Abstract
Semi-conserved exon boundaries in members of the CACNA1 gene family result in recurring pre-mRNA splicing patterns. The resulting variations in the encoded pore-forming subunit of the voltage-gated calcium channel affect functionally significant regions, such as the vicinity of the voltage-sensing S4 segments or the intracellular loops that are important for protein interaction. In addition to generating functional diversity, RNA splicing regulates the quantitative expression of other splice isoforms of the same gene by producing transcripts with premature stop codons which encode two-domain or three-domain channels. An overview of some of the known splice isoforms of the α1 calcium channel subunits and their significance is given.
There are only 10 genes in the human genome that encode pore-forming α1 subunits of voltage-gated calcium channels. In combination with accessory subunits, these 10 α1 subunits must mediate such diverse functions as: (i) intracellular calcium homeostasis, (ii) regulation of gene expression, and (iii) coupling of membrane potential changes to various downstream processes like neurotransmitter release or muscle contraction. Nature has chosen alternative pre-RNA splicing as a thrifty means to generate the required functional and structural diversity of the α1 subunits. This paper gives an overview of recurrent patterns in calcium channel α1 subunit RNA splicing and their functional significance. Reviews giving a general overview of a calcium channel family including information on splicing (Catterall, 2000; Lacinova et al. 2000; Abernethy & Soldatov, 2002; Perez-Reyes, 2002) or focusing especially on splicing in the N-type channel (Lipscombe et al. 2002) are available for further study.
Calcium channel α1 subunits
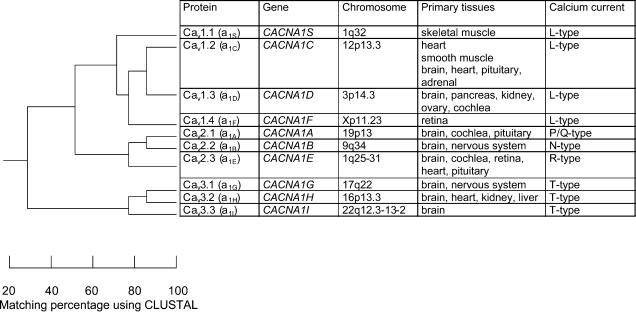
Voltage-gated calcium channels have been functionally differentiated according to their inactivation properties into either transient (T-type) or long lasting (l-type) currents. Additionally, N (neuronal), P (Purkinje cell), Q (granular cell) and R (toxin-resistant) channels can be distinguished depending on their tissue expression pattern and toxin sensitivity, respectively. Based on the phylogeny underlying these pharmacological and biophysical differences, Ertel et al. (2000) have suggested a more uniform nomenclature for the α1 subunits of calcium channels which is now commonly used (Fig. 1).
Figure 1. Calcium channel nomenclature (modified according to Ertel et al. 2000).
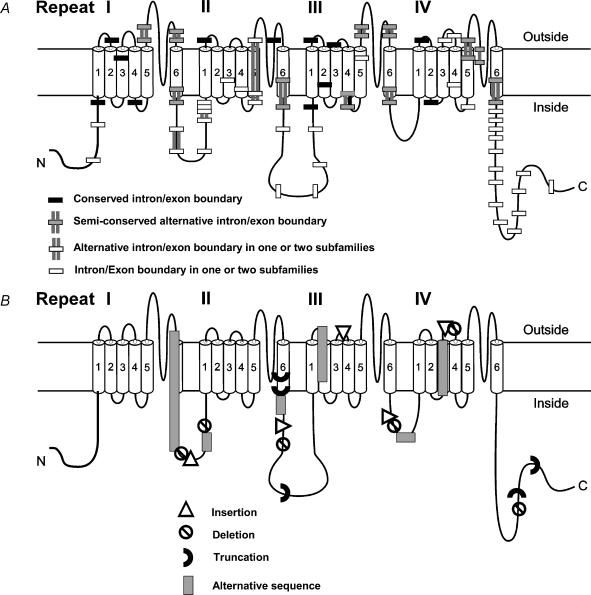
The α subunit of the channel, contains as a basic motif a tetrameric association of four domains each containing a series of six transmembrane α-helical segments, numbered S1–S6, which are connected by both intracellular and extracellular loops (Fig. 2. It comprises the ion-conducting pore and determines the main characteristics of the cation channel complex such as its ion selectivity, voltage sensitivity, pharmacology and binding characteristics for endogenous and exogenous ligands. The voltage sensitivity of cation channels is conveyed by the S4 segments, which are thought to move outward upon depolarization causing the channels to open. Calcium flows through the ion conducting pore, which is thought to be lined by the S5–S6 loops of all four domains. Whereas the localization of the activation gate may well be within the pore, the inactivation gate has not been unambiguously localized in calcium channels (for review see Catterall, 2000).
Figure 2. Scheme of the voltage-gated calcium channel α1 subunit.
The α1 subunit of voltage gated calcium channels consists of four domains (repeats) of six transmembrane segments connected by intracellular loops. A, conservation of the gene structure at the protein level as determined by protein alignments encoded by all exons of all 10 members of the CACNA1 family. Each protein region encoded by an exon is delineated by bars. White, black and grey bars indicate degree of conservation as noted in the figure. The following human reference sequences were used at NCBI: Cav2.1: NP_075461; Cav2.2: NP_000709; Cav1.2: NP_000710; Cav1.3: NP_000711; Cav2.3: NP_000712; Cav1.4: NP_005174; Cav3.1: NP_061496; Cav3.2: NP_066921; Cav3.3: NP_066919; and Cav1.1: NP_000060. B, regions of the protein that are affected by alternative splicing. Note that the changes now reflect the protein level only (i.e. deletions leading to frame shifts and early truncations are marked as truncation only). The diversity of the primary protein sequence due to insertions, deletions, truncations and alternative sequences is marked by the various symbols.
In order to form a functional calcium channel complex, the α1 subunit coassembles with at least three accessory subunits encoded by two gene families: an intracellular β subunit encoded by a CACNB gene, and an extracellular α2 subunit linked by a di-sulphide bond to the membrane-anchoring δ subunit both of which are encoded by a CACNA2D gene. In skeletal muscle, an additional accessory transmembranal γ subunit is part of the channel complex (Kang & Campbell, 2003; Wolf et al. 2003). In neuronal channels, its contribution to the channel complex is a matter of debate because coexpression did not regularly yield a functional change (Moss et al. 2003). The contribution of accessory subunit-mediated modulation to calcium channel diversity is reviewed elsewhere (Walker & De Waard, 1998; Birnbaumer et al. 1998). Briefly, the accessory α2/δ and β subunits increase the current amplitude (Brice et al. 1997), accelerate inactivation kinetics and facilitate gating (Singer et al. 1991), and shift the voltage dependence of inactivation in the hyperpolarizing direction (Singer et al. 1991).
