Abstract
Inbred strains of rodents have been used to study mammalian physiology and pathophysiology in an attempt to understand the contribution of genes in the pathogenesis of the disease process. In this review we focus on experimental animal models to identify quantitative trait loci (QTL) and possible strategies for identifying underlying genetic determinants responsible for hypertension. Confirmation of the existence of the QTL and dissection of the implicated region can be undertaken by production of either recombinant inbred, consomic or congenic strains. Despite complex interactions and the relatively few confirmed causative genes underlying QTL, recent developments in rat genome resources and advancement in statistical and bioinformatic methods will facilitate the identification of major gene(s) responsible for complex, polygenic traits.
Introduction
Model organisms have been utilized for over a century to understand biological processes (Barr, 2003). Inbred strains of rodents have been used to study mammalian physiology and pathophysiology in an attempt to understand the contribution of genes in the pathogenesis of the disease process (Beck et al. 2000; Stoll et al. 2001). Recent reviews have described mouse and other model organisms in some detail (Glazier et al. 2002; Svenson et al. 2003), and therefore this review will focus on the use of inbred rodent strains, with a special emphasis on the rat, to dissect genetic determinants of hypertension.
Human essential hypertension is a classic example of a complex, multifactorial, polygenic disease. It has been established that genetic determinants contribute between 30 and 50% of the blood pressure variation among individuals (Ward, 1990). Despite major recent advances in genome sequencing and statistical tools, the genetic dissection of essential hypertension still provides a formidable challenge (Colhoun, 1999). Several lines of investigation have been developed including linkage analysis in families segregating for rare, Mendelian forms of hypertension, candidate gene approaches and genome-wide scanning. Progress in identifying causative genes has been most successful in the Mendelian forms of hypertension in which several have now been characterized at the molecular level (Lifton et al. 2001). However, the contribution of these single gene defects to overall blood pressure variation in the general population is very small. Most traits with great impact on human health involve a number of genes that interact with one another and with environmental factors such as diet. Furthermore, candidate gene studies are by definition restricted to known variants and therefore cannot be used to identify new genes involved in the pathogenesis of hypertension. The methodological difficulties in studying the genetic determinants of human essential hypertension have given a major impetus for the development of similar, but inherently simpler paradigms in experimental models of genetic hypertension that remain under complex control (Rapp, 2000; Cowley, 2003). Genetic heterogeneity can be reduced by the use of inbred strains with complete control over environmental influences. Moreover, the ability to produce genetic crosses and analyse large numbers of progeny facilitate genetic analysis, including genetic dissection of complex phenotypes and gene–gene interactions (Sugiyama et al. 2001). We explain the basis of these techniques and discuss how they contribute to our understanding of the aetiology of hypertension.
Rat as a physiological model of hypertension
More than 30 years ago, researchers began selectively breeding rats to produce models with high, normal and low blood pressures (Dahl et al. 1962; Okamoto & Aoki, 1963). Many hypertensive rat strains have been produced ranging from the spontaneously hypertensive rat strains such as the spontaneously hypertensive rat (SHR) and stroke-prone spontaneously hypertensive rat (SHRSP) to strains where high dietary salt is necessary to induce hypertension such as the Dahl salt-sensitive and Sabra rats. These models also exhibit end-organ damage phenotypes similar to those seen in human essential hypertension, including left ventricular hypertrophy, stroke and renal failure. Most inbred rat strains were derived from Wistar-related stocks and others derived from Sprague-Dawley, but as these strains have a common origin there is relatively little genetic diversity. The exception is the Fawn-Hooded rat that may have a more distant origin (Lindsey et al. 1979).
Quantitative trait loci influencing blood pressure
The principal strategy in the rat for the search of genes involved in the development of hypertension has been the identification of quantitative trait loci (QTL) responsible for blood pressure regulation by genome wide scanning (Rapp, 2000). The principle difficulties in identifying QTL include epistasis, and a limited statistical power due to the number of hypotheses being tested. Moreover, any single QTL is responsible for only a fraction of the phenotypic variation and thus the phenotype–genotype correlation is low. Improvements in genomic resources and statistical analysis have led to the identification of a large number of QTL. However, despite the numerous complex traits that have been analysed by genome-wide linkage studies, few of the underlying genes have been identified (Glazier et al. 2002).
