Abstract
We tested the hypothesis that in primates, maternal melatonin restrains fetal and newborn adrenal cortisol production. A functional G-protein-coupled MT1 membrane-bound melatonin receptor was detected in 90% gestation capuchin monkey fetal adrenals by (a) 2-[125I] iodomelatonin binding (Kd, 75.7 ± 6.9 pm; Bmax, 2.6 ± 0.4 fmol (mg protein)−1), (b) cDNA identification, and (c) melatonin inhibition of adrenocorticotrophic hormone (ACTH)- and corticotrophin-releasing hormone (CRH)-stimulated cortisol but not of dehydroepiandrosterone sulphate (DHAS) production in vitro. Melatonin also inhibited ACTH-induced 3β-hydroxysteroid dehydrogenase mRNA expression. To assess the physiological relevance of these findings, we next studied the effect of chronic maternal melatonin suppression (induced by exposure to constant light during the last third of gestation) on maternal plasma oestradiol during gestation and on plasma cortisol concentration in the 4- to 6-day-old newborn. Constant light suppressed maternal melatonin without affecting maternal plasma oestradiol concentration, consistent with no effect on fetal DHAS, the precursor of maternal oestradiol. However, newborns from mothers under constant light condition had twice as much plasma cortisol as newborns from mothers maintained under a normal light–dark schedule. Newborns from mothers exposed to chronic constant light and daily melatonin replacement had normal plasma cortisol concentration. Our results support a role of maternal melatonin in fetal and neonatal primate cortisol regulation.
The fetal adrenal gland of primates is unique compared to that of other mammals because it has a large size relative to body weight and a distinct morphology that includes a prominent fetal zone devoid of the enzyme Δ5-3β-hydroxysteroid dehydrogenase (3β-HSD), required for cortisol production (Goldman et al. 1966; Serón-Ferré & Jaffe, 1981; Mesiano & Jaffe, 1997). As a result, during most of gestation the fetal primate adrenal produces limited amounts of cortisol and large amounts of Δ5-3β hydroxysteroid dehydroepiandrosterone sulphate (DHAS), the precursor for the placental synthesis of oestrogen. A high placental synthesis of oestrogen characterizes primate pregnancy (Siiteri & MacDonald, 1966; Serón-Ferré & Jaffe, 1981; Mesiano & Jaffe, 1997).
The regulation of the fetal primate adrenal gland is incompletely understood, involving the interaction of fetal and placental hormones. It is well established that fetal pituitary ACTH is a key regulator of adrenal growth and cortisol and DHAS production (Serón-Ferré & Jaffe, 1981; Mesiano & Jaffe, 1997). The primate placenta produces CRH that in vitro also stimulates DHAS and cortisol production (Smith et al. 1998; Parker et al. 1999). The role of maternal hormones in fetal adrenal regulation is limited by placental activity. Maternal ACTH does not cross the placenta (Allen et al. 1973), and most of maternal cortisol is metabolized, reaching the fetus in an inactive form (Murphy & Branchaud, 1983).
Maternal melatonin crosses the placenta unaltered, so that fetuses are exposed to the maternal melatonin rhythm (Yellon & Longo, 1988; McMillen & Nowak, 1989). This avenue for melatonin to mediate interactions between maternal and fetal physiological functions has been explored mostly at the level of the fetal suprachiasmatic nucleus (Naitoh et al. 1998), and in the control of fetal circadian rhythms (Serón-Ferréet al. 1989; McMillen et al. 1990; Houghton et al. 1993; Serón-Ferréet al. 1993). However, maternal melatonin may be involved in a wider array of fetal functions, given the presence of melatonin receptors in diverse tissues of the developing sheep (Helliwell & Williams, 1994), in the fetal human kidney (Drew et al. 1998) and in several areas of the fetal human brain (Thomas et al. 2002). One of these possibilities is that melatonin may act upon the fetal adrenal gland, inhibiting ACTH-induced cortisol production. Such an action of melatonin has been reported by us in the adrenal gland of the adult capuchin monkey, a New World primate (Torres-Farfan et al. 2003).
We hypothesize that maternal melatonin selectively decreases cortisol production in the fetal primate adrenal gland without interfering with DHAS production. We tested this hypothesis in the capuchin monkey by means of in vitro and in vivo experiments. In vitro we investigated, in the fetal adrenal gland, the expression of melatonin receptor isoforms and the direct effects of melatonin on ACTH- and CRH-stimulated cortisol and DHAS production and on 3β-HSD mRNA expression. In vivo we assessed the effect of chronic maternal melatonin suppression on maternal plasma oestradiol concentration (indirect reflection of fetal DHAS production) and on plasma cortisol concentration in the newborn. Maternal plasma melatonin was suppressed chronically by exposing the mothers to constant light during the last third of gestation; we complemented this study by replacing melatonin in the mothers exposed to constant light.
Methods
Animals
Twenty-six time-dated pregnant female capuchin monkeys were obtained from the Chilean Primate Center, Pontificia Universidad Católica de Chile. The females were maintained in individual cages in a room with controlled temperature and humidity, and water available ad libitum. Food was administered twice a day. The light–dark cycle in the facility was 14 : 10 h (lights on at 07.00 h). Animal handling and care was performed following the recommendations of the NIH Guide for Animal Experimentation Care. The study protocol was approved by the Commission on Bioethics and Biosafety of the Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile.
In vitro experiments
Collection of fetal tissues from pregnancies maintained in 14 : 10 h light–dark (LD)
Eight pregnant females at about 90% of gestation were anaesthetized with a mixture of 1% halothane in oxygen and the fetuses were delivered by hysterotomy performed at 14.00 h under sterile conditions. The fetuses were killed immediately by an i.p. overdose of sodium thiopental (100 mg (kg weight)−1) and the fetal adrenal glands were dissected and weighed. A small adrenal piece was embedded in OCT (Tissue-Tek, Ted Pella Inc, Redding, CA, USA) and stored at –20°C to obtain frozen sections. Adrenal glands from four fetuses (135.8 ± 3.3 days of gestation; 181.3 ± 7.7 g body weight; 416.5 ± 29.4 mg combined adrenal weight), as well as kidneys and diaphragm from three of the fetuses, were quickly frozen in liquid nitrogen and stored at –80°C for membrane preparation and RNA extraction. Adrenals from the remaining four fetuses (at 141.0 ± 1.9 days of gestation; 211.0 ± 7.7 g of body weight; 514 ± 24.7 mg of combined adrenal weight) were used fresh in culture experiments. The remaining fetal tissues were incorporated into the Colony Tissue Bank. After finishing the hysterotomy, the females were given an oral analgesic (Tramal, 1 drop kg−1, Grunental, Santiago, Chile), and i.m. anti-inflamatory (Ketofen 1%, 0.1 ml kg−1, Merial, Lyon, France) and antibiotics (Baytril 5%, 0.1 ml kg−1, Bayer S.A., Brazil, and Benacillin 1.5 ml, Troy Laboratories PTY Limited, NSW, Australia). Upon recovery from anaesthesia, the females were returned their cages. Tramal was given daily for the next 3 days, Ketofen and Baytril were given daily for 5 days and a second dose of Benacillin was given 48 h after surgery. The wound was cleaned daily for 5 days using sterile saline and Larvispray (Pfizer, Animal Health Division, Santiago, Chile).
To construct a curve of changes in adrenal weight with development, we obtained adrenal weights from two fetuses (90 days of gestation), eight newborns (3–30 days of age) and four young adults (4–5 years of age) from the records of the Chilean Primate Center.
In vivo experiments
Exposure to constant light during pregnancy and postpartum
From about 100 days of gestation up to the post-delivery conclusion of the experiment, 11 females were maintained with lights continuously on (2000 lux at the head level). Six females received a teaspoon of fruit juice daily at 1600 h as placebo (LL group) for 55.4 ± 2.2 days and the other five (LL + Mel group) received 250 μg (kg body weight)−1 of melatonin (Maver Ltda. Laboratory, Santiago, Chile) in fruit juice daily at 1600 h for 52.8 ± 1.7 days. Seven additional females remained in 14 : 10 h LD (LD control group). One pregnancy in each group ended in an intrapartum fetal death at term. All other pregnancies resulted in live term newborns, but one newborn in the LD group was not raised by the mother and therefore was not included in the experiment.
All maternal procedures were performed under i.m. ketamine (10 mg kg−1 of body weight; Ketaset, Laboratorios Wyeth Inc., Santiago, Chile). Starting at 110 days of gestation and about every 10 days, at 11.00 h, a routine clinical examination was performed in the mothers, maternal weight was recorded and fetal growth (biparietal diameter) and heart rate were measured by ultrasound (Corradini et al. 1998). Pregnancy length was recorded. Upon delivery, mothers and newborns were examined by the colony veterinarian. Newborns were weighed and measured (biparietal, fronto-occipital and chest diameters, and femur and crown-to-heel length).
Maternal and newborn blood sample collection
Maternal blood (0.6 ml) was drawn by saphena venipuncture under i.m. ketamine (10 mg kg−1) at each pregnancy follow up. Additionally, at about 140 days of gestation, blood samples were drawn at 20.00, 24.00, 04.00, and 08.00 h from three females from the LD and LL groups, and at 20.00 and 08.00 h from three females from the LL + Mel group. In the LD females the blood samples at 24.00 and 04.00 h were drawn under red light (<0.2 lux). All these females delivered healthy term newborns. Plasma was separated and stored in aliquots at –20°C until assayed to measure melatonin, cortisol and oestradiol concentrations by radioimmunoassay (RIA).
