Abstract
We explored whether hypoxic preconditioning minimizes oxidative injury induced by overdistension/emptying in the rat bladder. For hypoxic preconditioning, female Wistar rats were placed in a hypobaric chamber (380 Torr) 15 h day−1 for 28 days. Overdistension was induced by infusion of two times the threshold volume of saline into the bladder and was maintained for 1 or 2 h, followed by drainage/emptying. During overdistension (ischaemia) and emptying (reperfusion) periods, a bursting increase of reactive oxygen species (ROS) from the bladder was originated from the large numbers of infiltrating leucocytes and scattered resident cells, including urothelial, submucosal, and smooth muscle cells. ROS impaired the voiding function by a reduction of bladder afferent and efferent nerve activity and bethanecol- or ATP-induced detrusor contraction. ROS enhanced pro-apoptotic mechanisms, including increases in the Bax/Bcl-2 ratio, CPP32 expression, and poly(ADP-ribose) polymerase (PARP) fragments with subsequent apoptotic cell formation in the insulted bladders. Hypoxia preconditioning up-regulated Bcl-2 expression in the bladder and significantly reduced the levels of ROS and apoptosis detected in the overdistension/emptying bladders and preserved partial voiding function. Bcl-2 up-regulation by hypoxia preconditioning contributes protection against overdistension/emptying-induced oxidative stress and injury in the bladder.
Bladder overdistension occurs in patients with acute urinary retention secondary to bladder outlet obstruction (Carpenter, 1983). Overdistension, as a physiological or pathological stress, has been shown to result in contractile and metabolic dysfunction of the bladder (Mustonen et al. 1999; Chien et al. 2000a; Lee et al. 2000). Prolonged overdistension can result in injury to the neural pathways responsible for micturition (Tammela et al. 1990), reduce bladder elasticity, alter the biochemical and neuronal responsiveness of the bladders (Carpenter, 1983; Chien et al. 2000a,b), and subsequently lead to micturition problems. Acute urinary retention reduces renal haemodynamics by exaggerating the vesicovascular reflex and may impair renal function in the rat (Chien et al. 2000a).
There is increasing evidence that ischaemia followed by reperfusion may be responsible for the progression of bladder dysfunction associated with overdistension (Azadozi et al. 1996). In man and pigs, overdistension can induce significant ischaemia and hypoxia of the bladder wall, and immediately after bladder drainage/emptying there is a rebound in blood flow, allowing reperfusion to occur (Greenland & Brading, 2001; Kershen et al. 2002). In organs such as the heart, liver, brain and kidneys, ischaemia–reperfusion has been shown to lead to considerable tissue injury through nitric oxide and reactive oxygen species (ROS) production and multiple signalling pathways which ultimately culminate in inflammatory infiltrates and cell apoptosis/tissue necrosis (Kontos et al. 1992; Okuda et al. 1992; Schumer et al. 1992; Chien et al. 2001; Lieb et al. 2001; Schröder et al. 2001).
In the present study, we intend to demonstrate the existence of an overdistension/emptying (OD/E)-induced oxidative stress in rat bladders by direct demonstration of ROS production and apoptotic cells in tissues. Furthermore, human beings or animals subjected to long-term hypoxia could develop physiological adjustments to adapt the ischaemia–reperfusion oxidative damage in organs such as the heart and kidney (Shizukuda et al. 1992; Chien et al. 2000b; Bernardo et al. 1997; Piot et al. 1997). Thus, we also wanted to study whether hypoxic preconditioning (HP) might have a similar effect in protecting the bladder from OD/E-induced injuries. Attention was given to a possible up-regulation of antiapoptotic protein (i.e. Bcl-2) and/or a down-regulation of pro-apoptotic proteins in the preconditioned bladders. Our study showed that prolonged OD/E injury could potentiate the pro-apoptotic mechanisms by an increase in the Bax/Bcl-2 ratio, CPP32 and poly(ADP-ribose) polymerase (PARP) expression, and consequently, apoptosis formation in the insulted bladder tissue. HP could protect the overdistended bladder against ROS generation and voiding dysfunction in part by up-regulation of Bcl-2 in the urinary bladder.
Methods
Hypoxic preconditioning (HP)
Female Wistar rats (200–250 g) from the Experimental Animal Center of National Taiwan University were exposed to a hypoxic condition for 15 h day−1 (17.00 h to 08.00 h) in a hypoxic chamber equal to 5500 m in altitude for 28 days (Chien et al. 1997). The hypoxic chamber had the same constant temperature and light cycles as the rest of the animal room at sea level (SL). The level of 5500 m (380 mmHg) was selected because it represents the maximal altitude to which most rats can successfully adapt (Chien et al. 1997). The partial pressure of O2 in femoral arterial blood was at a consistent level of 39.3 ± 2.0 mmHg for rats maintained in the hypoxic chamber, as compared with a PO2 level of 99.4 ± 3.2 mmHg for control rats at sea level (measured with a Nova blood gas analyser, SP5, Hamburg, Germany).
The body weight of the animals was measured once a week. Food and water were freely available to the animals at all times.
Surgery and animal preparation
We used the preconditioned rats 3–4 weeks after exposure to the hypoxic condition. Previously, we had shown that a protective effect lasted at least for 4 weeks after hypoxic exposure (Chien et al. 2000b). The rats were anaesthetized by subcutaneous urethane (1.2 g kg−1). The maintenance of deep anaesthesia was determined by the persistence of miotic pupils as judged from frequent inspection and by the lack of heart rate and arterial blood pressure fluctuations in the absence of visceral stimuli. An experiment was terminated when the baseline mean arterial blood pressure was below 90 mmHg. Body temperature was kept at 36.5–37°C by an infrared light and was monitored with a rectal thermometer.
PE-50 catheters were placed in the left carotid artery for measurement of heart rate and arterial blood pressure and in the left femoral vein for administration of anaesthetics when needed. The arterial blood pressure and heart rate were recorded on an ADI system (PowerLab/16S, ADInstruments, Pty Ltd, Castle Hill, Australia) with a transducer (P23 1D, Gould-Statham, USA). A length of stretched PE-10 tubing (intra-arterial catheter) was inserted just above the bifurcation of the aorta from the right femoral artery, and was used for injection of drugs. This stretched PE-10 tubing had little effect on bladder blood flow (Chien et al. 2000c).
The animal care and experimental protocol were in accordance with the guidelines of the National Science Council of the Republic of China (NSC 1997). All efforts were made to minimize both animal suffering and the number of animals used throughout the experiment. At the end of each experiment, the animals were killed by an intravenous potassium chloride injection.
Experimental model of bladder overdistension
We inserted a PE-50 tube into the bladder through the urethra and tied it in place by a ligature around the urethral meatus (Chien et al. 2000a). The catheter was connected to a pressure transducer and an infusion pump (Infors AG, CH-4103, Bottmingen, Switzerland) via a T-tube connector. The bladder volume was increased by steady infusion of 0.9% saline (0.10 ml min−1) via the infusion pump. A threshold volume was defined as the infused volume at the point preceding eliciting of a micturition reflex (Chien et al. 2003). Overdistension was induced by injection of two times the threshold volume of saline into the bladder. The period of overdistension was set for 1 h (OD1h) or 2 h (OD2h). Drainage/emptying was done via the transurethral catheter. After one episode of OD/E, the rats were allowed to have a normal micturition cycle. The studies described below, if not specified, were generally done in rats 2 h post emptying.
