Abstract
Of those people who are anosmic to androstenone, a proportion can acquire sensitivity to it by repeated exposure and even those who are able to smell it can lower their threshold with this treatment. Using olfactory threshold testing, intranasal electrophysiology and EEG we show for the first time that: (1) the subjects' detection threshold is proportional to the amplitude of the olfactory evoked potential (EOG) recorded inside the nose; (2) the EOG amplitude is correlated with the amplitude of the olfactory event-related potential (OERP) recorded on the scalp; and (3) with repetitive exposure, human subjects acquire a reduced threshold for androstenone and, as they do so, their EOG and OERP increase. These observations support the existence of odourant-specific plasticity in the peripheral olfactory system.
In humans, repeated exposure to odours can have a number of different effects. First, it can result in a short-, medium-, or long-term reduction in responsiveness due to adaptation and/or habituation (Dalton & Wysocki, 1996; Dalton, 2000) or, second, and less frequently, it can lead to an increase in responsiveness (Wysocki et al. 1989; Dalton et al. 2002). Physiological and perceptual adaptation/habituation to odour have been correlated in humans and, while the physiological adaptation to repetitive stimulation with isoamyl acetate was incomplete, the response declining to around 25% with a time constant (τ) of 4–10 s depending on stimulus strength, the cognitive perception declined to zero with τ≈ 2.5 s and was not concentration dependent (Wang et al. 2002).
In rats, repeated exposure to odour has been shown to cause a gradual enhancement or sensitization of olfactory β-waves in the pyriform cortex which occurred while the mucosal receptor potential was decreasing (adapting) (Vanderwolf & Zibrowski, 2001), but sensitization to one odour transferred poorly to other odours. However, long exposure (several months) to an odourant has been shown to lead to mitral cell degeneration in the olfactory bulb of adult rats (Doving & Pinching, 1973) and, more recently, shorter exposures (<8 h) caused changes in the surface markers of olfactory receptor neurones (Yoshihara et al. 1993) and expression of immediate-early genes in cells in the olfactory bulb (Sallaz & Jourdan, 1993; Guthrie & Gall, 1995; Baba et al. 1997). Exposing rats for 20 min per day for six consecutive days to an odourant caused a decrease in responsiveness of the olfactory bulb both to the familiar odourant as well as to other, unfamiliar odourants, and the effect lasted for up to 10 days (Buonviso & Chaput, 2000). An increase in the inhibition of mitral/tufted cell responses by periglomerular cells, possibly mediated by dopamine, or by GABA from granular cells, has been invoked to explain this phenomenon (Buonviso & Chaput, 2000).
The acquisition or sensitization of smell perception in both humans and rats is a rarer event. It has been observed for one odour in particular – androstenone. Androstenone is a steroid that occurs in both urine and axillary secretions (sweat) and has been proposed as a human pheromone (Van Toller et al. 1983). A proportion of people cannot smell androstenone and those who can fall into two groups: (1) a very sensitive group, who can detect less than 10 parts per trillion and who find the odour extremely unpleasant (urinous); and (2) a group who are not only less sensitive but perceive the odour in different ways such as ‘sweet’, ‘musky’ or ‘perfume-like’ (Gower et al. 1998). The distribution of thresholds for androstenone, unlike most other odourants, is not normally distributed, but heavily skewed toward the high threshold end (see Gower & Ruparelia, 1993; their Fig. 9). Of those people who are initially anosmic to androstenone, a proportion can acquire sensitivity to it by repeated exposure (Wysocki et al. 1989) and even those who can smell it can lower their threshold by this treatment (Pause et al. 1999). Wang et al. (1993) reported that rats could acquire an increased odour-specific sensitivity to androstenone and isovaleric acid following repetitive exposure to these odourants and recently, such induction of olfactory sensitivity has been extended to other odourants (benzaldehyde, citralva) in humans, but only in women of reproductive age (Dalton et al. 2002).
How this phenomenon of induced sensitivity occurs is unclear. Yee & Wysocki (2001) provided evidence of the involvement of the olfactory epithelium by repetitively exposing mice with olfactory nerve transection to androstenone or amyl acetate and demonstrating increased sensitivity (compared to presurgery levels) upon regrowth of the olfactory nerves. However, Mainland et al. in a recent study (2002) on humans in which they exposed only one nostril to repetitive stimulation and showed acquisition of sensitivity by the other, unexposed nostril, suggested that this learning occurred in central brain regions of the olfactory system.
We set out to monitor the response of the olfactory system during the acquisition of increased androstenone sensitivity by measuring the evoked potentials of the olfactory epithelium (EOGs), simultaneously with the event-related potentials (OERPs) recorded on the scalp using EEG electrodes and correlating the results with the detection thresholds for androstenone. The EOG represents solely peripheral events whereas the OERP reflects the activity of both peripheral and central elements of the olfactory system and thus it is possible to dissect out the location of any induced changes.
