Abstract
Cataplexy, a symptom associated with narcolepsy, represents a unique dissociation of behavioural states. During cataplectic attacks, awareness of the environment is maintained, as in waking, but muscle tone is lost, as in REM sleep. We have previously reported that, in the narcoleptic dog, noradrenergic cells of the locus coeruleus cease discharge during cataplexy. In the current study, we report on the activity of serotonergic cells of the dorsal raphe nucleus. The discharge patterns of serotonergic dorsal raphe cells across sleep–waking states did not differ from those of dorsal raphe and locus coeruleus cells recorded in normal rats, cats and monkeys, with tonic discharge in waking, reduced activity in non-REM sleep and cessation of activity in REM sleep. However, in contrast with locus coeruleus cells, dorsal raphe REM sleep-off neurones did not cease discharge during cataplexy. Instead, discharge continued at a level significantly higher than that seen in REM sleep and comparable to that seen in non-REM sleep. We also identified several cells in the dorsal raphe whose pattern of activity was the opposite of that of the presumed serotonergic cells. These cells were maximally active in REM sleep and minimally active in waking and increased activity during cataplexy. The difference between noradrenergic and serotonergic cell discharge profiles in cataplexy suggests different roles for these cell groups in the normal regulation of environmental awareness and muscle tone and in the pathophysiology of narcolepsy.
Narcolepsy is characterized by persistent daytime sleepiness, disrupted night-time sleep, hallucinations before sleep onset, cataplexy and sleep paralysis (Aldrich, 1998). Cataplexy and sleep paralysis are unique conditions in which a complete loss of tone and stretch reflexes in the antigravity musculature occurs during alert waking. In normal animals such a loss of muscle tone occurs only in REM sleep. Cataplexy is typically triggered by strong, sudden emotions, such as that associated with laughter, or sudden anger. The narcoleptic is aware of his or her environment throughout the episode and has unimpaired memory for the attack (Guilleminault, 1976). Thus, cataplexy allows us to separate the neuronal events associated with the loss of muscle tone normally confined to REM sleep from those associated with the greatly reduced environmental awareness normally present in the REM sleep state despite the presence of an activated electroencephalogram.
Canine genetic narcolepsy is accompanied by persistent sleepiness and disrupted night-time sleep (Kaitin et al. 1986; Kilduff et al. 1986). Like human narcoleptics, narcoleptic dogs can move their eyes to track objects in the environment and appear awake during cataplectic episodes. The symptoms of canine narcolepsy have a similar response to drugs that exacerbate and ameliorate symptoms in human narcoleptics (Nishino & Mignot, 1997). Canine genetic narcolepsy is caused by a mutation in the hypocretin (orexin) receptor-2 gene (Lin et al. 1999). In contrast, human narcolepsy with cataplexy is caused in most cases by a degenerative loss of hypocretin neurones and consequent reduction in hypocretin release (Peyron et al. 2000; Thannickal et al. 2000).
Hypocretin neurones are located in the lateral hypothalamus and perifornical area (Peyron et al. 1998). This is the primary source of hypocretins in the brain. Hypocretin neurones have projections to many areas of the brain (Peyron et al. 1998; Chemelli et al. 1999; Date et al. 1999). There are heavy projections to several areas involved in sleep–wakefulness regulation, e.g. the locus coeruleus, the dorsal raphe and the tuberomammillary nucleus. Both hypocretin receptor-1 (HcrtR1) and receptor-2 (HcrtR2) exist on dorsal raphe neurones (Trivedi et al. 1998; Marcus et al. 2001). However, in genetically narcoleptic dogs, functional HcrtR2s are lacking (Lin et al. 1999). Some therapeutic effects of hypocretin administration have been reported in canine narcoleptics (John et al. 2000), presumably due to an ability of increased HcrtR1 signalling to compensate in part for an absence of signalling through HcrtR2.
Several groups of monoaminergic neurones show great variations in activity over the sleep cycle. Serotonergic, noradrenergic and histaminergic cells are all tonically active during waking, reduce discharge during non-REM sleep and cease or nearly cease discharge during REM sleep (McGinty & Harper, 1976; Trulson & Jacobs, 1979; Aston-Jones & Bloom, 1981; Vanni-Mercier et al. 1984; Steininger et al. 1999). Muscle tone suppression is closely linked to the cessation of monoamine release on to motoneurones (Lai et al. 2001; Mileykovskiy et al. 2000).
Jacobs and his collaborators have extensively studied the behavioural correlates of discharge of the raphe system. They found that cats in which REM sleep without atonia had been induced by pontine lesions had substantial dorsal raphe activity in REM sleep, in contrast to the normal cessation of activity during this state (Trulson et al. 1981). Microinjections of carbachol that induced muscle atonia during waking reduced the activity of dorsal raphe serotonergic neurones by 97%. Mephenesin, a centrally acting muscle relaxant, also reduced waking discharge, whereas succinylcholine or dantrolene, which reduce muscle tone through peripheral mechanisms, did not affect dorsal raphe serotonergic activity (Steinfels et al. 1983). Thus these results suggest that dorsal raphe activity is linked to motor control. In addition to its link to motor activity, there is also some evidence of a link between dorsal raphe activity and behavioural arousal and attentiveness (Trulson & Jacobs, 1979).
In the current study we sought to characterize the role of the serotonergic cells in the dorsal raphe nucleus in cataplexy, a state in which arousal and muscle tone are dissociated, to increase our understanding of the pathophysiology of cataplexy and the functional role of these cells in the regulation of sleep–waking states. Specifically, we tested the hypothesis that dorsal raphe REM-off neurones, like locus coeruleus neurones (Wu et al. 1999), were off in cataplexy to the same extent seen in REM sleep.
Methods
Surgery
Dorsal raphe cells were recorded from the midline dorsal mesopontine junction of four narcoleptic Doberman pinschers (1 M, 3 F; average age 8 years 5 months), using microwire recording techniques as previously described (Wu et al. 1999). All procedures were conducted in accord with the National Research Council's Guide for the Care and Use of Laboratory Animals (1996) and were approved by the Institutional Animal Care and Use Committees of the University of California at Los Angeles and the Department of Veterans Affairs.
