Abstract
TASK-1 and TASK-3 are functional members of the tandem-pore K+ (K2P) channel family, and mRNAs for both channels are expressed together in many brain regions. Although TASK-1 and TASK-3 subunits are able to form heteromers when their complementary RNAs are injected into oocytes, whether functional heteromers are present in the native tissue is not known. Using cultured cerebellar granule (CG) neurones that express mRNAs of both TASK-1 and TASK-3, we studied the presence of heteromers by comparing the sensitivities of cloned and native K+ channels to extracellular pH (pHo) and ruthenium red. The single-channel conductance of TASK-1, TASK-3 and a tandem construct (TASK-1/TASK-3) expressed in COS-7 cells were 14.2 ± 0.4, 37.8 ± 0.7 and 38.1 ± 0.7 pS (–60 mV), respectively. TASK-3 and TASK-1/TASK-3 (and TASK-3/TASK-1) displayed nearly identical single-channel kinetics. TASK-3 and TASK-1/TASK-3 expressed in COS-7 cells were inhibited by 26 ± 4 and 36 ± 2 %, respectively, when pHo was changed from 8.3 to 7.3. In outside-out patches from CG neurones, the K+ channel with single channel properties similar to those of TASK-3 was inhibited by 31 ± 7 % by the same reduction in pHo. TASK-3 and TASK-1/TASK-3 expressed in COS-7 cells were inhibited by 78 ± 7 and 3 ± 4 %, respectively, when 5 μm ruthenium red was applied to outside-out patches. In outside-out patches from CG neurones containing a 38 pS channel, two types of responses to ruthenium red were observed. Ruthenium red inhibited the channel activity by 77 ± 5 % in 42 % of patches (range: 72–82 %) and by 5 ± 4 % (range: 0–9 %) in 58 % of patches. When patches contained more than three 38 pS channels, the average response to ruthenium red was 47 ± 6 % inhibition (n= 5). These electrophysiological studies show that native 38 pS K+ channels of the TASK family in cultured CG neurones consist of both homomeric TASK-3 and heteromeric TASK-1/TASK-3.
TASK-1 and TASK-3 are cloned K+ channels that belong to the tandem-pore K+ channel family (Lesage & Lazdunski, 2000; Goldstein et al. 2001; Patel & Honore, 2001). When expressed in oocytes or mammalian cells, both TASK-1 and TASK-3 show constitutive channel activity and possess properties of a background or leak K+ channel. Native K+ channels with properties nearly identical to those of TASK-1 and TASK-3 have been identified in a variety of cell types (Kim et al. 1999; Czirjak & Enyedi, 2002b; Han et al. 2002; Han et al. 2003). In these cells, the K+ channels are also active at rest and therefore should help to stabilize the resting membrane potential. In addition to their role as background K+ channels, TASK-1 and TASK-3 have several other unique properties that may be important for regulation of cell excitability. TASK-1 and TASK-3 are highly sensitive to changes in extracellular pH (pHo), such that acidic conditions reduce and alkaline conditions augment the currents (Duprat et al. 1997; Kim et al. 2000). Changes in pHo are known to occur during nerve activity and this can have associated effects on neuronal excitability (Chesler 1990; Rose & Deitmer 1995; Ransom 2000). Neurotransmitters and hormones modulate TASK-1 and TASK-3 via Gq/11-coupled receptors. For example, angiotensin II and thyrotropin-releasing hormone have been shown to inhibit TASK-1 and TASK-3 via their specific receptors (Talley et al. 2000; Czirjak & Enyedi 2002b). In cerebellar granule cells, acetylcholine binding to M3 receptor produced inhibition of the standing outward K+ current that is made up of TASK-1 and TASK-3 (Millar et al. 2000; Han et al. 2002). In addition, TASK-1 and TASK-3 are targets of inhalation anaesthetic agents such as halothane that stimulate the K+ channel activity and cause depression of cell excitability (Patel et al. 1999; Sirois et al. 2000). Thus, TASK-1 and TASK-3 probably regulate cell excitability as background K+ channels and by responding to changes in pHo, receptor agonists and volatile anaesthetics.
Many K+ channels are now known to be heteromultimers made up of two different subunits. For example, the G protein-gated K+ channel (GIRK) is a heterotetramer consisting of two different GIRK subunits (Krapivinsky et al. 1995), certain delayed rectifier K+ channels are made up of KvLQT1 and mink (Barhanin et al. 1996; Sanguinetti et al. 1996), and certain neuronal M channels are made up of KCNQ2 and KCNQ3 (Wang et al. 1998). As TASK-1 and TASK-3 mRNAs are coexpressed in many brain regions, a heteromeric assembly of TASK subunits might also be possible and produce a channel that possesses novel properties that are different from TASK homomers. In a recent study, coexpression of TASK-1 and TASK-3 cRNAs into oocytes produced a K+ current with an intermediate pHo sensitivity (Czirjak & Enyedi 2002a). When a TASK-1/TASK-3 tandem construct, generated by joining the C-terminal end of TASK-1 to the N-terminal end of TASK-3, was expressed in oocytes a pHo sensitivity similar to that observed with coexpressed subunits was obtained (Czirjak & Enyedi 2002a). These results suggest that TASK-1/TASK-3 heteromers form functional K+ channels and show intermediate pHo sensitivity. Functional heteromerization of TASK-1 and TASK-3 has also been demonstrated in the HEK293 expression system using a tandem construct (Talley & Bayliss 2002). In oocytes, the TASK-3(G95E) mutant was shown to produce a dominant negative effect when coexpressed with TASK-1, and TASK-1(G95E) behaved as a dominant negative mutant when coexpressed with TASK-3 (Lauritzen et al. 2003). However, in two other studies the same coexpression experiment in oocytes using TASK-3(G95E) failed to reduce the TASK-1 current, suggesting that heteromeric channels did not form (Karschin et al. 2001; Pei et al. 2003). Regardless of whether heteromerization of TASK-1 and TASK-3 occurs in the expression systems, it is important to know whether these two subunits can assemble to form functional K+ channels in vivo. We have previously recorded K+ channels with electrophysiological properties similar to those of TASK-1 and TASK-3 in cardiac myocytes and neurones isolated from different brain regions (Kim et al. 1999; Han et al. 2002; Han et al. 2003). However, it is difficult to be certain whether they are homomers or heteromers, as single-channel kinetics of heteromers have not yet been studied.
