Abstract
L-type Ca2+ current (ICa−L) triggers Ca2+ release from the sarcoplasmic reticulum (SR) and both SR and ICa−L are potential sources of intracellular Ca2+ () for feedback regulation of ICa−L. bound to calmodulin (Ca2+–CaM) can inhibit ICa−L, while Ca2+–CaM can also activate Ca2+–CaM-dependent protein kinase II (CaMK) to increase ICa. However, it is not known whether ICa−L or the SR is the primary source of Ca2+ for ICa−L regulation. The L-type Ca2+ channel C terminus is implicated as a critical transduction element for ICa−L responses to Ca2+–CaM and CaMK, and the C terminus undergoes voltage-dependent steric changes, suggesting that control of ICa−L may also be regulated by cell membrane potential. We developed conditions to separately test the relationship of Ca2+–CaM and CaMK to ICa−L and SR release during voltage clamp conditions modelled upon time and voltage domains relevant to the cardiac action potential. Here we show that CaMK increases ICa−L after brief positive conditioning pulses, whereas Ca2+–CaM reduces ICa−L over a broad range of positive and negative conditioning potentials. SR Ca2+ release was required for both Ca2+–CaM and CaMK ICa−L responses after strongly positive conditioning pulses (+10 and +40 mV), while from ICa−L was sufficient for Ca2+–CaM during weaker depolarizations. These findings show that ICa−L responses to CaMK are voltage dependent and suggest a new model of L-type Ca2+ channel regulation where voltage-dependent changes control ICa−L responses to Ca2+–CaM and CaMK signalling.
L-type Ca2+ current (ICa−L) is a prominent feature of the cardiac action potential plateau where it triggers release of Ca2+ from the sarcoplasmic reticulum (SR) to modulate contraction (Tanabe et al. 1990), Ca2+-dependent gene transcription (Song et al. 2002) and is a source of inward current for arrhythmia-initiating afterdepolarizations (January et al. 1988; Wu et al. 2002). ICa−L is regulated by cytoplasmic Ca2+ () through binding to the -sensing protein calmodulin (Ca2+–CaM) (Peterson et al. 1999; Zuhlke et al. 1999), and Ca2+–CaM can bind to the l-type Ca2+ channel C terminus and inactivate ICa−L (Peterson et al. 1999; Zuhlke et al. 1999). However, Ca2+–CaM can also activate the Ca2+–CaM-dependent protein kinase II (CaMK) (Hudmon & Schulman, 2002), and CaMK increases ICa−L by shifting l-type Ca2+ channels into a high opening probability gating mode, through a phosphorylation-dependent mechanism (McCarron et al. 1992; Dzhura et al. 2000). The site of CaMK action for increasing ICa−L is uncertain, but CaMK can bind to the l-type Ca2+ channel C terminus in a region that overlaps with known Ca2+–CaM binding domains (Hudmon et al. 2002). Thus, the l-type Ca2+ channel C terminus appears to play a critical role for transducing ICa−L responses to . In this regard, it is of interest that the -sensing L-type Ca2+ channel C terminus is mobilized by cell membrane depolarization (Kobrinsky et al. 2003), suggesting the possibility that ICa−L regulation by may also be voltage dependent.
can increase as a direct consequence of ICa−L, or through secondary release of Ca2+ from the SR in heart. Some studies suggest that SR Ca2+ release is more important than ICa−L for activating CaMK, because CaMK-dependent phosphorylation of the SR regulatory protein phospholamban is reduced 50–90% when SR Ca2+ release is prevented by ryanodine (Kuschel et al. 1999; Bartel et al. 2000). On the other hand, from ICa−L is sufficient for engaging Ca2+–CaM-dependent ICa−L inactivation mechanisms in non-cardiac cells (Zuhlke et al. 1999; Peterson et al. 1999) where SR Ca2+ release is not anticipated to contribute to . Furthermore, it remains unknown whether for Ca2+–CaM-dependent ICa−L inactivation and for activating CaMK is primarily from ICa−L or the SR in cardiomyocytes during dynamic changes in cell membrane potential, as occur in the working heart.
We controlled CaMK activity with a Ca2+–CaM-independent form of CaMK and a CaMK inhibitory buffer (IB), previously shown to prevent ICa−L increases by endogenous CaMK (Wu et al. 2001a), and inhibited Ca2+–CaM with an inhibitory peptide (Wu et al. 2001b) or Ba2+ substitution to separately control CaMK and Ca2+–CaM activity in ventricular myocytes, in order to differentially test the effects of from ICa−L and SR for regulating ICa−L availability. ICa−L was measured in cardiomyocytes using a non-steady-state inactivation protocol that mimicked time and voltage conditions present during the cardiac action potential. Our findings support the novel concept that CaMK regulation of ICa−L in cardiomyocytes depends upon cell membrane potential. Both from ICa−L and the SR can recruit Ca2+–CaM for ICa−L inactivation, but SR Ca2+ release is required for CaMK effects, while SR Ca2+ release also predominates for Ca2+–CaM-dependent inactivation at strong depolarizations. A new model of voltage- and -dependent ICa−L regulation is proposed.