The scalp was clipped and disinfected, and an i.v. catheter was inserted into the cephalic vein for supplemental drug injections and fluid infusions (5% dextrose saline). The dog was then given a preanaesthetic dose of thiopental (12.5 mg kg−1, i.v.), intubated, maintained under isoflurane (1–2%) anaesthesia and put in a stereotaxic instrument. Fresh frozen plasma was thawed and given to the dog (8 ml kg−1, i.v.) before, during and after the surgery in infusions lasting 30 min, to prevent excessive bleeding due to von Willebrand's disease, a genetic blood clotting disorder affecting many narcoleptic dogs in our colony. The dog's temperature was maintained with water-circulated heating pads (Gaymar Industries, NY, USA). Oxygen saturation and heart rate were monitored continuously with an oxymeter and an EKG monitor. Rectal temperature and respiration were measured every 10 min.
Small holes were drilled on the skull to accommodate electrodes. Microdrives containing 25 and 64 μm stainless steel microwires (California Fine Wire Co., Grover Beach, CA, USA) aimed at the dorsal raphe area were implanted at a 10 deg angle laterally, with the tip of the electrodes 1 mm above the target. The electroencephalogram (EEG) and electrooculogram (EOG) were recorded with stainless steel screw electrodes placed over sensorimotor cortex and the posterior orbit, respectively. Tripolar twisted electrodes, made of three 256 μm formvar-insulated stainless steel wires (California Fine Wire Co.), were placed in the dorsal hippocampus for the recording of hippocampal EEG. Electromyogram (EMG) activity was recorded from the dorsal neck muscles with Teflon-coated multistranded stainless steel wires (Cooner Wire, Chatsworth, CA, USA). Anchoring screws were placed around the skull. Electrodes were connected to pin plugs and a mixture of sterile bone cement and Cefazolin antibiotic powder (West-Ward Pharmaceutical Corp, Eatontown, NJ, USA) was used to secure the electrodes and plugs to the skull along with anchoring screws. Animals were monitored continuously after surgery until they were ambulatory. Buprenorphine (0.01 mg kg−1, s.c.; twice/day; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA) and Cefazolin (35 mg kg−1, i.v.; thrice/day) were given to the animal for three days after surgery, followed by oral application of Cephalexin (10 mg kg−1; Biocraft Laboratories, Inc., Fair Lawn, NJ, USA) twice a day for additional five days. Drinking and eating were closely monitored, although no drinking or eating disorder has been observed. The area surrounding the implant was checked daily, cleaned with Prepodyne solution (West Agro Inc., Kansas City, MO, USA) then followed by application of Nolvasan aseptic ointment (Fort Dodge Animal Health, Fort Dodge, IA, USA). All the dogs appeared normal and healthy and no distress was ever observed during the course of recording.
Polygraphic recording in sleep and cataplexy
All the recordings were done at least two weeks after surgery with the dogs free to move around inside a large chamber. Unit recording was made differentially between the active and reference electrodes within the same bundle. Neuronal signals were filtered, with roll-off of signals below 300 Hz and above 10 kHz, and digitized at a frequency of 25 kHz. Cortical and hippocampal EEG as well as EOG signals were filtered between 1 and 100 Hz and sampled at a rate of 200 Hz. The EMG was filtered between 30 Hz and 3 kHz and sampled at 1 kHz. Electrodes were moved in 80 μm steps until a cell or cells with signal-to-noise ratio of at least 3 : 1 were isolated. The digitized action potentials, cortical and hippocampal EEG, EOG and EMG were recorded continuously on a PC using Spike2 software (Cambridge Electronic Design Ltd, Cambridge, UK). Multiple units were separated on-line with the Spike2 software using waveform templates. The activity of each cell was characterized throughout sleep–waking states and during cataplexy. Spike duration, from the onset of the negative component to the offset of the positive component, was determined using the averaged waveform taken over 200 spikes. The coefficient of variation of interspike intervals during quiet waking was calculated for each cell to compare the regularity of firing patterns among cells.
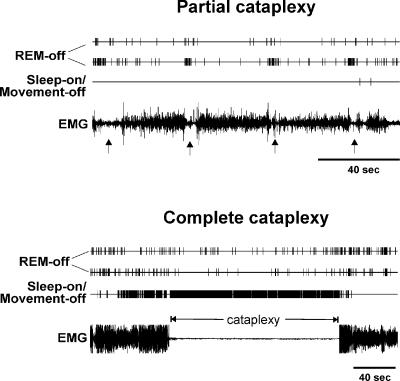
The baseline sleep–wake discharge profile for each cell was established across at least two complete sleep–wake cycles. Cataplexy was induced by the introduction of either soft food (Pedigree, Inc., Vernon, CA, USA), novel toys or play objects as previously described (Wu et al. 1999). A minimum of five cataplexy episodes was observed during the recording of each cell. Narcoleptic dogs display partial cataplexy attacks, in which only the hind limbs may collapse, and complete cataplexy attacks, in which all four limbs collapse and the whole body contacts the floor. Partial cataplexy attacks typically last less than five seconds, while complete cataplexy attacks may last from ten seconds to a few minutes. Because of the short duration of the partial cataplexy episodes, only complete cataplexy episodes were used for quantification of the change in unit discharge rate during this state. Cells were held for recording for as long as needed to complete the experimental protocol, on average for four days. Spike waveform, absence of abrupt changes in amplitude or optimal microwire derivations, consistency of sleep cycle discharge pattern and waking behavioural correlates were used to confirm that the same cells were recorded across multiple recording sessions.
8-Hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH DPAT) (Sigma-Aldrich, St Louis, MO, USA) was prepared fresh for each use (2–8 μg kg−1, dissolved in saline) and given intravenously. The effect of 8-OH DPAT on unit discharge was assessed 10–30 min after the injection during a period of quiet waking.
