Abstract
We report the characterization of BopE, a type III secreted protein that is encoded adjacent to the Burkholderia pseudomallei bsa locus and is homologous to Salmonella enterica SopE/SopE2. Inactivation of bopE impaired bacterial entry into HeLa cells, indicating that BopE facilitates invasion. Consistent with this notion, BopE expressed in eukaryotic cells induced rearrangements in the subcortical actin cytoskeleton, and purified BopE exhibited guanine nucleotide exchange factor activity for Cdc42 and Rac1 in vitro.
Burkholderia pseudomallei is the etiological agent of melioidosis, a severe invasive infection of humans and animals that is endemic in subtropical areas (3, 6). Melioidosis has a remarkable capacity for latency. Development of disease 26 years after geographical exposure has been reported (21), and relapse is common even in patients treated with antibiotics (4). This is believed to result from the ability of B. pseudomallei to invade nonphagocytic host cells and to survive and replicate within phagocytes, where antibiotics may be less effective (14, 16, 25). The mechanism by which B. pseudomallei enters epithelial cells is poorly understood.
We and others have identified a putative type III protein secretion apparatus in B. pseudomallei (Bsa) similar to the Salmonella enterica Inv/Spa/Prg and Shigella flexneri Ipa/Mxi/Spa systems (1, 26, 31). Type III secretion systems are key virulence determinants of Salmonella, Shigella, and other gram-negative facultative intracellular pathogens and serve to inject bacterial proteins into target cells (reviewed in references 5, 10, 13, and 29). A subset of type III secretion system secreted proteins (translocators) is believed to interact with the eukaryotic cell membrane and mediate the delivery of secreted effector proteins. Once inside host cells the effector proteins subvert host cell processes to the benefit of the bacteria (reviewed in references 5 and 13).
Research in our laboratory and elsewhere has identified a number of Salmonella Inv/Spa/Prg secreted effector proteins (Sops) and shown that several of these are delivered into eukaryotic cells by mechanisms dependent on secreted translocator proteins (Sips) (11, 34, 35). Mutations that disrupt the Inv/Spa/Prg apparatus and selected sip and sop genes inhibit bacterial invasion of epithelial cells and Salmonella-induced enteritis (reviewed in references 10, 33, and 36). Some Sop effector proteins possess eukaryote-like enzymatic activities. In particular, it has been shown that the SopE and SopE2 proteins promote bacterial invasion (2, 35) by acting as guanine nucleotide exchange factors (GEFs) for RhoGTPases that regulate the actin network (12, 27, 30). SopE acts as a GEF for Cdc42, Rac1, and Rab5 (8, 9, 12, 23, 27); however, SopE2 efficiently activates Cdc42 but not Rac1 (8, 30), indicating that SopE and SopE2 may activate different signaling cascades during Salmonella infection. Mutation of Salmonella sopE and sopE2 reduces the induction of intestinal inflammatory and secretory responses in calves, suggesting that they play a role in Salmonella-induced enteritis (36, 37).
Recently we reported that mutations affecting putative components of the B. pseudomallei Bsa secretion and translocation apparatus impair intracellular survival of B. pseudomallei in murine macrophage-like cells and prevent escape of the bacteria from endocytic vesicles (31). Here we have investigated the role of a putative Bsa-secreted protein (BopE) that shares homology with the Salmonella SopE/SopE2 proteins. BopE is 27% identical over 168 amino acids to SopE and 28% identical over 139 amino acids to SopE2.
BopE is secreted by the Bsa type III secretion apparatus.
To study expression and secretion of BopE, a BopE-glutathione-S-transferase fusion protein was generated and polyclonal antiserum was raised against BopE in rabbits. A DNA fragment encoding the domain of BopE proposed to be required for GEF activity (amino acid residues 78 to 261) was amplified using the primers BopEGexBam (5′-CGGCAGCTATGGATCCACGGGCGACGCGAAAC-3′) and BopEGexE1 (5′-CCACGCTGAATTCTCACGCGCCGTCC-3′) and the product cloned into pGEX-2T (Amersham Biosciences, Little Chalfont, Buckinghamshire, England) via EcoRI and BamHI sites (underlined) in the primers. Following expression in Escherichia coli BL21(DE3) under isopropyl-β-d-thiogalactoside induction, the fusion protein was purified using glutathione Sepharose 4B resin and BopE78-261 released from glutathione-S-transferase by thrombin digestion. A 12-week-old New Zealand White rabbit was immunized subcutaneously four times at 2-week intervals with ca. 100 μg of purified BopE78-261 in Freund's incomplete adjuvant and serum collected 12 days after the final booster.