Bladder haemodynamics of SL and HP rats
We first measured the bladder haemodynamics in response to a short period of bladder overdistension, mimicking a normal micturition cycle, in six rats each for the SL and HP groups. The bladder was gradually filled with 1.5 ml of saline for about 8 min and allowed to void spontaneously via a T tube. Total bladder arterial blood flow was measured by placement of an ultrasonic blood flow probe (1RB2098, Transonic System Inc., Ithaca, NY, USA) around the bladder vesical artery. The haemodynamic data were displayed in millilitres per minute in an Ultransonic System (T206, Transonic Systems Inc.). Parameters of arterial blood pressure, heart rate, intravesical pressure (IVP), whole-bladder arterial blood flow, and calculated bladder microvascular resistance in response to a graded bladder distension (from 0 mmHg at the empty stage to 100 mmHg at the distended period) were recorded on an ADI recording system (PowerLab/16S). To normalize blood flow for varying arterial blood pressure, we calculated the mean microvascular resistance by using the following formula (Kershen et al. 2002; Kozlowski et al. 2002): Bladder microvascular resistance = Mean arterial blood pressure (mmHg)/Mean total bladder flow (ml min−1).
In vivo chemiluminescence recording for ROS activity
The ROS generation in response to OD/E was measured from the bladder surface by a chemiluminescence detection method as previously described (Chien et al. 2003).
The rat was maintained on a respirator (tidal volume: 1.0–1.5 ml; rate, 80–90 cycles min−1; inspiratory pressure, 20–30 cm H2O) and a circulating water pad at 37°C in a dark box with a shielded plate. Only the bladder window was left unshielded and was positioned under a reflector, which reflected the photons from the exposed bladder surface on to the detector area. The measurement of ROS from the bladder was started by intravenous infusion of a superoxide anion probe, 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo-(1,2-a)-pyrazin-3-one-hydrochloride (MCLA) (0.2 mg ml−1 h−1, TCI-Ace, Tokyo Kasei Kogyo Co. Ltd, Tokyo, Japan) throughout the experiment by use of a Chemiluminescence Analysing System (CLD-110, Tohoku Electronic In. Co., Sendai, Japan). The MCLA-enhanced chemiluminescence counts were continuously recorded every 10 s during prefilling, overdistension, and postdrainage periods. The real-time displayed chemiluminescence signal was recognized as the ROS level from the bladder surface. To verify the specificity of the ROS-enhanced chemiluminescence activity, we added superoxide dismutase (500 U ml−1) to the intravenous solution. Superoxide dismutase is a known scavenger for superoxide anions (Chien et al. 2001).
In situ demonstration of superoxide generation and apoptosis formation
A nitroblue tetrazolium (NBT) perfusion method was used for localizing de novo ROS generation in the insulted bladder (Chien et al. 2001). Rats (n = 3 in each group) were killed at the end of overdistension, or 1 or 2 h post drainage. An 18-gauge needle connected to an infusion pump (Infors AG) was inserted into the lower abdominal aorta just below the level of the renal artery. The bladders were perfused with 37°C Hanks' balanced salt solution (flow rate, 10 ml min−1; pH 7.4), and the perfusate was allowed to drain from the inferior vena cava. Once blood had been removed, NBT (1 mg ml−1) was added to the solution, and the bladder was perfused for an additional 10 min at a flow rate of 5 ml min−1. All unreacted NBT was removed from the bladders by perfusion with Hanks' solution. The NBT-perfused bladder was removed and fixed in zinc/formalin (1% ZnSO4 in 10% formalin) for histologic examination for formazan deposits.
The method for the terminal deoxynucleotidyl transferase-mediated nick-end labelling method (TUNEL) was performed as previously described (Bardales et al. 1996). Sections of the bladder were prepared, deparaffinized, and stained by the methyl green and TUNEL–avidin–biotin-complex method. Twenty high-power (× 400) fields were randomly selected for each bladder section, and the value of apoptotic cells/(apoptotic cells and methyl green stained cells) was counted. For examination of inflammatory cell infiltrates, the sections were cut, stained with haematoxylin and eosin (H&E), and examined under light microcopy.
HP effect on Bax, Bcl-2, CPP32, and PARP expression in bladders
Six groups of HP and SL rat bladders with or without OD/E injury (n = 3 in each group) were used in this experiment. The rat bladders subjected to 1 h or 2 h of overdistension and bladder samples taken 0 min, 5 min, 60 min and 120 min post emptying were quickly frozen in liquid nitrogen, and stored at −70°C for protein isolation.
The expression of Bax/Bcl-2, caspase 3 and PARP of bladder tissues was evaluated by Western immunoblotting. Briefly, the tissues were homogenized with a prechilled mortar and pestle in extraction buffer, which consisted of 10 mm Tris-HCl (pH 7.6), 140 mm NaCl, 1 mm phenylmethylsulphonyl fluoride, 1% Nonidet P-40, 0.5% deoxycholate, 2%β-mercaptoethanol, 10 μg ml−1 pepstatin A, and 10 μg ml−1 aprotinin. The mixtures were homogenized completely by vortexing and kept at 4°C for 30 min. The homogenate was centrifuged at 12 000 g for 12 min at 4°C, the supernatant was collected, and the protein concentrations were determined by Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA).
Antibodies raised against Bax (human Bax synthetic peptide, a.a. 44–62, Chemicon, Temecula, CA, USA), Bcl-2 (human Bcl-2 peptide, a.a. 49–179, Transduction, Bluegrass-Lexington, KY, USA), the activation fragments of caspase 3 (CPP32/Yama/Apopain, Upstate Biotechnology, Lake Placid, NY, USA), PARP (N-terminal peptide from the p85 fragment, Promega, Madison, WI, USA), and β-actin (Clone AC-74, Sigma, Saint Louis, MO, USA) were used. All of these antibodies cross-react with the respective rat antigens.
Cystometrogram
We used a transcystometric model, as previously described (Chien et al. 2000c), to evaluate voiding pressure (i.e. maximal IVP during micturition in rats subjected to OD/E injury. The urinary bladder was exposed through a midline incision of the abdomen, and a PE-50 T-tube was inserted through the apex of the bladder dome. The bladders were filled by continuous infusion of saline (0.10 ml min−1) by an infusion pump (Infors AG) and allowed to void spontaneously via the urethra.
Exogenous ROS effect on voiding function
For demonstration of the effect of ROS on the bladder function, intra-arterial injection of a mixture of xanthine (100 nmol, Sigma) and xanthine oxidase (1 unit, Sigma) were performed in six SL rats (Cai & Ogawa, 1994). The reaction of xanthine and xanthine oxidase causes an elevation of ROS both in in vitro and in vivo conditions. For control, an intravenous superoxide dismutase (500 U ml−1) infusion was given before xanthine/xanthine oxidase administration to verify the direct involvement of ROS in voiding function.