QTL mapping is a phenotype driven approach that does not require prior knowledge of either causative genes or their function and can lead to the identification of novel genes involved in disease. In the case of blood pressure measurement, the Data Sciences telemetry system is the gold standard and is used for measurement of systolic, diastolic and mean arterial pressure, heart rate and motor activity (Davidson et al. 1995; Clark et al. 1996). The genomic resources available for the rat are considerable, including large numbers of microsatellite markers allowing high resolution genetic linkage map of the rat genome (Bihoreau et al. 1997; Steen et al. 1999). QTL can be detected by linkage of a phenotype to genetic markers spread evenly throughout the genome and tested on a large cosegregating population that has been phenotypically assessed. In rat genetics, this segregating population can be constructed by crossing two phenotypically different strains, for example a hypertensive and normotensive (or hypotensive) strain, to produce a first filial (F1) generation. The F1 hybrids are identical, as they have inherited 50% of their genetic material from each of their parents. The two breeding strategies that utilize the F1 progeny involve either backcrossing to one of the parental strains or an F1 intercoss to produce the second filial generation (F2) (Silver, 1995). F1 backcrossing is generally better suited for oligogenic dominant traits; however, given the polygenic nature of blood pressure, F1 intercross have been preferred. The F2 generation contains animals that are genetically and phenotypically segregated and therefore ideal for detection of QTL by way of linkage analysis (Lander & Schork, 1994). Computer software packages such as MAPMAKER (Lander et al. 1987) have been developed to firstly construct a genetic linkage map and then examine each marker for linkage to the trait and compare with adjacent markers. Statistical analysis of QTL mapping is typically carried out by interval mapping and uses maximum likelihood tests. The interval mapping procedure is based on an expectation that maximizes the likelihood of a single-gene genetic model. Despite this assumption, this approach has been successfully applied to detect multiple regions that underlie a wide range of complex, quantitative traits. The significance of genetic linkage is determined by the logarithm of the odds ratio (LOD score) of obtaining a set of linkage data (a QTL) over the odds of obtaining the same data by chance. Thresholds for suggestive and significant linkage for mapping loci involved in complex traits in both human and animal studies have been suggested (Lander & Kruglyak, 1995). These thresholds are dependent on the mode of inheritance, the crossover rate and the mapping method used.
The first genome-wide scans to identify QTL involved in blood pressure regulation in 1991 identified three QTL with a LOD score greater than 3 (Hilbert et al. 1991; Jacob et al. 1991). These genome-scans used the same SHRSPHeidelberg and the WKYHeidelberg cross and 240 minisatellite and microsatellite markers. They identified loci on rat chromosomes 10, 18 and X. After these pioneering results, many other genome-wide scans were undertaken to search for QTL contributing to blood pressure regulation (Rapp, 2000). These experiments used the wide variety of strains available and therefore a large number of chromosomal regions have been implicated in rat hypertension, a number of which are reproducible between strains in several independent studies (Stoll et al. 2000). The identification of a QTL being involved in blood pressure regulation can be considered a first step towards causative gene identification (Dominiczak et al. 1998). A rodent QTL region is often approximately 20–30 cM in size and is much too large to functionally test all putative candidate genes. Possible strategies to confirm the existence of the QTL and to allow genetic dissection of the implicated region include production of either recombinant inbred, consomic or congenic strains and substrains (Garrett & Rapp, 2002a). However, a further complication arises if QTL affecting a given phenotype are clustered in tight linkage on the same chromosome, because it may or may not result in QTL peaks depending on whether the alleles underlying the peak contribute to the phenotype (cis) or whether they have contrasting phenotypic effects (trans). In the first case, the contribution of individual QTL will explain an even smaller proportion of the phenotypic variance than the combined effects making it even more difficult to identify the underlying genetic determinants (Garrett & Rapp, 2002b). The analysis of QTL has been improved by recently developed tools, such as pseudomarker 0.9, for detecting gene–gene interactions (Sen & Churchill, 2001). Mapmaker/QTL tests one locus at a time, but multiple QTL models have an advantage over single QTL models because of their ability to separate linked QTL on the same chromosome and to detect interacting QTL that may otherwise be undetected (Broman et al. 2003).
