Abstract
Elevated intramyocellular triglyceride (IMTG) is strongly associated with insulin resistance, though a cause and effect relationship has not been fully described. Insulin sensitivity and IMTG content are both dynamic and can alter rapidly in response to dietary variation, physical activity and thermoregulatory response. Physically active humans (athletes) display elevated IMTG content, but in contrast to obese persons, are insulin sensitive. This paradox has created confusion surrounding the role of IMTG in the development of insulin resistance. In this review we consider the modern athlete as the physiological archetype of the Late Palaeolithic hunter–gatherer to whom the selection pressures of food availability, predation and fluctuating environmental conditions applied and to whom the genotype of modern man is virtually identical. As food procurement by the hunter–gatherer required physical activity, ‘thrifty’ genes that encouraged immediate energy storage upon refeeding after food deprivation (Neel, 1962) must have been of secondary importance in survival to genes that preserved physical capacity during food deprivation. Similarly genes that enabled survival during cold exposure whilst starved would be of primary importance. In this context, we discuss the advantage afforded by an elevated IMTG content, and how under these conditions, a concomitant muscle resistance to insulin-mediated glucose uptake would also be advantageous. In sedentary modern man, adiposity is high and skeletal muscle appears to respond as if a state of starvation exists. In this situation, elevated plasma lipids serve to accrue lipid and induce insulin resistance in skeletal muscle. Reversal of this physiological state is primarily dependant on adequate contractile activity, however, in modern Western society, physical inactivity combined with abundant food and warmth has rendered IMTG a redundant muscle substrate.
Introduction
An association exists between body adiposity and impaired carbohydrate metabolism (Coon et al. 1989). This association is such that overweight persons who become fatter develop an impaired ability to clear glucose from the blood (Rosenbaum et al. 2000) and obese persons losing body fat increase glucose tolerance (Coon et al. 1989; Colman et al. 1995). The prime cause of this impaired glycaemic control is a resistance in some tissues to the effects of insulin on glucose uptake. This insulin resistance when untreated often leads to type 2 diabetes, a ‘disease’ condition afflicting approximately 6% of the Western population (Black, 2002). Indeed obesity and the associated insulin resistance have been recently referred to as an ‘epidemic’ in both the United States and Australia (Zimmet et al. 2001).
The continuum between adiposity and impaired carbohydrate metabolism has led to the belief that the extra energy stored by the body as fat is causative in the development of insulin resistance. Consequently, much work has and is being done in an attempt to elucidate the mechanisms that underlie this association.
This review will focus on recent research highlighting the association between plasma free fatty acid concentration, intramuscular triglyceride (IMTG) content and insulin resistance. Using a teleological perspective, we discuss how both muscle insulin resistance and a concurrent elevation of IMTG would afford a survival advantage during periods of starvation by ensuring adequate fuel for necessary muscle function whilst protecting remaining blood glucose for the brain. Booth et al. (2000) have proposed that our (selected) genotype requires the stimulus of physical activity for optimal health, and it is in this context that strategies for the prevention and treatment of insulin resistance are briefly discussed.
‘Thrifty’ genes
The association between adiposity and insulin resistance and its high incidence in the developed world suggests that rather than being a ‘disease’ insulin resistance is a normal phenotypic response to protracted caloric surplus caused by excessive energy intake and/or physical inactivity. If so, it also implies that this genotype has, at some period in human evolution, been beneficial to survival and therefore selected for. From such reasoning sprung the ‘thrifty’ genotype hypothesis (Neel, 1962). This theory proposes that genes allowing conservation of glucose and efficient storage of energy as fat during periods of food abundance aid survival during subsequent food scarcity (Fig. 1). However, these previously beneficial genes become deleterious in affluent populations because the advent of agriculture and trade has removed prolonged caloric deficit as a selection pressure. Although detrimental in affluence, the ‘thrifty’ genes have survived the advent of agriculture and food preservation techniques because the deleterious phenotype is exposed well after sexual maturation.
Figure 1.
The survival advantage of a ‘quick insulin trigger’ as suggested by Neel (1962) and how this was proposed to aid survival in primitive man (A) but produce diabetes in modern man (B). (Diagram adapted from Reaven, 1998).
Factors affecting whole-body insulin sensitivity in healthy individuals
Sensitivity to insulin is a physiological process that, in healthy individuals, is rapidly altered in response to changing environmental pressures such as temperature fluctuations and food availability.
Whole-body insulin sensitivity is acutely improved following a single bout of exercise (Perseghin et al. 1996), but the effect is transient and appears to be proportional to exercise severity (Thompson et al. 2001). Chronic physical activity is also associated with improved insulin sensitivity (King et al. 1987; Rodnick et al. 1987) yet this adaptation is also rapidly diminished within six days of cessation of regular exercise (Mikines et al. 1989; Vukovich et al. 1996). Although resumption of training after short-term (10 days) inactivity restores glucose tolerance to near preinactivity levels (Heath et al. 1983), insulin sensitivity is not fully recovered (Dela et al. 1992), indicating a less plastic adaptive effect of training distinct from that of acute exercise.
In animals, whole-body and muscle insulin-mediated (Vallerand et al. 1990) and non-insulin-mediated (Shibata et al. 1989) glucose disposal improves with acute (48 h) exposure to cold. Although there has been little recent research in this area, and no equivalent studies have been performed on humans, these observations in animals may, akin to the effects of exercise, be a result of shivering-induced skeletal muscle contractile activity.
Dietary interventions are also known to induce rapid changes in insulin sensitivity. Insulin sensitivity is decreased in response to a short-term (3 day) high fat diet (Bachmann et al. 2001) or calorie restriction (Webber et al. 1994; Gazdag et al. 2000). The latter effect is evident as little as 14 h after the last meal (Horowitz et al. 2001).
Insulin sensitivity can also be rapidly altered in response to artificial manipulation of plasma free fatty acid (FFA) levels. An increase in FFAs via lipid/heparin infusion reduces insulin sensitivity within 4 h (Roden et al. 1996; Boden et al. 2001), whilst overnight administration of Acipimox lowers FFAs and improves insulin sensitivity in both lean and obese subjects (Santomauro et al. 1999).
A raised plasma FFA concentration has for sometime been implicated in dietary-induced insulin resistance. This was thought to occur in the liver and muscle via the glucose fatty-acid cycle. Glycolysis, and thence glucose uptake, is inhibited by increases in acetyl-CoA and NADH derived from lipid and ketone substrates (Randle et al. 1963). More recently, however, it has been shown that when FFA levels are acutely elevated following lipid/heparin infusion, skeletal muscle glucose-6-phosphate levels fall below control levels, indicating inhibition of glucose transport/phosphorylation (Roden et al. 1996; Krebs et al. 2001). Thus, raised plasma FFA availability may induce insulin resistance via inhibition of glucose transport, rather than inhibition of glycolysis via operation of the glucose fatty-acid cycle.
