Abstract
Flecainide, a class IC antiarrhythmic, was shown to improve myotonia caused by sodium channel mutations in situations where the class IB antiarrhythmic drug mexiletine was less efficient. Yet little is known about molecular interactions between flecainide and human skeletal muscle sodium (hNav1.4) channels. Whole-cell sodium currents (INa) were recorded in tsA201 cells expressing wild-type (WT) and mutant hNav1.4 channels (R1448C, paramyotonia congenita; G1306E, potassium-aggravated myotonia). At a holding potential (HP) of –120 mV, flecainide use-dependently blocked WT and G1306E INa equally but was more potent on R1448C channels. For WT, the extent of block depended on a holding voltage more negative than the activation threshold, being greater at –90 mV as compared to –120 and –180 mV. This behaviour was exacerbated by the R1448C mutation since block at –120 mV was greater than that at –180 mV. Thus flecainide can bind to inactivated sodium channels in the absence of channel opening. Nevertheless, all the channels showed the same closed-state affinity constant (KR∼480 μm) and the same inactivated-state affinity constant (KI∼18 μm). Simulations according to the modulated receptor hypothesis mimic the voltage-dependent block of WT and mutant channels by flecainide and mexiletine. All the results suggest similar blocking mechanisms for the two drugs. Yet, since flecainide exerts use-dependent block at lower frequency than mexiletine, it may exhibit greater benefit in all myotonic syndromes. Moreover, flecainide blocks hNav1.4 channel mutants with a rightward shift of availability voltage dependence more specifically than mexiletine, owing to a lower KR/KI ratio. This study offers a pharmacogenetic strategy to better address treatment in individual myotonic patients.
Excessive and sustained firing of action potentials in the skeletal muscle results in myotonia, a disorder characterized by long-lasting involuntary contractions leading to muscle stiffness. The genes responsible for inherited non-dystrophic myotonias have been identified as those encoding the skeletal muscle voltage-gated chloride or sodium channels, which lead to a loss or a gain, respectively, of gating function of the channel protein (Cannon, 2001; Jurkat-Rott et al. 2002). Chloride channel myotonias include the dominant myotonia congenita of Thomsen and the recessive generalized myotonia of Becker, while sodium channel myotonias include paramyotonia congenita (PMC) and potassium-aggravated myotonias (PAM) both with autosomal dominant inheritance patterns. Although clinically distinguishable by the nature of exacerbating factors, all these disorders share a similar phenotype and medication. The sodium channel blocker mexiletine is widely considered as the drug of choice to treat myotonic syndromes (Moxley, 2000). The rationale for the use of mexiletine is that this drug produces a use-dependent block of sodium channels, that is the higher the frequency of sarcolemma depolarization, the greater is the blocking action, which allows a selective action of the drug on myotonic discharges of action potentials. Yet, regarding the sodium channel myotonias, the mutations themselves can modify the sensitivity of the channel to mexiletine (Fan et al. 1996; Fleischhauer et al. 1998; Weckbecker et al. 2000; Desaphy et al. 2001; Takahashi & Cannon, 2001). These modifications may result from altered intrinsic affinity or from mutation-induced altered gating. For instance, since mexiletine binds inactivated sodium channels with much higher affinity than closed or open channels, a few myotonic mutations that shift the voltage dependence of channel inactivation toward more negative potentials with respect to wild-type channels increase mutant channel responsiveness to mexiletine, whereas most of the myotonic mutations decrease the proportion of inactivated channels at the resting potential (rightward shift of channel availability voltage dependence) and consequently reduce mutant channel block by the drug (Desaphy et al. 2001). Thus individual myotonic patients should benefit from drugs acting more specifically on mutant channels with respect to wild-type sodium channels (Griggs & Ptácek, 1999).
Interestingly, the antiarrhythmic drug flecainide was shown to improve muscle stiffness in patients with sodium channel myotonia and shorten Q–T intervals in patients with long-QT3 syndrome, an inherited life-threatening arrhythmia due to mutations in the cardiac sodium channel, in situations where mexiletine was less efficient (Rosenfeld et al. 1997; Wang et al. 1999; Benhorin et al. 2000; Abriel et al. 2000). Whereas mexiletine is a class IB antiarrhythmic drug, flecainide is considered as a paradigm for class IC antiarrhythmics. Both drugs have similar pKa (more than 95% of the drugs are protonated at physiological pH), but mexiletine binds to the inactivated channel from the intracellular side, whereas flecainide is referred to as an open-channel blocker and may reach its binding site from the extracellular side of the membrane, at least in cardiac Na+ channels (Nitta et al. 1992; Ragsdale et al. 1996; Grant et al. 2000; Nagatomo et al. 2000). Thus drug-specific molecular blocking mechanisms may influence individual patient response to antimyotonic therapy. Yet, nothing is known about the mechanism of block of skeletal muscle sodium channels by flecainide.
In the current study, we investigated the effects of flecainide on myotonic and wild-type human skeletal muscle sodium (hNav1.4) channels transiently expressed in tsA201 cells. We found that flecainide binds inactivated sodium channels with high affinity, as compared to closed channels. This process does not require channel opening, since voltage-dependent block develops at potentials more negative than the activation threshold. Using the model of modulated receptor, we show that the molecular mechanism of flecainide block is quite similar to that of mexiletine. Yet, there may be two main advantages in using flecainide against myotonic syndromes. First, use-dependent block develops at lower frequency as compared to mexiletine, which may offer a greater benefit in all myotonic syndromes, independently of the genetic origin. Second, flecainide block is less dependent on voltage-dependent channel availability as compared to mexiletine, owing to the smaller difference between affinities for the closed and the inactivated channels (Desaphy et al. 2001). Thus, for those mutations that produce a positive shift in the voltage dependence of sodium channel availability, the difference in flecainide block between mutant and WT channels is less with respect to mexiletine block, and the patients carrying these mutations may respond better to flecainide therapy. Overall, this study provides a framework for developing a pharmacogenetic therapy against sodium channel myotonias to address with enhanced specificity and efficiency the treatment in individual myotonic patients.
Methods
Full-length mutant hNav1.4 constructs were subcloned in the mammalian expression vector pRc/CMV as previously described (Yang et al. 1994). The tsA201 cells were cotransfected with 10 μg of plasmid DNA encoding the channels and lower amount of plasmid DNA encoding CD8 receptors, using the calcium phosphate coprecipitation method (Desaphy et al. 2001). For patch clamp recordings (36–72 h after transfection), successfully transfected cells were identified using Dynal microbeads coated with anti-CD8 antibody (Dynal A.S., Oslo, Norway).
