Abstract
Small conductance Ca2+-activated K+ channels (SK channels) contribute to the long lasting afterhyperpolarization (AHP) that follows an action potential in many central neurones. The biophysical and pharmacological attributes of cloned SK channels strongly suggest that one or more of them underlie the medium component of the AHP that regulates interspike interval and plays an important role in setting tonic firing frequency. The cloned SK channels comprise a distinct subfamily of K+ channels. Heterologously expressed SK channels recapitulate the biophysical and pharmacological hallmarks of native SK channels, being gated solely by intracellular Ca2+ ions with no voltage dependence to their gating, small unitary conductance values and sensitivity to the bee venom peptide toxin, apamin. Molecular, biochemical and electrophysiological studies have revealed that Ca2+ gating in SK channels is due to heteromeric assembly of the SK α pore-forming subunits with calmodulin (CaM). Ca2+ binding to the N-terminal E–F hands of CaM is responsible for SK channel gating. Crystallographic studies suggest that SK channels gate as a dimer-of-dimers, and that the physical gate of SK channels resides at or near the selectivity filter of the channels. In addition, Ca2+-independent interactions between the SK channel α subunits and CaM are necessary for proper membrane trafficking.
The afterhyperpolarization
Action potentials in many neurones are followed by a long-lasting afterhyperpolarization (AHP) during which the membrane potential is negative to the normal resting potential, and then slowly returns to baseline. During trains of action potentials, larger amplitude and longer duration AHPs develop resulting in increased interspike intervals and, ultimately, spike-frequency adaptation where the burst is terminated. Therefore, the AHP influences fundamental parameters of excitability (Alger & Nicoll, 1980; Hotson & Prince, 1980; Madison & Nicoll, 1984; Madison et al. 1987). The AHP has multiple components and has been particularly well studied in hippocampal CA1 neurones where three kinetic components may be distinguished. All three components are potassium conductances and are apparent in either current or voltage clamp recordings. The fast AHP (IfAHP) which decays with a time constant (τdecay) ∼50 ms is activated during voltage commands, or during the repolarizing phase of the action potential, and has been ascribed to either BK voltage- and calcium-gated K+ channels, or M-channels. The two longer lasting components are both due to voltage-independent, calcium-activated K+ conductances that require influx of Ca2+ through voltage-gated Ca2+ channels. The medium AHP (ImAHP), which has a τdecay of ∼250 ms, activates during the voltage command or action potential due to Ca2+ influx and is blocked by the bee venom peptide toxin, apamin (Fig. 1). By contrast, the slow AHP (sAHP) shows a prominent activation phase (τrise ∼ 600 ms) following a voltage pulse or action potential, and decays slowly, persisting for as long as several seconds. The sAHP is not blocked by apamin but is suppressed by cyclic AMP-dependent protein kinase A (PKA) permitting powerful modulation of excitability by neurotransmitters that bind to receptors coupled to adenylyl cyclase (Storm, 1987, 1989; 1990; Pedarzani & Storm, 1993; Stocker et al. 1999). Therefore, the three components can be pharmacologically isolated, and using this approach, it appears that the apamin-sensitive medium AHP regulates the number of action potentials per stimulus while the slow AHP underlies spike-frequency adaptation (Stocker et al. 1999; Fig. 1).
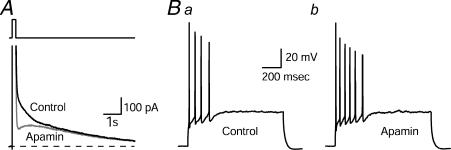
Figure 1. Blockade of the apamin-sensitive afterhyperpolarization (mAHP) in CA1 neurones increases excitability.
A, IAHPs were evoked in the whole-cell configuration by a 200 ms depolarizing pulse to –20 mV followed by a return to the –55 mV holding potential. IAHPs were obtained in the presence and absence of apamin (100 nm). After application of apamin, the medium-duration component (ImAHP) of the tail current was selectively inhibited. Broken line indicates zero current. B, apamin increased the number of action potentials. Ba, response of a pyramidal neurone to a 1 s depolarizing current pulse. Bb, response of the same neurone to the same depolarizing current pulse in the presence of apamin (control cells fired a mean ± s.e.m. of 4.7 ± 1.2 action potentials/depolarizing pulse, which increased to 6.7 ± 1.7 with apamin; n = 5; P = 0.04; paired Student's t test). From Stackman et al. 2002, with permission.