The rats were prepared as described in the previous section. Briefly, the urinary bladder was exposed through a midline incision of the abdomen, and urine was emptied by application of light pressure. A PE-50 T-tube was inserted through the apex of the bladder dome. The bladders were filled by continuous infusion of 0.9% saline (0.10 ml min−1) at room temperature and were allowed to drain/micturate repeatedly via the urethra (Chien et al. 2003). The exogenous ROS effect on IVP, bladder nerve activity, and urethral resistance was examined.
Bladder nerve activity
We measured bladder nerve activities in SL/HP rats subject to OD/E- or ROS-induced injury. Simultaneous measurement of multifibre bladder afferent and efferent nerve activities was performed as previously described (Chien et al. 2003). Two vesical branches originating from the left pelvic ganglion and attached to urinary bladder surface were dissected under dissecting microscopy. The amplified neural signals were continuously recorded on a magnetic tape and displayed on a Gould oscilloscope. The amplified signals were analysed with an ADI system (PowerLab/165) and were set to count the total voltage level. Nervous activity was amplified and transformed into voltage and was expressed as voltage per second.
Afferent firing of the bladder nerve was recorded after crushing of the nerve just central to the recording site, in order to eliminate efferent firing. Efferent firing was recorded when the bladder nerve was crushed distal to the recording site (Chien et al. 2000c, 2003). Afferent nerve activity was confirmed by its ability to show increased activity in response to small increments of IVP by saline infusion, whereas efferent nerve activity had minimal activity until a threshold volume/pressure in the filling bladder was reached which produced a bursting discharge causing a micturition contraction (Chien et al. 2003).
Urethral resistance
For measurement of urethral resistance, which also contributes to voiding function, a PE-50 catheter (bladder catheter) connected to an ultrasonic flow probe (1RB2098, Transonic Systems) was inserted from the bladder dome and was connected via a T-tube to an infusion pump and a pressure transducer. Another PE-50 catheter (urethral catheter) connected to an in-line flow probe (1NPXL, Transonic Systems) was inserted into the opening of the urethra and was immobilized by two to three sutures. IVP, infusion inflow, and urine outflow were continuously recorded on an ADI recording system (PowerLab/165). Urethral resistance was obtained from the formula: Urethral resistance = Peak IVP (mmHg)/Peak urine outflow (ml min−1).
Detrusor contractility
ATP and acetylcholine act as cotransmitters in the bladder nerves and are responsible for the amplitude of detrusor contraction (Theobald, 1995). Thus, an experiment was performed to evaluate the response of ATP- and acetylcholine-induced detrusor contraction after OD/E injury. The bladder was opened and divided into longitudinal strips, weighed, and placed in Krebs-Henseleit solution (37°C, pH 7.4) continuously gassed with 95% O2 and 5% CO2. The resting tension on the tissues was maintained at 1 g. Each strip was connected to a force-displacement transducer (Grass Medical Instruments, Quincy, MA, USA) for measuring isometric force, which was continuously displayed on a Grass 79D recording system. The contractile responses to a muscarinic agonist bethanecol (10−4 m), and to a purinergic agonist, ATP (1 mm), were constructed as previously described (Gosling et al. 2000).
Data acquisition and statistical analysis
All data are presented as the mean ± standard error of the mean. Data were subjected to analysis of variance, followed by Duncan's multiple-range test for assessment of the differences among groups. Student's paired t test was used for detecting differences between control and drug treatment groups. P < 0.05 was considered to indicate statistical significance.
Results
Hemodynamic changes in SL and HP bladders
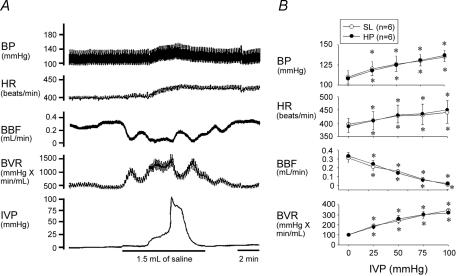
HP may affect the bladder microvasculature and lead to extreme ischaemic/hypoxic conditions and a decrease in reperfusion (Azadzoi et al. 1999). In our study, we used rats 3–4 weeks post HP. We did not find morphological and haemodynamic differences between SL and HP rats. As shown in Fig. 1, the SL bladder filled with up to 1.5 ml of saline triggered excitatory vesicovascular reflexes, showing a significant increase in IVP, heart rate, and arterial blood pressure. Notably, bladder overdistension can reduce bladder blood flow and increase bladder vascular resistance. Emptying of bladder fluids caused the bladder and systemic haemodynamics to recover to the predistended stage. Similar findings were noted in HP rats. The findings confirm the existence of ischaemia–reperfusion associated with bladder overdistension.
Figure 1. Response of systemic and urologic haemodynamics to overdistension.
A, acute infusion of 1.5 ml of saline into the bladder increases the intravesical pressure (IVP) and evokes an excitatory vesicovascular reflex including a significant elevation in blood pressure (BP) and heart rate (HR) in an SL rat. Bladder overdistension significantly reduces bladder blood flow (BBF) and increases bladder microvascular resistance (BVR). On emptying of bladder fluids, the bladder and systemic haemodynamics recovered to the predistended stage. The findings indicate the existence of ischaemia–reperfusion associated with bladder overdistension. B, mean changes of BP, HR, BBF, and BVR in response to increased IVP are similar in SL and HP rats. *P < 0.05 when compared to respective control value.
Bladder ROS in vivo
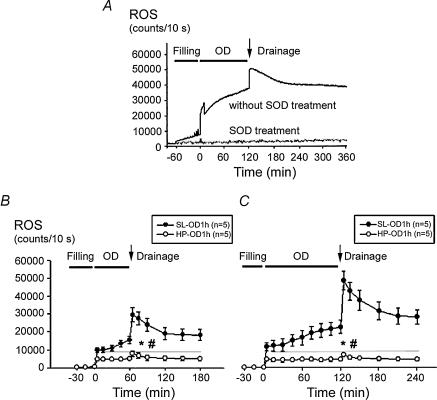
ROS generation was measured from the bladder surface by a chemiluminescence detection method. Continuous infusion of MCLA into an emptied bladder displayed a stable basal level of ROS chemiluminescence activity at about 1000–1400 counts (10 s)−1. A small, but gradual increase in ROS activity was noted in the bladder during the filling period (Fig. 2A). When the filling volume approached 1.5 ml, an abrupt increase in ROS activity in SL bladders was observed (up to 10 000 counts (10 s)−1 on average) and the activity kept increasing during the overdistension period. Shortly (1–2 min) after emptying/drainage, a further abrupt increase in ROS activity (up to 30 000 and 50 000 counts (10 s)−1 for SL-OD1h and SL-OD2h, respectively) was detected.
Figure 2. ROS detection from rat bladder surface in vivo.