Whole-cell sodium currents (INa) were recorded at room temperature (20–22°C) using an Axopatch 1D amplifier (Axon Instruments, Union City, CA, USA). Voltage clamp protocols and data acquisition were performed with pCLAMP 6.0 software (Axon Instruments) through a 12-bit A–D/D–A interface (Digidata 1200, Axon Instruments). The external solution contained (mm): 150 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 Hepes and 5 glucose, and the pH was set to 7.4 with NaOH. The pipette solution contained (mm): 120 CsF, 10 CsCl, 10 NaCl, 5 EGTA and 5 Hepes, and the pH was set to 7.2 with CsOH. With such solutions, pipettes made with Corning 7052 glass (Garner Glass, Claremont, CA, USA) had resistance ranged from 1 to 2 MΩ. Currents were low-pass filtered at 2 kHz (–3 dB) by the four pole Bessel filter of the amplifier and digitized at 10–20 kHz.
After rupturing the patch membrane, a 25 ms-long test pulse to –20 mV from a holding potential of –120 mV was applied to the cell at a low frequency until stabilization of INa amplitude and kinetics was achieved (typically 5 min). Data were considered for analysis only from cells exhibiting peak current amplitudes of 0.6–6 nA and series resistance errors <5 mV. Little (<5%) or no rundown was observed within the experiments. Specific voltage protocols and analysis procedures are described in the Results section.
Flecainide acetate was purchased from Sigma (Milan, Italy). QX-314 was a gift from Alomone Laboratories (Jerusalem, Israel). The R(–)-enantiomer of mexiletine was kindly provided by Professor V. Tortorella (Department of Medicinal Chemistry, University of Bari, Bari, Italy). The patched cell was continuously exposed to a stream of external solution flowing out of a plastic capillary. For extracellular application of flecainide, the superfusing external solution was supplemented with the drug at final concentration. For intracellular application, flecainide, QX-314 and mexiletine were dissolved in pipette solution at final concentration.
Average data are presented as means ± s.e.m. and statistical difference between the means was evaluated using Student's unpaired t test, with P < 0.05 considered as significant.
Results
Different sensitivity of WT and mutant sodium channels to flecainide
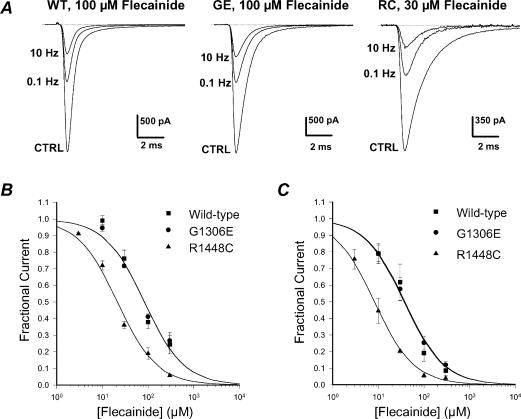
We have shown previously that paramyotonia congenita R1448C mutant channels and potassium-aggravated myotonia G1306E mutant channels are, respectively, more and less sensitive to mexiletine as compared to wild-type channels (Desaphy et al. 2001). To allow direct comparison, block of sodium channels by flecainide was evaluated by measuring the reduction of INa elicited from the holding potential (HP) of –120 mV to –30 mV at 0.1 Hz and 10 Hz. Applying this protocol in the absence of drug, there was no significant change (<5%) in current amplitude for WT or mutant channels (not shown). Figure. 1A illustrates examples of current traces recorded before (control) and at the steady-state of flecainide block, i.e. 3 min after drug application at 0.1 Hz and then between the 100th and 110th pulse at 10 Hz. For both WT and G1306E channels, 100 μm flecainide reduced peak INa by ∼60% at 0.1 Hz and by ∼80% at 10 Hz. In contrast, 30 μm flecainide was sufficient to obtain a similar block of R1448C peak INa as compared to WT. The concentration–response curves were fitted with a first-order binding function,
| (1) |
where IC50 (μm) is the half-maximum inhibitory concentration. At 0.1 Hz, the IC50 values calculated at the HP of –120 mV were 83.5 μm for WT channels, 82.8 μm for G1306E mutants, and 21.4 μm for R1448C mutants (Fig. 1B). At 10 Hz, the IC50 values were 36.6 μm, 38.2 μm, and 8.2 μm, respectively.
Figure 1. Frequency-dependent flecainide block of wild-type and mutant hNav1.4 channels at a holding potential of –120 mV.
A, block of sodium currents by flecainide was assessed 3 min after drug application by measuring the reduction of INa elicited from –120 to –30 mV at stimulation frequencies of 0.1 Hz and 10 Hz. B, concentration–response curves for flecainide block were constructed at 0.1 Hz using the protocol described in A and fitted with eqn (1) (see Results). Each data point is the mean ± s.e.m. of at least 3 cells. The calculated IC50 values ± s.e. of the fit were 83.5 ± 16.9 μm for WT, 82.8 ± 11.2 μm for G1306E, and 21.4 ± 2.2 μm for R1448C. C, concentration–response curves for flecainide block were constructed at 10 Hz using the protocol described in A and fitted with eqn (1) (see Results). Each data point is the mean ± s.e.m. of at least 3 cells. The calculated IC50 values ± s.e. of the fit were 36.6 ± 6.1 μm for WT, 38.2 ± 1.8 μm for G1306E, and 8.2 ± 0.4 μm for R1448C.
Closed state-dependent affinity of WT and mutant sodium channels for flecainide
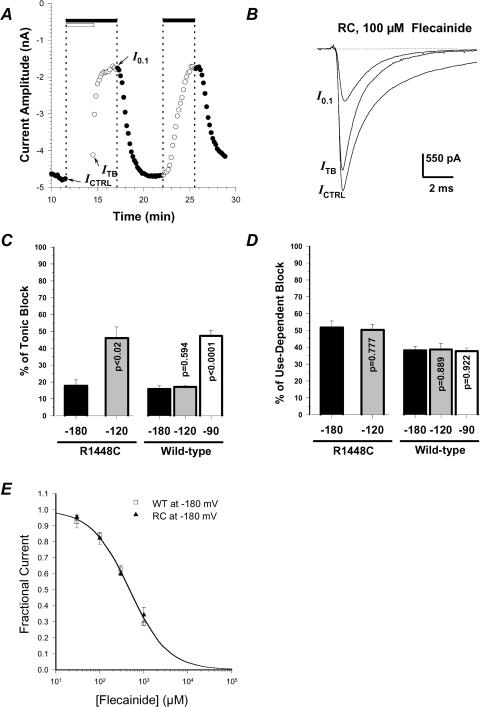
For an inactivated-channel blocker (e.g. mexiletine), apparent affinity measured at the HP of –120 mV reflected the combination of binding to resting (closed) and inactivated sodium channels (Wright et al. 1997; Desaphy et al. 2001). Indeed, a mutant channel (such as R1448C) showing greater inactivation at –120 mV is more sensitive to mexiletine than WT channels, whereas a mutation (such as G1306E) reducing inactivation at this potential is less sensitive. Although flecainide is generally reported as an open-channel blocker (Ragsdale et al. 1996; Grant et al. 2000), recent studies described binding of the drug to inactivated channels (Viswanathan et al. 2001; Liu et al. 2002, 2003). Thus to look at flecainide binding affinity for resting sodium channels (KR), we first measured block of WT and R1448C channels while maintaining the cell hyperpolarized at the HP of –180 mV for 180 s (prepulse) and, only after that, the cell was depolarized at 0.1 Hz frequency (Fig. 2A and 2B). At this holding potential, the entire population of WT and mutant channels should be in the closed state, ready to open in response to depolarization. In the presence of 100 μm flecainide, only ∼17% of R1448C INa reduction occurred during the prepulse at –180 mV, and this effect was quite similar for WT channels (Fig. 2C). The block further developed during stimulation at 0.1 Hz, revealing a large component of use-dependent block that was greater for R1448C channels as compared to WT (P < 0.05; Fig. 2D). The same drug effect was obtained using a 90 s-long prepulse, while the same protocol had no effect in the absence of the drug (data not shown), indicating that neither slow inactivation nor ultra-slow inactivation, which develop with time constants of ∼10 s and ∼100 s, was involved in INa reduction (Vilin & Ruben, 2001; Hilber et al. 2002). Altogether, these data suggested that the INa reduction during the prepulse was due to drug binding to closed channels and that flecainide has the same affinity for closed R1448C and WT channels, while the difference in sensitivity between these two channels observed at –180 mV and 0.1 Hz was mainly due to difference in use-dependent block. Thus we calculated the KR value for WT and R1448C sodium channels as the IC50 value of concentration–response curves for block occurring during the prepulse (KR∼480 μm, Fig. 2E).
