Abstract
Vascular endothelium constitutively generates nitric oxide (NO) in large vessels and induces a relaxation of smooth muscle cells. However, little is known about the production of NO in microvessels, where smooth muscle layers are thin or absent. In this study, we have compared the constitutive production of NO in bovine brain microvascular endothelial cells (BBECs) with that in bovine aortic endothelial cells (BAECs). ATP, acetylcholine (ACh) and A23187 induced Ca2+ transients both in BBECs and BAECs. In contrast, although ATP and A23187 evoked a similar degree of [Ca2+]i increase in both types of cell, they failed to induce NO production in BBECs, as measured with an NO-sensitive fluorescent dye DAF-2, whereas in BAECs there was an increase in DAF-2 fluorescence. Hypotonic stress induced ATP release and subsequent NO production in BAECs, but not in BBECs. We have developed an in vitro model vessel system that consists of aortic smooth muscle cells embedded in a collagen gel lattice and overlaid with endothelial cells. Precontracted gels showed relaxation in response to ACh, when BAECs were overlaid. However, ACh-induced relaxation was not observed in BBEC-overlaid gels. Expression of eNOS protein as well as cellular uptake of l-[3H]arginine were significantly lower in BBECs than in BAECs. These results indicate that Ca2+-dependent NO production is at an undetectable level in BBEC, for which at least two factors, i.e. low levels of eNOS expression and l-arginine uptake, are responsible.
Nitric oxide (NO) plays various physiological and pathological roles in variety of cell types (Moncada et al. 1991). It is well documented that vascular endothelium constitutively generates NO in response to the elevation of the intracellular Ca2+ concentration ([Ca2+]i). Ca2+ binds to calmodulin thereby stimulating endothelial NO synthase (eNOS) to produce NO, in combination with other cofactors such as NADPH and tetrahydrobiopterin (Lopez-Jaramillo et al. 1990). Endothelium-derived NO induces a relaxation of vascular smooth muscle cells, and prevents atherosclerosis and cell adhesion (Moncada et al. 1991). However, this view has been mainly developed in larger conducting vessels, and the relatively greater importance of endothelium-derived hyperpolarizing factor (EDHF) rather than NO has been suggested in smaller resistance arteries (Garland et al. 1995). Furthermore, a neural rather than an endothelial source of NO has been considered to regulate vascular flow in rat mesenteric arterioles (Kashiwagi et al. 2002).
It has been reported in cultured human cerebral microvascular endothelial cells that an exogenously applied NO donor inhibits endothelin-1-induced Ca2+ transients and down-regulates actin reorganization (Chen et al. 2003). Other reports have also shown that eNOS protein is expressed in rat brain microvascular endothelium and its expression level is altered by pathophysiological stimuli such as oestrogen (McNeill et al. 1999), perinuclear EP3 receptor stimulation (Gobeil et al. 2002) or angiotensin II (Yamakawa et al. 2003). However, if NO is constitutively generated by eNOS in cerebrocortical microvascular endothelium, it would affect the functions of neighbouring neurones directly or by changing cerebrocortical blood flow. Actually there has been no direct evidence reported so far showing the constitutive production of NO in cerebral microvascular endothelium. Furthermore, a recent report has shown that control of cerebral microcirculation is obtained by neurone-to-glia signals but not by vascular signals (Zonta et al. 2003).
The aim of this study was to clarify whether NO is constitutively generated in bovine brain microvascular endothelial cells (BBECs) or not. We have used two methods to detect NO production in BBECs and bovine aortic endothelial cells (BAECs). Firstly we measured the intracellular NO production with an NO-sensitive fluorescent dye, DAF-2 (Kojima et al. 1998). Secondly, we have developed a novel method to detect cultured endothelium-dependent vasorelaxation. We have previously reported that vascular smooth muscle cells embedded in collagen gels show contraction in response to Ca2+ mobilizing stimuli (Kimura et al. 2002). We therefore considered that the endothelial functions could be examined by overlaying cultured endothelium onto smooth muscle cell-embedded collagen gel. The results obtained indicate that Ca2+-dependent NO production is detectable using these methods in BAECs, but not in BBECs.
Methods
Cell culture
Thoracic aortas and brains of 1-year-old calves were obtained from the local slaughterhouse. BAECs were scraped off from the intima with the edge of a razor (Oike et al. 2000). BBECs were prepared following a Percoll gradient separation method as previously described (Kimura et al. 1998b). Both BAECs and BBECs were cultured in Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS, PAA Laboratories, Linz, Austria). Cells from primary cultures were subcultured at a split ratio of 1 : 3, and the harvested subcultured cells were used for the present experiments. Cells were grown on coverslips, which were coated with collagen type IA (Nitta Gelatin Inc., Osaka, Japan), for measuring [Ca2+]i and NO production. Endothelial identification of BAECs was confirmed by the specific uptake of fluorescence-labelled acetylated low-density lipoprotein (Dil-Ac-LDL) as previously reported (Kimura et al. 2001a). BBECs exhibited immunohistochemical staining for antifactor VIII antibody, indicating their endothelial nature. Endothelial cells obtained from 12 aortas and four brains were used in the present study.
