Abstract
Noxious stimuli inhibit inflammation by activating neuroendocrine stress axes, an effect that is potently attenuated by ongoing activity in subdiaphragmatic vagal afferents. Because this vagal afferent activity is carried in the coeliac and coeliac accessory branches of the subdiaphragmatic vagus, we tested the hypothesis that the activity arises from vagal afferents that innervate a proximal segment of the gastrointestinal tract. Surgical removal of the duodenum, but not the stomach, produces a marked (six orders of magnitude) leftward shift in the dose–response curve for intraplantar capsaicin-induced inhibition of synovial plasma extravasation induced by the potent inflammatory mediator bradykinin, in the knee joint; this is similar in magnitude to the inhibition produced by subdiaphragmatic or by coeliac plus coeliac accessory branch vagotomy. Fasting, to unload mechanically sensitive polymodal afferents in the proximal gastrointestinal tract, produces a similar leftward shift in the dose–response curve for the inhibitory effect of capsaicin, an effect that is reversed by balloon distension in the duodenum in fasted rats, while balloon distension postvagotomy had no effect. These results suggest that activation of mechanically sensitive vagal afferents in the duodenum contributes vagal afferent activity that modulates neuroendocrine control of the inflammatory response.
Noxious stimuli, such as that produced by an inflammatory lesion, inhibit the inflammatory response (Green et al. 1995; Miao et al. 1997b, 2000; Miao & Levine, 1999). This noxious stimulus-induced anti-inflammatory effect is mediated by neuroendocrine mechanisms, specifically a propriospinal pathway between afferent nociceptive inflow, a spino-bulbo-spinal pathway and preganglionic neurones projecting to the adrenal medullae (Miao et al. 1997b, 2000, 2001a; Miao & Levine, 1999). It is modulated by activity in subdiaphragmatic vagal afferents (Miao et al. 1997b); the spinal and spino-bulbo-spinal pathways involved are under powerful inhibitory control maintained by vagal activity and total subdiaphragmatic, or coeliac plus accessory coeliac branch vagotomy produces a marked (six orders of magnitude) leftward shift in the dose–response curve for noxious stimulus-induced inhibition of the plasma extravasation produced by bradykinin (Miao et al. 1997b), a potent inflammatory mediator (Regoli & Barabe, 1980). Such inhibitory effects of noxious stimulation can be reproduced by electrical stimulation of the proximal end of the cut subdiaphragmatic vagus nerve (F. Miao, unpublished observation). Similarly, the potency of spinal intrathecal nicotine, which inhibits the plasma extravasation by acting on central terminals of primary afferent nociceptors (Miao et al. 2003), is similarly enhanced following subdiaphragmatic vagotomy (Miao et al. 1994). In this study we have carried out experiments to determine the visceral organ from which the relevant vagal afferent activity that modulates bradykinin-induced plasma extravasation arises and physiological stimuli that activate these vagal afferents.
Methods
The experiments were performed on male Sprague-Dawley rats (300–400 g). For the surgical procedures and during the knee joint perfusion experiments, rats were anaesthetized by intraperitoneal injection of sodium pentobarbital (65 mg kg−1, Abbott Laboratory, Chicago, IL, USA). Animals were killed at the end of the experiments by sodium pentobarbital overdose (250 mg kg−1) followed by bilateral thoracotomy. Animal care and use conformed to NIH guidelines for the care and use of experimental animals. The University of California at San Francisco Committee on Animal Research approved the experimental protocol.
Perfusion of the knee joint
Knee joint perfusion was performed as previously described (Coderre et al. 1989; Miao et al. 1997b). In brief, after incision of the skin and connective tissue overlying the anterior aspect of the knee, Evans blue dye (50 mg kg−1), which binds to albumin and thus serves as a quantitative marker for plasma extravasation, was administered intravenously into the saphenous vein. Ten minutes later, a 30-gauge needle was inserted into the cavity of the knee joint for the infusion of fluid (250 μl min−1). After infusion of an initial volume of 100–200 μl of vehicle, a second needle (25-gauge), serving as an outflow cannula, was inserted into the knee joint, approximately 3 mm from the inflow needle. Fluid was withdrawn from the joint through the outflow cannula, using a second syringe pump. The fluid was infused and withdrawn at a constant rate of 250 μl min−1. Perfusate samples were collected every 5 min for a period of up to 145 min. Samples were analysed for the amount of Evans blue dye by spectrophotometric measurement of absorbance at 620 nm. The absorbance at this wavelength is linearly related to the dye concentration (Carr & Wilhelm, 1964).
After a baseline perfusion period of 15 min with vehicle, extravasation of plasma, into the knee joint, was stimulated by adding bradykinin to the perfusion fluid (160 ng ml−1) at a concentration that is in the range detected in inflamed tissues (Hargreaves et al. 1993; Swift et al. 1993). Both knee joints in the same rat were perfused simultaneously.