Inbred designer rats
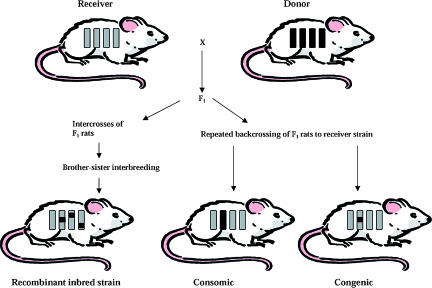
Recombinant inbred (RI) strains are genetic mosaics of the two founding strains that allow partitioning of individual complex traits into QTL with sufficient power to be studied as Mendelian loci (Fig. 1). RI strains are produced by crossing two inbred strains producing an F2 segregating population and selecting breeding pairs at random from the F2 population. Each pair then become founders for an inbred strain produced by 20 generations of brother × sister matings (Pravenec et al. 1989). It is possible to study the panel of strains in different environments and thus describe genotype–environment interactions, and similar to congenic strains it is possible to study animals of various ages revealing genetically controlled temporal effects. Although RI strains have proved to be useful tools for the analysis of complex traits (Pravenec et al. 1995), they are generally better suited for the mapping of Mendelian traits (Santos et al. 2001). More advanced forms of RI strains, such as second generation multiparental RI panels being developed for the mouse and recently for the rat, will be valuable for the study of complex traits (Printz et al. 2003).
Figure 1. Selected analytical tools in rat genetics.
The construction of recombinant inbred animals which are genetic mosaics of the two founding strains, consomic (chromosome substitution) and congenic strains as illustrated.
Marker-assisted ‘speed’ congenic breeding strategy
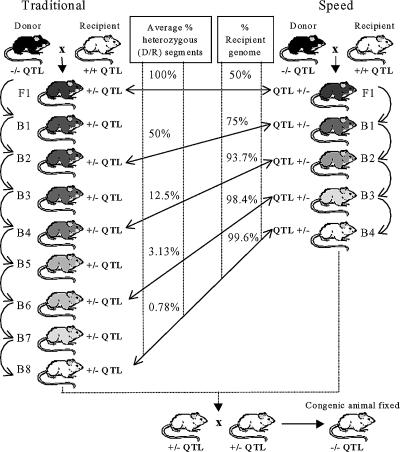
The traditional breeding strategy takes approximately three to four years to produce a congenic strain (Frantz et al. 1998). This time can be shortened by utilizing a marker-assisted ‘speed’ congenic breeding strategy previously tested in the mouse (Morel et al. 1996). Jeffs et al. (2000) were the first to show that this strategy can also be successful in the rat. Lander & Schork (1994) proposed that screening of polymorphic genetic markers covering the entire background of the genome could be used to select male offspring with fewest donor alleles in their background. In this way the next round of breeding using the ‘best’ male could dramatically reduce the time taken to clear the background of the recipient strain (Fig. 2). By four backcross generations it was calculated that donor genome contamination would be less than 1% if 60 background markers, spaced on average 25 cM apart, were used for screening 16 males at each generation. This speed congenic approach therefore reduces the average time required to produce strains to approximately two years (Jeffs et al. 2000).
Figure 2. Congenic strain construction to illustrate differences between the traditional and marker-assisted speed congenic approach.
Decreasing shades of grey to white represent the serial dilution of the donor genome in the genetic background. D, donor strain alleles; R, recipient strain alleles; B, backcross; F1, first filial generation.
Double congenic strains
As a first approximation, the effects of QTL are assumed to be additive, although, in the case of complex disease it is clear there may be epistatic interactions between or among QTL (Lander & Schork, 1994). A major interaction on blood pressure regulation was identified between QTL on chromosome 2 and 10 in an F2 population (Deng & Rapp, 1992). Comparison of the blood pressure response to a high salt diet of the double congenic, the two single congenic strains and Dahl salt-sensitive rats revealed a strong interaction (Rapp et al. 1998). Monti et al. (2003) analysed double and single congenic and parental strains and identified a significant epistatic interaction of systolic blood pressure QTL on chromosome 1 and 10. This indicates the feasibility of combining classical interval linkage analysis and double and single congenic strains to prove epistatic interactions of quantitative genetic factors regulating physiological systems such as blood pressure regulation.
