the mean size of the sections was dramatically reduced in hyperoxic versus control CBs, but the percentage of section area positively stained for TH was reduced by only ∼15% (Table 2). This finding indicates that hyperoxia has provoked a long-lasting general atrophy of the CB. No obvious differences were noticed in the apparent intensity of the immunostaining for TH in control versus hyperoxic animals (Fig. 2).
Figure 2. Immunostaining of central portion CB sections for TH with cresyl violet counterstaining.
Upper row, sections from a control CB. Lower row, sections from a hyperoxic CB. Both sections were immunostained for TH, the images recorded, then the sections were counterstained with cresyl violet to reveal the general histology, particularly the lumen of the capillaries. The middle sections correspond to magnified areas, demonstrating the intensity and localization of the immunostaining in type I cell cytoplasm.
Table 2.
Basic morphometric data of carotid bodles obtained from control and hyperoxic animals
| No. of sections (10 μm CB−1) | Mean area of sections (10 μm2/10−4) | Tyrosine hydroxylase-positive area (% total) | |
|---|---|---|---|
| Control carotid bodies (n= 2) | 45.0 ± 4.0 | 10.3 ± 0.8 | 25.1 ± 0.8 |
| Hyperoxic carotid bodies (n= 4) | 12.0 ± 2.9 | 2.2 ± 0.3 | 21.4 ± 1.7 |
| (P < 0.05) | (P < 0.01) | (P < 0.01) |
Content, synthesis and turnover of CA in normal and hyperoxic CBs
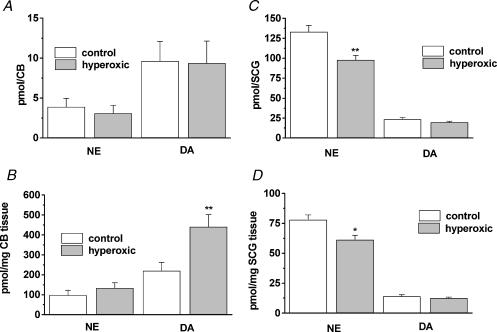
Following hyperoxia it has been reported a marked hypofunctionality of the CB that was evidenced by a null or minimal CSN increase in action potential frequency in response to hypoxia or asphyxia (Eden & Hanson 1986; Ling et al. 1997a). As there is a well established parallel between CSN activity and the catecholaminergic properties of chemoreceptor cells (Fidone et al. 1982; Gonzalez et al. 1992, 1994) we have explored the possible involvement of altered CA metabolism in CB hypofunctionality following hyperoxia. Figure 3A,B shows endogenous CA content in the 14 control and 14 hyperoxic CBs expressed as pmol per organ and as pmol (mg tissue)−1. When expressed per organ, the CA levels were not statistically different between groups (Fig. 3A). However, in the smaller hyperoxic CBs, CA levels per unit weight are significantly larger [hyperoxic: 441.19 ± 60.18 pmol (mg CB tissue)−1; control: 220.70 ± 40.54 pmol (mg CB tissue)−1; P < 0.01]. NE levels per mg of CB tissue were 98.62 ± 23.96 and 134.10 ± 26.64 (P > 0.05) in control and hyperoxic organs, respectively. For comparative purposes we also measured CA levels in the superior cervical ganglia of nine control and eight hyperoxic animals and expressed the contents by organ and by unit weight (Fig. 3C,D). NE levels were ∼25% smaller in hyperoxic than in control animals expressed by organ (P < 0.01) or unit weight (P < 0.05); DA levels were not statistically different.
Figure 3. Content of catecholamines in CB and superior cervical ganglia of control and hyperoxic animals.
A and B correspond to CB levels of dopamine (DA) and norepinephrine (NE) expressed, respectively, as pmol per CB and as pmol per mg tissue. C and D, DA and NE levels in superior cervical ganglion (SCG). Data are mean ±s.e.m. of 14 control and 14 hyperoxic CB, and nine control and eight hyperoxic SCG. *P < 0.05; **P < 0.01.
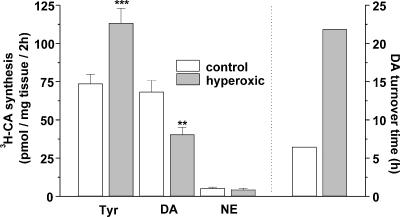
Figure 4 shows the rate of 3H-CA synthesis from the natural precursor 3H-tyrosine in CBs of control and hyperoxic animals. Hyperoxic CBs accumulated a significantly higher amount of free tyrosine in the intracellular space than control organs (P < 0.001). However, in spite of the higher concentration of tyrosine in the hyperoxic CBs their rate of 3H-DA synthesis was markedly reduced from 68.29 ± 7.54 (n= 30) in control organs to 40.41 ± 4.73 (n= 28) pmol (mg tissue)−1 in hyperoxic group (P < 0.01). There were no statistically significant differences in the rate of 3H-NE synthesis. However, the ratio 3H-DA/3H-NE was 12.64 in control CB and 9.22 in hyperoxic CB, implying a relative ∼25% faster turnover of NE in the CB of hyperoxic than in control animals. The calculated turnover time for DA [(pmol DA content)(mg tissue)−1/(pmol 3H-DA synthesis) hour−1 (mg tissue)−1], is nearly four times larger in hyperoxic than in control CB (21.84 versus 6.26 h; Fig. 4 right axis), implying a much smaller utilization of this putative neurotransmitter in the hyperoxic organs. In the initial characterization of the metabolism of CA in the rat CB (Vicario et al. 2000), the turnover time reported for control CBs was a comparable 5.8 h.
Figure 4. Rates of 3H-CA synthesis from the natural precursor 3H-tyrosine in CBs from control and hyperoxic rats.
The figure also shows the intracellular accumulation of free 3H-tyrosine. Data are expressed as pmol of 3H-DA and 3H-NE synthesized per mg of CB tissue in two hours. Data are mean ±s.e.m. and were obtained in 30 CBs from control animals and 28 CBs from hyperoxic animals. The right axis represents the calculated turnover time for DA in the control and hyperoxic CBs. The turnover time was calculated by dividing the DA content in the organ (data from Fig. 3; pmol per mg tissue) by the rate of synthesis (this figure, pmol per mg tissue per hour). **P < 0.01; ***P < 0.001.