Cells were classified as serotonergic based on the criteria adopted from dorsal raphe cells in the cat (Fornal & Jacobs, 1988). These criteria include: (1) slow (1–2 Hz) and regular firing in quiet waking, (2) greatly reduced firing in REM sleep, by at least 80% as compared to active waking rate, (3) suppression of firing by systemic administration of 8-OH DPAT, (4) relatively long duration action potentials, and (5) histological localization to the midline dorsal pontine region containing serotonin-immunoreactive (IR) cells. It is important that multiple criteria be fulfilled because it has been shown (Kirby et al. 2003) that some non-serotonergic cells also contain 5-HT1A serotonin receptors and hence may show reduced firing when 8-OH DPAT is applied.
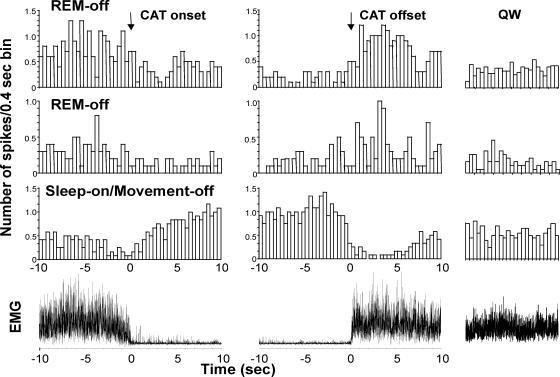
To characterize the temporal relationship between unit and EMG activity, both were averaged around the onset and offset of cataplexy. The times of cataplexy onset and offset were determined from the digitized EMG as previously described (Wu et al. 1999). The onset of cataplexy was defined as the point in time after which the EMG showed complete atonia for at least half a second. The offset of cataplexy was defined as the time point at which EMG activity resumed. Unit activity and EMG were averaged for 10 s before and after the onset and offset points. To prevent the contamination of our results with unwanted drug effects, we allowed a 3-h recovery from 8-OH DPAT before any further sleep or cataplexy observations.
Histology
At the completion of recording, dogs were first deeply anaesthetized with sodium pentobarbital (50 mg kg−1, i.v.), and a small lesion was then made by passing a 15 μA, 20 s anodal current through the electrodes from which cells had been recorded. Dogs were then euthanized, while still under deep anaesthesia, by perfusing transcardially with buffered saline followed by 4% paraformaldehyde.
Forty-micrometre brain sections were cut and processed for serotonin. Free floating sections were first rinsed in 0.1 m PBS five times for 10 min each at room temperature, then in a blocking buffer (0.1% Triton X-100, 0.1% bovine serum albumin, 2% normal goat serum diluted in 0.1 m phosphate buffered saline) for 1 hour. Sections were incubated with a rabbit antiserotonin (5-HT) primary antibody (DiaSorin, Stillwater, MN, USA) for 48 h at 4°C. Sections were rinsed in 0.1 m PBS five times for 10 min each. This was followed by a 2.5-h incubation in a 1: 500 dilution of biotinylated goat antirabbit IgG (Vector Laboratories, Burlingame, CA, USA). The tissue was incubated for 2 h with the avidin–biotin complex diluted 1: 100 (Vector Laboratories). Each incubation was followed by three rinses. Dilutions and rinses were with 1% normal goat serum in 0.1 m Trizma buffered saline. The sections were treated for 30 s to 10 min with a 0.05% solution of 3,3′-diaminobenzidine and 0.01% hydrogen peroxide, rinsed and mounted on gel coated slides. Adjacent, untreated, sections were stained with cresyl violet. Sections were cleared in xylene and coverslipped with Permount mounting medium. The electrode tracks and the serotonin-IR cells were visualized and plotted using a Nikon microscope equipped with NeuroLucida plotting software (MicroBrightField, Inc., Williston, VT, USA).
Results
Cells were recorded from a dorsal raphe region containing serotonin-IR cells
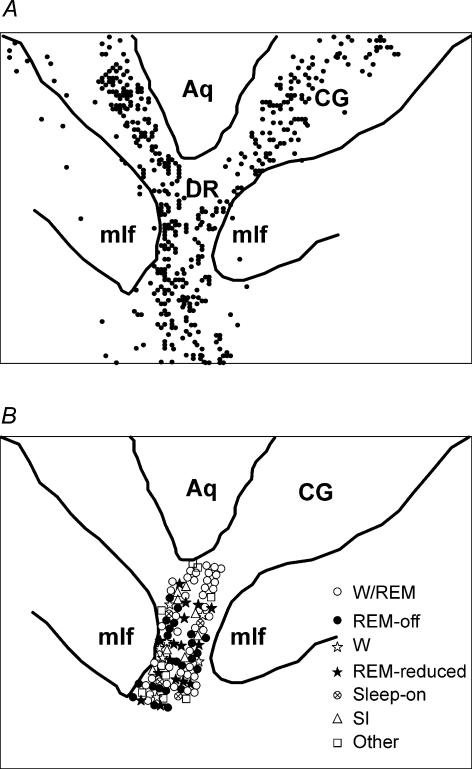
One hundred and ten cells were recorded in the medial and ventral part of the dorsal raphe nucleus. These cells were all recorded from electrodes that were histologically verified to have passed through the dorsal raphe nucleus, identified by 5-HT immunoreactive cells (Fig. 1). These cells were categorized into seven subtypes according to their sleep/wake pattern: REM sleep-off (REM-off), REM sleep-reduced (REM-reduced), Waking/REM sleep-active (W/REM), Wake-related (W), Sleep-on, State independent (SI). Cells that could not be placed in any of the above categories were labelled as ‘Other’ (Tables 1 and 2). Although Waking/REM sleep-active cells tended to be concentrated in the dorsal portions of the nucleus, there was no clear-cut pattern in the distribution of cell types (Fig. 2). When multiple cells were recorded from a single microwire bundle, they were typically of different types.
