Abstract
A transient increase in skin blood flow in response to an innocuous local pressure application, defined as pressure-induced vasodilatation (PIV), delays the occurrence of ischaemia, suggesting a protective feature against applied pressure. The PIV response depends on capsaicin-sensitive nerve fibres and calcitonin gene-related peptide (CGRP) has been shown to be involved. In these fibres, CGRP coexists with pituitary adenylate cyclase-activating polypeptide (PACAP). Three distinct receptors mediate the biological effects of PACAP: VPAC1 and VPAC2 receptors binding with the same affinity for PACAP and vasoactive intestinal peptide and PAC1 receptors showing high selectivity for PACAP. Because the receptors are widely expressed in the nervous system and in the skin, we hypothesized that at least one of them is involved in PIV development. To verify this hypothesis, we used [d-p-Cl-Phe6,Leu17]-VIP (nonspecific antagonist of VPAC1/VPAC2 receptors), PG 97-269 (antagonist of VPAC1 receptors), PACAP(6–38) (antagonist of VPAC2/PAC1 receptors) and Max.d.4 (antagonist of PAC1 receptors) in anaesthetized rodents. The blockade of VPAC1/VPAC2, VPAC1 or VPAC2/PAC1 receptors eliminated the PIV response, whereas PAC1 blockade had no effect, demonstrating an involvement of VPAC1/VPAC2 receptors in PIV development. Moreover, endothelium-independent and -dependent vasodilator responses were unchanged by the VPAC1/VPAC2 antagonist. Thus, the absence of a PIV response following VPAC1/VPAC2 blockade cannot be explained by any dysfunction of the vascular smooth muscle or endothelial vasodilator capacity. The involvement of VPAC1/VPAC2 receptors in the development of PIV seems to imply a series relationship in which each receptor type (CGRP, VPAC1, VPAC2) is necessary for the full transmission of the response.
We recently discovered a local neural control of skin vasodilatation, which is mediated by primary afferent fibres. It involves a significant transient increase in skin blood flow in response to an innocuous local pressure application and has been observed in humans (Fromy et al. 1998) and in rats (Fromy et al. 2000b). This transient increase in skin blood flow, defined as pressure-induced vasodilatation (PIV), delays the occurrence of ischaemia from direct pressure application, suggesting a protective feature against such applied pressure. This vasodilator response, activated by the stimulation of low threshold mechanical receptors in normal skin, disappears under local anaesthesia or following chronic capsaicin treatment, showing that PIV depends on capsaicin-sensitive nerve fibres in rats, as well as in humans (Fromy et al. 1998, 2000b). We also demonstrated that calcitonin gene-related peptide (CGRP) plays a major role, whereas neurokinins have no role in the development of PIV (Fromy et al. 2000b).
In these capsaicin-sensitive nerve fibres, CGRP coexists with pituitary adenylate cyclase-activating polypeptide (PACAP) (Moller et al. 1993; Mulder et al. 1994), another potent vasodilator neuropeptide reported in various organs, including the skin (Warren et al. 1992; Wilson & Warren, 1993; Wallengren, 1997; Dorner et al. 1998). These neuropeptides are known for their involvement in skin–nervous system interactions (Ansel et al. 1996). PACAP is a member of the secretin/glucagon/vasoactive intestinal peptide (VIP) superfamily of peptides, originally isolated from the ovine brain in 1989. Two biologically active forms of PACAP are derived from a single 176-amino acid precursor: PACAP-27 and PACAP-38, which is the predominant form in the tissues (Arimura et al. 1991; Arimura & Shioda, 1995). Particularly in the normal human skin, positive staining for PACAP-38, but not PACAP-27, was found in dermal sensory nerve fibres close to blood vessels and the epidermal border (Steinhoff et al. 1999).
Three distinct receptor subtypes mediate the biological effects of PACAP. They exhibit differential affinities for PACAP and VIP. The PAC1 receptor shows high selectivity for PACAP. In contrast, VPAC1 and VPAC2 receptors bind with the same affinity for PACAP-27, PACAP-38 and VIP (Zhou et al. 2002). A recent overview of the receptor nomenclature has been published (Harmar et al. 1998).
Expressed in the brain and peripheral tissues, the patterns of distribution of VPAC1, VPAC2 and PAC1 receptors are different, suggesting distinct functional roles (Vaudry et al. 2000). In rat cerebral blood vessels, evidence was provided for the abundance of VPAC1 in the media. Their presence in the endothelial layer is still controversial (Lundeberg & Nordlind, 1999; Fahrenkrug et al. 2000). In human skin, VPAC1 receptors are expressed in the epidermis and the dermis (Lundeberg & Nordlind, 1999). VPAC2 receptors are predominantly found in vessels and smooth muscles (Reubi, 2000). However, VPAC2 was not found in the vascular smooth muscle layer of cutaneous vessels (Fischer et al. 2001), whereas it was detected in de-endothelized aortic tissue and cultured vascular smooth muscle cells (Miyata et al. 1998). Also positive VPAC2 mRNA hybridization signals were localized to endothelial cells. In human skin, abundant expression of VPAC2 was reported in epidermal keratinocytes as well as cells of the germinative epithelium, matrix and medulla of the hair follicle (Lundeberg & Nordlind, 1999; Fischer et al. 2001). Particularly abundant in the brain (Zhou et al. 2002), the expression of PAC1 was also observed in human skin (Steinhoff et al. 1999).