Intra-plantar capsaicin
In order to examine the effect of nociceptor-induced inhibition of plasma extravasation, spinal afferents were excited from a site remote from the articular site at which the inflammatory response was being evaluated (i.e. the knee), by intraplantar injection of capsaicin. Capsaicin was injected in the paw at progressively higher doses (3–30 μg, at half-log dose increments in a volume of 10 μl per dose) at intervals of 20 min. To avoid a systemic effect of high-dose capsaicin, the dose–response relationship for the effect of intraplantar capsaicin was stopped at 30 μg (Miao et al. 2001b).
Subdiaphragmatic vagotomy
When the subdiaphragmatic vagus nerves were cut bilaterally, the inhibition of bradykinin-induced plasma extravasation by intrathecal nicotine was dramatically potentiated (Miao et al. 1993). The procedure has been previously described (Miao et al. 1993). Briefly, after incision of the lateral abdominal wall in the left upper quadrant, the subdiaphragmatic oesophagus was fully exposed. Approximately 3 mm of the vagus nerves were then dissected free from the oesophagus and removed bilaterally. Knee joint perfusion experiments began ∼1 h after this surgical intervention.
Gastrectomy and duodenectomy
The duodenum was studied because it is the most proximal structure in the gastrointestinal tract innervated by the coeliac and coeliac accessory branches of the subdiaphragmatic vagus, although minimal innervation of the stomach has not been excluded (Phillips & Powley, 1998); the stomach was used as a control, a neighbouring gastrointestinal structure. After a midline incision of the linea alba, the stomach or duodenum was first ligated at both ends, then resected between the ligatures (Hebel & Stromberg, 1976). Knee joint perfusion experiments were performed immediately after this surgical procedure.
Stimulation of mechanosensitive duodenal afferents
Vagal afferents can be activated by physiological stimuli, including pH, osmolarity, a variety of nutrients and mechanical distension (Mei, 1983; Andrews, 1986; Grundy, 1993; Schwartz et al. 1995; Schwartz & Moran, 1998; Phillips & Powley, 2000; Raybould, 2001); many vagal afferents are polymodal, responding to multiple stimulus modalities. To demonstrate if activation of mechanically sensitive duodenal afferent fibres can modulate the anti-inflammatory actions of capsaicin or nicotine a size 9 balloon (8.5 mm × 13 mm; Harvard Apparatus, Holliston, MA, USA) was inserted into the second section of the duodenum with a connecting catheter extending out of the duodenal cavity, through the wall of the intestine. The duodenum was sutured and the abdominal cavity closed. Injecting air into the catheter produced duodenal distension. Intraluminal pressure was digitally displayed, via a pressure transducer (Harvard Apparatus, Hollister, MA, USA), which was connected to the catheter. In preliminary studies bradykinin-induced plasma extravasation was measured under 10, 20 30, 40, 50, 60 or 70 cmH2O intraluminal pressure in the balloon (data not shown). We found that 30–40 cmH2O was the optimal (i.e. the lowest pressure able to produce the maximal effect) pressure to enhance bradykinin-induced plasma extravasation. This pressure is greater than the peak amplitude of normal peristaltic contractions (20–25 cmH2O) of the perfused rat duodenum (Fujimiya et al. 1997), and activates vagal afferents to decrease diastolic blood pressure (Moss & Sanger, 1990).
Electrical stimulation of vagal afferents
To study the contribution of afferents in the vagus nerves, the vagus was transected by dissecting out 1–2 mm of the distal end of the vagus. After cutting the vagus nerves, we applied continuous electrical stimulation (2 Hz, 0.5 ms, 3 V, 10 mA) to the proximal side of the vagus (i.e. close to the diaphragm) via a pair of platinum electrodes (Miao et al. 1994). The surgical field was filled with mineral oil. Body temperature was monitored via a rectal thermal probe that controlled a water-filled heating pad to maintain core body temperature at 37°C.
Fasting
Whilst the duodenum contains a large percentage of vagal afferents that are sensitive to solid food, fasting only eliminates mechanical stimuli produced by bulk volume in the gastrointestinal tract (Blackshaw & Grundy, 1990; Schwartz et al. 1995; Schwartz & Moran, 1998). Since transection of the subdiaphragmatic vagus dramatically potentiated the anti-inflammatory action of intraplantar capsaicin and intrathecal nicotine, reduced activity in a visceral vagal afferent should also produce similar potentiation. We tested this hypothesis by fasting rats for 48 h, i.e. restricting solid food but allowing ad libitum access to drinking water (Krolczyk et al. 2001).