Blood was drawn from five newborns (5.0 ± 0.4 days old) from LD mothers, five newborns (4.4 ± 0.5 days old) of LL mothers and four newborns (4.8 ± 0.5 days old) from LL + Mel mothers. These newborns were raised by their mothers and had similar gestational age and weight at birth (Table 1). To collect blood samples, the newborn was separated from the mother and placed inside a small clear plastic box containing a heating blanket and a furry teddy bear. Heparinized blood (0.2 ml) was drawn from the saphena vein by venipuncture every 3 h for 24 h starting at 08.00 h, after locally anaesthetizing the zone with lidocaine gel (4% Lidocaina gel, Laboratorio Chile SA, Santiago, Chile). The respective light schedules were maintained during blood sample collection, and lights-off blood samples in newborns from the LD group were drawn under red light. Plasma was separated and stored at –20°C until assayed for cortisol concentration. Bottle-feeding was provided every 3 h (NAN 1, Nestle, Vevey, Switzerland), immediately after completing the blood collection. Upon completion of the experiment, the newborn was returned to its mother and both, newborn and mother returned to the normal lighting conditions of the colony.
Table 1.
Maternal and neonatal variables (mean ±s.e.m.)
| Groups | LD (n= 5) | LL (n= 5) | LL + Mel (n= 4) | |
|---|---|---|---|---|
| Mothers | Weight gain (g)* | 345.0 ± 37.0 | 388.0 ± 4.0 | 335.0 ± 8.2 |
| Pregnancy length (days) | 158.2 ± 2.2 | 157.4 ± 1.3 | 156.0 ± 1.5 | |
| Newborns | Weight at birth (g) | 218.0 ± 10.9 | 237.0 ± 14.5 | 227.5 ± 14.5 |
| Biparietal diameter (cm) | 3.7 ± 0.2 | 4.0 ± 0.2 | 3.9 ± 0.1 | |
| Crown to heel length (cm) | 23.3 ± 0.3 | 23.6 ± 0.7 | 23.0 ± 0.7 |
n, number of animals. LD: mothers remained in 14 : 10 h light–dark cycle. LL, mothers remained with lights continuously on from about 100 days of gestation. LL + Mel, mothers remained with lights continuously on from about 100 days of gestation, receiving a daily oral dose of melatonin at 16.00 h.
Measured between 105 and 150 days of gestation.
Analysis protocols
2-[125I] iodomelatonin binding and competition studies
The 2-[125I] iodomelatonin binding assay was performed as described (Torres-Farfan et al. 2003). In brief, membranes were prepared by homogenizing the tissues in Tris buffer (25 mm Tris-HCl; 25 mm CaCl2; 0.2% BSA, pH 7.5), containing protease inhibitors. Triplicate aliquots of membrane preparations (50–400 μg of protein) were incubated with 25–300 pm 2-[125I] iodomelatonin (New England Nuclear, Life Science Products, Boston MA, USA; specific activity 2200 Ci mmol−1), in the presence (non-specific binding) or absence of 1 μm melatonin (total binding). We tested the effect of melatonin and its related indols 6-hydroxymelatonin, serotonin, tryptamine, d-l-tryptophan, of luzindole (an MT1/MT2 melatonin receptor antagonist; Dubocovich et al. 1998; Torres-Farfan et al. 2003) and of GTPγ-S (non-hydrolysable GTP analogue; Dubocovich, 1995) upon 2-[125I] iodomelatonin binding. Membrane preparations were incubated with 125 pm 2-[125I] iodomelatonin, and 1 pm to 1 μm of each compound. The maximum number of 2-[125I] iodomelatonin binding sites (Bmax) and dissociation constant (Kd) were determined by Scatchard analysis using GraphPad Prism (version 3.02; GraphPad Software Inc.). Fifty per cent inhibition (IC50) was determined by analysis of competition curves as reported (Torres-Farfan et al. 2003).
Cortisol and DHAS production by the fetal adrenal gland
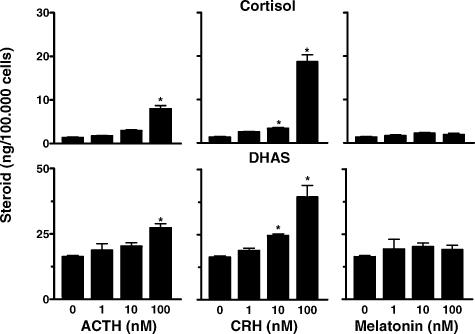
Immediately after necropsy, adrenal glands were weighed and processed. Dispersed cells were prepared from adrenal glands from one fetus to determine the maximal stimulatory doses of ACTH and CRH on cortisol and DHAS production, and the effect of melatonin alone on the production of these steroids. Cells were dispersed as described (Torres-Farfan et al. 2003). Triplicate aliquots of 100 000 cells well−1 were incubated during 48 h with 1–100 nm human ACTH 1–39 peptide, human CRH 1–24 peptide or melatonin (all from Sigma-Aldrich, St Louis MO, USA). At the end of the experiments, the supernatants were collected and stored at –20°C until assayed by radioimmunoassay (RIA). Fetal adrenal cells in culture produced cortisol and DHAS. Maximal stimulation of cortisol and DHAS was obtained with the 100 nm dose of ACTH or CRH (Fig. 1, left and middle panels). Treatment with increasing doses of melatonin alone (1–100 nm) had no effect upon cortisol or DHAS production (Fig. 1, right panel), as previously shown for the adult adrenal (Torres-Farfan et al. 2003). Based on these results, in the following experiments using fetal adrenal explants, 100 nm ACTH or 100 nm CRH were used to test the effects of melatonin on cortisol and DHAS production.
Figure 1. Effect of 1–100 nm ACTH (left panel), CRH (middle panel) and melatonin (right panel) upon cortisol and DHAS production by dispersed adrenal cells from a capuchin monkey fetus at 137 days gestation (term 158.2 ± 2.2 days).
Values are mean ±s.e.m. of triplicate incubations.* significantly different from basal production, P < 0.05, ANOVA for repeated measures and Tukey-Kramer test.
Fetal adrenal gland explants from three fetuses were prepared as described before (Torres-Farfan et al. 2003). In brief, fetal adrenal glands from each fetus were cut into small explants, which were mixed and suspended in culture medium (D-MEM/F12, 0.1% BSA, GIBCO, Grand Island, NY, USA). Triplicate 0.2 ml aliquots (containing 14.1 ± 0.74 mg explants), taken using a Gilson pipette with cut-off tip were incubated in 2 ml of culture medium for 48 h. Aliquots were incubated with medium alone (control); medium plus 100 nm ACTH; medium plus 100 nm ACTH and melatonin (0.1–10 nm); medium plus 100 nm ACTH, melatonin (0.1–10 nm), 1 μm luzindole; and medium plus 1 μm luzindole alone. Due to the limited amount of tissue available, we tested the effect of 100 nm ACTH plus 1 μm luzindole in two experiments only. A similar protocol was followed to test the effects of melatonin on CRH-stimulated cortisol and DHAS production. Melatonin solutions were prepared from a 10 mm range stock solution in absolute ethanol and kept at −20°C. Working solutions were prepared by successive dilutions (between 10 million and 100 000-fold) in D-MEM/F12 to reach the respective 0.1 nm and 100 nm concentrations used in the experiments. Luzindole solutions were prepared from a 10 mm range stock solution in dimethyl sulfoxide (DMSO) and kept at –20°C. Working solutions were prepared by successive dilutions (10 000-fold) in D-MEM/F12 to reach the 1 μm concentration used in the experiments. Fresh dilutions of melatonin and luzindole in D-MEM/F12 were prepared for each experiment. Adrenal explants from the three fetuses were used for the experiments with ACTH, whereas adrenal explants from two of these fetuses were used with CRH. At the end of each experiment, the medium was separated and stored at –20°C until assayed by RIA for cortisol and DHAS. The explants were weighed; steroid production was measured and corrected per milligram of tissue. Triplicate explants from each experimental point were pooled and stored in RNAlater (Ambion Inc., Austin, TX, USA).
Expression, cDNA sequencing and semiquantitative analyses of mRNAs using reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from frozen or RNAlater-preserved tissues using TRIZOL reagent (Life Technologies Inc., Rockville, MD, USA), according to the manufacturer's instructions. cDNAs were synthesized using random primers as described before (Torres-Farfan et al. 2003). cDNA pools of each tissue or experiment were utilized to establish lineal range of cycles for MT1, 3β-HSD and 18S-rRNA amplification. Amplification was lineal from 29 to 38 cycles for MT1 mRNA, from 24 to 29 cycles for 3β-HSD mRNA, and from 15 to 21 cycles for 18S-rRNA. All reverse transcription and PCR reactions were carried out in a Biometra thermocycler (Personal Cycler, Biometra Inc., Tampa, FL, USA). Aliquots of 15 μl of all PCR products were analysed by electrophoresis on a 1.5% agarose–ethidium bromide gel. Each gel was photographed with GelCam DS-34 (Polaroid Corporation, Allan D. Verch, 781, Cambridge, MA, USA); the photography was scanned using Snapscan e10 (Agfa-Gevaert N.V. Septestraat 27 B-2640 Mortsel, Belgium), and the density of the band was analysed with the software Scion Image (Scion Corporation, http://www.scioncorp.com). The mRNAs encoding for capuchin monkey MT1 and MT2 melatonin receptor isoforms were reverse transcribed and amplified as described (Torres-Farfan et al. 2003; accession numbers AY227380 and AY227381, respectively). The expected amplification product has a size of 435 bp for MT1 and 396 bp for MT2. The PCR program for MT1 and MT2 consisted of an initial denaturation step at 95°C for 5 min, followed by 35 cycles at 95°C for 30 s, 60°C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 10 min.