Bovine aortic smooth muscle cells (BASMCs) from thoracic aortas were cultured in DMEM with 10% FBS by the explant method as previously described (Kimura et al. 2002). Cells grown to confluency were harvested by trypsin digestion and stored at −80°C after one-step subculture. Smooth muscle α-actin was stained to confirm that the cells retained the nature of smooth muscle cells (not shown). The cells were embedded in collagen gel lattice for the gel contraction assay as described below. Smooth muscle cells from five aortas were used in the present study.
Measurement of [Ca2+]i
[Ca2+]i was measured from non-confluent BBECs and BAECs with fura-2 using an Attofluor digital fluorescence microscopy system (Atto Instruments, Rockville, MD, USA), as previously described (Oike et al. 2000).
Measurement of intracellular production of NO
For the measurement of NO with DAF-2, an NO-sensitive fluorescent dye (Kojima et al. 1998), non-confluent cells grown on coverslips were incubated with a diacetylated form of DAF-2 (10 μm, Daiichi Pure Chemicals, Co. Ltd, Tokyo, Japan) for 20 min at room temperature and for a subsequent 20 min at 37°C. DAF-2 was excited at a wavelength of 490 nm and emitted fluorescence at a wavelength of 515 nm was measured with an Attofluor fluorescence microscopy system. Since DAF-2 has single excitation and single emission wavelengths, conversion of DAF-2 fluorescence into intracellular NO concentrations is impossible (Kojima et al. 1998). However, since DAF-2 fluorescence increases almost linearly with NO concentration (Kojima et al. 1998), we have expressed the DAF-2 fluorescence relative to its initial values. Because NOS generates instead of NO in the absence of l-arginine (Xia et al. 1998), we added an excess concentration of l-arginine (3 mm) to all solutions used for NO measurement, except for the experiment with Nϖ-nitro-l-arginine methyl ester (l-NAME)-treated cells, where l-NAME (0.1 mm) was added during the last 30 min of the DAF-2 incubation period.
Gel contraction assay
Endothelial NO production was also assessed in an in vitro model vessel, which consists of a BASMC-embedded collagen gel and an overlaid endothelium. Cultured BASMCs were re-suspended in DMEM containing 0.2% collagen type IA at a density of 4 × 105 cells ml−1, poured into a 24-well culture plate and allowed to form a gel for 10 min at 37°C, as previously described. DMEM with 10% FBS was then poured on to the gel. After culturing for 24 h at 37°C, BBECs or BAECs were overlaid on the gel at a density of 2 × 104 cells cm−2.
After culturing for a further 24 h at 37°C, the gel was used for the contraction assay (see Fig. 4Ba). The lateral surface of the gel was carefully detached from the culture well with a fine needle. The culture plate was then placed on a hotplate (MP-10 Dm; Kitazato Supply, Shizuoka, Japan) and kept at 37°C. The gel images were captured with a digital camera (QV-800SX, Casio, Tokyo, Japan) every 1 min throughout the experiment. Contraction of the gel was then evaluated by measuring its surface area with image analysis software (Adobe Photoshop, Adobe Systems Inc., USA).
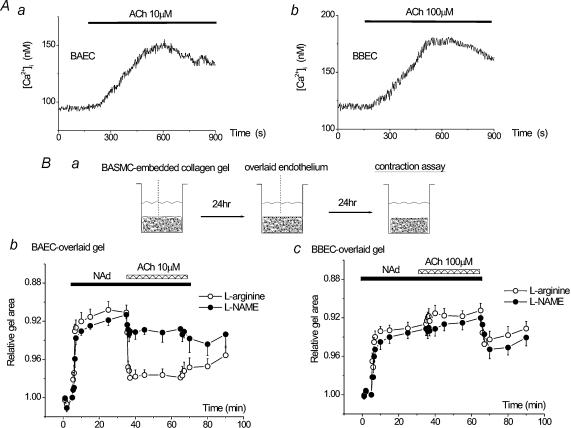
Figure 4. Endothelium-dependent relaxation of smooth muscle-embedded, endothelium-overlaid collagen gels.