We hypothesized that at least one of these receptors is involved in the development of the skin vasodilatation activated by the local application of a non-nociceptive pressure. To determine their involvement in PIV response, we used [d-p-Cl-Phe6,Leu17]-VIP (a non-specific antagonist of VPAC1/VPAC2 receptors), PG 97-269 (antagonist of VPAC1 receptors), PACAP(6–38) (antagonist of VPAC2/PAC1 receptors) and Max.d.4 (antagonist of PAC1 receptors) in anaesthetized rodents. To verify the endothelium-independent and -dependent capacities to vasodilate (Sloan & Soltani, 1986; Morris & Shore, 1996), we also tested the skin vasodilator response to iontophoretic application of sodium nitroprusside and acetylcholine, respectively, in complementary groups of rats.
Methods
Animal instrumentation
Experiments were performed in male Wistar rats weighing 250–320 g and in Swiss mice weighing 20–30 g. Prior to the experiments, animals had free access to standard laboratory food and water. Animals were housed in a regulated environment with a constant ambient temperature of 24°C. Procedures for the maintenance and use of the experimental animals were carried out in accordance with the principles of UK legislation.
To present a hairless area for the skin blood flow measurements, local pressure application and iontophoretic stimulation, hairs were removed from the skull to the back skin of the animals with a depilatory lotion. This was performed 2 days prior to the experiments to prevent skin irritation from confounding the results.
For the experiments, animals were anaesthetized by intraperitoneal injection of thiopental at a dose of 50 mg (kg body weight)−1 and 8 mg (kg body weight)−1 for rats and mice, respectively. The level of anaesthesia was determined by testing eye reflexes and tail pinch. Animals were placed in an incubator (MMS, Chelles, France) warmed to 30°C to maintain a stable body temperature, which was monitored with a rectal thermometer. A cutaneous thermocouple was placed on the skull near the pressure application site. The animals breathed spontaneously. The left femoral vein was cannulated for drug or vehicle administration. Mean arterial blood pressure (MABP) was measured by pressure transducer (Mallinckrodt, Medica, Dublin, Ireland) connected to the left femoral arterial catheter. The animals were placed in the prone position and the head was fixed on a frame.
PIV assessment
A weighbridge was adapted to hold a laser Doppler probe (PF408, Periflux, Perimed, Sweden). The probe has 12.6 mm2 and 7.0 mm2 circular contact surface areas for rat and mouse protocols, respectively. The measurement was performed in the centre of the hairless area of skull. The probe is connected to a laser Doppler flowmeter (PF4001 Master, Periflux, Perimed, Sweden), which provides a good indicator of the response pattern of skin blood flow from the region of illuminated skin (Saumet et al. 1986). The weighbridge was carefully equilibrated and the probe was then positioned perpendicular to the skin for the blood flow measurements at the local pressure application site. The technical details of this method have been described (Fromy et al. 2000a). Data collection began with a 1 min control period prior to the onset of increasing pressure. Externally applied pressure was then increased progressively at 11.1 Pa s−1 (5.0 mmHg min−1) for 30 min and 2.2 Pa s−1 (1 mmHg min−1) for 20 min in rat and mouse protocols, respectively.
Endothelium-independent and -dependent vasodilatation
Endothelium-independent vasodilatation was provoked by iontophoresis of the negatively charged nitric oxide (NO) donor, sodium nitroprusside, using cathodal current for 30 s. Endothelium-dependent vasodilatation in the cutaneous microcirculation was provoked by iontophoresis of the cation acetylcholine, using anodal current for 20 s. The iontophoretic application was performed over an area of 1.2 cm2 using a laser Doppler probe (481-1, Perimed, Sweden) positioned on the back skin of the rat. Indeed, this probe was specially designed to allow for simultaneous skin blood flow recording and current application (100 μA). Data collection began with a 1 min basal period prior to the onset of current application and was continuously recorded for 20 min.
Skin blood flows, MABP, rectal and skin temperatures were continuously recorded by a data acquisition system (Biopac, Santa Barbara, CA, USA) at the sample frequency of 500 Hz and analysed off-line by a soft (Acqknowledge Biopac, Santa Barbara, CA, USA). All animals were killed at the end of the experiment by an overdose of the anaesthetic agent. Skin blood flows and MABP were recorded for 1 min following the killing of the rat to assess the ‘physiological zero’ of the measurements, which was subsequently subtracted from corresponding measured values.
Protocol 1: VPAC1/VPAC2 receptor involvement
To investigate the involvement of VPAC1/VPAC2 receptors in the PIV development, we treated the rats with [d-p-Cl-Phe6,Leu17]-VIP, which was infused intravenously (3 μg kg−1 min−1), beginning 15 min prior to the onset of the local pressure application and continuing throughout the experiment (n = 10). The control rats (n = 10) received the same volume of vehicle as the treated rats. [d-p-Cl-Phe6,Leu17]-VIP is a competitive antagonist for both VPAC1 and VPAC2 receptors (Pandol et al. 1986; Rekik et al. 1998).
Protocol 2: VPAC1 receptor involvement
To examine the involvement of VPAC1 receptors in the PIV response, we treated the rats with PG 97-269, which was infused intravenously (6 μg kg−1 min−1) beginning 15 min prior to the onset of the local pressure application and continuing throughout the experiment (n = 8). Control rats (n = 10) received the same volume of vehicle as the treated rats. PG 97-269 is a chimeric VIP/growth hormone-releasing factor derivative that antagonizes rat VPAC1 receptors with high selectivity (200- to 1000-fold) relative to VPAC2 receptors (Gourlet et al. 1997).