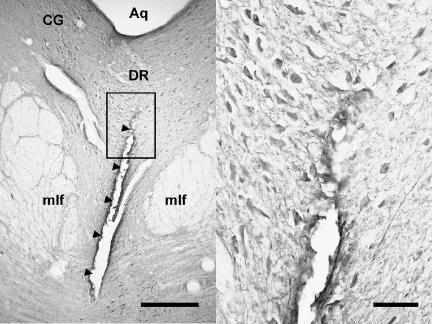
Figure 1. Coronal brain section showing the final position of the electrode bundle that had passed through a dense serotonergic cell region in the dorsal raphe.
Arrowheads in the left panel indicate the electrode track. The right panel is an enlargement of the framed area on the left. Serotonin-IR cells are stained dark around the electrode track. Aq, cerebral aqueduct; CG, central grey; DR, dorsal raphe nucleus; mlf: medial longitudinal fasciculus. Calibration: 0.5 mm and 0.1 mm for the left and right panel, respectively.
Table 1.
Criteria for classification of cell types. Discharge rates in AW were taken as 100% and the activity during other states were expressed as percentage of AW rate
| Cell Type | AW | QW | NREM | REM |
|---|---|---|---|---|
| REM-off | 100% | <1.5 Hz | — | <20% and <0.2 Hz |
| REM-reduced | 100% | — | — | 20–80% and >0.2 Hz |
| W | 100% | >5 Hz | <80% | <80% |
| W/REM | 100% | — | lowest | 20% > NREM rate |
| Sleep-on | 100% | — | >120% | >120% |
| SI | 100% | >80% and <120% | >80% and <120% | >80% and <120% |
REM-off, REM sleep-off; REM-reduced, REM sleep-reduced; W, wake-active; W/REM, wake- and REM sleep-active; SI, state independent. AW, active waking; QW, quiet waking; NREM, non-REM sleep; REM, REM sleep.
Table 2.
Spike width and discharge rate of dorsal raphe neurones during waking, sleep and cataplexy
| Discharge rate (spikes s−1) | |||||||
|---|---|---|---|---|---|---|---|
| Cell type | n | AW | QW | NREM | REM | CAT* | Spike width (ms) |
| REM-off | 19 | 1.18 ± 0.13 | 0.80 ± 0.11 | 0.32 ± 0.05 | 0.07 ± 0.02 | 0.26 ± 0.05 | 1.64 ± 0.06 |
| REM-reduced | 16 | 1.42 ± 0.18 | 1.24 ± 0.22 | 0.81 ± 0.20 | 0.57 ± 0.09 | 0.60 ± 0.10 | 1.52 ± 0.09 |
| W | 7 | 10.52 ± 4.73 | 9.30 ± 3.98 | 8.47 ± 4.28 | 6.64 ± 3.49 | 3.37 ± 0.84 | 1.44 ± 0.06 |
| W/REM | 45 | 8.22 ± 1.78 | 6.34 ± 1.49 | 4.62 ± 1.37 | 8.08 ± 1.74 | 6.21 ± 1.87 | 1.24 ± 0.06† |
| Sleep-on | 5 | 7.28 ± 6.28 | 8.46 ± 5.71 | 10.05 ± 6.07 | 11.04 ± 5.92 | 4.35 ± 2.71 | 0.92 ± 0.22† |
| SI | 9 | 2.55 ± 0.60 | 2.60 ± 0.62 | 2.60 ± 0.71 | 2.45 ± 0.63 | 2.51 ± 0.86 | 1.26 ± 0.13 |
| Other | 9 | 3.69 ± 1.94 | 5.60 ± 3.23 | 5.16 ± 2.92 | 4.71 ± 2.40 | 3.93 ± 1.51 | 1.12 ± 0.12 |
Discharge rates are mean ± s.e.m.; AW, active waking; QW, quiet waking; NREM, non-REM sleep; REM, REM sleep; CAT, cataplexy.
Not all cells were tested for cataplexy.
P < 0.05, Tukey-Kramer tests, compared with REM-off cells.
Figure 2. The distribution of serotonin-positive cells in the ventral central grey and dorsal raphe area (A) and the reconstruction of physiologically defined cells recorded in the same plane (B).
Aq, cerebral aqueduct; CG, central grey; DR, dorsal raphe nucleus; mlf, medial longitudinal fasciculus.
REM sleep-off cells did not cease discharge in cataplexy
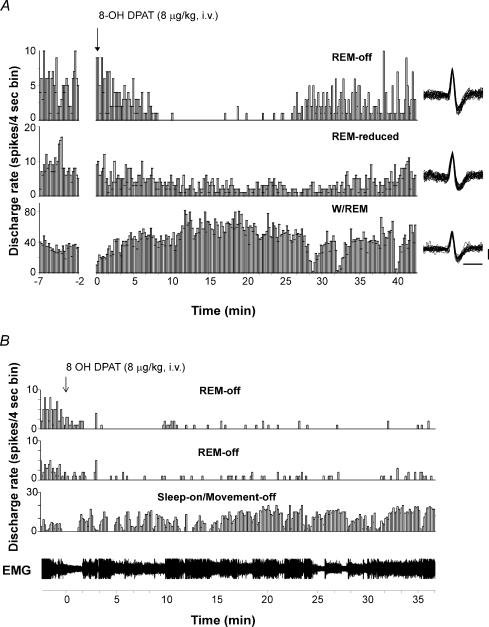
The REM sleep-off (REM-off) cells (n= 19) had waking–sleep discharge pattern similar to that of the locus coeruleus cells (Wu et al. 1999): slow and regular activity in waking, reduced activity in non-REM sleep and complete or near complete cessation of discharge in REM sleep (average of 94% reduction from AW) (Table 2; Figs 3–5). The activity of these cells during waking could be completely suppressed for 15–30 min with an intravenous injection of 8-OH DPAT at 8 μg kg−1 (Fig. 6).