Figure 2. Effect of holding potential on flecainide block of wild-type and R1448C hNav1.4 channels and flecainide affinity for closed channels.
A, time course evolution of peak INa amplitude in a tsA201 cell expressing R1448C channels. The cell was held at the HP of –180 mV and depolarized to –30 mV at 0.1 Hz frequency, except under the open bar where the HP was maintained with no depolarization for determination of tonic block (ITB). The filled bars indicate application of 100 μm flecainide. B, traces of R1448C INa measured at the times indicated by arrows in A. ICTRL was measured just before application of the drug, while I0.1 was measured when steady-state block was reached at 0.1 Hz stimulation frequency. C and D, tonic block is expressed as 100 × (ICTRL–ITB)/ICTRL, while use-dependent block is expressed as 100 × (ITB–I0.1)/ICTRL, measured as in A with HP =–180, –120 and –90 mV. Each bar corresponds to the mean ± s.e.m. of at least 4 cells. The P-values reported on bars were calculated using Student's unpaired t test versus respective block at HP =–180 mV. In addition, use-dependent block of R1448C channels was significantly greater than that of WT channels (at least P < 0.02). E, concentration–response curves were constructed for ITB/ICTRL measured as in A at HP =–180 mV and fitted with eqn (1). Each data point is the mean ± s.e.m. of at least 4 cells. The calculated IC50 values ± s.e. of the fit were 469.0 ± 31.8 μm for WT and 481.2 ± 21.4 μm for R1448C.
Inactivated state-dependent affinity of WT and mutant sodium channels for flecainide
To verify whether flecainide binds to sodium channels in the inactivated state, we repeated the same protocol as in Fig. 2A using various holding potentials more negative than the activation threshold. For WT channels, changing the HP from –180 to –120 mV produced no change in INa block, whereas tonic block was significantly greater at –90 mV (Fig. 2C and D). For R1448C channels, the extent of block was already significantly enhanced when depolarizing the cell to –120 mV. These results indicate that flecainide can bind to inactivated skeletal muscle channels without channel opening, i.e. through closed-state inactivation. Since there is no difference in slow inactivation between WT and R1448C channels (Hayward et al. 1999), the difference between the two channels in drug sensitivity observed at –120 and –90 mV is not imputable to slow inactivation. Also, it was not due to ultra-slow inactivation because we observed no difference between 90 s and 180 s prepulses (not shown). Thus the difference in drug sensitivity between the two channels observed at these potentials may result from difference in voltage dependence of fast inactivation.
In a previous study, we obtained a quite good estimate of the binding affinity constant to fast inactivated channels (KI) for clenbuterol by measuring the shift of steady-state availability curves induced by the drug (Bean et al. 1983; Desaphy et al. 2003). Thus we repeated this protocol for both R1448C and WT channels in the presence of various concentrations of flecainide. The steady-state availability relationships were fitted with a Boltzmann equation,
| (2) |
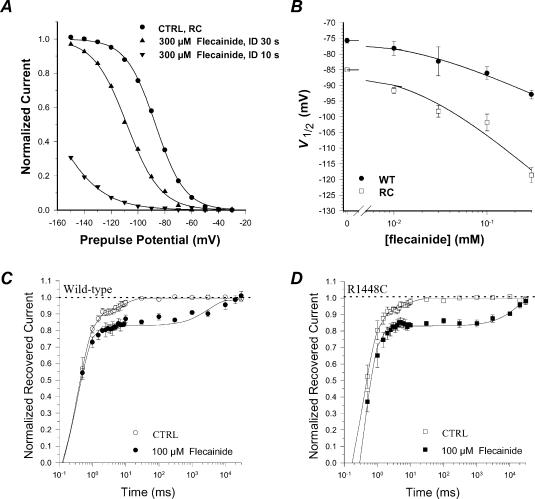
where V1/2 (mV) is the half-maximum inactivation potential and S (mV) is the slope factor. As already described, inactivation of R1448C INa occurred at ∼10-mV more negative potentials and S was lower as compared to WT INa (Desaphy et al. 2001). In the presence of flecainide, the availability curves of both channels were negatively shifted in a dose-dependent manner (Fig. 3A and B). Nevertheless the drug did not change the steepness factor of each channel, as expected from the modulated receptor model that forecasts a strong 1 : 1 binding to the inactivated channel with respect to binding to the closed channel (Hille, 1977; Bean et al. 1983). The half-maximum inactivation potential was reported as a function of flecainide concentration, and the relationships were fitted with the equation
| (3) |
where SCTRL and V1/2,CTRL were the mean values of S and V1/2 measured in control conditions (Bean et al. 1983; Fan et al. 1996; Desaphy et al. 2003). Although the shift was greater for R1448C channels, the KI value was quite similar for mutant and WT channels (KI= 18 μm). Indeed, the shift difference between the WT and R1448C channels was related to the different slope factors of availability curves between the two channels (see equation parameters of the fits in Fig. 3).
Figure 3. Flecainide affinity for inactivated wild-type and R1448C hNav1.4 channels and recovery from flecainide block.