The objective of this study is therefore to determine whether functional heteromeric TASK channels are present in a neurone that expresses both TASK-1 and TASK-3 mRNAs and TASK-3-like channel currents. To help identify the heteromer, we analysed in detail the single-channel kinetics of TASK-1, TASK-3 and the TASK-1/TASK-3 heteromer expressed in COS-7 cells, and compared them to those of native K+ channels in cerebellar granule neurones. The sensitivities of TASK-1, TASK-3 and the TASK-1/TASK-3 heteromer to pHo and ruthenium red were also determined and compared to those of native K+ channels. Our results show that heteromeric TASK-1/TASK-3 is present in cultured CG neurones, along with homomeric TASK-1 and TASK-3. The single channel kinetics of TASK-3 homomer and TASK-1/TASK-3 heteromer were nearly identical but they showed different sensitivities to inhibitors. The formation of TASK-1/TASK-3 in vivo should therefore increase the functional diversity of background K+ channels in neurones and probably in other cell types that express both TASK-1 and TASK-3.
Methods
Cerebellar granule neurone culture and isolation
All animals were used in accordance with the Guide for the Care and Use of Laboratory Animals (DHEW Publication no. NIH85-23). The cerebellum was isolated from rapidly decapitated P6-P8 rat pups, and washed with oxygenated physiological buffer solution (PBS) at 4°C. The cerebellar cortex was cut into 500 μm or thinner sections and incubated for 15 minutes in a solution containing papain (12 U ml−1; Worthington, Lakewood, NJ), albumin (0.2 mg ml−1) and dl-cysteine (0.2 mg ml−1). After digestion, the tissue was washed twice with PBS and resuspended in a solution containing DNase I (1000 kU ml−1; Worthington). After gentle trituration of the solution using a fire-polished glass pipette, the suspended cells were gently passed through a 3 CC/25 G syringe. The suspension was layered on top of sterilized fetal bovine serum and centrifuged at 110 g for 10 minutes. The pellet was resuspended in plating medium that contains NeuroBasal Media (Gibco), supplemented with B-27 (10 μl ml−1; Life Technologies, Rockville, MD), glutamic acid (2.5 mm), glutamine (20 mm), gentamicin (50 μg ml−1) and fungizone (2.5 μg ml−1). The cells were plated on glass coverslips coated with poly l-lysine at a density of 1 × 105 cells−2. After a 24 hour period for cell attachment, the medium was changed every 3 days with new plating medium containing B-27 (20 μl ml−1), glutamine, gentamicin and fungizone in NeuroBasal Medium. Cells were kept for 10 days at 37°C in a humidified incubator gassed with a 95 % air, 5 % CO2 mixture (v/v), and used during 1–3 days of culture.
Transfection in COS-7 cells
Rat TASK-1 and TASK-3 were cloned previously in this laboratory. The TASK-1/TASK-3 tandem construct was produced using PCR. This construct contains a three residue (Gly-Ser-Ala) linker region placed after the C-terminal amino acid of TASK-1 and fused to the starting methionine of TASK-3. The TASK-3/TASK-1 construct was produced in a similar way. The coding regions of rat TASK-1, TASK-1/TASK-3, TASK-3/TASK-1 and TASK-3 were subcloned into the vector pcDNA3.1 (Invitrogen, Carlsbad, CA) for transfection into COS-7 cells. COS-7 cells were seeded at a density of 2 × 105 cells per 35 mm dish 24 hours prior to transfection in 10 % (v/v) fetal bovine serum in Dulbecco's modified Eagle's medium (DMEM). COS-7 cells were cotransfected with a plasmid containing one of the K+ channel genes and pcDNA3.1/GFP using LipofectAMINE and OPTI-MEM I Reduced Serum Medium (Life Technologies). Green fluorescence from cells expressing green fluorescent protein (GFP) was detected with the aid of a Nikon microscope equipped with a mercury lamp light source. Cells were used 1–3 days after transfection.
Electrophysiological studies
Electrophysiological recording was performed using a patch clamp amplifier (Axopatch 200, Axon Instruments, Union City, CA). All recordings were performed at room temperature (24°C). Single channel currents were digitized with a digital data recorder (VR10, Instrutech, Great Neck, NY, USA), and stored on video tape. The recorded signal was filtered at 2 kHz using 8-pole Bessel filter (–3 dB; Frequency Devices, Haverhill, MA, USA) and transferred to a computer (Dell) using the Digidata 1200 interface (Axon Instruments) at a sampling rate of 20 kHz. Threshold detection of channel openings was set at 50 %. Whole cell currents were recorded after canceling the capacitive transients. Whole cell and single channel currents were analysed with the pCLAMP program (Version 8). For single channel analysis, the filter dead time was 100 μs (0.3/cutoff frequency) such that events shorter than 50 μs in duration would be missed. Data were analysed to obtain a duration histogram, amplitude histogram and channel activity (Npo, where N is the number of channels in the patch, and po is the probability of a channel being open). Npo was determined from ∼1–2 minutes of current recording. To compare the effect of pH on channel current, channel activity was multiplied by the amplitude (i) at that potential (I = Npoi). The single channel current tracings shown in the figures were filtered at 2 kHz. In experiments using cell-attached and outside-out patches, pipette and bath solutions contained 150 mm KCl, 1 mm MgCl2, 5 mm EGTA and 10 mm Hepes (pH 7.3). The pH was adjusted using HCl or KOH to the desired values. In whole cell recordings, the bath solution contained 135 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 5 mm glucose and 10 mm Hepes. The pH was adjusted to 7.3 using HCl or NaOH. Ruthenium red stock solution (20 mm) was prepared in distilled water, and diluted in the bath recording solution before using at 5 μm. All other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA). For statistics, Student's t test was used with P < 0.05 as the criterion for significance. Data are represented as means ±s.d.
