Abstract
A rise in extracellular potassium concentration in human skeletal muscle may play an important role in development of fatigue during intense exercise. The aim of the present study was to examine the effect of intense intermittent training on muscle interstitial potassium kinetics and its relationship to the density of Na+,K+-ATPase subunits and KATP channels, as well as exercise performance, in human skeletal muscle. Six male subjects performed intense one-legged knee-extensor training for 7 weeks. On separate days the trained leg (TL) and the control leg (CL) performed a 30 min exercise period of 30 W and an incremental test to exhaustion. At frequent intervals during the exercise periods interstitial potassium ([K+]I) was determined by microdialysis, femoral arterial and venous blood samples were drawn and thigh blood flow was measured. Time to fatigue for TL was 28% longer (P < 0.05) than for CL (10.6 ± 0.7 (mean ±s.e.m.) versus 8.2 ± 0.7 min). The amounts of Na+,K+-ATPase α1 and α2 subunits were, respectively, 29.0 ± 8.4 and 15.1 ± 2.7% higher (P < 0.05) in TL than in CL, while the amounts of β1 subunits and ATP-dependent K+ (KATP) channels were the same. In CL [K+]I increased more rapidly and was higher (P < 0.05) throughout the 30 W exercise bout, as well at 60 and 70 W, compared to TL, whereas [K+]I was similar at the point of fatigue (9.9 ± 0.7 and 9.1 ± 0.5 mmol l−1, respectively). During the 30 W exercise bouts and at 70 W during the incremental exercise femoral venous potassium concentration ([K+]v) was higher (P < 0.05) in CL than in TL, but identical at exhaustion (6.2 ± 0.2 mmol l−1). Release of potassium to the blood was not different in the two legs. The present data demonstrated that intense intermittent training reduce accumulation of potassium in human skeletal muscle interstitium during exercise, probably through a larger re-uptake of potassium due to greater activity of the muscle Na+,K+-ATPase pumps. The lower accumulation of potassium in muscle interstitium in the trained leg was associated with delayed fatigue during intense exercise, supporting the hypothesis that interstitial potassium accumulation is involved in the development of fatigue.
During exercise potassium is released from the intracellular to the extracellular space of human skeletal muscle and further into the blood stream. Accumulation of potassium in the muscle interstitium has been suggested to cause fatigue during intense exercise due to impaired membrane excitability (Fitts, 1994). Furthermore, several studies with isolated muscles have shown that extracellular potassium concentrations above 8 mmol l−1 reduce contractility (Renaud & Light, 1992; Cairns et al. 1995), and that interstitial potassium concentrations in human skeletal muscle can reach considerably higher levels during intense exercise (Juel et al. 2000a; Nordsborg et al. 2003; Nielsen et al. 2003). These observations suggest that accumulation of extracellular potassium might be important for the development of fatigue in human muscle. This is also supported by findings of similar femoral venous potassium concentrations at the point of exhaustion during two one-legged knee-extensor exercise bouts, even though the exercise times differed due to various manipulations (Bangsbo et al. 1992, 1996). Therefore, a reduction in release of potassium from the muscle cells and/or an increase in removal of potassium from the interstitium may delay the development of fatigue.
It is well-known that exercise training increases performance. However, it is unclear whether the accumulation of potassium in muscle interstitium during exercise is changed by training. McKenna et al. (1997) found that the femoral arterial–venous potassium difference during intense cycle exercise was the same before and after training, suggesting that the release of potassium to the blood stream was not changed by the training. Elevated performance after training could be due to a higher re-uptake of potassium by contracting muscle. Various types of exercise training have been shown to increase the amount of Na+,K+-ATPase measured by vanadate-facilitated ouabain binding. It is, however, unclear to what extent training affects Na+,K+-ATPase subunits and how such changes may influence muscle interstitium potassium kinetics and performance. During muscle activity, potassium is released to the interstitium via voltage-dependent K+ channels activated during propagation of action potentials. Potassium may also be released through the KATP channels during exercise. KATP channels have been identified in skeletal muscle from both frogs (Davies, 1990) and humans (Nielsen et al. 2003). It has been demonstrated that KATP channels are inhibited by ATP (Spruce et al. 1985) and that this effect is reversed by lowering pH (Davies, 1990). Furthermore, it was suggested that KATP channels are activated only in metabolically exhausted muscle fibres (Castle & Kaylett, 1987) and that the activity of the channels contributes to the decline in force during fatigue in frog muscle (Light et al. 1994). It is, however, not clear whether training induces changes in the amount of KATP channels.
Thus, the aim of the study was to examine the effect of intense intermittent training on muscle interstitial potassium kinetics during exercise and its relationship to the content of Na+,K+-ATPase subunits and KATP channels, as well as its relationship to performance during intense exercise in human skeletal muscle.
Methods
Subjects
Six healthy habitually active male subjects participated in the study. Age, height, weight and pulmonary maximal O2 uptake O2max were 25.3 ± 1.2 years, 185.0 ± 1.6 cm, 82.8 ± 4.8 kg and 50.2 ± 0.5 ml O2 min−1 kg−1, respectively (mean ±s.e.m.). The subjects were fully informed of any risks and discomforts associated with the experiment before giving their written informed consent to participate. The study conformed to the code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Ethics Committee of Copenhagen and Frederiksberg communities.
Experimental design
The subjects performed intense intermittent knee-extensor exercise training for 7 weeks with one leg (TL), while the other leg served as control (CL). Preliminary testing was performed to ensure that the subjects had no differences in the work capacity of the two legs prior to the training regime (see below). The leg for training was selected randomly. Muscle biopsies were obtained from each leg and muscle mass of each leg was determined before and after the training period. After the training period two identical main experiments were performed, during which femoral arterial and venous blood, as well as microdialysis samples, were collected before, during and after exercise at two submaximal intensities and an incremental exercise test.
Exercise model
Subjects performed dynamic one-legged knee-extensor exercise in the supine position on an ergometer that permitted the exercise to be confined to the quadriceps muscle (Andersen et al. 1985). Before the preliminary testing procedures the subjects practiced the exercise on more than three separate occasions. Visual and oral feedback was given to train the subjects to maintain a constant kicking frequency of 60 kicks min−1 and to avoid involvement of the hamstring muscles.
Preliminary testing
Each subject underwent preliminary exercise testing consisting of an incremental bicycle ergometer exercise in which maximal pulmonary oxygen uptake was determined using a gas analyser (Medical Graphics, Saint Paul, Minneapolis, USA) and two or more incremental knee-extensor exercise tests for each leg. The incremental knee-extensor exercise test consisted of 2 min at 40 W, after which the load was increased by 10 W every 2 min to exhaustion. The exercise test was terminated when the kicking frequency reached values below 55 r.p.m and the exercise time was recorded as the test performance. A strain gauge attached to the ergometer lever arm recorded frequency and force of the kicking. Subjects who had pulmonary peak oxygen uptakes O2max ranging from 45 to 55 ml O2 min−1 kg−1 and showed less than 15% leg-to-leg variability in knee-extensor exercise performance were included in the study. Three subjects trained with the right leg and three subjects trained with the left leg. The quadriceps muscle mass was 2.44 (range, 2.24–3.05) kg in TL and 2.48 (2.29–2.92) kg in CL prior to the training period. The incremental exercise test performance was 7.65 (5.49–9.22) and 7.80 (5.31–10.16) min in TL and CL, respectively.
