Abstract
Recent studies have shown that low-frequency repetitive transcranial magnetic stimulation (rTMS) to the left dorsal premotor cortex has a lasting influence on the excitability of specific neuronal subpopulations in the ipsilateral primary motor hand area (M1HAND). Here we asked how these premotor to motor interactions are shaped by the intensity and frequency of rTMS and the orientation of the stimulating coil. We confirmed that premotor rTMS at 1 Hz and an intensity of 90% active motor threshold (AMT) produced a lasting decrease in corticospinal excitability probed with single-pulse TMS over the left M1HAND. Reducing the intensity to 80% AMT increased paired-pulse excitability at an interstimulus interval (ISI) of 7 ms. Opposite effects occurred if rTMS was given at 5 Hz: at 90% AMT, corticospinal excitability increased; at 80% AMT, paired-pulse excitability at ISI = 7 ms decreased. No effects were seen if rTMS was applied at the same intensities to prefrontal or primary motor cortices. These findings indicate that the intensity of premotor rTMS determines the net effect of conditioning on distinct populations of neurones in the ipsilateral M1HAND, but it is the frequency of rTMS that determines the direction of the induced change. By selecting the appropriate intensity and frequency, premotor rTMS allows to induce a predictable up- or down-regulation of the excitability in distinct neuronal circuits of human M1HAND.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive method that can induce changes in excitability of the human cortex that outlast the stimulation for at least several minutes (for review, see Hallett et al. 1999). Most studies have focused on effects in the primary motor hand area (M1HAND) because changes in corticospinal excitability can be readily probed by measuring the size of motor evoked potentials (MEPs) in muscles of the contralateral upper limb (Siebner & Rothwell, 2003). The frequency of TMS has a substantial impact on the direction of excitability changes in the M1HAND. Stimulation for periods of up to 30 min at a frequency of around 1 Hz (referred to as low-frequency rTMS) results in a decrease in excitability that outlasts the stimulation by 30 min or so (Chen et al. 1997; Muellbacher et al. 2000; Fitzgerald et al. 2002; Touge et al. 2001). Conversely, rTMS at frequency of 5–20 Hz (referred to as high-frequency rTMS) results in an increase in MEP size for several minutes after completion of rTMS (Pascual-Leone et al. 1994; Maeda et al. 2000; Wu et al. 2000).
More recently, it has been shown that ‘focal’ rTMS is also capable of evoking after effects at distant sites that are interconnected with the stimulated cortex (Wassermann et al. 1998; Siebner et al. 2000; Gerschlager et al. 2001; Paus et al. 2001; Münchau et al. 2002). Remote after effects have been attributed to effective activation of output and input connections during rTMS (Ferbert et al. 1992; see for review: Rothwell et al. 1999). In previous studies with rTMS, we explored excitability changes that occur in the hand area of the left M1 after low-frequency rTMS of the left dorsal premotor cortex. When premotor rTMS was given at 90% of active motor threshold (AMT) for the M1HAND, 1 Hz rTMS caused a lasting reduction in excitability of the corticospinal system as indexed by a decrease in MEP size evoked in the right first dorsal interosseous (FDI) muscle (Gerschlager et al. 2001). At a slightly lower intensity (80% AMT), 1 Hz rTMS had no after effects on MEP size, but resulted in a selective modulation of a distinct set of cortico-cortical circuits in M1HAND as probed by a conditioning–test paradigm (Münchau et al. 2002).
In the present study, we explored whether the direction of after effects induced by rTMS to the left premotor cortex on excitability in ipsilateral M1HAND depends on the frequency of stimulation and/or the orientation of the rTMS coil. Considering the pattern of after effects found after direct rTMS of the M1HAND, we hypothesized that 5 Hz rTMS may result in opposite after effects compared with conditioning effects evoked by 1 Hz rTMS. If so, this would allow for a purposeful temporary modulation of premotor-to-motor connections and might open up unprecedented possibilities to shape functional interactions between premotor areas and M1HAND in the intact human brain.
Methods
Subjects
Eleven male healthy volunteers participated in the experiments (mean age ± s.d., 33.2 ± 6.7; range, 24–57 years). We studied only male subjects because Smith et al. (1999) reported that conditioning TMS pulses produced more inhibition in the luteal phase than in the follicular phase during the female cycle. As we were looking for subtle changes of motor cortex excitability, that involved repeating the experiments on the same subjects on different days, we decided to focus on men in whom the data from paired-pulse experiments is less variable. Subjects were seated in a comfortable reclining chair with the neck supported by a U-shaped pillow to avoid head movements during TMS. All participants gave their informed consent prior to participation. Experimental procedures were approved by the local Ethics Committee and were in accordance with the Declaration of Helsinki.
Design
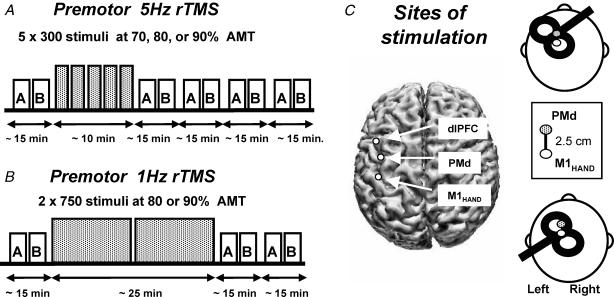
A repeated-measures within-subject design was used (see Fig. 1). The main factors investigated were the intensity, spatial specificity and the frequency of rTMS. All 11 subjects received 5 Hz rTMS over the left premotor cortex at 90% AMT. Each of these 5 Hz rTMS sessions consisted of five rTMS trains of 300 pulses, each separated by 1 min. The effect of intensity was explored in a subgroup of eight subjects who also received rTMS at 70 and 80% AMT. To explore the spatial specificity we compared the effect of premotor rTMS (90% AMT) with stimulation over M1HAND (n = 6) and over the dorso-lateral prefrontal cortex (n = 4). Finally, to explore frequency effects we compared premotor rTMS at 5 Hz with premotor rTMS at 1 Hz (two rTMS trains of 750 pulses; eight subjects at 80% and 90% of AMT). The rTMS was applied on separate days (at least 5 days between the rTMS sessions).