From congenic interval to candidate gene
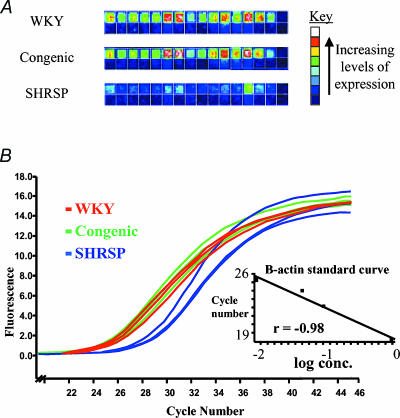
A combination of the strategies above is used to reduce the implicated region to the minimum as all genes in the transferred chromosomal region are, by definition, candidates for the phenotype observed. The most direct approach is to examine the appropriate genome sequence and annotation from human and mouse genome projects, to assess the gene content. Sequencing of these genes in the congenic and recipient strains can then be undertaken to identify functional mutations. Although, it would seem prudent to initially focus efforts on genes that may have relevance to the phenotype in question, the existence of genes with unknown or incompletely delineated function should not be forgotten. A particular useful approach may be microarray gene expression profiling to look for susceptibility genes whose action may be mediated at the level of variation in expression levels (Aitman et al. 1999). Aitman et al. (1999) used rat congenic strains derived from the spontaneously hypertensive (SHR) and Brown Norway (BN) strains, in combination with gene chip technology identifying a strong positional and physiological candidate gene, Cd36, although the congenic interval of the SHR.BN strain used was relatively large at 36 cM (Aitman et al. 1999). To detect differential expression the mRNA profiles should be generated from both parental and the congenic strains and genes underlying the QTL should be differentially expressed in both the hypertensive/congenic strain combination and in the interparental strain comparisons. More recent data from our laboratory utilized a similar strategy to search for blood pressure genes (McBride et al. 2003). The genome-wide mRNA expression was studied in the congenic animals derived from the stroke-prone spontaneously hypertensive (SHRSP) and Wistar Kyoto (WKY) and the two parental strains. Glutathione S-transferase (Gstm1) was identified as a putative positional and physiological candidate as it participates in the defence against oxidative stress. Furthermore, differential expression of Gstm1 was confirmed by real-time quantitative RT-PCR (Fig. 3).
Figure 3. Microarray and real-time PCR of rat Gstm1.
A, representative Affymetrix gene chip images for the 16 perfect match (upper) and mismatch (lower) probe set oligonucleotides for Gstm1 (X04229) from each of the WKY, congenic and SHRSP strains, showing decreased levels of Gstm1 expression in SHRSP. B, graph depicting the accumulation of fluorescence throughout the PCR for Gstm1; SHRSP (8.56 × 10−4 ± 1.6 × 10−4)versus congenic (3.67 × 10−3 ± 2.8 × 10−4); (95% C −3.9 × 10−3 to −1.8 × 10−3; P= 0.0034) and versus WKY (4.03 × 10−3 ± 5.1 × 10−4); (95% CI −5.4 × 10−3 to −8.9 × 10−4; P= 0.027). Inset graph indicates the β-actin standard curve.
The choice of tissue for gene expression profiling has been a matter of recent debate. It seems that for gene hunting experiments in hypertension the kidney is an ideal choice as several elegant transplantation experiments showed that hypertension ‘travels with the kidney’ (Rettig, 1993). The other important issue is the relative ease of interpretation of microarray data (Aitman et al. 1999). Whilst one would expect hundreds of differentially expressed genes between the two parental strains, this number is significantly reduced in the congenic and parental strain comparisons (McBride et al. 2003). Moreover, initially one would focus on genes that are differentially expressed and mapped to the congenic region, with a possibility of later analysis of genes mapping to other chromosomes but perhaps still of functional importance through the relevant physiological pathways (Wallace et al. 2002). However, sensitivity issues could be improved in situations where target tissue is more clearly defined by analysing cellular subpopulations of the tissue obtained by laser capture microdissection (Rekhter & Chen, 2001).