Statistics
Data are presented as mean ±s.e.m.; two-way (group × time) repeated measures analysis of variance (anova) was used, followed by Fisher or Bonferroni's post hoc test, to determine significant differences between pairs of curves. Differences were considered statistically significant at a P < 0.05.
Dose–response relationships
Dose–response relationships for intrathecal nicotine or intraplantar capsaicin inhibition of bradykinin-induced plasma extravasation were obtained by a cumulative dosing method (Miao & Lee, 1989; Miao et al. 1997a). The doses that produced 50% of the maximum inhibition (ED50 values) were determined for each joint. A 300 μg capsaicin dose, which induces maximal inhibition (i.e. Emax), was not included because this dose also produces systemic effects (Miao & Levine, 1999).
Materials
The chemicals used in the present experiments were obtained as follows: bradykinin acetate, naloxone hydrochloride, nicotine hydrogen tartrate, capsaicin and Tween 80 from Sigma Chemical Co., St Louis, MO, USA and, phentolamine hydrochloride from Ciba Pharmaceutical, Summit, NJ, USA. Capsaicin was first dissolved in a solution of ethanol and Tween 80 (1:1 ratio) and then diluted in normal saline (Baxter Laboratories, Inc., Deerfield, IL, USA). All other chemicals were dissolved in normal saline.
Results
Vagotomy enhances capsaicin effect
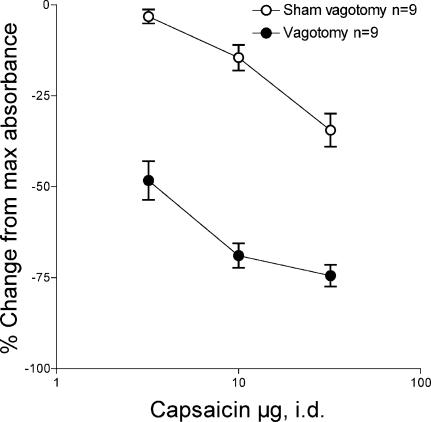
The perfusion of bradykinin through the knee joint produces a dose-related increase in plasma extravasation (Miao et al. 1996). This plasma extravasation reaches a stable level by approximately 30 min after onset of perfusion (data not shown). The intraplantar injection of capsaicin, in cumulatively higher doses, produces a dose-related suppression of bradykinin-induced plasma extravasation (Fig. 1, ○). In rats that have had subdiaphragmatic (Fig. 1, •) or coeliac plus coeliac accessory branch vagotomies, there is a marked enhancement of the inhibitory effect of capsaicin on bradykinin-induced plasma extravasation (Fig. 1 ○ versus •, F= 338.73, P < 0.01).
Figure 1. Effect of subdiaphragmatic vagotomy on the depression of bradykinin-induced plasma extravasation in the knee joint of the rat by intradermal capsaicin in the plantar skin of the hind paw.
Depression of bradykinin-induced plasma extravasation during stimulation of nociceptors in the plantar skin of the hind paw by capsaicin (3, 10 and 30 μg intradermal (i.d.)) in sham-vagotomised rats (○) and in rats in which the subdiaphragmatic vagus nerves were cut, immediately prior to the experiment (•).
Electrical stimulation reverses the effect of vagotomy
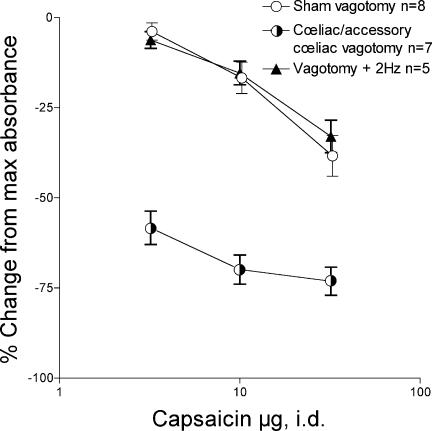
To confirm that loss of afferent neural activity in the subdiaphragmatic vagus nerve is responsible for vagotomy-induced enhancement of capsaicin-induced inhibition of bradykinin-plasma extravasation (Fig. 1), we electrically stimulated the proximal end of the cut subdiaphragmatic vagus nerve (Fig. 2, s). Acute coeliac plus coeliac accessory selective subdiaphragmatic vagotomy enhanced intradermal capsaicin-induced inhibition of bradykinin plasma extravasation (Fig. 2, half-filled circles versus○, F= 179.65, P < 0.01), an effect completely reversed by electrical stimulation of the proximal end of the cut subdiaphragmatic vagus nerve at a frequency of 2 Hz (Fig. 2, s versus half-filled circles, F= 211.26, P < 0.01).
Figure 2. Effect of electrical stimulation of the proximal end of the cut subdiaphragmatic vagus nerve on vagotomy-induced enhancement of the depression of bradykinin-induced plasma extravasation by cumulative dosing with intradermal capsaicin.