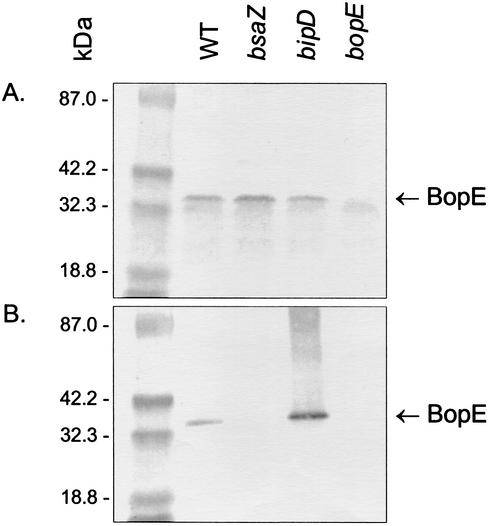
The BopE-specific antiserum was used to detect BopE in whole-cell and secreted protein fractions of B. pseudomallei strain 10276 and defined bsaZ, bipD, and bopE mutant strains described previously (31). BsaZ and BipD are homologous to the Salmonella SpaS and SipD proteins involved in secretion and translocation of Sop proteins, respectively. Bacteria were grown to stationary phase in Luria-Bertani broth, and culture supernatants were passed through 0.22-μm-pore-size filters prior to precipitation of secreted proteins with trichloroacetic acid (10% [vol/vol]). Approximately 25 μg of total protein (Fig. 1A) or secreted protein (Fig. 1B) was resolved by 4-to-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P membrane (Millipore, Bedford, Mass.). A 1:100 dilution of rabbit polyclonal antiserum to BopE78-261 was used, and bound antibody was detected with an anti-rabbit alkaline phosphatase conjugate. As expected, BopE was detected in whole-cell extracts of all strains except the 10276 bopE mutant (Fig. 1A). BopE secretion was dependent on the Bsa type III secretion apparatus, as no secretion was observed in a bsaZ mutant (Fig. 1B). In contrast, BopE secretion was elevated in B. pseudomallei lacking the putative translocator BipD (Fig. 1B). These data are consistent with the observation that Salmonella sip mutants secrete elevated levels of selected Sops (15, 35). Thus, our data suggest that the B. pseudomallei Bsa type III secretion apparatus is functional and that BopE is type III secreted.
FIG. 1.
Western blot analysis of BopE expression and secretion by B. pseudomallei 10276 wild type and bsaZ, bipD, and bopE mutant strains. Approximately 25 μg of total protein (A) or secreted protein (B) was probed with rabbit polyclonal antiserum to BopE78-261 and detected with an anti-rabbit alkaline phosphatase conjugate. Molecular mass markers are shown on the left.
BopE facilitates invasion of nonphagocytic cells.
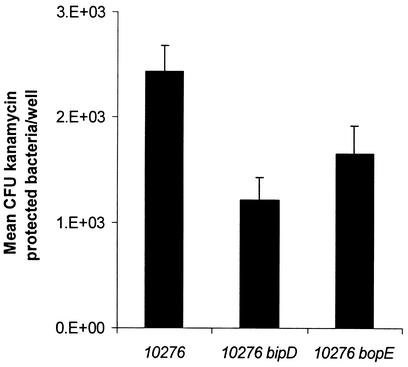
B. pseudomallei can invade and survive within nonphagocytic cells (14, 16). To assess the role of BopE in bacterial invasion, we quantified intracellular B. pseudomallei following infection of HeLa cells by strains 10276, 10276 bipD, and 10276 bopE by using a kanamycin protection assay. Previously we have been unable to detect significant invasion of HeLa cells by B. pseudomallei strain 10276 (31); however, we have found that invasion efficiency can be improved by centrifugation of the bacteria onto cell monolayers at 300 × g at the onset of infection. HeLa cells maintained in RPMI 1640 containing 10% (vol/vol) fetal calf serum were infected at a multiplicity of 10 with B. pseudomallei strains grown to stationary phase in Luria-Bertani broth at 37°C in a humidified 5% CO2 atmosphere. One hour after bacterial inoculation, monolayers were washed three times and overlaid with medium containing kanamycin (250 μg/ml) to kill extracellular bacteria. After 6 h viable intracellular bacteria were released by gentle lysis using 0.1% Triton X-100 and enumerated by plating of serial dilutions. We detected a statistically significant reduction in invasion of HeLa cells by the 10276 bopE mutant compared to that of the wild type (P = 0.0464) (Fig. 2), indicating that BopE, like Salmonella SopE/SopE2, facilitates bacterial invasion of nonphagocytic cells. SopE acts in concert with other type III secreted proteins to promote Salmonella invasion (38). Recently it was reported that the effector protein SopB, which possesses phosphatidylinositol phosphatase activity, influences Salmonella invasion (24, 39). It is likely that other type III secreted proteins influence invasion of nonphagocytic cells by B. pseudomallei. Consistent with this hypothesis we detected a highly significant reduction in invasion of HeLa cells by the 10276 bipD mutant (P = 0.0058). B. pseudomallei is a Centers for Disease Control and Prevention category B critical biological agent, and we were unable to trans-complement the bopE mutation owing to restrictions on genetic modification of the organism.