Training
Training lasted 7 (6.0–7.8) weeks. One training session consisted of 5 min of warm up at 10 W, 5 min of rest and 15 work intervals at an intensity of ∼150% of thigh O2max each lasting 1 min and separated by 3 min of rest. The load was selected to exhaust the subjects at the end of the last few bouts of each training session. The initial training load was 92 ± 3 (80–100) W. Every second week the training load was adjusted based on the result of an incremental exercise test finished at least 20 min prior to a training session. Training frequency was 3 times per week for weeks 1–2, 4 times per week for weeks 3–4, and 5 times per week for the last weeks. The total number of training sessions was 29 ± 3 (24–32).
Main experiment
The same main experiment (Fig. 1) was performed with TL and CL in randomized order on 2 days separated by at least 1 week. The experiment for TL was performed 2–3 days after the last training session. The subjects were asked to avoid strenuous physical activity and alcohol for 48 h, as well as caffeine and tobacco for 12 h, prior to the main experiment. On the morning of the experiment, subjects arrived at the laboratory after a light breakfast. After 30 min of rest in the supine position, a catheter was placed, under local anaesthesia using the Seldinger technique, in the femoral vein of the experimental leg with the tip positioned approximately 2 cm proximal to the inguinal ligament. This catheter was used for femoral venous blood sampling. A thermistor (Edslab, T.D. Probe, 94-030-2.5F, Baxter A/S, Allerød, Denmark) for measurement of femoral venous blood flow by thermodilution (Andersen & Saltin, 1985) was placed through the catheter and advanced 8–10 cm proximal to the tip of the catheter. A second catheter was inserted in the femoral artery of the resting leg. This catheter was used for arterial blood sampling. After insertion of the catheters, the subjects were moved to the one-legged ergometer model after which five microdialysis probes were inserted (for interstitial potassium determinations; see later). Then an incision to the lateralis muscle of the quadriceps group was made under local anaesthesia (1 ml of lidocaine (Xylocain); 20 mg ml−1 without adrenaline) for later extraction of resting and post-exercise muscle samples using the needle biopsy technique with suction (Bergström, 1962).
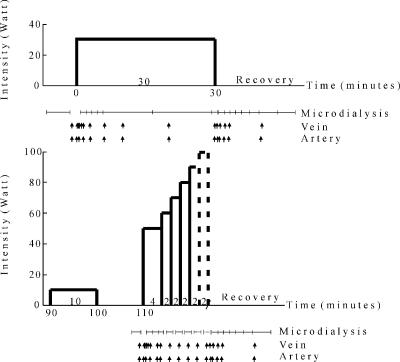
Figure 1. Protocol for the main experiment.
After a 7 week training period with one leg, subjects exercised in a one-legged knee extension ergometer with the control leg and trained leg on two different occasions. They performed a 30 min exercise period at an intensity of 30 W, followed by 1 h of rest. Then, they performed a 10 min exercise period at 10 W, followed by a 10 min rest period and an incremental exercise test consisting of 4 min at 50 W, then 2 min at 60 W, after which the load was increased by 10 W every 2 min to exhaustion. Fatigue was determined as the time at which the kicking frequency fell below 55 r.p.m. The work intensity at which the subjects fatigued varied, which is indicated by dashed vertical lines. Microdialysis samples and femoral venous and artery samples were obtained frequently and blood flow was measured prior to each blood sampling.
After more than 1 h of rest, the experimental leg carried out an exercise protocol consisting of 30 min of submaximal exercise at 30 W, followed by 1 h of rest. Thereafter exercise was performed at 10 W, followed by a 10 min rest period and an incremental exercise test consisting of 4 min at 50 W, then 2 min at 60 W, after which the load was increased by 10 W every 2 min to exhaustion (Fig. 1). The point of fatigue was determined as the time when the kicking frequency fell below 55 r.p.m.
Dialysate from the microdialysis probes and femoral arterial and venous blood samples were collected, and femoral venous blood flow was measured before each exercise bout, frequently during the initial phase and repeatedly throughout the rest of the bout (Fig. 1). These determinations were also performed repeatedly during the recovery period from the 30 W and the incremental exercise bouts (Fig. 1). An occlusion cuff placed below the knee was inflated to 250 mmHg 30 s prior to each exercise bout and remained inflated throughout exercise in order to avoid contribution of blood from the lower leg.
A biopsy sample from m. vastus lateralis was taken prior to and immediately after both the 30 W and incremental exercise tests. In addition, a muscle biopsy sample was taken from both legs before the first training session.
Microdialysis
Local anaesthesia (1 ml of lidocaine, 20 mg ml−1 without adrenaline) was administered to the skin and subcutaneous tissue. Then five microdialysis probes were inserted into the vastus lateralis muscle of the quadriceps femoris group in a direction estimated to be parallel with the muscle fibre orientation. Membrane length, outer diameter and molecular cut-off of the probes (CMA 60, CMA Microdialysis, Sweden) were 30 mm, 0.52 mm and 20 000 Da, respectively. The positions of all probes were marked, photographed and determined by distance and position from a line between the patella and the anterior superior iliac spine. The probes were placed in similar positions in TL and CL.
Approximately 1 h after insertion, the probes were perfused using a microdialysis pump (CMA 102, CMA Microdialysis, Sweden) at a rate of 5 μl min−1. The perfusate was an isotonic Ringer acetate solution (Pharmacia, Stockholm Sweden) in which 3 mmol−1 glucose, 1 mmol−1 lactate and 201Tl (thallous [201Tl] chloride injection, TDS1P, Nycomed Amersham, Buckinghamshire, UK) were dissolved, resulting in an activity of <7000 Bq ml−1. The probes were perfused >30 min before the experimental collection of microdialysate. Collection tubes were weighed before and after sampling, to determine the actual flow rate. Only probes with a calculated continuous perfusion rate in the range 4.5–5.5 μl min−1 were used.
During the first 5 min of the 30 W exercise bout, four microdialysis samples were collected (1.25 min per sample); during the next 23.5 min, two samples were collected (11.75 min per sample), and the last sample was collected over 1.25 min. During the first 5 min after the 30 W exercise bout, four samples were collected (1.25 min); for the next 4 min two samples were collected, and then two samples was collected during the following 8 min. During the incremental test, the times taken to collect microdialysis samples were 1.25 min for the first exercise load and 1.42 min for the following loads, starting 0.33 min into the increment (Fig. 1). Sample time for the last sample during the incremental test was shorter if the subject became exhausted before sampling was completed. The time for collection of the last sample was the same for TL and CL (1.19 ± 0.20 versus 1.17 ± 0.17 min, respectively). During recovery from the incremental exercise test, the time for collecting microdialysis samples was the same as during recovery from 30 W. A delay of 40 s when collecting microdialysis samples due to the volume of the outlet tubes is taken into account when the data are presented.
Five microlitres of each sample was transferred to a tube (NUNC, Minisorp 75 × 12 mm) containing 1 ml of a solution of 3 mmol−1 lithium chloride in distilled H2O, which was counted on a Packard autogamma counter and then stored at –20°C. The samples were analysed for potassium on a flame photometer (FLM3, Radiometer, Copenhagen, Denmark), running CL and TL samples from one subject on the same day. The potassium concentration in the perfusate was also measured.