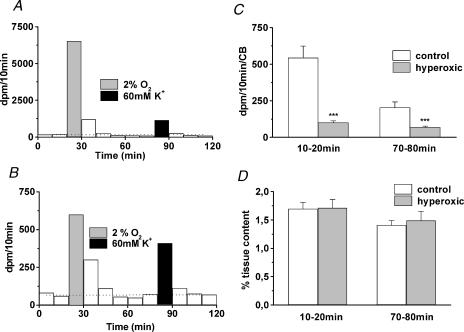
Release of 3H-CA in normoxia, hypoxia and high external K+
The release of 3H-CA in basal normoxic conditions (e.g. immediately prior to stimulation) represents a relative index of the turnover of CA. It should bear a close correspondence with the turnover parameters measured in the experiments of synthesis that described above. Therefore the quantification of the basal release is in itself an important parameter. Figure 5A,B shows a typical release experiment performed with a control and a hyperoxic CB, respectively. The basal or normoxic release throughout the experiment is represented by the horizontal dotted line across the histogram, and the evoked release is above this dotted line. In the samples collected after the application of the stimuli the release is above the basal level, due to washout of the 3H-CA released during the stimulus plus the 3H-catechol catabolites leaving the tissue more slowly (Fidone et al. 1982; Gonzalez et al. 1987). From the different scales in Fig. 5A,B it is evident that the control CB releases more 3H-CA than the hyperoxic one, both in normoxic conditions and in response to the stimuli. Figure 5C,D shows mean basal or normoxic 3H-CA release obtained in 27 control and 27 hyperoxic CB determined in the interval from 10 to 20 min and 70 to 80 min of the experiments. In Fig. 5C the data are expressed as absolute amount released per CB (d.p.m. per CB per 10 min) and in Fig. 5D as percentage of tissue content per 10 min. The lower turnover rate for DA estimated in the previous group of experiments corresponds to the lower rate of absolute 3H-CA release in basal conditions in CB of hyperoxic animals in comparison to CB of control animals (101 ± 11 and 69 ± 7 d.p.m. per 10 min per CB versus 544 ± 80 and 304 ± 39 d.p.m. per 10 min per CB at 10–20 and 70–80 min, respectively). Thus, each control CB synthesized an average 3.61 pmol 3H-CA per CB per two hours (73.7 pmol 3H-CA per mg tissue per two hours multiplied by the mean control CB weight 0.04897 mg) and each hyperoxic CB synthesized 0.89 pmol 3H-CA CB per two hours (44.72 pmol 3H-CA per mg tissue per two hours multiplied by the mean control CB weight 0.0199 mg). Consequently each control CB should release, as was the case, about four times more 3H-CA than hyperoxic organs. However, when the release is expressed as percentage of tissue content the release is, as expected, identical (see Discussion).
Figure 5. Typical release experiments in a control and a hyperoxic CB and mean normoxic release obtained in the entire population of control and hyperoxic CBs studied.
A, release of 3H-CA from a control CB in basal or normoxic conditions (white bars) and during hypoxic (grey bars) and high external K+ stimulation (black bars). Normoxic release throughout the experiment is represented by the dotted line crossing the bars. Evoked release, above the dotted line. The increased release in the 10 min fractions collected following stimulation represents the washout of the 3H-CA released during the stimulus plus their 3H-catechol catabolites. B, an identical experiment for a CB from a hyperoxic animal. Note the different scales in A and B. C, mean ±s.e.m. basal release obtained in 27 control CBs and in 27 hyperoxic CBs immediately prior to hypoxic (10–20 min fraction) and to high K+ (80–90 min fraction) stimulation; data are expressed in d.p.m. per 10 min per CB. D, the same data shown in C but expressed as percentage of tissue content (i.e, fraction of the total 3H-CA in the CB that are released in 10 min). ***P < 0.001.
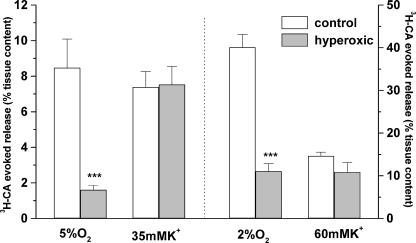
Figure 6 shows the mean evoked release elicited by hypoxia and high external K+ in groups of CBs obtained from control and hyperoxic animals. The left part of the figure corresponds to the release observed in response to stimuli of moderate intensity and middle right of the figure to the responses to intense stimuli. The release of 3H-CA induced by moderate hypoxia in CBs from control animals represented an 8.47 ± 1.6% (n= 15) of the total tissue content, and in the CBs from hyperoxic animals it was 1.61 ± 0.24% (n= 13) of the total tissue 3H-CA content. This implies a percentage decrease in the fractional release of 3H-CA in hyperoxic versus control CBs of 81% (P < 0.001). However, because 3H-CA synthesized in hyperoxic CBs was already reduced by 40%, the absolute amount of 3H-CA released from hyperoxic CBs represents only 11% of that released from control organs. In contrast, the fractional release induced by a nonspecific depolarizing stimulus (35 mm extracellular K+) was identical in control and hyperoxic CBs. A statistically identical result was obtained with the strong hypoxic and depolarizing stimuli. The percentage decrease in the fractional release of 3H-CA in hyperoxic versus control CBs in response to the intense hypoxic stimulus was 74% (P < 0.001). The fractional release in response to 60 mm K+ was not statistically different in control versus hyperoxic CBs.
Figure 6. Release of 3H-CA induced by hypoxia and high external K+ by CBs from control and hyperoxic rats.
The middle left of the figure shows the evoked release of 3H-CA elicited by a hypoxic stimulus and a depolarizing stimulus of moderate intensity (incubation of the CBs during 10 min in solutions equilibrated with 5% O2 or containing 35 mm K+). The middle right of the figure shows the responses to stimuli of higher intensity (2% O2 equilibrated or containing 60 mm K+). In both cases the evoked 3H-CA release is expressed as percentage of CB tissue content. Data are means ±s.e.m. (n= 13–15 for moderate stimuli and 10–11 for intense stimuli). ***P < 0.001.
The above results could be satisfactorily explained if CBs from hyperoxic animals were heterogeneous, with some chemoreceptor cells responding normally or near normally to hypoxia and other cells being poorly responsive to the natural hypoxic stimulus. We explored this possibility with two types of experiments: (1) in intact CB-CSN preparations we recorded action potential frequency in the nerve and free tissue CA concentration in response to hypoxia and to high external K+ and (2) in freshly dissociated chemoreceptor cells we monitored free [Ca2+]1 rises in response to hypoxia and high external K+.