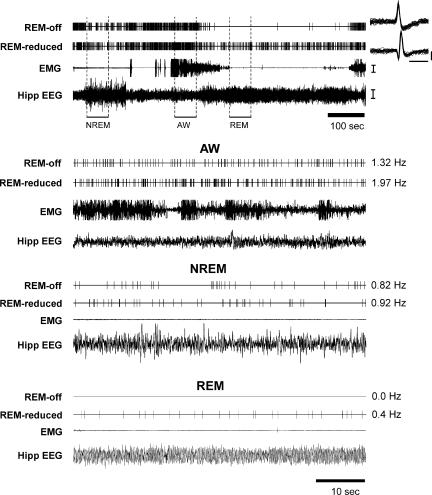
Figure 3. Sleep–wake activity of a REM-off and a REM-reduced cell recorded simultaneously.
The uppermost panel shows a continuous 15-min recording across the sleep–waking cycle. Units are displayed as computer triggered spike traces in this and all other panels. Twenty-five superimposed action potentials for REM-off and REM-reduced cells are displayed on the right. The lower three panels are expanded 60- s tracings during active waking (AW), non-REM sleep (NREM) and REM sleep (REM) during the time indicated by the bar under the uppermost panel. The number on the right of each unit trace is the firing rate during the corresponding state. EMG, electromyogram; Hipp EEG, hippocampal EEG. Calibration bars, Unit action potentials, 1 ms and 50 μV, respectively, for time and voltage; EMG, 100 μV; EEG, 50 μV.
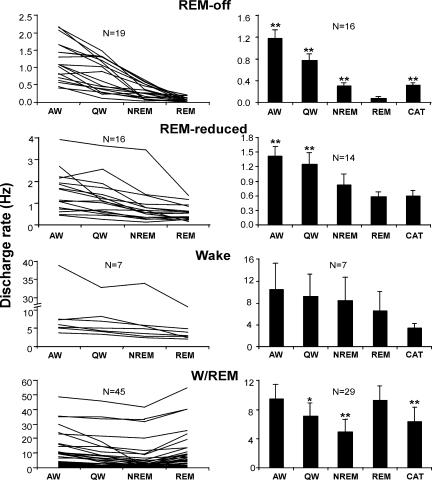
Figure 5. Discharge profiles of four cell groups during waking, sleep and cataplexy.
The left panel shows the discharge pattern of individual cells across sleep/wake states. The right panel shows the average discharge rate (± s.e.m.) during waking, sleep and cataplexy and includes only cells that were recorded during cataplexy.*P < 0.05, **P < 0.01, Bonferroni t test, compared with REM sleep.
Figure 6. Responses to the administration of 8-OH DPAT of cells of different types recorded simultaneously.
A, responses to 8-OH DPAT of a REM-off cell, a REM-reduced cell and a W/REM cell. 8-OH DPAT greatly suppressed the discharge of the REM-off cell for about 15 min. The activity of the REM-reduced cell was also suppressed but to a lesser extent. In contrast, the discharge of the W/REM type cell was greatly increased by 8-OH DPAT. Superimposed action potentials (n= 25) for the REM-off cell, REM-reduced cell and W/REM cell are displayed on the right. Calibration bars for unit action potentials are 1 ms and 50 μV. B, responses to the administration of 8-OH DPAT of two REM-off cells and a sleep-on/movement-off cell recorded simultaneously. The REM-off cells reduced discharge by 95% and 88%, respectively, while the sleep-on/movement-off cell increased discharge by 225% during the 30-min postdrug period. The animal was awake the entire time, as indicated by tonic EMG activity.
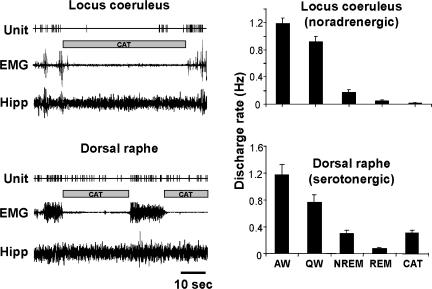
Unlike the locus coeruleus REM sleep-off neurones, which cease discharge both in REM sleep and cataplexy, dorsal raphe REM-off cells did not shut off during cataplexy, but rather, maintained an intermediate level of activity of on average 36% of their AW level (Figs 7, 8 and 9). The firing activity in cataplexy was similar to that in non-REM sleep and was significantly higher than that during REM sleep ( P < 0.01; Bonferroni t test; Fig. 5).
Figure 7. Changes in discharge during partial cataplexy (upper panel) and complete cataplexy (lower panel) of two REM-off cells and a sleep-on/movement-off cell recorded simultaneously.
The arrows in the upper panel indicate partial cataplexy episodes. REM-off cells reduced activity during both partial and complete cataplexy episodes, except for a brief burst of activity occurring at the beginning of the partial cataplexy episodes when the dog sat down as a result of weakness affecting the hindlimbs. The sleep-on/movement-off cell ceased discharge during and between brief partial cataplexy episodes, while it increased discharge during a prolonged complete cataplexy episode.
Figure 8. Averages of EMG and unit activity of three cells across 15 cataplexy episodes.
Data from these three cells, two REM-off cells and a sleep-on/movement-off cell were shown in Figs 4,7 and 8; these cells were recorded simultaneously.
Figure 9. Comparisons of discharge activity of REM-off cells in locus coeruleus (Wu et al. 1999) and dorsal raphe during sleep–waking states and cataplexy.
Left panel, discharge activity of cells in locus coeruleus and dorsal raphe during cataplexy episodes (hatched boxes). Noradrenergic LC cells completely cease discharge during cataplexy. In contrast, serotonergic DR cells retain activity during cataplexy. Right panel, histograms showing the average activity pattern during sleep–waking states and cataplexy of the two types of REM-off cells.
REM-reduced cells maintained REM sleep firing rate in cataplexy
REM-reduced cells, many of which were recorded simultaneously with REM-off cells, displayed a similar, but less dramatic reduction of discharge in REM sleep than did the REM-off cells (Table 2; Figs 3 and 5); REM-reduced cells displayed an average of 60% reduction of discharge during REM sleep, compared with AW. These cells also had a slightly higher discharge rate overall than did REM-off cells (Table 2; Figs 3 and 5). During cataplexy, REM-reduced cells maintained the same discharge rate as that seen in REM sleep (Fig. 5).
