Abstract
ATP released from damaged or inflamed tissues can act at P2X receptors expressed on primary afferent neurones. The resulting depolarization can initiate action potentials that are interpreted centrally as pain. P2X3 subunits are found in a subset of small-diameter, primary afferent neurones, some of which are also sensitive to capsaicin. They can form homo-oligomeric channels, or they can assemble with P2X2 subunits into hetero-oligomers. Studies with antagonists selective for P2X3-containing receptors, experiments with antisense oligonucleotides to reduce P2X3 subunit levels, and behavioural testing of P2X3 knock-out mice, all suggest a role for the P2X2/3 receptor in the signalling of chronic inflammatory pain and some features of neuropathic pain. The availability of such tools and experimental approaches promises to accelerate our understanding of the other physiological roles for P2X receptors on primary afferent neurones.
Introduction
It is about 25 years since Bleehen & Keele (1977) reported that ATP induced a sensation of pain when it was applied to a blister base in man. This much-cited study has given rise to considerable interest in the notion that ATP, released from damaged tissues, is an important player in the initiation of noxious signals, and that its actions are mediated by receptors expressed by primary afferent nerve fibres. Here I review the evidence that ATP and its analogues can elicit the sensation of pain, discuss the processes that might be involved in ATP release at the sites of pain generation, describe the effects of ATP on primary afferent neurones, and in particular assess the involvement of receptors for ATP that contain the P2X3 subunit. A more general review on P2X receptors and nociception is available (Chizh & Illes, 2000). Dunn et al. (2001) reviewed P2X receptors in peripheral neurones, including primary afferent cells.
ATP elicits pain and nocifensive behaviour
Other human studies have followed those by Bleehen & Keele (1977). Coutts et al. (1981) showed that local application of ATP elicited vascular changes in human skin and also caused a persistent sensation of pain. Hamilton et al. (2000) applied the ATP by ionophoresis into the skin of human fore-arms. They found that this elicited a mild sensation of pain, but that the pain was enhanced by other inflammatory mediators such as capsaicin, or by local ultraviolet irradiation. Infusion of ATP into trapezius muscle (9–36 μmol in 1 ml infused over 10 min) also elicits pain (Mork et al. 2003), as does the intracutaneous injection of 20 μl of 1 or 5 mm ATP (Hilliges et al. 2002).
In animals, nocifensive behaviour such as hindpaw licking and lifting follows subplantar or intraplantar administration of ATP (rat: Bland-Ward & Humphrey, 1997; Hamilton et al. 1999; Jarvis et al. 2001. In parallel with the studies in man, the effectiveness of ATP and related agonists to elicit nocifensive behaviour is increased when they are coapplied with prostaglandin E2, formalin or carrageenan (Sawynok & Reed, 1997; Hamilton et al. 1999).
ATP excites primary afferent neurones
Peripheral afferent fibres
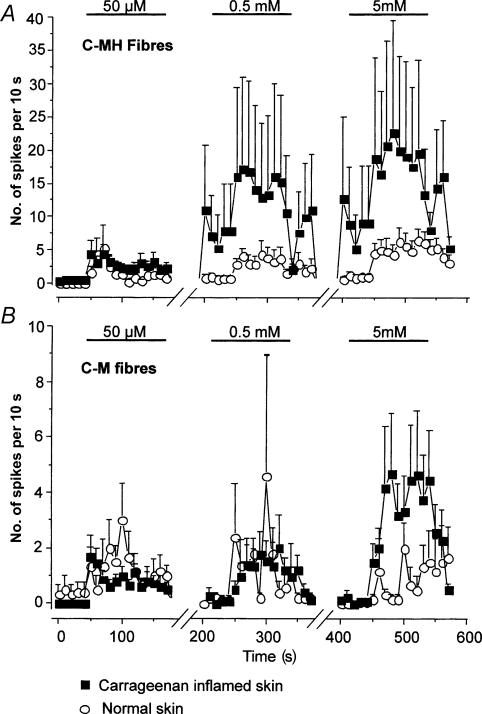
Both in vivo and in vitro experimental approaches have been used. Intracutaneous injections of ATP elicited discharges of C fibres monitored in the human peroneal nerve in vivo (Hilliges et al. 2002). Most of these fibres were heat-responsive, but responses to heat were not sensitized by ATP. Dowd et al. (1998) made recordings in vivo from anaesthetized rats and found that a subset of Aδ and C fibres in the medial articular nerve were excited by intra-articular ATP and αβmeATP; this effect was not obviously different in rats with chronically inflamed joints. Essentially similar results were found by Kirkup et al. (1999) in recordings from mesenteric primary afferents: it is not known whether such fibres are normally involved in sensation of noxious stimuli. In vitro recordings from skin nerves of the rat also show clear excitatory effects of ATP and αβmeATP (Hamilton et al. 2001) (Fig. 1). The fibres excited were predominately those classified as C-mechanoheat fibres; C-mechanonociceptors were much less responsive. Effective ATP concentrations were in the range 50 μm to 5 mm, and both the number of responsive fibres and the response magnitude were increased in preparations where the skin had been previously inflamed with carrageenan. The time course of these single unit excitations correlated well with that reported for pain in humans and nocifensive responses in rats. Taken together, these studies provide strong evidence that ATP is a physiologically relevant algogen.