A, effects of flecainide on voltage dependence of INa availability in a tsA201 cell expressing R1448C channels. INa was evoked by a 20 ms-long test pulse to –30 mV after a 50 ms-long conditioning pulses to potentials ranging from –150 to –30 mV in 10 mV increments. Pulses were delivered at 10 s or 30 s interval duration (ID) as indicated and HP was –180 mV. The peak INa recorded during the test pulse was plotted against the conditioning pulse potential. The relationships were fitted with eqn (2) (see Results). The values of the half-maximum inactivation potential, V1/2, along with the s.e. of the fit were –87.0 ± 0.2 mV in control, –108.2 ± 0.4 mV in the presence of 300 μm flecainide with 30 s ID, and –164.7 ± 0.8 in the presence of 300 μm flecainide with 10 s ID. The values of the slope factor, S, were 11.0 ± 0.2 mV in control, 12.6 ± 0.3 mV in the presence of 300 μm flecainide with 30 s ID, and 17.9 ± 0.6 mV in the presence of 300 μm flecainide with 10 s ID. Availability curves were normalized with respect to their own INa,max. B, the affinity of flecainide for inactivated channels (KI) was evaluated by plotting V1/2 values, determined as in A, against flecainide concentration. Each data point is the mean ± s.e.m. from at least 4 cells. The relationships were fitted with eqn (3) (see Results). The values of KI along with the s.e. of the fit were 17.1 ± 1.1 μm for WT (V1/2,CTRL was –75.7 mV and SCTRL was 5.8 mV in eqn (3)) and 17.8 ± 2.9 μm for R1448C channels (V1/2,CTRL was –85.0 mV and SCTRL was 11.1 mV). C and D, the recovery of WT and R1448C channels from inactivation and from flecainide block was measured at –180 mV. A recovery pulse at the HP of increasing duration was included between two test pulses at –30 mV. The peak INa recorded during the second test pulse was normalized with respect to the peak INa recorded during the first test pulse and means ± s.e.m. were calculated from at least 5 cells to be plotted against the recovery time. The relationships were fitted with eqn (4) (see Results). Fitted parameters are reported in Table 1.
Recovery from inactivation and from flecainide block
It should be noted that interpulse intervals in the availability protocol must be at least 30 s long to prevent accumulation of flecainide block and consequent over-estimation of the drug-induced shift (Fig. 3A). This is consistent with the development of use-dependent block we observed at 0.1 Hz frequency stimulation (see Fig. 2). To estimate more accurately recovery time from flecainide block, we included a recovery pulse of increasing duration at –180 mV between two test pulses at –30 mV (Fig. 3C and D). The amplitude of INa elicited by the second test pulse was normalized with respect to amplitude of first pulse INa and reported as a function of recovery pulse duration. The relationships were best fitted with a biexponential function:
| (4) |
where A1 and A2 are the relative contributions of the exponential time constants τ1 (ms) and τ2 (ms). The term A0 was introduced in eqn (4) to take into consideration the delay before recovery from inactivation that has been reported by others (Kuo & Bean, 1994; Groome et al. 1999). In drug-free condition, a 0.5 ms-long conditioning pulse allowed ∼50% of WT channels to recover from fast inactivation and full recovery was reached in ∼30 ms (Fig. 3C). In previous studies, several mutations at position 1448 were shown to accelerate recovery from inactivation, but this effect was less significant at hyperpolarized voltage (Fan et al. 1996; Ji et al. 1996; Bendahhou et al. 1999; Groome et al. 1999; Weckbecker et al. 2000). Accordingly, we found little difference in recovery from inactivation between R1448C and WT channels at –180 mV (Table 1). In the presence of flecainide, the time course of the fast component was similar to that observed without drug, and the relationships were fitted with eqn (4) using the τ1 value obtained in control conditions. For WT channels, flecainide increased A2 from ∼10% to ∼15% and drastically slowed τ2 from ∼7 ms to ∼3 s (Table 1). For R1448C channels, the effect of flecainide on A2 was similar, but the effect on τ2 was more pronounced (from ∼6 ms to ∼16 s). It is clear from Fig. 3 that an interval duration of 10 s (0.1 Hz) between two depolarizing pulses allowed fewer R1448C channels to recover from flecainide block as compared to WT, thereby explaining the greater use-dependent block of R1448C channels shown in Fig. 2.
Table 1.
Fit parameters of INa recovery at –180 mV from inactivation and from flecainide block of hNav1.4 and R1448C mutant occurring at –30 mV
| Channel | n | A0 | A1 | τ1 (ms) | A2 | τ2 (ms) | |
|---|---|---|---|---|---|---|---|
| Wild-type | CTRL | 5 | –0.37 ± 0.13 | 1.24 ± 0.13 | 0.36 ± 0.03 | 0.12 ± 0.01 | 7.3 ± 0.9 |
| Flecainide | 5 | –0.34 ± 0.10 | 1.17 ± 0.11 | 0.36 | 0.16 ± 0.02 | 3130 ± 1317 | |
| R1448C | CTRL | 6 | –0.51 ± 0.16 | 1.40 ± 0.15 | 0.37 ± 0.04 | 0.11 ± 0.01 | 6.1 ± 1.1 |
| Flecainide | 5 | –0.98 ± 0.09 | 1.81 ± 0.09 | 0.37 | 0.18 ± 0.03 | 16510 ± 8638 |
Parameters of the fit obtained with eqn (4) are expressed along with the s.e. of the fit. In the presence of the drug, the value of τ1 was fixed to the value found in CTRL.
Effect of flecainide on sodium current decay
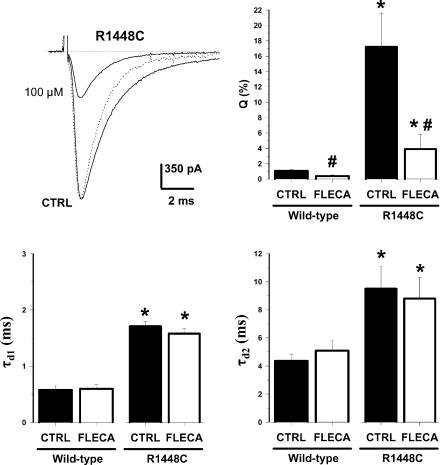
Since the slowing of INa decay rate is a common feature of myotonia-causing mutations, such a defect was proposed as a determinant of myotonic attacks (Yang et al. 1994). Counteracting this biophysical defect may therefore constitute a specific approach against myotonia. In Fig. 4, the superposition of control and flecainide-modified INa of R1448C channels, as well as the drug-modified INa normalized with respect to control peak INa (dashed line), indicates that the drug was able to accelerate current decay. To quantify such an effect, the current decays of WT and R1448C channels in control and in the presence of 100 μm flecainide were fitted with a biexponential function including a residual current (R),
| (5) |
Figure 4. Effects of flecainide on INa decay rate of WT and R1448C hNav1.4 channels.