Figure 1. Experimental design.
5 Hz rTMS (A) was given in five blocks of 300 stimuli, each separated by 1 min. MEP amplitude and SICI/ICF at rest (box A) and MEP amplitude during contraction and the duration of the cortical silent period (CSP) (box B) were determined before and four times afterwards in an alternating order. Stimulus intensity of rTMS is expressed as a percentage of the active motor threshold (AMT) for the ipsilateral M1HAND. 1Hz rTMS consisted of two sets of 750 stimuli with an intertrain interval of 1 min (B). In contrast to the experiments using 5Hz rTMS, only two measurements of motor cortex excitability were carried out after the end of the rTMS session. C, coil position and orientation for rTMS of the left dorsal premotor cortex (top left) and single-/paired-pulse TMS of the left primary motor hand area (M1HAND) (bottom left). The site for stimulation of the M1HAND was defined as being 2.5 cm rostral to the ‘motor hot spot’ for the FDI muscle (see Methods). A three-dimensional reconstruction of an individual brain shows the sites for stimulation of the premotor cortex, M1HAND, and the dorsolateral prefrontal cortex (dLPFC) projected onto the surface of the cortex.
Cortical excitability of the left M1HAND was assessed with single and paired-pulse TMS before (referred to as baseline) and up to 1 h after rTMS by evaluating EMG responses in the right FDI muscle both at rest (Fig. 1A) and during tonic contraction (Fig. 1B). In the initial experiment using premotor 5 Hz rTMS at 90% AMT, MEP measurements were repeated four times after rTMS. In all other experiments, MEP measurements were carried out twice after rTMS because we were mainly interested in the size and direction of the after effect rather than in its time course.
rTMS parameters
Focal rTMS was performed using a standard figure-of-eight coil with mean loop diameters of 9 cm, connected to a Magstim Rapid stimulator (Magstim Co., Whitland, Dyfed, UK). The magnetic stimulus had a biphasic waveform with a pulse width of ∼ 300 μs. During the first phase of the stimulus, the current in the centre of the coil flowed toward the handle. The coil was held tangentially to the skull with the handle pointing 45° antero-medially (Fig. 1C). For the Magstim Rapid stimulator, this coil orientation has been shown to have the lowest motor threshold for eliciting a MEP over the M1HAND (Kammer et al. 2001). Each individual's AMT over the M1HAND was determined prior to rTMS using the Magstim Rapid stimulator and this orientation.
The left dorsal premotor cortex was defined as being 2.5 cm anterior to M1HAND (see below) because recent functional imaging studies have demonstrated that the dorsal premotor cortex is located about 15–25 mm anterior to M1HAND (Fink et al. 1997; Picard & Strick, 2001). The left dorso-lateral prefrontal cortex was defined as lying 5 cm anterior to M1HAND.
Structural MRI images were available for five of the subjects, and confirmed the approximate site of premotor rTMS in relation to brain anatomy. The ‘motor hot spot’ was located functionally using TMS, then the premotor and the dorso-lateral prefrontal area were marked. These points, the nasion and the preauricular points were recorded using a Polhemus Fastrak digitizer and commercially available software (3-D space, Neurosoft Inc). Each individual's MRI was then segmented and a triangulated net of the cortical surface created (CURRY, Neurosoft Inc). The digitizer and MRI data were coregistered using the co-ordinates of the nasion and preauricular points. The points on the surface of the scalp were then projected onto the cortical surface using the centre of mass of the binary overlay created in the segmentation (CURRY, Neurosoft Inc). The points are displayed in Fig. 1C.
A total number of 1500 single stimuli were applied during a single rTMS session. The 5 Hz rTMS sessions consisted of five trains of 300 stimuli separated by intertrain intervals of 1 min (10 min in total), whereas 1 Hz sessions consisted of two trains of 750 stimuli with an intertrain interval of 1 min (25 min in total). The stimulation protocol was in accordance with published safety recommendations (Wassermann, 1998).
Control experiment: effect of current direction
To study the effects of the orientation of the coil on intracortical excitability, we gave rTMS over premotor cortex with the coil held tangentially to the skull and the handle pointing 45° postero-laterally in six subjects. Although we used the same relative intensity of conditioning rTMS as we had done in the main experiments (90% AMT), the absolute intensity in terms of stimulator output was slightly higher because AMT itself is higher with this orientation than it is using an antero-medial coil position (Kammer et al. 2001). Because of this, in four subjects, we repeated the experiments with postero-lateral stimulation but used the same (lower) intensity of rTMS as in the main set of experiments.