Muscle mass
The mass of the quadriceps femoris muscle group was estimated using measurements of thigh length, three circumferences of the thigh and skin-fold thickness, and a correction based on a comparison between anthropometric measurements and MRI-scan determinations (ratio 1: 0.8; Hellsten et al. 1999).
Blood analysis
All blood samples were immediately placed in ice-cold water until rapid centrifugation at 20 000 g for 30 s. Thereafter the plasma was collected and stored at –80°C until analysed for potassium in triplicate using the flame photometer (FLM3) with lithium as the internal standard. Haematocrit was determined from part of the sample using an ABL 600 analyser (Radiometer).
Biopsies
One part of the muscle biopsy sample was immediately frozen in liquid nitrogen and stored at –80°C for subsequent analysis. The frozen muscle samples were weighed before and after freeze-drying to determine water content. The freeze-dried sample was dissected free of blood, fat and connective tissue and about 1 mg dry weight of tissue was homogenized in 120 μl non-buffering solution containing 145 mmol l−1 KCl, 10 mmol l−1 NaCl and 5 mmol l−1 iodoacetic acid and used for measurements of muscle pH using a small glass electrode (Radiometer GK2801). The muscle biopsy sample obtained at rest before the training and before the 30 W exercise was used for determination of Na+,K+-ATPase subunits and KATP channels.
Na+,K+-ATPase subunits and KATP channels
A part of the muscle samples (30 mg) obtained at rest was homogenized in 1 ml sucrose buffer (mmol l−1: 250 sucrose, 30 Hepes, 2 EGTA, 40 NaCl, 2 phenylmethylsulphonyl fluoride, pH 7.4) with a Polytron 2100 and centrifuged at 1000 g for 5 min. The supernatant was spun at 190 000 g for 90 min at 4°C. The pellet was resuspended in Tris-SDS (mmol l−1: 10 Tris, 4% SDS, 1 EDTA, 2 phenylmethylsulphonyl fluoride, pH 7.4). Homogenate protein content was determined with a BSA standard (DC protein assay, Bio-Rad). Samples (10 μg protein) were run on SDS-PAGE (8–18% gradient gel) and electroblotted to a polyvinylidene difluoride membrane (Millipore Immobilon-P). Membranes were blocked in Tris-SDS buffer (containing 5% BSA + 2.5% dry milk + 0.1% Tween-20), washed, and incubated overnight at 4°C in primary antibodies diluted 1: 1000 in TS buffer containing 5% BSA + 0.1% Tween-20. Subsequently the membranes were washed twice in TS buffer and incubated in secondary antibodies (horseradish peroxidase goat antirabbit immunoglobulins, DAKO) 1: 1000 in TS buffer containing 5% BSA. After washing (TS buffer + 0.05% Tween-20) membranes were treated with enhanced chemiluminescent reagents (Amersham) and visualized on film. The quantification of the proteins was performed by scanning the film and by analysing band intensities (arbitrary units) with Sigma Gel software.
Specific α1 antibody (α6F) were obtained from Iowa Hybridoma Bank. Specific α2 antibodies produced from the rat sequence, were obtained from Upstate Biotechnology (Lake Placid, NY, USA). A polyclonal rabbit antiβ1-antibody was raised against the recombinant extracellular part (AA 58–302) of β1 from pig. The antigens were produced with a histidine tag in Escherichia coli. Antibodies were affinity purified before use in Western blots. Dr P. Amstrup Pedersen, University of Copenhagen, Denmark, kindly provided the β1 antibodies. The anti-Kir 6.2 (G-16, catalogue no. 11228) was from Santa Cruz Biotechnology, California, USA.
Calculations
The relative loss (RL) of 201Tl was calculated as RLTl= perfusate activity – perfusate activity/dialysate activity. The interstitial K+ concentration ([K+]I) was calculated from the dialysate samples, assuming that fractional loss of 201Tl from the perfusate equals fractional gain of K+ in the perfusate (Juel et al. 2000a):
Mean transit time correction
Corrections were made for the blood transit time from the capillaries to the collection points in the femoral artery and vein. This correction has significant importance in the initial phase of exercise, where blood flow rises progressively. Artery–venous mean transit times of 11, 7, 6 and 6 s after 5, 25, 65, 152 s were used for all exercise bouts, with 1/3 of the time representing the time from artery to capillary (Bangsbo et al. 2000).
Thigh potassium release
Potassium release (K+release) from the thigh was calculated by multiplying the venous (v)– arterial (a) potassium difference (v-adiff) by leg plasma flow, taking into account changes in haematocrit (Hct):
A continuous blood flow curve was constructed for each subject using a linear connection of consecutive data points in order to obtain time-matched values for blood flow and blood measurements. Total potassium release was calculated as the sum of the multiplied average potassium release between two measurements of release and the time between these two points.
Potassium accumulated in the interstitium was calculated by multiplying [K+]I by muscle volume, muscle water content and relative interstitial water volume, assumed to be 12 and 15% of muscle water at rest and after exhausting exercise, respectively (Sjøgaard et al. 1985). The total amount of intracellular potassium at exhaustion ([K+]i.exh) was calculated by subtracting the potassium released to the blood () and the potassium accumulated in the interstitium () at exhaustion from the amount of intracellular potassium at rest. Then, the intracellular potassium concentration at exhaustion was calculated by dividing the amount of intracellular potassium at exhaustion by muscle volume (MV), muscle water content (MW) and relative intracellular water content at exhaustion:
Statistics
Values are mean ±s.e.m. An average value for the microdialysis probes was calculated for each subject, and an overall mean was determined from these individual means. Differences between trained and untrained leg (TL versus CL) were tested using two-way repeated measures ANOVA. Differences within each leg were tested using a one-way repeated measures ANOVA. Since the subjects fatigued at different times the number of measurements was reduced towards the end of exercise. Only determinations where n was equal to or higher than five were analysed statistically. To test the time point with n= 5 the subject missing was excluded from all other time points. When running statistics including all subjects (n= 6), the time point with n= 5 was excluded. When a significant difference was found, a Student-Newman-Keuls post hoc test was performed to locate the difference. For testing differences in time to fatigue between TL and CL, as well as muscle variables before and after training, a paired t test was used. A significance level of 0.05 was chosen.
Results
Muscle mass
Before training the muscle mass of CL and TL was the same. After training no change was observed for CL (2.48 ± 0.10 versus 2.47 ± 0.09 kg), whereas the mass of the quadriceps of TL was 5%(P < 0.01) higher than before training (2.44 ± 0.13 versus 2.55 ± 0.13 kg).
Na+,K+-ATPase subunits and KATP channels
For TL, the levels of α1 and α2 Na+,K+-ATPase isoforms were 29.0 ± 8.4 and 15.1 ± 2.7% higher (P < 0.05) after training than before training whereas there was no significant increase in the amount of β1 (16.0 ± 13.0%). The level of the KATP channel subunit Kir6.2 was not significantly increased (10.7 ± 7.0%) in TL during the training period.
Performance
The mean power output during the final training session was 16% higher (P < 0.05) than during the first training session (106.3 ± 3.7 and 92.0 ± 3.3 W, respectively). Time to fatigue during the incremental test of TL was 27% longer (P < 0.05) after 6 weeks of training compared to before (9.68 ± 0.68 and 7.65 ± 0.47 min, respectively). In the main experiment time to fatigue for TL was 28% longer (P < 0.05) than for CL (10.56 ± 0.65 and 8.23 ± 0.70 min, respectively).