Evidence that REM sleep-off and REM sleep-reduced cells are serotonergic
All REM-off cells and the majority of REM-reduced cells tested with 8-OH DPAT (n= 9 tested of 19 recorded and 9 of 16, respectively) showed a reduction in rate after 8-OH DPAT administration (Fig. 6). However, the effect was dose dependent, and there was a significant interaction of cell type and dose ( F1,6= 12.56; P < 0.02; anova). At 4 μg kg−1, REM-off cells showed a 30.7 ± 8.3% reduction in discharge as compared with that during predrug QW, while REM-reduced cells showed a 27.7 ± 7.3% reduction, compared with predrug QW. At 8 μg kg−1, all REM-off cells showed a greater reduction in discharge (88.0 ± 9.5% reduction from predrug QW), with some showing complete suppression of firing. While most (4/6) REM-reduced cells showed reduced firing at 8 μg kg−1 (average 38.1 ± 6.1%), compared with predrug QW, two increased discharge at this dose.
Both REM-off and REM-reduced cells had significantly broader spike width than did other types of cells ( F6,97= 5.22; P < 0.0001; anova; Table 2 and Figs 3 and 4). These cells also tended to fire more regularly than other cell types during QW (coefficient of variation = 0.88 ± 0.05 and 0.95 ± 0.05, respectively, versus 1.00 ± 0.04 and 1.01 ± 0.05 for W/REM and state-independent cells). However, only three of the REM-off cells displayed the ‘clock-like’ discharge pattern (coefficient of variation <0.5) that has been reported for some dorsal raphe cells in behaving cats (Truson & Jacobs, 1979; Shima et al. 1986; Sakai & Crochet, 2001).
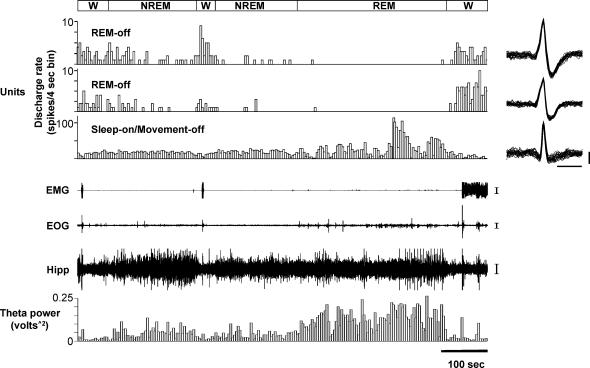
Figure 4. Sleep/wake activity of two REM-off cells and a sleep-on/movement-off cell recorded simultaneously.
The REM-off cells were most active in active waking and ceased discharge completely during REM sleep (as indicated by the silence in the EMG and the presence of hippocampal theta rhythm). The sleep-on/movement-off cell ceased discharge during movement (as indicated by EMG and EOG activities) and increased discharge during non-REM and REM sleep. Superimposed action potentials (n= 25) for the REM-off and the sleep-on/movement-off cell are displayed on the right. NREM, non-REM sleep. Calibration bars, Unit action potentials, 1 ms and 50 μV; EMG, 100 μV; EOG, 50 μV; EEG, 50 μV.
It is possible that REM-reduced cells are also serotonergic. The quantitative difference in firing rates during REM sleep and in response to 8-OH DPAT administration may reflect differences in cell physiology and in influences from different inputs. Similar types of cells have also been seen in normal cats (Type II-C), and these cells showed a complete suppression of discharge with microdialysis application of 8-OH DPAT at 500 μm (Sakai & Crochet, 2001).
Waking/REM sleep-active cells fired less in cataplexy than in REM sleep
The largest group (41%) of cells recorded in the dorsal raphe was active during both waking and REM sleep (W/REM type; Table 2; Fig. 5), with the lowest discharge rate occurring during non-REM sleep. For the majority (36/45) of these cells, there was no apparent relation between their discharge and any directionally specific movement, in contrast with adjacent mesopontine cells (Siegel & Tomaszewski, 1983; Siegel et al. 1983). The remainder of these W/REM cells (9/45) increased firing during certain movements. The W/REM cells are probably not serotonergic. On average, they increased firing by 27% after 8-OH DPAT administration, as compared with predrug QW (Fig. 6). These cells also had shorter action potentials than did REM-off and REM-reduced cells (P < 0.05; Tukey-Kramer post hoc tests). W/REM cells maintained a moderate firing rate during cataplexy, comparable to that during non-REM sleep but significantly less than that during REM sleep (P < 0.01; Bonferroni t test; Fig. 5).
Other cell types in the dorsal raphe nucleus have diverse firing patterns in cataplexy
A few cells (Waking type) were more active during waking than during sleep (Table 2; Fig. 5). Similar to REM-reduced cells, these wake-related cells also had their lowest discharge rate during REM sleep, compared with other normal sleep–wake states. However, these cells had much higher spontaneous discharge rates than did REM-off and REM-reduced cells. Wake-related cells fired less during cataplexy than during REM sleep (Fig. 5).
Five cells (Sleep-on type) increased firing during sleep (Table 2). Two of them were recorded during cataplexy, and the firing rates of both increased (>130%) during cataplexy, compared with AW firing rates. One of these Sleep-on cells ceased discharge during waking movements (Fig. 4). This sleep-on/movement-off cell was simultaneously recorded with two REM-off cells. Its lowest firing rate was during AW, and its highest firing rate was in REM sleep; this discharge pattern was the opposite of that observed for REM-off cells. Furthermore, the firing rate of this cell increased by 132% after the administration of 8-OH DPAT, in contrast to the decreased firing of REM-off cells (Fig. 6). This cell also had very narrow spike width (0.58 ms). It increased discharge (>200%) during complete cataplexy episodes arising out of waking states, in which discharge rates were low (Figs 7 and 8). It is interesting to note that this cell ceased discharge completely during partial cataplexy episodes during which neck and hind-limb muscles were weak but movements were prevalent: the animal sat down briefly and then quickly stood up again. On average, the sleep-on cells had the narrowest spike width of all types (Table 2; Fig. 4). The behavioural profile of these cells is similar to that of some non-serotonergic cells that were previously reported to increase firing in REM sleep (Guzman-Marin et al. 2000) and could possibly be GABAergic interneurones (Stamp & Semba, 1995; Nitz & Siegel, 1997; Varga et al. 2001).