Cloned SK channels
Clones encoding K+ channels with the gating characteristics of native small conductance Ca2+-activated K+ (SK) channels have been described. Four genes encode the family of SK channels. SK1, SK2, and SK3 encode apamin-sensitive, calcium-activated and voltage-independent channels with unit conductance values of ∼10 pS (Kohler et al. 1996; Hirschberg et al. 1998). The fourth member of the family is IK1, similarly voltage independent and calcium activated, but with a larger unitary conductance, ∼45 pS (Ishii et al. 1997b; Joiner et al. 1997). In addition, IK1 is not apamin sensitive but is blocked by charybdotoxin and clotrimazole. Indeed the biophysical and pharmacological profiles of heterologously expressed IK1 channels match those of the native Gardos channel from red blood cells, the first described calcium-activated K+ flux (Gardos, 1958; Christophersen, 1991; Ishii et al. 1997b). The mRNAs for SK1, SK2, and SK3 are expressed throughout the CNS in distinct and overlapping patterns (Stocker & Pedarzani, 2000). In addition, these genes are also expressed in a variety of peripheral tissues. IK1 is not expressed in the brain, but is widely expressed in the periphery. Despite the presence of more than one SK mRNA in numerous brain nuclei, it is not known whether the subunits form heteromeric channels in vivo, although they are capable of doing so in heterologous expression studies (Ishii et al. 1997a).
The primary amino acid sequences of the SK channel family members predict subunits with a serpentine architecture analogous to Shaker Kv channels, with the N- and C-termini residing within the cytoplasm, separated by six transmembrane domains. There is a re-entrant loop between the fifth and sixth transmembrane domains (TMs) bearing the hallmarks of the selectivity and conduction pathway for potassium ions (Heginbotham et al. 1994; Kohler et al. 1996). By analogy to other K+ channels, SK channels are probably tetramers of pore-forming subunits (MacKinnon, 1991). Within the subfamily of SK channels, the primary sequences of the subunits are highly similar, more so than within other K+ channel subfamilies The SK amino acid sequences vary most in their extreme termini, suggesting that these domains lend physiological specificity to the roles served by each of the different SK channels, either by being targets for second messengers, addresses for differential subcellular localization, or sites of association with other proteins that comprise the molecular neighbourhood of the channel.
Calcium gating
The essential feature of SK channels is their gating by intracellular calcium, thereby coupling the most ubiquitous second messenger with membrane potential. Calcium dose–response experiments with cloned channels revealed that the channels are highly calcium sensitive with submicromolar KD values (∼0.5 μm) and steep slopes (>4) (Fig. 2; Kohler et al. 1996; Hirschberg et al. 1998). Moreover, the calcium dose–response relationships for all four subtypes are virtually identical (Xia et al. 1998). However inspecting the primary sequences of the subunits does not reveal obvious Ca2+-binding motifs, such as E–F hands or C2 domains. How do Ca2+ ions gate SK channels? Two possibilities were considered: direct Ca2+ binding to the channel proteins, or Ca2+-activated second messenger molecules. In the first case, Ca2+-channel activation should occur rapidly with on-rate kinetics proportional to the concentration of applied Ca2+, while in the second case, gating may be slower, involving Ca2+ binding to a second protein, followed by binding to the channel and gating, and this process may be kinetically complex, perhaps limited by the expression levels of the Ca2+-sensing protein in the host cell. Fast application of Ca2+ revealed that channel gating was rapid, occurring in a few milliseconds upon introduction of saturating concentrations of Ca2+. This is similar to ionotropic receptors such as GABA or glutamate receptors in which a ligand binds directly to the receptor-channel protein and opens the gate (Lester et al. 1990; Maconochie et al. 1994). Since the Ca2+-dose–responses are the same for all SK subtypes, and Ca2+ acts from the inside of the cell, it seemed likely that Ca2+ binding would be mediated by negatively charged intracellular amino acids. Conserved intracellular glutamates and aspartates were identified, and individually changed to neutral amino acids. However, in no case was the Ca2+ sensitivity of the channels profoundly affected. As there are two clusters of conserved negatively charged residues, one within the intracellular loop between TMs two and three (2–3 loop), and the other in the proximal domain of the C-terminus, multiple neutralizations were introduced together in these domains. Channels with all four negatively charged residues substituted with neutral amino acids in the 2–3 loop functioned normally. However, multiple neutralizations in the proximal C-terminus destroyed channel activity. Closer inspection of this domain, ∼100 amino acids, revealed that even within the context of such conserved subunits, this region was the most conserved and, this domain could be modelled as a series of three or four highly positively charged α-helices.