We measured 3β-HSD mRNA expression by semiquantitative RT-PCR in adrenal pieces obtained at necropsy from three of the fetuses of the explant experiments and from two adults from a previous experiment (Torres-Farfan et al. 2003). In addition, we measured the effect of the different treatments on 3β-HSD mRNA expression in cultured capuchin monkey fetal adrenal explants. The sequence of the PCR primers, designed after annealing of 3β-HSD cDNA sequences published for human, rat and mouse (Genebank accession numbers NM_000198, M38179, NM_153193, respectively), were: forward, bases 342–365 (5′-CCATTCCTGAAGAGCGCCTGCCAG-3′) and reverse, bases 624–647 (5′-CGCCAGTACAGCCTTCTCAGCAAG-3′); base numbers according to the human mRNA for 3β-HSD2. The expected amplification fragment of 305 bp contains an insertion site for the intron located between exons II/III and was amplified with the PCR program for MT1 and MT2 but using 27 cycles. The PCR product obtained from capuchin monkey fetal adrenal (Genebank accession number AY223540) was sequenced by the sequencing facility of our Faculty at the Ecology Department and showed 90% identity to the cDNA for human 3β-HSD2. The 18S-rRNA housekeeping gene was amplified using the primers described by Einspanier et al. (2002); forward: 5′-TCAAGAACGAAAGTCGGAGG-3′, reverse: 5′-GGACATCTAAGGGCATCACA-3′, expected fragment of 488 bp. The PCR used 17 cycles in the program described above. Each 3β-HSD and 18S-rRNA cDNA sample was amplified in three separate assays. The ratio between the 3β-HSD and 18S-rRNA was calculated for each treatment (coefficient of variation was 24.6%).
Hormone assays
The melatonin concentration in maternal plasma was measured by RIA. Melatonin antiserum (G/S/704 8483, Stockgrand Ltd, Guildford, Surrey, UK) and [O-methyl-3H]melatonin (85 Ci mmol−1, Amersham Biosciences AB, SE-751 84, Uppsala, Sweden) as tracer were used following the manufacturer's recommendations. The range of the standard curve was 0.78–50 pg tube−1 (3.4–215 fmol tube−1) and the inter- and intra assay coefficients were less than 15%. Plasma samples were extracted with diethyl ether prior to assaying. Values were not corrected for recovery, as it was over 90%.
Cortisol and oestradiol concentrations in plasma and cortisol in culture medium were measured by RIA, using the reagents and methodology of the WHO Program for the Provision of Matched Assay reagents for RIA of Hormones in Reproductive Physiology Program. The inter- and intra-assay coefficients of variation were less than 15%. Assays were previously validated for capuchin monkey plasma (Recabarren et al. 1998).
The DHAS concentration in the culture medium was measured by RIA as described (Koritnik et al. 1983). As the antibody cross-reacts 100% with DHAS and DHA, [1,2,6,7-3H(N)]-dehydroepiandrosterone was used as tracer (New England Nuclear, Life Science Products, Boston MA, USA; specific activity 60 Ci mmol−1). The range of the standard curve was 10–800 pg tube−1 (25.6–2051 fmol tube−1). The inter- and intra-assay coefficients of variation were less than 15%. The proportion of DHAS in the culture medium was checked by assaying immunoreactivity before and after ether extraction. We found that less than 10% of immunoreactivity is extracted with ether, indicating that the steroid assayed in the culture is DHAS.
Data analysis
Data were expressed as means ±s.e.m. The effects of the treatments upon steroid production in each individual experiment (dispersed fetal adrenal cells and fetal adrenal explants) were analysed by ANOVA for repeated measures followed by the post hoc Tukey-Kramer test. Means of the three experiments stimulated with ACTH were analysed by one-way ANOVA followed by the post hoc Tukey-Kramer test. The mean ratio 3β-HSD mRNA/18S-rRNA was calculated after arcsin transformation and the effects of the treatments were compared by one-way ANOVA followed by the posthoc Tukey-Kramer test. Changes of melatonin concentration versus clock time and mean maternal concentrations of cortisol and oestradiol versus gestational age, were compared using anova for repeated measures followed by post hoc Tukey-Kramer test. Missing values were incorporated as the mean for the corresponding time interval. The concentration of cortisol in the newborns was calculated as the area under curve (AUC) in 24 h and expressed as μg ml−1× 24 h. Means of AUC in 24 h were compared by ANOVA and Tukey-Kramer test. Statistical analyses were performed using GraphPad Prism software (version 3.02; GraphPad Software Inc., San Diego, CA, USA). Results were considered significant when P values were < 0.05.
Results
Histology and growth pattern of the capuchin monkey adrenal gland
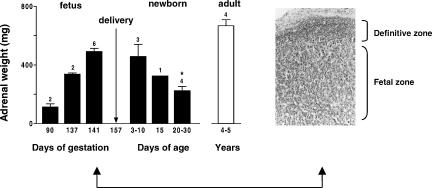
The capuchin monkey fetal adrenal gland was remarkably similar to the human and other primates in terms of development (Serón-Ferré & Jaffe, 1981; Mesiano & Jaffe, 1997; McNulty et al. 1981; Ducsay et al. 1991). The capuchin monkey fetal adrenal increased notably in size between 90 and 141 days of gestation (Fig. 2, left). This size was maintained during the first week after birth, decreasing to about half by 30 days of age. As shown in the figure, the fetal adrenal weight at late gestation was about 80% of the adrenal weight of the adult. At 141 days of gestation (about 90% gestation) the capuchin monkey fetal adrenal was composed of a rim of small basophilic cells located under the capsule (definitive zone) surrounding the zone of large eosinophilic cells (fetal zone) that makes up most of the gland at this age (Fig. 2, right).
Figure 2. Changes in adrenal gland weight from 90 days' gestation to adulthood in the capuchin monkey and morphology of the fetal adrenal gland.
Left panel: Combined adrenal weight. Number of animals is indicated over the bars. * Significantly different from 141–148 days of gestation, P < 0.05, one-way ANOVA and Tukey-Kramer test. Right panel: photomicrograph of an adrenal gland section at 140 days' gestation, stained with haematoxylin–eosin × 50.
In vitro experiments
Identification of melatonin receptors in the capuchin monkey fetal adrenal gland
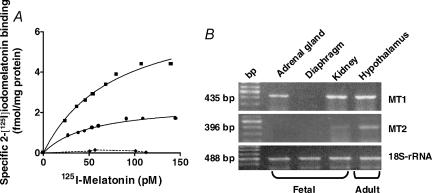
We found specific binding of 2-[125I] iodomelatonin in membrane preparations from whole fetal adrenal gland and fetal kidney, whereas no binding was detected in fetal diaphragm (Fig. 3, left panel). The specific 2-[125I] iodomelatonin binding to fetal adrenal gland membranes was displaced by melatonin and related indoles (melatonin > 6-OH melatonin >>> serotonin, tryptamine and tryptophan). Binding of 2-[125I] iodomelatonin was also displaced by the MT1/MT2 melatonin receptor antagonist luzindole and by GTPγ-S (Table 2). Taken together, these results indicate that the high affinity binding sites for 2-[125I] iodomelatonin present in the fetal adrenal gland correspond to a membrane-bound receptor coupled to a G-protein. We found that the fetal adrenal gland expresses only the MT1 melatonin receptor mRNA (Fig. 3, right panel). This receptor was also found in fetal kidney and adult capuchin monkey hypothalamus; the latter was used as positive control. There was a weak expression of the MT2 isoform mRNA in the fetal kidney whereas a strong expression of this isoform was detected in adult hypothalamus, as reported previously (Torres-Farfan et al. 2003). No expression of either melatonin receptor isoform mRNA was detected in fetal diaphragm (negative control). Amplification of traces of genomic DNA was ruled out by digesting the RNA samples with Dnase I, as well as by omitting the reverse transcriptase step or the RNA template (not shown).
Figure 3. Melatonin receptor in capuchin monkey fetal adrenal gland.
Left panel: binding in membrane preparations from fetal adrenal gland (•); kidney (▴) and diaphragm (***). Tissues were obtained from four capuchin monkey fetuses, gestational age 135.8 ± 3.3 days (term 158.2 ± 2.2 days). Right panel: RT-PCR products for MT1 (435 bp, upper panel), MT2 (396 bp, middle panel) and 18S-rRNA (488 bp, lower panel) from fetal adrenal gland, kidney and diaphragm (142 days' gestation) and from adult capuchin monkey hypothalamus. bp, DNA size marker.
Table 2.
Binding affinity (Kd), receptor density (Bmax) and IC50 displayed by melatonin and compounds competing with 2-[125I]iodomelatonin binding in membrane preparations from fetal capuchin monkey adrenal gland and kidney
| Fetal adrenal gland (n= 4) | Fetal kidney (n= 3) | ||
|---|---|---|---|
| Kd (pM) | 75.7 ± 6.9 | 130.2 ± 18.9 | |
| Bmax (fmol (mg protein)−1) | 2.6 ± 0.4 | 8.2 ± 1.0 | |
| IC50 (nm) | Melatonin | 0.4 ± 0.1 | 7.5 ± 0.3 |
| 6-Hydroxymelatonin | 4.5 ± 0.7 | 0.8 ± 0.3 | |
| Serotonin | >1000 | >1000 | |
| d-l-Tryptophan | >1000 | >1000 | |
| Tryptamine | >1000 | >1000 | |
| Luzindole | 12.5 ± 2.5 | 14.6 ± 3.0 | |
| GTPγ-S | 2.0 ± 0.6 | 1.5 ± 0.6 |
Mean ±s.e.m.; n, number of animals.