Subselective coeliac plus coeliac accessory branch vagotomy induces a marked enhancement of capsaicin (3, 10 and 30 μg, i.d.) induced inhibition of bradykinin plasma extravasation (half-filled circles) compared to sham vagotomy controls (○ from Fig. 1); the enhancement produced by coeliac plus coeliac accessory subdiaphragmatic vagotomy was similar to that produced by complete subdiaphragmatic vagotomy (• from Fig. 1). Electrical stimulation of the proximal end of the cut subdiaphragmatic vagus (s) completely reversed the effect of subdiaphragmatic vagotomy.
Duodenectomy enhances capsaicin effect
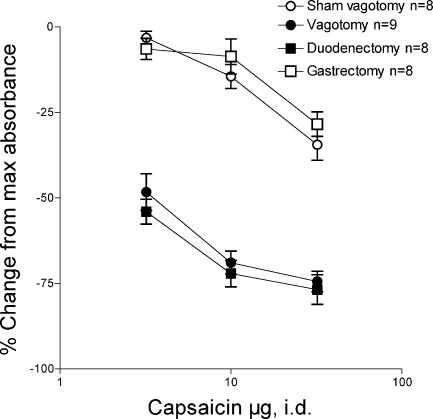
The coeliac plus coeliac accessory branches of the subdiaphragmatic vagus almost exclusively innervate the intestinal tract (Berthoud et al. 1991), although it has not been excluded that some fibres might innervate the stomach (Phillips & Powley, 1998), therefore we evaluated the effect of excision of the first two segments of the proximal gastrointestinal tract, the stomach and duodenum, on intraplantar capsaicin-induced inhibition of bradykinin-induced plasma extravasation. Surgical excision of the duodenum (▪), but not the stomach (gastrectomy, □), produced a marked leftward shift in the dose–response curve for capsaicin induced inhibition of bradykinin-induced plasma extravasation (Fig. 3, ▪versus○, two-way anovaF= 340.2, P < 0.01), similar in magnitude to that produced by subdiaphragmatic (Fig. 3, ▪versus•, two-way anovaF= 2.14, P > 0.05) or by coeliac plus accessory coeliac branch vagotomy (Fig. 3▪versusFigure 2 half-filled circles, F= 0.03, P > 0.05).
Figure 3. Effect of surgical excision of the stomach or duodenum on the depression of bradykinin-induced plasma extravasation by intradermal capsaicin.
Bradykinin-induced plasma extravasation during stimulation of nociceptors in the hind paw with capsaicin (3, 10, 30 and 100 μg i.d.) after gastrectomy (□) or after duodenectomy (▪), compared to sham vagotomy (○ from Fig. 1) and vagotomy (• from Fig. 1). Gastrectomy had no effect on the dose–response curve for capsaicin-induced inhibition of bradykinin plasma extravasation compared to control vagus intact rats, while duodenectomy enhanced capsaicin inhibition of bradykinin plasma extravasation. The magnitude of the enhancement of the effect of capsaicin by duodenectomy was similar to the magnitude of the effect of subdiaphragmatic vagotomy.
Fasting enhances capsaicin effect
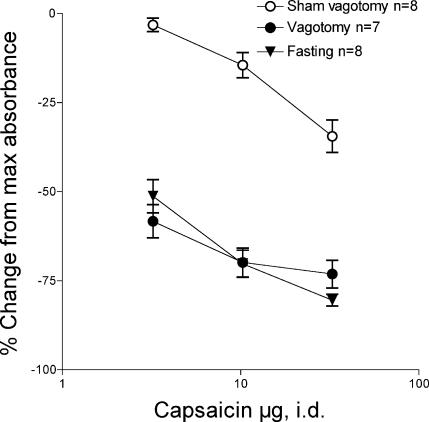
We then tested the hypothesis that a decrease in solid food in the gastrointestinal tract could mimic the effects of subdiaphragmatic vagotomy to potentiate the anti-inflammatory effect of intraplantar capsaicin. Fasting markedly enhanced the inhibitory effect of intraplantar capsaicin (Fig. 4, t versus○, F= 140.76, two-way anova, P < 0.01), similar in magnitude to that produced by vagotomy (Fig. 4, t versus•F= 0.01, P > 0.05).
Figure 4. Effect of 48 h fasting from solid food on the depression of bradykinin-induced plasma extravasation by intradermal capsaicin in the hind paw.
>The depression of bradykinin plasma extravasation by the intraplantar injection of capsaicin (3, 10 and 30 μg i.d.) in rats that were fasted for 48 h (t) was enhanced, compared to fed rats (○ from Fig. 1). The magnitude of enhancement induced by fasting was similar to the magnitude of that induced by subdiaphragmatic vagotomy (•; from Fig. 1).