Studies have confirmed concordance of rodent and human blood pressure QTL (Stoll et al. 2000; Sugiyama et al. 2001). However, identifying all genes either underlying QTL or in introgressed regions is not straightforward. A further source of gene information may be provided by comparative genome analysis between rats, mice and man. In silico maps have been produced by translating large QTL between rat and human, predicting 26 chromosomal locations in the human genome that may harbour genes influencing blood pressure (Stoll et al. 2000). Comprehensive maps can be produced across blood pressure QTL refining conserved synteny analysis. Comparative sequencing, in particular, can be extremely useful for confirming either functional annotations, computational gene-finding results or identifying novel genes (International Genome Sequencing Consortium, 2001). It also has unique potential to identify conserved sequences that reside outside of coding regions that may influence transcriptional control (Mouse Genome Sequencing Consortium, 2002).
Novel strategies for candidate gene identification
A further strategy has been developed that allows the power of linkage analysis, and QTL mapping to be combined with expression profiling (Jansen & Nap, 2001). As described, QTL detection is frequently used to identify large chromosomal regions that are statistically associated with the phenotypic trait of interest. All genes underlying the QTL are by definition candidates for the phenotype in question, but even QTL of a moderate size contain many hundreds of genes with fine mapping yielding too many candidates to proceed to appropriate validation experiments. The novel strategy utilizes genetic variation between segregating populations and adds the analytical tools available for molecular markers to the analysis of genome-wide expression profile data. The expression profiling of all individuals in a segregating population allows the profile of each gene in the population to be treated as a quantitative trait and can be resolved into the contributing loci and mapped as such. Brem et al. (2002) were the first to demonstrate the utility of this strategy by completing the first dissection of transcriptional regulation in budding yeast by showing that expression levels were complex and that loci identified by linkage fell into two distinct categories. The majority of loci were categorized as cis-acting modulators by looking at mRNA levels that were linked to markers within 10 kb of their own gene suggesting that polymorphisms affecting gene expression lies within the gene or its regulatory regions. However, a smaller number of trans-acting loci were also identified with widespread transcriptional effects. Comprehensive genetic screens of mouse, plant and human transcriptomes have now been undertaken in which gene expression values are considered as quantitative traits (Schadt et al. 2003). The identification of genetic loci that account for variation in the levels of gene expression were termed expression quantitative trait loci (eQTL). By focusing on a mouse model of obesity, specifically the mass of fat pads, they were initially able to divide the mice into high and low fat-pad mass; however, using gene expression profiling data they were also able to subdivide the high mass class into two subgroups. Subsequent genetic analysis of these two high fat-pad mass groups led to the identification of different QTL on different chromosomes indicating that these loci only affect a subset of the F2 population. In addition, stratification into functional groups with re-analysis led to the identification of further QTL not implicated in the original investigation suggesting another level of complexity underlying complex traits even in relatively ‘simple’ animal models of human disease. Despite these complex interactions and the relative paucity of confirmed causative candidate genes, it is concluded that the recent developments in genome resources including genome sequencing and genome-wide microarray expression profiling together with advancement in statistical and bioinformatic methods will facilitate the identification of major gene(s) responsible for complex, polygenic traits.
Acknowledgments
The BHF Glasgow Cardiovascular Research Centre group is funded by the British Heart Foundation Programme Grant (RG/02012) and the Wellcome Trust Functional Genomics Initiative (066780).