Effects of melatonin on ACTH-stimulated cortisol and DHAS production
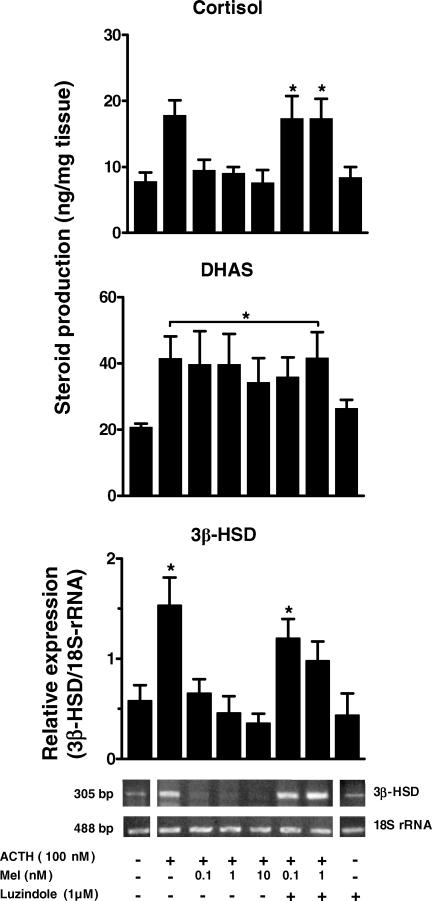
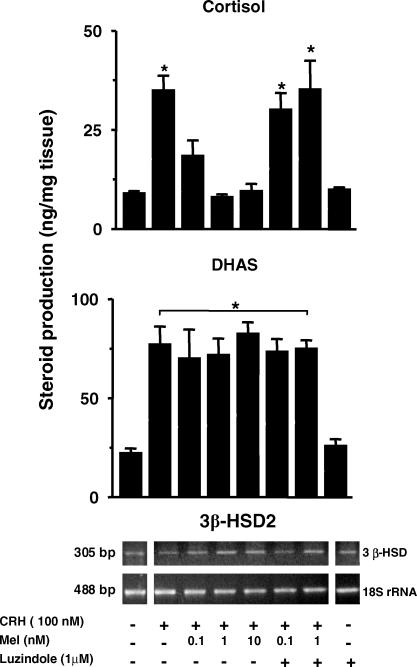
The production of both steroids increased about 2-fold after 48 h of ACTH stimulation (Fig. 4, upper and middle panels). Addition of melatonin (0.1, 1.0 and 10 nm) to the culture selectively inhibited the increase in cortisol production but had no effect upon DHAS production. The inhibitory effect of melatonin on ACTH-stimulated cortisol production was reversed by the addition of 1 μm luzindole (MT1/MT2 melatonin receptor antagonist). As shown in the figure, the antagonist alone did not change basal cortisol or DHAS production. No effect of luzindole was found in the presence of 100 nm ACTH.
Figure 4. Effect of melatonin upon ACTH-stimulated cortisol production (upper panel), DHAS production (middle panel) and 3β-HSD gene transcription (lower panel).
Values are mean ±s.e.m. of experiments performed in adrenal explants from three fetuses (gestational age 142.3 ± 1.9 days, term 158.2 ± 2.2 days). Representative agarose gels for 3β-HSD gene transcription and 18S-rRNA from a fetus at 140 days' gestation are included in the lower panel. The inhibitory effect of melatonin on ACTH-stimulated cortisol production and on 3β-HSD gene transcription was reversed by luzindole. * Significantly different from basal, P < 0.05, one-way ANOVA and Tukey-Kramer test.
Effects of melatonin on ACTH-stimulated 3β-HSD transcription in fetal adrenals
The ratio 3β-HSD/18S-rRNA in the fetal adrenal immediately after necropsy was about one-fifth lower than that detected in the adrenals of the two adults tested (0.24 ± 0.03 (n= 3) versus 1.33 and 1.43, respectively). After 48 h in culture, the basal ratio 3β-HSD/18S-rRNA in the fetal adrenal explants increased by 2- to 3-fold compared to that found in the same adrenals immediately after necropsy (0.58 ± 0.15 versus 0.24 ± 0.03, P < 0.1 >0.05 paired Student's t test; n= 3). Treatment with ACTH for 48 h increased 3β-HSD/18S-rRNA about 4-fold over basal (Fig. 4, lower panel); this effect was inhibited by the addition of melatonin. Luzindole reversed the inhibitory effect of 0.1 nm melatonin and partially inhibited the effect of 1 nm of melatonin. Luzindole alone did not change basal 3β-HSD/18S-rRNA. No effect of luzindole was found in presence of 100 nm ACTH.
Effects of melatonin on CRH-stimulated cortisol and DHAS production and on fetal adrenal transcription of 3β-HSD
We tested the effects of melatonin on CRH stimulated cortisol and DHAS production in adrenal explants from two fetuses. One of the experiments is shown in Fig. 5. In both experiments, 100 nm CRH stimulated cortisol and DHAS production (Fig. 5, upper and middle panels). Inhibitory effects of melatonin were observed on CRH-stimulated cortisol production, this effect was blocked by luzindole. No effects of melatonin were observed upon CRH-stimulated DHAS production. At variance with the results obtained for ACTH, CRH did not increase transcription of the 3β-HSD gene (see representative gel on Fig. 5).
Figure 5. Effect of melatonin upon CRH-stimulated cortisol production (upper panel), DHAS production (middle panel) and 3β-HSD gene transcription (lower panel).
The values are mean ±s.e.m. of triplicate incubations from one fetus (gestational age 141 days, term 158.2 ± 2.2 days). The inhibitory effect of melatonin on CRH-stimulated cortisol production was reversed by luzindole. * Significantly different from basal, P < 0.05, ANOVA for repeated measures and Tukey-Kramer test.
In vivo experiments
Effect of exposure to constant light during pregnancy on maternal plasma melatonin
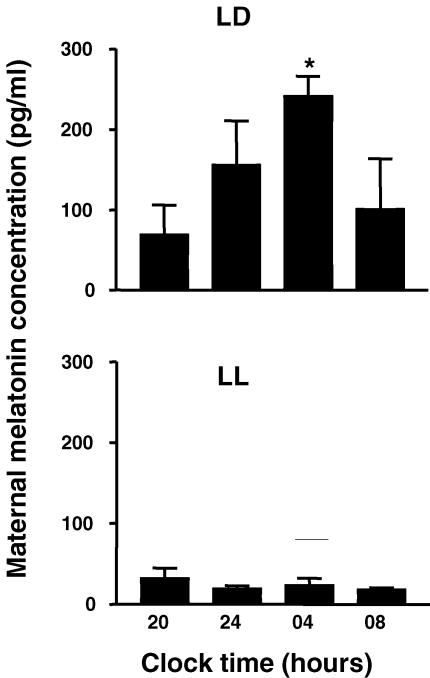
Constant light effectively suppressed maternal plasma melatonin concentration. Females maintained in 14 : 10 h LD showed a higher melatonin concentration during the lights-off hours. In contrast females maintained in LL had low melatonin at all time points (Fig. 6). Mean plasma melatonin concentrations measured throughout pregnancy at 11.00 h (see Methods) were also higher in LD females than in LL females (51.9 ± 3.1 versus 38.6 ± 10.5 pg ml−1, P < 0.05, respectively). Females that were in constant light but received a daily melatonin dose at 16.00 h had higher melatonin concentrations (328.3 ± 77.5 pg ml−1) than those females under LD and LL conditions.
Figure 6. Suppression by constant light of the nocturnal increase in plasma maternal melatonin concentration.
Values are mean ±s.e.m. (n= 3 in each group). Pregnant capuchin monkeys were exposed to a light : dark schedule of 14 : 10 h (LD), or to constant light (LL) from about 100 days of gestation (term 157 days). Plasma melatonin was measured at the indicated 24 h clock time at 140 days of gestation. * Significantly different from other times, P < 0.05, ANOVA for repeated measures and Tukey-Kramer test.
Effect of suppression of maternal melatonin during pregnancy on maternal plasma oestradiol and cortisol concentrations
The capuchin monkey, like other New World monkeys, has concentrations of plasma cortisol in the μg ml−1 range (Chrousos et al. 1982; Recabarren et al. 1998). In addition, it has concentrations of oestradiol during pregnancy in the ng ml−1 range found in human pregnancies (Patrick et al. 1979; Recabarren et al. 1998).
The decrease in plasma melatonin concentration caused by constant exposure to light during gestation had no effect on mean maternal plasma oestradiol levels. We did not detect any difference in the oestradiol concentration at any gestational age between these LL females and those maintained either under normal light–dark conditions or constant light conditions plus melatonin replacement (Table 3).
Table 3.
Mean ±s.e.m. maternal plasma oestradiol and cortisol concentration throughout gestation in capuchin monkeys
| n | Gestational age (days) 100–109 | 110–119 | 120–129 | 130–139 | 140–149 | ||
|---|---|---|---|---|---|---|---|
| Oestradiol (ng ml−1) | LD | 5 | 29.3 ± 0.8 | 30.9 ± 6.5 | 46.5 ± 7.3 | 46.6 ± 7.3 | 57.5 ± 10.2* |
| LL | 5 | 33.1 ± 6.0 | 40.2 ± 9.3 | 38.5 ± 15.0 | 39.9 ± 11.8 | 57.1 ± 7.8 | |
| LL + Mel | 4 | 20.5 ± 3.9 | 30.2 ± 3.4 | 26.6 ± 10.9 | 33.2 ± 6.4 | 38.4 ± 11.2 | |
| Cortisol (μg ml−1) | LD | 5 | 2.8 ± 0.3 | 2.5 ± 0.4 | 3.5 ± 0.5 | 3.4 ± 0.2 | 4.3 ± 0.3* |
| LL | 5 | 2.6 ± 0.3 | 3.5 ± 0.6† | 4.5 ± 0.4 † | 4.3 ± 0.3 † | 4.2 ± 0.6† | |
| LL + Mel | 4 | 3.4 ± 0.5 | 2.6 ± 0.5 | 4.8 ± 0.8 | 4.3 ± 0.4 | 4.6 ± 0.8 |
n, number of animals. LD, 14 h of light, 10 h of dark. LL, constant light. LL + Mel, LL plus melatonin supplementation. LL and LL + Mel treatments started at about 100 days of gestation. Blood samples were drawn at 11.00 h (see text for details). Mean values at 120–129 days of gestation were calculated from 4 animals in the LL group and 3 animals in the LL + Mel group; mean values at 140–149 days were calculated from 3 animals in the LL group.
Significantly different from 100–109 and 110–119 days of gestation
Significantly different from 100–109 days of gestation, P < 0.05 ANOVA for repeated measures and Tukey-Kramer multiple comparison test.