Splicing of CACNA1 transcripts
In order to obtain mature mRNA which can be directly translated into a protein sequence, the non-coding regions corresponding to introns of the DNA must be removed from the precursor pre-mRNA by the splicing process. The machinery performing this task, the spliceosome, recognizes introns by typical nucleotide sequences within the intron and adjacent exons (Wu & Krainer, 1999; Singh, 2002; Black & Grabowski, 2003). The probability for splicing out a sequence at any position in the pre-mRNA depends, among others, on the combination of different nucleotide sequences in key positions and the tissue-dependent spliceosome composition available. Therefore, the mRNA population resulting from one gene is not homogeneous in a given cell type, but rather shows the result of a variety of combinations of different single splicing events, each of which is present in a number of transcripts proportionate to its splicing probability.
In calcium channel transcripts, combinations of four types of alternative splicing are found: (i) splicing at alternative intron sequences near the 5′ intron end causing a possible elongation or shortening of the preceding exon (so-called alternative splice donor), (ii) splicing at alternative intron sequences near the 3′ intron end causing a possible elongation or shortening of the following exon (so-called alternative splice acceptor), (iii) optional splicing within an intron to retain an optional exon (so-called cassette exon) by use of less probable acceptor and donor splice signals flanking this exon, and (iv) alternative splicing of more than one cassette exon within a large intron that may result in a variety of combinations of these exons including the obligatory exclusion of one another. So far, cases of alternative donor and acceptor sites within an exon resulting in splicing out of central portions of that exon have not yet been described in CACNA1 transcripts.
Voltage-gated calcium channels are thought to have evolved by multiple gene duplication from a common ancestral channel gene encoding a one-domain potassium channel (Strong et al. 1993; Nelson et al. 1999; Anderson & Greenberg, 2001). The intron–exon boundaries or gene structures are therefore not only conserved from one CACNA1 gene to another (Huang et al. 1990; Barry et al. 1995; Wu & Krainer, 1999; Lipscombe et al. 2002), but also from one species to another, for example Drosophilaversus man (Peixoto et al. 1997). The gene structure within the CACNA1 gene family is generally well conserved at coding regions for segments S1–S5 of all four domains (Fig. 2A). The remaining regions, especially those coding for the domain interlinkers and the S6 segments of all four domains, show less conserved gene structure suggesting that the encoded protein areas are important for generating functional diversity. These less conserved coding regions coincide with the regions in which alternative splicing produces several different transcripts derived from a single gene (Fig. 2B). These transcripts generate proteins with similar function and sequence but different expression patterns, the so-called splice isoforms.
S3–S4 loops
The extracellular loops between S3 and S4 may influence S4 voltage sensor function because of their close vicinity to the S4 segments, which must move upon depolarization (Bezanilla, 2002). Effects on the voltage sensor function could therefore be achieved by alternative splicing in these areas while leaving the S4 itself unaffected. In the l-type Ca2+ channel family, the IS3–S4 loop of skeletal and cardiac channels helps to determine activation kinetics as has been demonstrated by the study of chimeras (Nakai et al. 1994). However, it is unlikely that this mechanism is taken advantage of in splicing regulation of IS3–S4 because it would require intraexonic splicing for all 10 hitherto known CACNA1 genes in which distal IS3, the whole IS3–S4 loop, and proximal IS4 are all encoded by one exon. For domain II, a similarly unlikely pattern of splicing would be required, but for domains III and IV, all 10 CACNA1 genes have an intron between genomic regions encoding S3 and S4. It is therefore not surprising that these regions are able to produce different splice isoforms. In domain III however, the only known variant is one with the insertion of 12 bases by cassette exon 24a leading to addition of four amino acid residues, SFMG, to IIIS3–S4 in the N-type Cav2.2 channel which does not, however, seem to have significant impact on the kinetics or voltage dependence of gating (Lin et al. 1999; Stea et al. 1999). This leaves only the variants generated by alternative splicing of regions encoding IVS3–S4 to be of functional significance.
In domain IV, the insertion of only six bases by a supplementary cassette exon 31a in both P/Q-type Cav2.1 and N-type Cav2.2 channels has a distinct functional effect even though it leads to the introduction of only two amino acid residues, NP or ET. The presence of ET in the N-type Cav2.2 channels results in a rightward shift of voltage dependence of activation and slowed activation kinetics (Lin et al. 1997 1999). Given that exon 31a is preferentially expressed in the peripheral but not in the central nervous system, this could lead to facilitated calcium entry selectively in cerebral neurones (Lin et al. 1999). In P/Q-type Cav2.1 channels, NP slows activation and inactivation and decreases affinity to ω-agatoxin IVA (Bourinet et al. 1999; Hans et al. 1999; Krovetz et al. 2000). The NP variant is generally thought to be present in the Q-type (low ω-agatoxin IVA affinity), while the variant lacking NP is thought to decisively contribute to the P-type (high affinity) calcium channel, even though secondary modifications and accessory subunits may also be contributing to the functional characteristics of P-type and Q-type (Mermelstein et al. 1999). Expression patterns suggest that the more rapidly gating P-type calcium channel is important for not only cerebellar Purkinje cells, but also pancreatic β cells (Ihara et al. 1995; Ligon et al. 1998).
In l-type channels, IVS3–S4 is altered by the splicing out of a short exon encoding part of this loop: for the skeletal muscle Cav1.1 channel it is exon 29 coding for TFLASSGGLYCLGGGCGNV, for the cardiac Cav1.2 channel exon 33 coding for PAEHTQCSPSM, and for the neuronal Cav1.3 channel exon 32 coding for PSENIPLPTATPG (Barry et al. 1995; Safa et al. 2001). In the cardiac Cav1.2 channel, this exon deletion is additionally accompanied by the replacement of exon 31 by exon 32 (exon 31 and 32 are mutally exclusive exons in the current nomenclature; Abernethy & Soldatov, 2002) encoding an altered sequence for part of IVS3 and IVS3–S4 (Perez-Reyes et al. 1990; Snutch et al. 1991; Diebold et al. 1992; Yu et al. 1992; Abernethy & Soldatov, 2002). The 11 amino acid IVS3–S4 deletion is present in several tissues making up about 12% of all transcripts while the IVS3 variation is tissue dependent and differentially regulated during development (Feron et al. 1994). For the skeletal muscle Cav1.1 channel, the 19 amino acid IVS3–S4 deletion makes up 10% of transcripts in adult muscle, but over 66% in regenerating muscle (unpublished data). For these three channels, the functional significance of these variants has not yet been clarified.