A, the basal ROS level before saline infusion was at about 1000–1400 counts (10 s)−1. A small and gradual increase in ROS level was noted when infusion began. When filling volume approached 1.5 ml, an abrupt increase in ROS was observed. The ROS activity remained elevated during the overdistension period. Shortly after emptying/drainage, a further abrupt increase in ROS was observed. Intravenous superoxide dismutase (SOD) administration abolished ROS activity, confirming the specificity of the chemiluminescence test. B and C, mean changes in bladder ROS from SL and HP rats (5 in each group) in response to 1 h (B) or 2 h (C) of overdistension are displayed. Prolonged overdistension significantly increased bladder ROS level in SL rats, but not in HP rats. *P < 0.05 when compared to respective control value. #P < 0.05 HP group versus SL group.
After emptying, the enhanced ROS activity gradually returned to the preoverdistension level over 12–15 h. The prolonged elevation of ROS chemiluminescence activity may be attributed to an increased leucocyte infiltration in the bladder. The effect of OD/E on the ROS chemiluminescence activity in HP rats was minimal, even with prolonged overdistension (i.e. in HP-OD2h) (Figs 2B and C). Intravenous administration of superoxide dismutase removed ROS and lowered the chemiluminescence activity; confirming the specificity of the test (Fig. 2A).
In situ localization of ROS formation and apoptotic cells
Increased numbers of leucocyte infiltrates were found in the SL-OD bladder 2 h post drainage. In HP-OD bladders, leucocyte infiltration was minimal.
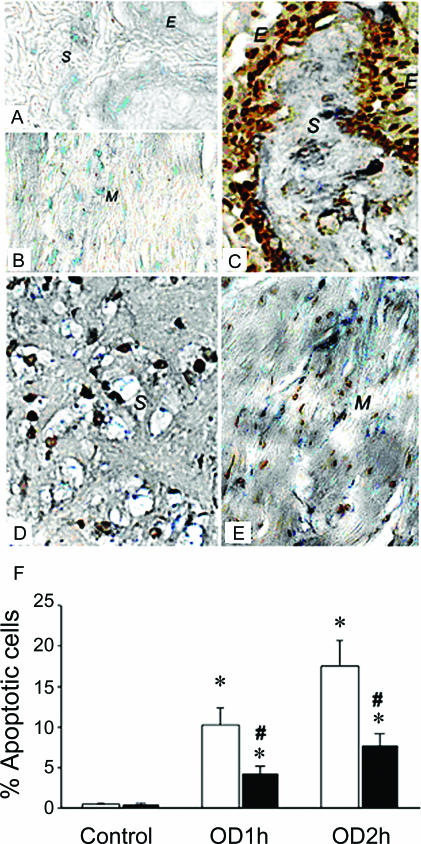
There was significant superoxide production (blue formazan deposits) in SL-OD2h bladders 2 h post drainage (Fig. 3). The NBT deposits were located mainly in the epithelium, submucosa, vessel walls, and, to a lesser degree, in smooth muscle. The blue formazan deposits were minimal in the HP-OD bladder, and were not readily detected in SL and HP bladders without OD/E injury.
Figure 3. In situ localization of ROS activity and apoptotic cells in OD/E bladder.
NBT deposits (blue precipitates) and apoptotic body staining (brownish-coloured nuclei) were used to demonstrate de novo production of ROS and to locate the apoptotic cells. The cellular source of ROS synthesis was mainly the neutrophils and resident cells in the OD/E bladder. A and B, NBT deposits and apoptotic bodies were absent in the control SL bladder. C–E, NBT deposits and apoptotic bodies were clearly visible in epithelium (E), submucosa (S), and smooth muscle layers (M) of SL-OD bladders. F, prolonged overdistension (OD2h) interval appeared to increase the number of apoptotic cells. HP treatment (filled bars) significantly reduced the formation of apoptotic nuclei in bladder tissues. Tissues were taken 2 h post drainage. * P < 0.05, when compared to respective control value. #P < 0.05, HP group versus SL group.
The spatial relationship of ROS production and apoptotic nucleus formation in damaged bladder tissues was determined by examination of tissue sections stained with both NBT and TUNEL methods. As shown in Fig. 3, apoptotic nuclei appeared in the epithelium, submucosa, and smooth muscle cells, coinciding with the sites of NBT deposits. HP treatment significantly reduced the formation of apoptotic nuclei in bladder tissues. The number of apoptotic cells in SL-OD1h and SL-OD2h bladder tissues was 10.3 ± 2.1% and 17.5 ± 3.2% per high-power field, respectively, whereas the apoptotic cell number was reduced to 4.2 ± 0.9% and 7.7 ± 1.5% per high power field in the HP-OD1h and HP-OD2h bladders (Fig. 3F).
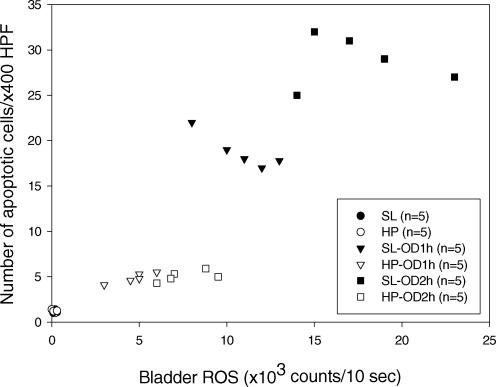
A scattergram illustrating the correlation between the bladder ROS activity and the number of apoptotic cells is shown in Fig. 4.
Figure 4. Relationship between ROS and apoptosis in the overdistension/emptying bladder.
Scattergram reveals a correlation between the number of apoptotic cells and ROS activity in SL and HP rats subjected to OD/E injury. Tissues were taken 2 h post drainage.
Apoptosis-regulated gene expression
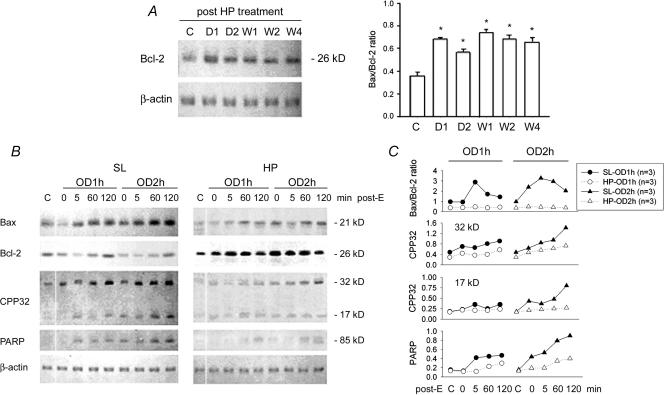
The expression of Bax, Bcl-2, CPP32, and PARP in the bladder samples was assessed by immunoblotting. The expression of Bcl-2 was detected in control SL bladder tissues and was increased after HP treatment. Four weeks post exposure to hypoxia, the expression of Bcl-2 in the rat bladder remained elevated (Fig. 5A).
Figure 5. Apoptosis-related protein expression in OD/E bladders.