Protocol 3: VPAC2/PAC1 receptor involvement
To examine the involvement of VPAC2/PAC1 receptors in the PIV development, we treated the rats with PACAP (6–38), which was injected intravenously as a bolus of 0.3 ml (60 μg kg−1), 4 min prior to the onset of the local pressure application (n = 10). Control rats (n = 10) received the same volume of vehicle as the treated rats. PACAP(6–38) shows a modest affinity for the VPAC2 receptor, but also has a high binding affinity for the PAC1 receptor (Dickinson et al. 1997; Moro et al. 1999). There are no satisfactory antagonists specific to VPAC2 receptors available at the present time (Laburthe et al. 2002). However, with the differing specificity among the antagonists used in the present study, we could infer the involvement of VPAC2 receptors in the development of PIV by subtraction using the results of protocols three and four.
Protocol 4: PAC1 receptor involvement
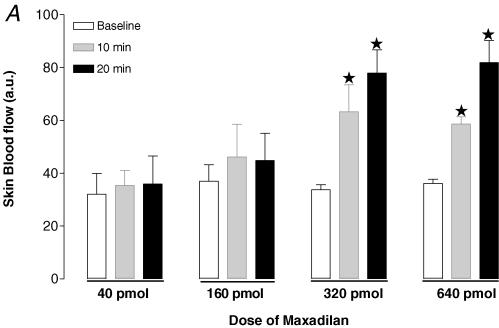
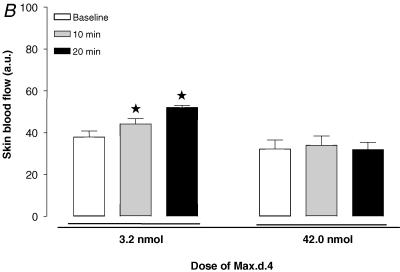
Recently, a novel PAC1 receptor selective agonist and an antagonist were reported. Maxadilan, a vasodilator 61-amino acid peptide isolated from the sand fly Lutzomyia longipalpis, was shown to activate PAC1 receptors with high affinity and did not have any affinity for VPAC1 or VPAC2 receptors (Moro & Lerner, 1997). Despite homologies between maxadilan and CGRP, it was found that maxadilan does not bind to CGRP receptors. Max.d.4, which was developed by the deletion of 19 amino acids of maxadilan, was demonstrated to be a specific antagonist for PAC1 receptors (Uchida et al. 1998; Moro et al. 1999). Because of the small amount of PAC1 antagonist available to us for the present study, we examined the involvement of PAC1 receptors in mice. Since there was no available data in vivo, we performed a preliminary study to determine the doses for both the agonist for PAC1 receptors (maxadilan) and the corresponding antagonist (Max.d.4) to be used in the present study. Firstly, we tested the effects of four different doses of maxadilan on skin blood flow: 40 pmol (n = 5), 160 pmol (n = 5), 320 pmol (n = 5) and 640 pmol (n = 5). A bolus of 0.1 ml maxadilan was injected intravenously and the skin blood flow was recorded for 20 min. A dose of 320 pmol was found to be sufficient to increase the skin blood flow (Fig. 1A). Using this dose of maxadilan, we tested the efficiency of two doses of the antagonist Max.d.4 (3.2 nmol, n = 5 and 42 nmol, n = 5) to block the skin blood flow increase in response to 320 pmol of maxadilan. The lower dose only partially inhibited the response, whereas a dose of 42 nmol of Max.d.4 proved effective in blocking the vasodilator response (Fig. 1B). Therefore, we used 42 nmol of Max.d.4 to examine the involvement of PAC1 receptors in the PIV development. The antagonist was injected intravenously as a bolus of 0.1 ml, 10 min prior to the onset of the local pressure application (n = 9). The control mice (n = 10) received the same volume of vehicle as the treated mice.
Figure 1.
A, effect of four different doses of the PAC1 receptor agonist maxadilan on skin blood flow. Skin blood flow (mean ± s.e.m.) was measured at baseline (open column), 10 min (grey column) and 20 min (filled column) after a bolus at 40 pmol (n = 5), 160 pmol (n = 5), 320 pmol (n = 5) and 640 pmol (n = 5) of maxadilan. B, effect of two doses of Max.d.4 (PAC1 receptor antagonist) on the skin blood flow increase in response to 320 pmol maxadilan. Skin blood flow (mean ± s.e.m.) was measured at baseline, 10 min and 20 min after a bolus of Max.d.4 (3.2 nmol, n = 5 and 42 nmol, n = 5).
Protocol 5: endothelium-independent and -dependent vasodilatation
To examine the endothelium-independent and -dependent vasodilator capacities following the VPAC1/ VPAC2 blockade using [d-p-Cl-Phe6,Leu17]-VIP (3 μg kg−1 min−1), we used the iontophoretic application of sodium nitroprusside (67 mm, n = 10) and acetylcholine (5.5 mm, n = 10), respectively. Control rats (n = 10 for nitroprusside application and n = 10 for acetylcholine) received the same volume of the vehicle for the antagonist as the treated rats.
Drugs
The substances used in this study were obtained from the following sources: [d-p-Cl-Phe6,Leu17]-VIP was purchased from Sigma-Aldrich (St Quentin Fallavier, France). PG 97-269 was kindly provided by Professor P. Robberecht (Universite libre de Bruxelles, Belgium). PACAP(6–38) was purchased from Neosystem company (Strasbourg, France). The antagonist Max.d.4 was kindly provided by Professor E. A. Lerner (Cutaneous Biology Research Center, Massachussets, USA). Sodium nitroprusside was obtained from Nitriate, SERB, France, and acetylcholine from Sigma.