The INa was evoked by 25 ms-long test pulses to –30 mV applied at 0.1 Hz from the V1/2 of –180 mV before (CTRL) and after application of 100 μm flecainide. To allow direct inspection of drug effect on current decay, INa measured during drug exposure was scaled with respect to peak amplitude of control INa (dashed line). The parameters τd1, τd2 and Q were calculated form the fit of current decay with eqn (5). Each bar represents the mean ± s.e.m. from 8 (WT) and 11 cells (R1448C). Statistical analysis was performed using Student's paired t test, * indicating at least P < 0.01 versus CTRL wild-type and # indicating at least P < 0.02versus relative control.
Such an equation allowed an excellent fit to experimental data in >90% of the cells; the other <10% of cells were discarded from analysis. As previously shown (Desaphy et al. 2001), the R1448C mutation significantly prolonged both τd1 and τd2, and drastically increased the contribution of τd2 (term Q in eqn (5)) to total current decay (Fig. 4). In the presence of flecainide, the acceleration of current decay was attributable mainly to a reduction of Q with respect to P, whereas R and both time constants remained unchanged (Fig. 4). The two time constants may be the macroscopic manifestation of channels gating in two inactivating modes, named M1 and M2 (Zhou et al. 1991). The M2 gating mode is largely repressed in wild-type channels expressed in mammalian cells, whereas it is exacerbated by myotonic mutations (Moran et al. 1999). The reduction of Q by flecainide therefore suggests that the drug may stabilize mutant channels in the M1 gating mode or may preferentially block M2 gating channels.
Access route of flecainide to its molecular binding site
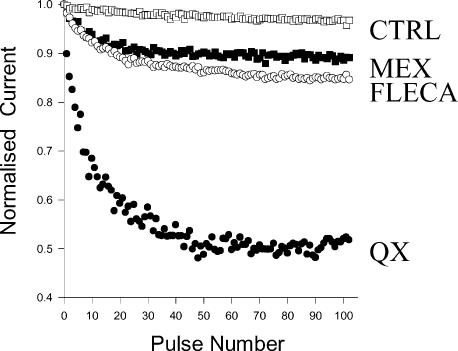
It is generally proposed that charged class I antiarrhythmic drugs reach their binding site from the intracellular side of the channel pore, but that cardiac sodium channels present also an external access path for membrane-impermeant quaternary amine local anaesthetics, which is not found in skeletal muscle and neuronal sodium channels (Frazier et al. 1970; Qu et al. 1995). By assessing use-dependent block in the presence of intracellular flecainide, two studies performed on native and heterologously expressed cardiac channels have proposed that flecainide may reach its binding site from an extracellular route (Nitta et al. 1992; Grant et al. 2000). To test this hypothesis in the skeletal muscle sodium channel, flecainide was included in the micropipette solution and, 5–10 min after achieving the whole-cell configuration, test pulses from –120 to –30 mV were applied at 10 Hz stimulation frequency to assess use-dependent block (Fig. 5). In drug-free conditions, such protocol produced less than 5% current reduction. With 300 μm QX-314 in the pipette, a membrane-impermeant quaternary lidocaine analogue, use-dependent block of INa developed to ∼50% of control. With 100 μm flecainide, use-dependent block was only ∼8% of control INa (not shown). Adding 1 mm flecainide to the pipette solution, use-dependent block reached ∼15% of control INa and was similar to that produced by 1 mm mexiletine (Fig. 5). This result suggests that flecainide can reach its binding site from the intracellular side. The reduced use-dependent block by internally applied, membrane-permeant drugs as compared to that obtained with external application of the same drugs is most probably due to the diffusion of the internally applied drug out of the cell because of the large difference in volume between the internal and external cell compartments. Accordingly, pronounced use-dependent block was observed with internal application of a membrane-impermeant, quaternary analogue of flecainide in a recent study performed on cardiac channels (Liu et al. 2003).
Figure 5. Effects of internal application of drugs on wild-type hNav1.4 channels.
Development of use-dependent block after internal diffusion of control pipette solution (CTRL, □), or pipette solution supplemented with 1 mm mexiletine (MEX, ▪), 1 mm flecainide (FLECA, ○), or 300 μm QX-314 (QX, •). The tsA201 cells expressing WT hNav1.4 channels were held at –120 mV and received a 25 ms-long depolarizing pulse to –30 mV every 0.1 s (10 Hz) to elicit INa. This protocol was applied about 5 min after achieving the whole-cell configuration to allow pipette solution to diffuse well within the cell. Peak INa measured at each test pulse was normalized with respect to the first pulse INa. Each data point is the mean from at least 3 cells.
Simulation of flecainide block according to the modulated receptor hypothesis
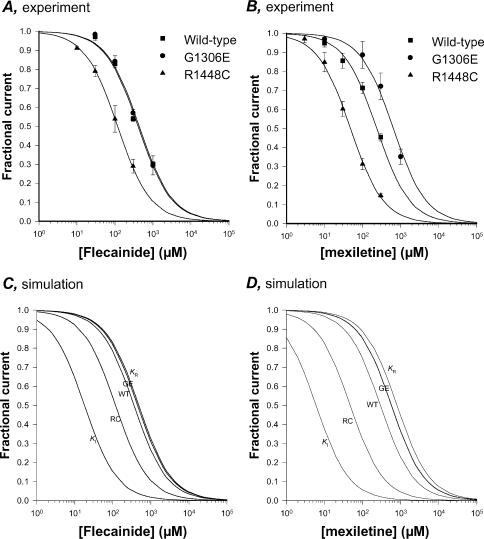
We performed simulations to test whether the modulated receptor hypothesis may account for the voltage dependence of flecainide block (Hille, 1977). Using the KR and KI values for mexiletine and flecainide we determined in previous and the present studies, we applied the modulated receptor hypothesis to block of WT, G1306E, and R1448C channels, using the equation
| (6) |
where KAPP is the apparent affinity constant at the potential considered and the terms h and (1 –h) are the proportions of closed and inactivated channels at this potential, as determined from steady-state availability curves (Bean et al. 1983). The IC50 values for tonic block obtained experimentally at the HP of –120 mV for both mexiletine and flecainide are quite similar to the theoretical values of KAPP calculated with eqn (6) (Table 2). The experimental and theoretical dose–response relationships are compared in Fig. 6. The model confirms that changing the HP from –180 to –120 mV has little effect on flecainide block of WT and G1306E channels but a pronounced effect on R1448C channel blockade (Fig. 6A and C). Conversely, because the affinity of mexiletine for inactivated channels was stronger (lower KI value), depolarizing the membrane to the HP of –120 mV has a marked effect also on WT channel blockade by mexiletine, in accord with the experimental data (Fig. 6B and D).
Table 2.