In all three groups, at 140 days' gestation, maternal oestradiol concentrations were higher at 20.00 h than at 08.00 h (P < 0.05, paired Student's t test). There was no difference between the groups in the values at 20.00 or at 08.00 h (Table 4). No differences in cortisol concentration were detected between groups at any of the gestational age intervals studied. However, the increase in cortisol occurring at 140–149 days of gestation in the LD group was observed earlier (110–119 days) in the LL group (Table 3). In the three groups, maternal cortisol concentrations were lower at 20.00 h than at 08.00 h (P < 0.05, paired Student's t test). There was no difference between the groups in the values at 20.00 or at 08.00 h (Table 4).
Table 4.
Mean ± s.e.m. maternal plasma oestradiol and cortisol concentration at 20.00 and 08.00 h in capuchin monkeys at 140 days gestation
| n | Oestradiol (ng ml−1) 20.00 h | 08.00 h | Cortisol (μg ml−1) 20.00 h | 08.00 h | |
|---|---|---|---|---|---|
| LD | 3 | 66.0 ± 8.7 * | 5.8 ± 1.3 | 1.3 ± 0.1 * | 4.8 ± 1.2 |
| LL | 3 | 62.2 ± 5.8 * | 8.5 ± 3.5 | 1.5 ± 0.3 * | 4.8 ± 0.8 |
| LL + Mel | 3 | 59.2 ± 10.2 * | 13.3 ± 4.1 | 1.8 ± 0.5 * | 5.4 ± 1.6 |
n, number of animals. LD, 14 h of light, 10 h of dark. LL, constant light. LL + Mel, LL plus melatonin supplementation. LL and LL + Mel treatments started at about 100 days of gestation (see text for details).
Significantly different from 08.00 h, P < 0.05, paired Student's t test.
Neither constant light nor constant light plus melatonin replacement treatments had any effect upon pregnancy outcome (see section headed Animals in Methods), maternal weight increase during pregnancy (Table 1) and fetal growth (data not shown), and pregnancy length or newborn weight at birth (Table 1). Moreover, newborn height (crown-to-heel length) and biparietal diameter, as well as the other measurements performed (data no shown), were similar to those of control animals (Table 1).
Effect of suppression of maternal melatonin during pregnancy on plasma cortisol concentration in the 4–6 days old newborn
The technical difficulty of obtaining repeated fetal blood samples prevented us from ascertaining the effects of the treatments upon fetal plasma cortisol. Instead, we measured the cortisol concentration in 4- 6-day-old capuchin monkey newborns.
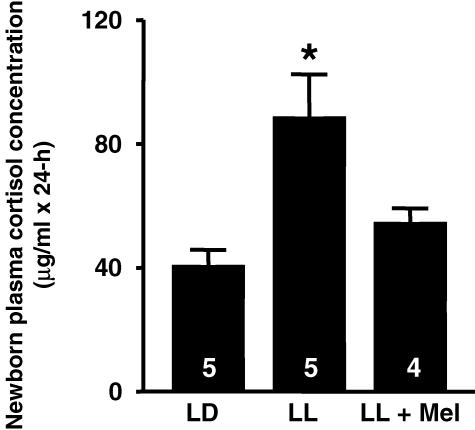
We detected profound effects of maternal melatonin suppression and maternal melatonin replacement on newborn plasma cortisol concentrations (Fig. 7). To minimize the effects of episodic cortisol secretion and time-of-day effects, cortisol concentration was measured in samples taken every 3 h for 24 h and cortisol concentration was calculated as the area under the curve in 24 h (AUC, expressed as μg ml−1× 24 h). The AUC cortisol concentration of newborns whose mothers had suppressed melatonin (LL) was nearly twice as high as that in newborns exposed to maternal melatonin (LD). In contrast, the newborns whose mothers were exposed to LL but received melatonin, had plasma AUC cortisol concentrations similar to those of the LD newborns (Fig. 7).
Figure 7. Effect of maternal melatonin suppression and replacement on plasma cortisol concentration of capuchin monkey newborns (4–6 days of age), measured as area under the curve.
Values are mean ±s.e.m. The number of newborns under each condition is indicated inside the bars. * Significantly different from LD and LL + Mel, P < 0.05, one-way ANOVA and Tukey-Kramer test.
Discussion
The results from this study support the hypothesis that maternal melatonin selectively decreases cortisol production in the capuchin monkey fetal adrenal gland without interfering with DHAS production. First, the capuchin monkey fetal adrenal gland expressed the membrane-bound melatonin receptor MT1. Second, melatonin in vitro had direct inhibitory effects on ACTH-stimulated cortisol production and on ACTH-stimulated 3β-HSD expression, whilst no effects were seen on ACTH-stimulated DHAS production. Finally, low maternal melatonin concentrations during the last 40 days of gestation, induced by chronic exposure of pregnant monkeys to constant light, did not affect maternal oestradiol or cortisol concentrations during pregnancy, but markedly increased cortisol concentrations in the neonate. The latter effect was reversed by melatonin administration to the mother.
The capuchin monkey fetal adrenal gland expresses a functional G-protein-coupled membrane-bound melatonin receptor, as shown by detection of specific high-affinity binding sites for 2-[125I]iodomelatonin. In membrane preparations of whole fetal adrenal gland, the 2-[125I]iodomelatonin binding sites had a Kd and a pharmacological profile similar to that previously reported for membrane-bound melatonin receptors (Torres-Farfan et al. 2003). 2-[125I]iodomelatonin binding was displaced by luzindole (an MT1/MT2 melatonin receptor antagonist; Dubocovich et al. 1998; Torres-Farfan et al. 2003) and by GTPγ-S, a-non-hydrolysable analogue of GTP (Dubocovich, 1995). Moreover, 6-hydroxymelatonin was a weak competitor for 2-[125I]iodomelatonin, whereas serotonin, d-l-tryptophan and tryptamine showed no effect. The Kd, 75.67 pm, was in the range reported in adult capuchin monkey adrenal cortex (Torres-Farfan et al. 2003) and in other tissues from humans and other species (Weaver et al. 1993; Reppert et al. 1995; Song et al. 1995; Woo et al. 2001; Vera et al. 1993; Witt-Enderby & Dubocovich, 1996). The binding sites were located in the whole fetal adrenal (whole mount autoradiography, data not shown). We identified the melatonin receptor subtype expressed by the fetal adrenal gland as the MT1 isoform by RT-PCR and cDNA sequencing, in line with our findings in the adult capuchin monkey adrenal gland (Torres-Farfan et al. 2003).
The late gestation capuchin monkey fetal adrenal gland secretes DHAS and cortisol, and increases the production of these steroids in response to ACTH and CRH treatment. This response to ACTH is similar to that reported for cultured mid-gestation human fetal adrenal explants (Simpson et al. 1979), dispersed cells (Fujieda et al. 1981), and superfused isolated fetal adrenal zones (Serón-Ferréet al. 1978); as well as for late-gestation superfused isolated fetal adrenal zones from rhesus monkey (Serón-Ferré & Jaffe, 1981), and dispersed fetal adrenal cells from baboon (Walker et al. 1988). In our experiments with capuchin monkey fetal adrenal cells and explants, stimulation of cortisol and DHAS production was achieved with 100 nm ACTH, a similar dose to that utilized by us to stimulate adult capuchin monkey adrenal cells and explants (Torres-Farfan et al. 2003) and by others in human fetal adrenal explants (Simpson et al. 1979). The late-gestation capuchin monkey fetal adrenal gland responded to 100 nm CRH by increasing cortisol and DHAS production to the same extent. The effects of CRH had been studied only in mid-gestation human fetal adrenal dispersed fetal zone cells. Smith et al. (1998) reported a preferential stimulation of DHAS over cortisol, whereas Parker et al. (1999), also investigating human fetal adrenal gland cells, reported similar effects of CRH on both steroids to the effects reported here in late-gestation capuchin fetal adrenal explants.
Melatonin selectively inhibited ACTH- and CRH-induced cortisol production by capuchin monkey fetal adrenal explants, without affecting DHAS production. Melatonin per se had no effect upon fetal adrenal steroidogenesis, in concordance with our findings in the adult adrenal gland (Torres-Farfan et al. 2003). The selective inhibition by melatonin of ACTH- and CRH-stimulated cortisol production in the capuchin monkey fetal adrenal suggests that melatonin interferes mainly with enzymes of the cortisol pathway. One such enzyme is 3β-HSD, which is indispensable for cortisol synthesis but is not required for DHAS synthesis. Decreases in cortisol production might result in increases in the alternative path from pregnenolone, leading to an increase in DHAS production. Since we observed no changes in DHAS production, minor compensatory adjustments at steps in the synthesis of DHAS involving Steroidogenic Acute Regulatory protein (StAR), or the enzymes cholesterol side-chain cleavage cytochrome P450 (P450scc) and cytochrome P450 17-hydroxylase (P450c17) may have occurred. This possibility needs to be investigated, given that melatonin decreases human chorionic gonadotropin (HCG)-stimulated mitochondrial StAR in a line of Leydig cells (Wu et al. 2001) and results in accumulation of 17-OH progesterone in gonadotropin releasing hormone (GnRH)-stimulated rat Leydig cell cultures (Valenti et al. 1999).