A, ACh (10 μm) induced Ca2+ transients in BAECs (a). A similar degree of [Ca2+]i elevation was obtained with a higher concentration of ACh in BBECs (100 μm, b). Representative traces of 30 (BAEC) or 28 (BBEC) cells are shown. Ba, in vitro model vessel consisting of bovine aortic smooth muscle cells (BASMC) embedded in a collagen gel lattice with overlaid endothelium. BASMC were embedded in type I collagen gel, and BAECs or BBECs were overlaid after 24 h. A gel contraction assay was performed after a further 24 h (right). Noradrenaline (NAd, 1 μm) induced a rapid contraction of the gels. Subsequent application of 10 μm ACh induced relaxation of precontracted gels when BAEC was overlaid (b, ○, n = 20). Note that l-NAME (0.1 mm) inhibited ACh-induced gel relaxation but did not affect initial gel contraction (b, •, n = 6). BBEC-overlaid gels did not show relaxation in response to 100 μm ACh both in control (○, n = 22) and l-NAME-treated gels (c, •, n = 6).
Measurement of ATP release with luciferase bioluminescence
For the measurement of the extracellular ATP concentration ([ATP]o), BAECs and BBECs were seeded on 96-well culture plates at densities of 5000 cells well−1. After culturing for 3 days, [ATP]o was measured using luciferin–luciferase chemiluminescence as previously described (Oike et al. 2000).
Western blotting of eNOS protein expression
Expression of eNOS protein in BAECs and BBECs was assessed by chemiluminescence Western blotting. Cells were lysed with 2% SDS and the lysate was electrophoresed through 7.5% polyacrylamide gel. Western blot analysis for eNOS protein was then performed using antieNOS polyclonal antibody (StressGen Biotechnologies Co., San Diego, CA, USA) and a chemiluminescence system (SuperSignal West Dura, Pierce Co., Rockford, IL, USA). Emitted chemiluminescence was detected and analysed with a lumino image analyser (FAS-1000, Toyobo, Osaka, Japan).
l-[3H]arginine uptake
Measurement of cellular uptake of l-[3H]arginine (Amershan, Uppsala, Sweden) was performed as previously reported (Nelin et al. 2001) with slight modifications. BBECs and BAECs were seeded on 6-well culture plates at a density of 25 000 cells well−1. After culturing for 3 days, the cells were washed three times with Hanks' balanced salt solution (HBSS, Sigma, St Louis, MO, USA). To determine total l-[3H]arginine uptake, 1 ml of HBSS with 1 μCi ml−1l-[3H]arginine was placed on each well. Non-specific uptake of l-[3H]arginine was determined with HBSS containing 1 μCi ml−1l-[3H]arginine and 10 mm unlabelled l-arginine. After 15 min of incubation at 37°C, the cells were washed three times with ice-cold HBSS, and lysed with 1 ml well−1 of 0.2 n NaOH. Aliquots were added to scintillation cocktail and radioactivity was quantified with a liquid scintillation spectrometer (LSC3500, Aloka Co., Tokyo, Japan).
Drugs and solutions
Krebs solution used in the present study contained (mm); NaCl 132.4, KCl 5.9, CaCl2 1.5, MgCl2 1.2, glucose 11.5, Hepes 11.5, and pH was adjusted to 7.4 by NaOH. Hypotonic solution (−30%) was prepared by adding distilled water to Krebs solution at a water : Krebs ratio of 3 : 7. We have previously shown that alterating the ionic composition of Krebs solution by adding water does not influence the cellular Ca2+ responsiveness (Oike et al. 2000). All drugs used in the present study were purchased from Sigma.
Statistics
Pooled data were expressed as means ± s.e.m. values. Statistic significance was examined with Student's unpaired t test. A probability below 0.05 (P < 0.05) was considered to show a significant difference.
Results
Effects of ATP and A23187 on NO production in BAECs and BBECs
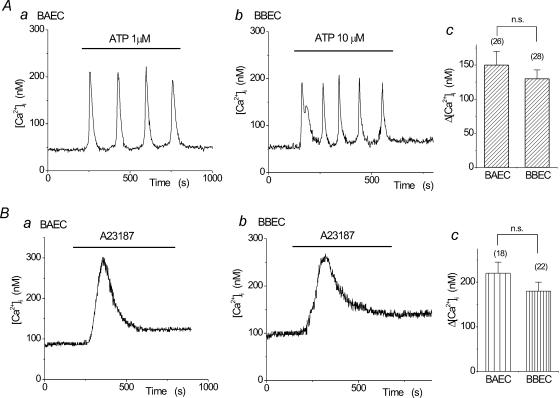
We have previously reported that ATP induced Ca2+ transients both in BBECs and BAECs, but the concentration–response relationship in BBECs was shifted to higher concentrations than that in BAECs (Kimura et al. 1998a, 2000b). This was confirmed in this study; i.e. ATP (1 μm) induced Ca2+ oscillations in Krebs solution in BAECs (Fig. 1Aa), and a similar Ca2+ response was obtained with 10 μm ATP in BBECs (Fig. 1Ab and c). The Ca2+ ionophore A23187 (1 μm) also induced [Ca2+]i elevation in both cell types (Fig. 1Ba and b). The net increments in 1 μm A23187-induced Ca2+ transients were not significantly different in BAECs and BBECs (Fig. 1Bc).