Triglyceride and insulin resistance in muscle
Skeletal muscle is the primary site of insulin action and is thus inherently linked to the development of whole-body insulin resistance. The mechanism by which raised plasma FFA concentrations may inhibit insulin-stimulated glucose uptake in muscle is not yet fully understood and is likely to be polygenic (Kraegen & Cooney, 1999). However, recent improvements in the ability to measure IMTG content in vivo have implicated these fatty acids stored within the muscle fibre as a ‘middle man’ in the insulin resistance–FFA relationship. In both normal and obese subjects, IMTG content is inversely associated with whole-body insulin sensitivity (Forouhi et al. 1999; Jacob et al. 1999; Krssak et al. 1999; Perseghin et al. 1999; Virkamaki et al. 2001; Goodpaster et al. 2001; Greco et al. 2002), inferring a causal relationship independent of whole-body adiposity. Support for this is found via the observation that, in healthy subjects, inducing an increase in IMTG content via 4–5 h lipid/heparin infusion reduces whole-body sensitivity to insulin (Boden et al. 2001; Brechtel et al. 2001).
The mechanism behind the close coupling of IMTG content and inhibition of insulin-stimulated glucose uptake in muscle is yet to be elucidated, although it appears unlikely that IMTG itself directly impairs insulin action. Rather, an increase in some related fatty acid moiety such as long chain acyl-CoA, ceramide or diacylglycerol, whose appearance is related to elevations in IMTG content, may disturb normal insulin-signalling pathways (Schmitz-Peiffer et al. 1999; Ellis et al. 2000; Itani et al. 2002). The mechanisms via which these may occur have been recently reviewed in detail elsewhere (Kelley et al. 2002; McGarry, 2002; Hegarty et al. 2003). However, that some homogenous groups characterisd by ethnicity (Forouhi et al. 1999; Misra et al. 2003) or high physical activity levels (Goodpaster et al. 2001; Thamer et al. 2003) fail to exhibit an inverse correlation between IMTG content and insulin sensitivity suggests that the conjunction is not simply linear and that other factors may influence the relationship. The precise mechanism notwithstanding, the weight of evidence suggests an elevated IMTG content almost certainly contributes to the development of muscle insulin resistance (McGarry, 2002) and type 2 diabetes (Kelley et al. 2002).
Physiology of IMTG
Cross-sectional studies of children (Ashley et al. 2002), adolescents (Sinh et al. 2002) and adults (Greco et al. 2002) show a direct correlation between whole-body adiposity and IMTG content. Furthermore, reductions in body fat are associated with a reduced IMTG content (Greco et al. 2002). Thus, there is a strong link between body fat content and muscle triglyceride.
Elevations in IMTG can be induced within hours with lipid/heparin infusion (Bachmann et al. 2001; Boden et al. 2001), while formation (in animals) can be curbed if lipolysis (and thus FFA concentration) is inhibited by nicotinic acid administration (Stankiewicz-Choroszucha & Gorski, 1978). Similarly, in healthy individuals, a high carbohydrate diet is a potent inhibitor of IMTG formation (Kiens, 1998; Coyle et al. 2001; Decombaz et al. 2001; Larson-Meyer et al. 2002), also probably via suppressing FFA availability. In contrast IMTG content is increased with a high fat diet (Bachmann et al. 2001; Decombaz et al. 2001) or fasting (Krssak et al. 2000; Stannard et al. 2002), conditions associated with elevated circulating lipid concentrations.
IMTG can be oxidized during exercise, though this is limited to the active musculature (Sacchetti et al. 2002). Although there is some evidence to the contrary (Jansson & Kaijser, 1982; Kayar et al. 1986; Kiens et al. 1993; Wendling et al. 1996), the majority of studies indicate that this endogenous lipid store is measurably reduced during prolonged contractile activity (Johnson et al. 2002; Watt et al. 2002a,b), and this is accentuated when dietary carbohydrate intake is restricted (Stankiewicz-Choroszucha & Gorski, 1978; Johnson et al. 2002).
Measurement of IMTG utilization or accretion using pre- and postintervention measurement (1H-MRS or biochemical and histological methods) are insensitive to the labile nature of the IMTG pool and are unable to measure IMTG turnover. Studies combining 14C-prelabelled IMTG and 13C-labelled FFAs reveal simultaneous hydrolysis and reesterification within muscle (Guo et al. 2000), favouring net hydrolysis during exercise (Dyck et al. 2001) and reesterification at rest provided FFA availability is adequate (Dyck et al. 2000). If these processes occur simultaneously at an equivalent rate, no absolute change in IMTG content would be observed (Dyck & Bonen, 1998; Guo et al. 2000), though fatty acids originally contained within IMTG stores may have been utilized.
Observations of net IMTG degradation, yet simultaneous glycogen repletion, in humans fed high carbohydrate in the postexercise period imply that a further role of IMTG may be to support muscle metabolism during recovery from exhaustive exercise, thereby enabling optimal carbohydrate preservation (Kiens, 1998). However, this finding is not replicated when no food (Krssak et al. 2000), high fat (Decombaz et al. 2000, 2001) or mixed (Decombaz et al. 2000) diets are given in recovery. Therefore, attenuation of IMTG repletion observed with a high carbohydrate recovery diet (Kiens, 1998; Coyle et al. 2001; Decombaz et al. 2001; Larson-Meyer et al. 2002) may result from suppressed FFA availability (Kiens, 1998), and if a stimulus for IMTG hydrolysis such as adrenaline (Watt et al. 2003) persists in the immediate postexercise period, IMTG levels could decrease.
In rodents, shivering-induced muscle contractile activity results in IMTG utilization during cold exposure (Gorski et al. 1981). Whilst no similar direct data is available in cold-exposed humans, indirect measurements suggest net muscle IMTG metabolism (Martineau & Jacobs, 1989), which like exercise, is accentuated when endogenous carbohydrate stores are low (Martineau & Jacobs, 1991).
Thus, the IMTG ‘pool’ is highly dynamic and represents a balance between the processes of formation (which is primarily a function of circulating fatty acid availability), and degradation (which is largely a function of contractile activity). The net effect of these opposing processes may range from a slow and subtle to a large and rapid change in IMTG content, during which time turnover may be fast or slow. Elevated IMTG in obesity may thus reflect a chronically elevated blood lipid profile, an alteration in triglyceride–fatty acid cycling within the muscle, and/or a chronically low muscle lipid oxidation in these individuals.