CSN activity and the release of endogenous CA
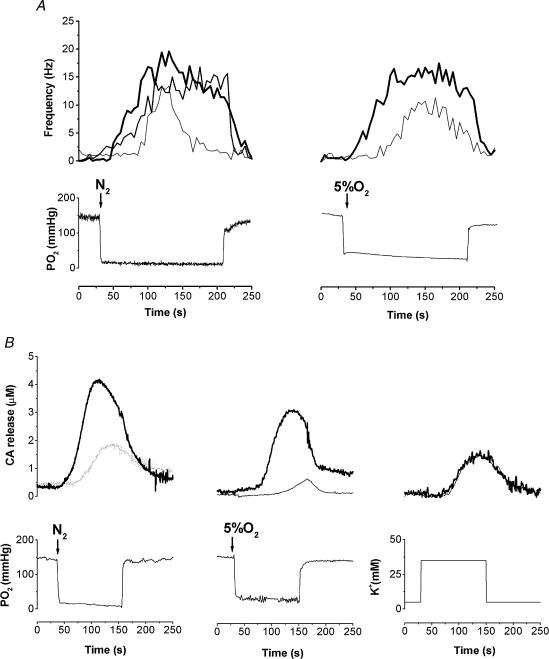
The recording of the electrical activity in the CSN was much more difficult in the preparations coming from hyperoxic than from control rats. Thus, with a hypoxic stimulus of moderate intensity (5% O2; PO2∼35 mmHg in the recording chamber) we were successful in recording CSN hypoxic response in five out of 28 preparations (18%) and when using a strong hypoxic stimulus [N2+ 5% (v/v) CO2-equilibrated solutions; PO2≈10–12 mmHg] we were successful in six out of 29 preparations (21%). In the preparations from control animals the success was approximately 80–85% for both stimuli. However, in the preparations from hyperoxic animals that responded to hypoxia, although the responses tended to have a later onset and to adapt sooner than in control preparations (Fig. 7A), the Δfrequency (peak hypoxic activity – basal activity) was not statistically different from control. With the strong hypoxic stimulus the peak responses in the hyperoxic preparations were 69.4 ± 17.5% of the control responses (100.0 ± 10.7%; n.s.).
Figure 7. Sample recordings of CSN action potential frequency in response to hypoxia and CB endogenous CA release elicited by hypoxia and high external K+ obtained in CBs from control and hyperoxic animals.
A, action potential frequency (Hz) response to N2- and 5% O2-equilibrated solutions in control and hyperoxic CB–CSN preparations (thick continuous traces, control; thin traces, hyperoxic). B, endogenous CA release responses to hypoxia and high external K+ in preparations from control (thick traces) and hyperoxic animals (thin traces); traces correspond to free CB tissue concentrations in μm. Lower traces from A and B correspond to the PO2 recordings, except for the last record that indicates the K+ concentration in the perfusing solutions.
The voltammetric recording of endogenous CA release induced by hypoxia was also more difficult in preparations from hyperoxic than from control animals. Using the moderate hypoxic stimulus we were able to record a release response in two out of 12 hyperoxic preparations (17%) and in 17 out of 18 control preparations (94%). The rate of success increased with the strong hypoxic stimulus to 55% (10 out of 18 preparations) but the release response in the successful preparations was statistically smaller in hyperoxic than in control preparations (46.0 ± 20.4%; n= 10; P < 0.05; Fig. 7B). In contrast to hypoxic stimuli, the response to high external K+ was comparable in all regards in control and hyperoxic CBs (Fig. 7B).
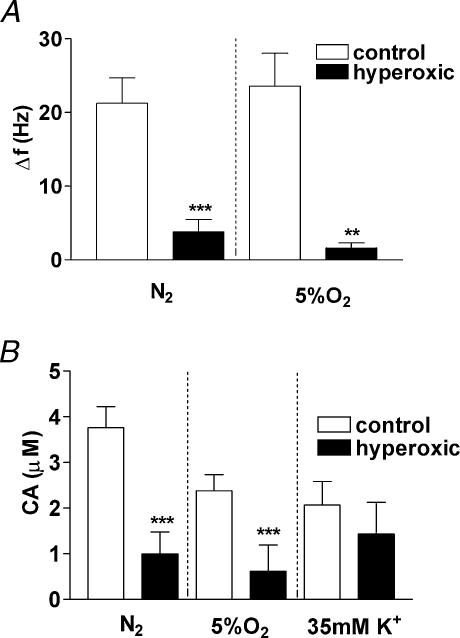
In Fig. 8 mean CSN and endogenous CA release responses are shown, including all experimental preparations whether or not they responded. As whole populations, the level of CSN activity and CA release were markedly reduced to less than 25% of the responses seen in control preparations (P < 0.001 in all cases, except for the release to 5% O2 where P < 0.01). Again, in contrast to hypoxic stimuli, the rate of success recording the endogenous CA release response to high external K+ (35 mm) was comparable in the controls (92%; 22 out of 24) and hyperoxic preparations (83%; 10 out of 12) and the amplitude of the responses was not statistically different (Fig. 8B).
Figure 8. Responses to hypoxia and high external K+ in CB-CSN preparations from control and hyperoxic animals.
A, Δ frequencies (Hz; peak discharge during stimulation minus basal discharge prior to stimulation) while perfusing with N2 and 5% O2-equilibrated solutions. The data are mean ±s.e.m.; n= 32 and 29 for N2, control (white bars) and hyperoxic (black bars), respectively, and 22 and 28 for 5% (v/v) O2 control (white bars) and hyperoxic (black bars). B, mean CA concentrations (μm) registered in the CB tissue from control (white bars) and hyperoxic animals (black bars). For N2, n= 28 (control) and 18 (hyperoxic); for 5% (v/v) O2, n= 18 and 12 for control and hyperoxic CBs, respectively. For 35 mm K+n= 24 and 12 for control and hyperoxic CBs, respectively.