Results
pHo Sensitivity of TASK expressed in COS-7 cells: whole-cell currents
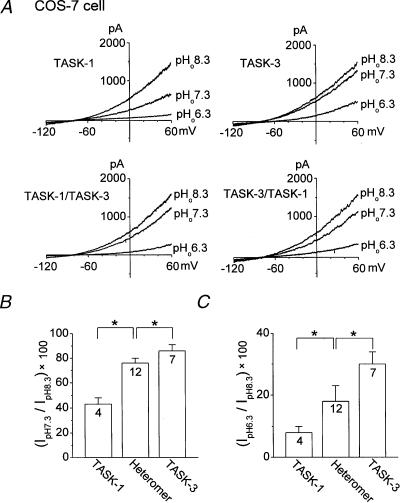
Both TASK-1 and TASK-3 are modulated by changes in pHo but a detectable difference in sensitivity has been observed previously (Czirjak & Enyedi 2002a). To confirm the difference in pHo sensitivity, we studied the effect of three pHo values on the whole-cell currents of TASK-1 and TASK-3 expressed in COS-7 cells. In a physiological bath solution containing 5 mm KCl, the membrane potential of cells under whole-cell configuration was held at −80 mV and then a voltage ramp (–120 to +60 mV; 1 second duration) was applied. The cells were first bathed with a bath solution buffered at pH 7.3, and switched to solutions buffered at pH 8.3 and 6.3. As shown in Fig. 1A (top), changes in pHo produced different degrees of current stimulation and inhibition of TASK-1 and TASK-3. TASK-1 showed large changes in current levels between pH 7.3 and 8.3 (233 ± 22 % increase), as well as between pH 7.3 and 6.3, as reported previously (Lopes et al. 2001). On the other hand, TASK-3 showed only a small increase in current when pHo of the solution was changed from 7.3 to 8.3 (116 ± 4 % increase), but showed a similar decrease as TASK-1 in the acidic range. The current measured at pHo 8.3 was taken as 100 %, and the decreases in current observed at pHo 7.3 and 6.3 were plotted as shown in Fig. 1B,C. COS-7 cells transfected with a plasmid containing GFP produced currents that were less than 80 pA. Thus, TASK-1 and TASK-3 could be distinguished based on their responses to pHo in the alkaline range alone.
Figure 1. Sensitivity of whole-cell currents of TASK-1, TASK-3 and heteromer channels (TASK-1/TASK-3, TASK-3/TASK-1) expressed in COS-7 cells.
A, whole-cell currents were recorded from COS-7 cells at three different pHo values. The bath solution contained 5 mm KCl. B, current observed at pH 7.3 as a percent of that observed at pH 8.3. C, current observed at pH 6.3 as a percent of that observed at pH 8.3. Current levels in both B and C were determined at +60 mV. An asterisk indicates a significant difference between two values (P < 0.05). The numbers inside the bar represent the number of cells used. The data obtained from COS-7 cells expressing TASK-1/TASK-3 and TASK-3/TASK-1 were combined (Heteromer).
To test the sensitivity of the TASK heteromer to pHo, two tandem constructs, TASK-1/TASK-3 and TASK-3/TASK-1, were generated by joining the carboxy terminal end of one subunit to the amino terminal end of the other subunit. COS-7 cells transfected with plasmids containing either TASK-1/TASK-3 or TASK-3/TASK-1 showed functional K+ currents with a pHo sensitivity somewhere between those observed with TASK-1 and TASK-3, as reported earlier in oocytes (Czirjak & Enyedi 2002a). In COS-7 cells, changing pHo from 8.3 to 7.3 decreased the current by 25 ± 4 % and 24 ± 5 % for TASK-1/TASK-3 and TASK-3/TASK-1 constructs, respectively. As the two tandem heteromers showed similar sensitivities to changes in pHo, the results obtained from the two heteromers were combined and shown in Fig. 1B,C. Although the responses to changes in pHo observed with the tandem heteromers were statistically significantly different from those of homomers, the differences were rather small.
Single-channel properties of TASK homomers and heteromers in COS-7 cells
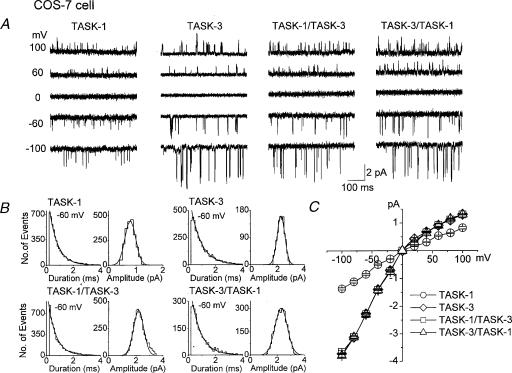
In order to identify the native K+ channel as a TASK-1/TASK-3 heteromer, it is important to know whether the single-channel properties of heteromers are different from those of homomers. Therefore, COS-7 cells were transfected with plasmids containing TASK-1, TASK-3, TASK-1/TASK-3 or TASK-3/TASK-1 and channel openings were recorded in cell-attached patches (Fig. 2A). The duration histogram obtained from patches containing only one level of opening could be fitted with a single exponential function. Single channel openings of TASK-1 and TASK-3 showed brief openings with mean open times of 0.8 ± 0.1 and 1.1 ± 0.2 ms (n= 5), respectively (Fig. 2B). The single-channel conductances of TASK-1 and TASK-3, determined from amplitude histograms obtained at –60 mV, were 14.2 ± 0.4 and 37.8 ± 0.7 pS, respectively, at an external divalent concentration of 1 mm. Both TASK-1 and TASK-3 exhibited weak inward rectification in symmetrical 150 mm KCl (Fig. 2C).
Figure 2. Single-channel kinetics of TASK-1, TASK-3 and heteromeric channels (TASK-1/TASK-3, TASK-3/TASK-1) expressed in COS-7 cells.
A, cell-attached patches were formed and single channel openings recorded at various membrane potentials. Pipette and bath solutions contained 150 mm KCl. Channel openings typical of those obtained in five other experiments are shown. B, amplitude and open time duration histograms of TASK-1, TASK-3 and heteromeric channels were obtained from openings at –60 mV, and fitted by a single exponential function and Gaussian function, respectively. C, single channel amplitudes were determined from amplitude histogram at each membrane potential to draw the current-voltage relationship. Each point is the mean ± S.D. of five determinations. Current–voltage relationships of TASK-3 and both heteromers could be superimposed.