It can be seen from the above discussion that situations in which IMTG is elevated, such as a high fat diet, fasting and artificial plasma FFA elevation, are also associated with insulin resistance. Conversely, muscular activity (via exercise or shivering), the only known non-pharmacological intervention that directly reduces IMTG, is associated with enhanced insulin sensitivity. However, the apparent relationship between elevated IMTG and insulin resistance becomes uncoupled when physical fitness is considered (Thamer et al. 2003). Physically active individuals (athletes), who have high insulin sensitivity (Goodpaster et al. 2001), paradoxically exhibit elevated IMTG concentrations (Hoppeler et al. 1973; Goodpaster et al. 2001), whereas the insulin resistant obese also display elevated IMTG (Goodpaster et al. 2001; Greco et al. 2002). This comparison has contributed to the confusion in the understanding of the physiology of IMTG and its role in insulin resistance.
It is well known that endurance training increases the ability of muscle to utilize lipid during subsequent exercise (Holloszy, 1967; Mole et al. 1971; Coggan et al. 1993; Martin et al. 1993; Poehlman et al. 1994; Helge et al. 1996; Phillips et al. 1996; Bergman et al. 1999), but there is dissonance over whether this extra lipid oxidized is of intra or extra-muscular origin (Saltin & Astrand, 1993; Martin, 1997). Studies employing a one-legged training model (Turcotte et al. 1992; Kiens et al. 1993) show a greater reliance on FFAs during exercise in the trained state. However, an increased reliance on FFA uptake and utilization is at odds with the blunted lipolytic hormone response (Green et al. 1991), and consequent reduction in FFA availability (Martin III et al. 1993), evident at the same absolute (two-legged) exercise intensity post-training. This incongruency may reflect the different training models used or the very low density lipoproteins (VLDL) triacylglycerol-derived fatty acid contribution (Kiens et al. 1987; Kiens et al. 1993; Helge et al. 2001), though it is argued that the latter remains small (Havel et al. 1967). Cross-sectional and longitudinal studies have demonstrated that endurance training imparts an increase in the capacity of the muscle to store IMTG (Hoppeler et al. 1973; Goodpaster et al. 2001; Schrauwen-Hinderling et al. 2003; Thamer et al. 2003) and hence an increase in its contribution as a substrate during exercise (Lithell et al. 1979; Hurley & Seals, 1986; Jansson & Kaijser, 1987; Martin III et al. 1993). If this is the case, the elevated IMTG content observed in endurance-trained athletes may signify an adaptation designed to ensure sufficient lipid substrate to support subsequent exercise in the presence of reduced FFA delivery (Martin III, 1997). This is supported by the observation that IMTG degradation is significantly attenuated during the later stages of prolonged exercise when plasma FFA availability has substantially increased (Watt et al. 2002a).
Physical capacity versus efficient energy storage in selection
It is proposed that the last significant period of selection pressure applied in the genetic development of man occurred during the Late Palaeolithic prior to the development of agriculture (Barnes, 1968; Washburn, 1976). The biology of Palaeolithic hunter–gatherer man surmised from fossil records has been used to illustrate the impact that changes in food type and availability (resulting from the development of agriculture and successful food preservation techniques) have had in the development of the modern ‘lifestyle’ ailments such as type 2 diabetes and cardiovascular disease (Eaton & Konner, 1985; Eaton et al. 1999, 2002; Booth et al. 2002; Cordain et al. 2002). Indeed, the original ‘thrifty’ genotype hypothesis (Neel, 1962) is based on the assumption that the hunter–gatherer experienced alternating periods of food surfeit and scarcity. Observations of remnant hunter–gatherer communities and the impact of their acculturation to Western lifestyle appear to strengthen the hypothesis that ancient genotypes and modern agriculture combine to result in a deleterious phenotype (Moodie, 1973; O'Dea, 1984).
In hunter–gatherer society, however, physical work and food procurement are inextricably linked (Booth et al. 2002; Eaton et al. 2002). Physical activity is required to obtain food before the energy in that food can be utilized or stored. The attributes of rapid and efficient storage of energy upon refeeding (the hypothesized role of ‘thrifty’ genes) are of little survival value if food cannot be first obtained whilst in the fasted state. That is, ‘thrifty’ genes (Neel, 1962) must have been of secondary importance in survival to genes that preserved physical capacity during food deprivation.
Athletes and hunter–gatherers
In their recent review, Booth et al. (2002) compare the physical activity levels of modern Western man with that of the Late Palaeolithic hunter–gatherer. The authors suggest that the de-trained muscles of the relatively sedentary modern man are an important aspect in the development type 2 diabetes. Their argument that habitual exercise in sedentary cultures restores perturbed mechanisms towards the ‘normal’ physiological range experienced by early man is supported by observations of enhanced insulin sensitivity and the preventative effects of chronic exercise against the development of type 2 diabetes. Booth et al. argue that there should be a paradigm shift in what is considered ‘normal’ physiology away from the sedentary majority towards the physiological characteristics of modern athletes. Such physiological characteristics include a proportionally high muscle and low fat mass and a high oxidative muscle capacity – the opposite to those observed in modern insulin-resistant humans. According to this proposition it is from physically active populations and not obese individuals that an understanding of the role of IMTG in the development of insulin resistance may be made. Thus, the elevated IMTG storage seen in athletes (and presumably hunter–gatherers) may represent part of a normal physiological fluctuation in IMTG levels. It is of note that the only situation in which these lean, healthy, normally insulin-sensitive individuals ‘naturally’ become insulin resistant is with short-term starvation (Mansell & Macdonald, 1990; Webber et al. 1994).
From a Darwinian perspective therefore, two questions need to be answered. Firstly, what selection pressures may have existed such that a greater ability to store IMTG was of advantage to survival and procreation, and secondly, what is the further advantage of a concomitant muscle insulin resistance?
The survival advantage of accumulating IMTG
From the results of their studies on fasting conducted in the 1960s (Cahill et al. 1966; Owen et al. 1967, 1969), Cahill and associates suggested that a crucial aspect of survival during starvation was the ability of the hunter–gatherer to maintain muscle protein (Cahill & Owen, 1968). Maintaining this functional tissue meant that the individual was able to hunt successfully at the first opportunity and also perhaps resist predation (Reaven, 1998). However, an issue not addressed by Cahill et al. is the necessity for the ‘protected’ muscle to contain a readily available substrate to fuel muscle contraction.
For the hunter–gatherer, the majority of energy expenditure in excess of maintenance would have occurred in the physical quest for food and for thermoregulation. To conserve energy, adult humans are inherently lazy, so physical activity would be largely restricted until food was scarce and real hunger was felt. Indeed the amount of energy expended in finding food was probably proportional to the hunger felt, the time since the last meal and, because carbohydrate stores are quickly depleted, inversely proportional to carbohydrate status. As energy deficit increased, physical ability to procure the next meal would have become even more important at the time when endogenous carbohydrate stores would have been challenged.