A characteristic of the primate adrenal gland in vivo is low 3β-HSD activity (Goldman et al. 1966; Serón-Ferré & Jaffe, 1981; Mesiano & Jaffe, 1997). As shown using microarrays, expression of 3β-HSD mRNA in the mid-gestation human fetal adrenal is much lower than in the adult adrenal gland (Rainey et al. 2001). We also found in capuchin monkeys that 3β-HSD mRNA expression in adrenal tissue from late-gestation fetuses is lower than the expression in the adult gland. In the human and other primates, 3β-HSD assessed by histochemistry, immunohistochemistry and in situ hybridization is restricted to the transitional zone of the fetal adrenal (Goldman et al. 1966; Mesiano et al. 1993; Narasaka et al. 2001). The early observation that human fetal adrenal cells differentiate morphologically in vitro, while increasing their capacity to synthesize cortisol in response to ACTH, led to the proposal that in vivo, placental/maternal factors inhibit 3β-HSD (Kahri et al. 1976; Fujieda et al. 1981). Several experiments established that the stimulatory action of ACTH upon cortisol production is accompanied by induction of 3β-HSD protein in explants of separated zones of the mid-gestation human fetal adrenal gland (Doody et al. 1990) and of 3β-HSD mRNA in dispersed human fetal zone cells (Voutilainen et al. 1991; Mason et al. 1993; Mesiano & Jaffe, 1993). In our experiments, 3β-HSD mRNA expression increased in untreated capuchin monkey explants after 48 h in culture, relative to the amount present in vivo. 3β-HSD mRNA expression was markedly increased after ACTH treatment, in line with the previous reports in the human fetal adrenal (Voutilainen et al. 1991; Mason et al. 1993; Mesiano & Jaffe, 1993). A novel finding was that ACTH-induced 3β-HSD mRNA expression in the capuchin monkey fetal adrenal gland was clearly inhibited by melatonin.
Inhibitory effects of melatonin upon CRH-stimulated cortisol production were also observed. However, in contrast to ACTH, we found that CRH does not induce 3β-HSD mRNA expression. This agrees with the lack of effect of CRH upon 3β-HSD mRNA expression reported in human fetal adrenal dispersed cells in culture (Smith et al. 1998). The mechanisms by which CRH stimulates cortisol production in the capuchin monkey fetal adrenal are presently unknown. They may include an increase in 3β-HSD activity, an increase in steroidogenic enzymes like cholesterol side-chain cleavage cytochrome P450 (P450scc) and cytochrome P450 17-hydroxylase (P450c17), as reported in the human fetal adrenal (Smith et al. 1998), which would provide more precursors for the small amounts of 3β-HSD present or even a decrease in potential intra-adrenal cortisol metabolism, since the capuchin monkey fetal adrenal, like the human fetal adrenal gland (Coulter et al. 1999), expresses 11β-hydroxysteroid dehydrogenase type II mRNA (Torres-Farfan, unpublished results). These possibilities remain to be investigated.
Our in vitro experiments showed inhibitory effects of melatonin upon ACTH- and CRH-stimulated cortisol production and ACTH-stimulated 3β-HSD mRNA expression in the capuchin monkey fetal adrenal gland. The fact that these effects were observed using a 0.1 nm concentration of melatonin (in the Kd range found for 2-[125I]iodomelatonin binding in membranes) and that were reversed by 1 μm luzindole (in the Ki range that displaced 2-[125I]iodomelatonin binding) supports melatonin activation of a membrane-bound receptor. Recent data suggest that luzindole also has inverse agonist activities (Ersahin et al. 2002), although these effects were not detected in the conditions of our experiments. At the concentration used in the present report, luzindole does not discriminate between MT1 and MT2 receptors (Dubocovich et al. 1998); however, we found that the adrenal gland expresses only the MT1 melatonin receptor mRNA. We conclude that the inhibitory effect of melatonin upon ACTH- and CRH-stimulated cortisol production and upon ACTH-stimulated 3β-HSD mRNA expression is exerted through a functional MT1 receptor.
Does melatonin play a role in late gestation fetal adrenal function in vivo? This question has not been addressed before. Fetuses are exposed to the maternal melatonin rhythm through the placenta (Yellon & Longo, 1988; McMillen & Nowak, 1989), while they seem to produce little melatonin. Maternal pinealectomy results in very low melatonin concentrations in the fetal circulation (Yellon & Longo, 1988; McMillen & Nowak, 1989). Moreover, melatonin concentrations are low in sheep, human and rat neonates, suggesting an inactive pineal (Reppert et al. 1984; Nowak et al. 1990; Kennaway et al. 1992). The main effect of melatonin on the fetal capuchin monkey adrenal gland in vitro was suppression of the stimulatory effects of ACTH and CRH upon fetal adrenal cortisol production and of ACTH upon 3β-HSD expression without interfering with DHAS production. Fetal pituitary ACTH is the main regulator of fetal adrenal function in the human and other primates (Serón-Ferré & Jaffe, 1981; Mesiano & Jaffe, 1997), and placental CRH is secreted into the fetal compartment in the human (Goland et al. 1986). We inferred, extrapolating from our in vitro findings, that chronic maternal melatonin suppression would result in unrestrained actions of fetal ACTH and CRH on the fetal adrenal and, in particular, would allow fetal ACTH to induce fetal adrenal 3β-HSD. The capuchin monkey adrenal gland experiences minor changes during the first week of life, as reported in other primates (McNulty et al. 1981; Serón-Ferré & Jaffe, 1981; Ducsay et al. 1991; Mesiano & Jaffe, 1997). Therefore an increased adrenal capacity to produce cortisol, acquired during fetal life because of chronic melatonin suppression leading to increased 3β-HSD expression, should be maintained in the early newborn. Melatonin had no effect upon DHAS production in vitro. In the capuchin monkey, as in the human and other primates (Siiteri & MacDonald, 1966; Serón-Ferré & Jaffe, 1981), fetal death rapidly decreases maternal oestradiol (Serón-Ferré, unpublished results), suggesting that fetal DHAS is the precursor for placental oestrogen synthesis. Therefore, chronic maternal melatonin suppression during pregnancy would not affect maternal oestradiol. These inferences were tested by measuring maternal plasma oestradiol throughout gestation and the 24 h plasma cortisol concentration in the 4- to 6-day-old newborn.
Maternal melatonin was suppressed by chronically exposing pregnant capuchin females to constant light during the last third of gestation. This treatment inhibited the nocturnal increase in melatonin as reported in pregnant rhesus monkeys using a 12 day constant light protocol (Matsumoto et al. 1991), and maintained lower daytime plasma melatonin concentrations during pregnancy than in the pregnant females exposed to LD. Conversely, LL mothers receiving melatonin had higher plasma melatonin levels than LL females and LD females. An initial concern in the present experiments were potential stressful effects of constant light and constant light plus melatonin upon maternal and fetal physiology. We observed that mothers in constant light and those receiving melatonin were inactive in segments of the 24 h, suggesting that they slept. A recent paper showed that melatonin advanced the beginning of the rest period when given 2 h in advance of lights off in non-pregnant monkeys (Macaca nemestrina, M. fascicularis and M. mulatta) kept in LD (Zhdanova et al. 2002). To study the effects of melatonin on maternal sleep were beyond the scope of the present experiments. Although potential effects of the treatments upon maternal sleep or other maternal physiological functions cannot be ruled out in this study, the maternal variables measured to assess pregnancy normality were not affected by constant light or constant light plus melatonin replacement. There were no effects upon maternal health, assessed clinically. Maternal weight gain throughout gestation was not altered; neither was fetal growth, gestational length nor pregnancy outcome. At birth, newborns had similar body weights and appeared clinically healthy, suggesting that melatonin deprivation or replacement did not cause major alterations of the fetal environment. This is consistent with observations in the fetal sheep, in which maternal pinealectomy (Yellon & Longo, 1988; McMillen & Nowak, 1989), maternal exposure to constant light (Stark & Daniel, 1989; Parraguez et al. 1996), or administration of melatonin (Serón-Ferréet al. 1989; Houghton et al. 1993) did not modify fetal cardiorespiratory variables.
As anticipated, suppression of maternal melatonin did not affect maternal oestradiol concentrations. The pregnant capuchin monkey has oestradiol concentrations in the ng ml−1 range found in human pregnancy (Patrick et al. 1979), but much higher than those found in rhesus and baboon pregnancies (Walsh et al. 1984; Wilson et al. 1991). No differences in maternal plasma oestradiol concentration were detected between females in the LD, LL and LL + Mel groups in samples taken at 11.00 h throughout gestation or in the samples taken at 08.00 and 20.00 h at 140 days of gestation. A lack of effect of constant light and constant light plus melatonin replacement on maternal oestradiol concentrations has been reported in rhesus monkeys (Matsumoto et al. 1991). In the capuchin monkey, maternal oestradiol increased almost 10-fold between 08.00 and 20.00 h in the three groups of animals. This suggests the presence of a circadian rhythm, as reported for oestriol in pregnant women (Patrick et al. 1979) and oestradiol in the pregnant baboon (Wilson et al. 1991). Whether this rhythm reflects a circadian oscillation of fetal DHAS in the capuchin monkey, similar to that reported in the rhesus fetus (Walsh et al. 1984), remains to be established.
Chronic maternal melatonin suppression and replacement had profound effects upon the 24 h plasma cortisol concentration in capuchin monkey newborns. At 4–6 days of age, the 24 h plasma cortisol concentration of LL newborns was about twice as high as that of the control newborns from LD mothers. Melatonin replacement in LL mothers resulted in a high maternal plasma concentration of melatonin. We do not know how much melatonin reached the fetus or how much melatonin was absorbed by the newborn, as melatonin is secreted in the milk (Illnerova et al. 1993). Nevertheless, maternal melatonin replacement restored newborn plasma cortisol to the levels present in control newborns, supporting the hypothesis that the effects upon plasma cortisol in the newborn were caused by maternal melatonin suppression. A strong possibility, currently under investigation is that, as suggested by our in vitro findings, deprivation of maternal melatonin resulted in early differentiation of the fetal adrenal gland towards cortisol production.
In addition to the proposed effects on fetal adrenal gland differentiation, melatonin deprivation during pregnancy may have affected the development of other fetal organs/systems that directly or indirectly lead to increased newborn plasma cortisol concentrations. Melatonin receptors have been detected in several organs in early-gestation fetal sheep (Helliwell & Williams, 1994), in the fetal human and capuchin monkey kidney (Drew et al. 1998; present report) and in several areas of fetal human hypothalamus including the paraventricular nucleus (Thomas et al. 2002). A possibility, not tested in the present study, but consistent with our results, is that LL newborns have an increased concentration of plasma ACTH or an increased ACTH response to stress.