Interestingly, both TASK-1/TASK-3 and TASK-3/TASK-1 heteromers displayed single-channel kinetics nearly identical to those of TASK-3. Thus, the mean open times of TASK-1/TASK-3 and TASK-3/TASK-1 were 0.9 ± 0.1 and 0.9 ± 0.1 ms, respectively, as estimated from duration histograms at –60 mV. The single-channel conductances measured at –60 and +60 mV were 38.1 ± 0.8 and 16.0 ± 0.7 pS for TASK-1/TASK-3, and 38.1 ± 0.6 and 16.2 ± 1.0 pS for TASK-3/TASK-1, respectively. The current–voltage relations of both heteromers were similar to that of TASK-3 but not to that of TASK-1 whose single-channel conductance is about half that of TASK-3 (16 pS). These results show that a native K+ channel that has single channel kinetics of TASK-3 could be either a TASK-3 homomer or a TASK heteromer. Clearly, other properties of TASK-3 and TASK-1/TASK-3 (or TASK-3/TASK-1) need to be explored to tell them apart.
pHo Sensitivity of TASK expressed in COS-7 cells: single-channel currents
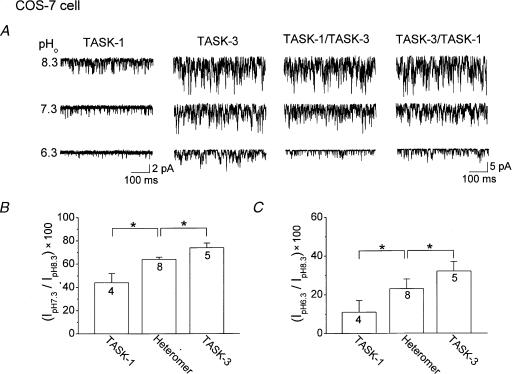
As TASK-3 homomer and TASK heteromers have slightly different pHo sensitivity (Fig. 1), we first chose to test whether such a difference can be observed at the single-channel level. K+ channels were recorded from outside-out patches formed from COS-7 cells expressing TASK-1, TASK-3, TASK-1/TASK-3 or TASK-3/TASK-1, and channel activities were measured at pHo 8.3, 7.3 and 6.3. Typical current tracings showing the inhibitory effect of acid are shown in Fig. 3A. As reported previously, TASK-1 and TASK-3 currents declined as the pH of the bath solution was decreased (Fig. 3). The acid-induced decrease in channel current was significantly greater for TASK-1 than TASK-3 (P < 0.05). These effects of protons on TASK-1 and TASK-3 are qualitatively similar to those observed at the whole-cell current level. In outside-out patches of COS-7 cells expressing TASK-1/TASK-3 and TASK-3/TASK-1, reductions in pHo also produced decreases in channel activity that were significantly less than that observed with TASK-1, but significantly greater than that observed with TASK-3. Thus, a change in pHo from 8.3 to 7.3 reduced TASK-1 by 56 ± 8 %, TASK-3 by 26 ± 4 %, TASK-1/TASK-3 by 36 ± 2 %, and TASK-3/TASK-1 by 35 ± 2 %. Because both heteromers were equally sensitive to pHo, the results from the two heteromers were combined (Fig. 3B). Qualitatively similar effects were observed when pHo was lowered to 6.3 (Fig. 3C). These results show that the pHo sensitivity of TASKs observed at the whole-cell level can be reproduced at the single-channel level, and provide the basis for testing whether the native K+ channel is a homomer or a heteromer.
Figure 3. pHo Sensitivity of single channel currents of TASK-1, TASK-3 and heteromeric channels (TASK-1/TASK-3, TASK-3/TASK-1) expressed in COS-7 cells.
A, single channel currents from outside-out patches are shown as inward currents recorded at –60 mV in symmetrical 150 mm KCl. Channel openings typical of those obtained in five other experiments are shown. B, current observed at pH 7.3 as a percent of that observed at pH 8.3. Channel current (channel activity × amplitude) was determined at –60 mV for TASK-1, TASK-3 and heteromers. The results obtained from both heteromers were combined. C, current observed at pH 6.3 as a percent of that observed at pH 8.3. An asterisk indicates a significant difference between two values (P < 0.05). The numbers inside the bar represent the number of cells (n) used.
pHo Sensitivity of TASK-3-like K+ channels in CG neurones
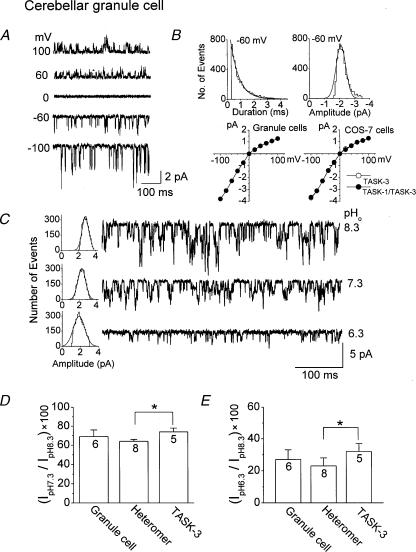
In CG neurones, a population of kinetically identical K+ channels with mean open time and conductance nearly identical to TASK-3 can be recorded from membrane patches without contamination by other ion channels. Although three other types of background K+ channels are present in CG neurones, they all have clearly different single-channel conductances and kinetics (Han et al. 2002). Figure 4A shows the single-channel openings of TASK-3-like K+ channels at different membrane potentials. The mean open time (1.1 ms) and the current–voltage relationship of this K+ channel, determined from duration and amplitude histograms, are indistinguishable from those of TASK-3 in COS-7 cells (Figs 2 and 4B). To determine whether the native K+ channel is a functional correlate of TASK-3 or TASK-1/TASK-3 heteromer, we determined the pHo sensitivity of the K+ channel using the same experimental method used above in COS-7 cells. Outside-out patches were formed from CG neurones and the effect of pHo was determined when only TASK-3-like channels were present in the patch, as judged by their mean open times (1.1 ms), single-channel conductance (∼38 pS at –60 and 16 pS at +60 mV) and the weak inwardly rectifying current voltage relationship (Fig. 4A,B). As shown in Fig. 4C, reduction in pHo was associated with a decrease in channel activity, similar to TASK-3 expressed in COS-7 cells. The decrease in channel current produced by a reduction of pHo from 8.3 to 7.3 was 31 ± 7 % for CG neurone K+ channel. This was not significantly different from the compared with that observed with TASK-3 or TASK-1/TASK-3 (Fig. 4D). Similarly, no significant difference could be observed with a reduction of pHo from 8.3 to 6.3 (Fig. 4E). Therefore, the single-channel studies on CG neurones failed to provide evidence to show that the native K+ channels are either TASK-3 homomers or TASK heteromers. The reasons for these results are discussed below.
Figure 4. Single-channel properties of K+ channels similar to TASK-3 cerebellar granule cells.