Figure 1. Ca2+ transients induced by ATP and A23187 in bovine aortic (BAECs) and brain microvascular (BBECs) endothelial cells.
A, representative traces of ATP-induced Ca2+ oscillations in BAECs (a) and BBECs (b). Similar levels of net [Ca2+]i elevation (Δ[Ca2+]i) were obtained with 1 μm and 10 μm ATP in BAECs and BBECs, respectively (c). B, A23187 (1 μm) induced Ca2+ transients in both BAECs (a) and BBECs (b) to a similar degree (c). n.s., P > 0.05.
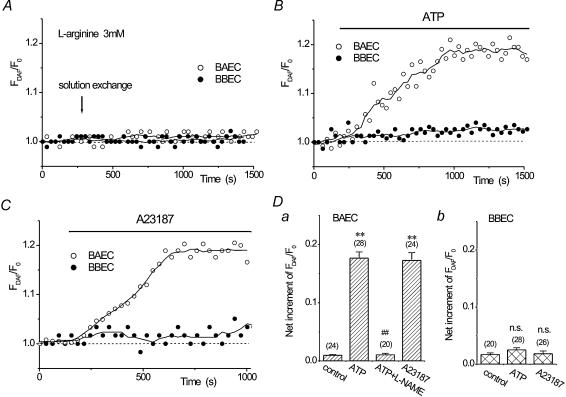
We then evaluated Ca2+-dependent NO production in BAECs and BBECs using DAF-2. We have previously demonstrated that Ca2+ mobilization evoked by ATP or A23187 induced an increase in NO production in BAECs (Koyama et al. 2002). As shown in Fig. 2A, a solution exchange alone did not induce any apparent change in DAF-2 fluorescence up to 20 min in both BAECs and BBECs, even in the presence of 3 mml-arginine. In BAECs, ATP (1 μm) induced a gradual increase in DAF-2 fluorescence (○, Fig. 2B), which was inhibited by pretreatment with 0.1 mm l-NAME (Fig. 2D), suggesting that DAF-2 fluorescence was properly linked to cellular NO production. In contrast, BBECs did not show any increase in DAF-2 fluorescence in response to 10 μm ATP (•, Fig. 2B), even though this concentration of ATP induced Ca2+ transients in BBECs (Fig. 1A). A23187 also induced an increase in DAF-2 fluorescence in BAECs, but not in BBECs (Fig. 2C). These results are summarized in Fig. 2D, and indicate that ATP and A23187 do not induce NO production in BBECs.
Figure 2. Effects of ATP and A23187 on NO production, assessed with DAF-2, in BAECs and BBECs.
A, solution exchange alone did not induce an increase in DAF-2 fluorescence in both BAECs (○) and BBECs (•). Representative results are shown. DAF-2 fluorescence (FDAF) is expressed relative to its initial value (t = 0, F0). Circles are the actual values of DAF-2 fluorescence and continuous lines were drawn by averaging the adjusting five points. Measurements were performed in the presence of 3 mM l-arginine. B, ATP (1 μm) induced an increase in DAF-2 fluorescence in BAECs (○), whereas 10 μm ATP did not show any apparent increase in DAF-2 fluorescence in BBECs (•). Representative results are shown. C, A23187 (1 μm) also increased DAF-2 fluorescence in BAECs (○) but not in BBECs (•). Representative results are shown. D, net increment of relative DAF-2 fluorescence at 10 min after solution exchange (control) or the application of ATP or A23187 in BAECs (a) and BBECs (b). Note that l-NAME (0.1 mm) inhibited ATP-induced DAF-2 fluorescence. **P < 0.01 vs. control. ##P < 0.01vs. ATP alone. n.s., P > 0.05vs. control.
Effects of hypotonic stress on NO production in BAECs and BBECs
We have previously reported that hypotonic stress (HTS), as an example of mechanical stress, induces NO production in a Ca2+-dependent manner due to ATP release in BAECs (Kimura et al. 2000a; Oike et al. 2000). So we then compared the HTS-induced, ATP-mediated NO production in BAECs and BBECs. HTS (−30%) induced ATP release in BAECs as previously reported (Oike et al. 2000). [ATP]o was elevated to 55.8 ± 5.9 nm after being exposed to HTS for 10 min (n = 14), whereas it was 28.8 ± 3.2 nm when the cells were kept in isotonic solution for the same period (n = 14, Fig. 3A). In contrast, a HTS-induced increase in [ATP]o was not observed in BBECs; i.e. [ATP]o was 25.6 ± 2.5 nm in isotonic solution (n = 13) and 28.1 ± 2.3 nm in hypotonic solution (n = 13, P > 0.05versus isotonic). As expected, HTS induced an increase in DAF-2 fluorescence in BAECs, but not in BBECs (Fig. 3B).