In contrast to the marked effects of maternal melatonin suppression upon newborn plasma cortisol, there were few effects on maternal plasma cortisol concentrations. At 140 days of gestation, regardless of the treatments, mean maternal cortisol concentrations were higher at 08.00 h than at 20.00 h, and there was no difference between the groups in cortisol concentration at these times. This suggests that a maternal cortisol rhythm is present in the capuchin pregnancy, as reported in human and other primates (Patrick et al. 1979; Walsh et al. 1984; Matsumoto et al. 1991). Neither the circadian rhythm nor the maternal plasma cortisol concentration was affected by chronic exposure to constant light or to constant light plus melatonin, similar to findings in the pregnant rhesus monkey (Matsumoto et al. 1991). Maintenance of the circadian rhythm of cortisol production was reported in a study in two non-pregnant human subjects exposed to constant light for 13 days (Krieger et al. 1969). In the capuchin monkey, maternal plasma cortisol increased with advancing gestational age, as reported for human pregnancy (Carr et al. 1981). This increase occurred about 20 days earlier in LL females than in LD females. This could be a initial response to constant light, as acute exposure to light increases cortisol in the human (Leproult et al. 2001). An effect of this premature increase in maternal cortisol on fetal adrenal function is not apparent from the present data. Maternal transfer of cortisol to the fetus is minimized by placental metabolism (Murphy & Branchaud, 1983), if this is overcome, the fetus would receive more maternal cortisol. However, it is well established that an increased transfer of maternal cortisol suppresses fetal ACTH and DHAS (Simmer et al. 1974), resulting in a decreased maternal oestradiol concentration (Simmer et al. 1974), a situation not observed in the present experiments.
Fetal glucocorticoids (cortisol or in some species corticosterone) are important for fetal homeostasis and the fetal organ maturation required for successful transition from fetus to neonate (Liggins, 1976). The present report provides evidence in a primate model of direct in vitro modulation by melatonin of ACTH- and CRH-stimulated cortisol production by the fetal adrenal gland and of effects of maternal melatonin suppression and replacement during gestation upon plasma cortisol concentration in the newborn. These results support a role for maternal melatonin in fetal and neonatal primate cortisol regulation.
Acknowledgments
We are very grateful to Alejandra Ortiz D.V.M. for expert animal care, Griselda Bravo for assistance in RIAs, Eliana Lira for help with histological sections and Francisco J. Valenzuela for help with 3β-HSD sequencing. This work was supported by Grants 2010140, 1030425 and Líneas Complementarias 8980006, from Fondo Nacional de Desarrollo Científico y Tecnológico, Chile, Grant 98/LABENDO/Resource Maintenance Grant-2 from the World Health Organization, and a grant from San Bernardino Medical Foundation. C.T.-F. is a recipient of a doctoral fellowship from Dirección de Investigación de la Pontificia Universidad Católica de Chile. P.R.-G. was a recipient of a postdoctoral fellowship from PROGRESAR, Chile.
References
- Allen JP, Cook DM, Kendall JW, McGilvra R. Maternal-fetal ACTH relationship in man. J Clin Endocrinol Metab. 1973;37:230–234. doi: 10.1210/jcem-37-2-230. [DOI] [PubMed] [Google Scholar]
- Carr BR, Parker CR, Jr, Madden JD, MacDonald PC, Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139:416–422. doi: 10.1016/0002-9378(81)90318-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Renquist D, Brandon D, Eil C, Pugeat M, Vigersky R, Cutler GB, Jr, Loriaux DL, Lipsett MB. Glucocorticoid hormone resistance during primate evolution: receptor-mediated mechanisms. Proc Natl Acad Sci U S A. 1982;79:2036–2040. doi: 10.1073/pnas.79.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini P, Recabarren M, Serón-Ferré M, Parraguez VH. Study of prenatal growth in the capuchin monkey (Cebus apella) by ultrasound. J Med Primatol. 1998;27:287–292. doi: 10.1111/j.1600-0684.1998.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Smith RE, Stowasser M, Sasano H, Krozowski ZS, Gordon RD. Expression of 11beta-hydroxysteroid dehydrogenase type 2 (11betaHSD-2) in the developing human adrenal gland and human adrenal cortical carcinoma and adenoma. Mol Cell Endocrinol. 1999;154:71–77. doi: 10.1016/s0303-7207(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Doody KM, Carr BR, Rainey WE, Byrd W, Murry BA, Strickler RC, Thomas JL, Mason JI. 3β-Hydroxysteroid dehydrogenase/isomerase in the fetal zone and neocortex of the human fetal adrenal gland. Endocrinology. 1990;126:2487–2492. doi: 10.1210/endo-126-5-2487. [DOI] [PubMed] [Google Scholar]
- Drew JE, Williams LM, Hannah LT, Barrett P, Abramovich DR, Morgan PJ. Melatonin receptors in the human fetal kidney, 2-[125I]iodomelatonin binding sites correlated with expression of Mel1a and Mel1b receptor genes. J Endocrinol. 1998;156:261–267. doi: 10.1677/joe.0.1560261. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin receptors: are there multiple subtypes. Trends Pharmacol Sci. 1995;16:50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–1220. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- Ducsay CA, Hess DL, McClellan MC, Novy MJ. Endocrine and morphological maturation of the fetal and neonatal adrenal cortex in baboons. J Clin Endocrinol Metab. 1991;73:385–395. doi: 10.1210/jcem-73-2-385. [DOI] [PubMed] [Google Scholar]
- Einspanier R, Schönfelder M, Müller K, Stojkovic M, Kosmann M, Wolf E, Schams D. Expression of the vascular endothelial growth factor and its receptors and effects of VEGF during in vitro maturation of bovine cumulus-oocyte complexes (COC) Mol Reprod Dev. 2002;62:29–36. doi: 10.1002/mrd.10068. [DOI] [PubMed] [Google Scholar]
- Ersahin C, Masana MI, Dubocovich ML. Constitutively active melatonin MT1 receptors in male rat caudal arteries. Eur J Pharmacol. 2002;439:171–172. doi: 10.1016/s0014-2999(02)01407-3. [DOI] [PubMed] [Google Scholar]
- Fujieda K, Faiman C, Reyes FI, Winter JSD. The control of steroidogenesis by human fetal adrenal cells in tissue culture. I. Response to adrenocorticotropin. J Clin Endocrinol Metab. 1981;53:34–38. doi: 10.1210/jcem-53-1-34. [DOI] [PubMed] [Google Scholar]
- Goland RS, Wardlaw SL, Stark RI, Brown LS, Jr, Frantz AG. High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. J Clin Endocrinol Metab. 1986;63:1199–1203. doi: 10.1210/jcem-63-5-1199. [DOI] [PubMed] [Google Scholar]
- Goldman AS, Yakovac WC, Bongiovanni AM. Development of activity of 3β-hydroxysteroid dehydrogenase in human fetal tissues and in two anencephalic newborns. J Clin Endocrinol Metab. 1966;26:14–22. doi: 10.1210/jcem-26-1-14. [DOI] [PubMed] [Google Scholar]
- Helliwell RJA, Williams LM. The development of melatonin-binding sites in the ovine fetus. J Endocrinol. 1994;142:475–484. doi: 10.1677/joe.0.1420475. [DOI] [PubMed] [Google Scholar]
- Houghton DC, Walker DW, Young IR, McMillen IC. Melatonin and the light-dark cycle separately influence daily behavioral and hormonal rhythms in the pregnant ewe and sheep fetus. Endocrinology. 1993;133:90–98. doi: 10.1210/endo.133.1.8319592. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Buresova M, Presl J. Melatonin rhythm in human milk. J Clin Endocrinol Metab. 1993;77:838–841. doi: 10.1210/jcem.77.3.8370707. [DOI] [PubMed] [Google Scholar]
- Kahri AI, Huhtaniemi I, Salmenpera M. Steroid formation and differentiation of cortical cells in tissue culture of human fetal adrenals in the presence and absence of ACTH. Endocrinology. 1976;98:33–41. doi: 10.1210/endo-98-1-33. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Stamp GE, Goble FC. Development of melatonin production in infants and the impact of prematurity. J Clin Endocrinol Metab. 1992;75:367–379. doi: 10.1210/jcem.75.2.1639937. [DOI] [PubMed] [Google Scholar]
- Koritnik DR, Laherty RF, Rotten D, Jaffe RB. A radioimmunoassay for dehydroepiandrosterone sulfate in the circulation of rhesus monkeys. Steroids. 1983;42:653–667. doi: 10.1016/0039-128x(83)90129-0. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Kreuzer J, Rizzo FA. Constant light: effect on circadian pattern and phase reversal of steroid and electrolyte levels in man. J Clin Endocrinol Metab. 1969;29:1634–1638. doi: 10.1210/jcem-29-12-1634. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, L'Hermite-Baleriaux M, Van Cauter E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab. 2001;86:151–157. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol. 1976;126:931–941. doi: 10.1016/0002-9378(76)90680-3. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Nowak R. Maternal pinealectomy abolishes the diurnal rhythm in plasma melatonin concentrations in the fetal sheep and pregnant ewe during late gestation. J Endocrinol. 1989;120:459–464. doi: 10.1677/joe.0.1200459. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Nowak R, Walker DW, Young IR. Maternal pinealectomy alters the daily pattern of fetal breathing in sheep. Am J Physiol. 1990;258:R284–R287. doi: 10.1152/ajpregu.1990.258.1.R284. [DOI] [PubMed] [Google Scholar]