A, channel openings of TASK-3-like channels in neurones are shown at five different membrane potentials in cell-attached patches in symmetrical 150 mm KCl. B, amplitude and duration histograms of the K+ channel were determined from openings at –60 mV. Current amplitudes were determined at each membrane potential and used to draw the current–voltage relationship. Current–voltage relationships for TASK-3 and TASK-1/TASK-3 channels expressed in COS-7 cells are also shown for comparison (n= 5). C, single channel currents from an outside-out patch of a cerebellar granule cell are shown at three different pHo values. Inward currents observed at –60 mV in symmetrical 150 mm KCl are shown. Corresponding amplitude histograms are also shown to indicate the decreases in amplitude and conductance by changes in pHo. D, current observed at pH 7.3 as a percent of that observed at pH 8.3. The results from both heteromers (TASK-1/3 and TASK-3/1) were combined. E, the same as D except that the pHo was changed from 8.3 to 6.3. An asterisk indicates a significant difference between two values (P < 0.05). The numbers inside the bar represent the number of cells (n) used.
Ruthenium red sensitivity of TASK expressed in COS-7 cells
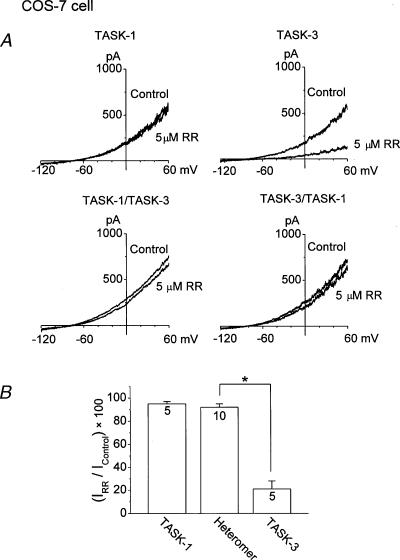
Ruthenium red has been reported to inhibit TASK-3 but not TASK-1 homomers and TASK-1/3 heteromers expressed in oocytes (Czirjak & Enyedi 2003). To help further distinguish between the TASK-3 homomer and TASK-1/TASK-3 heteromer, we re-examined the effect of ruthenium red on channels expressed in COS-7 cells first at the whole-cell level using a bath solution containing 5 mm KCl. The membrane potential of COS-7 cells expressing TASK-1 or TASK-3 was held at –80 mV and a voltage ramp was then applied from –120 mV to +60 mV for 1 second duration. As shown in Fig. 5, ruthenium red (5 μm) inhibited TASK-1 by 5 ± 2 % and TASK-3 by 79 ± 7 % (Fig. 5A). In COS-7 cells expressing TASK-1/TASK-3 and TASK-3/TASK-1 heteromers, ruthenium red (5 μm) inhibited the currents by 7 ± 2 %, and 8 ± 3 %, respectively (Fig. 5A). The results obtained from the two heteromers were similar and therefore combined in Fig. 5B. These results confirm earlier findings that ruthenium red is a selective inhibitor of TASK-3 homomers, among members of the TASK family (Czirjak & Enyedi 2003).
Figure 5. Sensitivity to ruthenium red of whole-cell currents of TASK-1, TASK-3 and heteromeric channels (TASK-1/TASK-3 and TASK-3/TASK-1) expressed in COS-7 cells.
A, whole-cell currents were recorded during a 1 second ramp pulse (–120 mV to +60 mV) in a bath solution containing 5 mm KCl. B, current observed in the presence of ruthenium red as a percent of control current measured at +60 mV. An asterisk indicates a significant difference between two values (P < 0.05). The numbers inside the bar represent the number of cells (n) used.
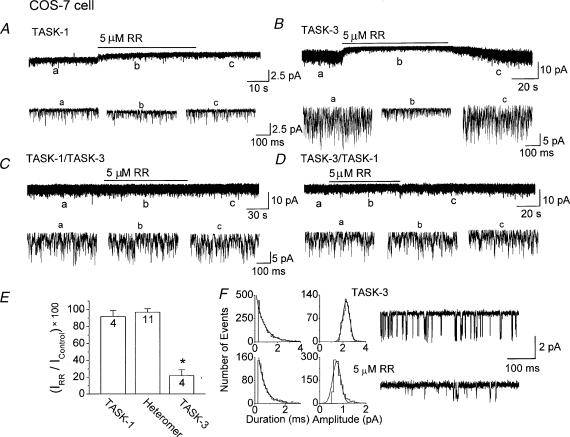
The effect of ruthenium red on TASK was studied in more detail at the single-channel level. Outside-out patches were formed from COS-7 cells expressing TASK-1 and TASK-3, and ruthenium red applied to the bath solution containing 150 mm KCl. As shown in Fig. 6A and 6B, ruthenium red (5 μm) had small inhibitory effect on TASK-1 activity (8 ± 7 %) but produced a 78 ± 7 % inhibition of TASK-3. The inhibition of TASK-3 by ruthenium red (5 μm) was associated with a marked decrease in single-channel amplitude as well as channel activity (Fig. 6F). Ruthenium red decreased the single channel conductance of TASK-3 by 62 % (38.3 pS to 14.3 pS) and the mean open time by 40 % (1.1 ms to 0.7 ms), producing an overall inhibition of TASK-3 current by ∼ 80 %. The inhibitory effect of ruthenium red on TASK-3 was fully reversible upon washout. As predicted from whole-cell current recordings, ruthenium red (5 μm) failed to produce significant effects (3 ± 3 % and 3 ± 4 % inhibition) on both TASK-1/TASK-3 and TASK-3/TASK-1 heteromers (Fig. 6C–E). These results confirm at the single channel levels that ruthenium red selectively inhibits TASK-3 among homomers and heteromers, and reveal that the inhibition is mainly due to the decrease in single-channel conductance and channel activity.
Figure 6. Sensitivity to ruthenium red of single-channel currents of TASK-1, TASK-3 and heteromeric channels (TASK-1/TASK-3 and TASK-3/TASK-1) expressed in COS-7 cells.
A-D, using outside-out patches, 5 μm ruthenium red was applied to the bath solution for 1–2 minutes and then washed off. Expanded current tracings are shown at indicated time points (lower case letters). E, effect of ruthenium red on the relative current of TASK-1, TASK-3, and the heteromers. The asterisk indicates a significant difference from the control observed before treatment of ruthenium red (P < 0.05). F, single channel openings from an outside-out patch showing only one TASK-3 channel are shown before and after application of ruthenium red. Amplitude and duration histograms obtained from these openings show the large inhibitory effect of ruthenium red on single channel TASK-3 conductance. TASK-3 control: 1.1 ± 0.2 ms and 2.3 ± 0.1 pA (–60 mV); after ruthenium red: 0.7 ± 0.1 ms and 0.9 ± 0.2 pA (– 60 mV).