Methods
Odorants
The odourants used were androstenone (5-α-androst-16-en-3-one) minimum 98% pure by TLC obtained from Steraloids (Newport, RI, USA) and amyl acetate (Sigma Chemical Co., Poole, UK), a substance with an apple-/banana-like odour. The binary androstenone dilutions were made from a stock solution of 3.67 mm (0.1% w/v) in silicone oil (Dow Corning, Midland, MI, USA) and binary dilutions of amyl acetate were made from a 4.28 mm (0.064% v/v) stock solution in the same silicone oil. These concentrations were chosen to follow the protocol of Wysocki et al. (1989).
Odour delivery
The olfactometer (described in detail by Wang et al. 2002) consisted of a filtered air supply delivery system of narrow tubes, a computer-controlled odour switching device, solenoid valves (Cole Palmer, Bishops Stortford, UK) and a water bath. A constant airflow was delivered to the nostril via a Teflon nasal canula inserted through a self-expanding bung (an Aearo Ear Protector, Stockport, UK) approximately 1.5 cm into the nostril. The self-expanding bung closes off the stimulated nostril, ensuring a unidirectional, constant airflow. The subjects were instructed to breathe through their mouths. Olfactory stimulation was achieved using computer-controlled valves to direct part of the airflow into either the amyl acetate or androstenone reservoirs without altering the pressure or flow rate. The concentration of androstenone and amyl acetate in the reservoirs was 0.1 and 0.064%, respectively. The switching mechanism was designed in such a way that during stimulation odourant pulses of pre-established concentrations (diluted 1:3 with humidified air) reached the olfactory region without altering the flow rate, thus reducing the chances of trigeminal activation, and during the interstimulus intervals (ISI) only non-odorous control air reached the nose.
On each test occasion, 20 pulses of odour stimulant were presented at a regular interstimulus interval (ISI) of 10 s, with a stimulus duration of 200 ms at a flow rate of 3 l min−1 to one nostril. The temperature of the air flowing into the nostril was regulated to 28.5°C by passing it through a coil immersed in a water bath. The relative humidity was maintained at 80% by passing the continuous air stream through a small glass reservoir containing water.
Ethical approval
The study conformed to the Declaration of Helsinki. Ethical approval for this study was granted by the Bro Taff Health Authority Local Research Ethics Committee, Temple of Peace and Health, Cathays Park, Cardiff, UK.
Subjects
The subjects were from the student population of the University and none had a history of olfactory dysfunction or respiratory disease. The average age was 24.4 ± 0.7 years (±s.e.m.; n= 33); there were 16 men (average age = 23.9 ± 0.9 years, range 18–31 years) and 17 women (average age 24.9 ± 1.1 years, range 18–30 years). Of the subjects tested (see ‘Threshold test’ below), those with the highest thresholds for androstenone were chosen for the trial group. The protocol was explained and written informed consent obtained. Of these, fifteen (7 males, 8 females) were allocated to the test group who would sniff androstenone daily and six (3 females, 3 males) were allocated to the ‘no sniffing’ control group. The test group were given a bottle containing 0.1% androstenone and were instructed to sniff it for 3 min 3 times daily. Both groups were then monitored for thresholds for both androstenone and amyl acetate, EOG and OERP at weekly intervals for three weeks.
A further group of subjects was allocated to the amyl acetate sniffing group. Six men and six women were assigned to sniff amyl acetate (4.28 mm in silicone oil; 0.064% v/v) for 3 min 3 times daily. They were tested for detection threshold, EOG and OERP on days 1, 8, 15 and 22.
Threshold test
The smell perception threshold protocol of Wysocki et al. (1989) was used. This involves a three-repetition, two-alternative forced choice test of an ascending series of 16 binary dilutions, starting concentration 0.1% w/v in silicone oil for androstenone and 0.064% v/v for amyl acetate. The threshold was set at the first dilution at which all the choices were correct.
Electrophysiological recording
(1) EOGs
The electro-olfactogram (EOG) was recorded using intranasal electrodes. The recording electrode was a silver–silver chloride electrode with a diameter of 0.8 mm (Harvard Apparatus Ltd., Edenbridge, UK). This electrode was covered by a 1 mm Teflon tube except for the silver chloride-coated tip. A measurement of the distance between the external nares and medial canthus of the eye was taken, which gives a rough guide to the level of the cribriform plate. This measurement was then noted and later marked on the freshly prepared electrode, giving an approximate idea of the position of the olfactory epithelium. Following initial guidance, the subjects then slowly introduced the intranasal electrode themselves. This was then secured to the upper lip using thin strips of surgical hypoallergenic tape (3M, USA). With training, subjects were able to perform this insertion by themselves without much discomfort.