Figure 2. SK channels are Ca2+ gated.
Application of increasing concentrations of Ca2+ applied to the inside face of patches excised from SK2-transfected cells yield increasing current amplitudes in response to voltage ramp commands. The channels have a KD of ∼0.5 μm and a steep Ca2+ dependence.
Could a second protein be involved in Ca2+ gating yet retain rapid gating? Such a protein would have to have a high Ca2+ affinity and be abundantly expressed in a wide variety of cell types, reflecting the large currents obtained by heterologous expression of the SK subunits. This possibility was investigated using the two-hybrid approach, and showed that the proximal domains of the C-termini of all four SK channel subtypes interact with the ubiquitous Ca2+ sensor, calmodulin (CaM). Biochemical experiments confirmed the two-hybrid result and revealed that the entire proximal C-terminal domain (CaM binding domain; CaMBD) interacts with CaM in the presence or absence of Ca2+, while a subdomain only interacts with CaM when Ca2+ is present. Indeed, the channel–CaM complex is extremely stable, requiring harsh denaturing to separate the two components. To demonstrate that CaM serves as the SK channel Ca2+sensor, SK channels were coexpressed with mutant CaMs that had reduced affinity for Ca2+. Currents evoked in the presence of Ca2+ appeared normal but the dose–response relationship was shifted ∼7-fold to higher Ca2+ concentrations and the slope was markedly shallower (Xia et al. 1998). CaM contains two E–F hand motifs within each of the globular N- and C-terminal regions, separated by a flexible linker. Mutagenesis experiments revealed that the two N-terminal E–F hands are necessary and sufficient to endow normal Ca2+ gating, and that the C-terminal E–F hands did not contribute to gating (Keen et al. 1999).
Therefore, SK channels are heteromeric complexes of four α, pore-forming subunits complexed with constitutively associated CaM. The high affinity of the association between CaM and the channel subunits provides the ability for rapid responses to changes in intracellular calcium concentrations, and the absolute Ca2+ affinity of CaM provides a perfect window for those responses within the physiological ranges experienced as a consequence of an action potential.
Structure of the CaMBD–Ca2+–CaM complex
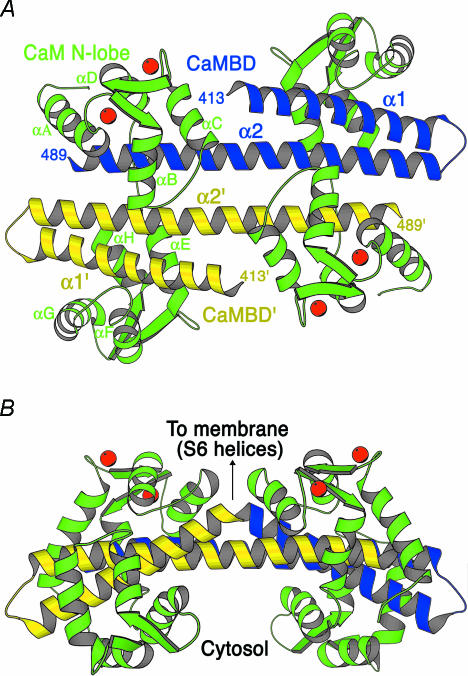
The interaction between the CaMBD and Ca2+–CaM was revealed by crystallographic studies that revealed a dimeric complex (Fig. 3). Two CaMBDs, each comprised of a short α-helix, a β-turn, and a longer extended α-helix are arranged with the longer helices in an antiparallel configuration and do not make direct contacts. Two CaMs are symmetrically woven around the CaMBDs with each CaM making multiple contacts with each of the two CaMBDs. The CaMs are in an unusual configuration and suggest several aspects of channel function. First, the N-terminal E–F hands are Ca2+ loaded while the C-terminal E–F hands are uncalcified, consistent with the structure–function analysis described above. The Ca2+-bound N-lobe E–F hands are folded into a structure similar to other Ca2+-bound forms of CaM, consolidating several hydrophobic residues into a hydrophobic patch that contacts the C-terminal region of the CaMBD, while the linker domain of CaM is extended, unlike other CaM structures, crossing the proximal part of both helices of the other CaMBD. Closer inspection of the C-lobe of CaM shows that the E–F hands are constrained by their multiple interactions with the CaMBD so that the negatively charged side chains that serve as the E–F hand fingers are spatially altered and unable to chelate a Ca2+ ion. These domains mediate the constitutive interactions between the two proteins. Biochemical experiments performed in the absence of Ca2+ were consistent with a monomeric structure. Taken together, the data suggest a model for Ca2+ gating in SK channels where CaM is constitutively bound through the C-lobe to the proximal portion of the CaMBD. Upon Ca2+ binding to the N-lobe E–F hands, a large-scale conformational rearrangement occurs in which the N-lobe of CaM contacts the distal domain of the CaMBD on a neighbouring subunit. This rearrangement is transduced to the associated S6 transmembrane domains and subsequent conformational changes open the ion-conducting pore. Therefore, the SK channels may gate as dimers-of-dimers (Schumacher et al. 2001).
