Abstract
The relative contributions of voltage- and Ca2+-dependent mechanisms of inactivation to the decay of L-type Ca2+ channel currents (ICaL) is an old story to which recent results have given an unexpected twist. In cardiac myocytes voltage-dependent inactivation (VDI) was thought to be slow and Ca2+-dependent inactivation (CDI) resulting from Ca2+ influx and Ca2+-induced Ca2+-release (CICR) from the sarcoplasmic reticulum provided an automatic negative feedback mechanism to limit Ca2+ entry and the contribution of ICaL to the cardiac action potential. Physiological modulation of ICaL by β-adrenergic and muscarinic agonists then involved essentially more or less of the same by enhancing or reducing Ca2+ channel activity, Ca2+ influx, sarcoplasmic reticulum load and thus CDI. Recent results on the other hand place VDI at the centre of the regulation of ICaL. Under basal conditions it has been found that depolarization increases the probability that an ion channel will show rapid VDI. This is prevented by β-adrenergic stimulation. Evidence also suggests that a channel which shows rapid VDI inactivates before CDI can become effective. Therefore the contributions of VDI and CDI to the decay of ICaL are determined by the turning on, by depolarization, and the turning off, by phosphorylation, of the mechanism of rapid VDI. The physiological implications of these ideas are that under basal conditions the contribution of ICaL to the action potential will be determined largely by voltage and by Ca2+ following β-adrenergic stimulation.
Introduction
Mutagenesis, chimera constructions and differential subunit expression have provided original insight into the processes of activation, permeation and inactivation of L-type Ca2+ channels (ICaL). The fundamental role of calmodulin mediating the process of Ca2+-dependent inactivation has been defined (Qin et al. 1999; Anderson, 2001) along with the action of calcium-calmodulin-dependent kinase II (Maier & Bers, 2002). The ensemble of this data accounts for variation in the behaviour of ICaL due to the expression in different tissues of splice variants, long and short forms of the α1c subunit and combinations with different β subunits. On the other hand, physiological modulation of the behaviour of ICaL by the means of phosphorylation via protein kinase A (PKA) which underlies sympathetic, parasympathetic and purinergic regulation of the Ca2+ current (McDonald et al. 1994; Kamp & Hell, 2000) has been less amenable to explanation by the methods of in vitro reconstruction. Few have succeeded in the reconstitution of the response of ICaL to stimulation via PKA (Gao et al. 1997; Naguro et al. 2001). Part of this problem has been found to be the assemblage of all of the elements required to affirm this reaction (Gao et al. 1997). The rest has been in determining what actually is or are the reactions of ICaL to agonist stimulation.
It is commonly held that the effect of β-adrenergic stimulation of ICaL is the increase in amplitude of the whole-cell current arising from an increase in the availability of the channels as well as an increase in their probability of opening; the voltage dependence of activation and inactivation of ICaL are shifted to more negative voltages (McDonald et al. 1994; Bers & Perez-Reyes, 1999). Inactivation of ICaL occurs via processes associated with membrane voltage and the increase in intracellular Ca2+. Initially, the relative extents of these two mechanisms was disputed (Kass & Sanguinetti, 1984; Mentrard et al. 1984; Lee et al. 1985; Hadley & Hume, 1987; Argibay et al. 1988). Today it is generally assumed that the dominant character determining the rapid decay of ICaL is inactivation consequent to the influx of Ca2+ (Linz & Meyer, 1998; Sun et al. 2000). The increase of inactivation following β-adrenergic stimulation can then readily be understood as a consequence of enhanced Ca2+ influx upon increased channel activity.
The physical mechanism of inactivation of ICaL is not known. In Ca2+ channels there is no obvious equivalent of the ‘ball-and-chain’ or rearrangement of the pore-lining segments which produce inactivation of voltage-gated Na+ or K+ channels (Antz & Fakler, 1998; Cantrell & Catterall, 2001). It is not known whether the processes of voltage-dependent inactivation and Ca2+-dependent inactivation have the same physical endpoint or whether they represent different mechanisms?
Recently a series of studies of ICaL in native cardiac myocytes have opened new perspectives upon the old problem of voltage- and Ca2+-dependent inactivation (Mitarai et al. 2000; Findlay, 2002a,b,c,d). This short review will summarize these novel ideas and attempt to integrate them into a simple framework. This framework is the switching on and the switching off of rapid voltage-dependent inactivation of the individual Ca2+ channel.
