Abstract
Fetal growth depends on the transplacental nutrient supply, which, in turn, is determined partially by the consumption and production of nutrients by the uteroplacental tissues. In fetal sheep, the rates of growth and umbilical glucose uptake decline coincidently towards term in parallel with the normal prepartum rise in plasma cortisol. While cortisol is known to reduce growth in fetal sheep, its effects on the uteroplacental handling and delivery of nutrients remain unknown. Hence, this study, quantified the rates of umbilical uptake and uteroplacental consumption of nutrients in preterm fetuses infused with cortisol for 5 days to mimic the prepartum cortisol surge. Umbilical uptakes of glucose and lactate, but not oxygen, were significantly lower in cortisol- than saline-infused fetuses, irrespective of whether values were expressed as absolute or weight-specific rates. The rate of uteroplacental consumption of glucose, but not oxygen, was significantly higher in cortisol- than saline-infused animals. Absolute rates of uteroplacental lactate production were lower in cortisol-infused animals. When all data were combined, fetal plasma cortisol levels were positively correlated to uteroplacental glucose consumption and inversely related to umbilical glucose uptake. Cortisol treatment had no apparent effect on placental mRNA expression for the glucose transporters, GLUT-1 and GLUT-3. The results demonstrate that cortisol is physiological regulator of uteroplacental metabolism and nutrient delivery to the sheep fetus. These observations have important implications for fetal growth both in late gestation and during adverse intrauterine conditions, which raise fetal cortisol levels earlier in gestation.
In all species studied to date, the major source of nutrients for the fetus is transplacental transfer from the mother with little, if any, endogenous nutrient production during normal nutritional conditions (Battaglia, 1986). For major oxidative substrates, such as glucose, transplacental transfer depends on facilitated diffusion, which, in turn, is determined by the transplacental glucose concentration gradient and the availability of carrier proteins, the glucose transporters (GLUTs). The amount of nutrient delivered to the fetus is also affected by the metabolic activity of the placenta itself. Ovine uteroplacental tissues have a 10-fold higher metabolic rate than the fetus, and consume 50% or more of the glucose taken up from the uterine circulation before it reaches the fetus (Simmons et al. 1979; Carver & Hay, 1995). These tissues also produce nutrients such as lactate and certain amino acids, which are released preferentially into the umbilical circulation for fetal use during late gestation (Sparks et al. 1983; Carter et al. 1991; Bell & Ehrhardt, 1998). However, the factors regulating uteroplacental nutrient production and the partitioning of nutrients between the fetal and uteroplacental tissues in pregnant sheep remain unknown.
As the fetus grows throughout gestation, its increased demand for nutrients is met by increasing the placental supply of glucose by widening the transplacental glucose concentration gradient and increasing GLUT expression in the placenta (Molina et al. 1991; Ehrhardt & Bell, 1997). However, in the last few days before birth, the growth rate of the fetus declines and the umbilical uptake of glucose falls by about 40% (Fowden et al. 1996; 1998b). These changes closely parallel the final prepartum escalation in the fetal plasma cortisol concentration (Fowden et al. 1998a). Indeed, glucocorticoids are known to decrease the growth rate of the sheep fetus and to suppress placental GLUT expression in humans and mice (Fowden et al. 1996; Derks et al. 1997; Hahn et al. 1999; Jensen et al. 2002). Yet, little is known about the direct effects of fetal cortisol on the glucose supply to the fetus or on the consumption and production of nutrients by the uteroplacental tissues during late gestation. Hence, the aim of this study was to quantify the effects of cortisol on the uteroplacental consumption, production and supply of nutrients in chronically catheterized pregnant ewes, with particular reference to the expression of the placental glucose transporters, GLUT-1 and GLUT-3.
Methods
Animals
A total of 16 Welsh Mountain ewes of known gestational age were used. All the ewes were housed in individual pens and fed concentrates (100 g twice a day; Sheep Nuts #6; H & C Beart Ltd, Kings Lynn, UK) and hay ad libitum. Food but not water was withheld for 18–24 h before surgery. Normal feeding regimes were restored within 24 h of surgery. All procedures were carried out under UK Animals (Scientific Procedures) Act, 1986.
Surgical procedures
Between 117 and 119 days of gestation (term 145 ± 2 days), anaesthesia was induced by bolus injection of thiopentone sodium (20 mg kg−1; Intraval Sodium; Rhone Mérieux, Dublin, Ireland) and, after intubation, was maintained with halothane (1.5–2.0% halothane in 50: 50 O2/N2O; Halothane Vet, Merial Animal Health Ltd, Harlow, UK). Catheters were inserted into the umbilical vein, fetal dorsal aorta and caudal vena cava via the tarsal veins, and into the uterine ovarian vein and the maternal aorta via a femoral artery using the surgical techniques previously described (Comline & Silver, 1972). Antibiotics were given intravenously into the fetus (100 mg ampicillin, Penbritin; SmithKline Beecham Animal Health, Surrey, UK) at the end of surgery and intramuscularly to the mother on the day of surgery and for 3 days there after (9–12 mg i.m. Depocillin; Mycofarm, Cambridge, UK).
Experimental procedures
Blood samples of 2 ml were taken daily throughout the experimental period from the catheterized fetuses to monitor fetal wellbeing, and to determine plasma cortisol. After at least 6 days of postoperative recovery, fetuses were assigned to one of two experimental groups. One group of eight fetuses was infused for 5 days with cortisol (1–3 mg kg−1 day−1 Efcortisol in 0.9% w/v NaCl, 2.4 ml day−1). The dose of cortisol was increased incrementally over the 5 days to mimic the normal prepartum cortisol surge and reached a maximum of 3 mg kg−1 day−1 for the final 24 h. The remaining eight fetuses were infused for 5 days with saline (0.9% NaCl, 2.4 ml day−1) to act as age-matched controls.
Blood samples (2.5 ml) were taken simultaneously from the fetal artey, umbilical vein, uterine vein and maternal artery before commencement of the metabolic study on the final day of infusion. Metabolite uptakes were measured using the Fick principle and steady-state infusion of antipyrine to determine umbilical and uterine blood flows (Meschia et al. 1966). Antipyrine (2.8–4.1 mg min−1 kg−1 in sterile 0.9% saline) was infused at a rate of 0.145 ml min−1 into a fetal vein catheter after an initial priming dose of 3–4 ml. After approximately 2 h when steady state had been established, four sets of blood samples (2.5 ml) were drawn simultaneously from the fetal umbilical vein, fetal artery, maternal artery and maternal vein. Blood samples were drawn at 20 min intervals (120, 140, 160 and 180 min, respectively).