Western blot analysis with specific antibodies to Bax, Bcl-2, CPP32, PARP, and β-actin of homogenates of rat bladder subjected to HP treatment and OD/E induced injury. A, Bcl-2 was significantly up-regulated in HP rats. The elevation persisted for at least 4 weeks (bar chart). B, left panel, in SL-OD bladders, the expression of Bcl-2 appeared to be decreased, but gradually returned to its preoverdistension level 120 min post emptying. Note the increased expression of Bax, CPP32 (32 kDa proenzyme and 17 kDa cleaved product), and PARP in SL-OD2h rats. B, right panel, note the markedly increased Bcl-2 in HP and HP-OD rats. The expression of Bax and, to a much lesser degree, of CPP32 and PARP was slightly increased in HP-OD rats. Equal protein loading was displayed by β-actin. C, mean changes in apoptosis-related protein expression are displayed. Note the increased Bax/Bcl-2 ratio in SL-OD rats. The ratio remains low in HP-OD rats. *P < 0.05 when compared to control value.
Bcl-2 was apparently depressed in SL-OD rats post OD/E injury, whereas Bcl-2 was already highly expressed and appeared not to be affected by OD/E in HP bladders.
The expression of Bax, PARP and the active form of 32 kDa CPP32 proenzyme was detected in control SL bladders. Their expression was increased 5 min post drainage and reached a high level at 120 min post drainage. Increased overdistension intervals seemed to potentiate their expression.
In HP rats before OD/E, expression of Bax, CPP32 and PARP was decreased as compared with that in SL rats. The expression of these three proteins was increased only slightly (Bax and CPP32), or not significantly changed (PARP) after OD/E injury (Fig. 5B).
Reduction of voiding pressure and detrusor contractility after OD/E-induced injury
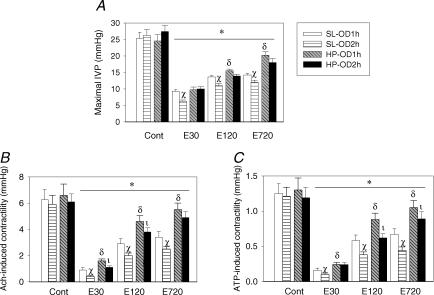
Figure 6A shows mean changes in bladder voiding contraction pressure (i.e. maximal IVP during micturition, mIVP) by cystometrogram in SL and HP rats after OD/E-induced injury. Bladder mIVPs were reduced in both SL-OD and HP-OD rats 30 min post emptying, and the reduction was particularly evident in OD2h rats. The reduced mIVP was partially recovered in HP rats 12 h after drainage. The delayed recovery of mIVP in the SL-OD bladder is correlated with persistent presence of infiltrated neutrophils in the bladder 12 h post OD/E-induced injury.
Figure 6. OD/E injury decreases in vivo voiding pressure and in vitro ATP- and bethanecol-induced detrusor contractility.
A, maximal intravesical pressures (mIVP, about 25 mmHg) during micturition were recorded by cystometrogram in SL-OD and HP-OD rats. The mIVP was significantly decreased after OD/E-induced injury. The decreased mIVP was slowly recovered in HP-OD rats 12 h post emptying. B and C, OD/E injury decreased bethanecol (Ach)- and ATP-induced detrusor muscle contractility for a prolonged period (> 12 h) in SL rats. Muscle strips taken from HP-OD rats 12 h after OD/E have near-normal contractility. Cont, control; E30, 30 min after emptying; E120, 120 min after emptying; E720, 720 min after emptying. *P < 0.05 when compared to each control value; α, P < 0.05 SL-OD2h versus SL-OD1h group; β, P < 0.05 HP-OD1h versus SL-OD1h group; γ, P < 0.05 HP-OD2h versus SL-OD2h group.
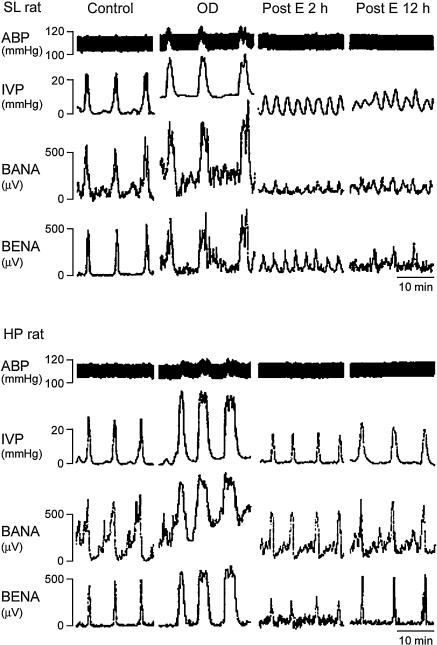
We found that bladder afferent and efferent nerve activities were reduced in rats subject to OD/E-induced injury (Fig. 7). A lesser depression of bladder nerve activity was observed in the HP-OD2h rats (about 45% and 34% reduction for afferent and efferent activity, respectively) when compared to SL-OD2h rats (77% and 85% reduction for afferent and efferent activity, respectively). The reduction of bladder nerve activity almost completely recovered in HP-OD rats 12 h post emptying. However, the bladder nerve activity remained depressed in SL-OD rats 12 h post emptying (Fig. 7).
Figure 7. OD/E injury impairs voiding function.
During the control stage, the bladders were filled with 0.9% saline (0.1 ml min−1) and allowed to void spontaneously. A rapid increase in intravesical pressure (IVP) was accompanied by activation of bladder afferent nerve activity (BANA) and bladder efferent nerve activity (BENA) in SL (upper) and HP (lower) rats. Overdistension (OD) significantly enhanced IVP, BANA, and BENA. The mIVP, BANA, and BENA remained greatly depressed in the SL rat 2 h and 12 h post emptying (E). In HP rats, the depression of mIVP, BANA, and BENA was restored 12 h post emptying.
The ATP- or bethanecol-induced detrusor muscle contractility was significantly decreased in muscle strips obtained from SL and HP rats subject to OD/E injury (Fig. 6B and C). The contractility of muscle strips from SL-OD rats remained depressed (about 50% of control) for 12 h. Muscle strips taken from HP-OD rats 12 h after OD/E had a near normal contractility (Figs 6B and C).
ROS directly impair bladder voiding contraction
OD/E evoked a bursting increase of ROS in the bladder. We explored whether the enhanced ROS impaired the voiding function by affecting bladder nerve activity and voiding activity. An elevated ROS level in the bladder was detected 5 min after intra-arterial xanthine/xanthine oxidase injection, and the elevated level lasted for 30–45 min. ROS chemiluminescence activities were 1244 ± 150 counts (10 s)−1 and 16 505 ± 1890 counts (10 s)−1 for control rats and xanthine/xanthine oxidase-treated rats, respectively (Table 1).