Ruthenium red sensitivity of K+ channels in CG neurones
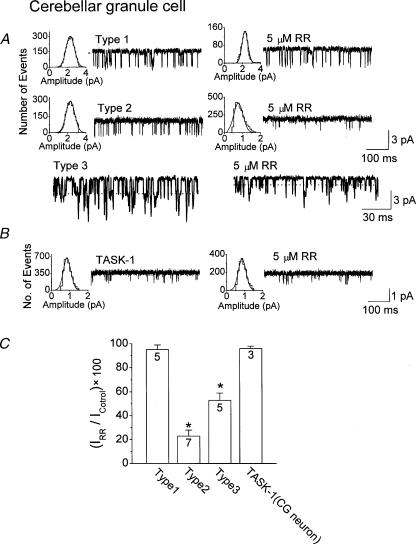
As ruthenium red strongly inhibits TASK-3 with only a small effect on TASK-1 and TASK heteromers, we predicted that this agent would help to identify the subunit composition of the native K+ channel. Unfortunately, a majority of outside-out patches formed showed more than one channel type and several channel openings, making it difficult to test for the presence of heteromeric TASK channels. However, because CG neurones grown in culture for only 1–2 days show a low level of channel expression, we were able to obtain outside-out patches that contained only one channel opening, although the success rate of getting only one channel was still very low (10 %). Based on the assumption that only one level of opening was present during the entire course of the experiment, it is probable that only one channel was present in the patch. Outside-out patches containing a K+ channel with single-channel kinetics and conductance similar to TASK-1 or TASK-3 were selected and tested for ruthenium red response. Surprisingly, in outside-out patches containing one open K+ channel with kinetics similar to those of TASK-3, we observed two types of responses (Fig. 7A). In the Type 1 response, application of ruthenium red (5 μm) to the bath solution produced almost no effect on the K+ channel current (95 ± 4 % of control; n= 5). In the Type 2 response, ruthenium red (5 μm) produced a large inhibition of the K+ current (23 ± 5 % of control; n= 7). Although ruthenium red (5 μm) inhibited the K+ current on average by 43 %, it was clear that there two population of responses to this agent. Based on the observed single-channel effects of ruthenium red (5 μm) on cloned TASK-3 and TASK-1/TASK-3 (Fig. 6), patches that show the Type 2 response probably contain only the TASK-3 homomer, and those that show the Type 1 response probably contain the TASK-1/TASK-3 heteromer. When patches contained several TASK-3-like channels, indicated by the number of openings in the current record, the inhibitory response to ruthenium red was always intermediate (47 ± 6 %; n= 5), suggesting that both TASK-3 homomers and TASK-1/TASK-3 heteromers were present (Type 3 response). In these patches, channel openings with amplitudes that are about half of that of TASK-3 could be seen after ruthenium red treatment, as indicated by the dotted lines in Type 3 response (Fig. 7A). In outside-out patches containing a K+ channel similar to TASK-1, ruthenium red (5 μm) did not produce a significant change in channel activity, as expected (Fig. 7B). These results summarized in Fig. 7C suggest that CG neurones in culture contain all three types of TASK isoforms. Based on the numbers of each type of responses to ruthenium red, the relative percentages of expression of TASK-3 homomer and TASK-1/TASK-3 heteromer in the plasma membrane of cultured CG neurones are estimated to be 56 % and 44 %, respectively. Therefore, approximately half of the native K+ channels that show single channel kinetics of TASK-3 are heteromers in CG neurones grown in culture for 1–3 days.
Figure 7. K+ channels similar to TASK-1 and TASK-3 in cerebellar granule neurones.
A, single channel currents from outside-out patches containing only K+ channels similar to TASK-3 are shown before and after treatment with ruthenium red. In patches containing one open K+ channel, two types of responses were observed. In Type 1 response, ruthenium red had a very small inhibitory effect (∼5 %). In Type 2 response, ruthenium red produced ∼ 80 % inhibition. In patches that contained several channels (Type 3 response), ruthenium red inhibited the channel activity by approximately half (48 %). The cell membrane potential was held at –60 mV. Pipette and bath solutions contained 150 mm KCl. B, single channel currents from outside-out patches containing only K+ channels similar to TASK-1, are shown before and after treatment with ruthenium red. C, summary of the effect of ruthenium red on K+ channel currents in CG neurones, showing the three types of responses. The asterisk indicates a significant difference from the Type 1 response (P < 0.05).
Discussion
TASK-1 and TASK-3 are members of the K2P channel family and are expressed in many regions in the brain as well as in certain peripheral tissues (Kim et al. 2000; Talley et al. 2001; Gu et al. 2002). They behave as background K+ channels, are modulated by pHo and are inhibited by neurotransmitters that are linked to Gq/11, indicating that both TASKs are important regulators of cell excitability and synaptic transmission. Native K+ channels with single channel kinetics similar to homomeric TASK-1 and TASK-3 have been identified in various cell types. However, whether the native K+ channel is a homomer or a heteromer is not yet known. The objective of this study was therefore to determine whether functional heteromeric TASK-1/TASK-3 is formed in vivo. Using two inhibitors of TASK channels, we demonstrated that functional TASK-1/TASK-3 heteromers are formed in a neuronal cell culture. Interestingly, we found that homomeric TASK-1 and TASK-3 are also present along with heteromeric TASK-1/TASK-3 in cultured CG neurones.
Single channel kinetics of TASK-3 homomer and TASK-1/TASK-3 heteromer
The primary difference between TASK-1 and TASK-3 is that the single-channel conductance of TASK-3 is about twice that of TASK-1 (Kim et al. 1999, 2000), making the distinction between these two channels relatively easy. Our study shows that the heteromeric TASK-1/TASK-3 has the current–voltage relationship and kinetics of opening nearly identical to those of TASK-3. Thus, native K+ channels previously thought to be TASK-3 could be either TASK-3 homomer or TASK-1/TASK-3 heteromer. To determine whether TASK-1/TASK-3 heteromers are present in a cell, at least two criteria need to be met. First, it is necessary to have a cell system that expresses both TASK-1 and TASK-3 mRNAs, and also express functional TASK-3-like K+ channels. Second, one requires an inhibitor or an activator that acts selectively on one TASK isoform rather than the other. The granular cell layer of the cerebellum is one of the brain regions that express both TASK-1 and TASK-3 mRNAs (Brickley et al. 2001; Han et al. 2002; Lauritzen et al. 2003). Functional K+ channels that are similar to TASK-1 and TASK-3 have also been recorded in CG neurones (Han et al. 2002). Therefore, the CG neuronal system is a good model to determine whether heteromeric TASKs are formed in vivo. To help distinguish between the TASK-3 homomer and TASK-1/TASK-3 heteromer, we employed two inhibitors that have been reported to be more selective for one isoform than the other, namely proton and ruthenium red (Czirjak & Enyedi 2002a, 2003). The effects of pHo and ruthenium red on TASK-1, TASK-3 and TASK-1/TASK-3 heteromer expressed in COS-7 cells and on K+ channels in CG neurones were therefore studied at both whole-cell and single-channel levels.