TMS measurements of the left primary motor hand area (M1HAND)
Excitability of the left M1HAND was assessed with single- and paired-pulse TMS before and up to 1 h after rTMS. Measurements were performed with a High Power Magstim 200 machine and a figure-of-eight-shaped coil with a mean loop diameter of 9 cm (Magstim Co., Whitland, Dyfed, UK). The magnetic stimulus had a nearly monophasic pulse configuration with a rise time of ∼100 μs, decaying back to zero over ∼0.8 ms. The coil current during the rising phase of the magnetic field flowed toward the handle. The coil was placed tangentially to the scalp with the junction region pointing backwards and laterally at a 45° angle away from the midline, approximately perpendicular to the line of the central sulcus inducing a posterior-anterior current in the brain (Fig. 1C). We chose this orientation as motor threshold is minimum when the induced electrical current in the brain flows approximately perpendicular to the line of the central sulcus (Brazil-Neto et al. 1992; Mills et al. 1992). We determined the optimum position for activation of the right FDI by moving the coil in 0.5 cm steps around the presumed M1HAND. The site at which stimuli of slightly suprathreshold intensity consistently produced the largest MEPs in the target muscle was marked with a grease pencil as the ‘motor hot spot’. Baseline and post-rTMS measurements were performed over this marked area.
Stimulus intensities for TMS were determined at the beginning of each experiment. RMT was defined as the minimum output of the stimulator that induced a reliable MEP (about 50 μV in amplitude) in at least 5 of 10 consecutive trials when the FDI muscle was completely relaxed. AMT was defined as the lowest stimulus intensity at which 5 of 10 consecutive stimuli elicited reliable MEP (about 200 μV in amplitude) in the tonically contracting FDI muscle.
MEPs were recorded from Ag-AgCl surface electrodes over the right FDI muscle, using a belly tendon montage. The signal was amplified and band-pass filtered (10–1000 Hz) by a Digitimer D150 amplifier (Digitimer Ltd, Welwyn Garden City, Herts, UK) and acquired at a sampling rate of 5 kHz on a personal computer for off-line analysis (SigAvg Software, Cambridge Electronic Design, Cambridge, UK). During the experiments EMG activity was continuously monitored with visual (oscilloscope) and auditory (speakers) feedback to ensure either complete relaxation at rest or a constant level of EMG activity during tonic contraction.
Motor cortex excitability at rest (Block A in Fig. 1)
Short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) were studied using the conditioning-test paradigm introduced by Kujirai et al. (1993). Two monophasic magnetic stimuli were given through the same stimulating coil over the M1HAND and the effect of the first (conditioning) stimulus on the second (test) stimulus was investigated. To avoid any floor or ceiling effect, we set the intensity of the conditioning stimulus to a relatively low value of 80% AMT. The test stimulus was adjusted to an intensity that, when given alone in control trials before rTMS, would evoke an EMG response of ∼1 mV peak to peak (about 115–125% RMT). The following interstimulus intervals (ISIs) were tested: 2, 4, 7, 9, and 12 ms. The six conditions (test pulse given alone and five conditioned pulses at different ISIs) were applied in a single block of 112 trials with an interval of 5 s between trials. In this block, that lasted approximately 8 min, the control condition (test pulse given alone) was tested 32 times and each of the conditioning-test stimuli 16 times. The order of the conditions was randomised. Measurements were made on each individual trial. The mean peak-to-peak amplitude of the conditioned MEP at each ISI was expressed as a percentage of the mean peak-to-peak size of the unconditioned test pulse in that block.
The peak-to-peak amplitude of the unconditioned MEP in the relaxed right FDI was used as a measure of corticospinal excitability. SICI was taken as the mean percentage inhibition of conditioned MEPs ISIs of 2 and 4 ms whilst ICF was taken as the mean facilitation at ISIs of 9 and 12 ms. An intermediate ISI of 7 ms was also included in each block as previous work has shown that premotor 1Hz rTMS at 80% AMT produces a selective after effect at ISIs of 6–7 ms (Münchau et al. 2002).
rTMS lead to changes in the amplitude of the test response, and it is possible that these could have affected the amount of SICI and ICF. However, as suggested by Ridding et al. (1995), we expected that the percentage SICI or ICF should remain approximately constant given the rather limited range in the amplitude of the test MEP that was observed. This was confirmed in on experiment on four subjects in which we examined paired-pulse interactions before and after 5 Hz rTMS at 90% AMT. Unfortunately, time considerations in the main series of experiments meant that we could not always incorporate a second block of trials in which the intensity of the test stimulus was adjusted to ensure that the test MEP was always in the 1 mV range.
Motor cortex excitability during contraction (Block B in Fig. 1)
We measured the peak-to-peak amplitude of 10 consecutive MEPs during slight (10–15% maximum) tonic contraction of the right FDI muscle using the same intensity as the control stimulus in the relaxed paired-pulse measurements (about 115–125% of RMT). In addition, we measured the duration of the cortically evoked silent period (CSP) which is a marker for the excitability of long-lasting intracortical inhibition. For CSP measurements, EMG traces were rectified but not averaged. The mean length of the SP was determined on the basis of measurements from each individual trial and defined as the interval between the onset of the MEP and the recovery of continuous EMG activity after the period of EMG suppression.
Statistical analysis
The effects of rTMS on the MEP amplitude (both under resting conditions and during slight voluntary muscle contraction) and the duration of cortical SP were evaluated by separate repeated-measures analyses of variance (ANOVA). For each dependent variable, we computed a two-way repeated-measures ANOVA with ‘TIME’ (pre-rTMS versus post-rTMS) and ‘FREQUENCY’ (5 Hz versus 1 Hz) as within-subject factors; TIME (pre-rTMS versus post-rTMS) and ‘SITE OF STIMULATION’ (motor versus premotor; prefrontal versus premotor) as within-subject factors; and TIME (pre-rTMS versus post-rTMS) and ‘COIL DIRECTION SPECIFITY’ (anterior-medial versus postero-lateral orientation) as within-subject factors. The Greenhouse–Geisser correction was used when necessary to correct for non-sphericity. The distribution of the data was assessed for normality by a Kolmogorov–Smirnov test. If it lay ouside this range, then we performed the statistics on log-transformed data. When an F-value was significant, post hoc paired-samples t tests were performed. Data are presented as means ± s.d. A P-value of <0.05 was considered significant.