At the conclusion of the experiments (128–130 days), all fetuses were delivered by Caesarian section under terminal anaesthesia (sodium pentobarbitone 200 mg kg−1i.v.). The position of all catheters was verified at delivery and the fetus and placenta were weighed. Placentomes representative of the general population in each animal were frozen in liquid nitrogen.
Biochemical analyses
The simultaneous blood samples were analysed immediately for blood pH, gas tension, packed cell volume (PCV) and O2 content (0.5 ml) using standard Radiometer (Radiometer, Copenhagen, Denmark) and Hemoximeter equipment (ABL 330 Radiometer) that had been calibrated with ovine blood (Owens et al. 1987). Fetal and maternal lactate concentrations were measured using a YSI 2300 Stat Plus (Yellow Springs Instruments, Farnborough, UK). The remainder of the blood samples (2 ml) were added to chilled tubes containing EDTA for subsequent analyses. An aliquot (0.5 ml) of the chilled EDTA-treated blood was immediately deproteinized with zinc sulphate (0.3 m) and barium hydroxide (0.3 m) and the supernatant used for determination of antipyrine and total concentrations of blood glucose. The remaining EDTA sample was centrifuged at 4°C and the plasma stored at –20°C for later use in hormone analysis.
Plasma cortisol concentrations were measured using a radioimmunoassay validated for use with ovine plasma (Robinson et al. 1983). The interassay coefficient of variation (%CV) was 7.3%. The concentration of deproteinized whole blood glucose was determined enzymatically with glucose oxidase (Sigma, UK) using a spectrophotometer (Unicam Helios α, Cambridge, UK). The interassay %CV was 4.4%. Fetal plasma insulin was measured using a commercially available double antibody 125I radioimmunoassay (RIA) using human insulin as standard (Pharmacia Insulin RIA 100, Pharmacia and Upjohn Diagnostics, Milton Keynes, UK). The interassay %CV was 6.2%. Plasma catecholamines concentrations were determined by high pressure liquid chromatography using electrochemical detection (Silver et al. 1987). The limits of sensitivity of the method were 70 pg ml−1 for adrenaline and 50 pg ml−1 for noradrenaline.
Glucose transporter abundance
GLUT-1 and GLUT-3 mRNA expression was measured using in situ hybridization (ISH) on frozen placentome sections from eight study animals (matched by treatment and sex of the fetus). The GLUT-1 and GLUT-3 sense and antisense oligonucleotide probes (45 mer) were based on a specific region of the ovine GLUT-1 and GLUT-3 cDNA sequence as isolated, cloned and sequenced by Currie et al. (1997). Probes used in the current study were synthesized by ‘The Microchemical Facility’ at the Babraham Institute, and were packaged as 1 μg μl−1 antisense and sense oligonucleotides in diethylpyrocarbonate-treated water (DEPC). The oligonucleotide probe sequence for GLUT-1 was: CTGC TGAGCG TCATCTTCAT CCCGCCCTG TTGCAGTGCA TCCTG. The oligonucleotide probe sequence for GLUT-3 was: TCTTCTGCGG ACTCTGCACA GGATTCGTGC CTATGTACAT TGGAG.
Frozen sections (6–8 μm) of placentomes were thaw mounted onto poly-l-lysine-coated slides, fixed in 4% (w/v) paraformaldehyde in PBS at 4°C for 5 min, rinsed in PBS, dehydrated in an ethanol series and stored at 4°C in 95% alcohol. The slides were air-dried before incubation with labelled probes. The oligonucleotide probes were end-labelled with (α-35S)dATP (N.E.N Research Products, UK) using terminal transferase (15–20 000 U ml−1, Pharmacia, UK), to catalyse the repetitive transfer of mononucleotide units from the deoxynucleoside triphosphate to the 3′-OH terminus. A small sample was counted on a β counter to assess specific activity. Approximately 3 × 105 d.p.m. of the probe in 100 μl hybridization buffer (50% (v/v) formamide, 10% (w/v) dextran sulphate, 5 × Denhardts (Sigma), 25 mm sodium phosphate (pH 7.0), 1 mm sodium polyadenylic acid, 4 × SSC, 200 μg ml−1 salmon sperm DNA), were added to each section. Hybridizations were performed at 42°C for 16 h, and sections were then washed for 2 × 1 h at room temperature and 1 h at 55°C in 1 × SSC containing 0.2% (w/v) sodium thiosulphate, followed by a 5 min rinse in 1 × SSC and dehydration in an ethanol series.
After air-drying the slides were placed in an autoradiography cassette along with a sheet of X-ray film (Kodac BioMax, Kodac, UK). The film was allowed to develop for 5 days, after which it was removed and developed in a imaging system (Xograft compact × 4, Tetbury, Gloustershire, UK). X-ray image transfer was undertaken using an adjustable illuminator and a camera attached to compatible software (MCID.M4 Version 3.0; Imagine Research Inc., Canada). This produced digital images of the X-rays that were then evaluated using integrated optical density (IOD) analysis software (Leica QWIN Version 1.0, Cambridge, UK).
Measurements of IOD were made using a selection frame that measured GLUT-1 and GLUT-3 mRNA density relative to an adjacent reference area, which contained no tissue. Non-specific binding (NSB) was measured as IOD relative to reference background using sense oligonucleotide-probed tissue on the same X-ray film. Values for NSB were subtracted from the antisense measurements to give the final IOD for GLUT abundance. At least five final IOD measurements were made on different areas of each probed section of tissue. A total of six sections from each placentome were probed and quantified for GLUT-1 and GLUT-3. Placentomes from four different animals in each treatment group were analysed for GLUT expression.
Calculations
All calculations were made using equations derived for steady-state kinetics. Umbilical and uterine blood flow was measured using the antipyrine steady-state diffusion technique (Meschia et al. 1966) and calculated using the following equations:
 |
(1) |
 |
(2) |
 |
(3) |
 |
(4) |
 |
(5) |
 |
(6) |
 |
(7) |
The percentage distributions of the net uterine uptake or uteroplacental production of substrate between fetal and either uteroplacental or maternal tissues were calculated as follows:
For glucose and oxygen:
| (8) |
 |
(9) |
For lactate
 |
(10) |
 |
(11) |
The sum of the percentage distribution to the fetal and either the uteroplacental or maternal tissues is therefore 100%.
Statistical analyses
Data were analysed using Microsoft Excel (Microsoft Corp., Seattle, USA) and SigmaStat (v2.0 SPSS, Chicago, USA). Results are presented as mean ± standard error of the mean (s.e.m.) throughout. Groups were compared using unpaired Student's t test if parametric distributions applied. Mann–Whitney rank sum tests were used for non-parametric distributions. For all analyses, statistical significance was accepted when P < 0.05.