Methods
Electrophysiology
Whole-cell mode configuration was used for voltage clamping isolated rabbit ventricular myocytes according to previously published methods (Wu et al. 1999a). Ventricular myocytes were isolated from New Zealand White rabbits killed by pentobarbital (50 mg kg−1, i.v.) overdose prior to excising the heart. The Vanderbilt University Animal Care Committee approved all experiments. Cells were held at –80 mV for >5 min for adequate dialysis with pipette solution before initiating experiments. ICa−L was conditioned by stepping the cell membrane from –80 mV to +40 mV in 10 mV increments from 30 to 500 ms at 0.1 Hz, and peak ICa−L was measured at a test potential of +10 mV (Fig. 1) and expressed as relative current. In some experiments ICa−L inactivation was quantified as the fraction of residual inward current present at the end of 30 ms (R30) and 80 ms (R80) conditioning pulses. All experiments were performed at 24°C. Adding Cs+ and TEA and reducing Na+ and K+ in the pipette and bath solutions eliminated Na+ and K+ currents. A Ca2+-activated Cl− conductance (ICl,Ca), known to be activated by SR Ca2+ release in rabbit ventricular myocytes (Wu & Anderson, 2000) at cell membrane potentials more positive than +20 mV (Wu et al. 1999b), was most clearly seen as a transient outward current in response to voltage command steps to +30 and +40 mV. ICl,Ca was eliminated by niflumic acid (10–20 μm, data not shown) and thapsigargin (Fig. 6A), and was not present at the test command potential of +10 mV. Thus, ICl,Ca was not likely to have significantly contributed to ICa−L measurements because the relationship of relative current (see below) to conditioning potential was not affected by niflumic acid (data not shown), and the relationship between relative current and R30 and R80 was not altered during positive voltage commands in IB (see below, Fig. 5A and B) or IB and thapsigargin (Fig. 7A and B). However, we cannot rule out the possibility that ICl,Ca did contribute to CaMK and Ca2+–CaM effects, especially at voltage command steps to +30 and +40 mV (Wu et al. 1999b). Elimination of the residual current by nifedipine (10 μm) or Cd2+ (100 μm) confirmed that the identity of active inward current was ICa−L (data not shown). The control pipette (intracellular) solution was (mm): CsCl 120.0, EGTA 10.0, Hepes 10.0, tetraethylammonium chloride (TEA) 10.0, phosphocreatine 5.0, CaCl2 3.0, MgATP 1.0, NaGTP 1.0, and pH was adjusted to 7.2 with 1.0 n CsOH. The ability of endogenous CaMK to facilitate ICa−L was eliminated by addition of an inhibitory buffer (IB) solution (see below) (Wu et al. 1999a,2001a). The bath (extracellular) solution was NMDG 137.0, CsCl 25.0, Hepes 10.0, glucose 10.0, CaCl2 (or BaCl2) 1.8, MgCl2 0.5, and pH was adjusted to 7.4 with 12 n HCl. Ryanodine (10 μm) or thapsigargin (1 μm) were added to the bath solution for some experiments. Myocyte contraction was eliminated under these conditions (data not shown).
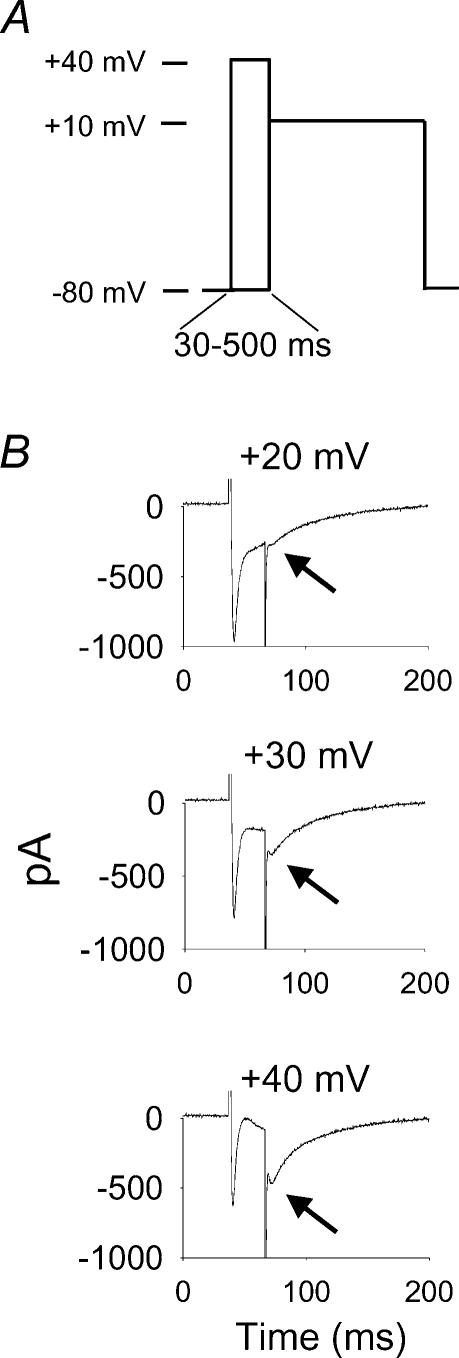
Figure 1. Non-steady-state inactivation voltage clamp protocol reveals increases in relative L-type Ca2+ current (ICa−L) after brief, positive conditioning steps.
A, a schematic representation of the voltage clamp protocol used for this study. Peak ICa−L was measured at a +10 mV test pulse, after conditioning steps from –80 to +40 mV in 10 mV steps, lasting from 30 to 500 ms. B, conditioning steps (30 ms) from +20 to +40 mV progressively increase available ICa−L at the test pulse of +10 mV (indicated by arrows). The conditioning prepulse potential is labelled above each panel. A transient outward Ca2+-activated Cl− current is superimposed on ICa−L during some conditioning steps positive to +10 mV (see Methods for details) (Wu et al. 1999b).
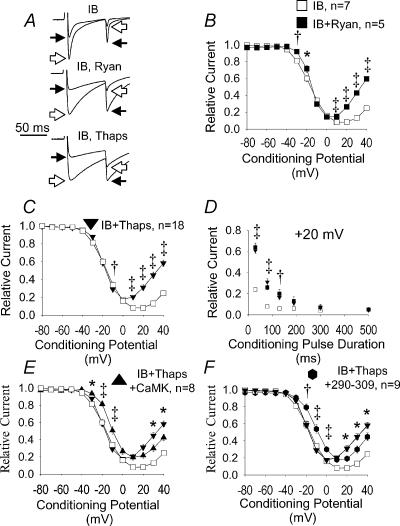
Figure 6. CaM and CaMK require SR after positive conditioning pulses.