Muscle pH during exercise
Muscle pH values at rest were 7.14 ± 0.03 and 7.16 ± 0.02 in TL and CL, respectively. Muscle pH did not change during the 30 W exercise bout. During the incremental exercise test, pH declined (P < 0.001) from 7.16 ± 0.04 to 6.69 ± 0.04 in CL and from 7.15 ± 0.03 to 6.82 ± 0.05 in TL, with no significant differences between TL and CL.
Potassium kinetics during and after exercise
Interstitial potassium
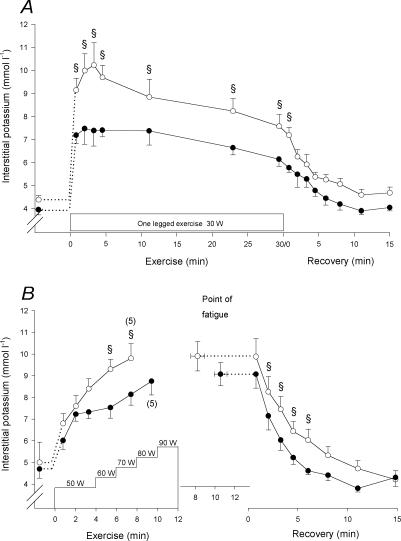
[K+]I increased more rapidly and was higher (P < 0.05) throughout the 30 W exercise bout in CL than in TL (Fig. 2A). In the initial 0.8 min of recovery from the 30 W exercise bout [K+]I remained higher (P < 0.05) in CL than in TL. After exercise [K+]I reached resting level after 4.5 min in both CL and TL.
Figure 2. Muscle interstitial potassium concentration.
Muscle interstitial potassium concentration measured by microdialysis in m. vastus lateralis of the control (○) and trained (•) legs before, during and after the 30 W (A) and the incremental (B) exercise tests. Due to early fatigue in one individual, n= 5 for the last measurement of the incremental exercise test. The value at the point of fatigue is extended by a dashed horizontal line to represent the start of recovery. § CL significantly different from TL (P < 0.05). Values are means ±s.e.m.
During the 60 and 70 W exercise in the incremental test, [K+]I in CL was higher (P < 0.05) than in TL (Fig. 2B), whereas no difference existed at the point of fatigue (9.9 ± 0.7 and 9.1 ± 0.5 mmol l−1 in CL and TL, respectively). No difference between CL and TL was observed 0.8 min into recovery, but after 2.2 min of recovery [K+]I became lower (P < 0.05) in TL than in CL. In CL, [K+]I reached resting level later (P < 0.05) than in TL (6.0 ± 3.0 versus 4.5 ± 3.1 min).
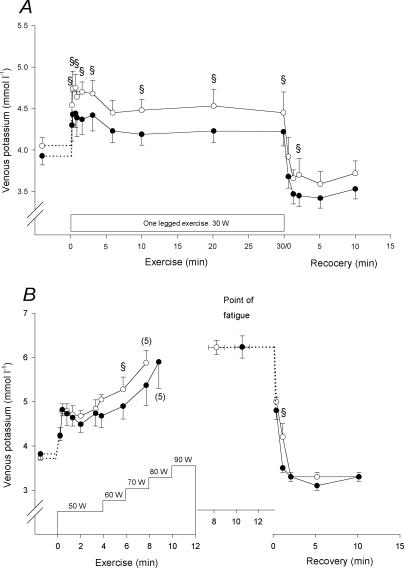
Femoral venous potassium
After 27 s and throughout the 30 W exercise bout (except after 6 min), the femoral venous potassium concentration ([K+]v) was higher (P < 0.05) in CL than in TL (Fig. 3A). After exercise [K+]v declined rapidly and in TL it was lower (P < 0.01) than at rest after 1 min (Fig. 3A). After 2.4 min of recovery, [K+]v was higher (P < 0.05) in CL than in TL.
Figure 3. Femoral venous potassium concentration.
Femoral venous potassium concentrations before, during and after the 30 W (A) and the incremental (B) exercise tests with the control (○) and trained (•) legs. Due to early fatigue in one individual, n= 5 for the last measurement of the incremental exercise. The value at the point of fatigue is extended by a dashed horizontal line to represent the start of recovery. § CL significantlt different from TL (P < 0.05). Values are means ±s.e.m.
At the end of the 70 W exercise during the incremental test, [K+]v was higher (P < 0.05) in CL than in TL, but was the same at exhaustion (6.2 ± 0.2 and 6.2 ± 0.2 mmol l−1 in CL and TL, respectively; Fig. 3B). After 1.2 min of recovery from the incremental test, [K+]v in CL was higher (P < 0.05) than in TL.
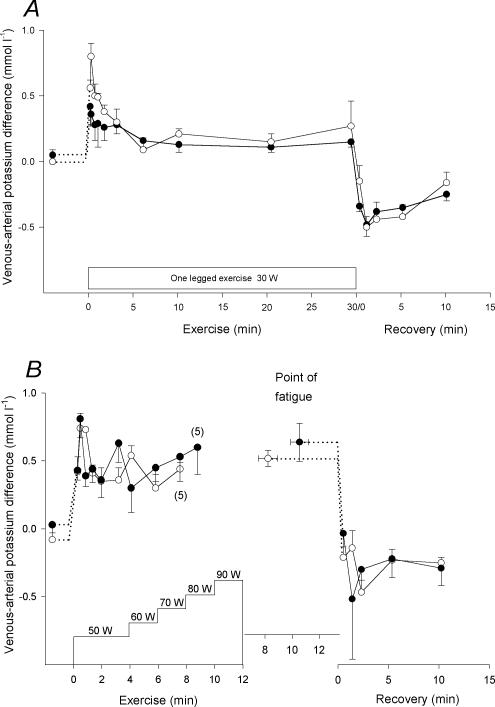
Venous–arterial difference
No potassium venous–arterial difference (v-adiff) was observed at rest, but after 20 s of the 30 W exercise bout it had increased (P < 0.05) to 0.6 ± 0.1 mmol l−1 in CL and 0.4 ± 0.2 mmol l−1 in TL, with no significant difference between the two legs (Fig. 4A). In the remaining part of exercise it decreased (P < 0.05) to 0.1 ± 0.0 mmol l−1. The potassium v-adiff after 1.3 min of recovery from the 30 W exercise bout was –0.5 ± 0.1 mmol l−1 in both CL and TL, and it remained negative during the first 10 min of recovery (Fig. 4A).
Figure 4. Femoral venous–arterial potassium difference.
Femoral venous–arterial potassium differences before, during and after the 30 W (A) and the incremental (B) exercise tests with the control (○) and trained (•) legs. Due to early fatigue in one individual, n= 5 for the last measurement of the incremental exercise. The value at the point of fatigue is extended by a dashed horizontal line to represent the start of recovery. § CL significantly different from TL (P < 0.05). Values are means ±s.e.m.