Nine cells were found to have state-independent firing patterns. These cells did not change discharge rate significantly during cataplexy (Table 2). Nine remaining cells, three movement-specific, could not be placed in any of the categories of cells discussed above and were labelled as ‘Other’. Four of these cells, all non-movement related, were recorded during cataplexy. Two (with highest firing rate in QW) fired least during cataplexy, among all states. One (with highest firing rate in REM sleep) reduced firing by 27% as compared with REM sleep, but its firing rate was nonetheless higher during cataplexy than in other states. The remaining cell (with highest firing rate in non-REM sleep) did not change firing rate significantly during cataplexy, compared with QW.
Discussion
We find that dorsal raphe serotonergic cells maintain a moderate level of discharge during cataplexy. The behaviour of these cells contrasts with that of the noradrenergic cells of the locus coeruleus, which show a complete cessation of discharge simultaneous with or immediately preceding cataplexy onset, with resumption of activity immediately prior to the end of the cataplexy period. Both of these cell groups receive potent hypocretinergic innervation (Peyron et al. 1998). The locus coeruleus contains predominantly hypocretin receptor-1, whereas the dorsal raphe has both receptor-1 and receptor-2 (Trivedi et al. 1998; Marcus et al. 2001). If hypocretin cells supply a facilitatory burst of activity during cataplexy eliciting situations in normal animals (Siegel, 1999; Li et al. 2002), one would expect the dorsal raphe neurones to receive less stimulation than locus coeruleus neurones during cataplexy in dogs lacking functional Hcrt receptor-2 proteins. However, this is not what was observed, suggesting that the loss of Hcrt receptor-2 has an indirect effect on the activity of hypocretin cells or of other cell groups afferent to the monoaminergic neurones. Given the symptomatic and pharmacological similarity of human and canine narcolepsy, similar indirect effects of reduced Hcrt signalling might occur in human narcolepsy.
Selective serotonin reuptake inhibitors (SSRIs) have been shown to be effective in the treatment of human cataplexy (Guilleminault & Anognos, 2000). However, the effectiveness of SSRIs is proportional to their potency in activating noradrenergic receptors, rather than due to any direct serotonergic action (Nishino et al. 1993). This finding is in accord with the cessation of noradrenergic but not dorsal raphe serotonergic neuronal discharge in canine cataplexy.
The differing pattern of discharge of dorsal raphe and locus coeruleus REM sleep-off neurones in cataplexy may not only be linked to differences between the roles of the noradrenergic and serotonergic systems in the regulation of muscle tone, but may also be related to the functional specialization of subregions of the serotonergic raphe system. It has been reported that spinal cord ventral horn serotonin release is proportional to the amount of motor activity (Gerin et al. 1995; Lai et al. 2001). However, although medullary raphe cells are strongly activated during treadmill walking, dorsal raphe neurones are not activated during this behaviour (Veasey et al. 1997). It has been shown that dorsal raphe cells are inactivated by treatments that reduce muscle tone, such as carbachol or mephenesin injection (Steinfels et al. 1983). Thus, the current study helps to define the range of motor activities that are and are not linked to activation of the dorsal raphe cell population, in comparison to more caudal serotonergic cell populations.
Considering the key role of the monoaminergic systems in mental health and motor control, it is interesting to note the differences between the noradrenergic and dorsal raphe serotonergic systems in cataplexy. Complete awareness of the environment and the ability to acquire sensory information and remember events, as manifested during cataplexy, apparently do not require any activity in locus coeruleus. In contrast, dorsal raphe activity accompanies these behaviours.
Our primary goal in the present study was to determine the nature of discharge rate changes in serotonergic cells in cataplexy in relation to the discharge pattern of the same cells across the sleep cycle. A much more difficult question to ask, which would also be time consuming to answer, would be whether the basal, waking firing rate of the serotonergic cell population varied between narcoleptic and normal dogs. Studies of breed-related variation in dogs would be necessary to separate possible strain differences in waking discharge rate from differences linked to the HcrtR2 mutation. This kind of question has not been addressed in any comparisons between breeds of rodents, cats or primates. But it is of some interest to compare the current results with prior studies in other species. The tonic waking and non-REM sleep discharge rates of the REM-off dorsal raphe cells in our narcoleptic dogs were considerably lower than those reported in several studies in normal cats, the only other carnivore species studied (Trulson & Jacobs, 1979; Fornal et al. 1987; Lydic et al. 1987; Veasey et al. 1997; Sakai & Crochet, 2001), but were comparable to those found in the two reports on rodents (Kocsis & Vertes, 1992; Guzman-Marin et al. 2000). Thus, although tonic raphe unit activity may be reduced in narcoleptic animals, the current data are not definitive on this point.
Several cells not fulfilling waveshape and 8-OH DPAT response criteria for serotonergic cells recorded within the dorsal raphe nucleus were found to increase discharge during REM sleep and decrease discharge during movement. We have previously reported that increased GABA release occurs within the dorsal raphe region during REM sleep (Nitz & Siegel, 1997). The identified cells may be the source of the GABA release (Gervasoni et al. 1998). However, the increased discharge of these cells in cataplexy is not accompanied by a corresponding decrease in dorsal raphe serotonergic cell discharge, suggesting that a lack of sufficient inhibition or the presence of other excitatory inputs may account for maintained serotonergic discharge.