Simulation parameters of INa blockade by flecainide and mexiletine according to the modulated receptor hypothesis
| Drug | KR (μM) | KI (μM) | Channel | V1/2 (mV) | S (mV) | h | KAPP (μM) | IC50 (μM) |
|---|---|---|---|---|---|---|---|---|
| Mexiletine | 800 | 6 | Wild-type | –79.1 ± 2.5 | 7.9 ± 0.2 | 0.987 ± 0.004 | 294.1 | 236 ± 14.8 |
| G1306E | –66.5 ± 1.8 | 8.4 ± 0.5 | 0.997 ± 0.007 | 572.7 | 642 ± 49.4 | |||
| R1448C | –89.9 ± 3.1 | 12.9 ± 0.7 | 0.880 ± 0.014 | 47.4 | 48 ± 1.9 | |||
| Flecainide | 480 | 18 | Wild-type | –79.1 ± 2.5 | 7.9 ± 0.2 | 0.987 ± 0.004 | 359.9 | 407 ± 39.1 |
| G1306E | –66.5 ± 1.8 | 8.4 ± 0.5 | 0.997 ± 0.007 | 445.7 | 435 ± 42.4 | |||
| R1448C | –89.9 ± 3.1 | 12.9 ± 0.7 | 0.880 ± 0.014 | 117.6 | 117 ± 2.8 |
The values of dissociation constants for closed channels (KR) and inactivated channels (KI) were calculated experimentally in the present study for flecainide and a previous study for mexiletine (Desaphy et al. 2001). Each drug showed the same state-specific affinities to all the three channels. The half-maximum inactivation potential (V1/2) and the slope factor (S) were determined from the fit of steady-state availability curves specific to each channel and are given along with the s.e. of the fit. The proportion of closed channels (h) at a holding potential (HP) of –120 mV is given as mean ± s.e.m. from 17–33 cells. The theoretical apparent affinities KAPP were calculated from eqn (6) (see Results) and were compared to the IC50 values (indicated along with the s.e.of the fit) calculated from dose–response curves obtained experimentally at the HP of –120 mV (see Fig. 6).
Figure 6. Simulation of flecainide and mexiletine effects on hNav1.4 channels using the modulated receptor model.
A and B, experimental concentration–response curves for flecainide and mexiletine effect on wild-type, R1448C, and G1306E hNav1.4 channels were constructed at a holding potential (HP) of –120 mV in absence of depolarization as described in Fig. 2. Each data point is the mean ± s.e.m. of at least 3 cells. The relationships were fitted with eqn (1) (see Results) and the IC50 values are reported in Table 2. C and D, the theoretical curves according to the modulated receptor hypothesis were built using eqn (1) (see Results) with the KAPP values calculated for WT, G1306E, and R1448C channels using eqn (6) at a HP of –120 mV and reported in Table 2. The lines labelled with KR and KI were obtained using KR and KI values reported in Table 2 for each drug and describe the theoretical relationships for a hypothetical pure block of closed channels (KR) and a hypothetical pure block of inactivated channels (KI).
Discussion
Molecular mechanism of flecainide block
Flecainide is a sodium channel blocker that has been studied in various animal models of arrhythmia and used against ventricular and supraventricular tachyarrhythmia in patients without structural heart disease (American Heart Association, 2000). Because of its potent inhibition of cardiac sodium channels and slow recovery kinetics, flecainide has been included in the class IC of antiarrhythmic drugs (Vaughan Williams, 1984). The molecular mechanism of flecainide block has been addressed on cardiac and neuronal sodium channels, but little is known about drug interaction with skeletal muscle sodium channels. Based on voltage dependence and kinetic analysis of whole-cell and single-channel current block of cardiac and brain sodium channels, flecainide is widely considered as an open channel blocker (Anno & Hondeghem, 1990; Nitta et al. 1992; Ragsdale et al. 1996; Nagatomo et al. 2000; Grant et al. 2000). However, such a view has been recently challenged by two studies using cardiac sodium channel mutants responsible for LQT3 and Brugada syndromes (Viswanathan et al. 2001; Liu et al. 2002). Both studies concluded that flecainide binds to inactivated states of the cardiac channels. Yet, one proposed that flecainide block occurs through closed-state inactivation that develops below the resting membrane potential, whereas the other retained the view that flecainide block requires channel opening.
Our study clearly demonstrates that closed-state inactivation is a determinant of flecainide block in cells expressing wild-type and mutant hNav1.4 channels. For WT channels, the extent of block was dependent on holding voltage below the activation threshold, being greater at –90 mV as compared to –120 and –180 mV. This behaviour was further exacerbated by the R1448C mutation that produces a negative shift in channel steady-state fast inactivation voltage dependence. These effects, as well as the differences between mexiletine and flecainide in voltage dependence of sodium channel blockade, were fully explained by the modulated receptor hypothesis that predicts the preferential binding of flecainide to inactivated channels as compared to closed channels. Thus flecainide can be considered as an inactivated-channel blocker of human skeletal muscle sodium channels.
Importantly, flecainide block developed at potentials that did not allow channels to open. We also verified that recovery of WT and R1448C channels from flecainide block does not require channel opening, since sodium current recovered control amplitude on return to drug-free solution in the absence of depolarization (not shown). Thus flecainide can access and leave its binding site without channel opening, although we cannot exclude that channel opening may favour transit of charged drug as previously suggested for the cardiac channel (Liu et al. 2003). Without single-channel recordings, it is hazardous to definitely exclude open-channel blockade by flecainide. However, flecainide did not modify the two decay time constants that describe the decay of WT and R1448C currents. This suggests that the channel mean open times were not modified by the drug, arguing against open channel blockade.
The mechanism of flecainide block we described on skeletal muscle sodium channels, including the internal access path toward the binding site, is in contrast with many of the studies performed with the cardiac sodium channels. The mechanistic basis that governs the differences in drug affinity between the two channel isoforms is still debated. Some studies proposed that it depends on differences in channel gating that secondarily alter drug effect (Wright et al. 1997; Nuss et al. 2000), whereas others proposed that it depends on structural differences in the drug receptor site or access (Nuss et al. 1995; Wang et al. 1996; Weiser et al. 1999). Elucidation of the mechanisms that account for the differences in flecainide block would require the direct comparison of drug effect between the two channels and is beyond the scope of this paper.
Effect of R1448C mutation on recovery from flecainide block
Recovery from flecainide block was assessed at –180 mV in an attempt to minimize any bias introduced by mutation-induced changes in inactivation voltage dependence. Interestingly, the time constant corresponding to recovery from flecainide block was larger for R1448C as compared to WT channels, which provides a rationale for increased use-dependent block of R1448C INa. A similar effect of mexiletine was described on R1448H channels (Weckbecker et al. 2000). The arginine at position 1448 is the outermost charged residue of the voltage sensor within domain IV and contributes approximately 2/3 of the gating charge of the S4 segment (Sheets & Hanck, 1999). Immobilization of S4 segments of domains III and IV in an outward position have been associated with the slow time course of recovery from inactivation, and binding of lidocaine to cardiac sodium channels has been shown to stabilize the gating charges of these two segments in the depolarized conformation (Cha et al. 1999; Sheets & Hanck, 2003). Accordingly, our results suggest that neutralization of the main gating charge in DIV-S4 through the R1448C mutation enhances voltage sensor immobilization by flecainide, thereby slowing the recovery time constant.