Data analysis
The signals were averaged every 30 s to reduce the effects of instantaneous variability due to vasomotion. Heart rate was determined by performing fast Fourier transform analysis on the blood pressure signal. Baseline values were calculated as the mean over the 1 min baseline period prior to the onset of increasing local pressure (protocols 1–4) or current application (protocol 5). Skin blood flow was expressed in the absolute units (a.u.) of the flowmeter output. All data were expressed as mean ± s.e.m. Unpaired t tests were used to evaluate the significance between two groups for specific data. Paired t tests were used to evaluate differences due to local applied pressure application or to iontophoresis within each group. A two-tailed P value less than 0.05 was regarded as statistically significant. Statistical evaluations were performed using Prism v.2.0 (Graphpad Software, San Diego, CA, USA).
Results
For all protocols there was no variation in haemodynamic parameters (MABP, heart rate), rectal and skin temperatures throughout the experiments.
Protocol 1: VPAC1/VPAC2 receptor involvement
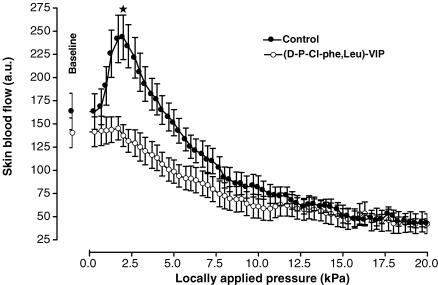
In control rats, baseline skin blood flow was 162 ± 19 a.u. With local pressure application, skin blood flow increased progressively and reached a maximal mean value of 243 ± 24 a.u. (+60 ± 15%, P < 0.001) at 2.0 kPa (Fig. 2). Over the same range of locally applied pressures (baseline and 2.0 kPa), skin blood flow did not change significantly (from 141 ± 17 a.u. to 137 ± 12 a.u.) in rats treated with [d-p-Cl-Phe6,Leu17]-VIP. At baseline, skin blood flow did not differ between controls and treated rats, whereas at a locally applied pressure of 2.0 kPa skin blood flow was significantly lower in treated rats compared to controls (P < 0.001).
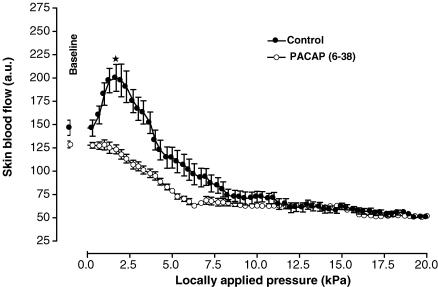
Figure 2.
Mean ± s.e.m. of skin blood flow obtained with 11.1 Pa s−1 local external pressure application in control rats (•, n = 10) and in rats treated with the non-specific VPAC1/VPAC2 receptor antagonist (d-p-Cl-Phe,Leu)-VIP (○, n = 10). The first point outside the scale of pressure corresponds to the mean baseline prior to the onset of local pressure application. At baseline, skin blood flow did not differ between controls and treated rats. At a locally applied pressure of 2.0 kPa, skin blood flow was significantly lower in treated rats compared to controls (*P < 0.001).
Protocol 2: VPAC1 receptor involvement
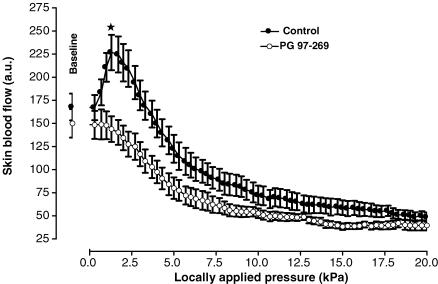
In control rats, baseline skin blood flow was 167 ± 14 a.u. With local pressure application, skin blood flow increased progressively and reached a maximal mean value of 227 ± 19 a.u. (+37 ± 6%, P < 0.001) at 1.3 kPa (Fig. 3). Over the same range of locally applied pressures (baseline and 1.3 kPa), skin blood flow did not change significantly (from 148 ± 15 a.u. to 144 ± 14 a.u.) in rats treated with PG 97-269. At baseline, skin blood flows were not different between controls and treated rats, whereas at 1.3 kPa skin blood flow was significantly lower in treated rats compared to controls (P < 0.001).
Figure 3.
Mean ± s.e.m. of skin blood flow obtained with 11.1 Pa s−1 local external pressure application in control rats (•, n = 10) and in rats treated with the specific VPAC1 receptor antagonist PG 97-269 (○, n = 8). At baseline, skin blood flows did not differ between controls and treated rats, whereas at 1.3 kPa skin blood flow was significantly lower in treated rats compared to controls (*P < 0.001).
Protocol 3: VPAC2/PAC1 receptor involvement
In control rats, baseline skin blood flow was 147 ± 9 a.u. With local pressure application, skin blood flow increased progressively and reached a maximal mean value of 200 ± 14 (+37 ± 8%, P < 0.01) at 1.7 kPa (Fig. 4). Over the same range of locally applied pressures (baseline and 1.7 kPa), skin blood flow did not change significantly (from 128 ± 4 a.u. to 119 ± 6 a.u.) in rats treated with PACAP(6–38). At baseline, blood flow values were not different between controls and treated rats. At 1.7 kPa, skin blood flow was significantly lower in treated rats compared to controls (P < 0.001).
Figure 4.