Cordain et al. (2002) argue that the diet of many hunter–gatherers was predominantly of animal origin. Physical activity, sometimes of a strenuous nature, would be required to trap an animal. Therefore, as real hunger is felt and starvation begins, the ability to maintain an endogenous substrate capable of immediately supporting physical activity would prove advantageous to survival.
Muscle glycogen is currently acknowledged as the most important substrate at the onset of exercise and during strenuous contractile activity because: it resides within the muscle fibre; its breakdown is catalysed by contraction; and anaerobic glycogenolysis can rapidly produce ATP. Perhaps for the same reasons, muscle glycogen has also been shown to be a significant substrate in support of shivering-induced thermogenesis (Martineau & Jacobs, 1988; Haman et al. 2002). Although fasting alone does not result in muscle glycogen depletion (Knapik et al. 1988), depletion during muscle activity followed by limited carbohydrate intake minimizes muscle glycogen accretion (Bergstrom et al. 1967; Pernow & Saltin, 1971). Thus, in the postexercised muscle of the hunter–gatherer, muscle glycogen content would remain low after a few days of starvation or a predominantly animal flesh diet. In contrast, plasma FFAs would be in abundant supply. However, FFAs alone could not then fuel the onset of further contractile activity because mobilization from remote depots is under humoral control and at this time lags muscle substrate requirement. In contrast, IMTG, which resides within the muscle fibre, is available for immediate utilization (Romijn et al. 1993) and like muscle glycogen its breakdown is activated by contraction (Langfort et al. 2000). A period of FFA elevation in a carbohydrate-lowered state provides an ideal environment for IMTG formation.
The survival advantage of elevated IMTG in prehistoric man may have been to provide muscle with an alternative to muscle glycogen as a readily available substrate to support muscle metabolism at the onset of exercise or cold exposure during early starvation or carbohydrate restriction. The recent finding that 72 h of complete food restriction results in a nearly three-fold increase in IMTG in physically trained men (Stannard et al. 2002) is salient evidence that under such conditions FFAs from adipose tissue may be re-distributed to skeletal muscle for storage in the form of IMTG. In an evolutionary context, 72 h of food or carbohydrate deprivation is not long and perhaps relatively often experienced. Internment of lipid within the muscle in these situations would allow immediate access of this substrate to fuel the muscle contraction required to obtain the next meal, resist predation and maintain body temperature during cold exposure. In support of this hypothesis are the observations that: (1) when leg muscles are depleted of glycogen by exercise followed by carbohydrate restriction, and FFA availability is simultaneously reduced via nicotinic acid ingestion, the majority of substrate supporting contraction is calculated to have come from IMTG (Pernow & Saltin, 1971); (2) the ability to utilize lipid stores to maintain body temperature via shivering-induced thermogenesis in the glycogen-depleted state is not compromised when FFA availability is simultaneously reduced via nicotinic acid administration (Martineau & Jacobs, 1991).
The survival advantage of reduced muscle glucose uptake
After a short period of starvation, lean individuals show a diminished ability to clear glucose from the bloodstream whether mediated by contraction (Knapik et al. 1988) or insulin (Webber et al. 1994). This ‘fasting diabetes’ provides a clear survival advantage in that circulating glucose is spared for use by the brain and other glucose-dependant tissues when liver glycogen stores are depleted. In turn, there is less pressure on the liver and kidney to manufacture glucose from amino acids, essential if functional tissue is to be maintained.
Resistance in muscle to the effects of insulin on glucose uptake may be particularly important during starvation or carbohydrate restriction. The ability of insulin to prevent rampant proteolysis and lipolysis is maintained during short-term starvation, yet its role in stimulating glucose uptake by muscle is prevented (Fryburg et al. 1990). This dissociation of insulin actions means the residual circulating insulin levels maintained in short-term starvation (Cahill et al. 1966) can attenuate proteolysis, ketosis and hepatic glucose release, whilst unnecessary utilization of blood glucose by muscle is prevented.
The whole-body effects of impaired muscle glucose disposal and insulin resistance during starvation or carbohydrate restriction would be relatively greater in the athlete/hunter–gatherer than in sedentary modern man because of their greater relative muscle mass and thus greater sink for glucose disposal.
The survival advantage of insulin resistance and elevated IMTG occurring in parallel
The beauty of these two physiological responses occurring in parallel, regardless of the existence of any cause-and-effect relationship, is revealed when muscle contractile activity occurs whilst in the fasted state.
Hepatic glucose release during intense exercise is centrally mediated and proportional to the muscle mass recruited (Vissing, 2000). Resistance to contraction-mediated glucose uptake in fasting allows a sparing of precious blood glucose without the contracting muscle taking advantage of any exercise-induced elevation in blood glucose concentrations (Sonne et al. 1987). The concomitant elevated levels of IMTG provide an alternate substrate to muscle glycogen to support contraction during exercise. Similarly, with cold exposure during fasting, IMTG is available as an adjunct to FFAs to support the involuntary muscle activity of shivering in the absence of significant carbohydrate stores. On cessation of exercise, any increased insulin secretion in response to residual elevated glucose (Marliss et al. 2000) would be less effective in promoting muscle glucose uptake. On the other hand, FFA concentrations will be extremely high and provide for rapid synthesis of IMTG, so that if food was not forthcoming, the ability to endure cold nights and hunt/gather could be maintained.
Development of insulin resistance in sedentary modern man
In the light of this discussion, how do these physiological phenomena relate to sedentary, obese modern man and the development of insulin resistance?
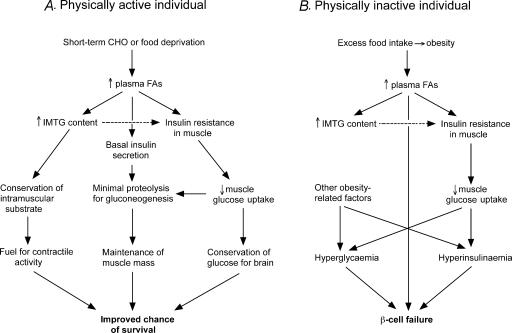
Obesity results in a chronically elevated blood lipid profile, largely because of the mass action effect of excessive adipose tissues stores. Therefore in obesity, an ‘unnatural’ situation for the hunter–gatherer, the elevated blood lipid profile, akin to the circulatory environment experienced in fasting or carbohydrate restriction, acts to trigger the physiological responses for such conditions (Fig. 2). The ‘misguided’ response is to protect circulating glucose via inhibition of muscle glucose transport whilst accumulating IMTG to ensure a readily available substrate for subsequent contractile activity.
Figure 2.
The survival advantage of muscle insulin resistance and elevated IMTG in physically active individuals during short-term starvation (A) and how this same genotype can lead to type 2 diabetes in sedentary individuals when caloric intake is excessive (B). Dotted arrow represents a strong association only.