After 40 s of the incremental exercise test, the potassium v-adiff had increased (P < 0.005) to a peak value of 0.8 ± 0.1 mmol l−1 in both CL and TL (Fig. 4B). During the remaining part of the exercise, it fluctuated insignificantly between 0.5 and 0.8 mmol l−1 in both CL and TL, with no significant differences between the two legs. In recovery, the potassium v-adiff decreased (P < 0.001) promptly, reaching –0.1 ± 0.1 mmol l−1 and –0.2 ± 0.2 mmol l−1 in CL and TL, respectively, 1 min after exercise, and remained negative (P < 0.005) during the first 10 min of recovery (Fig. 4B).
Potassium gradient
At the onset of the 30 W exercise bout, the difference between muscle interstitium potassium and artery potassium (potassium-gradientI-A) increased (P < 0.01) rapidly. Throughout the 30 W exercise bout, the potassium-gradientI-A was higher (P < 0.05) in CL than TL (Fig. 5A). In recovery, the potassium-gradientI-A remained higher (P < 0.05) in CL than in TL after 50 s, but thereafter no differences existed.
Figure 5. Muscle interstitial-arterial potassium gradient.
Muscle interstitial-arterial potassium differences before, during and after the 30 W (A) and the incremental (B) exercise tests with the control (○) and trained (•) legs. Due to early fatigue in one individual, n= 5 for the last measurement of the incremental exercise. The value at the point of fatigue is extended by a dashed horizontal line to represent the start of recovery. § CL significantly different from TL (P < 0.05). Values are means ±s.e.m.
The potassium-gradient I-A values in CL and TL rose (P < 0.01) within the first 2 min of the incremental exercise test to 3.3 ± 0.5 versus 3.1 ± 0.1 mmol l−1, respectively. The potassium-gradient I-A continued to increase in CL and after 6 min it was higher (P < 0.05) in CL than in TL (Fig. 5B). In recovery, the potassium-gradient I-A decreased and after 10 min of recovery in CL and 4.5 min in TL the potassium-gradient I-A was no longer different from pre-exercise levels.
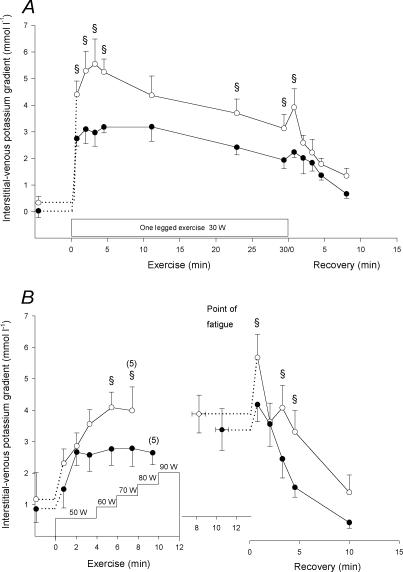
The potassium gradient between interstitium and vein (potassium-gradientI–V) also increased (P < 0.01) rapidly at the onset of the 30 W exercise bout. It was higher (P < 0.05) in CL than in TL throughout the 30 W exercise bout and during the first 50 s of recovery (Fig. 6A). Within the first 2 min of the incremental exercise test, potassium-gradientI–V values rose (P < 0.05) to 2.9 ± 0.4 and 2.7 ± 0.4 mmol l−1 in CL and in TL, respectively. The potassium-gradientI–V continued to increase in CL and after 6 min it was higher (P < 0.05) in CL than in TL (Fig. 6B). In recovery, the potassium-gradientI–V decreased and after 10 min of recovery in CL and 4.5 min of recovery in TL it was no longer different from pre-exercise values.
Figure 6. Muscle interstitial-femoral venous potassium gradient.
Muscle interstitial-femoral venous potassium difference before, during and after the 30 W (A) and the incremental (B) exercise tests with the control (○) and trained (•) legs. Due to early fatigue in one individual, n= 5 for the last measurement of the incremental exercise. The value at the point of fatigue is extended by a dashed horizontal line to represent the start of recovery. § CL significantly different from TL (P < 0.05). Values are means ±s.e.m.
Potassium release
During the 30 W exercise bout, net potassium release to the blood increased (P < 0.05) immediately and peaked after 1.1 and 0.7 min of exercise in CL and in TL, respectively, the peak values being 0.8 ± 0.1 and 0.6 ± 0.2 mmol min−1, respectively (Fig. 7A). No difference in potassium release between CL and TL was observed. The total amount of potassium released to the blood during exercise was 10.5 ± 3.3 and 7.8 ± 2.1 mmol in CL and TL, respectively, and the amount of potassium accumulated in the interstitium was 1.3 ± 0.2 and 1.1 ± 0.1 mmol, respectively. During the first 10 min of recovery the net uptake of potassium was 1.4 ± 0.5 and 1.4 ± 0.2 mmol in CL and TL, respectively.
Figure 7. Potassium release.
Potassium release before, during and after the 30 W (A) and the incremental (B) exercise tests with the control (○) and trained (•) legs. Due to early fatigue in one individual, n= 5 for the last measurement of the incremental exercise. The value at the point of fatigue is extended by a dashed horizontal line to represent the start of recovery. § CL significantly different from TL (P < 0.05). Values are means ±s.e.m.
The net release of potassium during the incremental exercise test was similar in CL and TL (Fig. 7B). The release of potassium was larger (P < 0.01) than at rest after 0.8 min in CL (1.8 ± 0.5 mmol min−1) and after 0.4 min in TL (1.8 ± 0.5 mmol min−1). The rates of potassium release at exhaustion were 1.5 ± 0.4 and 2.5 ± 0.6 mmol min−1 in CL and in TL, respectively (Fig. 7B). Total amounts of potassium released to the blood during exercise were 11.8 ± 1.6 mmol in CL and 13.1 ± 1.8 mmol in TL, while the amounts of potassium accumulated in the interstitium were 2.3 ± 0.4 and 2.3 ± 0.3 mmol, respectively. Together this corresponds to decreases in intracellular potassium of 21.1 ± 5.5 mmol l−1 in CL and 29.0 ± 5.5 mmol l−1 in TL when correcting for fluid movements. During recovery, a net potassium uptake (P < 0.001) was observed, and the total uptake during the first 10 min was 5.8 ± 2.8 mmol in CL and 4.5 ± 1.7 mmol in TL.
Thigh blood flow
At rest, thigh blood flow was 0.49 ± 0.06 l min−1 in CL and 0.55 ± 0.09 l min−1 in TL. Blood flow in TL was 11–15% higher (P < 0.05) than in CL from 0.6 to 3.5 min of the 30 W exercise bout, after which no differences were observed. Flow reached 3.95 ± 0.28 and 3.85 ± 0.27 l min−1 at the end of exercise in CL and TL, respectively, with no significant differences between legs to incremental exercise blood flow was 0.58 ± 0.09 l min−1 in CL and 0.38 ± 0.05 l min−1 in TL, respectively. During the 50 W exercise, blood flow was higher (P < 0.05) in TL than CL, reaching 4.98 ± 0.35 and 5.73 ± 0.53 l min−1 in CL and TL, respectively, whereas during the rest of the incremental test no differences in blood flow were observed between the two legs.