Effect of hypoxia and high external K+ on intracellular Ca2+ in dissociated chemoreceptor cells
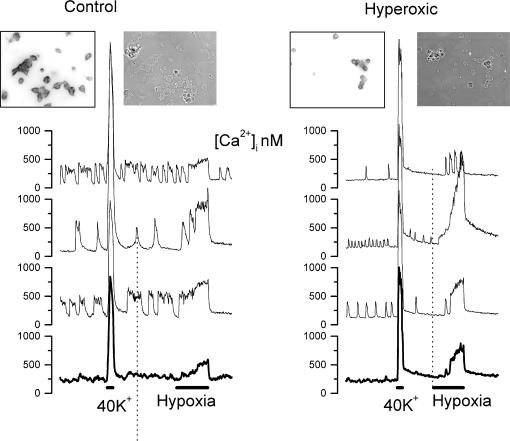
Tissue cultures with viable TH-positive chemoreceptor cells were also more difficult to obtain from CBs of hyperoxic animals compared with chemosensory organs from control animals. Due to their smaller size, usually six-to-eight hyperoxic CBs were used instead of the four that we normally dissociated to obtain four-to-six coverslips; yet the density of the cells was much lower than normal. Basal intracellular free Ca2+ concentration was not observed to be different in TH-positive cells obtained from control versus hyperoxic CBs, and diverse patterns of oscillations (difficult to analyse) were seen in both groups of cells. All TH-positive cells from both control and hyperoxic CBs, when challenged with a 30 s pulse of 40 mm K+, responded with a brisk and intense increase in [Ca2+]1 which reached comparable peaks, followed an almost identical time course and exhibited an overall similar integrated amplitude (Figs 9 and 10). In cultures from both control and hyperoxic CBs there were TH-positive cells that when challenged with a hypoxic pulse did not respond with an increase in [Ca2+]1; in control cultures a response was obtained in 85% of the cells, and in cultures from hyperoxic CBs only 58% responded. Due to this lower rate of response we examined a greater number of cultures from experimental versus control animals (eight versus four). Figure 9 shows sample images and responses obtained in a standard control culture and in a selected hyperoxic culture with a high density of cells. The responses of chemoreceptor cells from control CBs were uniform in shape, although their amplitudes were variable from cell to cell; the responses from the hyperoxic cultures were not uniform in shape nor in amplitude, and the onset of the response was slower and the time to reach peak larger. However, the overall amplitude of the integrated response was comparable (Fig. 10).
Figure 9. Intracellular Ca2+ responses to high external K+ and to hypoxia in chemoreceptor cells isolated from control and hyperoxic CBs.
Left part shows sample recordings obtained in three chemoreceptor cells of the microscope field (insets) and the lower trace is the response obtained in the entire field. Note the oscillatory behaviour of the intracellular Ca2+ levels in normoxia, the brisk increase in response to a short (30 s) pulse perfusion with 40 mm K+ and the moderate sustained increase elicited by perfusion with a hypoxic solution (PO2≈10 mmHg; 180 s). Right part shows an identical set of recordings obtained in a CB culture from hyperoxic animals. Note the greater variability of the response to hypoxia and the apparent slower onset of the response to hypoxia. The insets show phase contrast and tyrosine hydroxylase immunostaining of the dissociated CB tissue culture.
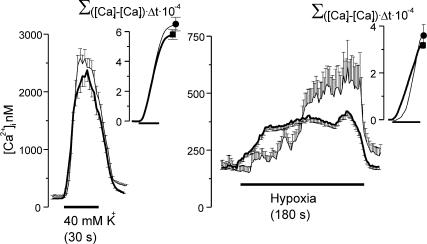
Figure 10. Integrated intracellular calcium responses to hypoxia and high external K+ in chemoreceptor cells isolated form control and hyperoxic animals.
Left and right: the mean time course of the intracellular Ca2+ response elicited by high external K+ (40 mm, 30 s) and hypoxia (PO2≈10 mmHg, 180 s), respectively. Chemoreceptor cells from CB of control animals (thick traces) and hyperoxic animals (thin traces) are shown. The inset shows the accumulative Ca2+ response: Σ([Ca]t–[ca]r) × Δt × 10-4 where t is time and r is the resting Ca2+ level. Data are mean ±s.e.m. of 158 control and 43 hyperoxic cells.
Discussion
In the present work we have evaluated the effects of perinatal exposure to hyperoxia (one week in utero and the first postnatal month; 55–60% O2) on the ventilatory response to hypoxia and hypercapnia and on the structure and function of the adult CB. Our main findings include the following: (1) The ventilatory frequency response elicited by hypoxia and hypercapnia is not different in control and hyperoxic animals. (2) The CB of the hyperoxic animals is hypotrophic, weighing about half of control organs, although the percentage of CB tissue representing chemoreceptor cells is near normal. (3) The concentration of DA (pmol per mg tissue) in hyperoxic CBs nearly doubles that of control organs, but their rate of 3H-DA synthesis (pmol per mg tissue per hour) from the natural precursor 3H-tyrosine is reduced by nearly 45%. The higher DA content combined with the lower turnover rate (turnover rate =3H-DA synthesis) indicate that the turnover time for DA in the CBs from hyperoxic animals is increased by a factor of around four. (4) Consistent with a slower DA turnover, the absolute basal release of newly synthesized 3H-CA is four-to-five times smaller in hyperoxic than in control animals. (5) The release of 3H-CA (normalized as percentage of the 3H-CA content in the tissues) induced by moderate and intense hypoxia is reduced by 75–80%, but the release elicited by high external K+ is not different in CBs from control and hyperoxic animals. (6) When monitoring the electrical activity in the intact CB–CSN preparations, only a small percentage of preparations from hyperoxic animals responded to hypoxia, but the amplitude of the responses in functional organs was not statistically different from controls. (7) In the intact CB–CSN preparations the release of endogenous CA elicited by hypoxia is also more difficult to obtain in hyperoxic preparations, and in addition, the magnitude of the responses is significantly reduced. (8) In contrast, the response to high external K+ was obtained in a comparable percentage in intact CB-CSN preparations of control and hyperoxic animals and the magnitude of the response itself is not different. (9) Comparable observations were made when monitoring intracellular Ca2+ levels in chemoreceptor cells freshly dissociated from hyperoxic CB in comparison to control: normal responses to high external K+ in all parameters, including percentage of responding cells, amplitude and time course; and altered responses to hypoxia with a smaller number of responding cells, slower time course of the response, but comparable integrated amplitudes of responses.