Therefore, irrespective of a causal relationship, concurrent elevation of IMTG and insulin resistance are part of a coordinated physiological response to elevations in circulating fatty acid availability mediated (in our hunter–gatherer ancestors at least) by short-term changes in food availability. Teleologically, these associated and probably related processes were designed to aid survival in an environment where food availability was sporadic. That these processes also occur simultaneously in sedentary Western man indicates an appropriate (or normal) physiological response, but in an inappropriate environment.
Implications in obesity and type 2 diabetes
If elevated IMTG does inhibit glucose transport into the muscle, whether directly (Keizer et al. 2001) or indirectly via elevated myoplasmic long-chain fatty acyl CoA (Cooney et al. 2002), with a chronically elevated blood lipid profile, such as is found in obesity, there will be a protracted period of muscle resistance to insulin-mediated glucose uptake. This in turn may result in hyperinsulinaemia and/or hyperglycaemia which might become chronic unless IMTG levels can be reduced.
This line of reasoning suggests that muscle insulin resistance can be prevented from developing or improved even when adiposity is high by ensuring an adequate rate of lipid oxidation. Indeed, it has been shown that in overweight persons increased rates of postabsorptive lipid oxidation are associated with normal IMTG content and insulin sensitivity (Perseghin et al. 2002). Furthermore, persons carrying larger amounts of adipose tissue improve whole-body insulin resistance with participation in an exercise-training programme without changes in body adiposity (Dengel et al. 1996), and finally, the inverse relationship between IMTG and insulin sensitivity in Pima Indians is independent of total adiposity (Pan et al. 1997). Together these results suggest that obesity alone does not directly cause insulin resistance, but that insulin resistance is prevalent in obesity because obesity elevates blood lipids and IMTG content. Even in obese persons, improving the ability of the muscles to oxidize lipid may prevent IMTG accrual and, in turn, insulin sensitivity can be increased.
A number of studies show a positive correlation between insulin resistance and the proportion of glycolytic muscle fibres in the quadriceps (Hickey et al. 1995; Kriketos et al. 1996; Lillioja et al. 1987). Although muscle fibre type distribution is not a direct measure of oxidative capacity, in the absence of exercise training this inherent characteristic may limit muscle lipid oxidation in some individuals (or homogenous groups), making them more susceptible to muscle insulin resistance.
In the light of our inheritance, how may muscle insulin resistance be reversed to prevent or treat whole-body insulin resistance? One answer may be acute exercise of a sufficient dose to decrease chronically elevated IMTG levels (Thompson et al. 2001). However, for an untrained individual whose oxidative capacity is limited, this would prove difficult because other fatigue factors may limit exercise duration prior to significant degradation of IMTG stores. Also, whilst the individual is still obese, an elevated blood lipid profile will remain to encourage reformation of IMTG.
Cold exposure, although shown in animals to potentiate the effects of insulin on muscle glucose uptake (Vallerand et al. 1990; Sano & Terashima, 2001) and reverse the diabetogenic effects of a high fat diet (Vallerand et al. 1986), would no doubt prove unpopular, and because subcutaneous adipose tissue depots provide insulation, this stimulus would be less effective in obese persons.
Perhaps the only non-pharmacological solution to prevent muscle insulin resistance is to engage in chronic physical activity and improve the capacity of the musculature to utilize fatty acids. In doing so modern man would regain the ‘normal’ physiology of our hunter–gatherer ancestors in the same way as the modern athlete and express the phenotype as it has evolved to be.
Acknowledgments
The authors wish to thank Martin Thompson for encouraging the virtues of critical thinking and a dogged work ethic. We also wish to thank John Brotherhood for his encouragement and critique of the manuscript.
References
- Ashley MA, Buckley AJ, Criss AL, Ward JA, Kemp A, Garnett S, Cowell CT, Baur LA, Thompson CH. Familial, anthropometric, and metabolic associations of intramyocellular lipid levels in prepubertal males. Pediatr Res. 2002;51:81–86. doi: 10.1203/00006450-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen CD, Schick F, Haring HU, Jacob S. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- Barnes F. The biology of pre-neolithic man. In: Boyden SV, editor. The Impact of Civilisation on the Biology of Man. Canberra: Australian National University Press; 1968. pp. 1–26. [Google Scholar]
- Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle metabolism. Am J Physiol. 1999;276:E106–E117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Black SA. Diabetes, diversity, and disparity: What do we do with the evidence? Am J Public Health. 2002;92:543–548. doi: 10.2105/ajph.92.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity. J Physiol. 2002;543:399–411. doi: 10.1113/jphysiol.2002.019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- Brechtel K, Dahl DB, Machann J, Bachman OP, Wenzel I, Maier T, Claussen CD, Haring HU, Jacob S, Schick F. Fast elevation of the intramyocellular lipid content in the presence of circulating free fatty acids and hyperinsulinaemia: a dynamic 1H-MRS study. Magn Reson Med. 2001;45:179–183. doi: 10.1002/1522-2594(200102)45:2<179::aid-mrm1023>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cahill GF, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45:1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GF, Owen OE. Survival and starvation. Trans Am Clin Climatol Assoc. 1968;79:13–20. [PMC free article] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, Kohrt WM, Holloszy JO. Effect of prolonged exercise on muscle citrate concentration before and after endurance training in men. Am J Physiol. 1993;264:E215–E220. doi: 10.1152/ajpendo.1993.264.2.E215. [DOI] [PubMed] [Google Scholar]
- Colman E, Katzel LI, Rogus E, Coon P, Muller D, Goldberg AP. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism. 1995;44:1502–1508. doi: 10.1016/0026-0495(95)90153-1. [DOI] [PubMed] [Google Scholar]
- Coon PJ, Bleecker ER, Drinkwater DT, Meyers DA, Goldberg AP. Effects of body composition and exercise capacity on glucose tolerance, insulin, and lipoprotein lipids in healthy older men: a cross-sectional and longitudinal intervention study. Metabolism. 1989;38:1201–1209. doi: 10.1016/0026-0495(89)90160-1. [DOI] [PubMed] [Google Scholar]