The electrode was connected to the input of the EEG machine (SLE 180TM, SLE Ltd, Croydon, UK) and referred to the earth electrode on the forehead. The high-pass filtering for this channel was removed, the low-pass filter was set to 150 Hz and notch filter at 50 Hz was used. Stable recordings of the baseline potentials in the computer traces suggested adequate contact with the olfactory mucosa, and accurate positioning of the electrode was signalled by a negative polarization in response to a pulse of odourant. During the experiment the subjects were seated in a comfortable chair in a test booth with a controlled environment. The subjects wore headphones through which white noise was played to eliminate auditory cues.
(2) OERPs
Olfactory event-related potentials (OERPs) were recorded using electroencephalography (SLE 180TM, SLE Ltd) as previously described (Wang et al. 2002). Electrodes were placed according to the international 10/20 system referred to A1 and an earth electrode was placed on the forehead. Only data from Cz are reported in this study. Traces contaminated with eye movement artefacts were discarded.
Analog data from all EEG channels were sent to a laboratory interface (CED1401), digitized at 100 Hz and analysed following signal averaging on a computer using ‘Signal’ analysis software (CED, Cambridge, UK).
Noise subtraction
The OERPs were measured from digitized records using the Signal analysis software. In conformity with other studies of OERPs (see Wang et al. 2002), the OERP was taken to be the N1–P2/P3 waveform. The peak value (amplitude) of the OERP was measured between manually set cursors. These were set just before the latency for N1 and just after the N1–P2/P3 waveform. Amplitude measurement could be semiautomated, removing subjectivity from the process. The noise, measured from the prestimulus baseline, was subtracted from this value.
Results
Androstenone threshold
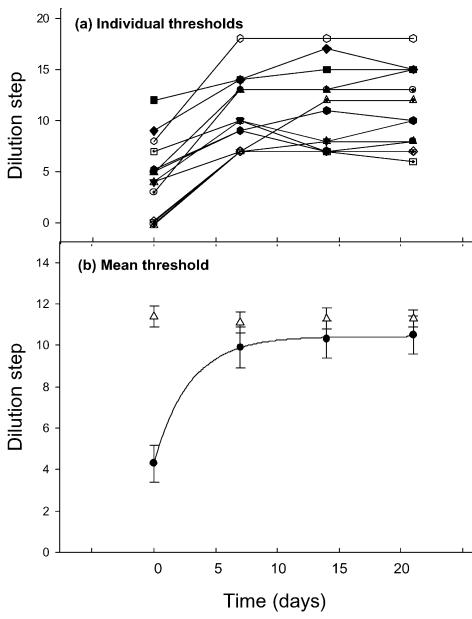
We found that most students between the ages of 18 and 31 years could smell androstenone. Only nine, out of 58 tested (15.5%), were unable to detect androstenone at the highest concentration (0.1%). We selected the poorest performers (highest thresholds), whose mean lowest detectable dilution was 4.3 ± 0.9 (mean ±s.e.m.; n= 15), which corresponds to a concentration of 2.2 ± 0.8 mm. They were then required to sniff 0.1% androstenone for 3 min, 3 times each day. After the first week of such exposure their detection ability had risen significantly to a mean dilution 9.9 ± 1.0 (Student's paired t test, P < 0.001) corresponding to a concentration of 0.14 ± 0.12 mm (Fig. 1). The thresholds for the following two tests were 10.3 ± 0.9 (0.03 ± 0.02 mm) at session 3 (14 days) and 10.5 ± 0.9 (0.04 ± 0.02 mm) at session 4 (21 days) (Fig. 1A). A single exponential was fitted to the data and the best fit (continuous line in Fig. 1A) was obtained with a time constant of 2.9 days although, because of the limited data in the rising phase of the curve, we cannot rule out the possibility of the process being better described by multiple exponentials.
Figure 1. Effect of sniffing androstenone on androstenone detection thresholds.
Measurements of individual thresholds (A) and mean thresholds (B) as a function of time. The mean thresholds ± standard error of the mean (bars; n= 15), to androstenone (•) and amyl acetate (Δ) were measured at 7 day intervals using a 3-repetition, 2-alternative forced choice test (Wysocki et al. 1989). Between the measurements the subjects were sensitized to androstenone by sniffing a 0.1% w/v solution in silicone oil for 3 min 3 times daily. In all subjects the dilution step at which the androstenone could be detected increased, corresponding to a threshold decrease. The dilution steps are binary dilutions from a starting concentration (0) of 0.1% for androstenone (3.67 mm) and 0.064% (4.28 mm) for amyl acetate. The continuous line represents the best fit of a single exponential to the threshold data with a time constant of 2.9 days.
To those who could not smell androstenone at the first session, it acquired a ‘sweaty’ smell when they became sensitive.
Control 1
For the control group who did not sniff androstenone each day there was no change in androstenone threshold. At the first session it was 6.0 ± 2.1 binary dilutions (or 2.5 ± 1.5 mm; n= 6, 3 males and 3 females) and at the second session, 7 days later, it was 6.2 ± 2.2 binary dilutions (or 2.5 ± 1.5 mm).