Mean ± s.e.m. of skin blood flow obtained with 11.1 Pa s−1 local external pressure application in control rats (•, n = 10) and in rats treated with PACAP(6–38), the VPAC2/PAC1 receptor antagonist (○, n = 10). At 1.7 kPa, skin blood flow was significantly lower in treated rats compared to controls (*P < 0.001), whereas no difference was observed at baseline.
Protocol 4: PAC1 receptor involvement
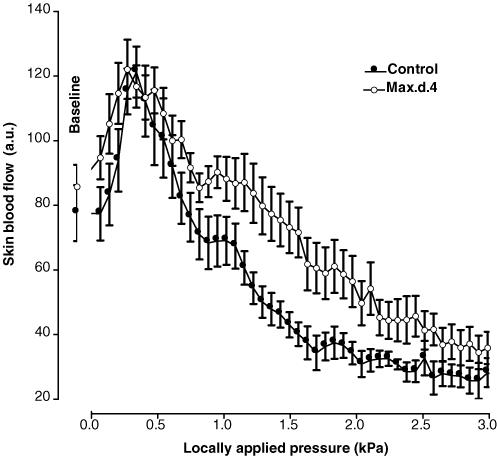
In control mice, baseline skin blood flow was 87 ± 8 a.u. With local pressure application, skin blood flow increased progressively and reached a maximal mean value of 122 ± 9 a.u. (+41 ± 8%, P < 0.01) at 0.3 kPa (Fig. 5). Over the same range of locally applied pressures (baseline and 0.3 kPa), skin blood flow increased from 71 ± 9 a.u. to 111 ± 12 a.u. (+63 ± 14%, P < 0.01) in mice treated with Max.d.4, the PAC1 receptor antagonist. At baseline and at 0.3 kPa, skin blood flows were not different between control and treated mice.
Figure 5.
Mean ± s.e.m. of skin blood flow obtained with 2.2 Pa s−1 local external pressure application in control mice (•, n = 10) and in mice treated with the specific PAC1 receptor antagonist Max.d.4, (○, n = 9). No difference was observed between control and treated mice.
Protocol 5: endothelium-independent and -dependent vasodilatation
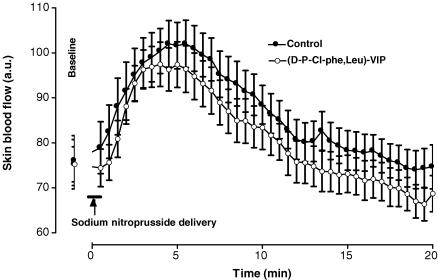
In control rats, baseline skin blood flow was 74 ± 5 a.u. Following sodium nitroprusside application, skin blood flow increased progressively and reached a maximal mean value of 102 ± 5 a.u. (+39 ± 5%, P < 0.01) 4.5 min after the start of the iontophoretic stimulation (Fig. 6). Over the same range of time, skin blood flow increased from 75 ± 4 a.u. to 96 ± 5 a.u. (+28 ± 4%, P < 0.01) in rats treated with the VPAC1/VPAC2 antagonist. At baseline and 4.5 min after the start of the iontophoretic stimulation, skin blood flow values were not different between control and treated rats.
Figure 6.
Mean ± s.e.m. of skin blood flow obtained with sodium nitroprusside iontophoretic delivery (cathode, 30 s; 10 μA) in control rats (○, n = 10) and in rats treated with the non-specific VPAC1/VPAC2 receptor antagonist (d-p-Cl-Phe,Leu)-VIP, (•, n = 10). The sodium nitroprusside-dependent vasodilatation was unchanged by the antagonist.
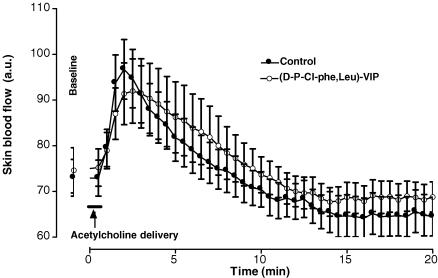
After iontophoretic delivery of acetylcholine, skin blood flow increased progressively from 73 ± 4 a.u. at baseline and reached a maximal mean value of 97 ± 7 a.u. (+22 ± 4 %, P < 0.01) 2.0 min after the start of the iontophoretic stimulation in controls (Fig. 7). Over the same range of time, skin blood flow increased from 74 ± 4 a.u. to 91 ± 7 a.u. (+25 ± 9 %, P < 0.01) in rats treated with the VPAC1/VPAC2 antagonist. At baseline and 2.0 min after the start of the iontophoretic stimulation, skin blood flows were not different between control and treated rats.
Figure 7.
Mean ± s.e.m. of skin blood flow obtained with acetylcholine iontophoretic delivery (anode, 20 s; 100 μA) in control rats (•, n = 10) and in rats treated with the non-specific VPAC1/VPAC2 receptor antagonist [d-p-Cl-Phe,Leu]-VIP (○, n = 10). The antagonist did not affect the acetylcholine-dependent vasodilatation as compared to controls.
Discussion
This study shows that both VPAC1 and VPAC2 receptors are involved in the development of PIV and that the absence of PIV following VPAC1/VPAC2 blockade cannot be explained by any dysfunction of the vasodilator capacity via the NO–cGMP pathway. This is supported by the findings that [d-p-Cl-Phe6,Leu17]-VIP, PG 97-269 and PACAP(6–38) antagonists led to the absence of the PIV development, whereas the specific antagonist of PAC1 receptors did not modify the PIV response. Moreover, the endothelium-independent and -dependent vasodilator responses were unchanged by the VPAC1/VPAC2 antagonist.