Therapeutic interest of flecainide in myotonic syndromes
Use of flecainide has appeared valuable in sodium channelopathies of heart and skeletal muscle where mexiletine was less efficient (Rosenfeld et al. 1997; Benhorin et al. 2000; Abriel et al. 2000). In the heart, such improvement may result from the different mechanisms of action of the two drugs on cardiac sodium channels (Nagatomo et al. 2000). In the skeletal muscle, molecular mechanisms of flecainide block are quite similar to those of mexiletine, and gating changes induced by myotonic mutations may account for the different drug sensitivities of encoded channels (Desaphy et al. 2001). Although some mutations may also affect more directly the binding site or the access path of the drugs (Fan et al. 1996; Takahashi & Cannon, 2001), we believe that, as for mexiletine, voltage dependence of channel availability may be considered as a general index of mutant channel responsiveness to flecainide therapy.
There may be two main motivations in using flecainide instead of mexiletine in myotonic syndromes. First, flecainide use-dependent block develops at frequencies lower than those required by mexiletine, which may help to prevent the development of myotonic runs of action potentials. Such mechanism should apply to all forms of myotonia, independently of the genetic origin. Second, for those mutations such as G1306E that produce a positive shift in sodium channel availability, flecainide may target more efficiently the mutated channel as compared to mexiletine. Indeed, we previously proposed that mexiletine most probably exerts its beneficial effect by blocking preferentially WT channels in the heterozygous patients carrying these mutations (Desaphy et al. 2001). Since flecainide block is less dependent on voltage-dependent channel availability, owing to a smaller difference between KI and KR, the difference in flecainide block between mutant and WT channels is less as compared with mexiletine block. Moreover flecainide is able to accelerate INa decay rate of myotonic mutants, which may represent a specific therapeutic approach toward sodium channel myotonias. Thus, flecainide appears to be a good candidate to improve the antimyotonic therapy in sodium channelopathies. For instance, carriers of the V445M mutation that suffer from painful myotonia are resistant to mexiletine and tocainide therapy, but respond dramatically to flecainide (Rosenfeld et al. 1997).
In conclusion, our findings provide a general framework for developing a pharmacogenetic therapy against sodium channel myotonia. Flecainide and mexiletine exhibit the same mechanism of block of skeletal muscle sodium channels, but flecainide blocks some mutant channels more efficiently than mexiletine. The choice of the drug can be addressed on the basis of gating defects induced by the mutation, especially the specific voltage dependence of channel availability.
Acknowledgments
We thank Luciano Coropulis for help with experiments and Dr Bruce P. Bean for help with eqn (3). This work was supported by Italian ‘Ministero dell'Istruzione, dell'Università e della Ricerca’ (FIRB RBNE01XMP4) to D. Conte Camerino.
References
- Abriel H, Wehrens XHT, Benhorin J, Kerem B, Kass RS. Molecular pharmacology of the sodium channel mutation D1790G linked to the long-QT syndrome. Circulation. 2000;102:921–925. doi: 10.1161/01.cir.102.8.921. [DOI] [PubMed] [Google Scholar]
- American Heart Association. International guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 6: Advanced Cardiovascular Life Support: Section 5: Pharmacology I: Agents for Arrhythmias. Circulation. 2000;102:I112–I128. [PubMed] [Google Scholar]
- Anno T, Hondeghem LM. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage-dependence of recovery. Circ Res. 1990;66:789–803. doi: 10.1161/01.res.66.3.789. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J General Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahhou S, Cummins TR, Kwiecinski H, Waxman SG, Ptacek LJ. Characterization of a new sodium channel mutation at arginine 1448 associated with moderate paramyotonia congenita in humans. J Physiol. 1999;518:337–344. doi: 10.1111/j.1469-7793.1999.0337p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhorin J, Taub R, Goldmit M, Kerem B, Kass RS, Windman I, Medina A. Effects of flecainide in patients with new SCN5A mutation. Mutation-specific therapy for long-QT syndrome. Circulation. 2000;101:1698–1706. doi: 10.1161/01.cir.101.14.1698. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Voltage-gated ion channelopathies of the nervous system. Clin Neurosci Res. 2001;57:772–779. [Google Scholar]
- Cha A, Ruben PC, George AL, Jr, Fujimoto E, Bezanilla F. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron. 1999;22:73–87. doi: 10.1016/s0896-6273(00)80680-7. [DOI] [PubMed] [Google Scholar]
- Desaphy J, De Luca A, Tortorella P, De Vito D, George AL, Jr, Conte Camerino D. Gating of myotonic Na channel mutants defines the response to mexiletine and a potent derivative. Neurology. 2001;57:1849–1856. doi: 10.1212/wnl.57.10.1849. [DOI] [PubMed] [Google Scholar]
- Desaphy J-F, Pierno S, De Luca A, Didonna D, Conte Camerino D. Different ability of clenbuterol and salbutamol to block sodium channels predicts their therapeutic use in muscle excitability disorders. Mol Pharmacol. 2003;63:659–670. doi: 10.1124/mol.63.3.659. [DOI] [PubMed] [Google Scholar]
- Fan Z, George AL, Jr, Kyle JW, Makielski JC. Two human paramyotonia congenita mutations have opposite effects on lidocaine block of Na+ channels expressed in a mammalian cell line. J Physiol. 1996;496:275–286. doi: 10.1113/jphysiol.1996.sp021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhauer R, Mitrovic N, Deymeer F, Lehmann-Horn F, Lerche H. Effect of temperature and mexiletine on the F1473S Na+ channel mutation causing paramyotonia congenita. Pflugers Arch. 1998;436:757–765. doi: 10.1007/s004240050699. [DOI] [PubMed] [Google Scholar]
- Frazier DT, Narahashi T, Yamada M. The site of action and active form of local anesthetics. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970;171:45–51. [PubMed] [Google Scholar]
- Grant AO, Chandra R, Keller C, Carboni M, Starmer CF. Block of wild-type and inactivation-deficient cardiac sodium channels IFM/QQQ stably expressed in mammalian cells. Biophys J. 2000;79:3019–3035. doi: 10.1016/S0006-3495(00)76538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs RC, Ptácek LJ. Mutations of sodium channels in periodic paralysis. Can they explain the disease and predict treatment. Neurology. 1999;52:1309–1310. doi: 10.1212/wnl.52.7.1309. [DOI] [PubMed] [Google Scholar]