Control 2
Those subjects in the experimental (androstenone-sniffing) group (n= 15) were also tested for their sensitivity to amyl acetate. While their androstenone threshold decreased, their amyl acetate thresholds remained constant during the course of the experiment (▵, Fig. 1).
Electrophysiological recording
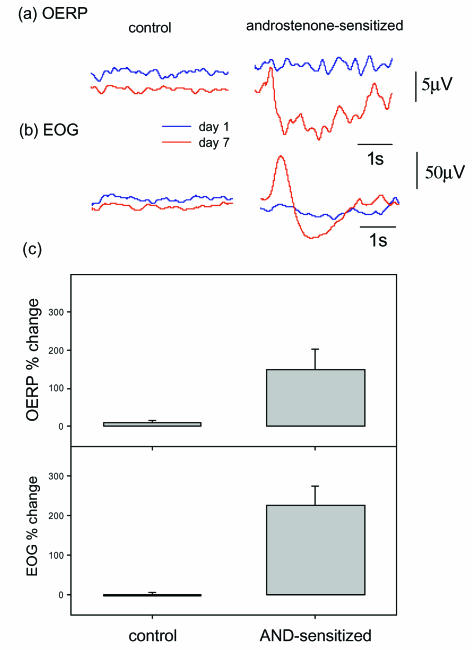
At the same time as their androstenone thresholds were measured, the subjects' OERP and EOG were recorded in response to androstenone pulses (see Methods) before and after androstenone sensitization. In the interval between the first and the second recording sessions (7 days apart), the OERP rose from 7.4 ± 0.6 to 15.0 ± 0.9 μV (Student's paired t test, P < 0.0001, n= 15) and the EOG increased from 18.8 ± 2.8 to 54.2 ± 7.2 μV (Student's paired t test, P < 0.0001) for the androstenone-sensitized group (Fig. 2). Those subjects who could not detect androstenone at its highest concentration gave the lowest EOGs (13.7 ± 1.8 μV, n= 4) and OERPs (5.3 ± 1.0 μV). However, no androstenone anosmic subject (unable to detect 0.1% androstenone) gave a zero EOG, while there was one OERP from one such subject that was indistinguishable from the noise. The observation that androstenone can induce an EOG and OERP without perception illustrates the principle that receptor response is not equivalent to perception (Aidley, 1998). The most likely explanations for this are: (1) that there were insufficient androstenone-sensitive receptor cells in androstenone anosmics to activate the mitral cells (the output neurones of the olfactory bulb); or (2) that descending inhibition, or a ‘neural gate’ (Bogen, 1995), prevents the signal from being transmitted to higher brain centres. The fact that there were cases of androstenone anosmics who gave OERPs (Fig. 3B), which contain components of central processing, favours the latter explanation. A similar observation has been made by Sobel et al. (1999), who demonstrated brain activation by an undetected airborne steroid, oestra-tetraenyl acetate, using fMRI.
Figure 2. Induced potentials.
A, The olfactory event related potential (OERP) was measured at Cz using scalp electrodes in response to androstenone (0.1% in silicone oil) on day 1 (blue traces) and on day 8 (red traces). The control responses were from a subject not given daily androstenone exposure and the androstenone-sensitized responses are from a subject exposed to androstenone daily. Traces are the average of 20 responses to 200 ms pulses of androstenone (see Methods). B, The electro-olfactogram (EOG) measured by intranasal Ag/AgCl electrodes was recorded as for the OERP in the same subjects. Measurements were taken on day 1 (blue traces) and day 8 (red traces). Only the subject exposed to androstenone daily showed any increase in the EOG. C, The mean results for the change in OERP (upper panel) and EOG (lower panel) amplitudes, expressed as the percent difference (±s.e.m.; bars), for the control group (no androstenone sniffing, n= 6) and the test group (daily androstenone sniffing, n= 15), between the measurements at day 1 and day 8. The OERP amplitude was taken as the difference between N1 (the first peak) and P2 (the first trough) (see Methods).
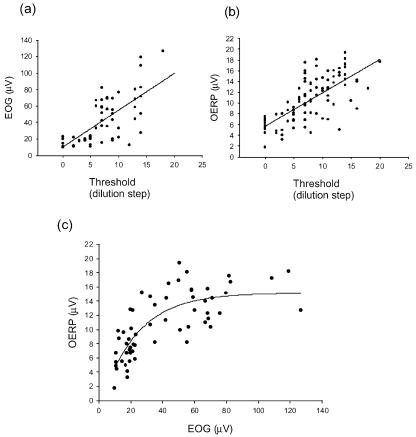
Figure 3. Correlation between EOG and OERP.