There are other procedures that could potentially distinguish TASK-1 homomers from TASK-1/TASK-3 heteromers. In oocytes, angiotensin II has been shown to inhibit TASK-1 and TASK-3 by ∼70 % and ∼20 %, respectively, whereas it inhibits TASK-1/TASK-3 heteromers by ∼60 % (Czirjak & Enyedi 2002a, 2003). Such differences in agonist-induced inhibition of different TASKs could be used to differentiate between TASK-3 and the TASK-1/TASK-3 heteromer for cloned channels. However, such a distinction in agonist-induced inhibition of TASK channels has not been observed in mammalian cell systems (Talley & Bayliss 2002). In the native system, it is critical to compare the effect of receptor agonists on single channel kinetics and activity in membrane patches, as measurement of whole-cell current is not useful due to the presence of other K+ channels. Because of the uncertainty in the signalling pathway and signalling molecules that inhibit native TASKs by receptor agonists, we were unable to employ receptor agonists as a method to identify the native K+ channels. It has been reported that extracellular divalent cations such as Ca2+ and Mg2+ inhibit TASK-3 but not TASK-1 (Derst et al. 2002). In our preliminary studies, 1 mm Ca2+ reduced the single-channel conductance of TASK-3 and TASK-1/TASK-3 by 31 ± 1 % and 32 ± 2 %, respectively, without an effect on TASK-1 conductance. Therefore, divalent cations are not useful in distinguishing between TASK-3 and TASK-1/TASK-3. Another molecule that has been reported to be a selective inhibitor of TASK-1 is anandamide (Maingret et al. 2001). We were unable to use anandamide because it easily activated TREK-2 channels that are abundant in CG neurones (Han et al. 2003), and pure TASK-3 channels were difficult to obtain in outside-out patches in the presence of anandamide. Whether anandamide inhibits TASK-1/TASK-3 was not studied.
Sensitivity of TASK-1, TASK-3 and heteromeric TASK-1/TASK-3 to pHo
TASK-1 and TASK-3 are both highly sensitive to pHo in the acidic range (pH 6.0–7.3), but the sensitivity differs more in the alkaline (pH 7.3–8.8) than in the acidic range. For example, a change in pHo from 7.3 to 8.3 increases TASK-1 by 133 % but increases TASK-3 by only 16 %. Such differences in pHo sensitivity have been observed in earlier studies (Czirjak & Enyedi 2002a). In both TASK-1 and TASK-3, the histidine residue located next to the pore amino acids that form the K+ channel signature sequence (GYGH) confers the pHo sensitivity, as mutation of this residue to other amino acids markedly reduces the pHo sensitivity (Kim et al. 2000; Rajan et al. 2000; Lopes et al. 2001; Morton et al. 2003). Although the difference in pHo sensitivity between TASK-1 and TASK-3 is easily measurable (Figs 1 and 3), the difference in pHo sensitivity between TASK-3 homomers and TASK-1/TASK-3 heteromers, although significant, is rather small. Therefore, we were not sure whether significant differences would be observed for native K+ channel in CG neurones, even if heteromers were present, because the experimental error would be larger at the single channel level when only a few channels can be studied. Indeed, we were unable to determine the identity of the native K+ channel using pHo sensitivity as the criterion. Between pH 6.3 and 8.3, the pHo sensitivity of the native K+ channel was not significantly different from that of either TASK-3 or TASK-1/TASK-3 (Fig. 4D,E). We now believe that the presence of both TASK-3 and TASK-1/TASK-3 in CG neurones is probably responsible for our results. In our experience, cultured or isolated neurones from various brain regions (hypothalamus, cortex, hippocampus) showed TASK-like K+ channel activities that were much lower than that observed in CG neurones. Therefore, the fact that we were unable to determine the subunit composition of the native K+ channels in CG neurones suggest that it will be difficult in general to use the ‘pHo sensitivity’ method to study the presence of heteromers in other cell types.
Sensitivity of TASK-1, TASK-3 and heteromeric TASK-1/TASK-3 to ruthenium red
Ruthenium red, an inhibitor of mitochondrial Ca2+ transporter and Ca2+ release channels, was recently found to selectively inhibit TASK-3 with little or no effect on TASK-1 (Czirjak & Enyedi 2003). Interestingly, although ruthenium red was able to strongly reduce TASK-3, it had a very small inhibitory effect on the TASK-1/TASK-3 heteromer. A recent study showed that ruthenium red inhibits TASK-3 by interconnecting the glutamate (E70) residue present in the extracellular region between M1 and P1 of one subunit with that of the second subunit (Czirjak & Enyedi 2003). Ruthenium red failed to tether the two subunits in TASK-1 homomers or TASK-1/TASK-3 heteromers because a glutamate residue at a similar region is not present in TASK-1 and only one glutamate residue is present in TASK-1/TASK-3 or TASK-3/TASK-1 heteromers. Therefore, TASK-1 and TASK-3 homomers, and the heteromer can be distinguished by their markedly different sensitivity to ruthenium red. Our result using COS-7 cells confirms the potent inhibitory action of ruthenium red on TASK-3 and its weak action on TASK-1 and TASK-1/TASK-3. In CG neurones, however, the effect of ruthenium red was variable. In outside-out patches from CG neurones containing only one open K+ channel with kinetics similar to TASK-3, ruthenium red showed a strong inhibition of channel activity in some patches as predicted for TASK-3 homomeric channels, but had a small effect in others as predicted for TASK-1/TASK-3 heteromeric channels. In patches containing several K+ channels, ruthenium red produced intermediate inhibition. The average inhibition elicited by ruthenium red was 43 %, a number that is inconsistent with the native K+ channel being either entirely TASK-3 or TASK-1/TASK-3, but consistent with a mixed population. Based on the numbers of patches showing different types of responses to ruthenium red, we conclude that approximately half (56 %) of the TASK-3-like channels are TASK-3 homomers and half (44 %) are TASK-1/TASK-3 heteromers in the soma of cultured CG neurones.