Results
No subject experienced any noticeable adverse effects during the course of the study other than mild local discomfort at the site of rTMS.
Premotor 5 Hz rTMS at 90% AMT
The mean RMT was 39.5 ± 1.1% and mean AMT 32.3 ± 1.1% of maximum stimulator output. Mean intensities used in the paired-pulse TMS paradigm were 48.1 ± 2.1% for the test pulse and 26.4 ± 0.9% for the conditioning stimulus. Mean AMT as determined with the rapid magnetic stimulator was 37 ± 1.3%. Mean rTMS intensity was 33.5 ± 1.2%.
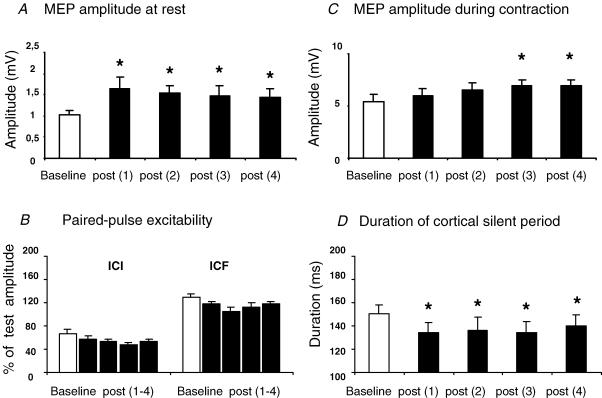
The after effects of 5 Hz rTMS at 90% AMT on excitability of the left M1HAND are summarized in Fig. 2. Premotor 5 Hz rTMS increased the amplitude of MEPs evoked by a single suprathreshold TMS stimulus in the relaxed FDI muscle. A repeated measures ANOVA demonstrated a significant effect of TIME on the mean MEP amplitude (F4,40= 5.3, P = 0.002). Post-hoc t tests revealed that the maximum increase in MEP amplitude was present in the first measurement after rTMS and that the increase lasted at least for 1 h (Fig. 2A). There was no lasting effect on the relative strength of SICI or ICF expressed as a percentage of unconditioned values (Fig. 2B).
Figure 2. Conditioning effects of premotor 5Hz rTMS at 90% AMT on MEP amplitude (A and C), the duration of the cortical silent period (D), and paired-pulse excitability (B).
Intracortical inhibition (SICI) was assessed using interstimulus-intervals (ISIs) of 2 and 4 ms (B, left). Intracortical facilitation (ICF) was estimated using ISIs of 9 and 12 ms (B, right). Error bars are standard error of the mean (s.e.m.). Asterisks denote a significant change relative to baseline.
Premotor 5 Hz rTMS also had a significant effect of TIME on MEPs evoked in the active FDI muscle (F4,40= 4.1, P = 0.031). As the same suprathreshold intensity was used for MEP recordings at rest and during contraction, MEP amplitudes were considerably higher in the preactivated FDI muscle as compared with MEPs in the relaxed FDI muscle (Fig. 2C). In some subjects this could have limited the post-rTMS increase in MEP amplitude. Despite this, MEP amplitude gradually increased during contraction over the first hour after rTMS. Accordingly, post hoc paired-samples t tests demonstrated a significant increase of MEP amplitude in active FDI muscle at 30 min (t =−2.3, P = 0.037) and 60 min after rTMS (t =−2.5, P = 0.04). There was also a significant reduction in the duration of the CSP (Fig. 2D). Repeated measures ANOVA showed a significant main effect for TIME (F4,40= 4.1, P = 0.03). Post-hoc paired-sample t tests showed that the CSP was shorter than control in all blocks after rTMS. (post 1 t =−3.1, P = 0.01; post 2 t =−2.15, P =0.028; post 3 t =−2.3, P = 0.021;post 4 t =−2, P = 0.048).
Premotor rTMS: effect of stimulus intensity at 5 Hz
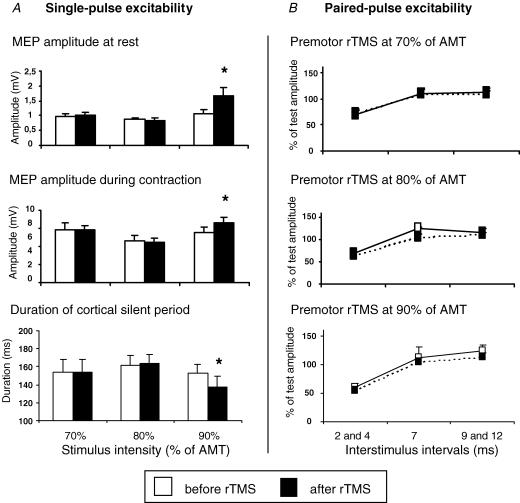
In eight subjects, premotor 5 Hz rTMS was given at 70, 80, or 90% AMT in three separate sessions performed on different days (Fig. 3). Baseline measures were well matched for all three sessions. In contrast to stimulation at 90% AMT, rTMS at 70 or 80% AMT had no lasting effect on MEP amplitude at rest or during contraction, and no effect on the duration of the CSP. This was confirmed by a repeated measure ANOVA showing a significant interaction between TIME and the INTENSITY OF STIMULATION for MEP amplitude at rest (F2,14= 7.5, P = 0.024), MEP amplitude during contraction (F2,14= 4.8, P = 0.041) and CSP duration (F2.14= 7.02, P = 0.008). Post-hoc paired-sample t tests demonstrated that only rTMS at 90% AMT produced a significant increase of MEP amplitudes (at rest t =–2.9, P = 0.021; during contraction t =–2.3, P = 0.033) and a significant decrease of CSP (t =–2.4, P = 0.046).