The correlation between EOG (A) and OERP (B) amplitude and detection threshold for androstenone. Threshold is given in binary dilution steps from a 0.1% solution of androstenone in silicone oil. Continuous lines are linear regression fits. As the detection threshold decreases (increase in dilution step) there is a corresponding increase in EOG (n= 60, r = 0.71, P < 0.0001) and OERP (n= 96, r = 0.71, P < 0.0001). In C EOG is plotted against OERP. There is a simple exponential relationship (regression fit, r = 0.79, P < 0.0001, n= 60). As EOG increases so does the OERP until it levels off at around 14–16 μV.
There was no change in the amplitudes of either the EOG or the OERP for the control group who were not exposed to androstenone between test sessions on day 1 and day 8 (Fig. 2). The control EOG was 44.5 ± 8.2 μV (n= 6) at day 1 and 46.0 ± 9.5 μV at day 8 and the OERP was 11.3 ± 2.0 μV (n= 6) at day 1 and 12.3 ± 2.3 μV at day 8.
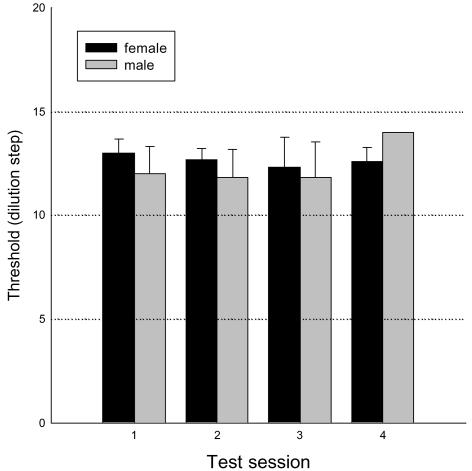
Gender differences
Over the first 7 day androstenone sensitization period the EOG increased from 17.6 ± 1.4 μV (n= 7) at day 1 to 42.5 ± 8.1 μV at day 8 in men and from 19.8 ± 5.3 μV (n= 8) to 64.4 ± 10.6 μV in women. This larger increase in women did not reach statistical significance (Student's paired t test, P= 0.133). However, with these rather low numbers of subjects the power of the experiment was only 0.45 (20 additional subjects would be required for an 80% chance of achieving significance at the 0.05 level). Over the same time period the OERP in women increased from 7.7 ± 0.8 to 14.4 ± 0.79 μV (n= 8) and from 7.1 ± 0.4 to 14.0 ± 0.4 μV (n= 7) in men.
Correlation between EOG, OERP and threshold
In Fig. 3A the threshold, represented as the binary dilution step from the 0.1% stock solution (step = 0), is plotted against the EOG evoked by the androstenone pulses. There is a linear correlation, over the range studied, between threshold and EOG (n= 60, r= 0.71, P < 0.0001) with EOG amplitude increasing with decreasing threshold; in other words, the more sensitive the individual, the lower the threshold and the larger the EOG. There is also a linear correlation between threshold and OERP (Fig. 3B; n= 96, r= 0.71, P < 0.0001).
Correlation between EOG and OERP
When EOG and OERP for each individual are plotted (Fig. 3C) a simple exponential relationship appears (n= 60, r= 0.79, P < 0.0001). Thus the larger the peripheral, receptor potential (EOG) the larger was the corresponding central potential (OERP). However, towards the higher values of the OERP, there is a decreasing increment for increasing values of the EOG.
Effect of amyl acetate sniffing on amyl acetate threshold, EOG and OERP
To test whether the phenomenon of olfactory ‘learning’ generalizes to other odourants we repeated the experimental protocol with amyl acetate. This was the control odour used in the original study of androstenone sensitization in humans (Wysocki et al. 1989).
Subjects were tested for their amyl acetate threshold, amyl acetate-induced EOG and OERP. They were then required to sniff amyl acetate 3 times daily for 3 min. Their threshold, EOG and OERP were monitored at weekly intervals for 3 weeks. Figure 4 shows that there were no significant changes in thresholds over this period, for either men or women. The thresholds were dilution step 12.5 ± 0.7 (n= 12, males and females; equivalent to 8.1 ± 5.58 μm), 12.3 ± 0.7 (13.0 ± 10.9 μm), 12.5 ± 0.9 (15.2 ± 10.1 μm) and 12.6 ± 0.7 (8.8 ± 5.2 μm) at sessions 1, 2, 3 and 4, respectively.
Figure 4. The effect of amyl acetate sniffing on the detection threshold for amyl acetate.
The detection threshold for amyl acetate is given in binary dilution steps from a stock solution of 4.28 mm. Thresholds were taken at weekly intervals (see Methods) for three weeks and between tests the subjects sniffed amyl acetate for 3-min 3-times daily. There was no significant change in the detection thresholds for either men of women over this period (n= 12; males and females). See text for values of threshold concentrations.