II–III loop
The task of the intracellular loop connecting domains II and III is to mediate interaction with effector proteins such as the calcium release channel for skeletal muscle excitation–contraction coupling (Dulhunty et al. 2002) or synaptic proteins such as syntaxin and SNAP25 (soluble attachment proteins of NSF –N-ethylmaleimide-sensitive fusion protein) or proteins for neuronal excitation–exocytosis coupling (Catterall, 1999; Mochida et al. 2003). Therefore, a functional modulation brought about by change or removal of these regions by alternative splicing may be expected. For example, in N-type Cav2.2 calcium channels, the first part of this loop may be altered by introducing an additional cassette following exon 18 (Pan & Lipscombe, 2000). This exon 18a is expressed in adult sympathetic ganglia, but not very abundantly in most of the regions of the neocortex except for monoaminergic neurones (Ghasemzadeh et al. 1999). This suggests that even though the putative synaptic protein binding site is not directly altered by alternative splicing, the 18a-encoded region may contribute to the targetting of the isoform to distinct synapses (Lipscombe et al. 2002). Likewise, even though the binding site of the β subunit is not affected, different β isoforms act differentially on the 18a variants (Scott et al. 1996; Pan & Lipscombe, 2000). Functionally, exon 18a causes a right-shift of the voltage-dependence of steady-state inactivation which may directly lead to hyperexcitability by affecting neurotransmitter release or to decreased excitability by activating Ca2+-dependent K+ channels indicating a possible higher degree of excitability of those neurones in which it is expressed (Pan & Lipscombe, 2000). In addition to the 18a variant, exons 19–21 may be spliced out with or without modification of the preceding splice donor site (Kaneko et al. 2002). These isoforms show not only the right shift of the steady-state inactivation curve, but also accelerated recovery from inactivation (Kaneko et al. 2002). Additionally, the sensitivity to ω-conotoxin GIVA is reduced, the reason for which could be the preferred binding of the toxins to the inactivated state, which is destabilized by the deletions (Lipscombe et al. 2002).
In the closely related P/Q Cav2.1 channels, exon 17, which corresponds to exon 18 of Cav2.2, is alternatively spliced at the splice donor site of intron 17 leading to a potential insertion/deletion of nine bases coding for VEA (Soong et al. 2002). No functional studies have yet been performed so that a possible effect on the synaptic protein binding site cannot be decided. However, rat and rabbit splice isoforms with sequence differences in the region encoded by human exon 19 showed different affinity to SNAP-25 and syntaxin (Kim & Catterall, 1997; Rettig et al. 1996) and different cellular expression patterns (Sakurai et al. 1996). This supports the idea of isoform-specific targeting as in the Cav2.2 channels (Catterall, 1999). Recently, exactly this region encoded by exon 19 was shown to mediate the synaptic protein interaction site (Mochida et al. 2003) confirming these assumptions. The II–III interlinker of the P/Q Cav2.1 channels also shows single amino acid substitutions caused by RNA editing which have been described in the mouse homologue affecting residues 886 and 1085, but the functional significance has not been clarified yet (Tsunemi et al. 2002).
In R-type Cav2.3 channels, exon 19, corresponding to exon 19 of Cav2.1 channels, can be spliced out, which results in the absence of calcium-dependent slowing of inactivation and acceleration of recovery from inactivation (Pereverzev et al. 2002). This isoform with decreased sensitivity to calcium influx shows differential distribution in murine cerebellum, the islets of Langerhans and kidney (Vajna et al. 1998; Grabsch et al. 1999). Even though the targeting to synapses has not been studied, the specific tissue distribution in other cell types may indicate changed protein interaction. Lastly, comparable to the N-type Cav2.2 channels and despite little sequential homology in II–III linker, in the T-type Cav3.1 channels, exon 16 may be spliced out leading to a right shift of steady state inactivation curve and slowing of inactivation kinetics (Mittman et al. 1999a; Chemin et al. 2001).
C-terminus
The C-terminus makes up a third of the channel protein and is not very well conserved within the CACNA1 gene family suggesting it to be a region of functional specialization. It is encoded by 3–14 exons, and the protein contains several regulatory elements such as binding sites for calcium, calmodulin and G-proteins (for reviews see Catterall, 2000; Hering et al. 2000; Abernethy & Soldatov, 2002; Perez-Reyes, 2002). Additionally, in Cav2.2 channels, the C-terminus is important for targeting the channels to synapses (Maximov & Bezprozvanny, 2002). This region is also relevant for a disease as indicated by the occurrence of a CAG repeat expansion in the coding region of Cav2.1 channels responsible for spinocerebellar atxia type 6 (Zhuchenko et al. 1997). The presence of at least two C-termini of different lengths is generally accepted for most calcium channels and is even now found in the NCBI database routinely. Perhaps the most well known are the P/Q Cav2.1 isoforms that are generated by alternative splice donor sites at the 5′ end of exon 47 resulting directly in a stop codon or insertion of five bases leading to a frame shift and an elongation of the C-terminus by 244 amino acids (Zhuchenko et al. 1997). Functionally, these isoforms and an additional one of intermediate length did not differ significantly when coexpressed with four different β subunits (Tsunemi et al. 2002). In the related N-type Cav2.2 channels of chicken, a very similar 5-bp insertion is present leading to an elongated C-terminus (Lu et al. 2001). In human N-type Cav2.2, a deletion of 187 bp in exon 46 has been described which changes the open reading frame and results in a 102-amino acid shorter translational product (Williams et al. 1992).
In all three types of Cav2 channels, there are one to two exons coding for distal parts of the C-terminus that may potentially be spliced out (exons 43 and 44 in P/Q-type Cav2.1, exon 46 in the N-type Cav2.2, and exon 45 in R-type Cav2.3). Generally, the shorter the C-terminus becomes, the greater the current amplitude and the stronger the calcium dependence of inactivation (Hell et al. 1994; Bourin et al. 1999; Krovetz et al. 2000; Sandoz et al. 2001; Soong et al. 2002; Pereverzev et al. 2002). For these isoforms, the reduction of current amplitude is most likely to be due to a reduced number of channels perhaps either by mRNA destablization or by reduction/hinderance of targeting signals in the C-terminus (Soong et al. 2002). It has been hypothesized that because the C-terminus mobility may contribute to removing the calcium–calmodulin complex from the inner mouth of the pore, a shorter C-terminus may contribute to this mobility and accelerate inactivation (Kobrinsky et al. 2003).