Using in vivo microdialysis techniques, we have found that loss of muscle tone triggered by brainstem stimulation is linked to a reduction of norepinephrine and serotonin release (Siegel et al. 1991; Wu et al. 1999; Lai et al. 2001) and an increase of GABA and glycine release (Kodama et al. 2003) on to motoneurones. We have recorded extensively from brainstem regions during cataplexy and have previously reported that noradrenergic and medial pontine reticular neurones reduce discharge prior to and during this state (Siegel et al. 1991; Wu et al. 1999), while medial medullary neurones increase discharge at these times (Siegel et al. 1991; Siegel et al. 1992). The coordinated activation of brainstem inhibitory systems and an inactivation of facilitatory systems appear to be responsible for both REM sleep atonia and the loss of muscle tone in cataplexy (Mileykovskiy et al. 2000; Chase & Morales, 2000). We hypothesize that the maintained activity of serotonergic neurones with ascending projections, perhaps together with the activity of forebrain arousal systems, is responsible for the maintained awareness of the environment in cataplexy.
Acknowledgments
The authors thank Dr Janusz Jawien for his excellent assistance in the care of the animals. This work was supported by the Medical Research Service of the Department of Veterans Affairs, NS14610, MH64109 and HL41370.
References
- Aldrich MS. Diagnostic aspects of narcolepsy. Neurology. 1998;50:S2–S7. doi: 10.1212/wnl.50.2_suppl_1.s2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MH, Morales FR. Control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W.B. Saunders; 2000. pp. 676–686. [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornal CA, Jacobs BL. Physiological and behavioral correlates of serotonergic single-unit activity. In: Osborne NN, Hamon M, editors. Neuronal Serotonin. New York: John Wiley & Sons; 1988. pp. 305–345. [Google Scholar]
- Fornal CA, Litto WJ, Morilak DA, Jacobs BL. Single-unit responses of serotonergic dorsal raphe nucleus neurons to environmental heating and pyrogen administration in freely moving cats. Exp Neurol. 1987;98:388–403. doi: 10.1016/0014-4886(87)90250-0. [DOI] [PubMed] [Google Scholar]
- Gerin C, Becquet D, Privat A. Direct evidence for the link between monoaminergic descending pathways and motor activity. I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res. 1995;704:191–201. doi: 10.1016/0006-8993(95)01111-0. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur J Neurosci. 1998;10:964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- Guilleminault C. Cataplexy. In: Guilleminault C, Dement WC, Passouant P, editors. Narcolepsy. New York: Spectrum; 1976. pp. 125–143. [Google Scholar]
- Guilleminault C, Anognos A. Narcolepsy. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W.B. Saunders; 2000. pp. 676–686. [Google Scholar]
- Guzman-Marin R, Alam MN, Szymusiak R, Drucker-Colin R, Gong H, McGinty D. Discharge modulation of rat dorsal raphe neurons during sleep and waking: effects of preoptic/basal forebrain warming. Brain Res. 2000;875:23–34. doi: 10.1016/s0006-8993(00)02561-0. [DOI] [PubMed] [Google Scholar]
- John J, Wu MF, Siegel JM. Systemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogs. Sleep Res Online. 2000;3:23–28. [PMC free article] [PubMed] [Google Scholar]
- Kaitin KI, Kilduff TS, Dement WC. Evidence for excessive sleepiness in canine narcoleptics. Electroenceph Clin Neurophysiol. 1986;64:447–454. doi: 10.1016/0013-4694(86)90078-7. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Bowersox SS, Kaitin KI, Baker TL, Ciaranello RD, Dement WC. Muscarinic cholinergic receptors and the canine model of narcolepsy. Sleep. 1986;9:102–106. doi: 10.1093/sleep/9.1.102. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Dorsal raphe neurons: synchronous discharge with the theta rhythm of the hippocampus in the freely behaving rat. J Neurophysiol. 1992;68:1463–1467. doi: 10.1152/jn.1992.68.4.1463. [DOI] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci. 2003;23:1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-a potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Kadotani H, Rogers W, Lin X, Qui X, de Jong P, Nishino S, Mignot E. The REM sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. Serotonin neurons and sleep I. Long term recordings of dorsal raphe discharge frequency and PGO waves. Arch Ital Biol. 1987;125:1–31. [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: Depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Kodama T, Lai YY, Siegel JM. Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. J Neurosci. 2000;20:8551–8558. doi: 10.1523/JNEUROSCI.20-22-08551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nishino S, Arrigoni J, Shelton J, Dement WC, Mignot E. Desmethyl metabolites of serotonergic uptake inhibitors are more potent for suppressing canine cataplexy than their parent compounds. Sleep. 1993;16:706–712. doi: 10.1093/sleep/16.8.706. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997;273:R451–455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neurosci. 2001;104:1141–1155. doi: 10.1016/s0306-4522(01)00103-8. [DOI] [PubMed] [Google Scholar]
- Shima K, Nakahama H, Yamamoto M. Firing properties of two types of nucleus raphe dorsalis neurons during the sleep-waking cycle and their responses to sensory stimuli. Brain Res. 1986;399:317–326. doi: 10.1016/0006-8993(86)91522-2. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Chiu C, Dement WC, Mignot E, Lufkin R. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J Neurosci. 1992;12:1640–1646. doi: 10.1523/JNEUROSCI.12-05-01640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer H, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science. 1991;262:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski C. Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J Neurophysiol. 1983;50:696–716. doi: 10.1152/jn.1983.50.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS, Wheeler RL. Behavioral organization of reticular formation: studies in the unrestrained cat. II. Cells related to facial movements. J Neurophysiol. 1983;50:717–723. doi: 10.1152/jn.1983.50.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Strecker RE, Jacobs BL. Raphe unit activity in freely moving cats is altered by manipulations of central but not peripheral motor systems. Brain Res. 1983;279:77–84. doi: 10.1016/0006-8993(83)90164-6. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL, Morrison AR. Raphe unit activity during REM sleep in normal cats and in pontine lesioned cats displaying REM sleep without atonia. Brain Res. 1981;226:75–91. doi: 10.1016/0006-8993(81)91084-2. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Jouvet M. [Specific neurons for wakefulness in the posterior hypothalamus in the cat] Neurones specifiques de l'eveil dans l'hypothalamus posterieur du chat. C R Acad Sci. 1984:195–200. [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurons in the cortical modulation of midbrain 5-hydroxytryptamine neurons. Neurosci. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neurosci. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- Wu MF, Gulyani S, Yao E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neurosci. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]