Figure 3. Structure of the CaMBD–Ca2+–CaM complex.
A, ribbon diagram of the CaMBD–Ca2+–CaM dimeric complex. CaMBD subunits are in blue and yellow, CaM molecules are in green and the Ca2+ ions are in red. Secondary structural elements, the CaM linker and the first and last observed residues in the CaMBD are labelled. B, the view is rotated 90°, showing the orientation of the complex relative to the membrane. Arrow indicates the positions of the first observed residue of each of the CaMBD monomers that are linked to the S6 pore helices. (Reproduced with permission from: Schumacher et al. 2001.)
Location of the SK channel activation gate
Crystallographic studies of the bacterial KcsA channel strongly suggest that the activation gate of these channels is formed by the distal domains of the four S6 helices that form a bundle crossing near the cytoplasmic interface that forms a physical occlusion for ion access to the pore in the closed state, and opens enough to permit ion entry in the open state (Doyle et al. 1998). More recent work also suggests that the gate of inwardly rectifying K+ channels may also reside in this same region of the channel structure (Kuo et al. 2003).
The crystallographic studies are consistent with functional studies of the Shaker voltage-gated channel in which state-dependent access to the pore from the inside face of the channel was probed by scanning cysteine accessibility mutagenesis (SCAM) which showed that positions near the cytoplasmic S6 boundary could be accessed in either the open or closed state of the channel, but residues predicted to lie internal to the proposed location of the bundle crossing gate were available to cysteine-modifying compounds only in the open state (Liu et al. 1997). Similar experiments using cysteine-substituted SK channels showed that relatively larger cysteine modifying reagents such as MTSET ([2-(trimethylammonium)ethyl[ methanethiosulfonate bromide) reported a similar pattern of state-dependent accessibility. However, smaller compounds such as MTSEA ](2-aminoethyl)methanethiosulfonate hydrobromide] were able to access positions deeper in the pore than predicted by the bundle-crossing model. Indeed, one position that showed a 1000-fold state-dependent difference to MTSEA modification in Shaker channel studies (I470C) was equally modified in either the open or closed state in SK channels (T387C). Further studies showed that even larger compounds, such as the known pore blocker tetrabutyl ammonium (TBuA), could protect T387C from MTSEA modification, demonstrating that MTSEA accessed T387C through the permeation pathway. Therefore, the SK channel activation gate is different from the Shaker channel gate in that it must reside deeper in the channel pore. Substituting SK residues into the KcsA crystal structure predicts that T387 is positioned near the top of the inner vestibule of the channel, just below the selectivity filter itself, where the K+ binding sites conduct the ions across the membrane. This suggests that conformational changes near or even in the selectivity filter itself may function to gate SK channels (Bruening-Wright et al. 2002).