References
- Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of CD 36 (Fat) as an insulin-resistance gene causing fatty acid and glucose metabolism in hypertensive rat. Nature Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- Barr MM. Supermodels. Physiol Genomics. 2003;13:15–24. doi: 10.1152/physiolgenomics.00075.2002. [DOI] [PubMed] [Google Scholar]
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Bihoreau MT, Gaugier D, Kato N, Hyne G, Lindpaintner K, Rapp JP, James MR, Lathrop GM. A linkage map of the rat genome derived from three F2 crosses. Genome Res. 1997;7:434–440. doi: 10.1101/gr.7.5.434. [DOI] [PubMed] [Google Scholar]
- Brem RB, Yvert G, Clinton R, Kruglyak L. Genetic dissection of transcriptional regulation in budding yeast. Science. 2002;296:752–755. doi: 10.1126/science.1069516. [DOI] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Charchar FJ, Tomaszewski M, Strahorn P, Champagne B, Dominiczak AF. Y is there a risk being male? Trends Endocrinol Metab. 2003;14:163–168. doi: 10.1016/s1043-2760(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Clark JS, Jeffs B, Davidson AO, Lee WK, Anderson NH, Bihoreau MT, Brosnan MJ, Devlin AW, Lindpaintner K, Dominiczak AF. Quantitative trait loci in genetically hypertensive rats. Possible sex specificity. Hypertension. 1996;28:898–906. doi: 10.1161/01.hyp.28.5.898. [DOI] [PubMed] [Google Scholar]
- Colhoun H. Confirmation needed for genes for hypertension. Lancet. 1999;353:1200–1201. doi: 10.1016/S0140-6736(99)00001-X. [DOI] [PubMed] [Google Scholar]
- Cowley AW. Genomics and homeostasis. Am J Physiol Regul Integr Comp Physiol. 2003;284:R611–627. doi: 10.1152/ajpregu.00567.2002. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Roman RJ, Kaldunski ML, Dumas P, Dickout JD, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rats. Hypertension. 2001;37:456–461. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- Dahl LK, Heine M, Tassinari L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess of salt ingestion. Nature. 1962;194:480–482. doi: 10.1038/194480b0. [DOI] [PubMed] [Google Scholar]
- Davidson AO, Schork NJ, Jaques BC, Kelman AW, Sutcliffe RG, Reid JL, Dominiczak AF. Blood pressure in genetically hypertensive rats. Influence of the Y chromosome. Hypertension. 1995;26:452–459. doi: 10.1161/01.hyp.26.3.452. [DOI] [PubMed] [Google Scholar]
- Deng AY, Rapp JP. Cosegregation of blood pressure with ACE and ANP receptor genes using Dahl salt-sensitive rats. Nature Genet. 1992;1:267–272. doi: 10.1038/ng0792-267. [DOI] [PubMed] [Google Scholar]
- Dominiczak AF, Clark JS, Jeffs B, Anderson NH, Negrin CD, Lee WK, Brosnan MJ. Genetics of experimental hypertension. J Hypertension. 1998;16:1859–1869. doi: 10.1097/00004872-199816121-00003. [DOI] [PubMed] [Google Scholar]
- Ely DL, Daneshvar H, Turner ME, Johnson ML, Salisbury RL. The hypertensive Y chromosome elevates blood pressure in F11 normotensive rats. Hypertension. 1993;21:1071–1075. doi: 10.1161/01.hyp.21.6.1071. [DOI] [PubMed] [Google Scholar]
- Frantz SA, Kaiser M, Gardiner S, Gauguier D, Vincent M, Thompson JR, Bennett T, Samani NJ. Successful isolation of a rat chromosome 1 blood pressure QTL in reciprocal congenic strain. Hypertension. 1998;32:639–646. doi: 10.1161/01.hyp.32.4.639. [DOI] [PubMed] [Google Scholar]
- Garrett MR, Rapp JP. Multiple blood pressure QTL on rat chromosome 2 defined by congenic Dahl rats. Mammalian Genome. 2002a;13:41–44. doi: 10.1007/s00335-001-2114-y. [DOI] [PubMed] [Google Scholar]
- Garrett MR, Rapp JP. Two closely linked interactive blood pressure QTL on rat chromosome 5 defined using congenic Dahl rats. Physiol Genomics. 2002b;8:81–86. doi: 10.1152/physiolgenomics.00080.2001. [DOI] [PubMed] [Google Scholar]