- Groome JR, Fujimoto E, George AL, Jr, Ruben PC. Differential effects of homologous S4 mutations in human skeletal muscle sodium channels on deactivation gating from open and inactivated states. J Physiol. 1999;516:687–698. doi: 10.1111/j.1469-7793.1999.0687u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LJ, Sandoval GM, Cannon SC. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology. 1999;52:1447–1453. doi: 10.1212/wnl.52.7.1447. [DOI] [PubMed] [Google Scholar]
- Hilber K, Sandtner W, Kudlacek O, Schreiner B, Glaser I, Schütz W, Fozzard HA, Dudley SC, Todt H. Interactions between fast and ultra-slow inactivation in the voltage-gated sodium channel. J Biol Chem. 2002;277:37105–37115. doi: 10.1074/jbc.M205661200. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J General Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S, George AL, Jr, Horn R, Barchi RL. Paramyotonia congenita mutations reveal different roles for segments S3 and S4 of domain D4 in hNav1.4 sodium channel gating. J General Physiol. 1996;107:183–194. doi: 10.1085/jgp.107.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K, Lerche H, Lehmann-Horn F. Skeletal muscle channelopathies. J Neurol. 2002;249:1493–1502. doi: 10.1007/s00415-002-0871-5. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Bean BP. Na+ channels must deactivate to recover from inactivation. Neuron. 1994;12:819–829. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Liu H, Atkins J, Kass RS. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: Evidence from experiments with neutral and quaternary flecainide analogues. J General Physiol. 2003;121:199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Tateyama M, Clancy CE, Abriel H, Kass RS. Channels openings are necessary but not sufficient for use-dependent block of cardiac Na+ channels by flecainide: evidence from the analysis of disease-linked mutations. J General Physiol. 2002;120:39–51. doi: 10.1085/jgp.20028558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran O, Nizzari M, Conti F. Myopathic mutations affect differently the inactivation of the two gating modes of sodium channels. J Bioenerg Biomembr. 1999;31:591–608. doi: 10.1023/a:1005473129183. [DOI] [PubMed] [Google Scholar]
- Moxley RT. Channelopathies. Curr Treatment Opt Neurol. 2000;2:31–47. doi: 10.1007/s11940-000-0022-1. [DOI] [PubMed] [Google Scholar]
- Nagatomo T, January CT, Makielski JC. Preferential block of late sodium current in the LQT3 ÄKPQ mutant by the class Ic antiarrhythmic flecainide. Mol Pharmacol. 2000;57:101–107. [PubMed] [Google Scholar]
- Nitta J-I, Sunami A, Marumo F, Hiraoka M. States and sites of actions of flecainide on guinea-pig cardiac sodium channels. Eur J Pharmacol. 1992;214:191–197. doi: 10.1016/0014-2999(92)90118-n. [DOI] [PubMed] [Google Scholar]
- Nuss HB, Kambouris NG, Marban E, Tomaselli GF, Balser JR. Isoform-specific lidocaine block of sodium channels explained by differences in gating. Biophys J. 2000;78:200–210. doi: 10.1016/S0006-3495(00)76585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss HB, Tomaselli GF, Marban E. Cardiac sodium channels (hH1) are intrinsically more sensitive to tonic block by lidocaine than are skeletal muscle (μ1) channels. J General Physiol. 1995;106:1193–1210. doi: 10.1085/jgp.106.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA. Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+ channel. Proc Natl Acad Sci U S A. 1995;92:11839–11843. doi: 10.1073/pnas.92.25.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J, Sloan-Brown K, George AL., Jr A novel muscle sodium channel mutation causes painful congenital myotonia. Ann Neurol. 1997;42:811–814. doi: 10.1002/ana.410420520. [DOI] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Gating of skeletal and cardiac muscle sodium channels in mammalian cells. J Physiol. 1999;514:425–436. doi: 10.1111/j.1469-7793.1999.425ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Molecular action of lidocaine on the voltage sensors of sodium channels. J General Physiol. 2003;121:163–175. doi: 10.1085/jgp.20028651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi MP, Cannon SC. Mexiletine-block of disease-associated mutations in S6 segments of the human skeletal muscle Na+ channel. J Physiol. 2001;537:701–714. doi: 10.1111/j.1469-7793.2001.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan Williams EM. Subgroups of class I antiarrhythmic drugs. Eur Heart J. 1984;5:96–98. doi: 10.1093/oxfordjournals.eurheartj.a061632. [DOI] [PubMed] [Google Scholar]
- Vilin YY, Ruben PC. Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem Biophys. 2001;35:171–190. doi: 10.1385/CBB:35:2:171. [DOI] [PubMed] [Google Scholar]
- Viswanathan PC, Bezzina CR, George AL, Jr, Roden DM, Wilde AAM, Balser JR. Gating-dependent mechanisms for flecainide action in SCN5A-linked arrhythmia syndromes. Circulation. 2001;104:1200–1205. doi: 10.1161/hc3501.093797. [DOI] [PubMed] [Google Scholar]
- Wang DW, Nie L, George AL, Bennett PB. Distinct local anesthetic affinities in Na+ channel subtypes. Biophys J. 1996;70:1700–1708. doi: 10.1016/S0006-3495(96)79732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, VanDeCarr D, Ruben PC, George AL, Jr, Bennett PB. Functional consequences of a domain I/S6 segment sodium channel mutation associated with painful congenital myotonia. FEBS Lett. 1999;448:231–234. doi: 10.1016/s0014-5793(99)00338-5. [DOI] [PubMed] [Google Scholar]
- Weckbecker K, Würz A, Mohammadi B, Mansuroglu T, George AL, Jr, Lerche H, Dengler R, Lehmann-Horn F, Mitrovic N. Different effects of mexiletine on two mutant sodium channels causing paramyotonia congenita and hyperkalemic periodic paralysis. Neuromuscul Disord. 2000;10:31–39. doi: 10.1016/s0960-8966(99)00060-7. [DOI] [PubMed] [Google Scholar]
- Weiser T, Qu Y, Catterall WA, Scheuer T. Differential interaction of R-mexiletine with the local anesthetic receptor site on brain and heart sodium channel α-subunits. Mol Pharmacol. 1999;56:1238–1244. doi: 10.1124/mol.56.6.1238. [DOI] [PubMed] [Google Scholar]
- Wright SN, Wang S, Kallen RG, Wang GK. Differences in steady-state inactivation between Na channel isoforms affect local anesthetic binding affinity. Biophys J. 1997;73:779–788. doi: 10.1016/S0006-3495(97)78110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Ji S, Zhou M, Ptacek LJ, Barchi RL, Horn R, George AL., Jr Sodium channel mutations in paramyotonia congenita exhibit similar biophysical phenotypes in vitro. Proc Natl Acad Sci U S A. 1994;91:12785–12789. doi: 10.1073/pnas.91.26.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JY, Potts JF, Trimmer JS, Agnew WS, Sigworth FJ. Multiple gating modes and the effect of modulating factors on the m1 sodium channel. Neuron. 1991;7:775–785. doi: 10.1016/0896-6273(91)90280-d. [DOI] [PubMed] [Google Scholar]