Voltage-dependent inactivation
The classical view of voltage-dependent inactivation (VDI) of ICaL is that it is slow and accelerates only slightly with depolarization (Bechem & Pott, 1985; Fukushima & Hagiwara, 1985; Matsuda, 1986). This vision resulted from experiments that were conducted under conditions that were the equivalent of β-adrenergic stimulation (see below). When attention was paid to record ICaL under basal conditions VDI strongly increased with progressive depolarization (Fig. 1) and was rapid at positive membrane potentials (Mitarai et al. 2000; Findlay, 2002a,d).
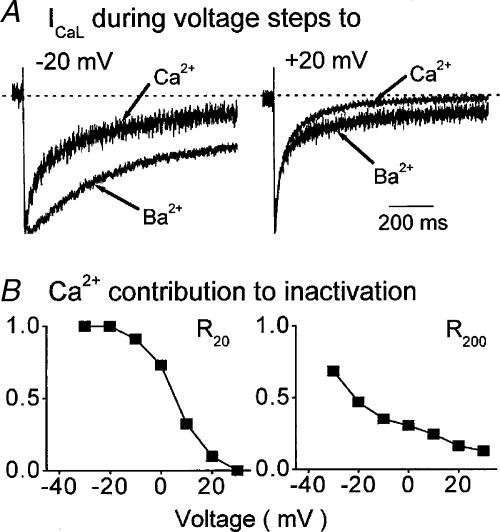
Figure 1. Inactivation of ICaL under basal conditions.
A, normalized ICaL carried by Ca2+ and Ba2+ in one ventricular myocyte. At negative voltages (left) Ca2+ current inactivates more rapidly than Ba2+ current. At positive voltages (right) the initial decay of Ba2+ current is as fast as that of Ca2+ current, only later does Ca2+ current show more inactivation. B, the contribution of Ca2+ to the inactivation of ICaL evaluated by the ratio of decay recorded with Ca2+ relative to that recorded with Ba2+, 20 ms (R20, left) and 200 ms (R200, right) after activation. At both times the contribution of Ca2+ declines with depolarization though there is clearly more of an effect of Ca2+ at positive voltages after 200 ms than after 20 ms. The figure has been redrawn from Findlay (2002a).
A detailed analysis of the time course of development and voltage dependence of VDI (Fig. 2A and B) (Findlay, 2002d) showed that at membrane potentials of –30 and –20 mV the whole-cell current could be divided into two components, a part which showed slow time-dependent decay and a part which showed essentially no decay. At –10 mV a third and rapidly decaying time-dependent component appeared and with further depolarization the amplitude of this rapid time-dependent component increased. At the same time, the amplitudes of slow time-dependent and time-independent components of the whole-cell current declined. Maximal VDI followed a biphasic time course (τf ∼30 ms and τs ∼300 ms) which was dominated by the rapid phase of inactivation.
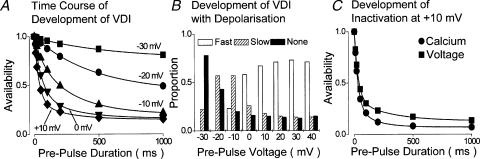
Figure 2. The development of rapid VDI under basal conditions.
A, the time course of the development of VDI was recorded in the absence of Ca2+, and in the absence of ion flux through the channels, between voltages which evoke minimal (–30 mV) and maximal (+10 mV) inactivation. The lines connecting data points represent the fitting of either a single (–30 and –20 mV) or a double (–10, 0 and +10 mV) exponential function to the data. B, the proportions of fast (open columns), slow (hatched columns) and no (filled columns) VDI which were recorded at different membrane potentials show that as the amount of current which showed fast VDI increased, that which showed no VDI declined. C, the relative contributions of Ca2+ and voltage to the decay of ICaL were assessed by measuring the time course of development of inactivation at +10 mV in the presence (circles) and the absence (squares) of extracellular Ca2+. The first recorded total inactivation, being the sum of VDI and CDI. The second recorded only VDI since no ion flux through ICaL occurred at this voltage under these conditions (see Findlay, 2002b for further details). It is clear that Ca2+ influx does little to increase the initial rapid phase of the development of inactivation. The results shown here were conducted in the presence of ryanodine. A and B have been redrawn from Findlay (2002d) and C from Findlay (2002b).
Biphasic decay of ICaL in cardiac myocytes has been traditionally assigned to the separate processes of Ca2+- (fast decay) and voltage- (slow decay) dependent inactivation (Adams & Tanabe, 1997; Ferreira et al. 1997; Sun et al. 1997). It was not expected that VDI alone would show a biphasic time course (Findlay, 2002d), though this can be explained by individual Ca2+ channel ‘modal’ behaviour (Hess et al. 1984; Tsien et al. 1986; Pietrobon & Hess, 1990). Thus an individual ion channel could either rapidly inactivate after the onset of opening or not (Plummer & Hess, 1991; Rose et al. 1992). This simple switch would convert an ion channel which did not show VDI into an ion channel which did show VDI. In mechanistic terms all that would be required is for VDI to be turned on by the voltage step and with an increasing probability of this occurring with depolarization. The stochastic nature of this mechanism would then account for the different kinetics of decay of ICaL and the graded development of rapid inactivation at the expense of slow or no inactivation in the whole-cell population of ion channels (Findlay, 2002d).