A, superimposed current tracings displayed as in Fig. 3A. Cells were dialysed with IB in all panels (IB), but ryanodine (IB, Ryan) or thapsigargin (IB, Thaps) were added to the bath solution to inactivate SR Ca2+ release in some experiments. Both ryanodine (B, Ryan) and thapsigargin (C, Thaps) significantly increased available ICa−L after 80 ms conditioning prepulses in cells dialysed with IB to inhibit endogenous CaMK. Pre-pulse potentials (abscissa) are plotted against peak ICa−L normalized to the maximum value (ordinate). D, normalized peak ICa−L values from B and C are plotted against conditioning prepulses to +20 mV for a range of prepulse durations (abscissa). No significant differences in peak ICa−L were present between ryanodine- and thapsigargin-treated cells. *P < 0.05, †P < 0.01 and ‡P < 0.001 for panels B–D. E, addition of Ca2+-independent CaMK failed to increase relative ICa−L in cells dialysed with IB and treated with thapsigargin after positive conditioning prepulses (IB + CaMK + Thaps). F, the Ca2+–CaM inhibitory peptide 290–309 failed to increase relative ICa−L in cells dialysed with IB and treated with thapsigargin (IB + Thaps + 290–309) after positive conditioning prepulses. ‡P < 0.001, †P < 0.01, *P < 0.05 for comparisons in panels E and F.
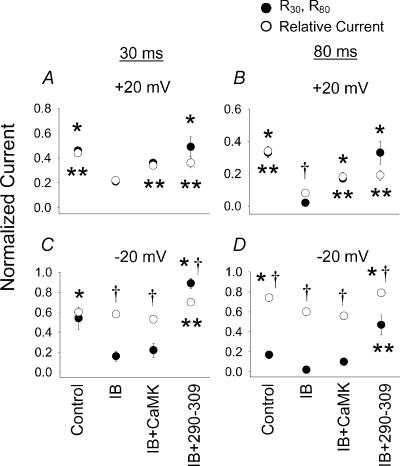
Figure 5. The effect of CaMK on relative and residual ICa−L.
The effect of CaMK on relative ICa−L (recorded during test pulses to +10 mV as in Fig. 1, shown as open circles) after conditioning prepulses to +20 mV (A and B) and –20 mV (C and D) and the residual ICa−L at the end of 30 ms (R30, A and C) and 80 ms (R80, B and D) conditioning prepulses (filled circles). The experimental pipette solutions are labelled below C and D, but are also valid for A and B. Cells were dialysed with control pipette solution (Control), IB solution (IB), IB containing a Ca2+-independent form of CaMK (IB + CaMK) or IB containing the CaM binding peptide 290–309 (IB + 290–309) and all data are from Figs 2 and 3. Significant differences in residual current (R30 and R80, *P < 0.05) or relative current (**P < 0.05) between the IB condition and Control, IB + CaMK or IB + 290–309 are indicated. Significant differences between residual currents and relative current measurements for each experimental condition are shown (†P < 0.05).
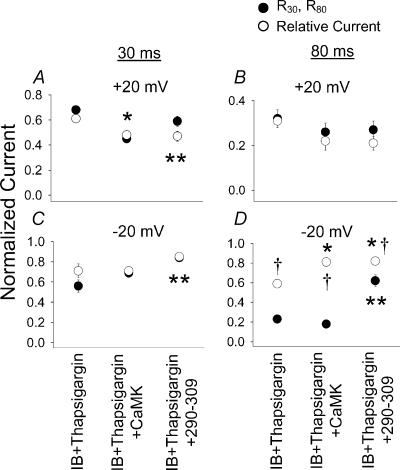
Figure 7. The effect of CaMK and Ca2+–CaM on relative ICa−L and R30 and R80 in cells treated with thapsigargin.
The data are displayed as in Fig. 5 except thapsigargin was included in the bath solution and cells were dialysed with IB (IB + thapsigargin), IB and Ca2+-independent CaMK (IB + thapsigargin + CaMK) or the Ca2+–CaM inhibitory peptide 290–309 (IB + thapsigargin + 290–309). The experimental conditions are labelled below C and D, but are also valid for A and B. The data sets were previously displayed for relative current in Fig. 6E and F. Significant differences between the IB + thapsigargin and other groups for residual current (R30 and R80, *P < 0.05) and relative current (**P < 0.05) are indicated. Significant differences between residual currents and relative currents for each experimental condition are shown (†P < 0.01).
Approaches to controlling CaM and CaMK activity
A recombinant monomeric truncation mutant of the mouse CaMK α isoform (amino acid residues 1–380) was expressed using baculovirus and then purified using CaM agarose affinity chromatography (Brickey et al. 1990). This CaMK was stored in IB (50 mm Hepes, pH 7.5, 1 mm EDTA, 1 mm DTT, 50% (v/v) glycerol, 10% (v/v) ethylene glycol) and activated by autophosphorylation in a 100 μl reaction containing 50 mm Hepes, pH 7.5, 2 mm magnesium acetate, 1.5 mm CaCl2, 18 μm CaM, 2 mm DTT and 100 μm ATPγ S. The reaction was initiated by addition of the (1–380) CaMK (9 μmol l−1 final subunit concentration), incubated at 30°C for 10 min, and stopped by the addition of EDTA (10 mm). IB was used without added CaMK, or after inactivation of enzymatically active CaMK by heating, to observe CaMK-independent effects of manipulating Ca2+–CaM and SR Ca2+ release. IB prevents ICa−L facilitation by endogenous CaMK (Wu et al. 2001a) and was used to separate CaMK from Ca2+–CaM activity and SR Ca2+ release. Ca2+–CaM-dependent autophosphorylation of the CaMK produces a constitutively active species that can phosphorylate substrates in the absence of Ca2+–CaM. Ca2+-independent activity was typically 35–50% of total activity in the presence of Ca2+–CaM using the peptide substrates syntide-2 or autocamtide. Constitutively active CaMK and IB were diluted 10-fold in the pipette solution (0.9 μm final) for use in voltage clamp studies and its activity confirmed in vitro, as described (Wu et al. 1999a). This dilution was chosen to approximate the physiological CaM kinase activity in heart (∼1–2 μm) derived from percentage yield calculations during purification (Iwasa et al. 1986; Gupta & Kranias, 1989).
The CaMK inhibitory peptide AC3-I (KKALHRQEAVDCL, IC50∼3 μm) (Braun & Schulman, 1995) (Macromolecular Resources, Fort Collins, CO, USA) is a modified CaMK substrate, which inhibits endogenous and thiophosphorylated constitutively active CaMK. AC3-I was included in the pipette solution at a final concentration of 20 μm. The CaM binding peptide 290–309 (Calbiochem) is modelled on the CaM binding domain of CaMK, and inhibits Ca2+–CaM signalling generally, and was added to the pipette solution at a final concentration of 50 μm. CaMK, 290–309 and AC3-I were dialysed into cells for 5–10 min prior to initiating experiments.