Table 1. Effects of intra-arterial administration of xanthine/xanthine oxidase on urodynamics, haemodynamics, and neural activity in urethane-anaesthetized rats.
| Before | After | |
|---|---|---|
| Amount of reactive oxygen species from bladder surface (counts (10 s)−1) | 1244 ± 150 | 16 505 ± 1890* |
| Amplitude of voiding bladder contractions (mmHg) | 32 ± 3 | 21 ± 2* |
| Duration of voiding bladder contractions (s) | 45 ± 4 | 27 ± 3* |
| Constant urine inflow (ml min−1) | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Maximal urine outflow (ml min−1) | 0.72 ± 0.11 | 0.42 ± 0.07* |
| Calculated urethral resistance (mmHg min ml−1) | 44.5 ± 5.2 | 50.9 ± 6.5 |
| Basal mean arterial pressure (mmHg) | 112 ± 4 | 110 ± 4 |
| Total BANA during a micturition cycle (μV s−1) | 795 ± 160 | 49 ± 14 |
| Total BENA during a micturition cycle (μV s−1) | 680 ± 130 | 167 ± 30 |
Values are means ± standard error of the mean. Comparisons are made before and after drug administration
P < 0.05. Six SL rats were used in this experiment. The micturition cycle is defined as extending from the end of the previous micturition contraction to the end of the next micturition contraction.
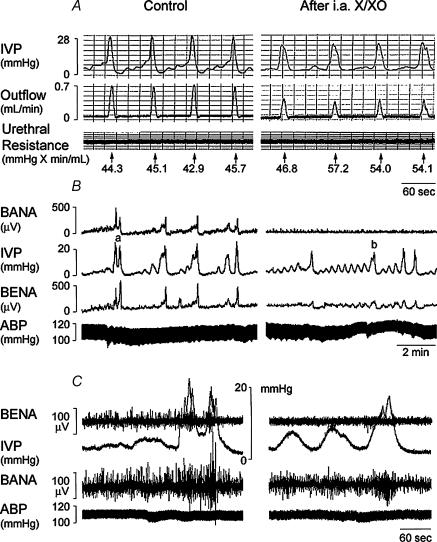
Xanthine/xanthine oxidase administration could reduce the amplitude of mIVP, duration of voiding contractions, peak value of urine outflow, and bladder afferent and efferent nerve activity, and increased bladder hyperactivity (Table 1 and Fig. 8). The urethral resistance was not changed in control and treated rats (Table 1). Superoxide dismutase treatment ameliorated the xanthine/xanthine oxidase effect, suggesting a role of ROS for the depressed voiding contraction and bladder nerve activity.
Figure 8. Superoxide generation by xanthine/xanthine oxidase impairs voiding function.
A, the bladders were filled with 0.9% saline with an inflow rate of 0.1 ml min−1 and allowed to void spontaneously. A rapidly increased IVP was accompanied by increased outflow. Through the micturition cycles, the urethral resistance remained the same. Note the decreased mIVP and outflow rate in xanthine/xanthine oxidase (X/XO)-treated rats (right panel). B and C, decreased bladder afferent (BANA) and efferent nerve activity (BENA) is displayed in X/XO-treated rat (right panel). SOD treatment abolished the X/XO effect (picture not shown). i.a. X/XO: intra-arterial xanthine/xanthine oxidase treatment. B: slow tracing; C: fast tracing.
Discussion
The rat model of HP in a hypobaric chamber represents whole body hypoxia. Therefore, HP-induecd protective changes may involve complex mechanisms including central, hormonal and local neuronal and metabolic changes. Using a rat model of bladder OD/E, we examined the effect of the overdistension and subsequent emptying from overdistension on oxidative stress and apoptosis in the bladder.
Overdistension resulted in incomplete ischaemia, and emptying from overdistension recovered bladder blood flow, initiated ROS production and inflammatory cell infiltration, and consequently led to apoptosis formation. Specifically, OD/E injury decreased voiding contraction by enhancing in vivo ROS amounts from damaged bladders, inhibiting both bladder afferent and efferent nerve activity, depressing ATP- and acetylcholine-induced detrusor contractility, and potentiating the apoptotic mechanism including the Bax/Bcl-2 ratio, CPP32, PARP and apoptotic cells in SL and to a lesser degree, in HP bladders. A prolonged overdistension interval increased oxidative stress and apoptotic injury in the damaged bladders. HP, which up-regulates Bcl-2 expression in bladder tissues, significantly protected the bladders' micturition function, and ameliorated the OD/E-induced ROS amounts and apoptotic injury.
OD/E clearly decreased voiding contraction, and the degree of voiding dysfunction was significantly increased after prolonged overdistension. The following factors may contribute to voiding dysfunction: decreased bladder elasticity and compliance (Lin et al. 1992), impairment of mitochondrial function (Lin et al. 1998), depletion of ATP (high energy phosphate) (Zderic et al. 1998) and impaired purinergic neurotransmission (Chien et al. 2000c), impairment of cholinergic neurotransmission, decreased cholinergic responses, and alterations in adrenergic neurotransmission (Sutherland et al. 1998). In addition, the contractile dysfunction of the bladder may be related to structural alteration, including loosely packed myofilaments and an irregular distribution of sarcoplasmic dense bodies in the damaged smooth muscle cells (Gosling et al. 2000). Our present results further indicated that OD/E-evoked ROS directly inhibit both bladder afferent and efferent nerve activity, and reduce bladder voiding contraction, including acetylcholine- and ATP-evoked detrusor contractility.
In published studies, both overdistension and bladder outlet obstruction resulted in significantly decreased local bladder blood flow in dogs (Azadozi et al. 1996; Lieb et al. 2001; Schröder et al. 2001). Acute overdistension resulted in decreased oxidative phosphorylation, decreased cellular concentrations of creatine phosphate and ATP, and increased glycolytic metabolism in the detrusor (Kato et al. 1990), suggesting that overdistension induced ischaemia–reperfusion damage. Our current study provides direct evidence for this hypothesis, i.e. that acute overdistension results in significantly decreased bladder blood flow and increased vascular resistance, and that relief from overdistension results in a significant increase in ROS formation and oxidative cellular (reperfusion) damage.
Several lines of evidence have shown that ischaemia followed by reperfusion results in the release of large amounts of ROS (Kontos et al. 1992; Okuda et al. 1992; Chien et al. 2001). In the present study, we used an enhanced CL method to study ROS production. This method has been applied to measurement of ROS production in cultured cells, in the whole-blood system (Chien et al. 2001), in isolated perfused organs (Okuda et al. 1992), and in in vivo kidney, bladder, and brain preparations (Kontos et al. 1992; Chien et al. 2001, 2003). Using this in vivo method, we showed that, after overdistension and OD/E injury, the level of ROS detected from the bladder surface was significantly increased. Using H&E stain and NBT perfusion, we localized the origin of the ROS of the OD/E bladders to leucocyte infiltration and within the uroepithelium, submucosa and smooth-muscle layers. Our results demonstrated that the largest amount of ROS occurred in the overdistended bladder within 5 min of drainage. Tsao et al. (1990) indicated that endothelial cells lost their function after 2.5 min of reperfusion injury by a possible mechanism of ROS formation.
In our experiments, haematouria usually occurred after bladder OD/E injury, and the release of Fe2+ from leakage of erythrocytes and haemoglobin into tissues can generate toxic ROS such as OH and lead to further bladder injury (Lin et al. 2000). It is generally known that organs and tissues subjected to ischaemia–reperfusion injury stimulate the generation and release of several forms of ROS during and shortly after the reperfusion stage (Kontos et al. 1992; Okuda et al. 1992). However, in this study, a large amount of ROS was generated in the bladder during the overdistension stage. We suggest that leakage of ROS from the incomplete blockade of bladder blood flow may explain the measurable ROS in the overdistended bladder.