Figure 3. Effects of hypotonic stress (HTS) on ATP release and subsequent NO production in BAECs and BBECs.
A, HTS (−30%) induced an increase in [ATP]o in BAECs but not in BBECs. Luciferin chemiluminescence was measured for 10 min, and converted into corresponding [ATP]o with [ATP]o–chemiluminescence standard curves obtained in each solution. **P < 0.01 vs. BAEC isotonic. n.s., P > 0.05vs. BBEC isotonic. Ba, gradual increase in DAF-2 fluorescence was evoked by HTS in BAECs (○) but not in BBECs (•) in the presence of 3 mml-arginine. Representative results are shown. Bb, Statistical analysis of net increment of relative DAF-2 fluorescence at 10 min after starting HTS. **P < 0.01vs. BAECs.
Endothelium-dependent relaxation of smooth muscle cell-embedded collagen gels
To further investigate NO production in BBECs and BAECs, we have developed a novel method for detecting cultured endothelium-dependent vasorelaxation. Endothelial cells were overlaid onto a BASMC-embedded collagen gel lattice as shown in the cartoon (Fig. 4Ba) so that endothelium-derived substances could affect the gel contraction.
In this assay, we used the classical endothelial agonist ACh (Furchgott & Zawadzki, 1980) as a Ca2+ mobilizing agent. ACh (10 μm) induced Ca2+ transients in BAECs (Fig. 4Aa). The same concentration of ACh (10 μm) induced smaller Ca2+ responses in BBECs (not shown), and a similar Ca2+ response was obtained with a higher concentration of ACh (100 μm, Fig. 4Ab).
BASMC-embedded collagen gel lattices showed a rapid contraction in response to noradrenaline (NAd, 1 μm) both in BAEC- and BBEC-overlaid gels (Fig. 4Bb and c, ○). Pretreatment with l-NAME did not affect the NAd-induced contraction both in BAEC- and BBEC-overlaid gels (Fig. 4Bb and c, •), thereby suggesting that NAd does not evoke NO production in these overlaid endothelia. Subsequent application of 10 μm ACh induced a relaxation of the precontracted gels in BAEC-overlaid gels (Fig. 4Bb, ○). The relaxation was not observed in the absence of overlaid BAECs (not shown) and was significantly inhibited when the gel was pretreated with l-NAME (Fig. 4Bb, •), thereby indicating that the relaxation of the gel was due to BAEC-derived NO. In contrast, when BBECs were overlaid on to a BASMC-embedded collagen gel, the precontracted gel did not show relaxation in response to 100 μm ACh (Fig. 4Bc, ○).
Therefore, these results indicate that [Ca2+]i elevation leads to the release of a significant amount of NO in BAECs but not in BBECs.
Western blotting of eNOS protein expression in BAECs and BBECs
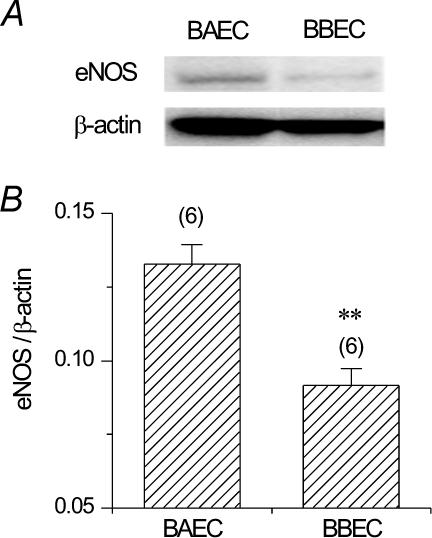
To explore the cellular mechanisms responsible for lower NO production in BBECs, we then examined the expression of eNOS protein in BAECs and BBECs with Western blotting. Though expression of eNOS protein was observed in BBECs, BAECs showed much a denser band of eNOS (Fig. 5A). Densitometric analysis revealed that the expression level of eNOS protein relative to that of the housekeeping β-actin protein was 0.133 ± 0.006 in BAECs (n = 6) but 0.092 ± 0.006 in BBECs (n = 6, Figs 5B; P < 0.01).
Figure 5. Expression of eNOS protein in BAECs and BBECs, assessed by Western blotting.