Results
Plasma hormone levels and morphometric measurements
Cortisol infusion for 5 days increased fetal cortisol levels to values that were similar to those seen close to term (Fowden et al. 1998a). On the 5th day of infusion, the mean plasma cortisol concentration was significantly higher in the cortisol-infused fetuses than in the saline-infused controls (P < 0.001; Table 1). The corresponding maternal concentrations of plasma cortisol in the saline- and cortisol-infused animals were 15.3 ± 4.7 ng ml−1 (n = 7) and 14.7 ± 4.2 ng ml−1 (n = 8), respectively. Fetal arterial concentrations of plasma insulin, noradrenaline and adrenaline were measured on the day of the metabolic study and were not significantly different between treatments (Table 1). There were no significant differences in body weight, total placental weight, average placentome weight, number of placentomes or in the fetal-to-placental weight ratio between cortisol- and saline-infused fetuses (Table 1, P > 0.05 all cases).
Table 1.
Mean (± s.e.m.) values of fetal arterial concentrations of plasma cortisol insulin, noradrenaline and adrenaline on the day of the metabolic study and of the morphometric measurements of the saline- (n = 8) and cortisol-infused (n = 8) fetuses at delivery at the end of the metabolic study
| Saline infused | Cortisol infused | ||
|---|---|---|---|
| Hormone concentrations | Cortisol (ng ml−1) | 15.7 ± 1.2 | 61.7 ± 5.3* |
| Insulin (μU ml−1) | 7.0 ± 0.7 | 5.7 ± 1.6 | |
| Noradrenaline (pg ml−1) | 514 ± 105 | 583 ± 174 | |
| Adrenaline (pg ml−1) | 126 ± 12 | 163 ± 21 | |
| Morphometric measurements | Fetal weight (g) | 3033 ± 146 | 2968 ± 88 |
| Total placentome weight (g) | 332 ± 29 | 309 ± 20 | |
| Total placentome number | 72 ± 4 | 72 ± 6 | |
| Average placentome weight (g) | 4.8 ± 0.6 | 4.6 ± 0.5 | |
| Fetal:placental wt ratio (g fetus (g placenta)−1) | 9.5 ± 0.7 | 9.8 ± 0.6 |
Significantly different from the value in the saline-infused fetuses (P < 0.01, t test).
Metabolite concentrations, and acid–base and blood gas status
Cortisol infusion had no effect on the arterial pH, PO2, PCO2, haemoglobin concentration, or percentage O2 saturation in either fetal or maternal blood (Table 2). Mean values of these variables did not change with cortisol or saline infusion and were similar in the two groups on the 5th day of treatment (Table 2). There were also no significant changes in fetal and maternal concentrations of plasma lactate during treatment in either group (Table 2). In contrast, fetal arterial concentrations of plasma glucose rose in response to infusion of cortisol but not saline (Table 2): mean values were therefore significantly higher in cortisol- than saline-treated fetuses on the 5th day of infusion (Table 2). Maternal arterial concentrations of plasma glucose were unaffected by treatment in both groups of animals (Table 2). Hence, the transplacental gradient in plasma glucose concentration was significantly less in animals treated with cortisol (2.26 ± 0.09 mmol l−1, n = 8) than those receiving saline (2.74 ± 0.13 mmol l−1, n = 8, P < 0.02). There were no significant differences in the fetal or maternal metabolite concentrations, acid–base status or in blood gas tensions between cortisol- and saline-infused animals before treatment began on Day 0 (Table 2). The transplacental plasma glucose concentration gradient was also similar in the cortisol- (2.68 ± 0.13 mmol l−1, n = 8) and saline-infused animals (2.58 ± 0.14 mmol l−1, n = 8) before treatment began.
Table 2.
Mean (± s.e.m.) values of pH, PO2, PCO2, haemoglobin (Hb) and percentage saturation of the haemoglobin in fetal and maternal blood and of arterial concentrations of glucose and lactate in fetal and maternal plasma before (Day 0) and on the 5th day (Day 5) of infusion of either saline (n = 8) or cortisol (n = 8) into the fetus
| Fetus | Mother | ||||
|---|---|---|---|---|---|
| Day 0 | Day 5 | Day 0 | Day 5 | ||
| pH | Saline | 7.35 ± 0.01 | 7.36 ± 0.01 | 7.51 ± 0.01 | 7.51 ± 0.01 |
| Cortisol | 7.35 ± 0.01 | 7.36 ± 0.01 | 7.51 ± 0.01 | 7.48 ± 0.01 | |
| PO2 (mmHg) | Saline | 22.0 ± 1.7 | 19.0 ± 0.9 | 97.0 ± 1.2 | 95.0 ± 1.1 |
| Cortisol | 21.0 ± 0.7 | 20.0 ± 1.0 | 93.0 ± 1.8 | 98.0 ± 2.4 | |
| PCO2 (mmHg) | Saline | 53 ± 2.5 | 52 ± 1.3 | 33.0 ± 2.0 | 34.0 ± 1.1 |
| Cortisol | 52 ± 0.7 | 52 ± 0.8 | 32.0 ± 0.5 | 34.0 ± 0.8 | |
| Hb (g dl−1) | Saline | 9.5 ± 0.6 | 9.9 ± 0.6 | 10.4 ± 0.6 | 9.6 ± 0.7 |
| Cortisol | 9.1 ± 0.2 | 9.0 ± 0.7 | 9.3 ± 0.4 | 8.7 ± 0.4 | |
| % sat Hb | Saline | 62.0 ± 3.3 | 58.0 ± 2.8 | 94.0 ± 0.8 | 94.0 ± 1.0 |
| Cortisol | 57.0 ± 1.5 | 57.0 ± 3.2 | 96.0 ± 1.2 | 97.0 ± 1.2 | |
| Plasma glucose (mmol l−1) | Saline | 0.85 ± 0.06 | 0.94 ± 0.06 | 3.38 ± 0.15 | 3.70 ± 0.13 |
| Cortisol | 0.90 ± 0.07 | 1.18 ± 0.08*† | 3.43 ± 0.11 | 3.41 ± 0.09 | |
| Plasma lactate (mmol l−1) | Saline | 1.30 ± 0.15 | 1.36 ± 0.16 | 0.40 ± 0.06 | 0.52 ± 0.09 |
| Cortisol | 1.28 ± 0.16 | 1.43 ± 0.16 | 0.40 ± 0.08 | 0.39 ± 0.07 | |
Significant increase during treatment (P < 0.01, paired t test).
Significantly different from value in saline-infused animals (P < 0.01, t test).