Discussion
The major findings of the present study were that intense intermittent training reduced the accumulation of potassium in the muscle interstitium during exercise, which was associated with an elevated performance level (delayed development of fatigue), and that at the point of fatigue muscle interstitial potassium was the same in the untrained and trained legs. Furthermore, the release of potassium from muscle to the blood stream during exercise was the same in both legs, although the concentration gradient between interstitium and blood was lowered after training. These findings suggest that re-uptake of potassium by contracting muscle cells is elevated after training, probably due to an increase in Na+,K+-ATPase activity as evidenced by the finding of higher amounts of the Na+,K+-ATPase α1 and α2 proteins after training. The present observations also support the hypothesis that accumulation of interstitial potassium is important for the development of fatigue during intense exercise.
Interstitial potassium kinetics during and after exercise
Throughout the 30 W exercise bout and during the later part of the incremental exercise test, [K+]I was lower in the trained leg than in the untrained leg. This could have been accomplished in three ways: (1) the release of potassium from the contracting muscle cells was lowered; (2) the re-uptake of potassium during exercise was elevated; (3) the release of potassium to the blood was higher after training.
It is difficult to evaluate whether the efflux of potassium from the muscle cells had changed with training. Release of potassium from the contracting muscle cells does occur through the delayed rectifier K+ channel during the repolarization phase of the action potential (Hocherman & Bezanilla, 1996). A difference in potassium release may have existed if more fast twitch fibres were recruited in the untrained leg, since release of potassium from fast twitch fibres has been suggested to be higher than from slow twitch fibres in rats. Nagaoka et al. (1994) observed that the change in intracellular K+ was faster in rat extensor digitorum longus muscle than soleus muscle, even when the Na+,K+-ATPase was inhibited with ouabain. In the present study there was no difference in the fraction of slow twitch and fast twitch fibres in the two legs (Juel et al. 2003), but a difference in fibre type recruitment may have existed. Vøllestad & Blom (1985) showed that during bicycling at 70 r.p.m. at 43% of O2max for 60 min few fast twitch fibres had been active and that the fraction of active fast twitch fibres was elevated with increasing work intensity. The 30 W exercise bout represented 48 ± 3 and 43 ± 2% of leg O2max for CL and TL, respectively. Calculated from the data of Vøllestad & Blom (1985), the relative number of active fast twitch fibres would be about 10 and 5% in CL and TL, respectively. Thus, the difference in fibre type recruitment between the two legs was probably of minor importance for potassium released from the muscle in the present study.
Potassium may also have been released through the KATP channels during exercise. There were, however, no changes in the amount of KATP channel protein. It is well known that the opening probability of KATP channels is increased by lowering pH (Davies, 1990), but the muscle pH at the end of exercise was not different between the untrained and trained legs. Taking this evidence together, it appears unlikely that the lowered accumulation of potassium in muscle interstitium after training can be explained by a reduced release of potassium from the contracting muscle cells. Therefore, and since the release of potassium to the blood during exercise was the same in the untrained and trained legs, it is reasonable to suggest that the uptake of potassium by the contracting muscle cells was elevated after training. It appears also to be the case during recovery, since the interstitial potassium concentration after exhaustive exercise was lower in the trained than in the untrained leg, whereas uptake of potassium from the blood to the muscle interstitium was the same.
A greater uptake of potassium during and after exercise may have been caused by an increase in Na+,K+-ATPase activity, since the relative amounts of α1 and α2 isoforms were significantly higher (29 and 15%, respectively) after the training period. This is in accordance with other studies, where a 10–20% increase in Na+,K+-ATPase content has been reported with different types of training (Green et al. 1993; Evertsen et al. 1997). In these studies, the total content of Na+,K+-ATPase was measured using vanadate-facilitated ouabain, which binds to the α subunits (Clausen et al. 1987; Kjeldsen et al. 1988). A functional Na+,K+-ATPase in human skeletal muscle consists of one β subunit in connection with either one α1 unit or one α2 unit (see McDonough et al. 2002). The isoforms are primarily located in the sarcolemma, though the α2 isoform is also located intracellularly (Hundal et al. 1994). Furthermore, it has been proposed that the α2 isoform can be translocated from the intracellular stores to the sarcolemma in response to exercise in humans (Juel et al. 2000b). These observations could lead to the suggestion that the higher level of Na+,K+-ATPase activity during exercise after training was partly due to a greater translocation of α2 isoform as a result of an increase in the amount of this isoform. However, further studies are needed to clarify this hypothesis.
There may be other factors that influenced the Na+,K+-ATPase activity during exercise. The activity is elevated by catecholamines (Clausen & Flatman, 1977) and by an elevated level of intracellular Na+ (Everts & Clausen, 1992). However, the catecholamines are not likely to have had a significant effect during the 30 W exercise bout, since during one-legged knee-extensor exercise at a power output of 30 W they are not elevated (Bangsbo et al. 1997), and in the present study a marked difference in interstitial potassium concentration was observed at 30 W between the untrained and trained legs. As discussed previously, a larger part of fast twitch fibres in CL may have been active during the 30 W exercise bout. This may have lead to a decrease in Na+-induced Na+,K+-ATPase activity during exercise. However, no difference in interstitial sodium concentration was observed between the untrained and trained legs (data not shown), suggesting that the difference in Na+ induced Na+,K+-ATPase activity during exercise was too small to explain the large difference in [K+]I.
After the incremental exercise test, there was a significant uptake of potassium from the blood in both the untrained and trained legs. The peak rates of uptake were 1.24 ± 0.47 and 1.44 ± 0.40 mmol min−1 in the untrained and trained legs, respectively. This corresponds to Na+,K+-ATPase-mediated uptake rates by the muscle cells of 0.52 ± 0.20 and 0.59 ± 0.17 mmol min−1 (kg muscle−1) in the untrained and trained legs, respectively, which are higher than the estimated uptake of 0.26 mmol min−1 (kg muscle−1) during bicycle exercise (Hallen et al. 1994). However, the values are considerably lower than the theoretical maximal Na+,K+-ATPase activity determined in vitro, which has been shown to be in the range of 6 mmol min−1 (kg wet weight−1) in rats (Clausen et al. 1987). It is noteworthy that the Na+,K+-ATPase-mediated uptake per kg of muscle was 13% higher in TL than in CL, a difference of the same magnitude as that observed for Na+,K+-ATPase α subunits.
Potassium release to the blood stream
An interesting finding in the present study was that the potassium concentration in the muscle interstitium during exercise was significantly higher than the femoral venous potassium concentration. One explanation of this observation is that femoral venous blood not only represents blood from the active muscles, but also blood that has perfused inactive tissue. However, this may only account for a minor part of the difference, since the blood flow to inactive muscle during exercise in the knee-extensor model is relatively low (Bangsbo et al. 1995; Radegran & Saltin, 1998). Thus, it appears that diffusion of potassium from the interstitium to capillaries is restricted. In agreement with this, Kajimura et al. (1998) observed in single perfused frog mesenteric capillaries a distinct potassium gradient between the inside and outside of the capillary and that the capillary permeability of K+ correlates linearly with flow. Additionally, Friedman & DeRose (1982) reported that the permeability of potassium, and other small solutes, increases with increasing flow rates in isolated microvessels. Apparently a potassium gradient through the capillary walls can be expected. In the trained leg the difference between interstitial and venous potassium concentrations was less than in the untrained leg (Fig. 6B), indicating that a higher degree of equilibrium between interstitium and blood was obtained after training. This is in accordance with the finding by Sexton et al. (1988) that the capillary filtration rate in isolated rat hindquarters was improved after the rats were intensely trained for 6–10 weeks. Also, the capillary diffusion capacity in human anterior tibialis muscles was shown to be higher in endurance-trained athletes than in sedentary subjects (Leinonen et al. 1978). The high degree equilibrium after training may be explained by a ∼20% higher capillary density in the trained compared to the untrained leg observed in this study (Höffner et al. 2002), which, together with the observation of no difference in blood flow in the main part of the 30 W exercise bout, indicates that the mean transit time of blood in the capillaries was elevated, thereby allowing more time for release of potassium from the interstitium to the capillaries. This can also explain the finding that the release of potassium to the blood was the same in the untrained and trained legs, even though the potassium gradient between the interstitium and the arterial blood in the trained leg was lower at the same blood flow (Fig. 6A). The finding that potassium release to the blood stream was the same for the untrained and trained legs is in agreement with the data of McKenna et al. (1997), who found no change in the potassium arterial–venous difference after a period of cycle sprint training. On the other hand, Kiens & Saltin (1986) observed that 8 weeks of one-legged endurance training (2 h) reduced potassium release during exercise. Furthermore, the rise in plasma potassium in dogs induced by exercise was lower in dogs that had performed endurance training (Knochel et al. 1985). Apparently, the intensity and duration of the exercise during training affects the potassium release during exercise.