Figure 1. Dose-dependent activation of C-MH and C-M fibres by αβmeATP in normal and inflamed skin.
In A the time course of C-MH fibre activation by αβmeATP (n = 16–21) is shown: spontaneous activity was not subtracted in this case. For clarity the activity between agonist doses is not shown (note the breaks in the abscissa). Note that in inflamed skin (filled squares) low doses of αβmeATP induce a long-lasting increase in the on-going activity in C-MH fibres that was not observed in normal skin (open circles). Thus the mean activity is already high in these fibres shortly before application of the second and third doses of α β meATP. In B the equivalent data from C-M fibres are plotted (n = 5–15). Note that these fibres are much less affected by carrageenan inflammation and no significant effect was observed of low doses of agonist on on-going activity. Reproduced, with permission, from Hamilton et al. (2001).
Cell bodies of primary afferents
There is an extensive literature on the actions of ATP on cell bodies of primary afferent fibres, whether in nodose, trigeminal or dorsal root ganglia (this is reviewed in detail by Dunn et al. 2001 and North, 2002). These experiments have been carried out by patch-clamp recording from dissociated cells – usually within a few hours but in some cases after several days in culture. Recording with conventional (sharp) electrodes from cells in the intact ganglion shows no effect of ATP and αβmeATP on the majority of neurones, reflecting either an absence of receptors on the cell surface or barriers to the diffusion of the nucleotides (Stebbing et al. 1998). The key question is whether the cells from which the recordings have been made are likely to be the cells involved in sensing pain, and the main difficulty in answering this question is that the properties and identities of neurones involved in pain sensing are likely to change markedly as pain persists (Snider & McMahon, 1998; Scholz & Woolf, 2002). The criteria that have usually been applied are one or more of (a) small (<30 μm) or medium (30–50 μm) diameter, (b) responsiveness to capsaicin, and (c) expression of the receptor for isolectin B4; these neurones require glia-derived neurotrophic factor (GDNF) for survival, and also express P2X3 subunits (see below) (Bradbury et al. 1998; Snider & McMahon, 1998). In some studies, the ganglion cells have been identified as those labelled by previous dye injection in their projection field: in the case of the tooth pulp and trigeminal ganglion this is considered to identify nociceptive neurones because pain is the only sensation reported when such fibres are stimulated in man (Cook et al. 1997).
Two kinds of response to ATP and αβmeATP are observed. The first is a rapidly rising inward current that desensitizes during a maintained application with a time constant of less than 100 ms. The second is an inward current that rises somewhat more slowly, and declines little if at all during agonist applications of 1–2 s. Some cells show a mixed response with both components. Considerable evidence supports the view that the first (desensitizing) response results from current through P2X receptors formed as homo-oligomers of P2X3 subunits. Thus, the response mirrors well in its kinetics and its sensitivity to antagonists the currents observed in HEK293 cells expressing P2X3 subunits (Lewis et al. 1995; North, 2002), and the response is not observed in neurones from mice with the P2X3 gene knocked out (Souslova et al. 2000; Cockayne et al. 2000; Zhong et al. 2001). The second (non-desensitizing) response closely resembles the currents observed when P2X2 and P2X3 subunits are expressed together in such heterologous systems (Lewis et al. 1995; Virginio et al. 1998); it is therefore assumed to result from currents through the hetero-oligomeric P2X2/3 receptor. The precise subunit composition of this native hetero-oligomeric channel is not known. However, coexpression of P2X subunits carrying individual cysteine substitutions has been used to engineer disulphide formation between the subunits of the ATP-activated channels; such studies indicate that the P2X2/3 hetero-oligomeric receptor probably contains one P2X2 and two P2X3 subunits (Jiang et al. 2003).