Figure 3. Intensity-dependent conditioning effects of premotor 5Hz rTMS on the excitability in ipsilateral M1HAND.
A, effects on single-pulse excitability of ipsilateral M1HAND The MEP amplitude at rest (top) and during tonic contraction (middle) and on the duration of the cortical silent period (bottom). B, the after effects on paired-pulse excitability are separately illustrated for intracortical inhibition at ISIs of 2 and 4 ms, intracortical facilitation at ISIs of 9 and 12 ms, and an intermediate interval of 7 ms. Error bars are standard error of the mean (s.e.m.). Asterisks denote a significant change relative to baseline.
The after effects of premotor 5 Hz rTMS on paired-pulse excitability also depended on the intensity of stimulation. A three-factorial repeated-measures ANOVA with TIME (pre versus post rTMS), INTENSITY of stimulation, and ISI as main factors. was performed on log-transformed data, as the raw data failed to satisfy criteria for normality. It revealed a significant main effect of TIME (F1,7= 8.3, P = 0.024) and a significant interaction between TIME and INTENSITY (F2,14= 3.8, P = 0.048), indicating that different intensities of rTMS had different effects on paired-pulse excitability. rTMS stimulation at both 80% and 90% tended to increase the level of ICI and decrease ICF. Follow up two-factorial ANOVAs at each intensity showed a marginal main effect of TIME at 80% and 90% AMT (F1,7= 5.1, P = 0.06; F1,7= 4.5, P = 0.07), respectively), but not at 70% AMT (F1,7= 1.8, P = 0.22) (Fig. 3B). The results were the same in a separate group of four subjects in whom we examined the effect of 5 Hz rTMS at 90% AMT after adjusting the intensity of the stimulus to match basal MEPs before and after rTMS.
Premotor rTMS: effect of frequency
Previous studies have shown that premotor rTMS at 1 Hz also leads to after effects on the excitability of M1HAND (Gerschlager et al. 2001; Münchau et al. 2002). To confirm these results in our group of subjects and for the coil position and orientation used in the present study, eight participants underwent an additional session of 1 Hz rTMS at 80% AMT. In six of these, we also explored the conditioning effects of premotor 1 Hz rTMS at 90% AMT.
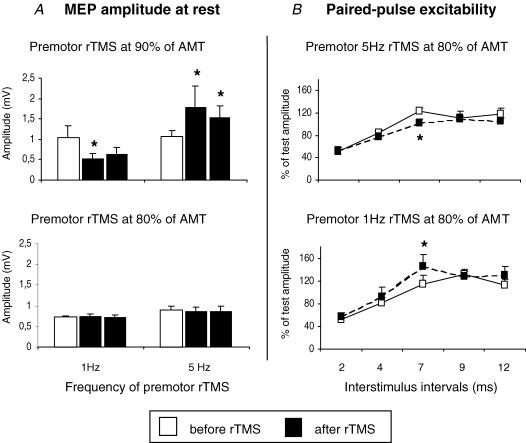
Figure 4 shows that 1 Hz and 5 Hz rTMS had opposing effects on MEPs evoked in the relaxed FDI muscle. A two-way repeated measures ANOVA demonstrated a significant interaction between the factors TIME and FREQUENCY of stimulation (F2,14= 5.8, P = 0.021). This was due to the fact that 1 Hz rTMS decreased MEP amplitudes, whereas 5 Hz rTMS facilitated them. rTMS at 80% AMT had no effect at either frequency.
Figure 4. Interaction between frequency and intensity of premotor rTMS on conditioning effects in the left M1HAND.
A, conditioning effects on corticospinal excitability as indexed by the MEP amplitude at rest. The columns indicate mean MEP amplitudes before (white) and after premotor rTMS (black). B, conditioning effects of premotor rTMS at 80% of AMT on paired-pulse excitability at an ISI of 7 ms. Error bars are standard error of the mean (s.e.m.). Asterisks denote a significant change relative to baseline.
The opposite effects on MEPs were complemented by opposing effects on paired-pulse interactions. As reported by Münchau et al. 2002), 1 Hz rTMS at 80% AMT (but not 90% AMT, not illustrated) increased paired-pulse excitability at an intermediate ISI of 7 ms even though there was no effect on the size of the test response alone. rTMS at 5 Hz and 80% AMT had the opposite effect (Fig. 4B). A three-factor repeated-measures ANOVA with TIME, ISI, and FREQUENCY as factors confirmed a significant interaction between the frequency of rTMS, the time of measurement, and the change in excitability at 7 ms (three-way interaction between TIME, FREQUENCY and ISI, F4,28= 4.06, P = 0.005).