- Glazier AM, Nadeau JH, Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–2349. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- Hilbert P, Lindpaintner K, Beckmann JS, Serikawa T, Soubrier F, Dubay C, Cartwright P, De Gouyon B, Julier C, Takahasi S. Chromosomal mapping of two genetic loci assoiated with blood pressure regulation in hereditary hypertensive rats. Nature. 1991;353:521–529. doi: 10.1038/353521a0. [DOI] [PubMed] [Google Scholar]
- International Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Jacob HJ, Lindpaintner K, Lincoln SE, Kusumi K, Bunker RK, Mao YP, Ganten D, Dzau VJ, Lander ES. Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell. 1991;67:213–224. doi: 10.1016/0092-8674(91)90584-l. [DOI] [PubMed] [Google Scholar]
- Jansen RC, Nap JP. Genetical genomics: the added value from segregation. Trends Genet. 2001;17:388–391. doi: 10.1016/s0168-9525(01)02310-1. [DOI] [PubMed] [Google Scholar]
- Jeffs B, Negrin CD, Graham D, Clark JS, Anderson NH, Gauguier D, Dominiczak AF. Applicability of a speed congenic strategy to dissect blood pressure QTL on rat chromosome 2. Hypertension. 2000;35:179–187. doi: 10.1161/01.hyp.35.1.179. [DOI] [PubMed] [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincolm SE, Newburg L. Mapmaker: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lander ES, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nature Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- Lindsey JR. The laboratory rat. Biology and diseases. In: Barker HJ, Lindsey JR, editors. Historical Foundations. New York: Academic Press; 1979. pp. 1–36. [Google Scholar]
- McBride MW, Carr FJ, Graham D, Anderson NH, Clark JS, Lee WK, Charchar FJ, Brosnan MJ, Dominiczak AF. Microarray analysis of rat chromosome 2 congenic strains. Hypertension. 2003;41:847–853. doi: 10.1161/01.HYP.0000047103.07205.03. [DOI] [PubMed] [Google Scholar]
- Meng H, Garrett MR, Dene H, Rapp JP. Localisation of a blood pressure QTL to a 2.4cM interval on rat chromosome 9 using congenic strains. Genomics. 2003;81:210–220. doi: 10.1016/s0888-7543(03)00003-x. [DOI] [PubMed] [Google Scholar]
- Monti J, Plehm R, Schultz H, Ganten D, Kreutz R, Hubner N. Interaction between blood pressure QTL in rats in which trait variation at chromosome 1 is conditional upon a specific allele at chromosome 10. Hum Mol Genet. 2003;12:435–439. doi: 10.1093/hmg/ddg041. [DOI] [PubMed] [Google Scholar]
- Morel L, Yu Y, Blenman KR, Caldwell RA, Wakeland EK. Production of congenic mouse strains carrying genomic intervals containing SLE-susceptibility genes derived from the SLE-prone NZM2410 strain. Mammalian Genome. 1996;7:335–339. doi: 10.1007/s003359900098. [DOI] [PubMed] [Google Scholar]
- Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nature Genet. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Negrin CD, McBride MW, Carswell HVO, Graham D, Carr FJ, Clark JS, Jeffs B, Anderson NH, Macrae IM, Dominiczak AF. Reciprocal consomic strains to evaluate Y chromosome effects. Hypertension. 2001;37:391–397. doi: 10.1161/01.hyp.37.2.391. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rat. Jap Circulation J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Pravenec M, Gaugier D, Schott JJ, Buard J, Kren V, Bila V, Szpirer C, Szpirer J, Wang JM, Huang H. Mapping of quantitaive trait loci for blood pressure and cardiac mass in the rat by genome scanning of recombinant inbred strains. J Clin Inves. 1995;96:1973–1978. doi: 10.1172/JCI118244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravenec M, Klir P, Kren V, Zicha J, Kunes J. An analysis of spontaneous hypertension in spontaneously hypertensive rats by means of new recombinant inbred strains. J Hypertension. 1989;7:217–221. [PubMed] [Google Scholar]
- Printz MP, Jirout M, Jaworski R, Alemayehu A, Kren V. HXB/BXH rat recombinant inbred strain platform: a newly enhanced tool for cardiovascular, behavioral, and developmental genetics and genomics. J Appl Physiol. 2003;94:2510–2522. doi: 10.1152/japplphysiol.00064.2003. [DOI] [PubMed] [Google Scholar]
- Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev. 2000;80:135–172. doi: 10.1152/physrev.2000.80.1.135. [DOI] [PubMed] [Google Scholar]
- Rapp JP, Garrett MR, Deng AY. Construction of a double congenic strain to prove epistatic interaction on blood pressure between rat chromosomes 2 and 10. J Clin Inves. 1998;101:1591–1595. doi: 10.1172/JCI2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekhter MD, Chen J. Molecular analysis of complex tissues is facilitated by laser capture microdissection: critical role of upstream tissue processing. Cell Biochem Biophys. 2001;35:10–113. doi: 10.1385/CBB:35:1:103. [DOI] [PubMed] [Google Scholar]
- Rettig R. Does the kidney play a role in the aetiology of primary hypertension? Evidence from renal transplantation studies in rats and humans. J Hum Hypertens. 1993;7:177–180. [PubMed] [Google Scholar]
- Santos J, Herranz M, Fernandez M, Vaquero C, Lopez P, Fernandez-Piqueras J. Evidence of a possible epigenetic inactivation mechanism operating on a region of mouse chromosome 19 in gamma-radiation-induced thymic lymphomas. Oncogene. 2001;20:2186–2189. doi: 10.1038/sj.onc.1204297. [DOI] [PubMed] [Google Scholar]
- Schadt EE, Monks SA, Drake TA, Lusis AJ, Colinayo V, Ruff TG, Milligan SB, Lamb JR, Cavet G, Linsley PS, Mao M, Stoughton RB, Friend SH. Genetics of gene expression surveyed in maize, mouse and man. Nature. 2003;422:297–302. doi: 10.1038/nature01434. [DOI] [PubMed] [Google Scholar]
- Sen S, Churchill GA. A statistical framework for quantitative trait mapping. Genetics. 2001;159:371–387. doi: 10.1093/genetics/159.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver LM. Mouse Genetics: Concepts and Applications. Oxford: Oxford University Press; 1995. [Google Scholar]
- Steen RG, Kwitek-Black AE, Glenn C, Gullings-Handley J, Van Etten W, Atkinson S, Appel D, Twigger S, Muir M, Mull T, Granados M, Kissebah M, Russo K, Crane R, Popp M, Peden M, Matise T, Brown DM, Lu J, Kingsmore S, Tonellato PJ, Rozen S, Slonim D, Young P, Knoblauch M, Provoost A, Ganten D, Colman SD, Rothberg J, Lander ES, Jacob HJ. A high-density integrated genetic linkage and radiation hybrid map of the laboratory rat. Genome Res. 1999;9:AP1–AP8. [PubMed] [Google Scholar]
- Stoll M, Cowley AW, Jr, Tonellato PJ, Greene AS, Kaldunski ML, Roman RJ, Dumas P, Schork NJ, Wang Z, Jacob HJ. A genomic-systems biology map for cardiovascular function. Science. 2001;294:1723–1726. doi: 10.1126/science.1062117. [DOI] [PubMed] [Google Scholar]
- Stoll M, Kwitek-Black AE, Cowley AW, Harris EL, Harrap SB, Krieger JE, Printz MP, Provoost AP, Sassard J, Jacob HJ. New target regions for human hypertension via comparative genomics. Genome Res. 2000;10:473–482. doi: 10.1101/gr.10.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama F, Churchill GA, Higgins DC, Johns C, Makaritsis KP, Gavras H, Paigen B. Concordance of murine quantitative trait loci for salt-induced hypertension with rat and human loci. Genomics. 2001;71:70–77. doi: 10.1006/geno.2000.6401. [DOI] [PubMed] [Google Scholar]
- Svenson KL, Bogue MA, Peters LL. Identifying new mouse models of cardiovascular disease: a review of high-throughput screens of mutagenized and inbred strains. J Appl Physiol. 2003;94:1650–1659. doi: 10.1152/japplphysiol.01029.2003. [DOI] [PubMed] [Google Scholar]
- Wallace CA, Ali S, Glazier AM, Norsworthy PJ, Carlos DC, Scott J, Freeman TC, Stanton LW, Kwitek AE, Aitman TJ. Radiation hybrid mapping of 70 genes from a data set differentially expressed genes. Mammalian Genome. 2002;13:194–197. doi: 10.1007/s00335-001-2140-9. [DOI] [PubMed] [Google Scholar]
- Ward R. Familial aggregation and genetic epidemiology of blood pressure. In: Laragh JH, Brenner BM, editors. Hypertension: Pathology, Diagnosis and Management. New York: Raven Press; 1990. pp. 811–1000. [Google Scholar]