Statistics
The null hypothesis was evaluated with Student's t test or ANOVA, as appropriate. Bonferroni's correction was applied for multiple comparisons.
Results
Enhanced ICa−L availability following brief, positive conditioning pulses
Brief, positive conditioning prepulses progressively increased relative ICa−L availability (Fig. 1), while IB significantly reduced relative ICa−L availability (Fig. 2A–C), compared to control pipette solution, suggesting that endogenous CaMK can increase ICa−L under action potential plateau conditions. The increase in ICa−L in response to brief (30–130 ms), positive conditioning prepulses was lost at longer (500 ms) prepulse durations when Ca2+ was the charge carrier (Fig. 2D), but persisted when Ba2+ was substituted for Ca2+ (Fig. 2E), in cells dialysed with IB. The persistence of Ca2+-dependent reduction in ICa−L availability in IB indicates that Ca2+–CaM-dependent inactivation is operative under these experimental conditions. These findings show that Ca2+ critically determines ICa−L availability during time and voltage conditions present during the ventricular action potential plateau, and serve as a starting point for dissecting the role of Ca2+–CaM, CaMK and SR Ca2+ release in determining ICa−L availability in cardiac myocytes.
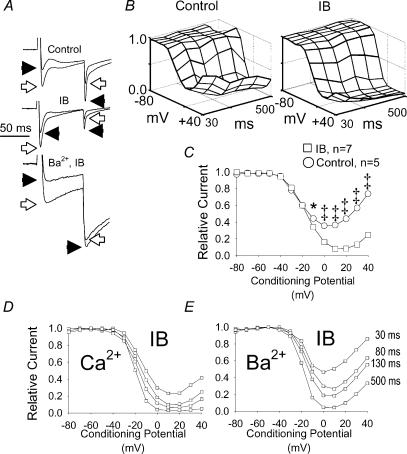
Figure 2. A CaMK inhibitory buffer (IB) reduces ICa−L at action potential plateau potentials.
A, current tracings recorded in control pipette solution with Ca2+ as charge carrier (Control), IB pipette solution with Ca2+ as charge carrier (IB) and IB pipette solution with Ba2+ as charge carrier (Ba2+, IB) are shown. Currents in response to +20 mV (open arrows) and +30 mV (filled arrow heads) conditioning prepulses are superimposed and normalized to the peak ICa−L at +20 mV for comparison. B, topology plots showing the combined effects of conditioning prepulse potentials (–80 to +40 mV) and conditioning prepulse durations (30–500 ms) on relative peak Ca2+ current (ICa) recorded during the test potential step to +10 mV in cells (n= 3 for each group) dialysed with control solution (Control) or IB solution (IB). The grid lines on the topology plots indicate conditioning potentials in +10 mV increments (evenly spaced from –80 to +40 mV) and conditioning prepulse durations (evenly spaced at 30, 80, 130, 190, 300 and 500 ms). C, peak ICa−L after 80 ms conditioning prepulses from –80 to +40 mV (abscissa) in cells dialysed with control buffer (n= 5) that permits activation of endogenous CaMK and with IB (n= 7, see Methods for details) that prevents activation of endogenous CaMK. This IB data set is also used in Figs 3–6 and this Control data set is used in Figs 4 and 5 for other comparisons. D and E show a family of non-steady-state inactivation relationships for a single cardiomyocyte dialysed with IB using (D) Ca2+ or (E) Ba2+ as charge carrier. The duration of the conditioning prepulse is indicated for each of the isochrones in C and D. Peak ICa−L is normalized to the maximum value in all panels. *P < 0.05, ‡P < 0.001.
CaMK increases ICa−L at action potential plateau conditions
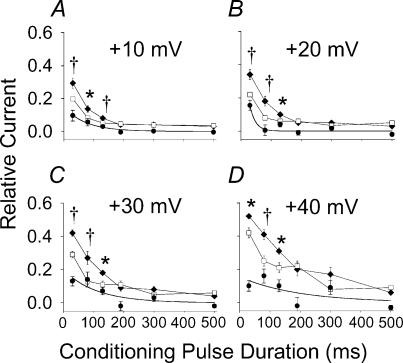
Previous work has shown that IB prevented CaMK mediated ICa increases in cardiac myocytes (Wu et al. 1999a, 2001a), strongly suggesting that CaMK inhibition was a critical feature of IB actions at ICa−L. On the other hand, the persistence of Ca2+-dependent ICa−L inactivation (Fig. 2A, D and E) suggested IB did not disrupt Ca2+–CaM signalling, generally. We created a topographical surface plot of relative ICa−L availability to better illustrate the effects of IB over a wide range of conditioning times and voltages (Fig. 2B). These plots reveal the functional targeting of significant IB actions to time and voltage durations relevant to the action potential plateau (P < 0.05 for all intergroup comparisons from conditioning potentials between 0 and +40 mV and conditioning pulse durations from 30 to 130 ms). In order to more thoroughly test the concept that IB was selective for CaMK and that reduction in ICa−L at action potential plateau potentials in IB was due to CaMK, we dialysed a Ca2+ independent form of CaMK into myocytes in the presence of IB (Fig. 3B). Ca2+–CaM-independent CaMK significantly restored reduced ICa−L availability in IB (Fig. 3B), indicating that inhibition of endogenous CaMK was the critical IB effect. The effects of Ca2+–CaM-independent CaMK on ICa−L were specifically due to the enzymatic activity of the exogenous kinase, as they were significantly reduced by coadministration of the CaMK inhibitory peptide AC3-I (data not shown), and eliminated by heat inactivation of the added CaMK (Fig. 3B). CaMK effects were confined to brief conditioning pulses (30–130 ms), and were lost after longer conditioning prepulses (190–500 ms, Fig. 4), confirming that CaMK actions on ICa−L were targeted to time and voltage domains relevant to the cardiac action potential plateau.