We were able to record K+ channels with single-channel properties similar to TASK-1 in some cell-attached and outside-out patches in earlier and present studies (Han et al. 2002), and our results show that TASK-1, TASK-3 and TASK1/TASK-3 heteromers are all present in CG neurones grown in culture for 1–3 days. The channel activity of K+ channels similar to TASK-1 does not change with growth, but that of TASK-3 increases steeply from day 3 to day 7 (Han et al. 2002). We speculate that TASK-1/TASK-3 heteromer formation increases as mRNA levels of both TASK-1 and TASK-3 increase during growth in culture. As TASK-3 and TASK-1/TASK-3 heteromer have identical channel kinetics, it would appear that only the expression level of TASK-3-like K+ channels is increased based on electrophysiology alone. The changes in mRNA levels that occur in culture and in vivo cannot be compared. Nevertheless, it is interesting that the expression of TASK-1 and TASK-3 mRNAs in vivo was found to increase during days 10–14 after birth in rats (Lauritzen et al. 2003), a time period the same as those in culture (7-day-old rat and 3 days in culture). It is quite plausible that the expression levels of TASK-1, TASK-3 and TASK-1/TASK-3 heteromer also change in vivo during growth and contribute to various cellular functions.
Physiological significance of TASK-1/TASK-3 heteromer formation
The formation of TASK-1/TASK-3 heteromers clearly increases the functional diversity of the TASK family. Both TASK-1 and TASK-3 mRNAs are expressed in many brain regions examined, as judged by PCR and in situ hybridization (Meadows & Randall 2001; Medhurst et al. 2001; Talley et al. 2001), suggesting that TASK-1/TASK-3 is probably formed in cells from these regions as well. TASK channels have been reported to be involved in various cell functions including growth and proliferation, apoptosis, and chemosensitivity (Buckler et al. 2000; Hartness et al. 2001; Trimarchi et al. 2002; Lauritzen et al. 2003; Mu et al. 2003; Pei et al. 2003). The heteromers along with TASK-3 homomers are probably important in these processes. With respect to pHo sensitivity, it seems reasonable to hypothesize that a cell possessing several TASK isoforms with different pKa values would be more adaptive to a wide range of pHo variations by responding faster and more accurately than a cell having one pHo sensor. Such fast responses could be important in cell excitability in response to acute changes in pHo. Similarly, a cell that has several TASK isoforms with different sensitivity to receptor agonists may have a greater control of membrane potential than a cell having only one TASK isoform. In a recent study comparing the expression of TASK-1 and TASK-3 mRNAs in P7, P14 and adult rats, it was found that TASK-1 mRNA expression in the granular layer of the cerebellum increased from P7 to P14 stage but declined afterwards (Brickley et al. 2001). In contrast, TASK-3 mRNA expression was high at P7 but declined by P14 and then increased again. Although the relative expressions of TASK-1, TASK-3 and heteromeric TASK have not been measured, these results suggest that the amount of TASK isoforms expressed may be important for normal cell growth and proliferation. Only TASK-1 is expressed in rat cardiac myocytes (Kim et al. 1999), whereas TASK-3 may be predominantly expressed in adrenal glomerulosa cells (Czirjak & Enyedi 2002b). Therefore, the formation of TASK heteromers is not a requirement for all cells, but may provide a tighter regulation of cell excitability in those cells that express it.
Acknowledgments
This work was supported by a grant-in-aid from the American Heart Association and National Intitute of Health (D.K.) and by the postdoctoral fellowship program of Korea Science and Engineering Foundation (Dawon Kang and Jaehee Han).
References
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Part 1):135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002a;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002b;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Ruthenium red inhibits TASK-3 potassium channel by interconnecting glutamate 70 of the two subunits. Mol Pharmacol. 2003;63:646–652. doi: 10.1124/mol.63.3.646. [DOI] [PubMed] [Google Scholar]
- Derst C, Liu GX, Musset B, Rajan S, Preisig-Muller R, Daut J. Sensitivity of TASK channels to extracellular divalent cations. Biophys J. 2002;82:636a. (Abstract) [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Gu W, Schlichthörl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Gnatenco C, Sladek CD, Kim D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2003;546:625–639. doi: 10.1113/jphysiol.2002.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartness ME, Lewis A, Searle GJ, Kelly I, Peers C, Kemp PJ. Combined antisense and pharmacological approaches implicate hTASK as an airway O2 sensing K+ channel. J Biol Chem. 2001;276:26499–26508. doi: 10.1074/jbc.M010357200. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am J Physiol. 1999;277:H1669–H1678. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honore E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis: Role of TASK leak K+ channels. J Biol Chem. 2003 doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Zilberberg N, Goldstein SA. Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J Biol Chem. 2001;276:24449–24452. doi: 10.1074/jbc.C100184200. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows HJ, Randall AD. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology. 2001;40:551–559. doi: 10.1016/s0028-3908(00)00189-1. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Barrat L, Southan AP, Page KM, Fyffe REW, Robertson B, Mathie A. A Functional Role for the Two-pore Domain Potassium Channel TASK-1 in Cerebellar Granule Neurons. Proc Natl Acad Sci USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton MJ, Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and -2. Pflugers Arch. 2003;445:577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C, Tong JJ, Spiegel L, Nguyen KC, Servoss A, Peng Y, Pei L, Marks JR, Lowe S, Hoey T, Jan LY, McCombie WR, Wigler MH, Powers S. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell. 2003;3:297–302. doi: 10.1016/s1535-6108(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci USA. 2003;100:7803–7807. doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Muller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Ransom BR. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis revisited. Prog Brain Res. 2000;125:217–228. doi: 10.1016/S0079-6123(00)25012-7. [DOI] [PubMed] [Google Scholar]
- Rose CR, Deitmer JW. Stimulus-evoked changes of extra- and intracellular pH in the leech central nervous system. II. Mechanisms and maintenance of pH homeostasis. J Neurophysiol. 1995;73:132–140. doi: 10.1152/jn.1995.73.1.132. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K (V) LQT1 and minK (IsK) proteins to form cardiac I (Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C, III, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JR, Liu L, Smith PJ, Keefe DL. Apoptosis recruits two-pore domain potassium channels used for homeostatic volume regulation. Am J Physiol Cell Physiol. 2002;282:C588–C594. doi: 10.1152/ajpcell.00365.2001. [DOI] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]