It was mentioned earlier that skin nerves are more sensitive to ATP in conditions of acute inflammation (Hamilton et al. 2001). A possible basis for this has been proposed at the level of the cell body, by studying the effect of two other inflammatory mediators on the currents elicited by ATP. Neurokinin B potentiates the ATP-evoked currents in dorsal root ganglion cells, acting through NK3 receptors (Wang et al. 2001): block of this effect by intracellular H-7 implicated protein phosphorylation. Paukert et al. (2001) showed that substance P and bradykinin potentiate the ATP-evoked currents in oocytes expressing P2X3 or P2X2/3 channels, along with the appropriate neuropeptide receptor. The effect was mimicked by phorbol esters and blocked by protein kinase inhibitors. Point mutations suggested that direct phosphorylation of the receptor at the conserved protein kinase C consensus site in the N terminus domain was the most likely explanation for these results. Longer term inflammation of the receptive field, by injections of complete Freund's adjuvant, resulted in a two- to three-fold increase in the currents elicited by ATP in dorsal root ganglion cells removed from treated rats (Xu & Huang, 2002).
Central terminals of primary afferent fibres
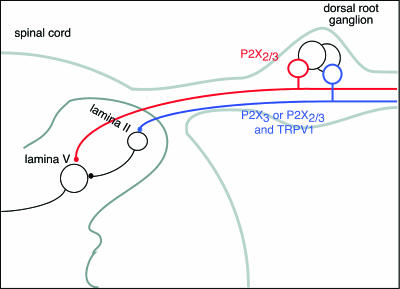
The presence of P2X receptors on the central terminations of primary afferent fibres has been inferred by measuring the increase in spontaneous excitatory postsynaptic currents (EPSCs) elicited by αβmeATP in dorsal horn neurones (Li et al. 1998; Nakatsuka & Gu, 2001; Nakatsuka et al. 2002). When such currents persist in the presence of tetrodotoxin, they are assumed to reflect direct activation of presynaptic terminals expressing P2X3 subunit-containing channels. Such studies on rat spinal cord slices indicate that neurones in superficial lamina receive inputs from glutamate-releasing terminals endowed with both P2X3 subunit-containing and TRPV1 receptors, because both capsaicin and αβmeATP are able to increase EPSC frequency. EPSCs in lamina V cells are increased in frequency by αβmeATP and by capsaicin, but the effect of capsaicin is secondary to excitation of more superficial cells because it disappeared in tetrodotoxin, or in slices in which the superficial layers of the cord had been removed (Nakatsuka et al. 2002). The effect of αβmeATP in lamina V is blocked by PPADS (10 μm) but not (2′, 3′-trinitrophenyl)-ATP (1μm) suggesting a novel hetero-ogliomeric receptor (Nakatsuka et al. 2003; Fig. 2).
Figure 2. Schematic representation of primary afferent fibres that express P2X3 subunit-containing channels.
P2X3 subunits confer sensitivity to αβmeATP. Small- and medium-sized dorsal root ganglion cells sensitive to αβmeATP are either sensitive (blue) or insensitive (red) to capsaicin (TRPV1 receptors). Capsaicin-sensitive cells show predominately fast-desensitizing (∼100 ms) currents when αβmeATP is applied, consistent with homo-oligomeric P2X3 receptors. These fibres terminate mostly in inner lamina II. Capsaicin-insensitive cells show slow-desensitizing (≫ 1 s) currents when αβmeATP is applied, consistent with other hetero-oligomeric channels. Some of these fibres terminate in lamina V. There is also evidence for other P2X receptors (not sensitive to αβmeATP) on terminals of GABA- and glycine-releasing interneurones (not shown) and on some dorsal horn cell bodies (see North, 2002).