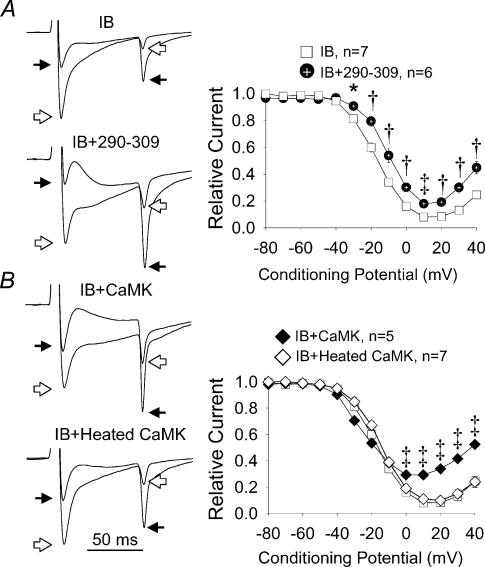
Figure 3. The effects of Ca2+–CaM and CaMK on ICa−L.
A, the left panels show superimposed current tracings (as in Fig. 2A) in response to conditioning prepulses to +20 mV (open arrows) and +40 mV (filled arrows) in cells dialysed with IB (IB) or a combination of IB and the Ca2+–CaM inhibitory peptide 290–309 (IB + 290–309). The right panel shows summary findings for peak ICa−L after 80 ms conditioning prepulses from –80 to +40 mV (abscissa) in cells treated as described in the left panels. B, the left panels show superimposed current tracings labelled as in A (above), but these cells were dialysed with IB and a Ca2+-independent form of CaMK (IB + CaMK) or IB and heat-inactivated CaMK (IB + heated CaMK). The right panel shows summary findings for peak ICa−L (as in A), but from cells treated as in the left panels. Dialysis with a Ca2+–CaM-independent form of CaMK that is resistant to IB increases relative ICa−L (80 ms prepulses) compared to IB alone after positive conditioning pulses. CaMK activity is ablated by heat inactivation and peak ICa−L is normalized to the maximum value. *P < 0.05, †P < 0.01, ‡P < 0.001 for A and B.
Figure 4. The effect of exogenous Ca2+–CaM-independent CaMK on ICa−L availability after positive conditioning prepulses.
A–D, each shows peak ICa−L responses to conditioning prepulses of varying durations (abscissa) at four positive membrane potentials (indicated above each plot). Cells were dialysed with IB to inhibit endogenous CaMK activity in the presence (filled diamonds, n= 5) or absence (open squares, n= 7) of Ca2+-independent CaMK that is resistant to IB. Filled circles represent the difference in relative ICa−L in IB + CaMK and IB alone and the contiuous lines are exponential fits of these differences. *P < 0.05, †P < 0.01.
We next considered the relationship between ICa−L inactivation during brief conditioning voltage pulses (R30 and R80) and relative ICa−L availability (relative current) in the subsequent test pulse (Fig. 1), in order to better understand the apparent voltage dependence of CaMK signalling to l-type Ca2+ channels (Dzhura et al. 2000). At a positive conditioning potential (+20 mV) R30 and R80 accurately predicted relative current under control conditions, in IB and in IB after CaMK replacement (Fig. 5A and B). CaMK replacement significantly restored R30, R80 and relative current under these conditions. In contrast, IB significantly reduced R30 compared to relative current after a weakly depolarizing conditioning step to –20 mV, but CaMK replacement failed to equalize R30 and relative current (Fig. 5C). IB significantly reduced both R80 and relative current after a –20 mV conditioning potential, but R80 was significantly less than relative current even under control conditions (Fig. 5D). These comparisons between ICa−L inactivation and relative (ICa−L) current suggest that a greater fraction of L-type Ca2+ channels were available to open at the +10 mV test voltage following inactivation of ICa−L during the –20 mV than the +20 mV conditioning step. Taken together, results from experiments using both CaMK inhibition and replacement strategies indicate that CaMK can significantly increase ICa−L by a time- and voltage-dependent mechanism in cardiac myocytes.
Ca2+–CaM reduces ICa−L availability at action potential plateau potentials
The finding that cells dialysed with IB retained Ca2+-dependent inactivation (Figs 2A, D and E) suggested that Ca2+–CaM remained operative under these experimental conditions. Cellular dialysis with the Ca2+–CaM binding peptide 290–309 did significantly increase relative ICa−L availability. In contrast to CaMK that was only effective after positive conditioning potentials (Figs 3B and 5), 290–309 was effective after weakly and strongly depolarizing conditioning potentials (Figs 3A and 5). These data show that endogenous Ca2+–CaM was a significant signal transduction element for grading ICa−L in the presence of IB. However, 290–309 did not fully increase ICa−L availability to levels present with Ba2+ as charge carrier (Fig. 2E), perhaps indicating that a constitutively bound pool of CaM (Erickson et al. 2001; Pitt et al. 2001) was inaccessible to the peptide.
In contrast to the effects of 290–309 on ICa−L availability, peak ICa−L during the conditioning pulse in IB + 290–309 (4.1 ± 0.4 pA pF−1, n= 5) was significantly less than peak ICa−L recorded in IB alone (7.1 ± 0.9 pA pF−1, n= 7) or in IB + CaMK (8.1 ± 0.5 pA pF−1, n= 5), whereas IB + 290–309 did not decrease peak ICa−L compared to control solution (6.2 ± 0.5 pA pF−1, n= 5).
SR Ca2+ release selectively reduces ICa−L after brief, positive conditioning pulses
The results of experiments so far show that the CaMK-dependent component of signalling to L-type Ca2+ channels is voltage dependent (Figs 3B and 4); however, they do not distinguish between ICa−L and SR Ca2+ release as dominant sources of signalling for grading ICa−L. Both ryanodine and thapsigargin significantly increased ICa−L after positive conditioning potentials in the presence of IB, indicating that Ca2+-induced Ca2+ release is present under these experimental conditions (Fig. 6A–C). Reduction in SR Ca2+ release by either ryanodine or thapsigargin targeted ICa−L increases over similar cell membrane potential (Fig. 6B and C) and temporal domains (Fig. 6D) as CaMK (Figs 3B and 4), suggesting the possibility that activation of endogenous CaMK is predominately due to SR Ca2+ release, and that Ca2+–CaM-dependent inactivation relies on SR under action potential plateau conditions.