During the metabolic study on the 5th day of infusion, fetal, but not maternal, arterial concentrations of blood glucose were significantly higher in cortisol- than saline-infused animals (Table 3). There were no significant differences in the fetal or maternal concentrations of blood lactate or in blood O2 content between the two treatment groups during the metabolic study (Table 3).
Table 3.
Mean (± s.e.m.) values of umbilical and uterine blood flow, fetal and maternal arterial metabolite concentrations, the absolute rates of umbilical and uterine uptakes or outputs of substrates and of the absolute rates of uteroplacental consumption or production of substrates in animals in which the fetus was infused with either saline (n = 8) or cortisol (n = 8) for 5 days before the measurements were made
| Saline infused | Cortisol infused | P | ||
|---|---|---|---|---|
| Umbilical blood flow (ml min−1) | 619 ± 33 | 691 ± 46 | n.s. | |
| Uterine blood flow (ml min−1) | 1344 ± 75 | 1546 ± 135 | n.s. | |
| Glucose | Umbilical venous blood glucose (mmol l−1) | 1.09 ± 0.05 | 1.28 ± 0.09 | n.s. |
| Fetal arterial blood glucose (mmol l−1) | 0.93 ± 0.04 | 1.17 ± 0.09 | 10.024 | |
| Maternal arterial blood glucose (mmol l−1) | 2.69 ± 0.13 | 2.77 ± 0.13 | n.s. | |
| Uterine venous blood glucose (mmol l−1) | 2.55 ± 0.14 | 2.63 ± 0.13 | n.s. | |
| Umbilical glucose uptake (μmol min−1) | 99.1 ± 5.9 | 72.9 ± 4.6 | 0.003 | |
| Uterine glucose uptake (μmol min−1) | 177.3 ± 8.6 | 207.4 ± 26.4 | n.s. | |
| Uteroplacental glucose consumption (μmol min−1) | 78.3 ± 10.5 | 134.5 ± 24.3 | 0.050 | |
| Lactate | Umbilical venous blood lactate (mmol l−1) | 1.29 ± 0.13 | 1.36 ± 0.15 | n.s. |
| Fetal arterial blood lactate (mmol l−1) | 1.21 ± 0.13 | 1.31 ± 0.12 | n.s. | |
| Maternal arterial blood lactate (mmol 1−1) | 0.49 ± 0.10 | 0.39 ± 0.08 | n.s. | |
| Uterine venous blood lactate (mmol l−1) | 0.56 ± 0.09 | 0.44 ± 0.11 | n.s. | |
| Umbilical lactate uptake (μmol min−1) | 49.1 ± 2.2 | 37.9 ± 3.8 | 0.022 | |
| Uterine lactate output (μmol min−1) | 41.1 ± 4.0 | 34.1 ± 4.1 | n.s. | |
| Uteroplacental lactate production (μmol min−1) | 90.2 ± 4.8 | 72.0 ± 6.3 | 0.037 | |
| Oxygen | Umbilical venous blood oxygen content (mmol l−1) | 4.77 ± 0.19 | 4.84 ± 0.31 | n.s. |
| Fetal arterial oxygen content (mmol l−1) | 3.41 ± 0.27 | 3.30 ± 0.15 | n.s. | |
| Maternal arterial oxygen content (mmol l−1) | 5.59 ± 0.32 | 5.25 ± 0.41 | n.s. | |
| Uterine venous blood oxygen content (mmol l−1) | 3.00 ± 0.21 | 2.89 ± 0.27 | n.s. | |
| Umbilical oxygen uptake (μmol min−1) | 841 ± 31 | 951 ± 54 | n.s. | |
| Uterine oxygen uptake (μmol min−1) | 1602 ± 97 | 1632 ± 169 | n.s. | |
| Uteroplacental oxygen consumption (μmol min−1) | 794 ± 80 | 692 ± 164 | n.s. |
P = probability using unpaired Student's t test. n.s. = not significant, P > 0.05.
Metabolic rates
On the 5th day of treatment, substrate uptakes from the uterine circulation and substrate delivery to the umbilical circulation were calculated using the Fick principle and the respective uterine and umbilical blood flows (see eqns (3)–(5)). Cortisol infusion into the fetus had no apparent effect on either the uterine or umbilical blood flow: mean values for each of these flows were similar in the saline- and cortisol-infused animals (Table 3).
Umbilical substrate uptake
Cortisol infusion appeared to have no effect on the umbilical uptake of O2; mean rates were similar in the saline- and cortisol-infused fetuses, irrespective of whether values were expressed in absolute terms (Table 3) or per kilogram fetal body weight (Fig. 1A). In contrast, the rates of umbilical uptake of glucose and lactate were significantly lower in cortisol- than saline-infused fetuses both as absolute values (Table 3) and when calculated on a weight-specific basis (Fig. 1A). When all the data were combined irrespective of treatment, there was a significant inverse correlation between the fetal plasma cortisol concentration at the time of the metabolic study and the rate of umbilical uptake of glucose (Fig. 2A), but not lactate (r =–0.419, n = 16, P > 0.05).
Figure 1. Rates of (A) umbilical uptake and (B) uteroplacental consumption or production of glucose (i) lactate (ii) and oxygen(iii).
Rates were expressed as weight-specific values (μmol min−1 kg−1) per kg fetal weight (A) or kg placenta (defined as total placentome mass) (B). Fetuses were infused with either saline (n = 8) or cortisol (n = 8). Significant differences are: *P < 0.02; †P < 0.05; ‡P < 0.039 (Student's unpaired t test).
Figure 2. Relationships between the fetal plasma cortisol concentration (ng ml−1) and either umbilical glucose uptake (A) or uteroplacental glucose consumption (B).
Uptake expressed in μmol min−1 (kg fetus)−1; y =–0.162x+ 35.374, n = 16, r = 0.52, P < 0.037); consumption expressed in μmol min−1 (kg placenta)−1; y = 4.232x+ 174.520, n = 16, r = 0.60, P < 0.014). Fetuses were infused with either saline (•, n = 8) or cortisol (○, n = 8).
Uterine substrate uptake
The mean rates of uptake of O2 and glucose from the uterine circulation were not significantly different in the saline- and cortisol-infused animals (Table 3). There was a significant output of lactate from the uteroplacental tissues into the uterine circulation in both groups of animals, which did not differ in absolute rate with treatment (Table 3).