We believe that OD/E-induced ROS generation directly reduces bladder voiding function by one or more of the following mechanisms: impairment of contractile proteins (Jackson, 1990), impairment of Ca2+ pump activity (Temsah et al. 1999), injury of membrane and DNA integrity of mitochondria (Sohal et al. 1995), lipid peroxidation (Freeman & Crapo, 1982), and expression and activation of calpain (calcium-activated protease) (Zhao et al. 1994). Tarcan et al. (2000) reported that products of oxidative stress, including isoprostane, are made in the healthy bladder and modulate bladder contractility.
In our study, HP resulted in a significant protection of voiding function and detrusor contractility and reduced ROS generation. This protection may be partly related to the up-regulation of Bcl-2 and the increased Bcl-2/Bax ratio. Several reports have demonstrated that HP or temporary ischaemia can afford protection against subsequent severe ischaemic injury (Murry et al. 1986; Shizukuda et al. 1992). Exposure of cells in vitro to environmental stresses such as heat, anoxia, and hypoxia has been shown to induce protective proteins such as catalase, Bcl-2 (Shimizu et al. 2001), and stress or heat shock proteins (Murry et al. 1986; Marber et al. 1993; Chien et al. 2000b). This protective function could also be raised by the mechanisms of adenosine A1 receptor activation (Heurteaux et al. 1995), ATP dependent potassium channel activation (Heurteaux et al. 1995), and nitric oxide induction (Hotter et al. 1996). Among these, Bcl-2 has a role in suppressing the production of ROS, lipid peroxidation, and apoptosis and a regulatory effect on intracellular calcium levels (Engelman et al. 1995). In the heart, Bcl-2 antisense oligodeoxynucleotide treatment blocked the induction of tolerance by preconditioning ischaemia, suggesting that, in this model of induced tolerance to focal ischaemia, Bcl-2 appears to be a major determinant.
Our study further showed that prolonged overdistension resulted in increases in apoptotic cell number and in pro-apoptotic proteins such as Bax, CPP32, and PARP. Bcl-2 expression was significantly depressed by overdistension and OD/E injury in unprotected rats, resulting in an increased Bax/Bcl-2 ratio. HP appeared to prevent the pro-apoptotic mechanisms by an abundant and prolonged increase in Bcl-2 expression, despite an increased expression of pro-apoptotic proteins in bladders subject to OD/E. This resulted in a decreased Bax/Bcl-2 ratio in HP-OD rats, as compared to that in unprotected SL-OD rats.
In summary, in view of the fact that hypoxia–reoxygenation and ischaemia–reperfusion lead to the generation of ROS, the induction of Bcl-2 protein expression by HP appears to reflect the bladder's up-regulation of the endogenous antioxidant defense system, enabling it to survive a subsequent ischaemic stress by reducing an oxidative insult and preserving bladder nerve activity and contractile function. The present study indicates that ROS are produced in significant amounts in response to overdistension and OD/E, and the increased ROS may contribute to the voiding dysfunction by inhibition of bladder afferent and efferent nerve activity and ATP- and acetylcholine-mediated contraction, and bladder cell apoptosis. These studies also support the use of antioxidants in the treatment of overdistension injury.
Acknowledgments
The authors thank Mr Young Kuen-Chung and Miss Tsai Jin-Yue for expert technical assistance. This work was supported by the National Science Council of the Republic of China (NSC90-2320-B-002-096, NSC90-2314-B-002-446, and NSC-91–2320-B-002-118) to C.T.C. and H.J.Y.
References
- Azadozi KM, Pontari M, Vlachiotis J, Siroky MB. Canine bladder blood flow and oxygenation: changes induced by filling, contraction and outlet obstruction. J Urol. 1996;155:1459–1465. doi: 10.1016/s0022-5347(01)66307-9. [DOI] [PubMed] [Google Scholar]
- Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999;162:1768–1778. [PubMed] [Google Scholar]
- Bardales RH, Hailey LS, Xie SS, Schaefer RF, Hsu SM. In situ apoptosis assay for the detection of early acute myocardial infarction. Am J Pathol. 1996;149:821–830. [PMC free article] [PubMed] [Google Scholar]
- Bernardo NL, Chelleya J, D'Angelo M, Loesser KB, Grant S, Kukreja RC. The second window of protection induces expression of proto-oncogene Bcl-2 and inhibits apoptosis in rabbit heart. Circulation. 1997;Suppl. 96:I-553. (Abstract) [Google Scholar]
- Cai M, Ogawa R. Effects of free radical scavengers, methylprednisolone, and ulinastatin on acute xanthine and xanthine oxidase-induced lung injury in rats. Circ Shock. 1994;43:71–78. [PubMed] [Google Scholar]
- Carpenter FG. Impairment and restoration of rat urinary bladder responsiveness following distension. Am J Physiol. 1983;244:R106–R113. doi: 10.1152/ajpregu.1983.244.1.R106. [DOI] [PubMed] [Google Scholar]
- Chien CT, Fu TC, Wu MS, Chen CF. Attenuated response of renal mechanoreceptors to volume expansion in chronically hypoxic rats. Am J Physiol. 1997;273:F712–F717. doi: 10.1152/ajprenal.1997.273.5.F712. [DOI] [PubMed] [Google Scholar]
- Chien CT, Hsu SM, Chen CF, Lee PH, Lai MK. Hypoxic preconditioning reduces ischemia/reperfusion-induced apoptosis cell death in rat kidney. Transplant Proc. 2000b;32:1653–1664. doi: 10.1016/s0041-1345(00)01434-2. [DOI] [PubMed] [Google Scholar]
- Chien CT, Lee PH, Chen CF, Ma MC, Lai MK, Hsu SM. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol. 2001;12:973–982. doi: 10.1681/ASN.V125973. [DOI] [PubMed] [Google Scholar]
- Chien CT, Yu HJ, Cheng YJ, Wu MS, Chen CF, Hsu SM. Reduction in renal haemodynamics by exaggerated vesicovascular reflex in rats with acute urinary retention. J Physiol. 2000a;526:397–408. doi: 10.1111/j.1469-7793.2000.t01-1-00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CT, Yu HJ, Lin TB, Chen CF. Neural mechanisms of impaired micturition reflex in rats with acute partial bladder outlet obstruction. Neuroscience. 2000c;96:221–230. doi: 10.1016/s0306-4522(99)00508-4. [DOI] [PubMed] [Google Scholar]
- Chien CT, Yu HJ, Lin TB, Lai MK, Hsu SM. Substance P via NK1 receptor facilitates hyperactive bladder afferent signaling via action of ROS. Am J Physiol Renal Physiol. 2003;284:F840–F851. doi: 10.1152/ajprenal.00187.2002. [DOI] [PubMed] [Google Scholar]