Effect of perinatal hyperoxia on ventilatory responses to hypoxia
At the outset of this discussion we want to state that the interpretation of our data on ventilation has the limitation derived from the fact that we have monitored ventilatory frequency, and not minute ventilation, which represents the most correct index of the CB-driven ventilatory response to hypoxia. Also, although we have not seen differences in the increase in ventilatory frequency elicited by hypoxia, it is not possible to exclude differences in tidal volumes, and thereby a reduction in the minute ventilation response to hypoxia in hyperoxic animals. However, as stated above (see Materials and methods section) in normal (Ling et al. 1996; Roux et al. 2000; Fernandez et al. 2003) as well as in hyperoxic rats (Ling et al. 1996) the increase in minute ventilation induced by hypoxia (∼70–87.5% in the experiments of Ling et al. 1996) is primarily due to an increase in respiratory frequency, making this parameter a reasonable index to evaluate hypoxic ventilatory response. In this context, our findings that perinatal hyperoxic treatment does not alter ventilatory frequency agree with the original observations reported by Eden & Hanson (1986). These authors found that chronically hyperoxic rats developed a sustained increase in ventilation in response to hypoxia [8% (v/v) and 12% (v/v) O2, five minutes] by day five, as in normoxic rats after days 5–14. In a subsequent and independent study devoted to explore the effects of chronic perinatal hypoxia, the same authors (Eden & Hanson, 1987b) showed that 8% (v/v) and 12% (v/v) O2 produced a comparable ventilatory response in their control rats, indicating that breathing 12% (v/v) O2 is a stimulus capable of producing a maximal ventilation. Our observations also agree with the findings of Ling et al. (1996) regarding ventilatory responses to hypercapnic stimulus, but there are some apparent discrepancies regarding the responses to hypoxia. Ling et al. (1996) observed that after 20 min of hypoxia, when control and hyperoxic rats have an identical arterial blood PO2 of ∼47.5 mmHg [animals breathing 12.5–14.2% (v/v) O2], hypoxic ventilatory response was markedly reduced in the perinatally treated rats. However, lowering blood PO2 in perinatally treated animals to ∼41.5 mmHg produced ventilatory responses (frequency, tidal volume and minute ventilation) that were statistically identical to those of control animals at ∼47.5 mmHg. After 60 min of hypoxia a comparable reduction in hypoxic ventilatory response was observed at a PO2 of ∼47.5 mmHg; however, with a lower level of arterial PO2 (∼41.5 mmHg), ‘the perinatal-treated rats increased minute ventilation to levels rivaling control rats, although still significantly lower’. They concluded that ventilatory response to hypoxia was attenuated by perinatal hyperoxia, but not abolished, ‘since with a lower arterial PO2 (41.5 mmHg), perinatal-treated rats increased minute ventilation to levels only slightly below control rats at ∼47.5 mmHg’. Nearly identical results were obtained in a subsequent paper (Ling et al. 1997b) measuring phrenic nerve responses to isocapnic hypoxias (arterial blood PO2 40 and 50 mmHg; these two levels of hypoxia were achieved by switching the inspired gas mixture to a level of FIO2 between 11 and 19% for three to five minutes). At 50 mmHg hyperoxic animals exhibited attenuated burst frequencies and integrated phrenic activity as well as minute phrenic activity. At 40 mmHg however, burst frequency and integrated phrenic activity were not different than in control animals, but the product (minute phrenic activity) was statistically smaller. They also noticed that in control animals a hypoxia of PO2 50 mmHg produced responses identical to one of 40 mmHg of PO2, leading them to conclude that both responses are maximal, and that while control animals need a less intense hypoxia to generate maximal responses (50 mmHg), hyperoxic animals only reach maximal responses with the more intense hypoxia (40 mmHg). Considering the levels of hypoxia used by Eden & Hanson (1986) and by the Wisconsin group (Ling et al. 1996, 1997a,b) and the stronger hypoxic level used in our experiments [10% (v/v) O2] it appears that we are measuring maximal increases in respiratory frequency, and therefore the responses are indistinguishable in control versus perinatally hyperoxic animals. In summary, a conclusion that appears to be supported by the studies of Eden & Hanson (1986) and Ling et al. (1996 1997a,b) and by our own findings is that perinatal hyperoxic animals are capable of generating a ventilatory drive to produce maximal or near maximal responses to hypoxia if hypoxic stimuli are sufficiently intense. An additional conclusion based on the original findings of Ling et al. (1996) with nonanaesthetized animals breathing spontaneously (i.e. hypocapnic hypoxia) is that the perinatal hyperoxic treatment produces impaired responses to hypoxic stimuli of moderate intensity. This last conclusion is supported by subsequent studies of the Wisconsin group using anaesthetized animals, isocapnic hypoxia and monitorization of phrenic activity (Ling et al. 1997a,b; Fuller et al. 2001, 2002; Bavis et al. 2002, 2003).
Origin of ventilatory responses to hypoxia and hypercapnia
Because the ventilatory response to CO2 is generated both at the level of the CB and central chemoreceptors (Berkenbosch et al. 1979; Gonzalez et al. 2002a) and there are no differences in the responses to this stimulus following hyperoxia, it would appear that the CO2-sensing mechanism in the CB and central chemoreceptors, as well as the central projections of the CSN and mechanisms of integration of the CB respiratory drive, are functioning properly. An alternative possibility would be that the CB and CB chemoreceptor pathways and integration mechanisms are damaged or altered, but that the central chemoreceptors are exactly compensating the deficit of the peripheral chemoreceptors. However, electrical stimulation of the central stump of the sectioned CSN generates an identical phrenic motor output in control and hyperoxic animals (Ling et al. 1997c), suggesting that peripheral chemoreceptor pathways and central integrating mechanisms are intact. Therefore, any eventual deficit in the ventilatory response to hypoxia can be attributed to a deficit in the O2 chemoreception mechanisms at the level of the CB. Additionally, the normality of the central connections and functioning of the CSN imply that the sensory fibers present in the CSN, and responsible for the genesis of the phrenic responses, are being exposed to a normal level of activity. It is well known that the presence of normal neural activity is essential for the formation and maintenance of a mature system of neural connections (Kaas 1995; Katz & Shatz 1996; Crair 1999; Personius & Balice-Gordon 2000; Penn 2001).
Eden & Hanson (1986) observed that hyperoxic animals developed a ventilatory response to hypoxia by postnatal day five, which was equivalent to normoxic animals at days 5–14. They also found that in six hyperoxic rats of 5–10 weeks of age the CB was totally unresponsive to hypoxia. They concluded that hyperoxia accelerated the maturation of the central nervous component of the response to acute hypoxia, and that the hypoxic ventilatory response observed must have originated outside the CB. In the studies of the Winsconsin group (Ling et al. 1996,1997a,b,c) hyperoxic rats have a parallel reduction in the ventilatory response to hypoxia measured in intact animals and in the output of the phrenic nerve during isocapnic hypoxic stimulation measured in anaesthetized animals that have been previously vagotomized. Because vagotomy produces denervation of the aortic and abdominal chemoreceptors and the phrenic response to isocapnic hypoxia disappeared after sectioning of the CSN, their findings imply that the ventilatory and phrenic response to hypoxia is generated in the CB. Ling et al. (1997a) also found that the CSN response to short asphyxic tests (10 s ventilator off) was detected in only five out 16 hyperoxic preparations with a mean response of approximately 18% (n= 16) of control animals. As this 18% of the response was generated by 30% of preparations (five out of 16) it follows that the responding preparations should have on average a response of ∼60–65%. In our study we found that hyperoxic animals have normal increase in ventilatory frequency in response to hypoxia, a CSN response to hypoxia recorded in only one of five or six preparations, and that in these responding animals the response tends to be smaller than in control animals but not statistically different.