ATP is released by damaged tissue?
The above studies have dealt with exogenous ATP and its analogues, and obviously a key question is whether stimuli to tissue that produce pain also release endogenous ATP. There are several circumstances in which ATP is released. First, it can be released from primary afferent nerves themselves (Holton, 1959). There is also much evidence for ATP release from sympathetic nerves, where it appears to be copackaged with noradrenaline (Sneddon & Burnstock, 1984; Starke et al. 1991; Evans & Surprenant, 1992). In some of these cases ATP is the main neuro-effector transmitter, such as the innervation of the vas deferens and some arteriolar smooth muscle. However, the more pertinent relation to pain is likely those conditions in man in which neuropathic pain is sympathetically maintained, and may have as its anatomical substrate the pathological connections that form between sympathetic fibres and primary sensory neurones (Ramer et al. 1999; McLachlan et al. 1993; Marchettini et al. 2000).
Second, endothelial and epithelial cells can release ATP. In the former case, the release is elicited by the flow of solution over the surface of the cells (Bodin & Burnstock, 1995, 2001; Schweibert et al. 2002; Yamamoto et al. 2003); the synergistic effect of hypoxia on the flow-induced release prompts the speculation that this underlies hypoxia-induced pain (Bodin & Burnstock, 1995). Release of ATP from epithelia of the bladder in response to distension has been shown by Ferguson et al. (1997). It is likely that this ATP acts on P2X3 subunit-containing receptors on primary afferent nerves lying beneath the urothelium, because in P2X3 knock-out mice the bladder must be distended to a larger volume before the voiding reflex is initiated (Cockayne et al. 2000). Whether or not such distensions are noxious remains to be shown.
Third, ATP may simply be released by lysed cells. The concentrations within the cytoplasm (>1 mm) are certainly sufficient to excite P2X receptors, there is evidence that tumour cells have even higher concentrations than normal cells (Maehara et al. 1987), and rheumatoid joints are reported to have higher concentrations than normal joints (Ryan et al. 1991). A direct excitation of P2X receptors on sensory neurones by the ATP released from a mechanically lysed cell has recently been shown in a coculture system (Cook & McCleskey, 2002). These observations illustrate the potential for ATP release from damaged tissues, but the best evidence that it carries a noxious signal to the primary afferent nerve would come from studies with antagonists (see below).
P2X3 receptor subunits
Distribution
cDNAs encoding P2X3 subunits were originally isolated from dorsal root ganglia (Lewis et al. 1995; Chen et al. 1995); these and subsequent studies have shown that the mRNA distribution is essentially restricted to primary afferent neurones (Collo et al. 1996). Chen et al. (1995) reported that the P2X3 RNA was limited in its expression to a subset of primary afferent neurones likely to be nociceptors, on the basis of size and expression of peripherin. Subsequent studies at the protein level have confirmed this view. Nociceptive neurones can be divided according to their sensitivity to nerve growth factor (NGF) or GDNF (Snider & McMahon, 1998); P2X3 immunoreactivity corresponds to the second of these groups (Vulchanova et al. 1997; Bradbury et al. 1998). The cells also express the binding site for isolectin B4, and at least some of them also express TRPV1, the receptor for capsaicin (Guo et al. 1999; Hubscher et al. 2001; Petruska et al. 2000a, 2000b, 2002). The axons project centrally to end in the inner part of lamina II of the dorsal horn of the spinal cord, the corresponding region of the trigeminal complex (from the Vth nerve), and the nucleus tractus solitarius (from the vagus nerve)(Vulchanova et al. 1997, 1998; Bradbury et al. 1998). Lesion studies show that P2X3 immunoreactivity traffics both centrally and peripherally into the sciatic nerve. As for the peripheral projections, P2X3 immunoreactivity has been reported in fine unmyelinated fibres in tongue, viscera, tooth pulp and skin (Bradbury et al. 1998). Many of the P2X3 positive cells also stain for P2X2 subunits, including almost all cells in the nodose ganglion (Vulchanova et al. 1997), providing a histological substrate for the hetero-oligomeric receptors described above.