For l-type channels, variants with shortened C-terminus in heart and skeletal muscle Cav1.2 and Cav1.1 have long been known (Beam et al. 1992; Gerhardstein et al. 2000; Gao et al. 2001), but the truncation takes place at the protein level rather than being the result of RNA splicing. In the cardiac Cav1.2 channel, exons 40–43 show combinations of usage of an alternative splice donor site at the 3′ end of exon 40 or alternative splice acceptor sites at the 5′ ends of exons 42 and 43, inclusion of a supplementary cassette exon following exon 40 or skipping of exon 42 (Klockner et al. 1997; Soldatov et al. 1997). As in neuronal channels, the shorter the C-terminus becomes, the greater the current amplitude and the greater the calcium-dependent inactivation (Soldatov et al. 1997). Likewise, in neuronal Cav1.3 channels, replacement of exon 41 by a mutually exclusive exon 41a leads to an early stop codon truncating over 500 amino acid residues encoded by exons 42–49, which results in a twofold increase of current amplitude without change of voltage dependence of gating (Safa et al. 2001).
Alternatively spliced C-termini in T-type channels have been described for Cav3.3 (Mittman et al. 1999a; Murbartian et al. 2002). They are produced by combinations of alternative splice acceptor sites in exons 33 and 34 which shorten these exons to different lengths. One of the variants produces a frame shift leading to a premature truncation of the C-terminus. In this variant, it is the presence of the shortened exon 33 in the transcript and not the lack of the subsequent exons that produces the observed functional consequences of slowed activation, accelerated inactivation, and slowed recovery from inactivation (Murbartian et al. 2002). The presumed changes to calcium current kinetics may be expected to influence neuronal function in such a manner that the slowly inactivating variants would be liable to sustain firing patterns. Analogous splice variants are present in T-type Cav3.1-encoding genes affecting exons 34, 35 and 38 (Mittman et al. 1999b), but functional studies are still lacking.
Two-domain or three-domain truncations
Several two-domain variants consisting of domains I and II exist in l-type calcium channels, possibly a relict of the second gene duplication thought to have taken place during gene evolution. In the cardiac Cav1.2 channel either exons 17 and 18 or exon 19 is deleted leading to premature stop codons with C-terminal tails of 62 or 19 amino acid residues, respectively, found specifically in cardiac sarcoplasmic reticulum (Wielowieyski et al. 2001). The same channel in neuronal and fibroblast tissue shows two additional isoform possibilities: an alternative splice donor site at the 5′ end of exon 15 generating 75% of the transcripts and a rare 12 bp insertion at the 3′ end of exon 16 both leading to premature stop codons in or following IIS6 (Soldatov, 1992; Soldatov, 1994). In rabbit skeletal neonatal muscle, a two-domain channel generated by the splicing of IIS2 onto IVS2 and thus consisting of domain I and chimeric domain IV of the skeletal muscle Cav1.1 channel has been detected (Malouf et al. 1992). None of these have been functionally expressed as yet.
In synaptic membranes, there is evidence for an alternative isoform of the neuronal P/Q-type Cav2.1 channel consisting of domains I and II at the protein level which may be due to RNA splicing or proteolysis (Scott et al. 1998). Later, a deletion of exons 16 and 17 encoding part of IIS6 and the II–III interlinker leading to a frame-shift and generating an early stop codon has actually been described in these channels, but not functionally expressed (Soong et al. 2002). A functional hypothesis for the significance of such two-domain channels can be deduced from a study on the closely related N-type Cav2.2 channels by Raghib et al. (2001) who demonstrated that a channel consisting of domains I and II is not functional when expressed alone but only when coexpressed with a construct forming domains III and IV. However, this study demonstrates that coexpression of these isoforms with the full length channel markedly reduced protein quantity and current density mediated by the latter. Similarly, a three-domain channel which lacked the first domain and a part of the second domain by using an alternative promotor does not produce a measurable calcium current but instead, inhibits the functional expression of the full-length form (Okagaki et al. 2001). Therefore, this type of splicing may represent a simple possibility to transiently down-regulate a specific calcium channel without influencing promotor regulation.
Other domain interlinkers and transmembrane segments
The domain I–II interlinker encoded by three to five exons is important for G-protein modulation, inactivation and possibly β subunit interaction (for review see Catterall, 2000). In the trout l-type Cav1.3 channels, there is a 26 amino acid insertion encoded by an accessory cassette exon 9a for which a human homologue has not yet been described (Ramakrishnan et al. 2002); in man, however, exon 11 may be spliced out and/or exon 12 replaced by an alternative exon 12b (Safa et al. 2001). In T-type Cav3.3 channels, there is an alternative exon 9a (Mittman et al. 1999b). For the P/Q-type Cav2.1 channels, alternative splice acceptor sites at the 5′ prime end of exon 10 allow insertion of either G or VG residues (Bourinet et al. 1999; Soong et al. 2002). The isoform containing V showed slowed inactivation but enhanced G-protein inhibition and protein kinase C up-regulation compared to the isoform without V and is thought to also contribute to P-type characteristics of Cav2.1 calcium currents (Bourinet et al. 1999).
In contrast, the domain III–IV interlinker is encoded by only two to three exons and its functional significance is unclear. Even so, in the T-type Cav3.1 channel gene, an alternative splice donor site of exon 25 has been described that leads to skipping of seven amino acid residues, KAKQMA, and generates a right shift of activation and inactivation and slowing of activation kinetics (Monteil et al. 2000; Chemin et al. 2002). In the same channel, the skipping of exon 26 leads to an 18-amino-acid deletion with a left shift of inactivation and accelerated activation kinetics. Additional alternative splicing events in this loop occur in neuronal R-type Cav2.3 channels that may contain a 15 amino acid insertion following exon 29 (Takimoto et al. 1997) suggesting an important role of III–IV interlinker isoforms for excitation regulation.