Uteroplacental substrate consumption and production
The rates of uteroplacental consumption of O2 and glucose were calculated as the difference between the uterine and umbilical uptakes (eqn (6), Methods), while the uteroplacental production of lactate was estimated as the sum of the rates of umbilical lactate uptake and uteroplacental lactate output into the uterine circulation (eqn (5), Methods). The uteroplacental consumption of glucose, but not O2, was significantly higher in cortisol- than saline-infused animals, both in absolute terms (Table 3) and when expressed per kilogram placenta (total placentome weight, Fig. 1B). Absolute rates of uteroplacental lactate production were significantly less in cortisol- than saline-infused animals (Table 3). However, when production was expressed per kilogram placental weight, this difference was no longer significant (Fig. 1B(ii), P > 0.05). When all data were combined, there was a significant positive correlation between the fetal plasma cortisol concentration at the time of the metabolic study and the rate of uteroplacental glucose consumption (Fig. 2B). No significant correlations were observed between fetal plasma cortisol and the rates of O2 consumption and lactate production by the uteroplacental tissues expressed either as absolute or weight-specific values (P > 0.05, all cases)
Nutrient partitioning
The distributions of the glucose and O2 taken up from the uterine circulation between the fetal and uteroplacental tissues in the cortisol- and saline-infused animals and are shown in Fig. 3 together with the partitioning of uteroplacental lactate production between the fetus and mother in the two treatment groups (eqns (8)–(11), Methods). The percentage distribution of uterine glucose uptake to the fetus was significantly less in cortisol- than saline-infused animals (Fig. 3). There were no significant differences in the partitioning of uterine O2 uptake or uteroplacental lactate production between the two groups of animals (Fig. 3).
Figure 3. The effects of cortisol on the distribution of nutrients.
The mean (± s.e.m.) percentage distribution of the uterine uptake of glucose and oxygen between fetal (black columns) and uteroplacental tissues (grey columns) and of uteroplacental lactate production between fetal (black columns) and maternal tissues (grey columns) in saline- (n = 8) and cortisol-infused fetuses (n = 8). *P < 0.008, compared to saline-infused controls (Student's unpaired t test). 100%= total uterine uptake or uteroplacental production, irrespective of absolute value.
Placental GLUT-1 and GLUT-3 mRNA expression
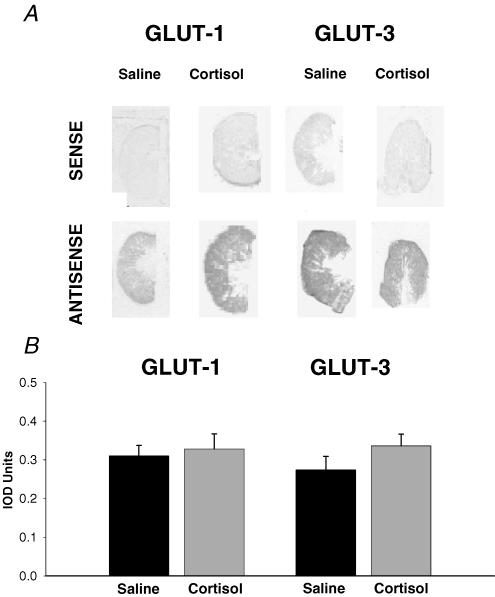
Using in situ hybridization and X-ray densitometry, GLUT-1 and GLUT-3 mRNA were detected readily in all placentomes studied, and were evenly distributed throughout the fetal and maternal tissue in the placentomes (Fig. 4A). Cortisol had no apparent effect on the placental abundance of GLUT-1 or GLUT-3 mRNA; mean values of GLUT-1 and GLUT-3 mRNA abundance in the saline-infused fetuses were not significantly different from the corresponding values in the cortisol-infused animals (P > 0.05 all cases, Fig. 4B).
Figure 4. The effect of cortisol on placental GLUT expression.
In situ hybridization analysis of GLUT-1 and GLUT-3 mRNA expression shown as typical sections through placentomes from cortisol- and saline-infused fetuses visualized with the sense and antisense oligionucleotide probes (A) and mean (± s.e.m.) values of integrated optical density (IOD) units of placentome sections from saline- (n = 4) and cortisol-infused fetuses (n = 4) (B).
Discussion
The results demonstrate that uteroplacental metabolism is regulated by cortisol in pregnant sheep during late gestation. Cortisol infusion reduced the delivery of glucose and lactate from the uteroplacental tissues to the umbilical circulation both in absolute terms and when expressed per kilogram fetal body weight. It had no effect on the uteroplacental delivery of oxygen. The fall in umbilical glucose uptake in the cortisol-infused fetuses was accompanied by an elevated fetal glucose concentration, a lower transplacental glucose concentration gradient and an increased uteroplacental consumption of glucose compared to the saline-infused controls, but was not associated with any apparent changes in placental GLUT abundance. Uteroplacental glucose consumption was significantly increased in the cortisol-infused fetuses, both in absolute terms and when expressed per kilogram placental weight. In contrast, uteroplacental lactate production was reduced in absolute terms, but not when expressed per gram placental weight. Cortisol infusion had no effect on the rate of uteroplacental consumption and uterine uptake of O2. Nor did it affect the uterine uptake and output of glucose and lactate, respectively. Cortisol infusion into the fetus therefore appears to alter both uteroplacental substrate utilization and uteroplacental delivery of nutrients to the fetus. Indeed, the reciprocal correlations observed between fetal plasma cortisol and the uteroplacental consumption and fetal delivery of glucose suggest that cortisol may be a physiological regulator of uteroplacental glucose handling over the normal range of cortisol concentrations found in the sheep fetus during late gestation.
Glucose metabolism
The reduced rate of umbilical glucose uptake measured after 5 days of fetal cortisol infusion in the present study is the first unequivocal demonstration that cortisol regulates the uteroplacental delivery of glucose to the fetus. Previous studies have shown no effect of short-term (4 h) infusion of cortisol on umbilical glucose uptake by fetal sheep at 124 days of gestation (Milley, 1996). More long-term treatment (24 h) of sheep fetuses with the synthetic glucocorticoid dexamethasone provided indirect evidence of a glucocorticoid-mediated reduction in placental glucose delivery by using the modified Widdas equation to estimate umbilical glucose uptake from the fetal and maternal glucose concentrations (see Widdas, 1952; Hay et al. 1990). The rates of umbilical glucose uptake measured in the saline-infused animals in the current study were similar to those reported previously for fetuses at the same stage of gestation in this and other laboratories (Owens et al. 1987; Hay et al. 1990; Jensen et al. 1999). In addition, the rates of umbilical glucose uptake in the cortisol-infused fetuses were within the range of values observed in older control fetuses with high endogenous cortisol levels close to term (Fowden et al. 1998b). Umbilical glucose uptake therefore appears to be reduced at high concentrations of cortisol, whether of exogenous or endogenous origin.