Balloon distension reverses effect of fasting
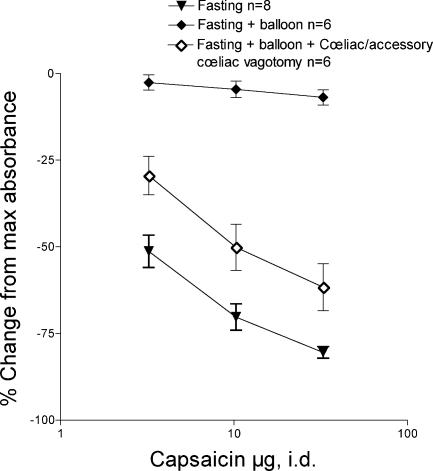
To provide evidence that the effect of fasting on capsaicin-induced inhibition of bradykinin-induced plasma extravasation could be due to a decrease in activity in mechanically sensitive vagal afferents in the duodenum, we evaluated the ability of distension of an intraluminal latex balloon, in the duodenum, to a pressure of 30–40 cmH2O, to reverse the effect of the fasting. Balloon distension completely reversed the effect of fasting on capsaicin-induced inhibition of bradykinin-induced plasma extravasation (Fig. 5, t versus♦, F= 550.7, two-way anova, P < 0.01); in control naïve rats, duodenal distension did not significantly affect bradykinin-induced plasma extravasation (data not shown). To demonstrate neural signals generated by the balloon distension is transmitted via the coeliac and accessory coeliac branches of the vagus, we repeated the above experiment in selective vagotomised rats. The effect of balloon distension on fasting in these rats was markedly attenuated in rats with lesioned coeliac and accessory coeliac branches (Fig. 5. ⋄versus♦, F= 118.95, two-way anova, P < 0.01).
Figure 5. Effect of duodenal balloon distension, in 48-h fasted rats.
Depression of bradykinin plasma extravasation by intraplantar capsaicin (3, 10 and 30 μg I.d.) in 48-h fasted rats (t from Fig. 4) was reversed by distension of a balloon in the duodenum (♦). Selective coeliac and accessory coeliac branch vagotomy (⋄) markedly reduced the effect of balloon distention.
Discussion
While there have been many studies of the modulation of inflammation (Miao et al. 1994, 1997b), nociception, body temperature and other illness-related symptoms (Bluthe et al. 1994; Watkins et al. 1994a; Simons et al. 1998; Gaykema et al. 2000; Goehler et al. 2000; Jänig et al. 2000; Kirchner et al. 2000; Hansen et al. 2001; Ness et al. 2001) by vagal afferent activity, little is known about the visceral organ from which the stimuli arise and what type of stimuli activate relevant subdiaphragmatic vagal afferents. In this study we have identified both a visceral organ, the duodenum, from which vagal afferent activity arises to potently modulate noxious stimulus-induced inhibition of bradykinin plasma extravasation, as well as a specific physiological stimulus, mechanical stimulation, which modulates the inflammatory response by activity in subdiaphragmatic vagal afferents. While it is not known whether other stimuli, alone or by interacting with mechanical stimuli, also contribute to this effect of vagal afferent activity on inflammation, it seems likely since most duodenal vagal afferents are polymodal, responding to nutrients, osmotic stimuli and inflammatory mediators, as well as to mechanical stimuli; in fact, some are silent to mechanical stimuli until exposed to inflammatory mediators (Cottrell & Iggo, 1984; Grundy, 1988; Grundy & Scratchard, 1989; Berthoud et al. 1992; Wei & Wang, 2000; Zhu et al. 2001). Our observed effects of a 48 h fast could be due to changes independent of physical removal of luminal contents (e.g. changes in mediator release and receptor expression). However, we have reported that 48 h fasting-induced enhanced nociception is not prevented by providing 5% glucose in the drinking water, but is reversed by gavage administration of petrolatum to provide bulk without nutrition (Khasar et al. 2003). It should also be noted that whatever the mechanism, the fasting effect is abolished by vagotomy, which strongly supports our hypothesis that vagal afferents in the duodenum modulates mediate the neuroendocrine control of the inflammatory response. While it has not been tested whether visceral organs other than the duodenum might also contribute, their contribution is likely to be significantly smaller, since not only is the effect of subdiaphragmatic vagotomy very similar in magnitude to the effect produced by duodenectomy, but also gastrectomy was without effect. In addition, since vagotomy markedly attenuated the ability of duodenal distension to affect capsaicin-induced modulation of inflammation, the signal arising from the duodenum is probably neural rather than humoral.