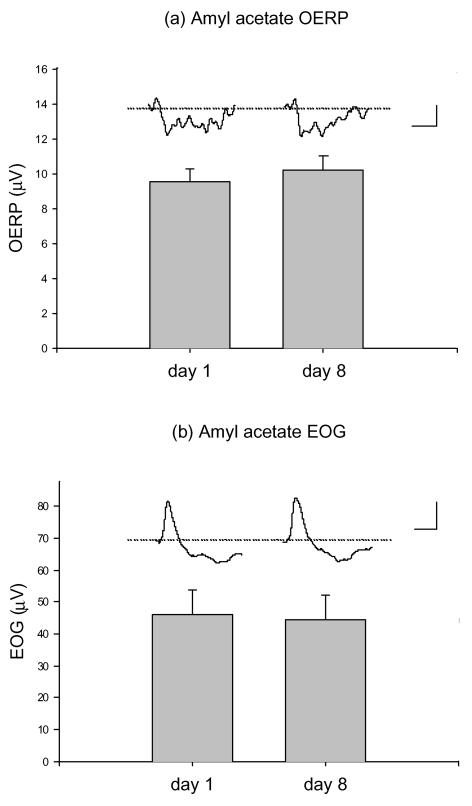
There was no effect of amyl acetate exposure on either the amyl acetate-induced OERP (Fig. 5A) or on the EOG (Fig. 5B). The OERP was 9.6 ± 0.7, 10.2 ± 0.8, 10.1 ± 0.7 and 9.6 ± 0.8 μV (n= 12) at sessions 1, 2, 3 and 4, respectively. The EOG was only measured on sessions 1 and 2 and was 45.9 ± 8.0 and 44.5 ± 7.4 μV (n= 9), respectively.
Figure 5. Amyl acetate exposure does not alter EOG or OERP.
The OERP (A) and EOG (B) in response to amyl acetate odour pulses before (day 1) and after (day 8) exposure to amyl acetate (3 min sniffing 3-times daily). Amyl acetate pulses (200 ms) of 4.28 mm (diluted 1: 3) were delivered at 10 s intervals by an olfactometer (see Methods) and 20 responses were averaged. The N1–P2 amplitudes were then measured. There were no changes in the amplitudes of either the OERP (n= 12) or the EOG (n= 9). Scale bars represent 5 μV (vertical), 1 s (horizontal) and 50 μV, 1 s for the OERP (A) and EOG (B), respectively.
Discussion
Our results demonstrate that, upon sensitization of the olfactory system to androstenone by repetitive exposure, there is an increase in the amplitude of both the EOG and the OERP in response to androstenone. This was not a generalized sensitization since there was no concurrent change in the threshold for amyl acetate. These increases in the EOG and OERP were correlated (see Fig. 3C), with larger EOGs giving rise to larger OERPs.
The key finding is therefore peripheral plasticity in the human olfactory system. Since the EOG reflects the summed generator potentials of the olfactory receptor neurones (ORNs; Ottoson, 1956; Getchell, 1974), this means an increased response from the ORNs and we can rule out release from descending inhibition and removal of adaptation/habituation as mechanisms mediating this phenomenon.
Similar androstenone-induced plasticity, possibly involving stimulus-controlled gene expression (Wang et al. 1993) at the level of the olfactory epithelium (Yee & Wysocki, 2001), has been demonstrated in mice. Yee & Wysocki, (2001), showed that this sensitization phenomenon occurred in mice in which the olfactory nerve was sectioned and allowed to recover. However, support for such changes occurring more centrally came from a human detection experiment by Mainland et al. (2002), in which they repeatedly exposed one nostril to androstenone and tested the unexposed nostril. They found that both the exposed nostril and the naïve nostril learnt to recognize the smell and suggested that this demonstrates that olfactory plasticity is a central phenomenon, although they could not rule out a contribution from the peripheral components of the olfactory system. Peripheral receptors may be induced in the unexposed nostril in response to a central signal (direct, or hormonal, for example; Mainland et al. 2002). Crossover in the olfactory system occurs at the level of the anterior commissure, thus a pathway for contralateral, efferent signalling from primary olfactory cortex to olfactory bulbs exists – although the findings of Yee & Wysocki (2001) militate against such centrifugal signalling. It is also possible that androstenone gained access to the blocked nostril via solution in saliva or the bloodstream, for instance by absorption via the lungs. Bloodborne odourants reach olfactory regions and can be perceived. Intravenous injection of odourants and subsequent perception is frequently used as a means of testing hyposmia (Furukawa et al. 1988). Our results demonstrate that there are peripheral receptor changes in response to repetitive exposure to androstenone. There are at least two possibilities: (1) existing androstenone-sensitive olfactory receptor neurones (ORNs) might express more androstenone receptors per ORN; or (2) additional androstenone-sensitive ORNs might be formed from basal or immature cells. The signal for this increase in receptor expression could originate in the periphery or be generated centrally.
Does olfactory ‘learning’ generalize to other odourants?
In the original study of inducible sensitivity to androstenone (Wysocki et al. 1989) it was found that the phenomenon did not extend to other odours. Daily exposure to amyl acetate and pyridine did not enhance sensitivity to these odours (Wysocki et al. 1989). It this present study we also found that the sensitization effect did not occur with amyl acetate. The threshold and OERP remained constant, in both men and women, for four tests over 21 days following daily sniffing of amyl acetate and the EOG remained constant for the two tests 7 days apart.