A, expression of eNOS and β-actin proteins in BAECs and BBECs. Same amount of total cellular protein (5 μg) was applied to each lane. Note that eNOS protein expression was lower in BBECs than in BAECs. B, densitometric analysis of the eNOS protein bands. Values are normalized to β-actin band density. **P < 0.01 vs. BAECs.
Cellular l-[3H]arginine uptake in BBECs and BAECs
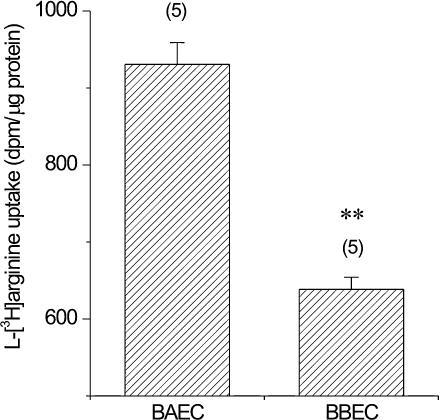
To further examine the possible cause of the reduction of NO production in BBECs, we finally examined the cellular l-arginine uptake in BAECs and BBECs. As shown in Fig. 6, uptake of l-[3H]-arginine over 15 min was significantly lower in BBECs than in BAECs (BAECs, 930.6 ± 28.4 d.p.m. (μg protein)−1; BBECs, 638.4 ± 15.7 d.p.m. (μg protein)−1, n = 5 for both cell types, P < 0.01).
Figure 6. Uptake of l-[3H]arginine in BAECs and BBECs.
Cells were incubated with l-[3H]arginine for 15 min at 37°C, and the incorporated l-[3H]arginine was measured as described in Methods. Results are shown as radioactive disintegrations per minute (d.p.m.) per μg cell protein (mean ±s.e.m., n = 5). **P < 0.01vs. BAECs.
Discussion
We have previously reported that ATP induces Ca2+ oscillations in BAECs and BBECs with different concentration–response relationships (Kimura et al. 1998a, 2000b). The present study showed that Ca2+ transients induced by 1 μm ATP in BAECs were similar to those induced by 10 μm ATP in BBECs (Fig. 1A). Furthermore, the Ca2+ ionophore A23187 (1 μm) induced Ca2+ transients in both BAECs and BBECs (Fig. 1B). In spite that the similar degree of [Ca2+]i elevation was induced by ATP and A23187 in BAECs and BBECs, these agents induced an increase in DAF-2 fluorescence only in BAECs (Fig. 2), thereby suggesting that [Ca2+]i elevation does not lead to detectable NO production in BBECs. In addition, HTS induced NO production in BAECs but not in BBECs (Fig. 3B), so we suppose that mechanical stresses that can be mimicked by HTS do not evoke NO production in BBECs. We have previously shown that HTS-induced NO production is due to endogenous ATP release (Kimura et al. 2000a), which was also absent in BBECs (Fig. 3A). Anion channels (Sabirov et al. 2001; Hisadome et al. 2002) and vesicular exocytosis (Bodin & Burnstock, 2001) have been suggested as components of the mechanical stress-induced ATP release pathway, and tyrosine kinases and the RhoA/Rho-kinase cascade have been reported as cellular mechanisms for HTS-induced ATP release (Koyama et al. 2001). Therefore it can be speculated that BBECs lack some of these or any other as yet unknown mechanisms that are involved in HTS-induced ATP release.
The absence of detectable Ca2+-dependent NO production in BBECs may contradict previous reports showing the expression of eNOS protein in cerebral microvessels in mouse (Ishii et al. 2002), rat (McNeill et al. 1999; Yamakawa et al. 2003) and pig (Gobeil et al. 2002). We have also observed that though the expression level is significantly lower than in BAECs, eNOS protein is certainly expressed in BBECs (Fig. 5). However, no previous studies have directly shown the production of NO in cerebral microvessels. Because the amount of NO generated by eNOS is relatively smaller than that produced by inducible NOS (Stoclet et al. 1999), very few methods have been introduced to measure NO production in cultured endothelium, i.e. a porphyrinic-based microsensor (Malinski & Taha, 1992) and the NO-sensitive fluorescent dye DAF-2 (Kojima et al. 1998). Though DAF-2 has been successfully used to measure NO production in cultured endothelium (Kimura et al. 2001b; Koyama et al. 2002), the specificity of DAF-2 fluorescence to NO has been questioned, because it is influenced by micromolar concentrations of Ca2+ (Broillet et al. 2001). Therefore, we have developed a novel method in this study for detecting NO production in cultured endothelium (Fig. 4Ba). Endothelium-overlaid, BASMC-embedded gels showed relaxation in response to ACh, when BAECs were overlaid (Fig. 4Bb). ACh-induced relaxation was inhibited by l-NAME and was not observed in the absence of BAECs, thereby indicating that the relaxation of the gel was due to NO generated by the overlying BAECs. Thus we suppose that this method is applicable for detecting NO production in cultured endothelium. Using this method, we observed that BBECs did not induce vasorelaxation in response to 100 μm ACh (Fig. 4Bc), while this concentration of ACh induced considerable Ca2+ transients in BBECs (Fig. 4Ab). Pretreatment with l-NAME did not affect the initial gel contraction induced by NAd, thereby eliminating the possibility that NAd induced NO production and therefore subsequent ACh failed to generate further NO in BBECs.