PIV is related to the integrity of vascular and endothelial vasodilator capacities (Fromy et al. 2000b; Fizanne et al. 2003). On the one hand, sodium nitroprusside causes vasodilatation by directly relaxing vascular smooth muscle cells. In the present study, the responses to iontophoretically delivered sodium nitroprusside were not different between treated rats and controls, demonstrating an intact capacity of the vascular smooth muscle to vasodilate following the blockade of VPAC1/VPAC2 receptors. On the other hand, acetylcholine interacts with the endothelium and mediates its endothelium-dependent vasodilatation via a muscarinic receptor on the endothelial surface. This leads to a rise in intracellular calcium concentration and increases the synthesis and release of endothelial factors, mainly NO. In the present study, acetylcholine-induced vasodilatation was unaffected by the non-specific VPAC1/VPAC2 antagonist, showing the integrity of the NO–cGMP pathway following antagonist administration. Thus, the absence of the PIV response following the blockade of VPAC1/VPAC2 receptors was not related to an impairment of vasodilator capacities via the NO–cGMP pathway. However, the absence of PIV following the VPAC1/VPAC2 blockade could be explained by an inability to activate the NO–cGMP pathway activation, since we demonstrated in a previous investigation that this pathway plays a crucial role in the development of PIV (Fromy et al. 2000b). Further studies are needed to investigate this hypothesis.
The prostaglandins also play a major role in the development of PIV (Fromy et al. 2000b). However, although PACAP stimulated PGF2 release, it did not affect other cyclo-oxygenase metabolites (Anzai et al. 1995). Therefore the absence of PIV following the blockade of VPAC1/VPAC2 receptors is unlikely to be due to an inhibitory action on prostaglandin synthesis.
Although the PIV response is activated by innocuous local pressure, it is not found in skin that has been locally anaesthetized or chronically treated with capsaicin, showing that PIV depends on C fibres in rats, as in humans (Fromy et al. 1998, 2000b). Dickinson et al. (1999) showed that sustained sensory-evoked activity of multireceptive dorsal horn neurones can clearly be promoted or mediated, at least in part, by the three PACAP receptors. Indeed, the PACAP receptor antagonists appear to exert a generalized modulation of dorsal horn neurone responses in normal anaesthetized rats, inhibiting both innocuous brush-evoked activity as well as sustained C fibre-evoked activity to similar extents (Dickinson et al. 1999). Thus, an alternative explanation for the absence of PIV development following the VPAC1/VPAC2 blockade could be due to an inhibition of C fibre activity and that VPAC1 and VPAC2 receptors play an important regulatory role in the transmission of sensory information of non-nociceptive mechanical stimulation in physiological conditions. However further studies are needed to clarify this point. This is in total agreement with ‘the evidence that PACAP receptors are functionally active in normal animals, suggesting that their roles might not simply be restricted to episodes of chronic pain’ (Dickinson & Fleetwood-Walker, 1999). In contrast, mice treated with a specific PAC1 receptor antagonist and PAC1-/- mice (L. Fizanne, unpublished observations) have a PIV response, showing that the specific PACAP receptor has little or no role in the sensory information activated by a non-nociceptive mechanical stimulation.
It is generally reported that both forms of PACAP relax vascular smooth muscle in vitro and in vivo (Warren et al. 1992; Wilson & Warren, 1993) and play a major role in plasma extravasation in rat skin (Cardell et al. 1997). Similarly, VIP is a potent vasodilator in cerebral arteries (Seebeck et al. 2002). The present results indicate the involvement of VPAC1 and VPAC2 receptors in the cutaneous vasodilatation in response to the local application of pressure. Since the two peptides (PACAP and VIP) share common receptors, the respective role of either PACAP or VIP in this mechanism remains to be determined.
In the nervous system, VIP or PACAP induces neuronal calcium flux, facilitates membrane depolarization, and increases spike frequency (Tatsuno et al. 1992; Tanaka et al. 1996; Lai et al. 1997; May et al. 1998), suggesting a neuromodulatory function of the neuropeptide (Moller et al. 1993). Since the release of neurotransmitters critically depends on the rise in intracellular calcium inducing exocytosis, the blockade of VPAC1/VPAC2 receptors could prevent the exocytosis process limiting the neurotransmitter release. Lioudyno et al. (1998) showed that PACAP induced an increased immunoreactivity for CGRP in dorsal root ganglion explant cultures, showing that PACAP was able to modulate the CGRP expression within the ganglia. Since CGRP plays a major role in the development of PIV (Fromy et al. 2000b), it could explain the absence of PIV following the blockade of VPAC1/VPAC2 receptors in the present study. Yet, no direct relationship has been established between VPAC1/VPAC2 receptors and the consequent CGRP secretion. However, the synergism between CGRP and PACAP was reported in the isolated porcine ophthalmic artery (Elsas & White, 1997). Therefore, either an inhibitory effect on CGRP secretion or a synergism between CGRP and VIP or PACAP could also explain the absence of PIV development following the VPAC1/VPAC2 blockade. Further studies will be needed to establish the exact link between VPAC1/VPAC2 receptors and CGRP.
In conclusion, the blockade of VPAC1/VPAC2 receptors prevents the development of pressure-induced vasodilatation, demonstrating the involvement of VPAC1 and VPAC2 receptors in this mechanism. However, the absence of a PIV response following VPAC1/VPAC2 blockade cannot be explained by any dysfunction of the vascular smooth muscle or endothelial vasodilator capacity. Thus the involvement of VPAC1/VPAC2 receptors in PIV development seems to imply a series relationship in which each receptor type is necessary for the full transmission of the response. This could be at the endothelial level (NO pathway activation) or at the neuronal level (either in the transmission of sensory information or in the efferent function via a direct or indirect action on CGRP).