The distribution of P2X3 subunits is altered in experimental models of chronic pain. Novakovic et al. (1999) found an increase in the number of P2X3-positive small- and medium-sized neurones after chronic constriction injury to the sciatic nerve. The dorsal root fascicles in such animals also have an increased sensitivity to αβmeATP, where it enhances the ectopic discharge observed in many such fibres (Chen et al. 1999; Zhou et al. 2001). Fourteen days after spinal nerve ligation, there is an overall reduction in the P2X3 immunoeactivity in the dorsal root ganglia, and fewer small neurones respond to αβmeATP (Kage et al. 2002). These findings indicate that there is a selective decrease in expression of P2X3 subunits (probably as homo-oligomers) in the small diameter neurones, but no change in the expression of the subunit on larger cells, that mediate mechanical allodynia (Ossipov et al. 1999). On these cells the subunit appears to be present as a P2X2/3 hetero-oligomer. Chronic inflammation (complete Freund's adjuvant administered to the hindpaw) results in an increased expression of P2X3 protein expression in the corresponding dorsal root ganglia and larger than normal ATP-induced currents (Xu & Huang, 2002).
Antagonists
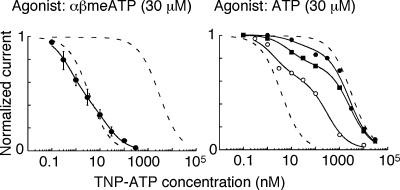
The cloning of P2X3 subunit cDNAs has allowed the development of selective antagonists (North & Surprenant, 2000). Virginio et al. (1998) identified 2′3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) as a nanomolar blocker of homo-oligomeric P2X1, homo-oligomeric P2X3 and hetero-oligomeric P2X2/3 receptors. These subtypes are almost 1000 times more sensitive to block than P2X2, P2X4 and P2X7 receptors (Virginio et al. 1998). The fast component of the ATP-evoked and sustained current evoked by αβmeATP (through hetero-oligomeric P2X2/3 receptors) are also completely blocked by TNP-ATP in neurones of dorsal root (Burgard et al. 1999) and nodose ganglia (Thomas et al. 1998). TNP-ATP appears to act as a competitive antagonist at the P2X3 and P2X2/3 receptors (Burgard et al. 2000; Spelta et al. 2002). In primary afferent neurones of the nodose ganglion, two populations of P2X receptor can be distinguished from the biphasic inhibition curve for TNP-ATP (Fig. 3). One of these corresponds in affinity (KD ∼3 nm) to the P2X2/3 hetero-oligomeric channel, but the other has a much lower affinity (KD ∼ 3 nm) which is close to that for the P2X2 homo-oligomeric channel.
Figure 3. Individual primary afferent neurones express more than one P2X receptor.
Left, inhibition by TNP-ATP of the current evoked by αβmeATP in nodose ganglion neurones (n = 6). The monophasic inhibition suggests a single population of receptors, and the IC50 is about 3 nm. The dashed lines correspond to the inhibition curves for TNP-ATP in cells expressing P2X2/3 and P2X2 receptors: IC50 values are 3 nm and 3 μm. Right, inhibition by TNP-ATP of the current evoked by ATP in three different neurones. In these cells, the biphasic inhibition curve suggest that each cell expresses two populations of receptors. The continuous lines indicate the fits to a mixture of sites, 35% with IC50 3 nm and 65% with IC50 3 μm. Reproduced, with permission, from Thomas et al. (1998).
Jarvis et al. (2002) have described a non-nucleotide antagonist with selectivity for P2X3 and P2X2/3 receptors. A-317491 competitively blocks these receptors, as measured by whole-cell currents or calcium uptake in cells expressing recombinant subunits and in rat dorsal root ganglion cells. The dissociation equilibrium constant (from Schild plots) is in the range 10–100 nm. The action of A-317491 is not shared by its R enantiomer A-317344, and the compound has no activity at a wide range of other membrane receptors and ion channels.
Evidence for P2X3 subunit involvement in nocifensive behaviour
Three kinds of tool have been used to assess the role of P2X3 subunit-containing receptors in pain sensation. These are selective receptor antagonists, subunit depletion by antisense oligonucleotides or small interfering RNA, and gene knock-outs.