We hypothesize that mechanosensitive duodenal afferents play a critical role in the body's defence against toxic insults. The gastrointestinal tract is an early line of defence against ingested infectious agents and toxic substances. As a site for toxins as well as nutrients to enter the body, the gastrointestinal tract must serve protective as well as digestive functions, including signalling to the rest of the body when toxic exposures occur in the lumen of the gastrointestinal tract. In this regard, the stomach, the first segment of the gastrointestinal tract that orally ingested substances stay in contact with for any period of time, deals with toxic insults by having a high hydrogen ion concentration (i.e. acidic pH) (Sun et al. 2003) and an ability to undergo stasis (Hermann et al. 2003), helping to prevent toxic materials from passing through the pylorus as well as retropulsion to facilitate elimination of the infectious or toxic insult from the mouth (Carpenter, 1990). In addition, the stomach does not have the absorption mechanisms observed in the subsequent segments of the proximal gastrointestinal tract. The duodenum, on the other hand, is the first segment of the gastrointestinal tract that has an absorptive function and is therefore also a potential portal for the entry of toxic agents into the body (Kumagai, 1989). While some orally ingested toxins can be rapidly eliminated by hypermotility, many cause a pronounced decrease in gastrointestinal activity (ileus) (Cullen et al. 1996), which may lead to increased absorption of toxins or infectious agents from the gastrointestinal tract. While only mechanical stimulation was used in this study, to localize the stimulus to the proximal duodenum, ischaemically sensitive afferents may also have been activated by the pressure stimulus we used, and toxins or inflammatory mediators may be more relevant stimuli in such a signalling pathway, since it is important that the gastrointestinal tract can send signals to the rest of the body to prepare it for responding to noxious or toxic insults occurring at the gastrointestinal tract lumen. The most rapid way to communicate such signals is via afferent neurones, which can, in turn, orchestrate specific neural and endocrine signals that can differentially regulate vascular and immune function in different parts of the body (Elenkov et al. 2000; Webster et al. 2002). A secondary decrease in activity of mechanosensitive afferents, following the decrease in gastrointestinal motility would lead to a generalized increase in the body's response to inflammatory stimuli (Miao et al. 1994; Miao et al. 1997b). Further studies are needed to evaluate the altered modulation of the inflammatory response induced by decreased vagal afferent activity of the proximal gastrointestinal tract and how it might affect the body's response to a systemic noxious state such as might be produced by a toxic insult that enters the body via the gastrointestinal tract.
In the present study we have elucidated a visceral organ from which vagal afferent activity arises that can potently modulate at least one important component of the inflammatory response, microvascular permeability, and a physiological stimulus capable of modifying the potent modulation of noxious stimulus-induced inhibition of inflammation by activity in subdiaphragmatic vagal afferents (Miao et al. 1997b). How these signals may integrate with others from abdominal viscera remains to be examined, as does the role of vagal afferent input from other segments of the gastrointestinal tract. Since vagal afferent activity also contributes to modulation of pain, fever, and other signs and symptoms, collectively referred to as ‘illness symptoms’ (Bluthe et al. 1994; Simons et al. 1998; Gaykema et al. 2000; Kirchner et al. 2000; Hansen et al. 2001; Ness et al. 2001), it will be important to examine the role of polymodal duodenal afferents in illness symptoms. Of note in this regard, the hepatic branch of the subdiaphragmatic vagus provides more sensory fibres that innervate the gastrointestinal tract than the liver (Berthoud et al. 1992; Phillips et al. 1997), possibly helping to explain, at least in part, why illness behaviour has also been reported to be dependent on the hepatic branch of the subdiaphragmatic vagotomy (Watkins et al. 1994a; Watkins et al. 1994b). It will also be of interest to elucidate how mechanical stimulation in the duodenum interacts with other stimuli that activate duodenal afferents, and with subdiaphragmatic vagal afferents from other segments of the proximal gastrointestinal tract, such as the hepatic branch, which monitors plasma glucose level (Niijima, 1983, 1984) and from the jejunum and ileum.
Acknowledgments
The authors wish to express their appreciation to Professor Wilfrid Jänig for many helpful discussions. This work was supported by a grant from the National Institutes of Health (AR32634).