Sympathetic and parasympathetic regulation of ICaL in cardiac muscle follows activation of adenylate cyclase and PKA (Kamp & Hell, 2000). There are several prospective sites for the action of PKA upon α1C and β2 subunits (Bunemann et al. 1999) and the close association of PKA with the Ca2+ channel in a supramolecular complex is required for in vitro reconstitution of the β-adrenergic response (Gao et al. 1997). The isolation of VDI following β-adrenergic stimulation has not been easy. The effect of β-adrenergic stimulation upon the apparently voltage-dependent inactivation of ICaL carried by Ba2+ or Sr2+ has given rather mixed results with clear indications of the contribution of an ion-dependent process (Tiaho et al. 1991; Ferreira et al. 1997; Findlay, 2002a). On the other hand, following β-adrenergic stimulation ICaL carried by Na+ clearly retains the character of VDI without ion-dependent inactivation (Mitarai et al. 2000; Findlay, 2002a). In these circumstances VDI is slow and shows little development with depolarization. These recent results therefore correspond to the classical view of VDI (Bechem & Pott, 1985; Fukushima & Hagiwara, 1985; Matsuda, 1986) which for diverse reasons were conducted in fact under experimental conditions that were likely to lead to activation of PKA.
Analysis of the effects of agonists upon VDI (Mitarai et al. 2000; Findlay, 2002c) revealed that the number of ion channels which showed rapid VDI was reduced by the β-adrenergic agonist isoprenaline in a dose-dependent manner (Fig. 4). At the same time the number of ion channels which did not show VDI was increased. At each dose of isoprenaline, the addition of the muscarinic agonist carbachol increased the number of channels which showed rapid VDI and reduced the number of channels which did not show VDI (Findlay, 2002c). There was clearly a reciprocal relationship between the number of channels which did show rapid VDI and the number of channels which did not show rapid VDI. Since these β-adrenergic and muscarinic agonists are considered to act upon the Ca2+ channels by, respectively, increasing and decreasing their phosphorylation via PKA, this would suggest that phosphorylation turns off or otherwise prevents the mechanism of rapid VDI. This simple switch would convert ion channels which did show rapid VDI into channels which did not show VDI.
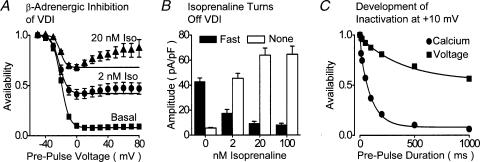
Figure 4. Inhibition of rapid VDI by β-adrenergic stimulation.
A, dose-dependent reduction of the availability–voltage relationship of ICaL by Isoprenaline (Iso). Experiments were conducted in the absence of extracellular Ca2+ with a double-pulse voltage-clamp protocol with 1000 ms duration prepulse voltage steps. B, the effects of isoprenaline upon the amount of ICaL which showed fast VDI (filled columns) and no inactivation (open columns). These experiments were conducted in the absence of extracellular Ca2+ and Findlay (2002c) should be consulted for further details. C, the relative contributions of Ca2+ and voltage to the decay of ICaL were assessed as described in Fig. 2C in the presence (circles) and the absence (squares) of extracellular Ca2+ in the presence of 100 nm isoprenaline. It is clear that Ca2+ influx dramatically increases the rate of development of inactivation, and comparison with Fig. 2C reveals that this is due to the suppression of fast VDI. These experiments were conducted in the presence of ryanodine. A and B have been redrawn from Findlay (2002c) and C from Findlay (2002b).
Thus a single and simple mechanism can be proposed to account for the development of VDI with depolarization and the modulation of VDI by agonists. The stochastic operation of this on–off switch for VDI in the single channel would account for the multiphasic nature of the behaviour of the whole-cell population of ion channels.
In historical terms, it is not the drastic reduction of VDI of ICaL in cardiac myocytes which is evoked by β-adrenergic stimulation that would be considered to be an unusual result. Though it should be mentioned that this effect of agonist modulation of a voltage-gated ion channel is unique to ICaL. Agonist modulation of voltage-gated Na+ and K+ channel currents is confined to shifting activation and inactivation curves along the voltage axis (Cantrell & Catterall, 2001). They do not result in the reduction of the minimum of the inactivation curve (Fig. 4A). The novel aspect of this analysis of VDI of ICaL in cardiac myocytes is the discovery of rapid VDI under basal conditions in the absence of agonists (Findlay, 2002a,b,c,d). The decay of ICaL carried by Ca2+ in cardiac myocytes is rapid and when it was thought that VDI was slow, it was inferred that Ca2+-dependent inactivation was the dominant physiological mechanism of inactivation (Linz & Meyer, 1998; Sun et al. 2000). This conclusion is called into question with the discovery of rapid VDI.