Spatial specifity of rTMS at 5 Hz
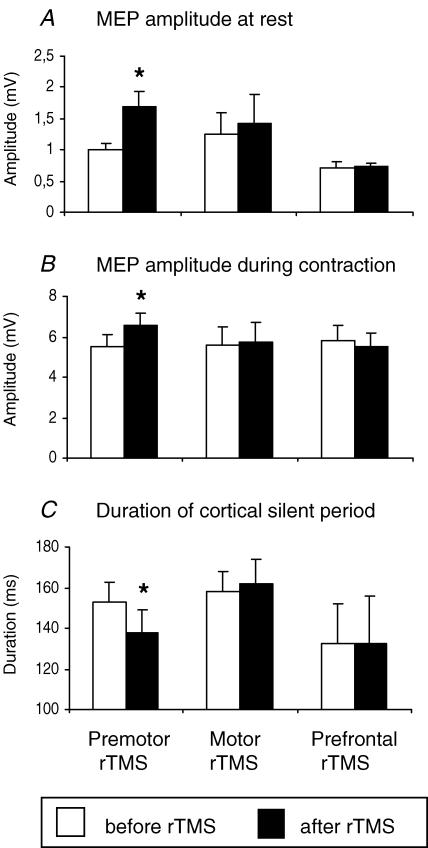
Figure 5 compares the effect on MEP amplitude (rest and active) and CSP of applying 5 Hz rTMS at 90% AMT to premotor, motor and prefrontal cortex in six subjects. Repeated-measures ANOVA revealed a significant interaction between TIME and SITE OF STIMULATION for all three measures (MEP at rest, F2,10= 4.5, P = 0.04; MEP during contraction, F2,10= 3.8, P = 0.043; duration of CSP, F2,10= 4.1, P = 0.046). Post-hoc paired-sample t tests showed that only premotor rTMS had an after effect on any of these variables. It increased MEP amplitudes at rest (t =–3.2, P = 0.022, Fig. 5A) and during contraction (t =–2.8, P = 0.034, Fig. 5B) and decreased the duration of CSP (t =–2.7, P = 0.040, Fig. 5C). These data are consistent with the idea that the conditioning effect of premotor rTMS was not due to a spread of stimulation to adjacent cortical areas.
Figure 5. Comparison of the effect of giving 5 Hz rTMS at 90% AMT over premotor (PMd, left pair of columns), motor (M1HAND, middle pairs of columns) and prefrontal (dLPFC, right pairs of columns) cortical sites.
A, mean MEP amplitudes at rest. B, mean MEP amplitudes during tonic contraction. C, duration of the cortical silent period. Error bars are standard error of the mean (s.e.m.). Asterisks denote a significant change relative to baseline.
Control experiment: effect of current direction on premotor 5 Hz rTMS
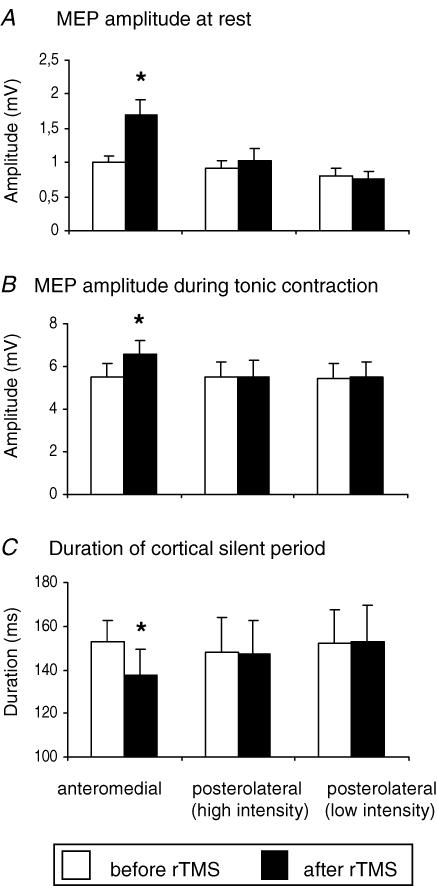
The mean data of six subjects who participated in this experiment are illustrated in Fig. 6. In this experiment, we compared the effect of holding the rTMS coil with the handle of the coil pointing 45° postero-laterally rather than antero-medially as in the main series of experiments. Two intensities of stimulation were used for rTMS: (a) the same absolute value as used in the main experiments with the antero-medial handle position (38.5 ± 5% stimulator output for this group of subjects), and (b) 90% AMT estimated with the coil handle pointing postero-laterally (45 ± 4.3%). When the premotor coil was held tangentially to the skull with the handle pointing postero-laterally, the MEP size at rest and during contraction (Fig. 6A and B) and the duration of SP (Fig. 6C) were not affected by rTMS at either intensity of stimulation.
Figure 6. Effect of coil orientation during premotor rTMS on changes in MEP amplitude of the relaxed (A) or active (B) hand muscle and on the duration of the cortical silent period (C).
Error bars are standard error of the mean (s.e.m.). Asterisks denote a significant change relative to baseline. Posterolateral (low intensity) refers to stimulation with the handle of the TMS coil pointing posterolaterally, at an intensity equal to 90% AMT assessed with the handle pointing anteromedially (as in the main experiment) (mean of 38% maximum stimulator output). High intensity posterolateral stimulation refers to stimulation at 90% AMT as assessed with a posterolateral coil orientation (mean of 45% maximum stimulator output).
Discussion
The present results show that 1 and 5 Hz rTMS over left dorsal premotor cortex have opposite after effects on the excitability of ipsilateral motor cortex. The effects depend on the intensity of rTMS and are spatially specific to the premotor cortex. Thus, MEPs evoked by single pulse stimulation of the M1HAND are increased for up to 1 h after 1500 pulses of 5 Hz rTMS at 90% AMT, whereas they are reduced after 1 Hz rTMS. Similarly, paired-pulse facilitation at an ISI of 7 ms is decreased by 5 Hz rTMS at 80% AMT, whereas it is increased after 1 Hz rTMS. We propose that these effects are caused by cortico-cortical interactions between premotor and motor areas. They extend the previous studies of Gerschlager et al. (2001) and Münchau et al. (2002) by showing that the direction of change is governed by the frequency of rTMS.