- Cooney GJ, Thompson AL, Furler SM, He J, Kraegen EW. Muscle long-chain CoA esters and insulin resistance. Ann N Y Acad Sci. 2002;967:196–207. doi: 10.1111/j.1749-6632.2002.tb04276.x. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Brand Miller J, Mann N, Hill K. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56:S42–S52. doi: 10.1038/sj.ejcn.1601353. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Jeukendrup AE, Oseto MC, Hodgkinson BJ, Zderic TW. Low-fat diet alters intramuscular substrates and reduces lipolysis and fat oxidation during exercise. Am J Physiol Endocrinol Metab. 2001;280:E391–E398. doi: 10.1152/ajpendo.2001.280.3.E391. [DOI] [PubMed] [Google Scholar]
- Decombaz J, Fleith M, Hoppeler H, Kreis R, Boesch C. Effect of diet on the replenishment of intramyocellular lipids after exercise. Eur J Clin Nutr. 2000;39:244–247. doi: 10.1007/s003940070002. [DOI] [PubMed] [Google Scholar]
- Decombaz J, Schmitt B, Ith M, Decarli B, Diem P, Kreis R, Hoppeler H, Boesch C. Postexercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. Am J Physiol. 2001;281:R760–R769. doi: 10.1152/ajpregu.2001.281.3.R760. [DOI] [PubMed] [Google Scholar]
- Dela F, Mikines KJ, Von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol. 1992;263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol. 1996;81:318–325. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Bonen A. Muscle contraction increases palmitate esterification and oxidation and triacylglycerol oxidation. Am J Physiol. 1998;275:E888–E896. doi: 10.1152/ajpendo.1998.275.5.E888. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Miskovic D, Code L, Luiken JJFP, Bonen A. Endurance training increases FFA oxidation and reduces triacylglyceride utilization in contracting rat soleus. Am J Physiol. 2000;278:E778–E785. doi: 10.1152/ajpendo.2000.278.5.E778. [DOI] [PubMed] [Google Scholar]
- Dyck DJ, Steinberg G, Bonen A. Insulin increases FA uptake and esterification but reduces lipid utilization in isolated contracting muscle. Am J Physiol. 2001;281:E600–E607. doi: 10.1152/ajpendo.2001.281.3.E600. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Eaton SB, Konner MJ. Paleolithic nutrition revisited. In: Trevathan W, Smith EO, McKenna JJ, editors. Evolutionary Medicine. Oxford: Oxford University Press; 1999. pp. 313–332. [Google Scholar]
- Eaton SB, Konner MJ. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Strassman BI, Nesse RM, Neel JV, Ewald PW, Williams GC, Weder AB, Eaton SB, Lindeberg S, Konner MJ, Mysterud I, Cordain L. Evolutionary health promotion. Prev Med. 2002;34:109–118. doi: 10.1006/pmed.2001.0876. [DOI] [PubMed] [Google Scholar]
- Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, Cooney GJ. Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol. 2000:E554–E560. doi: 10.1152/ajpendo.2000.279.3.E554. [DOI] [PubMed] [Google Scholar]
- Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsli U, McKeigue PM, Bell JD. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia. 1999;42:932–935. doi: 10.1007/s001250051250. [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Barrett EJ, Louard RJ, Gelfand RA. Effect of starvation on human muscle protein metabolism and its response to insulin. Am J Physiol. 1990;259:E477–E482. doi: 10.1152/ajpendo.1990.259.4.E477. [DOI] [PubMed] [Google Scholar]
- Gazdag AC, Wetter TJ, Davidson RT, Robinson KA, Buse MG, Yee AJ, Turcotte LP, Cartee GD. Lower calorie intake enhances muscle insulin action and reduces hexosamine levels. Am J Physiol. 2000;278:R504–R512. doi: 10.1152/ajpregu.2000.278.2.R504. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- Gorski J, Kuryliszyn A, Wereszczynska U. Effect of acute cold exposure on the mobilization of intramuscular glycogen and triglycerides in the rat. Acta Physiol Polon. 1981;32:755–759. [PubMed] [Google Scholar]
- Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- Green HJ, Jones S, Ball-Burnett M, Fraser I. Early adaptations in blood substrates, metabolites, and hormones to prolonged exercise training in man. Can J Physiol Pharmacol. 1991;69:1222–1229. doi: 10.1139/y91-179. [DOI] [PubMed] [Google Scholar]
- Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol. 2000;89:2057–2064. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- Haman F, Peronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, Weber J-M. Effect of cold exposure on fuel utilization in humans. plasma glucose, muscle glycogen, and lipids. J Appl Physiol. 2002;93:77–84. doi: 10.1152/japplphysiol.00773.2001. [DOI] [PubMed] [Google Scholar]
- Havel RJ, Pernow B, Jones NL. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. J Appl Physiol. 1967;23:90–99. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- Heath GW, Gavin JR, 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol. 1983;55:512–177. doi: 10.1152/jappl.1983.55.2.512. [DOI] [PubMed] [Google Scholar]
- Hegarty BD, Furler SM, Yee AJ, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373–383. doi: 10.1046/j.1365-201X.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- Helge JW, Richter EA, Kiens B. Interaction of training and diet on metabolism and endurance during exercise in man. J Physiol. 1996;492:293–306. doi: 10.1113/jphysiol.1996.sp021309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helge JW, Watt PW, Richter EA, Rennie MJ, Kiens B. Fat utilization during exercise. Adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J Physiol. 2001;537:1009–1020. doi: 10.1111/j.1469-7793.2001.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MS, Weidner MD, Gavigan KE, Zheng D, Tyndall GL, Houmard JA. The insulin action-fiber type relationship is humans is muscle group specific. Am J Physiol. 1995;269:E150–E154. doi: 10.1152/ajpendo.1995.269.1.E150. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Hoppeler H, Luthi P, Claasen H, Weibel ER, Howald H. The ultrastructure of human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflugers Arch. 1973;344:217–232. doi: 10.1007/BF00588462. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Coppack SW, Klein S. Whole-body and adipose tissue glucose metabolism in response to short-term fasting in lean and obese women. Am J Clin Nutr. 2001;73:517–522. doi: 10.1093/ajcn/73.3.517. [DOI] [PubMed] [Google Scholar]
- Hurley BF, Seals DR. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60:562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C and IκB-α. Diabetes. 2002;51:11. doi: 10.2337/diabetes.51.7.2005. 2005–. [DOI] [PubMed] [Google Scholar]
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maeker E, Matthaei S, Schick F, Claussen CD, Haring HU. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- Jansson E, Kaijser L. Effect of diet on the utilization of blood-borne and intramuscular substrates during exercise in man. Acta Physiol Scand. 1982;115:19–30. doi: 10.1111/j.1748-1716.1982.tb07041.x. [DOI] [PubMed] [Google Scholar]