Ca2+-dependent Inactivation
Ca2+-dependent inactivation (CDI) of ICaL is mediated by the interaction of the Ca2+-binding protein calmodulin with the proximal region of the cytoplasmic C-terminus of the α1C subunit of the Ca2+ channel (Anderson, 2001; Maier & Bers, 2002). Calmodulin is attached to the Ca2+ channel in the presence of basal intracellular Ca2+ concentrations (Erickson et al. 2001; Pitt et al. 2001). An increase in intracellular Ca2+ activates calmodulin which reorientates its attachments and in consequence is thought to alter the 3D structure of the C-terminus (Petersen et al. 2000). In an unknown manner, this procedure provokes inactivation of ICaL. The implication of calmodulin with the process of CDI of ICaL has several interesting consequences. The first of these is that calmodulin is not selective and there is no obligation for Ca2+ which has entered via a Ca2+ channel to provoke inactivation. Any increase of Ca2+ in the vicinity of the internal surface of the channel will suffice to trigger CDI irrespective of whether the channel has opened or not. Thus Ca2+-induced Ca2+-release (CICR) from the cardiac sarcoplasmic reticulum can cause CDI (Adachi-Akahane et al. 1996; Sham, 1997). The opening of a neighbouring Ca2+ channel can cause CDI (Imredy & Yue, 1992). The triggering of CICR by chemical means can cause CDI (Lipp et al. 1987; Adachi-Akahane et al. 1996). The photolytic release of Ca2+ from intracellular caged compounds can cause CDI (Hadley & Lederer, 1991; Bates & Gurney, 1993). In cardiac myocytes it is not clear to what extent Ca2+ which enters via a given Ca2+ channel causes CDI of that channel. Otherwise it would be difficult to account for the significant effects of CICR upon CDI within the local-control domain (Stern, 1992; Wier et al. 1994) which suggests that while sufficient Ca2+ can enter via the surface Ca2+ channel to provoke CICR, this is insufficient to locally provoke CDI. Also, in cells which lack the geometry of close association between surface membrane and junctional sarcoplasmic reticulum, CDI is related more closely to the overall cell current density of ICaL rather than the driving force for Ca2+ entry via the single channel (Argibay et al. 1988). The second point which arises from the association of calmodulin with the process of CDI is that the interaction between Ca2+ and calmodulin is readily reversible and entirely dependent upon the local Ca2+ concentration. But it is dogma that inactivation of voltage-gated ion channels is an absorptive state that requires a period at a hyperpolarized potential to recover (Imredy & Yue, 1994) while several studies have shown that CDI might be reversible without repolarization (Sipido et al. 1995; Brette et al. 2003). It was therefore in the context of this complexity of the kinetics and sources of CDI that recent studies which wished to evaluate the relative contributions of CDI and VDI to the decay of ICaL in native cardiac myocytes chose to block CICR with ryanodine (Findlay, 2002a,c). In this manner, CDI where it occurred would result entirely from the influx of Ca2+ through sarcolemmal Ca2+ channels.
Two methods were used to assess the relative contributions of CDI and VDI in isolated cardiac myocytes. The first compared the decay of ICaL carried by Ca2+ with that of ICaL carried by Ba2+, Sr2+ and Na+ (Findlay, 2002a). The second utilized the technique developed by Hadley & Hume (1987) which enables the recording of ICaL in the absence of cation influx when ICaL is blocked by extracellular Mg2+ (Findlay, 2002b). In basal conditions at positive membrane potentials the development of inactivation followed a biphasic time course and there was little or no difference between the initial rapid phase of inactivation of ICaL when this resulted from the influx of Ca2+, Ba2+, Sr2+, Na+ or no ion flux (Figs 1A and 2C). The second, slow phase of inactivation was less when ICaL was carried by Ca2+ (Fig. 1A). These results quite clearly showed that the initial rapid phase of inactivation of ICaL was independent of CDI and therefore due to VDI. This represents the reverse of the traditional description of fast decay of ICaL due to CDI and slow decay of ICaL due to VDI (Adams & Tanabe, 1997; Ferreira et al. 1997; Sun et al. 1997). At negative membrane potentials the distinction between the decay of ICaL carried by Ca2+ and the other cations was larger (Fig. 1A) and it was clear that CDI did play an important role in determining the decay of ICaL (Fig. 1B). When these experiments were repeated following maximal β-adrenergic stimulation with isoprenaline ICaL carried by Ca2+ decayed more rapidly than ICaL carried by the other cations at all voltages (Fig. 3A). In these circumstances CDI dominated the decay of ICaL (Figs 3B and C).