The mechanisms by which cortisol reduced the umbilical glucose uptake remain unclear, but may have involved changes in the transplacental glucose concentration gradient, the placental glucose transfer capacity and in the rate of uteroplacental glucose consumption. Previous studies of fetal glucocorticoid overexposure induced by maternal administration of synthetic glucocorticoids have shown a decrease in the transplacental glucose concentration gradient which is associated with increases in both fetal and maternal glycaemia during late gestation (Barbera et al. 1997; Bennett et al. 1999). In the present study, the transplacental glucose concentration gradient was reduced by 15–20% in response to fetal cortisol infusion, primarily as a result of a rise in the fetal arterial plasma glucose concentration. This suggests that, in the current study, the fall in the transplacental gradient is predominantly of fetal origin, and may arise either from decreased glucose utilization and/or a rise in glucose production by the fetus. In late gestation, the rise in endogenous cortisol level is not accompanied by any change in the rate of fetal glucose utilization (Fowden et al. 1998b). Cortisol also had no effect on the fetal concentration of insulin, a major factor regulating glucose utilization by the fetus (Fowden et al. 1998b). The rise in fetal glycaemia in response to cortisol is therefore unlikely to be due to a fall in the fetal glucose utilization. An increase in fetal glucose production is a more likely explanation as cortisol is known to increase the glucogenic capacity of the fetus, and has been shown to activate hepatic gluconeogenesis in sheep fetuses close to term (Townsend et al. 1989; Fowden et al. 1993). An increase in endogenous glucose production also occurs in normal fetuses with high cortisol levels close to term (Fowden et al. 1998b). Since catecholamines are known to stimulate hepatic glucose production in fetal sheep (Apatu & Barnes, 1991), the tendency for higher total catecholamine concentrations during cortisol infusion may favour endogenous glucose production in these fetuses.
Whatever the cause of the rise in fetal glucose level, the increase in fetal glycaemia relative to maternal values will decrease the driving force for facilitated diffusion of glucose across the placenta. Using the modified Widdas equation (Widdas, 1952; Hay et al. 1990), this decrease in transplacental glucose concentration gradient would account for about half the reduction in the rate of umbilical glucose uptake observed during cortisol infusion in the present study. However, the modified Widdas equation predicts an umbilical glucose uptake of about 28–30 μmol min−1 kg−1 for the glucose levels observed in the current cohort of cortisol-infused animals, which is higher than the value actually observed (24 μmol min−1 kg−1, Fig. 1Ai). This suggests that other factors such as changes in the placental glucose transfer capacity or uteroplacental glucose consumption contribute to the reduced umbilical glucose uptake observed during cortisol infusion.
The capacity for placental glucose transfer at specific glucose concentration gradients is determined by the placental area for exchange, the number of transporters and by the rate of placental glucose consumption. All these factors contribute to the increase in placental glucose transfer capacity that occurs with increasing gestational age in pregnant sheep (Molina et al. 1991). Previous studies have also shown down-regulation of GLUT-1 expression in response to fetal hypoglycaemia in ovine placentomes and suppression of both GLUT-1 and GLUT-3 by glucocorticoids in human and mouse placentae (Das et al. 1998; Hahn et al. 1999). In the current study, there were no apparent differences in the abundance or localization of GLUT-1 and GLUT-3 mRNA in placentomes following cortisol infusion. In situ hybridization is widely accepted as a sufficiently robust and accurate method for quantifying gene expression and has the added advantage of establishing the cellular localization of the specific mRNAs (Gadd et al. 2000; Mitchell et al. 2002). Although further studies are required to determine whether cortisol affects expression of GLUT proteins in ovine placentomes, the current observations suggest that changes in transporter abundance are unlikely to make a major contribution to the cortisol-induced decrease in umbilical glucose uptake.
The rate of uteroplacental glucose consumption was 80% higher in cortisol- than saline-infused animals. Since there was no significant difference in uterine glucose uptake with treatment, the fractional distribution of the maternally derived glucose between the fetal and uteroplacental tissues was altered by cortisol infusion, with significantly more of the uterine glucose uptake being used by the uteroplacental tissues in the cortisol-infused animals. In normal animals, experimental manipulation of the fetal and maternal glucose levels has shown that uteroplacental glucose consumption is a function of the fetal glucose concentration, and is virtually independent of the maternal glucose concentration (Hay et al. 1990). The rise in the fetal glucose level observed in response to cortisol infusion would therefore be expected to increase uteroplacental glucose consumption (Hay, 1995). Using the equation relating uteroplacental glucose consumption to fetal glycaemia derived by Hay et al. (1990), the current cortisol-induced rise in fetal glycaemia would be predicted to increase uteroplacental glucose consumption by 25–50 μmol min−1, which is within the range of values actually observed (Table 3). The rise in uteroplacental glucose consumption may therefore be the consequence, rather than the cause, of the reduced umbilical glucose uptake.
Lactate metabolism
Much less is known about the regulation of the production and transfer of lactate by the ovine uteroplacental tissues. Lactate production by these tissues normally increases with increasing gestational age towards term (Aldoretta & Hay, 1999). This ontogenic change is accompanied by a switch in the distribution of uteroplacental lactate production, from delivery preferentially into the uterine circulation at midgestation, to output predominantly into the umbilical circulation close to term (Sparks et al. 1983). The current rate of uteroplacental lactate production and the distribution between the maternal and fetal tissues in the saline-infused animals were similar to those reported previously for sheep fetuses at the same stage of gestation (Sparks et al. 1982). Cortisol infusion decreased both the rates of umbilical lactate uptake and absolute uteroplacental lactate production, but not the weight-specific rate of uteroplacental lactate production. There was also no change in the fetal lactate concentration, or in the distribution of uteroplacental lactate production between the fetal and maternal tissues.
In normal animals, the uteroplacental tissues provide about one-third of the lactate used by the fetus (Sparks et al. 1982). Since fetal lactate levels were unaffected by cortisol infusion, lactate production by the fetal tissues may rise to compensate for the decreased uteroplacental supply. This suggestion is consistent with the rise in fetal glucose levels observed during cortisol infusion, as previous studies have shown that fetal lactate production is directly related to glucose availability in the fetal circulation (Sparks et al. 1983). In normal well-fed animals, uteroplacental lactate production increases directly with the rate of uteroplacental glucose consumption (Aldoretta & Hay, 1999). Hence, increased rather than decreased uteroplacental lactate production would have been expected during cortisol infusion. Previous studies have shown that fetal insulin-like growth factor-I (IGF-I) infusion decreases umbilical uptake and uteroplacental production of lactate in association with a tendency for increased uteroplacental glucose consumption (Harding et al. 1994; Jensen et al. 1999). Although fetal cortisol infusion has no effect on fetal IGF-I concentrations (Li et al. 1996), it does induce tissue-specific changes in IGF-I gene expression (Li et al. 1996; 2002). Cortisol may therefore reduce placental lactate production through up-regulation of placental IGF-I expression, independently of its effects on uteroplacental glucose consumption.