SK channel and CaM: non-constitutive associations and regulated channel trafficking
The crystal structure of the CaMBD–Ca2+–CaM complex predicts that negatively charged residues in the CaM linker region form strong electrostatic interactions with positively charged residues on the CaMBD contributing to the constitutive interactions between the two proteins (Keen et al. 1999). Consistent with this interpretation, reversing the charges at R464 and K467 of the CaMBD resulted in a R464E; K467E CaMBD that was no longer able to interact with CaM in Ca2+-free conditions. Whole-cell recordings from cells transfected with SK2R464E; K467E (SK2:64/67) in which calcium was dialysed into the cell through the patch pipette failed to yield SK currents, while cells cotransfected with SK2:64/67 and CaM yielded robust SK currents. Inside-out patches from SK2-transfected cells, excised into a bath solution containing Ca2+ yielded SK currents that disappeared upon changing to Ca2+-free solution, and reactivated upon subsequent exposure to Ca2+ solution. In contrast, patches excised from SK2:64/67-transfected cells into Ca2+ solution yielded currents that rapidly decayed despite continued exposure to Ca2+. However, application of Ca2+ and recombinant, purified CaM resulted in reactivation of SK2:64/67 channel activity, suggesting that CaM was initially associated with SK2:64/67 channels from cells cotransfected with CaM, but rapidly dissociated upon exposure to CaM-free bath solution upon patch excision; Ca2+–CaM trans-associated with CaM-free SK2 channels. In contrast, Ca2+–CaM application to patches from cells transfected only with SK2:64/67 did not yield currents. Incubating patches from SK2:64/67-transfected cells in Ca2+-free solution containing CaM followed by Ca2+ application showed that CaM associated with SK2:64/67 channels even in the absence of Ca2+. Therefore, the SK2:64/67 mutations appear to reduce the affinity of the channels for CaM. To further test this model, charge reversals were introduced into CaM at E84 and E87 (CaM:8487). Patches from cells cotransfected with SK2:64/67 and CaM:84/87 showed robust currents when excised into Ca2+ solution. These currents did not decay, and channel activity was repeatedly evoked by application of Ca2+ solution following channel closure in the absence of Ca2+. Therefore, the charge reversals at E84 and E87 in CaM were compensatory for the charge reversals in SK2, consistent with salt bridges between these positions stabilizing the constitutive interactions between the two proteins.
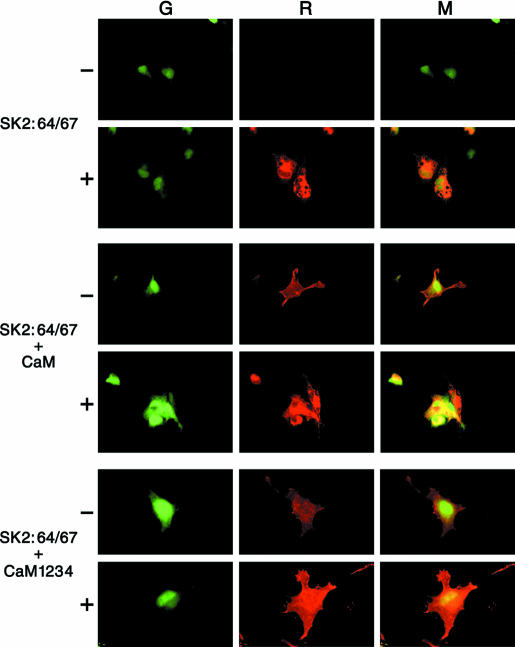
Why was channel activity not re-established by CaM application to patches from cells transfected only with SK2:64/67? One possibility is that CaM is required for cell-surface expression of SK channels. To test this hypothesis, a myc epitope was inserted into the extracellular loop between TMs 3 and 4, permitting assessment of SK2 surface expression. Immunostaining of cells transfected with SK2:64/67(myc) showed that the protein was expressed but did not get inserted into the plasma membrane, while cotransfection with CaM resulted in plasma membrane localization (Fig. 4). Further experiments showed that cotransfecting SK2:64/67(myc) with CaM1,2,3,4, a CaM in which the ability of all four of the E–F hands to bind Ca2+ was abolished by mutations, also yielded channels in the plasma membrane. Patches excised from these cells into Ca2+ solution did not yield currents, but application of recombinant Ca2+–CaM did restore channel activity, suggesting that the channels were associated with mutant CaM when the patches were excised, and as the mutant CaM dissociated following excision, it was replaced by applied wild-type CaM. These results suggest that Ca2+-independent interactions between SK channels and CaM are necessary for cell surface expression (Lee et al. 2003).
Figure 4. CaM is required for surface expression.
Immunocytochemistry of COS cells transfected with the indicated combinations of SK2:64/67, CaM, and a GFP expression plasmid. SK2:64/67 harbours three tandem copies of the myc epitope in the external loop between transmembrane domains 3 and 4. For each panel, transfected cells were visualized by expression of GFP (G; left), channel protein was detected with an anti-myc mouse monoclonal antibody and visualized by Texas Red-conjugated horse antimouse secondary antibody (R; middle) and the signals were merged (M; right). In each case, cells were examined either without (–) or with (+) the membrane permeabilization. Top, SK2:64/67 was not detected on the cell surface (–) but channel protein was detected inside permeablized cells (+). Middle, SK2:64/67 was detected on the cell surface (–) as well as inside the cell (+) when transfected with wild-type CaM or bottom, the Ca2+-independent CaM mutant, CaM1,2,3,4. (Reproduced with permission from: Lee et al. 2003.)