A unitary interpretation for these sets of apparently inconsistent data may be justified. First, the available evidence indicates that the CSN of all animals responds to hypoxia, and the negative results are due almost exclusively to technical difficulties for recording from a hypoplasic CSN with a marked reduction in the number of chemoreceptor fibers (Erickson et al. 1998). These conclusions are based on the observation of Ling et al. (1997b) that the entire population of hyperoxic animals tested exhibited a phrenic nerve response to isocapnic hypoxia that resisted vagotomy and was totally abolished by CSN transection, indicating that the response had been generated in the CB and carried by the CSN. Second, given the rate of success in recording a CSN response to hypoxia (asphyxia), five out of 16 preparations in Ling et al. (1997a) and 11 out of 57 preparations in the present study, it is conceivable that the absence of CSN activity the Eden and Hanson study (1986) is a statistical consequence of the small sample size (six preparations) of their work. Third, from the preceding conclusions it would follow that the reduced number of chemoreceptor units in the CSN of hyperoxic animals are capable (if stimulated with sufficiently intense hypoxia; see above) of generating maximal or near maximal ventilatory responses comparable to those seen in control animals, even if each of the surviving units exhibits a hypoxic response about 60–70% of that found in control animals. This conclusion is not surprising because it is well known that the information travelling in the CSN is redundant. An identical ventilatory response is obtained in several animal species after reducing the brainstem input from the CB by 50% by sectioning one of the CSN (Busch et al. 1983; Vizek et al. 1987; Cragg & Khrisanapant 1994). Additionally, the experiments of Ling et al. (1997a,c) support our conclusion. First, the phrenic response elicited by electrical stimulation of the CSN is identical in control and hyperoxic animals despite the marked reduction in the number of chemosensory fibers in the CSN of the experimental group (Erickson et al. 1998). Second, in both control and hyperoxic animals the electrical stimulation of the CSN at 5 Hz produced above 80% of the phrenic response elicited at the highest frequency of stimulation tested (20 Hz; the phrenic response tends to a plateau at frequencies of 10 and 20 Hz). The incapacity of the CBs of hyperoxic animals to generate normal ventilatory responses at submaximal intensities of hypoxic stimulation would imply, as suggested by Ling et al. (1996,1997b; see above also), that the surviving units have a shifted sensitivity to hypoxia. In fact, the smaller slopes of the CSN response (Fig. 7) and of Ca2+ increase in chemoreceptor cells (Fig. 10) during stimulation with hypoxia are fully compatible with this shift in the sensitivity to hypoxia.
Effect of perinatal hyperoxia on CA metabolism in carotid body
Our data on CA metabolism support the interpretation given above. The CBs of the hyperoxic animals contain high concentrations of CA, and a low rate of 3H-CA synthesis and release in normoxic conditions, implying a slow utilization of CA by chemoreceptor cells. Previous studies have established a strong correlation between the level of CB stimulation, CA utilization and chemoreceptor activity in the CSN (Hanbauer & Hellstrom 1978; Fidone et al. 1982; see Gonzalez et al. 1992, 1994). Thus, low utilization of CA implies that activity in chemoreceptor cells is much lower in the CB of hyperoxic versus control animals in normoxic conditions. However, a decrease in the metabolic rate of CA in the intact CB can be the result of (1) a generalized lower activity in the entire population of chemoreceptor cells in the CBs of hyperoxic animals in comparison with control animals, or (2) also due to a heterogeneous situation with some chemoreceptor cells having an activity comparable to that of normal animals and other cells having a much lower activity. Considering the arrangement of the CB in functional units or glomoids (De Castro 1940; Seidl 1975; see Fidone & Gonzalez 1986) (composed of a cluster of parenchymatous cells, the sensory fibre innervating the chemoreceptor cells of the cluster and the surrounding net of capillaries) our findings support the second alternative, in which some glomoids (probably most, due to the increase in the turnover time by a factor of four) are markedly hypofunctional with a small percentage functioning within the normal range. In those intact CB–CSN preparations obtained from hyperoxic animals in which we were able to record the activity in the CSN, the peak activity was near normal, and in those chemoreceptor cells responding to hypoxia with an increase in intracellular free Ca2+ the response was again near normal. It might be more than a mere coincidence that the CA turnover time is four times larger in the CBs from hyperoxic than from control animals and that the overall frequency of success in recording CSN in response to hypoxia in preparations from hyperoxic animals computed from Eden & Hanson (1986), Ling et al. (1997a) and the present study is almost exactly one fourth of that in control animals.
Control CB weight averaged 48.97 μg, and were found to synthesize 73.7 pmol 3H-CA per mg tissue per two hours, i.e. each control CB accumulates 3.61 pmol 3H-CA per CB. The same calculations for hyperoxic CBs yield 0.89 pmol 3H-CA per CB. Although the absolute normoxic release in hyperoxic CBs is around 20% of that found in controls, the normoxic release expressed as percentage of tissue content is nearly identical, as expected (Fig. 5D). The percentage of tissue content released by a nonspecific depolarizing stimulus is also identical in control and hyperoxic CBs. In contrast, the release induced by hypoxia expressed as percentage of tissue content is 20–25% of that found in control CBs, suggesting once again that the hypoxia-evoked release is due to activity in 20–25% of chemoreceptor cells. The release data obtained in the intact CB–CSN preparations with voltammetric measurements are also consistent with this interpretation. With high external K+ as stimulus, the tip of the recording electrode detects a comparable free tissue CA concentration in control and hyperoxic animals, suggesting that CAs are reaching the electrode from all surrounding chemoreceptor cells. Similarly, hypoxic stimulation in control CBs presumably elicits CA release from all chemoreceptor cells, but in the case of hyperoxic preparations only some glomoids are releasing CA. Therefore, in situations where the electrode is located in the vicinity of a responding glomoid the recorded concentration appears normal. However, if the tip of the recording electrode is remote and/or upstream from a responding glomoid, the recorded concentration may be zero (basal). Consistent with this notion, the free tissue CA concentrations recorded in hyperoxic CBs during hypoxic stimulation are, as it was the case with the isotopic method, around 25% of those seen in control preparations.