CaMK actions at ICa−L are determined by SR Ca2+ release
Previous studies suggest SR Ca2+ is important for activating endogenous CaMK in cardiac myocytes (Kuschel et al. 1999; Wu et al. 1999a, 2001b; Bartel et al. 2000), so we reasoned that Ca2+-independent CaMK would circumvent the requirement for activation of endogenous CaMK by SR Ca2+ release. Surprisingly, thapsigargin-treated cells did not show increases in ICa−L availability at action potential plateau potentials in response to Ca2+-independent CaMK, but did show a significant depolarizing shift in ICa−L availability in response to weaker depolarizations (Fig. 6E). This result was in striking contrast to the significant increases in ICa−L after positive conditioning pulses when myocytes with intact SR Ca2+ release were supplemented with Ca2+-independent CaMK (Figs 3B and 4). In contrast to CaMK-dependent increases in R30, R80 and relative ICa−L at +20 mV (Fig. 5A and B), CaMK replacement was ineffective for increasing these parameters after thapsigargin at +20 mV (Fig. 7A and B). CaMK replacement also failed to evoke a consistent response in R80 and relative current after a –20 mV conditioning step (Fig. 7D). These data underscore the close relationship between SR Ca2+ release and L-type Ca2+ channel function, and suggest the possibility that the Ca2+-independent form of CaMK acts to increase ICa−L availability through a SR-dependent mechanism.
SR Ca2+ release significantly determines Ca2+–CaM responses after strong depolarizations
In contrast to CaMK (Fig. 3B), Ca2+–CaM effects on ICa−L appear to be voltage independent because they operate over a broad range of physiological conditioning potentials (Fig. 3A). SR Ca2+ release does contribute to Ca2+-dependent ICa−L inactivation (Balke & Wier, 1991; Wu et al. 2001b), and reduction of SR Ca2+ release by ryanodine (Fig. 6B) or thapsigargin (Fig. 6C) significantly increased relative ICa−L in cells dialysed with IB only after positive conditioning steps, suggesting that the source of activator for Ca2+–CaM-dependent inactivation may vary in a voltage-dependent manner in cardiac myocytes. In order to better understand the contribution of ICa−L and SR to Ca2+–CaM for regulating ICa−L responses, we dialysed 290–309 into myocytes after thapsigargin. Ca2+–CaM inhibition with 290–309 significantly increased relative ICa−L (Fig. 6F) and R30 (Fig. 7C) and R80 (Fig. 7D) at –20 mV, but not at +20 mV (Fig. 7A and B), supporting previous findings that ICa−L is sufficient for Ca2+–CaM-dependent inactivation, but suggesting that normal SR Ca2+ release significantly determines Ca2+–CaM signalling to L-type Ca2+ channels at strongly depolarized conditioning potentials, present during the action potential plateau.
Discussion
CaMK, ICa−L and SR Ca2+ release
The present studies use a combination of approaches to inhibit endogenous CaMK-dependent ICa−L increases (with IB) or control CaMK activity (with exogenous Ca2+–CaM independent CaMK) while SR Ca2+ release is preserved or eliminated (with ryanodine or thapsigargin) in cardiomyocytes. The central finding of these experiments is that CaMK increases in ICa−L are functionally targeted over time and voltage domains that are directly relevant to the cardiac action potential plateau. This finding suggests CaMK actions are analogous to protein kinase A, which can also regulate L-type Ca2+ channels by a voltage-dependent mechanism (Sculptoreanu et al. 1993). Previous investigations established that CaMK can directly increase L-type Ca2+ channel openings in excised membrane patches from ventricular myocytes (Dzhura et al. 2000), but these experiments did not test for temporal- or voltage-dependent features of CaMK signalling and could not measure effects of SR Ca2+ release. The dramatic loss of CaMK signalling effects after elimination of SR Ca2+ release (Figs 6E and F and 7) shows those CaMK actions are reliant upon SR Ca2+ release. One possible unifying explanation for these observations is that an important CaMK action on ICa−L in intact myocytes may be indirect, via modulation of SR Ca2+ release (Li et al. 1997; Wu et al. 2001a). The concept that CaMK exerts important actions on ICa−L by influencing SR Ca2+ release may reconcile earlier reports showing that ICa−L increases were linked to dynamic reduction in SR Ca2+ release (Delgado et al. 1999), but were eliminated by inactivation of SR Ca release (Wu et al. 1999a, 2001b). Interestingly CaMK slightly, but significantly, increased relative ICa−L availability at weakly depolarized potentials in the absence of SR Ca2+ release (Figs 6E and 7D), raising the possibility that CaMK may be capable of regulating ICa−L by a SR-independent pathway under these voltage clamp conditions. The finding that ICa−L availability responses to CaMK (Fig. 6E) and 290–309 (Fig. 6F) are very similar in thapsigargin, after weak and strong depolarizations, suggests that in the absence of SR Ca2+ release, CaMK and Ca2+–CaM may compete for shared molecular machinery, such as the L-type Ca2+ channel C terminus. Thus, the present studies add important new information to our understanding of how CaMK may contribute to ICa−L regulation in the working heart.