References
- Andrews PL. Vagal afferent innervation of the gastrointestinal tract. Prog Brain Res. 1986;67:65–86. doi: 10.1016/s0079-6123(08)62757-0. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol. 1992;186:431–442. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (cck-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. [PubMed] [Google Scholar]
- Carpenter DO. Neural mechanisms of emesis. Can J Physiol Pharmacol. 1990;68:230–236. doi: 10.1139/y90-036. [DOI] [PubMed] [Google Scholar]
- Carr J, Wilhelm DL. The evaluation of increased vascular permeability in the skin of guinea pigs. Aust J Exp Biol Med Sci. 1964;42:511–522. doi: 10.1038/icb.1964.48. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Basbaum AI, Levine JD. Neural control of vascular permeability: Interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989;62:48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- Cottrell DF, Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol. 1984;354:457–475. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JJ, Ephgrave KS, Caropreso DK. Gastrointestinal myoelectric activity during endotoxemia. Am J Surg. 1996;171:596–599. doi: 10.1016/s0002-9610(96)00037-2. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997;108:105–113. doi: 10.1007/s004180050151. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE, Hansen MK, Maier SF, Watkins LR. Subdiaphragmatic vagotomy blocks interleukin-1beta-induced fever but does not reduce il-1beta levels in the circulation. Auton Neurosci. 2000;85:72–77. doi: 10.1016/s1566-0702(00)00222-8. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Green PG, Miao FJ, Janig W, Levine JD. Negative feedback neuroendocrine control of the inflammatory response in rats. J Neurosci. 1995;15:4678–4686. doi: 10.1523/JNEUROSCI.15-06-04678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. J Auton Nerv Syst. 1988;22:175–180. doi: 10.1016/0165-1838(88)90104-x. [DOI] [PubMed] [Google Scholar]
- Grundy D. Mechanoreceptors in the gastrointestinal tract. J Smooth Muscle Res. 1993;29:37–46. doi: 10.1540/jsmr.29.37. [DOI] [PubMed] [Google Scholar]
- Grundy D, Scratchard T. Sensory afferents from the gastrointestinal tract. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of physiology The Gastrointestinal System. Vol 1. Bethesda, MD: American Physiological Society; 1989. pp. 593–620. [Google Scholar]
- Hansen MK, O'Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol. 2001;280:R929–934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Roszkowski MT, Swift JQ. Bradykinin and inflammatory pain. Agents Actions. 1993;41:65–73. [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy of the Laboratory Rat. Baltimore: Williams & Wilkins; 1976. [Google Scholar]
- Hermann GE, Tovar CA, Rogers RC. Tnfalpha-stimulation of cfos-activation of neurons in the solitary nucleus is suppressed by tnfr: Fc adsorbant construct in the dorsal vagal complex. Brain Res. 2003;976:69–74. doi: 10.1016/s0006-8993(03)02687-8. [DOI] [PubMed] [Google Scholar]
- Jänig W, Khasar SG, Levine JD, Miao FJ. The role of vagal visceral afferents in the control of nociception. Prog Brain Res. 2000;122:273–287. doi: 10.1016/s0079-6123(08)62145-7. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Reichling DB, Green PG, Isenberg WM, Levine JD. Fasting is a physiological stimulus of vagus-mediated enhancement of nociception in the female rat. Neuroscience. 2003;119:215–221. doi: 10.1016/s0306-4522(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2000;55:1167–1171. doi: 10.1212/wnl.55.8.1167. [DOI] [PubMed] [Google Scholar]
- Krolczyk G, Zurowski D, Sobocki J, Slowiaczek MP, Laskiewicz J, Matyja A, Zaraska K, Zaraska W, Thor PJ. Effects of continuous microchip (mc) vagal neuromodulation on gastrointestinal function in rats. J Physiol Pharmacol. 2001;52:705–715. [PubMed] [Google Scholar]
- Kumagai S. Intestinal absorption and excretion of aflatoxin in rats. Toxicol Appl Pharmacol. 1989;97:88–97. doi: 10.1016/0041-008x(89)90057-4. [DOI] [PubMed] [Google Scholar]
- Mei N. Recent studies on intestinal vagal afferent innervation. Functional implications. J Auton Nerv Syst. 1983;9:199–206. doi: 10.1016/0165-1838(83)90141-8. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Benowitz NL, Levine JD. Endogenous opioids suppress activation of nociceptors by sub-nanomolar nicotine. Br J Pharmacol. 2001a;133:23–28. doi: 10.1038/sj.bjp.0704031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao FJ, Dallman MF, Benowitz NL, Basbaum AI, Levine JD. Adrenal medullary modulation of the inhibition of bradykinin-induced plasma extravasation by intrathecal nicotine. J Pharmacol Exp Ther. 1993;264:839–844. [PubMed] [Google Scholar]