In summary, the data on the electrophysiological recording of the CSN activity indicate firstly that only about 25% of the fibres in the CSN of hyperoxic animals respond to hypoxia and secondly that this limited complement of functional fibres are sufficient to develop a normal increase in respiratory frequency when the animals are exposed to intense hypoxia, and a normal phrenic response when the CSN is electrically stimulated. The CA data indicate that only about 25% of chemoreceptor cells are capable of responding to hypoxia with a release of CA. Thus, responsive chemoreceptor cells are apparently innervated by fibres that convey an increase in action potential frequency during hypoxia. Stated differently, the CBs of hyperoxic animals, with about 25% functional units or glomoids capable of responding to hypoxia, generate a nearly normal increase in respiratory frequency or ventilation in response to intense hypoxic challenges. Additionally, considering that at submaximal intensities of hypoxic stimulation the ventilatory responses are smaller in hyperoxic than in control animals (Ling et al. 1996), it should be postulated that responding cells present a dose–response curve to hypoxia shifted to the right.
Mechanisms of chemoreceptor cell damage by hyperoxia
Further consideration of the metabolism of CA and intracellular Ca2+ concentration provides some insight into the nature of the processes damaged by the hyperoxic exposure. Two different levels of damage produced by hyperoxia can be distinguished. First, the damage that leads to general atrophy of the CB, including a proportional reduction in the number of chemoreceptor cells, and second, the damage produced in the majority (75–80%) of surviving chemoreceptor cells, impeding the development of their capacity to respond to hypoxia. Our present data do not provide an insight regarding the first type of damage (Ling et al. 1996, 1997a,b,c; Erickson et al. 1998; Fuller et al. 2002), but allow us to make some considerations regarding the second level of damage. They appear to exclude a deficit in the communication between cells because there are isolated cells that respond to hypoxia with a normal increase in Ca2+. Therefore we should consider which steps in the intracellular O2-transduction cascade are affected. According to the plasma membrane model (Gonzalez et al. 1992) oxygen transduction involves the following steps: low PO2→ O2-sensing mechanism of chemoreceptor cells → coupling to specific K+ channels → inhibition of K+ channels → chemoreceptor cell depolarization → activation of voltage-dependent Ca2+ channels → Ca+ entry and increase in intracellular free Ca2+ concentration → exocytosis of neurotransmitters. The increased rate of neurotransmitters leads to activation of CSN terminals, increased action potential frequency in the CSN fibers and increased ventilatory drive in the brainstem respiratory controllers. From this cascade, it is obvious that hypoxia and high external K+ share mechanisms that elicit the release of neurotransmitters downstream from depolarization. As the release induced by high external K+ is normal following hyperoxia, the chemoreceptor cells that are unresponsive to hypoxia must have damaged (or intensely underexpressed) steps located upstream to depolarization, i.e. the O2-sensing mechanism or the molecular machinery coupling the O2-sensing mechanism to K+ channels. Although in theory K+ channels themselves could be damaged, the fact that the responses to both 35 and 60 mm K+ are unaffected suggests that there is not a marked decrease in the overall K+ conductance of the cells, although it is possible that the specific K+ channels supporting the hypoxic response are underexpressed and an alternative subtype of channel is compensating. Whatever the step affected, it is conceivable that hyperoxic damage could occur in other O2-sensing systems, such as pulmonary artery smooth muscle and erythropoietin-producing cells, which may utilize O2-transducing mechanisms similar to chemoreceptor cells (Bunn & Poyton, 1996; Richalet, 1997; Gonzalez et al. 2002b). Future experiments will be designed to explore this possibility.
Acknowledgments
We want to express our gratitude to Maria de los Llanos Bravo for her technical support. We want to thank Dr Bruce Dinger for his critical reading of the manuscript. This work has been supported by Spanish DGICYT grant BFI 2001– 1713, by J.C. y L. grant VA092/03 and by a FISS Grant to the Red Respira-SEPAR.
References
- Barros RC, Branco LG. Central dopamine modulates anapyrexia but not hyperventilation induced by hypoxia. J Appl Physiol. 2002;92:975–981. doi: 10.1152/japplphysiol.00852.2001. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol. 2002;92:1013–1018. doi: 10.1152/japplphysiol.00859.2001. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J Appl Physiol. 2003;95:1550–1559. doi: 10.1152/japplphysiol.01043.2002. [DOI] [PubMed] [Google Scholar]
- Berkenbosch A, Van Dissel J, Olievier CN, De Goede J, Herringa J. The contribution of the peripheral chemoreceptors to the ventilatory response to CO2 in anaesthetized cats during hyperoxia. Respir Physiol. 1979;37:381–390. doi: 10.1016/0034-5687(79)90083-5. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Busch MA, Bisgard GE, Mesina JE, Forster HV. The effects of unilateral carotid body excision on ventilatory control in goats. Respir Physiol. 1983;54:353–361. doi: 10.1016/0034-5687(83)90078-6. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Altose MD. Central chemoreceptors. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung. PA USA: Lippincott-Raven; 1997. pp. 1767–1776. [Google Scholar]
- Cragg PA, Khrisanapant W. Is the second carotid body redundant. Adv Exp Med Biol. 1994;360:297–299. doi: 10.1007/978-1-4615-2572-1_51. [DOI] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive. Curr Opin Neurobiol. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- De Castro F. Nuevas observaciones sobre la innervación de la region carotidea. Los quimio y preso-receptores. Trab Laboratory Invest Biol Uni Madrid. 1940;32:297–384. [Google Scholar]
- Donnelly DF. Function of the Carotid Body Intra-utero and in the Postnatal Period. In: Gonzalez C, editor. The Carotid Body Chemoreceptors. Heidelberg Germany: Springer-Verlag; 1997. pp. 193–203. [Google Scholar]
- Donnelly DF, Doyle TP. Developmental changes in hypoxia-induced catecholamine release from rat carotid body, in vitro. J Physiol. 1994;475:267–275. doi: 10.1113/jphysiol.1994.sp020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effect of hyperoxia from birth on the carotid chemoreceptor and ventilatory responses of rats to acute hypoxia. J Physiol. 1986;374:24P. [Google Scholar]
- Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987a;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol. 1987b;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, JR, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol. 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez R, Arriagada I, Garrido AM, Larrain C, Zapata P. Ventilatory chemosensory drive in cats, rats and guinea-pigs. Adv Exp Med Biol. 2003;536:485–489. doi: 10.1007/978-1-4419-9280-2_62. [DOI] [PubMed] [Google Scholar]
- Fidone S, Gonzalez C. Catecholamine synthesis in rabbit carotid body in vitro. J Physiol. 1982;333:69–79. doi: 10.1113/jphysiol.1982.sp014439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S, Gonzalez C. Peripheral chemoreceptors: initiation and control of discharges. In: Fishman AP, editor. Handbook of Physiology. The Respiratory System II. Bethesda MD USA: Am Physiol Soc; 1986. pp. 247–312. [Google Scholar]
- Fidone S, Gonzalez C, Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bavis RW, Vidruk EH, Wang ZY, Olson EB, JR, Bisgard GE, Mitchell GS. Life-long impairment of hypoxic phrenic responses in rats following 1 month of developmental hyperoxia. J Physiol. 2002;538:947–955. doi: 10.1113/jphysiol.2001.012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Wang ZY, Ling L, Olson EB, Bisgard GE, Mitchell GS. Induced recovery of hypoxic phrenic responses in adult rats exposed to hyperoxia for the first month of life. J Physiol. 2001;536:917–926. doi: 10.1111/j.1469-7793.2001.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Lawson EE. Developmental influences on carotid body responses to hypoxia. Respir Physiol. 2000;121:199–208. doi: 10.1016/s0034-5687(00)00128-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992;15:146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Arterial chemoreceptors. In: Bittar EE, editor. Pulmonary Biology in Health and Disease. New York USA: Springer-Verlag; 2002a. pp. 114–141. [Google Scholar]
- Gonzalez C, Kwok Y, Gibb JW, Fidone SJ. Effects of hypoxia on tyrosine hydroxylase activity in rat carotid body. J Neurochem. 1979;33:713–719. doi: 10.1111/j.1471-4159.1979.tb05216.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Sanz-Alfayate G, Agapito MT, Gomez-Nino A, Rocher A, Obeso A. Significance of ROS in oxygen sensing in cell systems with sensitivity to physiological hypoxia. Respir Physiol Neurobiol. 2002b;132:17–41. doi: 10.1016/s1569-9048(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Rigual R, Fidone SJ, Gonzalez C. Mechanisms for termination of the action of dopamine in carotid body chemoreceptors. J Auton Nerv Syst. 1987;18:249–259. doi: 10.1016/0165-1838(87)90123-8. [DOI] [PubMed] [Google Scholar]
- Hanbauer I, Hellstrom S. The regulation of dopamine and noradrenaline in the rat carotid body and its modification by denervation and by hypoxia. J Physiol. 1978;282:21–34. doi: 10.1113/jphysiol.1978.sp012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M, Kumar P. Chemoreceptor function in the fetus and neonate. Adv Exp Med Biol. 1994;360:99–108. doi: 10.1007/978-1-4615-2572-1_9. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The reorganization of sensory and motor maps in adult mammals. In: Gazzanige MS, editor. The Cognitive Neurosciences. Cambridge, MA, USA: The Mit Press, Massachusetts Institute of Techology; 1995. pp. 51–71. [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol. 1997a;110:261–268. doi: 10.1016/s0034-5687(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Phrenic responses to isocapnic hypoxia in adult rats following perinatal hyperoxia. Respir Physiol. 1997b;109:107–116. doi: 10.1016/s0034-5687(97)00045-5. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Integrated phrenic responses to carotid afferent stimulation in adult rats following perinatal hyperoxia. J Physiol. 1997c;500:787–796. doi: 10.1113/jphysiol.1997.sp022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T. Biochemistry of Catecholamines. The Biochemical Method. Tokyo, Japan: University Park Press; 1973. [Google Scholar]
- Nakano H, Lee SD, Ray AD, Krasney JA, Farkas GA. Role of nitric oxide in thermoregulation and hypoxic ventilatory response in obese Zucker rats. Am J Respir Crit Care Med. 2001;164:437–442. doi: 10.1164/ajrccm.164.3.2010142. [DOI] [PubMed] [Google Scholar]
- Penn AA. Early brain wiring: activity-dependent processes. Schizophr Bull. 2001;27:337–347. doi: 10.1093/oxfordjournals.schbul.a006880. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia MT, Obeso A, Lopez-Lopez JR, Herreros B, Gonzalez C. Characterization of cultured chemoreceptor cells dissociated from adult rabbit carotid body. Am J Physiol. 1992;263:1152–1159. doi: 10.1152/ajpcell.1992.263.6.C1152. [DOI] [PubMed] [Google Scholar]
- Personius KE, Balice-Gordon RJ. Activity-dependent editing of neuromuscular synaptic connections. Brain Res Bull. 2000;53:513–522. doi: 10.1016/s0361-9230(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Prieto-Lloret J, Caceres AI, Rigual R, Obeso A, Rocher A, Bustamante R, Castañeda J, Lopez-Lopez JR, Perez-Garcia T, Agapito T, Gonzalez C. Effects of perinatal hyperoxia on carotid body chemoreceptor activity in vitro. Adv Exp Med Bio. 2003;536:517–524. doi: 10.1007/978-1-4419-9280-2_65. [DOI] [PubMed] [Google Scholar]
- Richalet JP. Oxygen sensors in the organism: examples of regulation under altitude hypoxia in mammals. Comp Biochem Physiol a Physiol. 1997;118:9–14. doi: 10.1016/s0300-9629(96)00370-2. [DOI] [PubMed] [Google Scholar]
- Rigual R, Almaraz L, Gonzalez C, Donnelly DF. Developmental changes in chemoreceptor nerve activity and catecholamine secretion in rabbit carotid body: possible role of Na+ and Ca2+ currents. Pflugers Arch – Eur J Physiol. 2000;439:463–470. doi: 10.1007/s004249900198. [DOI] [PubMed] [Google Scholar]
- Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol. 2000;522:493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl E. On the morphology of the vascular system of the carotid body of cat and rabbit and its relation to the glomus Type I cells. In: Purves MJ, editor. The Peripheral Arterial Chemoreceptors. Oxford, UK: Cambridge University Press; 1975. pp. 293–299. [Google Scholar]
- Verna A. The mammalian carotid body: morphological data. In: Gonzalez C, editor. The Carotid Body Chemoreceptors. Heidelberg, Germany: Springer-Verlag; 1997. pp. 1–29. [Google Scholar]
- Vicario I, Rigual R, Obeso A, Gonzalez C. Characterization of the synthesis and release of catecholamine in the rat carotid body in vitro. Am J Physiol Cell Physiol. 2000;278:490–499. doi: 10.1152/ajpcell.2000.278.3.C490. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J Appl Physiol. 1987;63:2403–2410. doi: 10.1152/jappl.1987.63.6.2403. [DOI] [PubMed] [Google Scholar]