Oxygen metabolism
Cortisol infusion had no effects on the umbilical uptake, uterine uptake or uteroplacental consumption of O2. Nor did it affect the distribution of uterine oxygen uptake between the uteroplacental and fetal tissues. All rates of uteroplacental consumption, uterine and umbilical uptake of O2 measured in the current study were within the range of values reported previously for normal sheep fetuses at the same stage of gestation in this and other laboratories (Harding et al. 1994; Jensen et al. 1999). Since O2 uptake is flow mediated, and dependent on the diffusion distance across the placenta, the current observations suggest that cortisol had little effect on the vascular architecture or diffusion distance within the placentomes. In contrast, the absence of cortisol-induced changes in fetal and uteroplacental O2 consumption despite the concomitant alterations in carbohydrate metabolism indicates that cortisol may alter relative use of substrates for oxidative metabolism by both the fetal and uteroplacental tissues. In the fetus, less placentally derived carbohydrate is available for oxidation and, hence, oxidation must be maintained either by other carbohydrate sources (e.g. glucogenesis in the fetal liver and kidney), or by use of non-carbohydrate substrates such as amino acids or lipid. This will reduce the availability of substrates for tissue accretion and may explain, in part, the decreased growth rate observed in cortisol-infused fetuses (Fowden et al. 1996). In the uteroplacental tissues, more glucose is available during cortisol infusion, although the fate of these extra glucose molecules remains unknown. In normal animals, increasing the glucose supply to the uteroplacental tissues increases the oxidative use of glucose, but even at high glucose availability, only 34% of the uteroplacental glucose consumption is oxidized by these tissues (Aldoretta & Hay, 1999). A significant amount of the glucose taken up by the uteroplacental tissues is therefore used to synthesize other molecules such as lactate, fructose, proteins, lipids and amino acids (Fowden, 1993). In addition, since uteroplacental O2 consumption was unaffected by cortisol, increased oxidative use of glucose will increase the availability of the alternate oxidative substrates, e.g. lipids and amino acids. Taken together, these changes in uteroplacental substrate availability are likely to have major effects on processes such as tissue differentiation and hormone synthesis that occur in the placenta when fetal cortisol levels are raised (Challis et al. 2000).
Conclusions
The current results indicate that cortisol affects many aspects of uteroplacental metabolism. It influences the consumption, production, fetal delivery and oxidative use of glucose and lactate. However, the extent to which its actions are direct, or mediated via changes in fetal metabolism remain unclear. Cortisol appeared to have little direct effect on the placental glucose transfer capacity, as there were no apparent changes in mRNA abundance for the placental glucose transporters GLUT-1 and GLUT-3. The effects of cortisol on the rate of umbilical uptake and uteroplacental consumption of glucose appeared to be indirect, and mediated through the rise in the fetal, relative to maternal glucose levels. The primary effect of cortisol may therefore have been to activate fetal glucose production (Townsend et al. 1989; Fowden et al. 1993; 1998b). In turn, the increase in fetal glycaemia reduced the transplacental glucose concentration gradient and increased uteroplacental glucose consumption. Hence, less glucose was transferred from the uteroplacental tissues to the fetus. These changes in fetal, relative to maternal glycaemia, and in uteroplacental glucose handling may have led to the observed alterations in feto-placental lactate metabolism. Further studies measuring the fetal rates of utilization and production of glucose during cortisol infusion are required before the mechanisms by which cortisol acts on feto-placental metabolism can be fully elucidated.
Acknowledgments
The authors wish to acknowledge Mr Paul Hughes for his technical assistance during surgery and Mrs Sue Nicholls for the routine care of the animals used in this study. This work was supported by the Avrith Research studentship (J.W.W.).
References
- Aldoretta PW, Hay WW. Effect of glucose supply on ovine uteroplacental glucose metabolism. Am J Physiol. 1999;277(4):R947–R958. doi: 10.1152/ajpregu.1999.277.4.R947. [DOI] [PubMed] [Google Scholar]
- Apatu RS, Barnes RJ. Release of glucose from the liver of fetal and postnatal sheep by portal vein infusion of catecholamines or glucagon. J Physiol. 1991;436:449–468. doi: 10.1113/jphysiol.1991.sp018560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera A, Wilkening RB, Teng C, Battaglia FC, Meschia G. Metabolic alterations in the fetal heaptic and umbilical circulations during glucocorticoid-induced parturition in sheep. Ped Res. 1997;41:242–248. doi: 10.1203/00006450-199702000-00015. [DOI] [PubMed] [Google Scholar]
- Battaglia FC. An Introduction to Fetal Physiology. Orlando: Academic Press; 1986. [Google Scholar]
- Bell AW, Ehrhardt RA. Placental Regulation of Nutrient Partitioning During Pregnancy. In: Bray GA, Hansel W, Ryan DH, editors. Nutrition & Reproduction. Baton Rouge: Louisiana. State University Press; 1998. pp. 229–254. [Google Scholar]
- Bennett L, Kozuma S, Mcgarrigle HHG, Hanson MA. Temporal changes in fetal cardiovascular, behavioural, metabolic and endocrine responses to maternally administered dexamethasone in the late gestation fetal sheep. Br J Obstr Gyn. 1999;106:331–339. doi: 10.1111/j.1471-0528.1999.tb08270.x. [DOI] [PubMed] [Google Scholar]
- Carter BS, Moores RR, Battaglia FC. Placental transport and fetal and placental metabolism of amino acids. J Nutr Biochem. 1991;2:4–13. [Google Scholar]
- Carver TD, Hay WW. Uteroplacental carbon substrate metabolism and O2 consumption after long-term hypoglycemia in pregnant sheep. Am J Physiol. 1995;269:E299–E308. doi: 10.1152/ajpendo.1995.269.2.E299. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthew SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Comline RS, Silver M. The composition of foetal and maternal blood during parturition in the ewe. J Physiol. 1972;222:233–256. doi: 10.1113/jphysiol.1972.sp009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie MJ, Bassett NS, Gluckman PD. Ovine glucose transporter-1 and -3: cDNA partial sequences and developmental gene expression in the placenta. Placenta. 1997;18:393–401. doi: 10.1016/s0143-4004(97)80039-2. [DOI] [PubMed] [Google Scholar]