It should be noted that, despite the femoral venous potassium concentration being lower than the interstitial concentration during exercise, the venous potassium concentration reflected the interstitial potassium level. For example, the femoral venous potassium concentration was lower in the trained compared to the untrained leg during the 30 W and the incremental exercise bouts, as were the interstitial concentrations. Thus, the femoral venous concentrations during knee-extensor exercise can be used to evaluate differences in the concentrations of potassium in the muscle interstitium.
Development of fatigue
At the point of fatigue, the interstitial potassium concentration was around 10 mmol l−1, which is at the same level as in another recent study using the microdialysis technique and intense knee-extensor exercise (Nordsborg et al. 2003). It should be noted that the average time for the final microdialysis sample was 1.2 min and it is therefore likely, that peak [K+]I at the time of exhaustion was even higher. Potassium concentrations at this level decrease membrane potential significantly (Renaud & Light, 1992; Cairns et al. 1995). The effect of the changes in potassium concentrations on membrane potential can be estimated from the Goldman-Hodgkin-Katz (GHK) equation (Sejersted & Sjøgaard, 2000). According to this equation membrane potential is primarily based on the ratio between extra- and intracellular potassium concentrations. Assuming [K+]intracellular was 165 mmol l−1 before exercise (Sjøgaard et al. 1985) and using the amount of potassium released to blood and interstitium, the [K+]intracellular values at exhaustion were calculated to be 144 ± 5 and 136 ± 5 mmol l−1 in the untrained and trained leg, respectively. Further, using [K+]I prior to the incremental exercise and at exhaustion, this corresponds to a change in membrane potential of 28 and 27% in the untrained and trained legs, respectively. The changes in membrane potential were probably underestimated, since the contribution from changes in sodium concentrations has not been taken into account. It should be noted, however, that the calculation does not include the electrogenic effect of the Na+,K+-ATPase. Anyway, a change in membrane potential of this magnitude will have a marked effect on muscle excitability and contractility (Renaud & Light, 1992; Cairns et al. 1995).
The notion that the accumulated potassium and the concomitant decrease in the membrane potential may have caused fatigue is supported by the finding that [K+]I at exhaustion and the calculated membrane potential were the same in the untrained and trained legs, despite the time to exhaustion being longer in the trained leg. It is also worth noticing that, although significantly lower than the interstitial concentration, the arterial and femoral venous potassium concentrations at the time of exhaustion were identical for the untrained and trained legs, which suggests that previous observations of the same femoral potassium concentration at the point of fatigue during intense knee-extensor (Bangsbo et al. 1996) and bicycle exercise (Hallen et al. 1994) under different conditions also represents equal interstitial potassium concentrations under those conditions. Therefore, it also seems valid to conclude from such studies that fatigue is associated with a given accumulation of potassium in the muscle interstitium.
Other possible fatigue agents should be considered. Lowered pH has in a number of in vitro studies been shown to impair muscle performance (Balog & Fitts, 2001). However, although muscular pH at exhaustion in the present study was not significant different in the untrained compared to the trained leg, findings in a number of in vitro (Westerblad et al. 1997) and in vivo studies (Bangsbo et al. 1996; Krustrup et al. 2003) have suggested that lowered muscle pH per se is not a plausible cause of fatigue during intense exercise. Furthermore, muscle lactate at exhaustion tended (P = 0.06) to be different (95.5 ± 14.5 and 59.0 ± 15.1 mmol (kg dry weight−1) in trained and untrained legs, respectively), supporting the finding by Posterino & Fryer (2000) that muscular lactate was not a primary cause of fatigue. It should be noted that a number of other parameters, which were not measured in the present study, such as intracellular accumulation of inorganic phosphate or adenosine diphosphate (ADP) and low levels of creatine phosphate in some muscle fibres (Fitts, 1994) may have contributed to the development of fatigue during the incremental exercise test.
Total net K+ uptake from the blood during the first 10 min of recovery from incremental exercise was 5.8 ± 2.8 mmol in the untrained leg and 4.5 ± 1.7 mmol in the trained leg, which corresponds to 51 ± 24 and 35 ± 15% of the net release of potassium to the blood during the incremental exercise test, respectively. Apparently, 10 min is not enough time to re-establish potassium homeostasis after exercise. Using the mean rate of potassium uptake during the last 5 min, it can be calculated that in the untrained and trained legs [K+]intracellular reaches pre-exercise levels approximately 30 and 60 min after exercise, respectively, providing that the intracellular water content is also re-established. The slow recovery of intracellular potassium may provide an explanation for why performance during repeated intense exercise is reduced even when the recovery time is long (Bangsbo et al. 1992), since the membrane potential will start at higher level when exercise is repeated.
Summary
The present study showed that a period of intense intermittent training lead to a higher amount of Na+,K+-ATPase α1 and α2 isoforms but no change in the amount of KATP channels. Interstitial and venous potassium concentrations during exercise were lower, whereas potassium release was the same. These findings suggest that the rate of re-uptake of potassium is elevated after training as a result of an increase in activity of the Na+–K+ pumps. The improvement in performance and the decrease in accumulation of interstitial potassium in the trained leg, in association with the lack of a difference in the interstitial potassium level at exhaustion in the untrained and the trained legs, support the hypothesis that accumulation of potassium in muscle interstitium during exercise is causing fatigue.
Acknowledgments
We would like to express our gratitude to Ingelise Kring, Winnie Taagerup and Merete Vannby for excellent technical assistance, Helle Walas for preparing the protein analysis, David Hudson for participating in the training and experiments, as well as the subjects for their great spirit during the demanding training and experiments. The study was supported by grants from Team Denmark and the Danish National Research Foundation (501-14).