Lastly, regions encoding a few transmembrane segments are alternatively spliced: IS6 of cardiac l-type Cav1.2 channels encoded by exon 8 or 8a, producing tissue-specific dihydropyridine sensitivity and putative changes of inactivation characteristics (Welling et al. 1997; Goodwin et al. 1999); IS6 of l-type Cav1.3 channels encoded by mutually exclusive exons 8a or 8b which result in a six-amino-acid difference in the pore region (Koschak et al. 2001); IIIS2 in cardiac l-type Cav1.2 channels encoded by mutually exclusive exons 21 and 22 which result in a seven-amino-acid difference influencing the voltage-dependent action of dihydropyridines (Soldatov et al. 1995); IVS3 in the same channels encoded by mutually exclusive exons 31 and 32 which work as a developmentally regulated switch coinciding with major changes in excitation (Diebold et al. 1992); and IVS3 encoded by mutually exclusive exons 31a or 31b which influence dihydropyridine sensitivity (Safa et al. 2001).
Perspective
Mutations in voltage-gated calcium channels are responsible for the pathogenesis of several hereditary ion channelopathies such as hemiplegic migraine, periodic paralysis, stationary night blindness, episodic and progressive spinocerebellar ataxias (for review of these see Lehmann-Horn & Jurkat-Rott, 1999) and, only recently reported, absence seizures (Chen et al. 2003). Given the above reviewed spectrum of functional and regulatory changes of the α1 subunits generated by alternative splicing already under normal physiological conditions, a simple mechanism to cause change of function of the voltage gated calcium channels would be to alter the splicing probability and therefore splice isoform distribution. Supporting this hypothesis is the fact that mutations in independent genes are capable of changing ion channel splicing and thus contribute to the phenotype (Charlet et al. 2002; Mankodi et al. 2002).
Neurotransmitters such as dopamine and glutamate (Berke et al. 2001) as well as anti-inflammatory (Vogiagis et al. 2001) and antipsychotic (Meshul et al. 1996) drugs have been shown to alter splicing patterns of several proteins including ligand-gated ion channels such as glutamate receptors. Even though not yet shown, it is highly likely that voltage-gated calcium channels may also be altered by drug intake, especially if there is an effect on excitability (Fields, 1998; Fass et al. 1999; Vigues et al. 1999). Therefore, the understanding of function and regulation of the splicing isoforms will be important for determining successful therapeutic strategies in the future, possibly representing an alternative to gene therapy.
Acknowledgments
This work was supported by the German Research Foundation (DFG) (JU 470/1) and the network on Excitation-contraction coupling and calcium signalling in health and disease of the IHP Program funded by the European Community.
References
- Abernethy DR, Soldatov NM. Structure-functional diversity of human l-type Ca2+ channel: perspectives for new pharmacological targets. J Pharmacol Exp Ther. 2002;300:724–728. doi: 10.1124/jpet.300.3.724. [DOI] [PubMed] [Google Scholar]
- Anderson PA, Greenberg RM. Phylogeny of ion channels: clues to structure and function. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:17–28. doi: 10.1016/s1096-4959(01)00376-1. [DOI] [PubMed] [Google Scholar]
- Barry EL, Gesek FA, Froehner SC, Friedman PA. Multiple calcium channel transcripts in rat osteosarcoma cells: selective activation of α1D isoform by parathyroid hormone. Proc Natl Acad Sci U S A. 1995;92:10914–10918. doi: 10.1073/pnas.92.24.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam KG, Adams BA, Niidome T, Numa S, Tanabe T. Function of a truncated dihydropyridine receptor as both voltage sensor and calcium channel. Nature. 1992;360:169–171. doi: 10.1038/360169a0. [DOI] [PubMed] [Google Scholar]
- Berke JD, Sgambato V, Zhu PP, Lavoie B, Vincent M, Krause M, Hyman SE. Dopamine and glutamate induce distinct striatal splice forms of Ania-6, an RNA polymerase II-associated cyclin. Neuron. 2001;32:277–287. doi: 10.1016/s0896-6273(01)00465-2. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. Voltage sensor movements. J General Physiol. 2002;120:465–473. doi: 10.1085/jgp.20028660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel β subunits. J Bioenerg Biomembr. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- Black DL, Grabowski PJ. Alternative pre-mRNA splicing and neuronal function. Prog Mol Subcell Biol. 2003;31:187–216. doi: 10.1007/978-3-662-09728-1_7. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarization-sensitive α1A antibody. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann NY Acad Sci. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. Review. [DOI] [PubMed] [Google Scholar]
- Charlet BN, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. Alternatively spliced αlG (Cav3.1) intracellular loops promote specific T-type Ca2+ channel gating properties. Biophys J. 2001;80:1238–1250. doi: 10.1016/S0006-3495(01)76100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Nargeot J, Lory P. Neuronal T-type α1H calcium channels induce neuritogenesis and expression of high-voltage-activated calcium channels in the NG108-15 cell line. J Neurosci. 2002;22:6856–6862. doi: 10.1523/JNEUROSCI.22-16-06856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, Ding K, Lo WH, Qiang B, Chan P, Shen Y, Wu X. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54:239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- Diebold RJ, Koch WJ, Ellinor PT, Wang JJ, Muthuchamy M, Wieczorek DF, Schwartz A. Mutually exclusive exon splicing of the cardiac calcium channel α1 subunit gene generates developmentally regulated isoforms in the rat heart. Proc Natl Acad Sci U S A. 1992;89:1497–1501. doi: 10.1073/pnas.89.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF, Haarmann CS, Green D, Laver DR, Board PG, Casarotto MG. Interactions between dihydropyridine receptors and ryanodine receptors in striated muscle. Prog Biophys Mol Biol. 2002;79:45–75. doi: 10.1016/s0079-6107(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Fass DM, Takimoto K, Mains RE, Levitan ES. Tonic dopamine inhibition of l-type Ca2+ channel activity reduces α1D Ca2+ channel gene expression. J Neurosci. 1999;19:3345–3352. doi: 10.1523/JNEUROSCI.19-09-03345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O, Octave JN, Christen MO, Godfraind T. Quantification of two splicing events in the l-type calcium channel α1 subunit of intestinal smooth muscle and other tissues. Eur J Biochem. 1994;222:195–202. doi: 10.1111/j.1432-1033.1994.tb18857.x. [DOI] [PubMed] [Google Scholar]