Therefore, these results strongly suggest that BBECs do not generate significant amounts of NO in response to [Ca2+]i elevation. However, since we have examined NO production only in non-stimulated BBECs, this study does not exclude the possibility that eNOS in cerebral microvascular endothelium may generate NO under some pathophysiological environments, as previously suggested (McNeill et al. 1999; Gobeil et al. 2002; Yamakawa et al. 2003). For instance, McNeill et al. (1999) reported that chronic treatment of rat cerebral microvessels with oestrogen increased the expression of eNOS and discussed the possibility that this might be involved in the neuroprotective effect of oestrogen and sex-related differences in cerebrovascular events. Furthermore, it is well known that shear stress generates NO in a Ca2+-independent manner in endothelium (Fleming et al. 1998), and we have only observed the absence of ATP-mediated, Ca2+-dependent mechanosensitive NO production in BBECs (Fig. 3). Therefore it should be noted that the present study does not rule out the presence and importance of shear stress-induced, Ca2+-independent eNOS activation in BBECs.
Expression of eNOS alone is not enough to induce Ca2+-dependent NO production but its substrate l-arginine and cofactors such as calmodulin, tetrahydrobiopterin and NADPH are also essential (Moncada et al. 1991). In this study we propose two possible mechanisms for the absence of detectable NO production in spite of a sufficient [Ca2+]i elevation in BBECs, namely lower expression of eNOS protein (Fig. 5) and lower cellular l-arginine uptake (Fig. 6). Cellular uptake of l-arginine in vascular endothelium is achieved by cationic amino acid transport systems such as y+ and y+L, and it is known that the efficiency of endothelial amino acid transport is markedly site specific (for a review see Mann et al. 2003). However, we cannot conclude from the present results that these mechanisms are solely responsible for the absence of detectable NO production in BBECs. Another possibility would be that eNOS protein is not coupled properly with other cofactors in BBECs. Therefore, it remains to be elucidated whether significant NO production could be obtained if the expression of eNOS protein is increased in pathological conditions.
It has been suggested that EDHF rather than NO plays a significant role in endothelial control of vascular tone in smaller vessels (Garland et al. 1995). The results of the present study have further clarified that [Ca2+]i elevation in brain microvascular endothelium does not lead to considerable NO production, and may support a recent report showing that cerebral microcirculation is controlled by neurone-to-astrocyte signals (Zonta et al. 2003). In conclusion, the present study has revealed that constitutive Ca2+-dependent and HTS-induced NO production is not detectable in BBECs, and suggests that there may be a marked site specificity in the generation of NO in endothelium.
Acknowledgments
The authors thank Dr M. Hirakawa for his initial support with Western blotting experiments. This study was carried out as a part of ‘Ground Research Announcement for the Space Utilization’ promoted by National Space Development Agency of Japan and Japan Space Forum. This study was also supported in part by a grant-in-aid from the Japan Society for the promotion of Science (No. 14570081).