Ca2+–CaM, ICa−L and SR Ca2+ release
CaM is a ubiquitous -sensing protein that is required for -dependent ICa−L inactivation in cardiac L-type Ca2+ channels (Peterson et al. 1999; Zuhlke et al. 1999). CaMK and CaM colocalize with L-type Ca2+ channels and ryanodine receptors (Wu et al. 1999a; Pate et al. 2000; Balshaw et al. 2001; Pitt et al. 2001; Dzhura et al. 2002; Erickson et al. 2003), and are capable of regulating both of these proteins. Ca2+–CaM also activates other proteins, including CaMK, so that myriad effects potentially complicate interpretation of Ca2+–CaM inhibition experiments. The present experiments used IB dialysis and a Ca2+–CaM inhibitory peptide (290–309) to separately control Ca2+–CaM and CaMK signalling. These findings support the concept that Ca2+–CaM reduces available ICa−L in a voltage-independent manner (Fig. 3A). However, the critical source of for Ca2+–CaM is determined by cell membrane voltage because ryanodine (Fig. 6B) and thapsigargin (Fig. 6C) only increased ICa−L at plateau potentials and because 290–309 was ineffective at increasing ICa−L at plateau potentials in the absence of SR Ca2+ release (Figs 3A and 6F). In contrast, CaM sequestration with the 290–309 peptide enhanced ICa availability after weakly depolarizing prepulses, independent of SR Ca2+ release (Figs 6F and 7D and E), suggesting that ICa−L alone is a sufficient source of for Ca2+–CaM-dependent inactivation of ICa−L at weakly depolarized cell membrane potentials. The finding that Ca2+–CaM competition by 290–309 significantly increased relative currents and slowed ICa−L inactivation in IB (Fig. 5) is consistent with a recent report showing marked slowing of ICa−L inactivation and action potential prolongation in cardiomyocytes transfected with Ca2+-binding deficient, dominant-negative CaM mutants (Alseikhan et al. 2002). A potential limitation to studies with 290–309 is highlighted by the finding that peak ICa−L during the conditioning pulse was reduced in IB + 290–309 compared to IB alone, raising the possibility that an outward current, such as ICl,Ca, may be activated by 290–309 and complicate ICa−L measurements during this experimental condition. Taken together, these results reveal the interdependence of CaM, SR Ca2+ release and cell membrane potential and add to other recent work highlighting the importance of CaM as a -driven signalling element for regulating ICa in heart.
The relationship between ICa−L inactivation and availability
The present experiments show that it is possible to distinguish between SR Ca2+, CaM and CaMK signalling effects on relative ICa−L availability in cardiac myocytes. Both CaM (Fig. 3A) and CaMK (Fig. 3B) can separately regulate ICa−L availability under non-steady-state conditions. Relative ICa−L availability is closely related to ICa−L inactivation after positive conditioning pulses (Figs 5A and B, and 7A and B). This relationship is consistent with the concept that ICa−L inactivation (R30 and R80) during the conditioning pulse directly determines ICa−L availability during the subsequent test pulse. Experiments to inhibit or replace CaMK (Fig. 5), eliminate SR Ca2+ release, or reduce Ca2+–CaM (Fig. 7) did not alter this relationship at +20 mV. In contrast, R30 was significantly less than relative ICa−L after CaMK inhibition with IB (Fig. 5C), suggesting that CaMK could reduce this measure of ICa−L inactivation at –20 mV without altering the pool of L-type Ca2+ channels available for opening in response to the +10 mV test pulse. SR Ca2+ also reduced R30 and R80 during CaMK inhibition at –20 mV (compare IB in Fig. 5C and D with IB + thapsigargin in Fig. 7C and D). However, from ICa−L was sufficient for significant Ca2+–CaM actions on R30 and R80 at –20 mV, because these measures were both increased by 290–309 in the combined presence of IB and thapsigargin (Fig. 7D).
A model for voltage- and -dependent regulation of ICa−L in heart
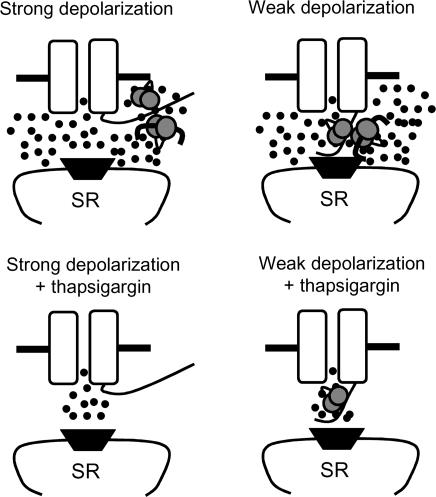
The recent finding that the L-type Ca2+ channel C terminus undergoes significant voltage-dependent movement (Kobrinsky et al. 2003) provides a potentially important context for understanding our findings. The C terminus is now accepted to be richly endowed with sensing machinery, and three distinct Ca2+–CaM binding domains have been identified (Zuhlke et al. 1999; Pate et al. 2000; Pitt et al. 2001). On the other hand, the C terminus is also capable of binding activated CaMK (Hudmon et al. 2002). Given that both Ca2+–CaM and CaMK can converge upon the C terminus and given that the C terminus is significantly mobile over the cell membrane potential ranges used in our study, it is possible that the C terminus could be variably positioned to differentially respond to from ICa−L (when positioned near the pore region) or the SR (when positioned outside of the pore region). Based upon these considerations and upon our finding that SR Ca2+ release was required for complete Ca2+–CaM and CaMK actions at +20 mV (Figs 5 and 7), we hypothesize that the C terminus moves away from the pore region during strong depolarizations. Because from ICa−L was sufficient for Ca2+–CaM at –20 mV, we further hypothesize that the C terminus is close enough to the pore region during weak depolarizations to sense directly from ICa−L (Fig. 8).
Figure 8. Schematic depiction of the hypothesized relationship between cell membrane potential, L-type Ca2+ channel C terminus motion and ‘sensing’ by CaM and CaMK.
The L-type Ca2+ channel pore forming subunit (α1C) is shown as a pair of open rectangles with a central pore. The C terminus protrudes into the cytoplasmic space from the right rectangle and the pore region and SR Ca2+ release channel (ryanodine receptor, dark trapezoid) are en face. Ca2+–CaM is depicted as a pair of stippled circles linked by a curved segment and activated CaMK is shown as a thick bar with curved ends bound to Ca2+–CaM. According to the hypothesized model, strong depolarizations motivate the C terminus to move away from the α1C pore so that sensing is primarily from the SR. Weak depolarizations leave the C terminus in the vicinity of the pore where sensed is directly from ICa−L. Inactivation of SR Ca2+ release (by thapsigargin) results in significant impairment of sensing through CaM and CaMK during strong depolarizations, while from ICa−L is sufficient for Ca2+–CaM at weak depolarizations in the absence of SR Ca2+ release.