Summary and perspectives
SK channels are important regulators of excitability throughout the CNS. The cloned SK channels comprise a remarkably conserved subfamily of K+ channels, yet the sequence differences in their extreme intracellular termini suggest that they participate in distinct physiological functions. The cloned SK channels are efficient calcium signalling machines, transducing changes in intracellular Ca2+ concentrations into altered membrane potentials. Structurally, the SK channels are heteromeric complexes with constitutively associated CaM that serve as the intrinsic Ca2+ sensors for the channels. Ca2+-independent interactions with CaM are necessary for proper subcellular trafficking and deposition in the plasma membrane. In contrast to bacterial K+ channels, and voltage-gated K+ channels, the gating cue for SK channels opens a channel gate that is much closer to the selectivity filter itself than predicted for the other channels.
The unique partnership between SK channels and CaM permits rapid responses to physiological fluctuations in the cytoplasmic Ca2+ concentration. The submicromolar KD and the steep slope of the Ca2+ sensitivity of SK channels suggests that the subcellular localization of SK channels relative to Ca2+ sources, such as voltage-gated Ca2+ channels or intracellular Ca2+ stores will affect neuronal excitability. This, in turn, indicates that there may be molecular mechanisms to strictly control the relative placement of SK channels and Ca2+ sources. Indeed, in hippocampal CA1 neurones, somatic SK channels are localized to within ∼100 nm of L-type Ca2+ channels (Marrion & Tavalin, 1998). A similar strict colocalization occurs in cochlear outer hair cells between Ach receptors and SK2 channels, with profound consequences for hair cell excitability (Oliver et al. 2000). In addition, CaM is subject to multiple levels of regulation, for example by protein kinases that may shift the ability of CaM to respond to Ca2+ binding and thus affect SK channel gating. Therefore, determining the identities of other proteins that reside in a molecular neighbourhood with SK channels will illuminate new intricacies to the mechanisms that regulate SK channel-mediated effects on neuronal function.
Finally, it is interesting to note that the gating and pharmacological characteristics of the cloned SK channels correspond well with the profile for channels underlying the medium AHP in CA1 neurones, highlighting at least one of the important roles for these channels. However, the channels responsible for the slow AHP may not correspond to the cloned SK channels. Defining the molecular identity of the calcium-activated K+ channels underlying the slow AHP remains an important future goal.
References
- Alger BE, Nicoll RA. Epileptiform burst after hyperpolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980;210:1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Bruening-Wright A, Schumacher MA, Adelman JP, Maylie J. Localization of the activation gate for small conductance Ca2+-activated K+ channels. J Neurosci. 2002;22:6499–6506. doi: 10.1523/JNEUROSCI.22-15-06499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophersen P. Ca2+-activated K+ channel from human erythrocyte membranes: single channel rectification and selectivity. J Membr Biol. 1991;119:75–83. doi: 10.1007/BF01868542. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity [see comments] Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg B, Maylie J, Adelman JP, Marrion NV. Gating of recombinant small conductance Ca-activated K+ channels by calcium. J Gen Physiol. 1998;111:565–581. doi: 10.1085/jgp.111.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson JR, Prince DA. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980;43:409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem. 1997a;272:23195–23200. doi: 10.1074/jbc.272.37.23195. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA. 1997b;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Wang L-Y, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci USA. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, Janowsky A, Fakler B, Adelman JP, Maylie J. Domains responsible for constitutive and Ca2+–dependent interactions between calmodulin and small conductance Ca2+-activated potassium channels. J Neurosci. 1999;19:8830–8838. doi: 10.1523/JNEUROSCI.19-20-08830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain [see comments] Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal Structure of the Potassium Channel KirBac1.1 closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- Lee WS, Ngo-Anh TJ, Bruening-Wright A, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin: cell surface expression and gating. J Biol Chem. 2003;278:25940–25946. doi: 10.1074/jbc.M302091200. [DOI] [PubMed] [Google Scholar]
- Lester RAJ, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open. Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Madison DV, Lancaster G, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA1 pyramidal neurons in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/s0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993;11:1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channelsmodulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramdial neurons. Proc Natl Acad Sci USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca(2+) -activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Br Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Xia X-M, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]