However, Wang et al. (1993) have demonstrated the induction of olfactory receptor sensitivity in mice to isovaleric acid as well as to androstenone, which they explained by stimulus-controlled gene expression. They found that the induction required a specific anosmia to the inducing odourant. However, we have found in humans that those already able to smell androstenone are able to increase their sensitivity.
Dalton et al. (2002) have reported enhanced olfactory sensitivity in women of reproductive age to benzaldehyde and citralva. The effect did not occur in men or prepubertal or postmenopausal women. The odours used in by Dalton et al. (2002), benzaldehyde and citralva, were different from our study (androstenone and amyl acetate) and the studies of Wysocki et al. (1989) (androstenone, amyl acetate and pyridine) and Wang et al. (1993) (androstenone and isovaleric acid in mice) and there were methodological differences. In the Dalton et al. (2002) study the thresholds were taken every 2 days compared to once a week in both our study and that of Wysocki et al. (1989), and the subjects did not sniff the odourants between tests, so there was greater emphasis on the test itself. Attending to olfactory stimuli decreases latency and increases the amplitude of the OERP (Krauel et al. 1998; Masago et al. 2001), which may influence threshold, but this does not explain the gender dimorphism. The androstenone-induced increase in the EOG was greater in women than men in our study, although this difference did not achieve significance. This is one area that requires further study.
Neither Dalton et al. (2002) nor Wysocki et al. (1989) reported the age of their participants. Age has a profound affect on threshold (Van Toller et al. 1985) and there were large differences in the thresholds reported in these studies; Dalton et al. (2002) reported a range of thresholds for amyl acetate from about 6.3 μm (females) to 63 μm (males), whereas Wysocki et al. (1989) reported a range of 8.3–16.7 μm (males and females); these compare with our findings of 0.9–11.7 μm (females) and 0.3–14.3 μm (males). Induction of sensitivity may depend to some extent upon the initial degree of insensitivity (Wang et al. 1993). It has been suggested (Wang et al. 1993) that the phenomenon of induced sensitivity to androstenone is most likely to be related to the continual turnover of ORNs (Farbman, 1992) and the expansion of the androstenone-sensitive population of receptor cells in response to exposure to the odour in a manner analogous to the immune system (Wysocki et al. 1989). This process could well be influenced by hormonal levels, thereby explaining the gender differences found by Dalton et al. (2002).
In conclusion, we have demonstrated for the first time peripheral plasticity in the human olfactory system as measured by an increase in the EOG. The EOG increase occurs concomitant with the decrease in detection threshold during androstenone-induced olfactory sensitization.
Acknowledgments
Funded by RAM Research Ltd, London, UK. We would like to thank Ernest Polak for his advice, support and critical reading of the manuscript.
References
- Aidley DJ. The Physiology of Excitable Cells. 4. Cambridge, UK: Cambridge University Press; 1998. p. 239. [Google Scholar]
- Baba K, Ikeda M, Houtani T, Nakagawa H, Ueyama T, Sato K, Sakuma S, Yamashita T, Tsukahara Y, Sugimoto T. Odour exposure reveals non-uniform expression profiles of c-Jun protein in rat olfactory bulb neurones. Brain Res. 1997;774:142–148. doi: 10.1016/s0006-8993(97)81697-6. [DOI] [PubMed] [Google Scholar]
- Bogen JE. On the neurophysiology of consciousness. I. An overview. Conscious Cogn. 1995;4:52–62. doi: 10.1006/ccog.1995.1003. (review) [DOI] [PubMed] [Google Scholar]
- Buonviso N, Chaput M. Olfactory experience decreases responsiveness of the olfactory bulb in the adult rat. Neuroscience. 2000;95:325–332. doi: 10.1016/s0306-4522(99)00450-9. [DOI] [PubMed] [Google Scholar]
- Dalton P. Psychophysical and behavioral characteristics of olfactory adaptation. Chem Senses. 2000;25:487–492. doi: 10.1093/chemse/25.4.487. [DOI] [PubMed] [Google Scholar]
- Dalton P, Doolittle N, Breslin PAS. Gender-specific induction of enhanced sensitivity to odors. Nature Neuroscience. 2002;5:199–202. doi: 10.1038/nn803. [DOI] [PubMed] [Google Scholar]
- Dalton P, Wysocki CJ. The nature and duration of adaptation following long-term odor exposure. Percept Psychophys. 1996;58:781–792. doi: 10.3758/bf03213109. [DOI] [PubMed] [Google Scholar]