- McNulty WP, Novy MJ, Walsh SW. Fetal and postnatal development of the adrenal glands in Macaca mulatta. Biol Reprod. 1981;25:1079–1089. doi: 10.1095/biolreprod25.5.1079. [DOI] [PubMed] [Google Scholar]
- Mason JI, Ushijima K, Doody KM, Nagai K, Naville D, Head JR, Milewich L, Rainey WE, Ralph MM. Regulation of expression of the 3 beta-hydroxysteroid dehydrogenases of human placenta and fetal adrenal. J Steroid Biochem Molec Biol. 1993;47:151–159. doi: 10.1016/0960-0760(93)90069-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Hess DL, Kaushal KM, Valenzuela GJ, Yellon SM, Ducsay CA. Circadian myometrial and endocrine rhythms in the pregnant rhesus macaque: Effects of constant light and timed melatonin infusion. Am J Obstet Gynecol. 1991;165:1777–1784. doi: 10.1016/0002-9378(91)90032-m. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Coulter CL, Jaffe RB. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17alpha-hydroxylase/17,20-lyase, and 3beta-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J Clin Endocrinol Metab. 1993;77:1184–1189. doi: 10.1210/jcem.77.5.8077311. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. Interaction of insulin-like growth factor II and estradiol directs steroidogenesis in the human fetal adrenal toward dehydroepiandrosterone sulfate production. J Clin Endocrinol Metab. 1993;77:754–758. doi: 10.1210/jcem.77.3.8396578. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- Murphy BEP, Branchaud CTL. Fetal metabolism of cortisol. In: Martini L, James VHT, editors. Fetal Endocrinology and Metabolism. Current Topics in Experimental Endocrinology. Vol. 5. New York: Academic Press; 1983. pp. 197–229. [Google Scholar]
- Naitoh N, Watanabe Y, Matsumura K, Murai I, Kobayashi K, Imai-Matsumura K, Ohtuka H, Takagi K, Miyake Y, Satoh K, Watanabe Y. Alteration by maternal pinealectomy of fetal and neonatal melatonin and dopamine D1 receptor binding in the suprachiasmatic nuclei. Biochem Biophys Res Commun. 1998;253:850–854. doi: 10.1006/bbrc.1998.9819. [DOI] [PubMed] [Google Scholar]
- Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol. 2001;174:111–120. doi: 10.1016/s0303-7207(00)00445-7. [DOI] [PubMed] [Google Scholar]
- Nowak R, Young IR, McMillen IC. Emergence of the diurnal rhythm in plasma melatonin concentrations in newborn lambs delivered to intact or pinealectomized ewes. J Endocrinol. 1990;125:97–102. doi: 10.1677/joe.0.1250097. [DOI] [PubMed] [Google Scholar]
- Parker CR, Jr, Stankovic AM, Goland RS. Corticotropin-releasing hormone stimulates steroidogenesis in cultured human adrenal cells. Mol Cell Endocrinol. 1999;155:19–25. doi: 10.1016/s0303-7207(99)00118-5. [DOI] [PubMed] [Google Scholar]
- Parraguez VH, Valenzuela GJ, Vergara M, Ducsay CA, Yellon SM, Serón-Ferré M. Effects of constant light on fetal and maternal prolactin rhythms in sheep. Endocrinology. 1996;137:2355–2361. doi: 10.1210/endo.137.6.8641186. [DOI] [PubMed] [Google Scholar]
- Patrick J, Challis J, Natale R, Richardson B. Circadian rhythms in maternal plasma cortisol, estrone, estradiol, and estriol at 34–35 weeks' gestation. Am J Obstet Gynecol. 1979;135:791–798. doi: 10.1016/0002-9378(79)90393-4. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Wang Z-N, Parker CR., Jr Gene profiling of human fetal and adult adrenals. J Endocrinol. 2001;171:209–215. doi: 10.1677/joe.0.1710209. [DOI] [PubMed] [Google Scholar]
- Recabarren MP, Valenzuela GJ, Serón-Ferré M. Proteic-caloric restriction during pregnancy affects the adrenal-placenta axis and decreases birth weight in a primate, Cebus apella, without affecting gestational length. Prenat Neonat Med. 1998;3:309–313. [Google Scholar]
- Reppert SM, Coleman RJ, Heath HW, Swedlow JR. Pineal N-acetyltransferase activity in 10-day-old rats: a paradigm for studying the developing circadian system. Endocrinology. 1984;115:918–925. doi: 10.1210/endo-115-3-918. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serón-Ferré M, Ducsay CA, Valenzuela GJ. Circadian rhythms during pregnancy. Endocr Rev. 1993;14:594–609. doi: 10.1210/edrv-14-5-594. [DOI] [PubMed] [Google Scholar]
- Serón-Ferré M, Jaffe RB. The fetal adrenal gland. Ann Rev Physiol. 1981;43:141–162. doi: 10.1146/annurev.ph.43.030181.001041. [DOI] [PubMed] [Google Scholar]
- Serón-Ferré M, Lawrence CC, Siiteri PK, Jaffe RB. Steroid production by definitive and fetal zones of the human fetal adrenal gland. J Clin Endocrinol Metab. 1978;47:603–609. doi: 10.1210/jcem-47-3-603. [DOI] [PubMed] [Google Scholar]
- Serón-Ferré M, Vergara M, Parraguez VH, Riquelme R, Llanos AJ. Fetal prolactin levels respond to a maternal melatonin implant. Endocrinology. 1989;125:400–403. doi: 10.1210/endo-125-1-400. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, MacDonald PC. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab. 1966;26:751–761. doi: 10.1210/jcem-26-7-751. [DOI] [PubMed] [Google Scholar]
- Simmer HH, Tulchinsky D, Gold EM, Frankland M, Greipel M, Gold AS. On the regulation of estrogen production by cortisol and ACTH in human pregnancy at term. Am J Obstet Gynecol. 1974;119:283–296. doi: 10.1016/0002-9378(74)90287-7. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Carr BR, Parker R, Jr, Milevich L, Porter JC, Macdonald PC. The role of serum lipoproteins in steroidogenesis by the human fetal adrenal cortex. J Clin Endocrinol Metab. 1979;49:146–148. doi: 10.1210/jcem-49-1-146. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83:2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- Song Y, Tam PC, Poon AM, Brown GM, Pang SF. 2-[125I]iodomelatonin-binding sites in the human kidney and the effect of guanosine 5′- O J Clin Endocrinol Metab. 1995;80:1560–1565. doi: 10.1210/jcem.80.5.7745000. -(3-thiotriphosphate) [DOI] [PubMed] [Google Scholar]
- Stark RI, Daniel SS. Circadian rhythm of vasopressin levels in cerebrospinal fluid of the fetus: effect of continuous light. Endocrinology. 1989;124:3095–3101. doi: 10.1210/endo-124-6-3095. [DOI] [PubMed] [Google Scholar]
- Thomas L, Purvis CC, Drew JE, Abramovich DR, Williams LM. Melatonin receptors in human fetal brain: 2-[125I]iodomelatonin binding and MT1 gene expression. J Pineal Res. 2002;33:218–224. doi: 10.1034/j.1600-079x.2002.02921.x. [DOI] [PubMed] [Google Scholar]
- Torres-Farfan C, Richter HG, Rojas-García P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Serón-Ferré M. mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J Clin Endocrinol Metab. 2003;88:450–458. doi: 10.1210/jc.2002-021048. [DOI] [PubMed] [Google Scholar]
- Valenti S, Thellung S, Florio T, Giusti M, Schettini G, Giordano G. A novel mechanism for the melatonin inhibition of testosterone secretion by rat Leydig cells: reduction of GnRH-induced increase in cytosolic Ca2. J Mol Endocrinol. 1999;23:299–306. doi: 10.1677/jme.0.0230299. [DOI] [PubMed] [Google Scholar]
- Vera H, Tijmes M, Ronco AM, Valladares LE. Melatonin binding sites in interstitial cells from immature rat testes. Biol Res. 1993;26:337–340. [PubMed] [Google Scholar]
- Voutilainen R, Ilvesmaki V, Miettinen PJ. Low expression of 3beta-hydroxy-5-ene-steroid dehydrogenase gene in human fetal adrenals in vivo; adrenocorticotropin and protein kinase C-dependent regulation in adrenocortical cultures. J Clin Endocrinol Metab. 1991;72:761–767. doi: 10.1210/jcem-72-4-761. [DOI] [PubMed] [Google Scholar]
- Walker ML, Pepe GJ, Albrecht ED. Regulation of baboon fetal adrenal androgen formation by pituitary peptides at mid- and late gestation. Endocrinology. 1988;122:546–551. doi: 10.1210/endo-122-2-546. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Ducsay CA, Novy MJ. Circadian hormonal interactions among the mother, fetus and amniotic fluid. Am J Obstet Gynecol. 1984;150:745–753. doi: 10.1016/0002-9378(84)90679-3. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Stehle JH, Stopa EG, Reppert SM. Melatonin receptors in human hypothalamus and pituitary: implications for circadian and reproductive responses to melatonin. J Clin Endocrinol Metab. 1993;76:295–301. doi: 10.1210/jcem.76.2.8381796. [DOI] [PubMed] [Google Scholar]
- Wilson L, Jr, Parsons MT, Flouret G. Forward shift in the initiation of the nocturnal estradiol surge in the pregnant baboon: Is this the genesis of labor. Am J Obstet Gynecol. 1991;165:1487–1498. doi: 10.1016/0002-9378(91)90396-9. [DOI] [PubMed] [Google Scholar]
- Witt-Enderby PA, Dubocovich ML. Characterization and regulation of the human ML1A melatonin receptor stably expressed in Chinese hamster ovary cells. Mol Pharmacol. 1996;50:166–174. [PubMed] [Google Scholar]
- Woo MM, Tai CJ, Kang SK, Nathwani PS, Pang SF, Leung PC. Direct action of melatonin in human granulosa-luteal cells. J Clin Endocrinol Metab. 2001;86:4789–4797. doi: 10.1210/jcem.86.10.7912. [DOI] [PubMed] [Google Scholar]
- Wu C-S, Leu S-F, Yang H-S, Huang B-M. Melatonin inhibits the expression of Steroidogenic Acute Regulatory protein and steroidogenesis in MA-10 cells. J Androl. 2001;22:245–254. [PubMed] [Google Scholar]
- Yellon SM, Longo LD. Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy. Biol Reprod. 1988;39:1093–1099. doi: 10.1095/biolreprod39.5.1093. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Rosene DL, Moss MB, Madras BK. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002;75:523–529. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]