- Fields RD. Effects of ion channel activity on development of dorsal root ganglion neurons. J Neurobiol. 1998;37:158–170. doi: 10.1002/(sici)1097-4695(199810)37:1<158::aid-neu12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Eick RT, Hosey MM. C-terminal fragments of the α1C (Cav1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated α1C subunits. J Biol Chem. 2001;276:21089–21097. doi: 10.1074/jbc.M008000200. [DOI] [PubMed] [Google Scholar]
- Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C terminus of the α1C subunit of l-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Pierce RC, Kalivas PW. The monoamine neurons of the rat brain preferentially express a splice variant of αlB subunit of the N-type calcium channel. J Neurochem. 1999;73:1718–1723. doi: 10.1046/j.1471-4159.1999.731718.x. [DOI] [PubMed] [Google Scholar]
- Goodwin LO, Leeds NB, Guzowski D, Hurley IR, Pergolizzi RG, Benoff S. Identification of structural elements of the testis-specific voltage dependent calcium channel that potentially regulate its biophysical properties. Mol Hum Reprod. 1999;5:311–322. doi: 10.1093/molehr/5.4.311. [DOI] [PubMed] [Google Scholar]
- Grabsch H, Pereverzev A, Weiergraber M, Schramm M, Henry M, Vajna R, Beattie RE, Volsen SG, Klockner U, Hescheler J, Schneider T. Immunohistochemical detection of α1E voltage-gated Ca2+ channel isoforms in cerebellum, INS-1 cells, and neuroendocrine cells of the digestive system. J Histochem Cytochem. 1999;47:981–994. doi: 10.1177/002215549904700802. [DOI] [PubMed] [Google Scholar]
- Hans M, Urrutia A, Deal C, Brust PF, Stauderman K, Ellis SB, Harpold MM, Johnson EC, Williams ME. Structural elements in domain IV that influence biophysical and pharmacological properties of human α1A-containing high-voltage-activated calcium channels. Biophys J. 1999;76:1384–1400. doi: 10.1016/S0006-3495(99)77300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Elliott EM, Catterall WA. Differential phosphorylation, localization, and function of distinct α1 subunits of neuronal calcium channels. Ann NY Acad Sci. 1994;747:282–293. [PubMed] [Google Scholar]
- Hering S, Berjukow S, Sokolov S, Marksteiner R, Weiss RG, Kraus R, Timin EN. Molecular determinants of inactivation in voltage-gated Ca2+ channels. J Physiol. 2000;528:237–249. doi: 10.1111/j.1469-7793.2000.t01-1-00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Temizer D, Quertermous T. Polymerase chain reaction cloning of L-type calcium channel sequences from the heart and the brain. FEBS Lett. 1990;274:207–213. doi: 10.1016/0014-5793(90)81365-u. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Yamada Y, Fujii Y, Gonoi T, Yano H, Yasuda K, Inagaki N, Seino Y, Seino S. Molecular diversity and functional characterization of voltage-dependent calcium channels (CACN4) expressed in pancreatic β-cells. Mol Endocrinol. 1995;9:121–130. doi: 10.1210/mend.9.1.7760845. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Cooper CB, Nishioka N, Yamasaki H, Suzuki A, Jarvis SE, Akaike A, Satoh M, Zamponi GW. Identification and characterization of novel human Cav2.2 (α1B) calcium channel variants lacking the synaptic protein interaction site. J Neurosci. 2002;22:82–92. doi: 10.1523/JNEUROSCI.22-01-00082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MG, Campbell KP. γ subunit of voltage-activated calcium channels. J Biol Chem. 2003;278:21315–21318. doi: 10.1074/jbc.R300004200. [DOI] [PubMed] [Google Scholar]
- Kim DK, Catterall WA. Ca2+-dependent and -independent interactions of the isoforms of the αlA subunit of brain Ca2+ channels with presynaptic SNARE proteins. Proc Natl Acad Sci U S A. 1997;94:14782–14786. doi: 10.1073/pnas.94.26.14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockner U, Mikala G, Eisfeld J, Iles DE, Strobeck M, Mershon JL, Schwartz A, Varadi G. Properties of three COOH-terminal splice variants of a human cardiac L-type Ca2+-channel αl subunit. Am J Physiol. 1997;272:H1372–H1381. doi: 10.1152/ajpheart.1997.272.3.H1372. [DOI] [PubMed] [Google Scholar]
- Kobrinsky E, Schwartz E, Abernethy DR, Soldatov NM. Voltage-gated mobility of the Ca2+ channel cytoplasmic tails and its regulatory role. J Biol Chem. 2003;278:5021–5028. doi: 10.1074/jbc.M211254200. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Krovetz HS, Helton TD, Crews AL, Horne WA. C-Terminal alternative splicing changes the gating properties of a human spinal cord calcium channel α1A subunit. J Neurosci. 2000;20:7564–7570. doi: 10.1523/JNEUROSCI.20-20-07564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacinova L, Klugbauer N, Hofmann F. Low voltage activated calcium channels: from genes to function. General Physiol Biophys. 2000;19:121–136. [PubMed] [Google Scholar]
- Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79:1317–1371. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- Ligon B, Boyd AE, 3rd, Dunlap K. Class A calcium channel variants in pancreatic islets and their role in insulin secretion. J Biol Chem. 1998;273:13905–13911. doi: 10.1074/jbc.273.22.13905. [DOI] [PubMed] [Google Scholar]
- Lin Z, Haus S, Edgerton J, Lipscombe D. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron. 1997;18:153–166. doi: 10.1016/s0896-6273(01)80054-4. [DOI] [PubMed] [Google Scholar]
- Lin Z, Lin Y, Schorge S, Pan JQ, Beierlein M, Lipscombe D. Alternative splicing of a short cassette exon in αlB generates functionally distinct N-type calcium channels in central and peripheral neurons. J Neurosci. 1999;19:5322–5331. doi: 10.1523/JNEUROSCI.19-13-05322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Pan JQ, Gray AC. Functional diversity in neuronal voltage-gated calcium channels by alternative splicing of Cavα1. Mol Neurobiol. 2002;26:21–44. doi: 10.1385/MN:26:1:021. [DOI] [PubMed] [Google Scholar]