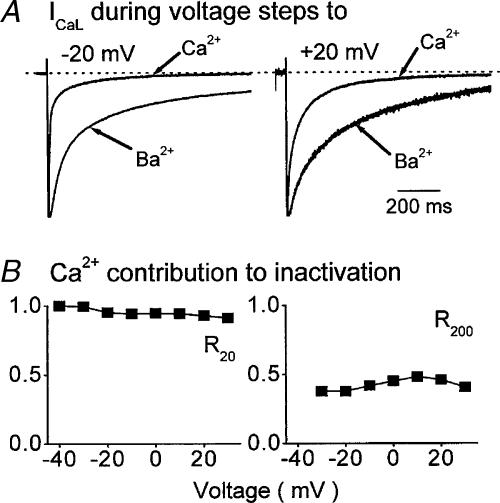
Figure 3. Inactivation of ICaL following β-adrenergic stimulation.
A, normalized ICaL carried by Ca2+ and Ba2+ in one ventricular myocyte. At negative (left) and positive (right) voltages Ca2+ current inactivates more rapidly than Ba2+ current. B, the contribution of Ca2+ to the inactivation of ICaL 20 ms (R20, left) and 200 ms (R200, right) after activation is clearly sustained at all membrane voltages. The figure has been redrawn from Findlay (2002a).
The results which had been obtained by directly comparing total inactivation recorded during Ca2+ influx with isolated VDI show an interesting relation between these two phenomena. Under basal conditions the contribution of CDI to the decay of ICaL progressively declined with depolarization (Fig. 1B). VDI increased with depolarization and in particular the number of channels which show fast VDI increased (Fig. 2B). CDI dominated decay of ICaL at all voltages following β-adrenergic stimulation (Fig. 3B) when rapid VDI had been suppressed (Fig. 4B and 4C).
Conclusions and perspectives
It is not necessary to look for complicated explanations for the variation of the contributions of VDI and CDI to the decay of ICaL under a range of physiological conditions. Physiological modulation of inactivation of ICaL is proposed to act at a single point, the triggering of rapid voltage-dependent inactivation. This is turned on with depolarization, and turned off by phosphorylation. When it is on, CDI is ineffective; when it is off, CDI is effective. The graded influence of VDI and CDI and the effects of β-adrenergic and muscarinic agonists are then a consequence of the variation in the number of individual ion channels which show VDI. It is proposed that at the level of the single L-type Ca2+ channel rapid VDI will either occur or not. That single Ca2+ channels are capable of switching between rapid VDI and no inactivation has already been shown for a neuronal N-type Ca2+ channel (Plummer & Hess, 1991). The probability of this occurring in the L-type Ca2+ channel would increase with increasing depolarization. Since a channel which has already been inactivated by voltage cannot show inactivation which is caused by Ca2+ it is clear that the on–off switch for the occurrence of fast VDI determines the visible contribution of Ca2+ to the overall time course of decay of ICaL. Thus, as the number of channels which adopt rapid VDI increases, the number of channels which are inactivated by Ca2+ decreases. The prevention of fast VDI by phosphorylation of the channel either at the α1C or at the β2 subunit will then permit CDI.
It seems likely that CDI is a process that has a certain latency. This does not necessarily arise from the interaction of calmodulin with the C-terminus of the channel, in particular since calmodulin can be expected to be tethered to the channel under all physiological conditions (Erickson et al. 2001; Pitt et al. 2001). But the rise in ‘fuzzy space’ Ca2+ to the level required to trigger the transformation of calmodulin might require a certain time, in particular since Ca2+ from several sources is known to provoke CDI and may be required to provoke CDI (Imredy & Yue, 1994; Adachi-Akahane et al. 1996).
The physiological consequences of these observations reside in the observation of the dominance of VDI at positive membrane potentials under basal conditions which is replaced by the dominance of CDI following β-adrenergic stimulation (Findlay, 2002b). These results predict that should there be an error in the process of CDI this would have little effect upon ICaL and its contribution to the action potential under basal conditions. On the other hand, this would cause a drastic slowing of the decay of ICaL and probably massive elongation of the action potential following β-adrenergic stimulation. Alseikhan et al. (2002) have expressed mutant Ca2+-insensitive calmodulin in isolated cardiac myocytes. The action potentials recorded from these cells were drastically increased. This clearly illustrates the importance that CDI could have to limit the contribution of ICaL to the electrophysiology of cardiac myocytes. These experiments were conducted in the absence of agonists and thus presumably under basal conditions, which would contradict the importance of VDI which is suggested here. But it was noted that the adenovirus infection technique by itself evoked an increase in the density of ICaL (Alseikhan et al. 2002). It is therefore possible that PKA was activated in these cells. It remains to be seen whether, with a normal basal density of ICaL, the duration of the cardiac action potential would have been elongated by the expression of mutated calmodulin.