References
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Broillet M, Randin O, Chatton J. Photoactivation and calcium sensitivity of the fluorescent NO indicator 4,5-diaminofluorescein (DAF-2): implications for cellular NO imaging. FEBS Lett. 2001;491:227–232. doi: 10.1016/s0014-5793(01)02206-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, McCarron RM, Golech S, Bembry J, Ford B, Lenz FA, Azzam N, Spatz M. ET-1- and NO-mediated signal transduction pathway in human brain capillary endothelial cells. Am J Physiol. 2003;284:C243–C249. doi: 10.1152/ajpcell.00305.2002. [DOI] [PubMed] [Google Scholar]
- Fleming I, Bauersachs J, Fisslthaler B, Busse R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ Res. 1998;82:686–695. doi: 10.1161/01.res.82.6.686. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Gobeil F Jr, Dumont I, Marrache AM, Vazquez-Tello A, Bernier SG, Abran D, Hou X, Beauchamp MH, Quiniou C, Bouayad A, Choufani S, Bhattacharya M, Molotchnikoff S, Ribeiro-Da-Silva A, Varma DR, Bkaily G, Chemtob S. Regulation of eNOS expression in brain endothelial cells by perinuclear EP3 receptors. Circ Res. 2002;90:682–689. doi: 10.1161/01.res.0000013303.17964.7a. [DOI] [PubMed] [Google Scholar]
- Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J General Physiol. 2002;119:511–520. doi: 10.1085/jgp.20028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Shimizu S, Shiota K, Yamamoto S, Kiuchi Y, Yamamoto T. Stimulation of tetrahydrobiopterin synthesis by cyclosporin A in mouse brain microvascular endothelial cells. Int J Biochem Cell Biol. 2002;34:1134–1141. doi: 10.1016/s1357-2725(02)00033-x. [DOI] [PubMed] [Google Scholar]
- Kashiwagi S, Kajimura M, Yoshimura Y, Suematsu M. Nonendothelial source of nitric oxide in arterioles but not in venules: alternative source revealed in vivo by diaminofluorescein microfluorography. Circ Res. 2002;91:e55–64. doi: 10.1161/01.res.0000047529.26278.4d. [DOI] [PubMed] [Google Scholar]
- Kimura C, Cheng W, Hisadome K, Wang YP, Koyama T, Karashima Y, Oike M, Ito Y. Superoxide anion impairs contractility in cultured aortic smooth muscle cells. Am J Physiol. 2002;283:H382–H390. doi: 10.1152/ajpheart.00574.2001. [DOI] [PubMed] [Google Scholar]
- Kimura C, Koyama T, Oike M, Ito Y. Hypotonic stress-induced NO production in endothelium depends on endogenous ATP. Biochem Biophys Res Commun. 2000a;274:736–740. doi: 10.1006/bbrc.2000.3205. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Ito Y. Acute glucose overload abolishes Ca2+ oscillation in cultured endothelial cells from bovine aorta: a possible role of superoxide anion. Circ Res. 1998a;82:677–685. doi: 10.1161/01.res.82.6.677. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Ito Y. Hypoxia-induced alterations in Ca2+ mobilization in brain microvascular endothelial cells. Am J Physiol. 2000b;279:H2310–H2318. doi: 10.1152/ajpheart.2000.279.5.H2310. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Kashiwagi S, Ito Y. Effects of acute glucose overload on histamine H2 receptor-mediated Ca2+ mobilization in bovine cerebral endothelial cells. Diabetes. 1998b;47:104–112. doi: 10.2337/diab.47.1.104. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Koyama T, Ito Y. Alterations of Ca2+ mobilizing properties in migrating endothelial cells. Am J Physiol. 2001a;281:H745–H754. doi: 10.1152/ajpheart.2001.281.2.H745. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Koyama T, Ito Y. Impairment of endothelial nitric oxide production by acute glucose overload. Am J Physiol. 2001b;280:E171–E178. doi: 10.1152/ajpendo.2001.280.1.E171. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kimura C, Park SJ, Oike M, Ito Y. Functional implications of Ca2+ mobilizing properties for nitric oxide production in aortic endothelium. Life Sci. 2002;72:511–520. doi: 10.1016/s0024-3205(02)02246-4. [DOI] [PubMed] [Google Scholar]
- Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol. 2001;532:759–769. doi: 10.1111/j.1469-7793.2001.0759e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Jaramillo P, Gonzalez MC, Palmer RM, Moncada S. The crucial role of physiological Ca2+ concentrations in the production of endothelial nitric oxide and the control of vascular tone. Br J Pharmacol. 1990;101:489–493. doi: 10.1111/j.1476-5381.1990.tb12735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill AM, Kim N, Duckles SP, Krause DN, Kontos HA. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke. 1999;30:2186–2190. doi: 10.1161/01.str.30.10.2186. [DOI] [PubMed] [Google Scholar]
- Malinski T, Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992;358:676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Nelin LD, Nash HE, Chicoine LG. Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol. 2001;281:L1232–L1239. doi: 10.1152/ajplung.2001.281.5.L1232. [DOI] [PubMed] [Google Scholar]
- Oike M, Kimura C, Koyama T, Yoshikawa M, Ito Y. Hypotonic stress-induced dual Ca2+ responses in bovine aortic endothelial cells. Am J Physiol. 2000;279:H630–H638. doi: 10.1152/ajpheart.2000.279.2.H630. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J General Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoclet JC, Muller B, Gyorgy K, Andriantsiothaina R, Kleschyov AL. The inducible nitric oxide synthase in vascular and cardiac tissue. Eur J Pharmacol. 1999;375:139–155. doi: 10.1016/s0014-2999(99)00221-6. [DOI] [PubMed] [Google Scholar]
- Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Jezova M, Ando H, Saavedra JM. Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J Cereb Blood Flow Metab. 2003;23:371–380. doi: 10.1097/01.WCB.0000047369.05600.03. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]