- Lu Q, Atkisson MS, Jarvis SE, Feng ZP, Zamponi GW, Dunlap K. Syntaxin 1A supports voltage-dependent inhibition of α1B Ca2+ channels by Gβγ in chick sensory neurons. J Neurosci. 2001;21:2949–2957. doi: 10.1523/JNEUROSCI.21-09-02949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf NN, McMahon DK, Hainsworth CN, Kay BK. A two-motif isoform of the major calcium channel subunit in skeletal muscle. Neuron. 1992;8:899–906. doi: 10.1016/0896-6273(92)90204-q. [DOI] [PubMed] [Google Scholar]
- Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, Cannon SC, Thornton CA. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell. 2002;10:35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- Maximov A, Bezprozvanny I. Synaptic targeting of N-type calcium channels in hippocampal neurons. J Neurosci. 2002;22:6939–6952. doi: 10.1523/JNEUROSCI.22-16-06939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Foehring RC, Tkatch T, Song WJ, Baranauskas G, Surmeier DJ. Properties of Q-type calcium channels in neostriatal and cortical neurons are correlated with β subunit expression. J Neurosci. 1999;19:7268–7277. doi: 10.1523/JNEUROSCI.19-17-07268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, Bunker GL, Mason JN, Allen C, Janowsky A. Effects of subchronic clozapine and haloperidol on striatal glutamatergic synapses. J Neurochem. 1996;67:1965–1973. doi: 10.1046/j.1471-4159.1996.67051965.x. [DOI] [PubMed] [Google Scholar]
- Mittman S, Guo J, Agnew WS. Structure and alternative splicing of the gene encoding αlG, a human brain T calcium channel αl subunit. Neurosci Lett. 1999a;274:143–146. doi: 10.1016/s0304-3940(99)00716-8. [DOI] [PubMed] [Google Scholar]
- Mittman S, Guo J, Emerick MC, Agnew WS. Structure and alternative splicing of the gene encoding α1I, a human brain T calcium channel αl subunit. Neurosci Lett. 1999b;269:121–124. doi: 10.1016/s0304-3940(99)00319-5. [DOI] [PubMed] [Google Scholar]
- Mochida S, Westenbroek RE, Yokoyama CT, Zhong H, Myers SJ, Scheuer T, Itoh K, Catterall WA. Requirement for the synaptic protein interaction site for reconstitution of synaptic transmission by P/Q-type calcium channels. Proc Natl Acad Sci U S A. 2003;100:2819–2824. doi: 10.1073/pnas.262787699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil A, Chemin J, Bourinet E, Mennessier G, Lory P, Nargeot J. Molecular and functional properties of the human αlG subunit that forms T-type calcium channels. J Biol Chem. 2000;275:6090–6100. doi: 10.1074/jbc.275.9.6090. [DOI] [PubMed] [Google Scholar]
- Moss FJ, Dolphin AC, Clare JJ. Human neuronal stargazin like proteins, γ2, γ3 and γ4; an investigation of their specific localization in human brain and their influence on Cav2.1 voltage-dependent calcium channels expressed in Xenopus oocytes. BMC Neurosci. 2003;4:23. doi: 10.1186/1471-2202-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murbartian J, Arias JM, Lee JH, Gomora JC, Perez-Reyes E. Alternative splicing of the rat Cav3.3 T-type calcium channel gene produces variants with distinct functional properties (1) FEBS Lett. 2002;528:272–278. doi: 10.1016/s0014-5793(02)03341-0. [DOI] [PubMed] [Google Scholar]
- Nakai J, Adams BA, Imoto K, Beam KG. Critical roles of the S3 segment and S3–S4 linker of repeat I in activation of L-type calcium channels. Proc Natl Acad Sci U S A. 1994;91:1014–1018. doi: 10.1073/pnas.91.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RD, Kuan G, Saier MH, Jr, Montal M. Modular assembly of voltage-gated channel proteins: a sequence analysis and phylogenetic study. J Mol Microbiol Biotechnol. 1999;1:281–287. [PubMed] [Google Scholar]
- Okagaki R, Izumi H, Okada T, Nagahora H, Nakajo K, Okamura Y. The maternal transcript for truncated voltage-dependent Ca2+ channels in the ascidian embryo: a potential suppressive role in Ca2+ channel expression. Dev Biol. 2001;230:258–277. doi: 10.1006/dbio.2000.0119. [DOI] [PubMed] [Google Scholar]
- Pan JQ, Lipscombe D. Alternative splicing in the cytoplasmic II–III loop of the N-type Ca channel α1B subunit: functional differences are β subunit-specific. J Neurosci. 2000;20:4769–4775. doi: 10.1523/JNEUROSCI.20-13-04769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto AA, Smith LA, Hall JC. Genomic organization and evolution of alternative exons in a Drosophila calcium channel gene. Genetics. 1997;145:1003–1013. doi: 10.1093/genetics/145.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereverzev A, Leroy J, Krieger A, Malecot CO, Hescheler J, Pfitzer G, Klockner U, Schneider T. Alternate splicing in the cytosolic II–III loop and the carboxy terminus of human E-type voltage-gated Ca2+ channels: electrophysiological characterization of isoforms. Mol Cell Neurosci. 2002;21:352–365. doi: 10.1006/mcne.2002.1179. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2002;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Wei XY, Castellano A, Birnbaumer L. Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes. J Biol Chem. 1990;265:20430–20436. [PubMed] [Google Scholar]
- Raghib A, Bertaso F, Davies A, Page KM, Meir A, Bogdanov Y, Dolphin AC. Dominant negative synthesis suppression of voltage-gated calcium channel Cav2.2 induced by truncated constructs. J Neurosci. 2001;21:8495–8504. doi: 10.1523/JNEUROSCI.21-21-08495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan NA, Green GE, Pasha R, Drescher MJ, Swanson GS, Perin PC, Lakhani RS, Ahsan SF, Hatfield JS, Khan KM, Drescher DG. Voltage-gated Ca2+ channel Cav1.3 subunit expressed in the hair cell epithelium of the sacculus of the trout Oncorhynchus mykiss: cloning and comparison across vertebrate classes. Brain Res Mol Brain Res. 2002;109:69–83. doi: 10.1016/s0169-328x(02)00522-3. [DOI] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA. Isoform-specific interaction of the α1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci U S A. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa P, Boulter J, Hales TG. Functional properties of Cav1.3 (α1D) L-type Ca2+ channel splice variants expressed by rat brain and neuroendocrine cells. J Biol Chem. 2001;276:38727–38737. doi: 10.1074/jbc.M103724200. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Westenbroek RE, Rettig J, Hell J, Catterall WA. Biochemical properties and subcellular distribution of the BI and rbA isoforms of α1A subunits of brain calcium channels. J Cell Biol. 1996;134:511–528. doi: 10.1083/jcb.134.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Bichet D, Cornet V, Mori Y, Felix R, De Waard M. Distinct properties and differential β subunit regulation of two C-terminal isoforms of the P/Q-type Ca2+-channel α1A subunit. Eur J Neurosci. 2001;14:987–997. doi: 10.1046/j.0953-816x.2001.01728.x. [DOI] [PubMed] [Google Scholar]