- Doving KB, Pinching AJ. Selective degeneration of neuronesin the olfactory bulb following prolonged odour exposure. Brain Res. 1973;52:115–129. doi: 10.1016/0006-8993(73)90653-7. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell Biology of Olfaction. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- Furukawa M, Kamide M, Miwa T, Umeda R. Significance of intravenous olfaction test using thiamine propyldisulphide (Alinamin) in olfactometry. Auris Nasus Laryx. 1988;15:25–23. doi: 10.1016/s0385-8146(88)80006-3. [DOI] [PubMed] [Google Scholar]
- Getchell TV. Electrogenic sources of slow voltage transients recorded from frog olfactory epithelium. J Neurophysiol. 1974;37:115–130. doi: 10.1152/jn.1974.37.6.1115. [DOI] [PubMed] [Google Scholar]
- Gower DB, Nixon A, Mallet AI. The significance of odorous steroids in axillary odour. In: Van Toller S, Dodd GH, editors. Perfumery. London: Chapman & Hall; 1998. pp. 47–75. [Google Scholar]
- Gower DB, Ruparelia BA. Olfaction in humans with special reference to odorous 16-androstenes: their occurrence, perception, possible social, psychological and sexual impact. J Endocrinol. 1993;137:167–187. doi: 10.1677/joe.0.1370167. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall CM. Odours increase Fos in olfactory bulb neurones including dopaminergic cells. Neuroreport. 1995;6:2145–2149. doi: 10.1097/00001756-199511000-00012. [DOI] [PubMed] [Google Scholar]
- Krauel K, Pause BM, Sojka B, Schott P, Ferstl R. Attentional modulation of central odor processing. Chem Senses. 1998;23:423–432. doi: 10.1093/chemse/23.4.423. [DOI] [PubMed] [Google Scholar]
- Mainland JD, Bremner EA, Young N, Johnson BN, Khan RM, Bensafi M, Sobel N. One nostril knows what the other learns. Nature. 2002;419:802. doi: 10.1038/419802a. [DOI] [PubMed] [Google Scholar]
- Masago R, Shimomura Y, Iwanaga K, Katsuura T. The effects of hedonic properties of odors and attentional modulation on the olfactory event-related potentials. J Physiol Anthropol Appl Human Sci. 2001;20:7–13. doi: 10.2114/jpa.20.7. [DOI] [PubMed] [Google Scholar]
- Ottoson D. Analysis of the electrical activity of the olfactory epithelium. Acta Physiol Scand. 1956;35:1–83. [PubMed] [Google Scholar]
- Pause BM, Rogalski KP, Sojka B, Ferstl R. Sensitivity to androstenone in female subjects is associated with an altered brain response to male body odor. Physiol Behav. 1999;68:129–137. doi: 10.1016/s0031-9384(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Sallaz M, Jourdan F. C-fos expression and 2-deoxyglucose uptake in the olfactory bulb of odour-stimulated awake rats. Neuroreport. 1993;4:55–58. doi: 10.1097/00001756-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Glover GH, Sullivan V, Gabrieli JDE. Blind smell: brain activation induced by an undetected air-borne chemical. Brain. 1999;122:209–217. doi: 10.1093/brain/122.2.209. [DOI] [PubMed] [Google Scholar]
- Van Toller C, Dodd GH, Billing A. Ageing and the Sense of Smell. IL, USA: Charles C Thomas, Springfield; 1985. [Google Scholar]
- Van Toller C, Kirk-Smith M, Wood N, Lombard J, Dodd GH. Skin conductance and subjective assessments associated with the odour of 5-α-androstan-3-one. Biol Psychol. 1983;16:85–107. doi: 10.1016/0301-0511(83)90056-x. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Zibrowski EM. Pyriform cortex β-waves: odor-specific sensitization following repeated olfactory stimulation. Brain Res. 2001;892:301–308. doi: 10.1016/s0006-8993(00)03263-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Walker VE, Sardi H, Fraser C, Jacob TJC. The correlation between physiological and psychological responses to odour stimulation in human subjects. Clin Neurophysiol. 2002;113:542–551. doi: 10.1016/s1388-2457(02)00029-9. [DOI] [PubMed] [Google Scholar]
- Wang HW, Wysocki CJ, Gold GH. Induction of olfactory receptor sensitivity in mice. Science. 1993;260:998–1000. doi: 10.1126/science.8493539. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Dorries KM, Beauchamp GK. Ability to perceive androstenone can be acquired by ostensibly anosmic people. Proc Natl Acad Sci U S A. 1989;86:7976–7978. doi: 10.1073/pnas.86.20.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KK, Wysocki CJ. Odorant exposure increases olfactory sensitivity: olfactory epithelium is implicated. Physiol Behav. 2001;72:705–711. doi: 10.1016/s0031-9384(01)00428-0. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Katoh K, Mori K. Odour stimulation causes disappearance of R4B12 epitope on axonal surface molecule of olfactory sensory neurones. Neuroscience. 1993;53:101–110. doi: 10.1016/0306-4522(93)90288-q. [DOI] [PubMed] [Google Scholar]