Cortical area activated by rTMS
It is difficult to be completely certain about the precise site of our premotor stimulus. Following previous imaging studies (e.g. Picard & Strick, 2001), we placed the centre of the junction region of the figure-of-eight coil 2.5 cm anterior to the M1HAND. This was confirmed to be over the precentral gyrus/sulcus in five subjects by coregistering the scalp position of the coil with an MRI of the individual's brain. If, as in motor cortex, the maximum stimulation occurs under the centre of the coil then it seems likely that we activated a proportion of the dorsal premotor cortex. This would be consistent with the fact that we saw no after effects if the rTMS coil was moved 2.5 cm anterior or posterior to this position.
The after effects of rTMS
We used three tests to monitor the after effects of rTMS: MEP amplitude, the duration of the CSP and paired-pulse interactions in the SICI/ICF curve. The question we address here is whether rTMS affected these measures because it produced: (a) changes in the excitability of spinal circuits due to activation of premotor-spinal projections, (b) changes in motor cortex excitability due to activation of premotor-motor projections, or (c) local effects on the excitability of premotor cortex itself.
We deal first with changes in MEP. Changes in the MEP can occur because of changes in cortical as well as spinal excitability. Is it possible that rTMS of premotor cortex either activates directly, or causes a change in the ongoing activity of premotor-spinal projections that then affects the excitability of spinal motoneurones? Activation of corticospinal neurones from the premotor area seems unlikely in view of the fact that the electrical threshold of this projection is much higher than that of the projection from the primary motor cortex (Cerri et al. 2003), yet the intensity of rTMS was only around the threshold for activating the output from the primary motor cortex (maximum of 90% AMT). Indeed, when the rTMS was applied direct to the M1HAND there was no after effect on MEPs.
If the changes in MEP amplitude reflect changes in cortical excitability, then can we be sure that these involved M1HAND, or could they have been limited to premotor cortex itself? The stimulus intensity we used to evoke MEPs was relatively large (115–125% RMT), and it is conceivable that even though the coil was placed over M1HAND, some of the stimulus spread to premotor cortex and recruited activity in premotor-spinal connections that contributed to the MEP. A change in the excitability of this premotor-spinal projection after rTMS could therefore affect the MEP without any need to invoke effects on M1HAND. At the present time we cannot be certain of the contribution, if any, of corticospinal projections from premotor cortex to the MEP in hand muscles. There is no electrophysiological data in primates on the relative strength and conduction velocity of corticospinal projections from premotor and motor cortex to intrinsic hand motoneurones. However, data does exist from another secondary motor area, the SMA. Here, Maier et al. (2002) have shown that the SMA projection is much weaker and slower than that from motor cortex. If the same is true for the premotor cortex in human, we conclude it is likely that at least some of the after effects of rTMS on MEP amplitude were caused by changes in excitability of M1HAND.
A similar line of argument can be used to account for the effects of premotor rTMS on the CSP. The latter part of the CSP is thought to be primarily cortical in origin. H-reflex studies of spinal cord excitability (Fuhr et al. 1991), comparison of TMS to the motor cortex with direct stimulation of the descending corticospinal tract at the cervicomedullary junction (Inghilleri et al. 1993) and direct recordings of descending corticospinal volleys from the epidural space (Chen et al. 1999) all suggest that, apart from its first 50–75 ms, the duration of the CSP is determined by activation of cortical inhibitory connections. Whether these are limited to M1HAND or also involve circuits in premotor cortex is not known. However, if the contribution of premotor-spinal connections to the MEP is small, then the likelihood is that the main effects of rTMS occurred because of changes in excitability of motor cortical circuits.
We can be a little more certain about the locus of rTMS effects on the SICI/ICF curve. Because the latter employs a conditioning stimulus that is below the threshold for evoking any descending corticospinal activity, it is thought to test excitability of intracortical circuits within the primary motor cortex (DiLazzaro et al. 1998). The changes in paired-pulse interactions after premotor rTMS must therefore have been due to direct effects on the excitability of these motor cortical circuits.
We propose that the effects on all three measures, MEP, CSP and SICI/ICF were due to cortico-cortical interactions between the area of stimulation in the premotor cortex and the M1HAND. This is in consistent with the dense interconnections and strong functional links between the premotor and motor cortex as revealed by functional imaging data in humans (Fink et al. 1997) and anatomical (Morecraft & van Hoesen, 1993) and electrophysiological experiments (Ghosh & Porter, 1988; Tokuno & Nambu, 2000) in primates.
Effect of stimulus intensity
There was a clear threshold intensity for effects on the MEP and CSP after conditioning with 5 Hz rTMS. MEPs were facilitated and the duration of the CSP was reduced after rTMS at 90% AMT, whilst there was no effect on either at 70% or 80% AMT. This is consistent with the results of Gerschlager et al. (2001) who found that the threshold for effects on the MEP was 90% AMT when they used 1 Hz rTMS. The situation was less clear for SICI/ICF: there was a tendency for the time course to be depressed after 5 Hz rTMS at both 80% and 90% AMT, but this was not individually significant at either intensity. Further work is needed to establish this threshold with certainty. However, it is interesting to note that Münchau et al. (2002) found the threshold to be 80% when they used 1 Hz rTMS.
Effects of stimulus frequency
In addition to intensity, the frequency of stimulation had a profound impact on the after effects of premotor rTMS and determined the direction of excitability changes (i.e. inhibition versus facilitation in the M1HAND). At an intensity of 90% AMT, premotor 5Hz rTMS increased corticospinal excitability in the M1HAND, whereas 1 Hz rTMS had the opposite effect. A frequency-dependent reversal was also observed for the modulation of paired-pulse excitability at 80% AMT. Although such frequency-dependent effects have been observed previously following direct stimulation over primary motor cortex (reviewed by Siebner & Rothwell, 2003), this is the first time that such effects have been described at sites distant from the point of stimulation.