Our findings show that CaMK responses are very different during non-steady-state time and voltage domains that approximate action potential plateau conditions than during steady-state conditions. While most studies have understandably focused on the effects of prolonged voltage clamp command pulses, in order to measure steady-state responses, steady-state behaviours may not always be relevant to the physiology of the action potential. These experiments highlight the importance of considering non-steady-state measurements for understanding the effects of cellular signals on ionic current responses.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NHLBI, HL62494, HL70250, HL46881). M.E.A. is an Established Investigator of the American Heart Association. J.T.K. is partially funded by a NHI training grant. We thank Ms Martha Bass and Ms Jinying Yang for expert technical assistance, and Dr Lou DeFelice for criticisms and insightful comments.
References
- Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke CW, Wier WG. Ryanodine does not affect calcium current in guinea pig ventricular myocytes in which Ca2+ is buffered. Circ Res. 1991;68:897–902. doi: 10.1161/01.res.68.3.897. [DOI] [PubMed] [Google Scholar]
- Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J Biol Chem. 2001;276:20144–20153. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- Bartel S, Vetter D, Schlegel WP, Wallukat G, Krause EG, Karczewski P. Phosphorylation of phospholamban at threonine-17 in the absence and presence of beta-adrenergic stimulation in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2000;32:2173–2185. doi: 10.1006/jmcc.2000.1243. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. A non-selective cation current activated via the multifunctional Ca(2+)–calmodulin-dependent protein kinase in human epithelial cells. J Physiol. 1995;488:37–55. doi: 10.1113/jphysiol.1995.sp020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickey DA, Colbran RJ, Fong YL, Soderling TR. Expression and characterization of the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II using the baculovirus expression system. Biochem Biophys Res Commun. 1990;173:578–584. doi: 10.1016/s0006-291x(05)80074-9. [DOI] [PubMed] [Google Scholar]
- Delgado C, Artiles A, Gomez AM, Vassort G. Frequency-dependent increase in cardiac Ca2+ current is due to reduced Ca2+ release by the sarcoplasmic reticulum. J Mol Cell Cardiol. 1999;31:1783–1793. doi: 10.1006/jmcc.1999.1023. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu YJ, Colbran RJ, Corbin JD, Balser JR, Anderson ME. Cytoskeletal disrupting agents prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels. J Physiol. 2002;545:399–406. doi: 10.1113/jphysiol.2002.021881. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Kranias EG. Purification and characterization of a calcium-calmodulin-dependent phospholamban kinase from canine myocardium. Biochemistry. 1989;28:5909–5916. doi: 10.1021/bi00440a030. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Pitt GS, Tsien RW, Schulman H. Molecular mechanism and regulation of the interaction between calcium–calmodulin dependent protein kinase II and the L-type calcium channel. Biophys J. 2002;82:172a. [Google Scholar]
- Hudmon A, Schulman H. Structure/function of the multifunctional Ca2+/Calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa T, Inoue N, Fukunaga K, Isobe T, Okuyama T, Miyamoto E. Purification and characterization of a multifunctional calmodulin-dependent protein kinase from canine myocardial cytosol. Arch Biochem Biophys. 1986;248:21–29. doi: 10.1016/0003-9861(86)90396-6. [DOI] [PubMed] [Google Scholar]
- January CT, Riddle JM, Salata JJ. A model for early afterdepolarizations: induction with the Ca2+ channel agonist Bay K 8644. Circ Res. 1988;62:563–571. doi: 10.1161/01.res.62.3.563. [DOI] [PubMed] [Google Scholar]
- Kobrinsky E, Schwartz E, Abernethy DR, Soldatov NM. Voltage-gated mobility of the Ca2+ channel cytoplasmic tails and its regulatory role. J Biol Chem. 2003;278:5021–5028. doi: 10.1074/jbc.M211254200. [DOI] [PubMed] [Google Scholar]
- Kuschel M, Karczewski P, Hempel P, Schlegel WP, Krause EG, Bartel S. Ser16 prevails over Thr17 phospholamban phosphorylation in the beta-adrenergic regulation of cardiac relaxation. Am J Physiol. 1999;276:H1625–H1633. doi: 10.1152/ajpheart.1999.276.5.H1625. [DOI] [PubMed] [Google Scholar]
- Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca(2+)-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron JG, McGeown JG, Reardon S, Ikebe M, Fay FS, Walsh JV., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992;357:74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- Pate P, Mochca-Morales J, Wu Y, Zhang JZ, Rodney GG, Serysheva II, Williams BY, Anderson ME, Hamilton SL. Determinants for calmodulin binding on voltage-dependent Ca2+ channels. J Biol Chem. 2000;275:39786–39792. doi: 10.1074/jbc.M007158200. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Song LS, Guia A, Muth JN, Rubio M, Wang SQ, Xiao RP, Josephson IR, Lakatta EG, Schwartz A, Cheng H. Ca(2+) signaling in cardiac myocytes overexpressing the [alpha](1) subunit of L-type Ca(2+) Channel. Circ Res. 2002;90:174–181. doi: 10.1161/hh0202.103230. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Mikami A, Numa S, Beam KG. Cardiac-type excitation–contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990;344:451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- Wu Y, Anderson ME. Ca2+-activated non-selective cation current in rabbit ventricular myocytes. J Physiol. 2000;522:51–57. doi: 10.1111/j.1469-7793.2000.0051m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Colbran RJ, Anderson ME. Calmodulin kinase is a molecular switch for cardiac excitation–contraction coupling. Proc Natl Acad Sci U S A. 2001a;98:2877–2881. doi: 10.1073/pnas.051449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dzhura I, Colbran RJ, Anderson ME. Calmodulin kinase and a calmodulin-binding ‘IQ’ domain facilitate L-type Ca(2+) current in rabbit ventricular myocytes by a common mechanism. J Physiol. 2001b;535:679–687. doi: 10.1111/j.1469-7793.2001.t01-1-00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol. 1999a;276:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- Wu Y, Roden DM, Anderson ME. Calmodulin kinase inhibition prevents development of the arrhythmogenic transient inward current. Circ Res. 1999b;84:906–912. doi: 10.1161/01.res.84.8.906. [DOI] [PubMed] [Google Scholar]
- Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble RW, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]