Antagonists
Suramin was originally introduced as an antileprotic agent, but there are reports that it reduces pain in man (e.g. in therapy of hormone-resistant prostate cancer: Kehinde et al. 1995): it has actions at so many different receptors that this finding cannot be interpreted as implicating P2X receptors. TNP-ATP attenuates the nocifensive behaviour elicited by intraplantar injection of formalin (Jarvis et al. 2001) and by intra-abdominal injection of acetic acid (Honore et al. 2002). In the latter case, the abdominal constriction assay, TNP-ATP was almost as potent as morphine; like morphine, it abolished completely the behavioural response to intraperitoneal acetic acid. TNP-ATP also blocks the actions of ATP at P2X1 receptors at nanomolar concentrations (Virginio et al. 1998), and the lack of effectiveness of di-inosine pentaphosphate, which is a selective blocker of P2X1 receptors, indicates that its action indeed results from blocking P2X3 subunit-containing receptors (Honore et al. 2002).
A-317491 is a potent and selective blocker of P2X receptors containing the P2X3 subunit (see above: Jarvis et al. 2002). This compound also reduces certain aspects of chronic inflammatory (Freund's adjuvant-induced thermal hyperalgesia) and neuropathic (mechanical allodynia) pain in the rat (Jarvis et al. 2002). The compound gave full block of mechanical allodynia and thermal hyperalgesia in the chronic constriction injury model, and reduced to 50% the tactile allodynia after spinal nerve ligation. It had no obvious effect on motor or cardiovascular function. A stereo-isomer of A-317491 which is ineffective at blocking P2X3 receptors (A-317344) was also ineffective at reducing the nocifensive behaviour.
P2X3 subunit knock-down with antisense oligonucleotides
The level of expression of P2X3 subunits in dorsal root ganglion cells can be greatly reduced by repeated administration of antisense oligonucleotides into the intrathecal space. After 7 days of such treatment the levels are reduced to about 50% of control, as measured by Western blotting (Barclay et al. 2002; Honore et al. 2002). Concomitant with the reduction in P2X3 subunit expression, these rats show marked reductions in chronic (but not acute) inflammation-induced thermal (Honore et al. 2002) and mechanical (Barclay et al. 2002) hyperalgesia, and spinal nerve ligation-induced mechanical allodynia (Honore et al. 2002).
P2X3 gene knock-outs
Two groups have bred mice with ablated P2X3 subunit genes (Souslova et al. 2000; Cockayne et al. 2000). The homozygous P2X3(–/–) mice have no detectable P2X3 protein in their primary afferent nerves, and dorsal root and nodose ganglia do not show any currents elicited by αβmeATP (i.e. they have no homo-oligomeric P2X3 receptors and no P2X2/3 hetero-oligomeric receptors). In both studies, the mice showed a modest reduction in hindpaw licking and lifting after intraplantar formalin. Cockayne et al. (2000) found a paradoxical potentiation of thermal hyperalgesia following complete Freund's adjuvant. The most stiking phenotpye reported by Cockayne et al. (2000) was the apparent impairment in the ability to sense bladder filling. This is concluded to result from the absence of P2X3 receptors on terminals of primary nerves lying in the bladder wall beneath the urothelium: in anaesthetized mice ATP released from the urothelium of the distended bladder normally activates these fibres through their P2X3 receptors as the initial step of the voiding reflex.
Conclusions
The unusually limited distribution of the P2X3 subunit first prompted the suggestion of a unique role in sensation, and its expression on a subset of smaller-diameter primary afferent cells sustained this view. We now know that this subunit forms both homo-oligomeric and hetero-oligomeric ion channels: currents through the latter form (the P2X2/3 hetero-oligomer) are more sustained and seem likely to play a more significant physiological role. Experiments with antagonists selective for P2X3 subunit-containing channels, studies with antisense oligonucleotides that reduce the level of expression of P2X3 subunits in primary afferent cells, and observations on P2X3 gene knock-out mice, all substantiate the view that extracellular ATP has a part to play in the generation and transmission of the signals in chronic inflammation and in nerve damage. The details of this role, as well as the wider roles in sensing visceral distension, are now being worked out.
Acknowledgments
The author's work is supported by the Wellcome Trust.