- Miao FJ, Green PG, Benowitz N, Levine JD. Vagal modulation of spinal nicotine-induced inhibition of the inflammatory response mediated by descending antinociceptive controls. Neuropharmacology. 2003;45:605–611. doi: 10.1016/s0028-3908(03)00224-7. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Janig W, Dallman MF, Benowitz NL, Heller PH, Basbaum AI, Levine JD. Role of vagal afferents and spinal pathways modulating inhibition of bradykinin-induced plasma extravasation by intrathecal nicotine. J Neurophysiol. 1994;72:1199–1207. doi: 10.1152/jn.1994.72.3.1199. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Janig W, Green PG, Levine JD. Inhibition of bradykinin-induced synovial plasma extravasation produced by intrathecal nicotine is mediated by the hypothalamopituitary adrenal axis. J Neurophysiol. 1996;76:2813–2821. doi: 10.1152/jn.1996.76.5.2813. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Janig W, Green PG, Levine JD. Inhibition of bradykinin-induced plasma extravasation produced by noxious cutaneous and visceral stimuli and its modulation by vagal activity. J Neurophysiol. 1997a;78:1285–1292. doi: 10.1152/jn.1997.78.3.1285. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Janig W, Jasmin L, Levine JD. Spino-bulbo-spinal pathway mediating vagal modulation of nociceptive-neuroendocrine control of inflammation in the rat. J Physiol. 2001b;532:811–822. doi: 10.1111/j.1469-7793.2001.0811e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao FJ, Janig W, Levine JD. Vagal branches involved in inhibition of bradykinin-induced synovial plasma extravasation by intrathecal nicotine and noxious stimulation in the rat. J Physiol (Lond) 1997b;498(2):473–481. doi: 10.1113/jphysiol.1997.sp021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao FJ, Janig W, Levine JD. Nociceptive neuroendocrine negative feedback control of neurogenic inflammation activated by capsaicin in the rat paw: Role of the adrenal medulla. J Physiol. 2000;527:601–610. doi: 10.1111/j.1469-7793.2000.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao FJ, Lee TJ. Effects of bilirubin on cerebral arterial tone in vitro. J Cereb Blood Flow Metab. 1989;9:666–674. doi: 10.1038/jcbfm.1989.94. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Levine JD. Neural and endocrine mechanisms mediating noxious stimulus-induced inhibition of bradykinin plasma extravasation in the rat. J Pharmacol Exp Ther. 1999;291:1028–1037. [PubMed] [Google Scholar]
- Moss HE, Sanger GJ. The effects of granisetron, ics 205–930 and ondansetron on the visceral pain reflex induced by duodenal distension. Br J Pharmacol. 1990;100:497–501. doi: 10.1111/j.1476-5381.1990.tb15836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, Randich A, Fillingim R, Faught RE, Backensto EM. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2001;56:985–986. doi: 10.1212/wnl.56.7.985. [DOI] [PubMed] [Google Scholar]
- Niijima A. Glucose-sensitive afferent nerve fibers in the liver and their role in food intake and blood glucose regulation. J Auton Nerv Syst. 1983;9:207–220. doi: 10.1016/0165-1838(83)90142-x. [DOI] [PubMed] [Google Scholar]
- Niijima A. The effect of d-glucose on the firing rate of glucose-sensitive vagal afferents in the liver in comparison with the effect of 2-deoxy-d-glucose. J Auton Nerv Syst. 1984;10:255–260. doi: 10.1016/0165-1838(84)90021-3. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. J Comp Neurol. 1997;384:248–270. [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Gastric Volume detection after selective vagotomies in rats. Am J Physiol. 1998;274:R1626–R1638. doi: 10.1152/ajpregu.1998.274.6.R1626. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: Re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Raybould H. Primary afferent response to signals in the intestinal lumen. J Physiol. 2001;530:343. doi: 10.1111/j.1469-7793.2001.0343k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol. 1998;274:R1236–1242. doi: 10.1152/ajpregu.1998.274.5.R1236. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Tougas G, Moran TH. Integration of vagal afferent responses to duodenal loads and exogenous CCK in rats. Peptides. 1995;16:707–711. doi: 10.1016/0196-9781(95)00033-g. [DOI] [PubMed] [Google Scholar]
- Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Szekely M, Romanovsky AA. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis. Am J Physiol. 1998;275:R63–R68. doi: 10.1152/ajpregu.1998.275.1.R63. [DOI] [PubMed] [Google Scholar]
- Sun FJ, Kaur S, Ziemer D, Banerjee S, Samuelson LC, De Lisle RC. Decreased gastric bacterial killing and up-regulation of protective genes in small intestine in gastrin-deficient mouse. Dig Dis Sci. 2003;48:976–985. doi: 10.1023/a:1023068116934. [DOI] [PubMed] [Google Scholar]
- Swift JQ, Garry MG, Roszkowski MT, Hargreaves KM. Effect of flurbiprofen on tissue levels of immunoreactive bradykinin and acute postoperative pain. J Oral Maxillofac Surg. 1993;51:112–116. doi: 10.1016/s0278-2391(10)80002-3. discussion. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994a;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994b;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wei JY, Wang YH. Effect of cck pretreatment on the cck sensitivity of rat polymodal gastric vagal afferent in vitro. Am J Physiol Endocrinol Metab. 2000;279:E695–706. doi: 10.1152/ajpendo.2000.279.3.E695. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]