The simplest explanation for these effects is that there is some change in synaptic behaviour at these two frequencies although its mechanism is for the moment unclear. However, there is one fact that suggests the situation may be more complex. The effect of 5 Hz rTMS on MEP amplitude was sensitive to reversal of the stimulating current, whereas a previous study of Gerschlager et al. 2001) had found that rTMS at 1 Hz was unaffected by reversing the orientation of the coil. Indeed, this was confirmed in the present study: the 1 Hz results were the same as those reported by Gerschlager et al. (2001) even though we used the opposite coil orientation. The tentative conclusion must be that the effects on the MEP might be produced by two subpopulations of neurones in premotor cortex that have similar thresholds. One of them is orientation insensitive and produces inhibitory effects on motor cortex MEPs at 1 Hz. The other is best activated with a posterior–anterior current flow, and has facilitatory effects on motor cortex MEPs with 5 Hz stimulation.
Mechanisms mediating remote plasticity in the M1HAND
The exact mechanisms that are responsible for these patterns of stimulation-induced plasticity remain to be clarified. Touge et al. (2002) suggested that there may be two broad mechanisms that underlie long-term effects of rTMS: (i) changes in synaptic efficacy such as seen in long-term potentiation and depression, and (ii) overall changes in neural excitability caused by changes in resting membrane potentials. In their experiment with rTMS over primary motor cortex, the after effects were present only in subjects at rest and disappeared when tested during voluntary muscle contraction. They concluded that changes in resting membrane potentials were likely to have contributed to the effects seen at rest, and that these were effectively normalized during voluntary contraction.
In the present experiments, the changes in MEP amplitude persisted during tonic voluntary contraction, and therefore we suggest that some of the after effects are probably caused by changes in the effectiveness of synaptic transmission. Indeed, circuit-specific changes in synaptic transmission would also explain why the duration of the CSP decreased after 5 Hz premotor rTMS whilst the MEP increased. Under normal circumstances the CSP increases with MEP amplitude, and to see such dissociation implies specificity of effects on facilitatory and inhibitory circuits that are most readily explained at a synaptic level. Similar specificity is needed to account for the selective effects on SICI/ICF at ISI = 7 ms.
Finally we must ask whether rTMS of premotor cortex caused effects on M1HAND because it produced repetitive activity in pathways linking the two areas that perhaps led to remote changes in synaptic efficacy in the M1HAND itself. Alternatively, it is possible that the intensity of rTMS was insufficient to evoke any activity in cortico-cortical pathways, and that its direct effects were limited to the premotor cortex under the point of stimulation. In this case, after effects on M1HAND could have been due to changes in the tonic level of activity in premotor-motor connections.
The present data cannot distinguish these possibilities directly, although functional imaging studies of metabolic changes during rTMS might provide useful information in the future. We speculate that as premotor cortex is likely to have a lower threshold for TMS activation than M1HAND, stimulation of premotor cortex at 90% AMT may well be sufficient to activate cortico-cortical outputs. If so, at this intensity long-term after effects may well be induced in circuits outside of the premotor area. In contrast, stimulation at 80% AMT may be below that needed to activate premotor output cells. If so, then at this intensity, effects would be limited to the site of stimulation.
Conclusions
By selecting the appropriate intensity and frequency, premotor rTMS provides a non-invasive tool to tune the excitability in distinct neuronal circuits of human M1HAND. The possibility to modulate temporarily the primary cortex via premotor-to-motor connections opens up new possibilities to shape functional interactions between premotor cortex and M1HAND in the intact human brain. Our data also shows that the conditioning effects of rTMS on the stimulated cortex may be seen as ‘the tip of the iceberg’ and distant conditioning effects may need to be taken into account, when using rTMS to induce representational plasticity.
Acknowledgments
We would like to thank Mr Peter Asselman for his invaluable help in maintaining and building the equipment used in these experiments. V. Rizzo was supported by the European Commission Improving Human Potential programme (contract number HPRI-CT-1999–00025), H.R. Siebner was supported by the Deutsche Forschungsgemeinschaft (grant SI 738/1–1 and the BMBF grant GO 1337400), A. Münchau and H.R. Siebner by Volkswagen Stiftung.
References
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:132–136. [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Br Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- DiLazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Collebath JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002;113:1136–1141. doi: 10.1016/s1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Porter S. Corticocortical synaptic influences on morphologically identified pyramidal neurone in the motor cortex of the monkey. J Physiol. 1988;400:617–629. doi: 10.1113/jphysiol.1988.sp017139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Wassermann EM, Pascual-Leone A, Valls-Sole J. Repetitive transcranial magnetic stimulation. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Supplement. 1999;52:105–113. [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Hoesen GW. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. J Comp Neurol. 1993;337:669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Münchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolatral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potential. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Supplement. 1999;52:97–103. [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell JC. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg DB, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM. Menstrual effects on cortical excitability. Neurology. 1999;53:2069–2072. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization on nonprimary motor cortical inputs on pyramidal and non pryramidal tract neurons of primary motor cortex: an electrophysiological study in the macaque monkey. Cereb Cortex. 2000;10:58–68. doi: 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1 Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250:141–144. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- Wu T, Sommer M, Tergau F, Paulus W. Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett. 2000;287:37–40. doi: 10.1016/s0304-3940(00)01132-0. [DOI] [PubMed] [Google Scholar]