- Jansson E, Kaijser L. Substrate utilization and enzymes in skeletal muscles of extremely endurance-trained men. J Appl Physiol. 1987;62:999–1005. doi: 10.1152/jappl.1987.62.3.999. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Stannard SR, Mehalski K, Trenell MI, Sachinwalla T, Thompson CH, Thompson MW. Intramyocellular triacylglycerol in prolonged cycling with high and low carbohydrate availability. J Appl Physiol. 2002;94:1365–1372. doi: 10.1152/japplphysiol.00833.2002. [DOI] [PubMed] [Google Scholar]
- Kayar S, Hoppeler H, Howald H, Claassen H, Oberholzer F. Acute effects of endurance exercise on mitochondrial distribution and skeletal muscle morphology. Eur J Appl Physiol. 1986;54:578–584. doi: 10.1007/BF00943344. [DOI] [PubMed] [Google Scholar]
- Keizer HA, Hesselink M, Schaart G, Borghouts L, van Kranenburg G. Triglyceride content and fatty acid translocase (FAT/CD36) expression in individual muscle fibres of vastus lateralis muscles in type II diabetic patients. In: Mester J, King G, Struder H, Tsolakidis E, Osterburg A, editors. Sport und Buch Strauss. Cologne: 6th Ann Congr Eur Coll Sports Sci; 2001. p. 573. [Google Scholar]
- Kelley DE, Goodpaster B, Storlein L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–346. doi: 10.1146/annurev.nutr.22.010402.102912. [DOI] [PubMed] [Google Scholar]
- Kiens B. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol. 1998;275:E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: Effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Gad P, Lithell H. Lipoprotein lipase activity and intramuscular triglyceride stores after long-term high-fat and high-carbohydrate diets in physically trained men. Clin Physiol. 1987;7:1–9. doi: 10.1111/j.1475-097x.1987.tb00628.x. [DOI] [PubMed] [Google Scholar]
- King DS, Dalsky GP, Staten MA, Clutter WE, Van Houten DR, Holloszy JO. Insulin action and secretion in endurance-trained and untrained humans. J Appl Physiol. 1987;63:2247–2252. doi: 10.1152/jappl.1987.63.6.2247. [DOI] [PubMed] [Google Scholar]
- Knapik JJ, Meredith CN, Jones BH, Suek L, Young VR, Evans WJ. Influence of fasting on carbohydrate and fat metabolism during rest and exercise in men. J Appl Physiol. 1988;64:1923–1929. doi: 10.1152/jappl.1988.64.5.1923. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Cooney GJ. The role of free fatty acids in muscle insulin resistance. Diabetes Ann. 1999;12:141–159. [Google Scholar]
- Krebs M, Krssak M, Nowotny P, Weghuber D, Gruber S, Mlynarik V, Bischof M, Stingl H, Furnsinn C, Waldhausl W, Roden M. Free fatty acids inhibit the glucose-stimulated increase of intramuscular glucose-6-phosphate concentration in humans. J Clin Endocrinol Metab. 2001;86:2153–2160. doi: 10.1210/jcem.86.5.7488. [DOI] [PubMed] [Google Scholar]
- Kriketos AD, Pan DA, Lillioja S, Cooney GJ, Baur LA, Milner MR, Sutton JR, Jenkins AB, Bogardus C, Storlein LH. Interrelationships between muscle morphology, insulin action, and adiposity. Am J Physiol. 1996;270:R1332–R1339. doi: 10.1152/ajpregu.1996.270.6.R1332. [DOI] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- Krssak M, Petersen KF, Bergeron R, Price T, Laurent D, Rothman DL, Roden M, Shulman GI. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab. 2000;85:748–754. doi: 10.1210/jcem.85.2.6354. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Hunter GR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. Am J Physiol Endocrinol Metab. 2002;282:E95–E106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- Lillioja S, Young A, Culter C, Ivy JL, Abbott WGH, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80:415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell H, Orlander J, Schele R, Sjodin B, Karlsson J. Changes in lipoprotein-lipase activity and lipid stores in human skeletal muscle with prolonged heavy exercise. Acta Physiol Scand. 1979;107:257–261. doi: 10.1111/j.1748-1716.1979.tb06471.x. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Mansell PI, Macdonald IA. The effect of starvation on insulin-induced glucose disposal and thermogenesis in humans. Metabolism. 1990;39:502–510. doi: 10.1016/0026-0495(90)90009-2. [DOI] [PubMed] [Google Scholar]
- Marliss EB, Kreisman SH, Manzon A, Halter JB, Vranic M, Nessim SJ. Gender differences in glucoregulatory responses to intense exercise. J Appl Physiol. 2000;88:457–466. doi: 10.1152/jappl.2000.88.2.457. [DOI] [PubMed] [Google Scholar]
- Martin WH., III Effect of endurance training on fatty acid metabolism during whole body exercise. Med Sci Sports Exerc. 1997;29:635–639. doi: 10.1097/00005768-199705000-00008. [DOI] [PubMed] [Google Scholar]
- Martin WH, III, Dalsky GP, Hurley BF, Matthews DE, Bier DM, Hagberg JM, Rogers MA, King DS, Holloszy JO. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol. 1993;265:E708–E714. doi: 10.1152/ajpendo.1993.265.5.E708. [DOI] [PubMed] [Google Scholar]
- Martineau L, Jacobs I. Muscle glycogen utilization during shivering thermogenesis in humans. J Appl Physiol. 1988;65:2046–2050. doi: 10.1152/jappl.1988.65.5.2046. [DOI] [PubMed] [Google Scholar]
- Martineau L, Jacobs I. Free fatty acids availability and temperature regulation in cold water. J Appl Physiol. 1989;67:2466–2472. doi: 10.1152/jappl.1989.67.6.2466. [DOI] [PubMed] [Google Scholar]
- Martineau L, Jacobs I. Effects of muscle glycogen and plasma FFA availability on human metabolic responses in cold water. J Appl Physiol. 1991;71:1331–1339. doi: 10.1152/jappl.1991.71.4.1331. [DOI] [PubMed] [Google Scholar]
- Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of acute exercise and detraining on insulin action in trained men. J Appl Physiol. 1989;66:704–711. doi: 10.1152/jappl.1989.66.2.704. [DOI] [PubMed] [Google Scholar]
- Misra A, Sinha S, Kumar M, Jagannathan NR, Pandey RM. Proton magnetic resonance spectroscopy study of soleus muscle in non-obese healthy and Type 2 diabetic Asian Northern Indian males: high intramyocellular lipid content correlates with excess body fat and abdominal obesity. Diabet Med. 2003;20:361–367. doi: 10.1046/j.1464-5491.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- Mole PA, Oscai LB, Holloszy JO. Adaptation of muscle to exercise. Increase in levels of palmityl CoA synthetase, carnitine palmityltransferase, and palmityl CoA dehydrogenase, and the capacity to oxidize fatty acids. J Clin Invest. 1971;50:2323–2330. doi: 10.1172/JCI106730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie PM. Aboriginal Health. Canberra: Australian National University; 1973. [Google Scholar]
- Neel JV. Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- O'Dea K. Marked improvement in carbohydrate and lipid metabolism in diabetic Australian aborigines after temporary reversion to traditional lifestyle. Diabetes. 1984;33:596–603. doi: 10.2337/diab.33.6.596. [DOI] [PubMed] [Google Scholar]
- Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF. Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969;48:574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlein LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- Pernow B, Saltin B. Availability of substrates and capacity for prolonged heavy exercise in man. J Appl Physiol. 1971;31:416–422. doi: 10.1152/jappl.1971.31.3.416. [DOI] [PubMed] [Google Scholar]
- Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, Danna M, Battezzati A, Benedini S, Meneghini E, Del Maschio A, Luzi L. Normal insulin sensitivity and IMCL content in overweight humans are associated with higher fasting lipid oxidation. Am J Physiol Endocrinol Metab. 2002;283:E556–E564. doi: 10.1152/ajpendo.00127.2002. [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H–13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetics. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol. 1996;81:2182–2191. doi: 10.1152/jappl.1996.81.5.2182. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Gardner AW, Arciero PJ, Goran MI, Calles-Escandon J. The effects of endurance training on total fat oxidation. J Appl Physiol. 1994;76:2281–2287. doi: 10.1152/jappl.1994.76.6.2281. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Hypothesis: muscle insulin resistance is the (‘not-so’) thrifty genotype. Diabetologia. 1998;41:482–484. doi: 10.1007/s001250050933. [DOI] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Falk Petersen K, Rothman DL, Cline GW. Mechanism of free-fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnick KJ, Haskell WL, Swislocki ALM, Foley JE, Reaven GM. Improved insulin action in muscle, liver and adipose tissue in physically trained human subjects. Am J Physiol. 1987;253:E489–E495. doi: 10.1152/ajpendo.1987.253.5.E489. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71:1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J Physiol. 2002;540:387–395. doi: 10.1113/jphysiol.2001.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Astrand P. Free fatty acids and exercise. Am J Clin Nutr. 1993;57:752–758. doi: 10.1093/ajcn/57.5.752S. [DOI] [PubMed] [Google Scholar]
- Sano H, Terashima Y. Effects of dietary protein level and cold exposure on tissue responsivenes and sensitivity to insulin in sheep. J Anim Physiol Anim Nutr. 2001;85:349–355. doi: 10.1046/j.1439-0396.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Craig D, Biden T. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Schrauwen P, Hesselink MK, van Engelshoven JM, Nicolay K, Saris WH, Kessels AG, Kooi ME. The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab. 2003;88:1610–1616. doi: 10.1210/jc.2002-021464. [DOI] [PubMed] [Google Scholar]
- Shibata H, Perusse F, Vallerand AL, Bukowiecki L. Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. Am J Physiol. 1989;257:R96–R101. doi: 10.1152/ajpregu.1989.257.1.R96. [DOI] [PubMed] [Google Scholar]
- Sinh R, Dufour S, Falk Petersen K, LeBon V, Enoksson S, Ma Y-Z, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessement of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- Sonne B, Mikines KJ, Galbo H. Glucose turnover in 48-hour-fasted running rats. Am J Physiol. 1987;252:R587–R593. doi: 10.1152/ajpregu.1987.252.3.R587. [DOI] [PubMed] [Google Scholar]
- Stankiewicz-Choroszucha B, Gorski J. Effect of decreased availability of substrates on intramuscular triglyceride utilization during exercise. Eur J Appl Physiol. 1978;40:27–35. doi: 10.1007/BF00420986. [DOI] [PubMed] [Google Scholar]
- Stannard SR, Thompson MW, Fairbairn K, Huard B, Sachinwalla T, Thompson CH. Fasting for 72 h increases intramyocellular lipid content in non-diabetic, physically-fit men. Am J Physiol Endocrinol Metab. 2002;283:E1185–E1191. doi: 10.1152/ajpendo.00108.2002. [DOI] [PubMed] [Google Scholar]
- Thamer C, Machann J, Bachman OP, Haap M, Dahl DB, Wietek B, Tschritter O, Niess A, Brechtel K, Fritsche A, Claussen CD, Jacob S, Schick F, Haring HU, Stumvoll M. Intramyocellular lipids. Anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1785–1791. doi: 10.1210/jc.2002-021674. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33:S438–S445. S452–453. doi: 10.1097/00005768-200106001-00012. discussion. [DOI] [PubMed] [Google Scholar]
- Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs untrained humans. Am J Physiol. 1992;262:E791–E799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Lupien J, Bukowiecki L. Cold exposure reverses the diabetogenic effects of high-fat feeding. Diabetes. 1986;35:329–334. doi: 10.2337/diab.35.3.329. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Perusse F, Bukowiecki L. Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol. 1990;259:R1043–R1049. doi: 10.1152/ajpregu.1990.259.5.R1043. [DOI] [PubMed] [Google Scholar]
- Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001;50:2337–2343. doi: 10.2337/diabetes.50.10.2337. [DOI] [PubMed] [Google Scholar]
- Vissing J. Muscle reflex and central motor control of neuroendocrine activity, glucose homeostasis and circulation during exercise. Acta Physiol Scand. 2000;170:5–26. [PubMed] [Google Scholar]
- Vukovich MD, Arciero PJ, Kohrt WM, Racette SB, Hansen PA, Holloszy JO. Changes in insulin action and GLUT-4 with 6 days of inactivity in endurance runners. J Appl Physiol. 1996;80:240–244. doi: 10.1152/jappl.1996.80.1.240. [DOI] [PubMed] [Google Scholar]
- Washburn SL. Foreword. In: Lee RB, DeVore I, editors. Kalahari Hunter-Gatherers. Cambridge: Harvard University Press; 1976. pp. xv–xvii. [Google Scholar]
- Watt MJ, Heigenhauser GF, Dyck DJ, Spriet LL. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol. 2002a;541:969–978. doi: 10.1113/jphysiol.2002.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJF, Spriet LL. Intramuscular triacylglycerol utilization in human skeletal muscle during exercise: is there a controversy? J Appl Physiol. 2002b;94:1185–1195. doi: 10.1152/japplphysiol.00197.2002. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Stellingwerff T, Heigenhauser GJF, Spriet LL. Effect of plasma adrenaline on hormone-sensitive lipase at rest and during moderate exercise in human skeletal muscle. J Physiol. 2003;550:325–332. doi: 10.1113/jphysiol.2003.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Taylor J, Greathead H, Dawson J, Buttery PJ, Macdonald IA. Effects of fasting on fatty acid kinetics and on the cardiovascular, thermogenic and metabolic responses to the glucose clamp. Clin Sci. 1994;87:697–706. doi: 10.1042/cs0870697. [DOI] [PubMed] [Google Scholar]
- Wendling PS, Peters SJ, Heigenhauser GJF, Spriet LL. Variability of triacylglycerol content in human skeletal muscle biopsy samples. J Appl Physiol. 1996;81:1150–1155. doi: 10.1152/jappl.1996.81.3.1150. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]