References
- Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, Abdel'al S, Natt F, Hall J, Winter J, Bevan S, Wishart W, Fox A, Ganju P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002;22:8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland-Ward PA, Humphrey PPA. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br J Pharmacol. 1997;122:366–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen T, Keele CA. Observation on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia. 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoceptors in sensory neurons: effects of axotomy and glial derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Niforatos W, van Biesen T, Lynch KJ, Kage KL, Touma E, Kowaluk EA, Jarvis MF. Competitive antagonism of recombinant P2X2/3 receptors by 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP) Mol Pharmacol. 2000;58:1502–1510. doi: 10.1124/mol.58.6.1502. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Niforatos W, van Biesen T, Lynch KJ, Touma E, Metzger RE, Kowaluk EA, Jarvis MF. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J Neurophysiol. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shu Y, Zhao Z. Ectopic purinergic sensitivity develops at sites of chronic nerve constriction injury in rat. Neuroreport. 1999;10:2779–2782. doi: 10.1097/00001756-199909090-00015. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2000;53:553–568. [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu Q-M, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APDW. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Jorizzo JL, Eady RA, Greaves MW, Burnstock G. Adenosine triphosphate-evoked vascular changes in human skin: mechanism of action. Eur J Pharmacol. 1981;76:391–401. doi: 10.1016/0014-2999(81)90110-2. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992;106:242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure – a possible sensory mechanism. J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol. 1999;126:326–332. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- Hilliges M, Weidner C, Schmelz M, Schmidt R, Orstavik K, Torebjork E, Handwerker H. ATP responses in human C nociceptors. Pain. 2002;98:59–68. doi: 10.1016/s0304-3959(01)00469-9. [DOI] [PubMed] [Google Scholar]
- Holton P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Mikusa J, Bianchi B, McDonald H, Cartmell J, Faltynek C, Jarvis MF. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002;96:99–105. doi: 10.1016/s0304-3959(01)00434-1. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Petruska JC, Rau KK, Johnson RD. Co-expression of P2X receptor subunits on rat nodose neurons that bind the isolectin GS-I–B4. Neuroreport. 2001;12:2995–2997. doi: 10.1097/00001756-200109170-00048. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage KYuH, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Wismer CT, Schweitzer E, Yu H, Van Biesen T, Lynch KJ, Burgard EC, Kowaluk EA. Modulation of BzATP and formalin induced nociception: attenuation by the P2X receptor antagonist, TNP-ATP and enhancement by the P2X3 allosteric modulator, cibacron blue. Br J Pharmacol. 2001;132:259–269. doi: 10.1038/sj.bjp.0703793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L-H, Kim M, Spelta V, Bo X, Surprenant A, North RA. Subunit arrangement in P2X receptors. J Neurosci. 2003;23:8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage K, Niforatos W, Chu CZ, Lynch KJ, Honore P, Jarvis MF. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res. 2002;147:511–519. doi: 10.1007/s00221-002-1263-x. [DOI] [PubMed] [Google Scholar]
- Kehinde EO, Terry TR, Mistry N, Horsburgh T, Sandhu DP, Bell PR. UK studies on suramin therapy in hormone resistant prostate cancer. Cancer Surv. 1995;23:217–229. [PubMed] [Google Scholar]
- Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurones. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li P, Calejesan AA, Zhuo M. ATP P2x receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J Neurophysiol. 1998;80:3356–3360. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- Maehara Y, Kusumoto H, Anai H, Kusumoto T, Sugimacji K. Human tumour tissues have higher ATP content than normal tissues. Clin Chim Acta. 1987;169:341–344. doi: 10.1016/0009-8981(87)90337-8. [DOI] [PubMed] [Google Scholar]
- Marchettini P, Lacerenza M, Formaglio F. Sympathetically maintained pain. Curr Rev Pain. 2000;4:99–104. doi: 10.1007/s11916-000-0042-2. [DOI] [PubMed] [Google Scholar]