- Das UG, Sadiq HF, Soares MJ, Hay WW, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol. 1998;274:R339–R347. doi: 10.1152/ajpregu.1998.274.2.R339. [DOI] [PubMed] [Google Scholar]
- Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GH, Padbury JF, Nathanielsz PW. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. J Physiol. 1997;499:217–226. doi: 10.1113/jphysiol.1997.sp021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt RA, Bell AW. Developmental increases in glucose transporter concentration in the sheep placenta. Am J Physiol. 1997;273:R1132–R1141. doi: 10.1152/ajpregu.1997.273.3.R1132. [DOI] [PubMed] [Google Scholar]
- Fowden AL. Fetal metabolism and energy balance. In: Harding R, Thorburn GD, editors. Textbook of Fetal Physiology. Melbourne: Oxford Medical Publications; 1993. pp. 70–82. [Google Scholar]
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance. Proc Nutr Soc. 1998a;57:113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Mijovic J, Silver M. The effects of cortisol on hepatic and renal gluconeogenic enzyme activities in the sheep fetus during late gestation. J Endo. 1993;137:213–222. doi: 10.1677/joe.0.1370213. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Mundy L, Silver M. Developmental regulation of glucogenesis in the sheep fetus during late gestation. J Physiol. 1998b;508:937–947. doi: 10.1111/j.1469-7793.1998.937bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Szemere J, Hughes P, Gilmour RS, Forhead AJ. The effects of cortisol on the growth rate of the sheep fetus during late gestation. J Endo. 1996;151:97–105. doi: 10.1677/joe.0.1510097. [DOI] [PubMed] [Google Scholar]
- Gadd TS, Osgersby JC, Wathes DC. Regulation of insulin-like growth factor binding protein-6 expression in the reproductive tract throughout the estrous cycle and during the development of the placenta in the ewe. Biol Reprod. 2000;67:1756–1762. doi: 10.1095/biolreprod67.6.1756. [DOI] [PubMed] [Google Scholar]
- Hahn T, Barth S, Graf R, Engelmann M, Beslagic D, Reul JM, Holsboer F, Dohr G, Desoye G. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endo Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- Harding JE, Liu L, Evans PC, Gluckman PD. Insulin-like growth factor 1 alters feto-placental protein and carbohydrate metabolism in fetal sheep. Endo. 1994;134:1509–1514. doi: 10.1210/endo.134.3.8119193. [DOI] [PubMed] [Google Scholar]
- Hay WW. Regulation of placental metabolism by glucose supply. Reprod Fert Dev. 1995;7:365–375. doi: 10.1071/rd9950365. [DOI] [PubMed] [Google Scholar]
- Hay WW, Molina RA, DiGiacomo JE, Meschia G. Model of placental glucose consumption and glucose transfer. Am J Physiol. 1990;258:R569–R577. doi: 10.1152/ajpregu.1990.258.3.R569. [DOI] [PubMed] [Google Scholar]
- Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late-gestation fetal sheep. J Endo. 2002;174:27–36. doi: 10.1677/joe.0.1740027. [DOI] [PubMed] [Google Scholar]
- Jensen EC, Harding JE, Bauer MK, Gluckman PD. Metabolic effects of IGF-I in the growth retarded fetal sheep. J Endo. 1999;161:485–494. doi: 10.1677/joe.0.1610485. [DOI] [PubMed] [Google Scholar]
- Li J, Forhead AJ, Dauncey MJ, Gilmour RS, Fowden AL. Control of growth hormone receptor and insulin-like growth factor-I expression by cortisol in ovine fetal skeletal muscle. J Physiol. 2002;541:581–589. doi: 10.1113/jphysiol.2002.016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Owens JA, Owens PC, Saunders JC, Fowden AL, Gilmour RS. The ontogeny of hepatic growth hormone receptor and insulin-like growth factor I gene expression in the sheep fetus during late gestation: developmental regulation by cortisol. Endo. 1996;137:1650–1657. doi: 10.1210/endo.137.5.8612497. [DOI] [PubMed] [Google Scholar]
- Meschia G, Cotter JR, Makowski EL, Barron DH. Simultaneous measurement of uterine and umbilical blood flows and oxygen uptakes. Quart J Exp Physiol. 1966;52:1–18. [Google Scholar]
- Milley JR. Fetal substrate uptake during increased ovine fetal cortisol concentration. Am J Physiol. 1996;271:E186–E191. doi: 10.1152/ajpendo.1996.271.1.E186. [DOI] [PubMed] [Google Scholar]
- Mitchell SE, Robinson JJ, King ME, McKelvey WAC, Williams LM. Interleukin 8 in the cervix of non-pregnant ewes. Reprod. 2002;124:409–416. [PubMed] [Google Scholar]
- Molina R, Meschia G, Battagli FC, Hay WW. Gestational maturation of placental glucose transfer capacity in sheep. Am J Physiol. 1991;261:R697–R704. doi: 10.1152/ajpregu.1991.261.3.R697. [DOI] [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Effect of restriction of placental growth on oxygen delivery to and consumption by the pregnant uterus and fetus. J Dev Physiol. 1987;9:137–150. [PubMed] [Google Scholar]
- Robinson PM, Comline RS, Fowden AL, Silver M. Adrenal cortex of fetal lamb: changes after hypophysectomy and effects of Synacthen on cytoarchitecture and secretory activity. Quart J Exp Physiol. 1983;68:15–27. doi: 10.1113/expphysiol.1983.sp002697. [DOI] [PubMed] [Google Scholar]
- Silver M, Fowden AL, Knox J, Ousey JC, Franco R, Rossdale PD. Sympathoadrenal and other responses to hypoglycaemia in the young foal. J Reprod Fert Suppl. 1987;35:607–614. [PubMed] [Google Scholar]
- Simmons MA, Battaglia FC, Meschia G. Placental transfer of glucose. J Dev Physiol. 1979;1:227–243. [PubMed] [Google Scholar]
- Sparks JW, Hay WW, Bonds D, Meschia G, Battaglia FC. Simultaneous measurements of lactate turnover rate and umbilical lactate uptake in the fetal lamb. J Clin Invest. 1982;70:179–192. doi: 10.1172/JCI110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JW, Hay WW, Meschia G, Battaglia FC. Partition of maternal nutrients to the placenta and fetus in the sheep. Eur J Obstr Gynec Reprod Biol. 1983;14:331–340. doi: 10.1016/0028-2243(83)90009-6. [DOI] [PubMed] [Google Scholar]
- Townsend SF, Rudolph CD, Wood CE, Rudolph AM. Perinatal onset of hepatic gluconeogenesis in the lamb. J Dev Physiol. 1989;12:329–335. [PubMed] [Google Scholar]
- Widdas WF. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J Physiol. 1952;118:23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]