It is interesting also to consider that abolition of the process of VDI of ICaL might be without obvious consequence upon the electrophysiology of the cardiac myocyte. VDI is already essentially abolished by β-adrenergic stimulation under which circumstances rapid decay of ICaL is undertaken by CDI (Fig. 4C: Findlay, 2002b). There is therefore no reason to suppose that this would not also occur should VDI be impeded under basal conditions. In any case, CDI already contributes to the decay of ICaL under basal conditions at negative membrane potentials where VDI has little effect (Findlay, 2002a).
Notwithstanding that chimera constructions of the α1 subunit and the expression of different β subunits of voltage-gated Ca2+ channels have been able to indicate regions of the channel that can be involved in VDI (Stotz & Zamponi, 2001b), none have had to account for the physiological modulation of this process. Three sites upon the L-type Ca2+ channel are known to be phosphorylated by PKA: two sites upon the β2 subunit and one site distal to the calmodulin binding regions of the C-terminus of the α1C subunit (Puri et al. 1997). Although it is now clear that the proximal region of the C-terminus of the α1C subunit is responsible for CDI (Erickson et al. 2001; Pitt et al. 2001), it is not known how this is achieved and it is not known what effect PKA-induced phosphorylation of this region (Puri et al. 1997) has upon either CDI or VDI. None of the experiments conducted by either Mitarai et al. (2000) or Findlay (2002a,b,c,d) suggest that CDI is a process that could be influenced directly by phosphorylation of the Ca2+ channel.
β2 subunits which influence, amongst other things, the rate of inactivation of Ca2+ channels (Birnbaumer et al. 1998) are attached to the α1C subunit at the I–II intracellular linker (Pragnell et al. 1994). This linker has been suggested to be responsible for VDI via a ‘hinged-lid’ mechanism which would occlude the internal vestibule of the channel pore (Stotz & Zamponi, 2001a). If movement of the I–II linker and attached β2 subunit was responsible for the mechanism of VDI it would not be difficult to imagine that phosphorylation of the β2 subunit might physically affect this process. There may be analogy to be drawn with the effect of phosphorylation of the N-terminal inactivation particle of voltage-gated K+ channels altering its configuration and affinity for the vestibule receptor (Covarrubias et al. 1994; Antz et al. 1999). Alternatively, phosphorylation or dephosphorylation of the β2 subunit might be responsible for dissociation of the β2 subunit from the I–II linker (Bichet et al. 2000; Restituito et al. 2001). Finally, it is not to be excluded that the distal region of the C-terminus of α1C which is truncated by post-translational proteolysis, but which can remain attached to the channel complex (Gerhardstein et al. 2000; Gao et al. 2001) may play a role which could be influenced by phosphorylation of Ser 1928 (Gao et al. 1997).
In conclusion, it is clear that the concept of physiological modulation of VDI of ICaL (CaV1.2) which has arisen from studies of the native channel in its physiological context poses some direct questions that remain to be answered with the tools of molecular biology and mutagenesis. The description of a very loose, open and flexible structure of a voltage-gated K+ channel (Jiang et al. 2003a,b) may provide impetus towards the decryption of the processes of activation and inactivation of the more complex supramolecular structure of the mammalian Ca2+ channel. This review has concentrated attention upon the behaviour of ICaL in cardiac myocytes in the absence of CICR. The influence of CICR upon the inactivation of ICaL in native cardiac myocytes is an important component of the physiology of the regulation of the cardiac action potential. But this geometric arrangement is particular to cardiac myocytes. By concentrating attention upon the influence of Ca2+ influx upon inactivation of ICaL it is hoped that the concepts which have been developed here from work upon cardiac myocytes may find application to a wider range of tissues.