- Mork H, Ashina M, Bendtsen L, Olesen J, Jensen R. Experimental muscle pain and tenderness following infusion of endogenous substances in humans. Eur J Pain. 2003;7:145–153. doi: 10.1016/S1090-3801(02)00096-4. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Furue H, Yoshimura M, Gu JG. Activation of central terminal vanilloid receptor-1 receptors and alpha beta-methylene-ATP-sensitive P2X receptors reveals a converged synaptic activity onto the deep dorsal horn neurons of the spinal cord. J Neurosci. 2002;22:1228–1237. doi: 10.1523/JNEUROSCI.22-04-01228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21:6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Tsuzuki K, Ling JX, Sonobe H, Gu JG. Distinct roles of P2X receptors in modulating glutamate release at different primary sensory synapses in rat spinal cord. J Neurophysiol. 2003;89:3243–3252. doi: 10.1152/jn.01172.2002. [DOI] [PubMed] [Google Scholar]
- North RA. The molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Bain D, Malan TP, Lai J, Porreca F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–133. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, Grunder S. Inflammatory mediators potentiate ATP-gated channels through the P2X3 subunit. J Biol Chem. 2001;276:21077–21082. doi: 10.1074/jbc.M101465200. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Cooper BY, Gu JG, Rau KK, Johnson RD. Distribution of P2X1, P2X2, and P2X3 receptor subunits in rat primary afferents: relation to population markers and specific cell types. J Chem Neuroanat. 2000a;20:141–162. doi: 10.1016/s0891-0618(00)00080-6. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neuroscience. 2002;115:15–30. doi: 10.1016/s0306-4522(02)00409-8. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000b;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Thompson SW, McMahon SB. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain. 1999;6:S111–S120. doi: 10.1016/S0304-3959(99)00144-X. [DOI] [PubMed] [Google Scholar]
- Ryan LM, Rachow JW, McCarty DJ. Synovial fluid ATP: a potential substrate for the production of inorganic pyrophosphate. J Rheumatol. 1991;18:716–720. [PubMed] [Google Scholar]
- Sawynok J, Reed A. Peripheral adenosine 5′-triphosphate enhances nociception in the formalin test via activation of a purinergic P2X receptor. Eur J Pharmacol. 1997;330:115–121. doi: 10.1016/s0014-2999(97)01001-7. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. Can we conquer pain. Nat Neurosci. 2002;5:1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- Schweibert LM, Rice WC, Kudlow BA, Taylor AL, Schweibert EM. Extracellular ATP signalling and P2X nucleotide receptodrs in monolayers of primary hauman vascular endothelial cells. Am J Physiol Cell Physiol. 2002;282:C289–C301. doi: 10.1152/ajpcell.01387.2000. [DOI] [PubMed] [Google Scholar]
- Sneddon P, Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984;106:149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenius-Oosthuizen D, Smith AJH, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Spelta V, Jiang LH, Surprenant A, North RA. Kinetics of antagonist actions at rat P2X2/3 heteromeric receptors. Br J Pharmacol. 2002;135:1524–1530. doi: 10.1038/sj.bjp.0704591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K, von Kugelgen I, Bulloch JM, Illes P. Nucleotides as cotransmitters in vascular sympathetic neuroeffector transmission. Blood Vessels. 1991;28:19–26. doi: 10.1159/000158839. [DOI] [PubMed] [Google Scholar]
- Stebbing MJ, McLachlan EM, Sah P. Are there functional P2X receptors on cell bodies in intact dorsal root ganglia of rats. Neuroscience. 1998;86:1235–1244. doi: 10.1016/s0306-4522(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Thomas S, Virginio C, North RA, Surprenant A. The antagonist trinitrophenyl-ATP reveals co-existence of distinct P2X receptor channels in rat nodose neurones. J Physiol. 1998;509:411–417. doi: 10.1111/j.1469-7793.1998.411bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Xiong SH, Li ZW. Neurokinin B potentiates ATP-activated currents in rat DRG neurons. Brain Res. 2001;923:157–162. doi: 10.1016/s0006-8993(01)03211-5. [DOI] [PubMed] [Google Scholar]
- Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J. Endogenously released ATP mediates shear-stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H793–803. doi: 10.1152/ajpheart.01155.2002. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Bardini M, Ford AP, Cockayne DA, Burnstock G. Changes in P2X receptor responses of sensory neurons from P2X3-deficient mice. Eur J Neurosci. 2001;14:1784–1792. doi: 10.1046/j.0953-816x.2001.01805.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Chung K, Chung JM. Development of purinergic sensitivity in sensory neurons after peripheral nerve injury in the rat. Brain Res. 2001;915:161–169. doi: 10.1016/s0006-8993(01)02845-1. [DOI] [PubMed] [Google Scholar]