Acknowledgments
The present study was supported by the French National Scientific Institute INSERM and the Region Pays de la Loire (ESPRI 2002, Georges Leftheriotis). We thank Dr Ethan A. Lerner (Cutaneous Biology Research Center, Massachussets General Hospital, Charlestown, MA, USA) for the generous supply of maxadilan and Max.d.4, Dr Patrick Robberecht (Department of Biochemistry and Nutrition, Medical School, Université de Bruxelles, Belgium) for providing PG 97-269, Dr Joël Bockaert for the gift of PAC1-/- mice and Céline Baron for her technical assistance. We thank the local Animal Care Unit of the University of Angers for facilities.
References
- Ansel JC, Kaynard AH, Armstrong CA, Olerud J, Bunnett N, Payan D. Skin–nervous system interactions. J Invest Dermatol. 1996;106:198–204. doi: 10.1111/1523-1747.ep12330326. [DOI] [PubMed] [Google Scholar]
- Anzai M, Suzuki Y, Takayasu M, Kajita Y, Mori Y, Seki Y, Saito K, Shibuya M. Vasorelaxant effect of PACAP-27 on canine cerebral arteries and rat intracerebral arterioles. Eur J Pharmacol. 1995;285:173–179. doi: 10.1016/0014-2999(95)00404-9. [DOI] [PubMed] [Google Scholar]
- Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Cardell LO, Stjarne P, Wagstaff SJ, Agusti C, Nadel JA. PACAP-induced plasma extravasation in rat skin. Regul Pept. 1997;71:67–71. doi: 10.1016/s0167-0115(97)00027-x. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM. VIP and PACAP: very important in pain? Trends Pharmacol Sci. 1999;20:324–329. doi: 10.1016/s0165-6147(99)01340-1. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM, Mitchell R, Lutz EM. Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides. 1997;31:175–185. doi: 10.1016/s0143-4179(97)90087-1. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Mitchell R, Robberecht P, Fleetwood-Walker SM. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology. 1999;38:167–180. doi: 10.1016/s0028-3908(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Dorner GT, Wolzt M, Eichler HG, Schmetterer L. Effect of pituitary adenylate cyclase activating polypeptide 1–27 on ocular, cerebral and skin blood flow in humans. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:657–662. doi: 10.1007/pl00005308. [DOI] [PubMed] [Google Scholar]
- Elsas T, White LR. Evidence for a possible synergism between pituitary adenylate cyclase activating polypeptide and calcitonin gene-related peptide in porcine ophthalmic artery. Acta Ophthalmol Scand. 1997;75:159–161. doi: 10.1111/j.1600-0420.1997.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J, Tams J, Georg B. Immunohistochemical localization of the VIP1 receptor (VPAC1R) in rat cerebral blood vessels: relation to PACAP and VIP containing nerves. J Cereb Blood Flow Metab. 2000;20:1205–1214. doi: 10.1097/00004647-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Fischer TC, Hartmann P, Loser C, Springer J, Peiser C, Dinh QT, Fischer A, Groneberg DA. Abundant expression of vasoactive intestinal polypeptide receptor VPAC2 mRNA in human skin. J Invest Dermatol. 2001;117:754–756. doi: 10.1046/j.0022-202x.2001.01449.x. [DOI] [PubMed] [Google Scholar]
- Fizanne L, Fromy B, Preckel MP, Sigaudo-Roussel D, Saumet JL. The effect of isoflurane on the skin pressure-induced vasodilation. J Vasc Res. 2003;40:416–422. doi: 10.1159/000072890. [DOI] [PubMed] [Google Scholar]
- Fromy B, Abraham P, Saumet JL. Non-nociceptive capsaicin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Res. 1998;811:166–168. doi: 10.1016/s0006-8993(98)00973-1. [DOI] [PubMed] [Google Scholar]
- Fromy B, Abraham P, Saumet JL. Progressive calibrated pressure device to measure cutaneous blood flow changes to external pressure strain. Brain Res Protoc. 2000a;5:198–203. doi: 10.1016/s1385-299x(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Fromy B, Merzeau S, Abraham P, Saumet JL. Mechanisms of the cutaneous vasodilator response to local external pressure application in rats: Involvement of CGRP, neurokinins, prostaglandins and. Br J Pharmacol. 2000b;131:1161–1171. doi: 10.1038/sj.bjp.0703685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Vertongen P, Rathe J, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. In vitro Properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Receptors Channels. 2002;8:137–153. [PubMed] [Google Scholar]
- Lai CC, Wu SY, Lin HH, Dun NJ. Excitatory action of pituitary adenylate cyclase activating polypeptide on rat sympathetic preganglionic neurons in vivo and in vitro. Brain Res. 1997;748:189–194. doi: 10.1016/s0006-8993(96)01297-8. [DOI] [PubMed] [Google Scholar]
- Lioudyno M, Skoglosa Y, Takei N, Lindholm D. Pituitary adenylate cyclase-activating polypeptide (PACAP) protects dorsal root ganglion neurons from death and induces calcitonin gene-related peptide (CGRP) immunoreactivity in vitro. J Neurosci Res. 1998;51:243–256. doi: 10.1002/(SICI)1097-4547(19980115)51:2<243::AID-JNR13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lundeberg L, Nordlind K. Vasoactive intestinal polypeptide in allergic contact dermatitis: an immunohistochemical and radioimmunoassay study. Arch Dermatol Res. 1999;291:201–206. doi: 10.1007/s004030050394. [DOI] [PubMed] [Google Scholar]