- Engelman DT, Chen CZ, Watanabe M, Engelman RM, Rousou JA, Flack JE, 3rd, Deaton DW, Maulik N, Das DK. Improved 4- and 6-hour myocardial preservation by hypoxic preconditioning. Circulation. 1995;92 (Suppl.):II417–422. doi: 10.1161/01.cir.92.9.417. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Biology of disease: free radical and tissue injury. Laboratory Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C, Levin RM. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol. 2000;163:1349–1356. [PubMed] [Google Scholar]
- Greenland JE, Brading AF. The effect of bladder outflow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol. 2001;165:245–248. doi: 10.1097/00005392-200101000-00072. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci U S A. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MJ. Free radicals and skeletal muscle disorders. In: Das DK, editor. Oxygen Radicals: Systemic Events and Disease Process. New York: Karger; 1990. pp. 149–171. [Google Scholar]
- Kato K, Wein AJ, Radzinski C, Longhurat PA, McGuire Miller LF, Elbadawi A, Levin RM. Short-term functional effects of bladder outlet obstruction in the cat. J Urol. 1990;143:1020–1025. doi: 10.1016/s0022-5347(17)40175-3. [DOI] [PubMed] [Google Scholar]
- Kershen RT, Azadzoi KM, Siroky MB. Blood flow, pressure and compliance in the male human bladder. J Urol. 2002;168:121–125. [PubMed] [Google Scholar]
- Kontos CD, Wei EP, Williams J, Kontos HA, Polvisshock JT. Cytochemical detection of superoxide in cerebral inflammation and ischemia in vivo. Am J Physiol. 1992;263:H1234–H1242. doi: 10.1152/ajpheart.1992.263.4.H1234. [DOI] [PubMed] [Google Scholar]
- Kozlowski R, Siroky MB, Krane RJ, Azadzoi KM. Regulation of blood flow and microcirculation resistance in rabbit bladder. J Urol. 2002;168:1608–1614. doi: 10.1016/S0022-5347(05)64529-6. [DOI] [PubMed] [Google Scholar]
- Lee TM, Su SF, Chen MF, Tsai CH. Acute effects of urinary bladder distention on the coronary circulation in patients with early atherosclerosis. J Am Coll Cardiol. 2000;36:453–460. doi: 10.1016/s0735-1097(00)00751-8. [DOI] [PubMed] [Google Scholar]
- Lieb J, Kogan B, Das AK, Leggett RE, Schroeder A, Levin RM. The effect of urine volume and nitric oxide on basal bladder blood flow: response to catheterization and drainage. Neurourol Urodyn. 2001;20:115–124. doi: 10.1002/1520-6777(2001)20:1<115::aid-nau13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lin AT, Chang LA, Chen MT, Yang CH, Shiao MS, Chen CJ, Levin RM. Energetics of detrusor contraction: effects of outlet obstruction. Neurourol Urodyn. 1992;11:605–614. [Google Scholar]
- Lin AT, Chen KK, Yang CH, Chang LS. Effects of outlet obstruction and its reversal on mitochondrial enzyme activity in rabbit urinary bladders. J Urol. 1998;160:2258–2262. doi: 10.1097/00005392-199812010-00096. [DOI] [PubMed] [Google Scholar]
- Lin AT, Chen KK, Yang CH, Chang LS. Mannitol facilitates rabbit urinary bladder recovery from overdistension injury. Urology. 2000;56:702–707. doi: 10.1016/s0090-4295(00)00702-0. [DOI] [PubMed] [Google Scholar]
- Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Mustonen S, Ala-Houhala I, Tammela TL. Proteinuria and renal function during acute urine retention. J Urol. 1999;161:1781–1784. [PubMed] [Google Scholar]
- Okuda M, Lee HC, Kumar C, Chance B. Oxygen radical generation during ischemia-reperfusion in the isolated perfused rat liver monitored by enhanced chemiluminescence. Circ Shock. 1992;38:228–237. [PubMed] [Google Scholar]
- Piot C, Padmanaban D, Ursell PC, Sievers RE, Wolfe CE. Ischemic preconditioning decreases apoptosis in rat hearts in vivo. Circulation. 1997;96:1598–1604. doi: 10.1161/01.cir.96.5.1598. [DOI] [PubMed] [Google Scholar]
- Schröder A, Lieb J, Kogan BA, Levin RM. Increased blood flow after catheterization and drainage in the chronically obstructed rabbit urinary bladder. Urology. 2001;58:295–300. doi: 10.1016/s0090-4295(01)01142-6. [DOI] [PubMed] [Google Scholar]
- Schumer M, Colombel MC, Sawczuk IS, Gobe G, Connor J, O'Toole KM, Olsson CA, Wise GJ, Buttyan R. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;40:831–838. [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Nagayama T, Jin KL, Zhu L, Loeffert JE, Watkins SC, Graham SH, Simon RP. Bcl-2 Antisense treatment prevents induction of tolerance to focal ischemia in the rat brain. J Cereb Blood Flow Metab. 2001;21:233–243. doi: 10.1097/00004647-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Shizukuda Y, Mallet RT, Lee SC, Downey HF. Hypoxic preconditioning of the ischemic canine myocardium. Cardiovasc Res. 1992;26:534–542. doi: 10.1093/cvr/26.5.534. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic Biol Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- Sutherland RS, Baskin LS, Kogan BA, Cunha G. Neuroanatomical changes in the rat bladder after bladder outlet obstruction. Br J Urol. 1998;82:895–901. doi: 10.1046/j.1464-410x.1998.00873.x. [DOI] [PubMed] [Google Scholar]
- Tammela T, Lasanen L, Waris T. Effect of distention on adrenergic innervation of the rat urinary bladder. Urol Res. 1990;18:345–348. doi: 10.1007/BF00300785. [DOI] [PubMed] [Google Scholar]
- Tarcan T, Siroky MB, Krane RJ, Azadzoi KM. Isoprostane 8-epi PGF2alpha, a product of oxidative stress, is synthesized in the bladder and causes detrusor smooth muscle contraction. Neurourol Urodyn. 2000;19:43–51. doi: 10.1002/(sici)1520-6777(2000)19:1<43::aid-nau6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Temsah RM, Netticadan T, Chapman D. Alterations of sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am J Physiol. 1999;277:H584–H594. doi: 10.1152/ajpheart.1999.277.2.H584. [DOI] [PubMed] [Google Scholar]
- Theobald RJ. Purinergic and cholinergic components of bladder contractility and flow. Life Sci. 1995;56:445–454. doi: 10.1016/0024-3205(94)00909-0. [DOI] [PubMed] [Google Scholar]
- Tsao PS, Aoki N, Lefer DJ, Johnson G, III, Lefer AM. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 1990;82:1402–1412. doi: 10.1161/01.cir.82.4.1402. [DOI] [PubMed] [Google Scholar]
- Zderic SA, Wein A, Rohrman D, Gong C, Nigro D, Haugaard N, Levin RM. Mechanisms of bladder smooth muscle hypertrophy and decompensation: lessons from normal development and the response to outlet obstruction. World J Urol. 1998;16:350–358. doi: 10.1007/s003450050079. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chacko S, Levin RM. Expression of stress proteins (Hsp-70 and Hsp-90) in the rabbit urinary bladder subjected to partial outlet obstruction. Mol Cell Biochem. 1994;130:49–55. doi: 10.1007/BF01084267. [DOI] [PubMed] [Google Scholar]