- Scott VE, De Waard M, Liu H, Gurnett CA, Venzke DP, Lennon VA, Campbell KP. (subunit heterogeneity in N-type Ca2+ channels. J Biol Chem. 1996;271:3207–3212. doi: 10.1074/jbc.271.6.3207. [DOI] [PubMed] [Google Scholar]
- Scott VE, Felix R, Arikkath J, Campbell KP. Evidence for a 95 kDa short form of the αlA subunit associated with the ω-conotoxin MVIIC receptor of the P/Qtype Ca2+ channels. J Neurosci. 1998;18:641–647. doi: 10.1523/JNEUROSCI.18-02-00641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Singh R. RNA–protein interactions that regulate pre-mRNA splicing. Gene Expr. 2002;10:79–92. [PMC free article] [PubMed] [Google Scholar]
- Snutch TP, Tomlinson WJ, Leonard JP, Gilbert MM. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991;7:45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- Soldatov NM. Molecular diversity of L-type Ca2+ channel transcripts in human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:4628–4632. doi: 10.1073/pnas.89.10.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatov NM. Genomic structure of human L-type Ca2+ channel. Genomics. 1994;22:77–87. doi: 10.1006/geno.1994.1347. [DOI] [PubMed] [Google Scholar]
- Soldatov NM, Bouron A, Reuter H. Different voltage-dependent inhibition by dihydropyridines of human Ca2+ channel splice variants. J Biol Chem. 1995;270:10540–10543. doi: 10.1074/jbc.270.18.10540. [DOI] [PubMed] [Google Scholar]
- Soldatov NM, Zuhlke RD, Bouron A, Reuter H. Molecular structures involved in L-type calcium channel inactivation. Role of the carboxyl-terminal region encoded by exons 40–42 in α1C subunit in the kinetics and Ca2+ dependence of inactivation. J Biol Chem. 1997;272:3560–3566. doi: 10.1074/jbc.272.6.3560. [DOI] [PubMed] [Google Scholar]
- Soong TW, DeMaria CD, Alvania RS, Zweifel LS, Liang MC, Mittman S, Agnew WS, Yue DT. Systematic identification of splice variants in human P/Q-type channel α12.1 subunits: implications for current density and Ca2+-dependent inactivation. J Neurosci. 2002;22:10142–10152. doi: 10.1523/JNEUROSCI.22-23-10142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A, Dubel SJ, Snutch TP. (1B N-type calcium channel isoforms with distinct biophysical properties. Ann N Y Acad Sci. 1999;868:118–130. doi: 10.1111/j.1749-6632.1999.tb11282.x. [DOI] [PubMed] [Google Scholar]
- Strong M, Chandy KG, Gutman GA. Molecular evolution of voltage-sensitive ion channel genes: on the origins of electrical excitability. Mol Biol Evol. 1993;10:221–242. doi: 10.1093/oxfordjournals.molbev.a039986. [DOI] [PubMed] [Google Scholar]
- Takimoto K, Li D, Nerbonne JM, Levitan ES. Distribution, splicing and glucocorticoid-induced expression of cardiac α1C and α1D voltage-gated Ca2+ channel mRNAs. J Mol Cell Cardiol. 1997;29:3035–3042. doi: 10.1006/jmcc.1997.0532. [DOI] [PubMed] [Google Scholar]
- Tsunemi T, Saegusa H, Ishikawa K, Nagayama S, Murakoshi T, Mizusawa H, Tanabe T. Novel Cav2.1 splice variants isolated from Purkinje cells do not generate P-type Ca2+ current. J Biol Chem. 2002;277:7214–7221. doi: 10.1074/jbc.M108222200. [DOI] [PubMed] [Google Scholar]
- Vajna R, Schramm M, Pereverzev A, Arnhold S, Grabsch H, Klockner U, Perez-Reyes E, Hescheler J, Schneider T. New isoform of the neuronal Ca2+ channel α1E subunit in islets of Langerhans and kidney-distribution of voltage-gated Ca2+ channel α1 subunits in cell lines and tissues. Eur J Biochem. 1998;257:274–285. doi: 10.1046/j.1432-1327.1998.2570274.x. [DOI] [PubMed] [Google Scholar]
- Vigues S, Gastaldi M, Chabret C, Massacrier A, Cau P, Valmier J. Regulation of calcium α1A subunit splice variant mRNAs in kainate-induced temporal lobe epilepsy. Neurobiol Dis. 1999;6:288–301. doi: 10.1006/nbdi.1999.0248. [DOI] [PubMed] [Google Scholar]
- Vogiagis D, Brown W, Glare EM, O'Brien PE. Rat colorectal tumours treated with a range of non-steroidal anti-inflammatory drugs show altered cyclooxygenase-2 and cyclooxygenase-1 splice variant mRNA expression levels. Carcinogenesis. 2001;22:869–874. doi: 10.1093/carcin/22.6.869. [DOI] [PubMed] [Google Scholar]
- Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels: role in channel function. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the α1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–532. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- Wielowieyski PA, Wigle JT, Salih M, Hum P, Tuana BS. Alternative splicing in intracellular loop connecting domains II and III of the α1 subunit of Cav1.2 Ca2+ channels predicts two-domain polypeptides with unique C-terminal tails. J Biol Chem. 2001;276:1398–1406. doi: 10.1074/jbc.M006868200. [DOI] [PubMed] [Google Scholar]
- Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Wolf M, Eberhart A, Glossmann H, Striessnig J, Grigorieff N. Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. J Mol Biol. 2003;332:171–182. doi: 10.1016/s0022-2836(03)00899-4. [DOI] [PubMed] [Google Scholar]
- Wu Q, Krainer A. AT-AC pre-mRNA splicing mechanisms and conservation of minor introns in voltage-gated ion channelgenes. Mol Cell Biol. 1999;19:3225–3236. doi: 10.1128/mcb.19.5.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AS, Hebert SC, Brenner BM, Lytton J. Molecular characterization and nephron distribution of a family of transcripts encoding the pore-forming subunit of Ca2+ channels in the kidney. Proc Natl Acad Sci U S A. 1992;89:10494–10498. doi: 10.1073/pnas.89.21.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the α1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]