References
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as a model for the study of an isolated exercising muscle in man. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balog EM, Fitts RH. Effects of depolarization and low intracellular pH on charge movement currents of frog skeletal muscle fibers. J Appl Physiol. 2001;90:228–234. doi: 10.1152/jappl.2001.90.1.228. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Aagaard T, Olsen M, Kiens B, Turcotte LP, Richter EA. Lactate and H+ uptake in inactive muscles during intense exercise in man. J Physiol. 1995;488:219–229. doi: 10.1113/jphysiol.1995.sp020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Graham TE, Kiens B, Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol. 1992;451:205–227. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Juel C, Hellsten Y, Saltin B. Dissociation between lactate and proton exchange in muscle during intense exercise in man. J Physiol. 1997;504:489–499. doi: 10.1111/j.1469-7793.1997.489be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, González-Alonso J, Bouschel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol. 2000;279:R899–R906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scand J Clin Laboratory Invest. 1962;68(suppl.):1–101. [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflugers Arch. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Castle NA, Kaylett DG. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol. 1987;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Everts M, Kjeldsen K. Quantification of the maximum capacity for active sodium-potassium transport in rat skeletal muscle. J Physiol. 1987;388:163–181. doi: 10.1113/jphysiol.1987.sp016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Flatman JA. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J Physiol. 1977;270:383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NW. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990;343:375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Everts ME, Clausen T. Activation of the Na-K pump by intracellular Na in rat slow- and fast-twitch muscle. Acta Physiol Scand. 1992;145:353–362. doi: 10.1111/j.1748-1716.1992.tb09375.x. [DOI] [PubMed] [Google Scholar]
- Evertsen F, Medbo JI, Jebens E, Nicolaysen K. Hard training for 5 months increases Na+-K+ pump concentration in skeletal muscle of cross-country skiers. Am J Physiol. 1997;272:R1417–R1424. doi: 10.1152/ajpregu.1997.272.5.R1417. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Friedman J, DeRose NE. The effects of flow and of hyperosmolal superfusion on the K+ permeability of single capillaries. Microvasc Res. 1982;24:68–76. doi: 10.1016/0026-2862(82)90043-7. [DOI] [PubMed] [Google Scholar]
- Green HJ, Chin ER, Ball-Burnett M, Ranney D. Increases in human skeletal muscle Na+-K+-ATPase concentration with short-term training. Am J Physiol. 1993;264:C1538–C1541. doi: 10.1152/ajpcell.1993.264.6.C1538. [DOI] [PubMed] [Google Scholar]
- Hallen J, Gullestad L, Sejersted OM. K+ shifts of skeletal muscle during stepwise bicycle exercise with and without beta-adrenoceptor blockade. J Physiol. 1994;477:149–159. doi: 10.1113/jphysiol.1994.sp020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Richter EA, Kiens B, Bangsbo J. AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J Physiol. 1999;520:909–920. doi: 10.1111/j.1469-7793.1999.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocherman SD, Bezanilla F. A patch-clamp study of delayed rectifier currents in skeletal muscle of control and mdx mice. J Physiol. 1996;493:113–128. doi: 10.1113/jphysiol.1996.sp021368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffner L, Krustrup P, Klarskov C, Hudson D, Juel C, Bangsbo J, Hellsten Y. Four weeks of anaerobic training induces capillarization in human skeletal muscle. FASEB J. 2002;16–4:384.3. [Google Scholar]
- Hundal HS, Maxwell DL, Ahmed A, Darakhshan F, Mitsumoto Y, Klip A. Subcellular distribution and immunocytochemical localization of Na,K-ATPase subunit isoforms in human skeletal muscle. Mol Membr Biol. 1994;11:255–262. doi: 10.3109/09687689409160435. [DOI] [PubMed] [Google Scholar]
- Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol. 2003 doi: 10.1152/ajpendo.00303.2003. 10.1152/ajpendo.00303.2003. [DOI] [PubMed] [Google Scholar]
- Juel C, Nielsen JJ, Bangsbo J. Exercise-induced translocation of Na+-K+ pump subunits to the plasma membrane in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2000b;278:R1107–R1110. doi: 10.1152/ajpregu.2000.278.4.R1107. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000a;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Kajimura M, Head SD, Michel CC. The effects of flow on the transport of potassium ions through the walls of single perfused frog mesenteric capillaries. J Physiol. 1998;511:707–718. doi: 10.1111/j.1469-7793.1998.707bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B, Saltin B. Endurance training of man decreases muscle potassium loss during exercise. Acta Phys Scand. 1986;126:20A. abstract. [Google Scholar]
- Kjeldsen K, Bjerregaard P, Richter EA, Thomsen PE, Nørgaard A. Na+,K+-ATPase concentration in rodent and human heart and skeletal muscle: apparent relation to muscle performance. Cardiovasc Res. 1988;22:95–100. doi: 10.1093/cvr/22.2.95. [DOI] [PubMed] [Google Scholar]
- Knochel JP, Blachley JD, Johnson JH, Carter NW. Muscle cell electrical hyperpolarization and reduced exercise hyperkalemia in physically conditioned dogs. J Clin Invest. 1985;75:740–745. doi: 10.1172/JCI111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Mohr M, Amstrup T, Rysgaard T, Johansen J, Steensberg A, Pedersen PK, Bangsbo J. The Yo-Yo intermittent recovery test: physiological response, reliability, and validity. Med Sci Sports Exerc. 2003;35:697–705. doi: 10.1249/01.MSS.0000058441.94520.32. [DOI] [PubMed] [Google Scholar]
- Leinonen H, Salminen S, Peltokallio P. Capillary permeability and maximal blood flow in skeletal muscle in athletes and non-athletes measured by local clearence of 133Xe and 131I- Scand J Clin Laboratory Invest. 1978;38:223–227. doi: 10.3109/00365517809108415. [DOI] [PubMed] [Google Scholar]
- Light PE, Comtois AS, Renaud JM. The effect of glibenclamide on frog skeletal muscle: evidence for K+ ATP channel activation during fatigue. J Physiol. 1994;475:495–507. doi: 10.1113/jphysiol.1994.sp020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol. 2002;282:F967–F974. doi: 10.1152/ajprenal.00360.2001. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJ, McKelvie RS, MacDougall JD, Jones NL. Sprint training enhances ionic regulation during intense exercise in men. J Physiol. 1997;501:687–702. doi: 10.1111/j.1469-7793.1997.687bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka R, Yamashita S, Mizuno M, Akaike N. Intracellular Na+ and K+ shifts induced by contractile activities of rat skeletal muscles. Comp Biochem Physiol a Physiol. 1994;109:957–965. doi: 10.1016/0300-9629(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J, Juel C. Localization and function of ATP-sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2003;284:R558–R563. doi: 10.1152/ajpregu.00303.2002. [DOI] [PubMed] [Google Scholar]
- Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285:R143–R148. doi: 10.1152/ajpregu.00029.2003. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Fryer MW. Effects of high myoplasmic 1-lactate concentration on E-C coupling in mammalian skeletal muscle. J Appl Physiol. 2000;89:517–528. doi: 10.1152/jappl.2000.89.2.517. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol. 1998;274:H314–H322. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- Renaud JM, Light P. Effects of K+ on the twitch and tetanic contraction in the sartorius muscle of the frog, Rana pipiens. Implication for fatigue in vivo. Can J Physiol Pharmacol. 1992;70:1236–1246. doi: 10.1139/y92-172. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Sexton WL, Korthuis RJ, Laughlin MH. High-intensity exercise training increases vascular transport capacity of rat hindquaters. Am J Physiol. 1988;254:H274–H278. doi: 10.1152/ajpheart.1988.254.2.H274. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985;248:R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Blom PC. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125:395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]