- May V, Beaudet MM, Parsons RL, Hardwick JC, Gauthier EA, Durda JP, Braas KM. Mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP)-induced depolarization of sympathetic superior cervical ganglion (SCG) neurons. Ann N Y Acad Sci. 1998;865:164–175. doi: 10.1111/j.1749-6632.1998.tb11175.x. [DOI] [PubMed] [Google Scholar]
- Miyata A, Sato K, Hino J, Tamakawa H, Matsuo H, Kangawa K. Rat aortic smooth-muscle cell proliferation is bidirectionally regulated in a cell cycle-dependent manner via PACAP/VIP type 2 receptor. Ann N Y Acad Sci. 1998;865:73–81. doi: 10.1111/j.1749-6632.1998.tb11165.x. [DOI] [PubMed] [Google Scholar]
- Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, Sundler F. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–732. doi: 10.1016/0306-4522(93)90018-b. [DOI] [PubMed] [Google Scholar]
- Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- Moro O, Wakita K, Ohnuma M, Denda S, Lerner EA, Tajima M. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem. 1999;274:23103–23110. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996;496:531–542. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder H, Uddman R, Moller K, Zhang YZ, Ekblad E, Alumets J, Sundler F. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63:307–312. doi: 10.1016/0306-4522(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pandol SJ, Dharmsathaphorn K, Schoeffield MS, Vale W, Rivier J. Vasoactive intestinal peptide receptor antagonist [4Cl-D-Phe6, Leu17] VIP. Am J Physiol. 1986;250:G553–G557. doi: 10.1152/ajpgi.1986.250.4.G553. [DOI] [PubMed] [Google Scholar]
- Rekik M, Delvaux M, Frexinos J, Bueno L. Role of vasoactive intestinal polypeptide in the adaptation of intestinal smooth muscle cells to mechanical distension. J Pharmacol Exp Ther. 1998;287:832–838. [PubMed] [Google Scholar]
- Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann N Y Acad Sci. 2000;921:1–25. doi: 10.1111/j.1749-6632.2000.tb06946.x. [DOI] [PubMed] [Google Scholar]
- Saumet JL, Dittmar A, Leftheriotis G. Non-invasive measurement of skin blood flow: comparison between plethysmography, laser-Doppler flowmeter and heat thermal clearance method. Int J Microcirc Clin Exp. 1986;5:73–83. [PubMed] [Google Scholar]
- Seebeck J, Lowe M, Kruse ML, Schmidt WE, Mehdorn HM, Ziegler A, Hempelmann RG. The vasorelaxant effect of pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in isolated rat basilar arteries is partially mediated by activation of nitrergic neurons. Regul Pept. 2002;107:115–123. doi: 10.1016/s0167-0115(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Sloan JB, Soltani K. Iontophoresis in dermatology. A review. J Am Acad Dermatol. 1986;15:671–684. doi: 10.1016/s0190-9622(86)70223-5. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, McGregor GP, Radleff-Schlimme A, Steinhoff A, Jarry H, Schmidt WE. Identification of pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP type 1 receptor in human skin: expression of PACAP-38 is increased in patients with psoriasis. Regul Pept. 1999;80:49–55. doi: 10.1016/s0167-0115(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Shibuya I, Nagamoto T, Yamashita H, Kanno T. Pituitary adenylate cyclase-activating polypeptide causes rapid Ca2+ release from intracellular stores and long lasting Ca2+ influx mediated by Na+ influx-dependent membrane depolarization in bovine adrenal chromaffin cells. Endocrinology. 1996;137:956–966. doi: 10.1210/endo.137.3.8603609. [DOI] [PubMed] [Google Scholar]
- Tatsuno I, Yada T, Vigh S, Hidaka H, Arimura A. Pituitary adenylate cyclase activating polypeptide and vasoactive intestinal peptide increase cytosolic free calcium concentration in cultured rat hippocampal neurons. Endocrinology. 1992;131:73–81. doi: 10.1210/endo.131.1.1319331. [DOI] [PubMed] [Google Scholar]
- Uchida D, Tatsuno I, Tanaka T, Hirai A, Saito Y, Moro O, Tajima M. Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP type 1 receptor. Ann N Y Acad Sci. 1998;865:253–258. doi: 10.1111/j.1749-6632.1998.tb11185.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Wallengren J. Vasoactive peptides in the skin. J Invest Dermatol Symp Proceedings. 1997;2:49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- Warren JB, Larkin SW, Coughlan M, Kajekar R, Williams TJ. Pituitary adenylate cyclase activating polypeptide is a potent vasodilator and oedema potentiator in rabbit skin in vivo. Br J Pharmacol. 1992;106:331–334. doi: 10.1111/j.1476-5381.1992.tb14336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Warren JB. Adenylate cyclase-mediated vascular responses of rabbit aorta, mesenteric artery and skin microcirculation. Br J Pharmacol. 1993;110:633–638. doi: 10.1111/j.1476-5381.1993.tb13858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Shioda S, Yada T, Inagaki N, Pleasure SJ, Kikuyama S. PACAP and its receptors exert pleiotropic effects in the nervous system by activating multiple signaling pathways. Curr Protein Pept Sci. 2002;3:423–439. doi: 10.2174/1389203023380576. [DOI] [PubMed] [Google Scholar]