References
- Adachi-Akahane S, Leeman L, Morad M. Cross-signalling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J General Physiol. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B, Tanabe T. Structural regions of the cardiac Ca channel alpha subunit involved in Ca-dependent inactivation. J General Physiol. 1997;110:379–389. doi: 10.1085/jgp.110.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci USA. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME. Ca2+-dependent regulation of cardiac L-type Ca2+ channels: is a unifying mechanism at hand. J Mol Cell Cardiol. 2001;33:639–650. doi: 10.1006/jmcc.2000.1354. [DOI] [PubMed] [Google Scholar]
- Antz C, Bauer T, Kalbacher H, Frank R, Covarrubias M, Kalbitzer HR, Ruppersberg JP, Baukrowitz T, Fakler B. Control of K+ channel gating by protein phosphorylation: structural switches of the inactivation gate. Nature Struc Biol. 1999;6:146–150. doi: 10.1038/5833. [DOI] [PubMed] [Google Scholar]
- Antz C, Fakler B. Fast inactivation of voltage-gated K+ channels: from cartoon to structure. News Physiol Sci. 1998;13:177–182. doi: 10.1152/physiologyonline.1998.13.4.177. [DOI] [PubMed] [Google Scholar]
- Argibay JA, Fischmeister R, Hartzell HC. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SE, Gurney AM. Ca2+-dependent block and potentiation of L-type calcium current in guinea-pig ventricular myocytes. J Physiol. 1993;466:345–365. [PMC free article] [PubMed] [Google Scholar]
- Bechem M, Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985;404:10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Bers DM, Perez-Reyes E. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc Res. 1999;42:339–360. doi: 10.1016/s0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- Bichet D, Lecomte C, Sabatier J-M, Felix R, De Waard M. Reversibility of the Ca2+ channel α1–β subunit interaction. Biochem Biophys Res Commun. 2000;277:729–735. doi: 10.1006/bbrc.2000.3750. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel β subunits. J Bioenerg Biomemb. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- Brette F, Lacampagne A, Sallé L, Findlay I, Le Guennec J-Y. Internal Cs+ activates the PKA pathway revealing a fast, reversible, Ca2+-dependent inactivation of L-type Ca2+ current. Am J Physiol Cell Physiol. 2003;285:C310–C318. doi: 10.1152/ajpcell.00368.2002. [DOI] [PubMed] [Google Scholar]
- Bunemann M, Gerhardstein BL, Gao T, Hosey MM. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the β2 subunit. J Biol Chem. 1999;26:33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nature Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Wei A, Salkoff L, Vyas TB. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron. 1994;13:1403–1412. doi: 10.1016/0896-6273(94)90425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- Ferreira G, Yi J, Rios E, Shirokov R. Ion-dependent inactivation of Ba2+ current through L-type calcium channels. J General Physiol. 1997;109:449–461. doi: 10.1085/jgp.109.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. Voltage- and cation-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J Physiol. 2002a;541:731–740. doi: 10.1113/jphysiol.2002.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. β-Adrenergic stimulation modulates Ca2+- and voltage-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J Physiol. 2002b;541:741–751. doi: 10.1113/jphysiol.2002.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. β-Adrenergic and muscarinic agonists modulate inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. J Physiol. 2002c;545:375–388. doi: 10.1113/jphysiol.2002.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. Voltage-dependent inactivation of L-type Ca2+ currents in guinea-pig ventricular myocytes. J Physiol. 2002d;545:389–397. doi: 10.1113/jphysiol.2002.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Ten Eick R, Hosey MM. C-terminal fragments of the α1C (CaV1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated α1C subunits. J Biol Chem. 2001;276:21089–21097. doi: 10.1074/jbc.M008000200. [DOI] [PubMed] [Google Scholar]
- Gao T, Yatani A, Dell'acqua ML, Sako H, Green SA, Dascal N, Scott JT, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C-terminus of the α1C subunit of the L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- Hadley RW, Hume JR. An intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J Physiol. 1987;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RW, Lederer WJ. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Imredy JP, Yue DT. Submicroscopic Ca2+ diffusion mediates inhibitory coupling between individual Ca2+ channels. Neuron. 1992;9:197–207. doi: 10.1016/0896-6273(92)90159-b. [DOI] [PubMed] [Google Scholar]
- Imredy JP, Yue DT. Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-gated K+ channel. Nature. 2003a;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature. 2003b;423:42–48. doi: 10.1038/nature01581. [DOI] [PubMed] [Google Scholar]
- Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- Kass RS, Sanguinetti MC. Inactivation of calcium channel current in the calf cardiac Purkinje fibre: evidence for voltage- and calcium-mediated mechanisms. J General Physiol. 1984;84:705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Marban E, Tsien RW. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz KW, Meyer R. Control of L-type calcium current during the action potential of guinea-pig ventricular myocytes. J Physiol. 1998;513:425–442. doi: 10.1111/j.1469-7793.1998.425bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Mechmann S, Pott L. Effect of calcium release from sarcoplasmic reticulum on membrane currents in guinea-pig atrial cardioballs. Pflugers Arch. 1987;410:121–131. doi: 10.1007/BF00581904. [DOI] [PubMed] [Google Scholar]
- Maier LS, Bers DM. Calcium, calmodulin and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919–939. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Sodium conductance in calcium channels of guinea-pig ventricular cells induced by removal of external calcium ions. Pflugers Arch. 1986;407:465–475. doi: 10.1007/BF00657502. [DOI] [PubMed] [Google Scholar]
- McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Mentrard D, Vassort G, Fischmeister R. Calcium-mediated inactivation of the Ca conductance in caesium-loaded frog heart cells. J General Physiol. 1984;83:105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitarai S, Kaibara M, Yano K, Taniyama K. Two distinct inactivation processes related to phosphorylation in cardiac L-type Ca2+ channel currents. Am J Physiol Cell Physiol. 2000;279:C603–C610. doi: 10.1152/ajpcell.2000.279.3.C603. [DOI] [PubMed] [Google Scholar]
- Naguro I, Nagao T, Adachi-Akahane S. Ser1901 of α1C subunit is required for the PKA-mediated enhancement of L-type Ca2+ channel currents but not for the negative shift of activation. FEBS Lett. 2001;489:87–91. doi: 10.1016/s0014-5793(01)02079-8. [DOI] [PubMed] [Google Scholar]
- Petersen BZ, Lee JS, Mulle JG, Wang T, de Leon M, Yue DT. Critical determinants of Ca2+-dependent inactivation within an E-F hand motif of L-type Ca2+ channels. Biophys J. 2000;78:1906–1920. doi: 10.1016/S0006-3495(00)76739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D, Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990;346:651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- Plummer MR, Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991;351:657–659. doi: 10.1038/351657a0. [DOI] [PubMed] [Google Scholar]
- Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- Puri TS, Gerhardstein BL, Zhao X-L, Ladner MB, Hosey MM. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry. 1997;36:9605–9615. doi: 10.1021/bi970500d. [DOI] [PubMed] [Google Scholar]
- Qin N, Olese R, Bransby M, Lin T, Birnbaumer L. Ca2+-induced inhibition of the cardiac Ca2+ channel depends upon calmodulin. Proc Natl Acad Sci USA. 1999;96:2435–2438. doi: 10.1073/pnas.96.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restituito S, Cens T, Rousset M, Charnet P. Ca2+ channel inactivation heterogenecity reveals physiological unbinding of auxiliary β subunits. Biophys J. 2001;81:89–96. doi: 10.1016/S0006-3495(01)75682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose WC, Balke CW, Weir WG, Marban E. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992;456:267–284. doi: 10.1113/jphysiol.1992.sp019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham JSK. Ca2+ release-induced inactivation of Ca2+ current in rat ventricular myocytes: evidence for local Ca2+ signalling. J Physiol. 1997;500:285–295. doi: 10.1113/jphysiol.1997.sp022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Callewaert G, Carmeliet E. Inhibition and rapid recovery of Ca2+ current during Ca2+-release from sarcoplasmic reticulum in guinea-pig ventricular myocytes. Circ Res. 1995;76:102–109. doi: 10.1161/01.res.76.1.102. [DOI] [PubMed] [Google Scholar]
- Stern M. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz SC, Zamponi GW. Identification of inactivation determinants in the domain IIS6 region of high voltage-activated calcium channels. J Biol Chem. 2001a;276:33001–33010. doi: 10.1074/jbc.M104387200. [DOI] [PubMed] [Google Scholar]
- Stotz SC, Zamponi GW. Structural determinant of fast inactivation of high voltage-activated Ca2+ channels. Trends Neurosci. 2001b;24:176–181. doi: 10.1016/s0166-2236(00)01738-0. [DOI] [PubMed] [Google Scholar]
- Sun L, Fan J-S, Clark JW, Palade PT. A model of the L-type Ca2+ channel in rat ventricular myocytes: ion selectivity and inactivation mechanisms. J Physiol. 2000;529:139–158. doi: 10.1111/j.1469-7793.2000.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Leblanc N, Nattel S. Mechanisms of inactivation of L-type calcium channels in human atrial myocytes. Am J Physiol Heart Circ Physiol. 1997;272:H1625–H1635. doi: 10.1152/ajpheart.1997.272.4.H1625. [DOI] [PubMed] [Google Scholar]
- Tiaho F, Nargeot J, Richard S. Voltage-dependent regulation of L-type cardiac Ca channels by isoproterenol. Pflugers Arch. 1991;419:596–602. doi: 10.1007/BF00370301. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by β-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Wier WG, Egan TM, Lopez-Lopez JR, Balke CW. Local control of excitation–contraction coupling in rat heart cells. J Physiol. 1994;474:463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]