FIG. 2.
Invasion of HeLa cells by B. pseudomallei 10276 wild type and bipD and bopE mutant strains. HeLa cells (5 × 105) were infected at a multiplicity of infection 10 in triplicate for each assay and the results represent the arithmetic means (error bars show standard errors of the means) of results of four independent assays.
BopE expressed in eukaryotic cells induces rearrangements in the subcortical actin cytoskeleton.
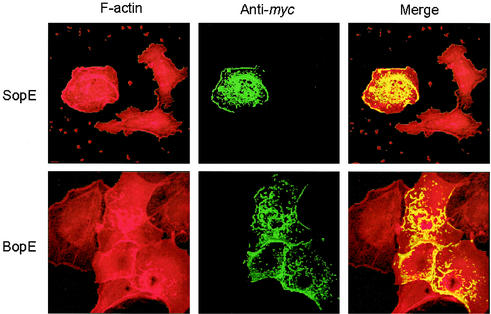
To assess the activity of BopE in eukaryotic cells, we amplified the entire coding sequence of bopE using the primers BopEpRKBam (5′-CTCGGATCCATGACTTACAACCCGAGAATCGGCGG-3′) and BopEpRKE1 (5′-CTCGAATTCTCACGCGCCGTCCGCCGCGTTCGTCGC-3′) and cloned the product into pRK5myc (17) via EcoRI and BamHI (underlined) sites. This created a BopE fusion protein with a myc tag at the N terminus. As a control the Salmonella enterica serovar Typhimurium sopE gene was cloned into pRK5myc in the same way using the primers SopEpRKBam (5′-CTCGGATCCGTGACAAAAATAACTTTATTTCC-3′) and SopEpRKE1 (5′-CTCGAATTCTCAGGGAGTGTTTTGGATATATT-3′). HeLa cells were transfected with pRK5myc-BopE or pRK5myc-SopE by using Lipofectamine (Invitrogen Life Technologies, Paisley, United Kingdom). Twenty-four hours after transfection, cells were stained for the presence of the myc-tagged protein with a mouse monoclonal myc-specific antibody (Invitrogen) detected with anti-mouse Alexa488 conjugate (Molecular Probes, Leiden, The Netherlands). Filamentous actin was stained using tetramethyl rhodamine isothiocyanate-conjugated phalloidin and the cells were viewed using a Leica TCS NT confocal laser scanning microscope. In cells expressing the myc-tagged BopE and SopE proteins filamentous actin was abundant under the membrane and was associated with areas of “ruffling” (Fig. 3), suggesting that the proteins interfere with actin dynamics in eukaryotic cells. Such rearrangements were not detected in nontransfected cells present in the same field. Some SopE and BopE appeared to colocalize with regions of intense F-actin staining in membrane ruffles (Fig. 3).
FIG. 3.
Confocal micrographs showing rearrangement of the subcortical actin cytoskeleton following expression of BopE and SopE in transiently transfected HeLa cells. HeLa cells were transfected with pRK5myc-SopE or pRK5myc-BopE. F-actin was stained red with tetramethyl rhodamine isothiocyanate-conjugated phalloidin, and myc-tagged protein was stained green with a myc-specific monoclonal antibody detected with anti-mouse Alexa488 conjugate. Nontransfected cells are present in each field for comparison. Magnification, ×500.
BopE is a GEF.
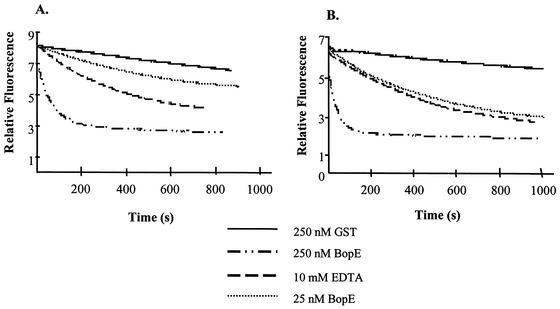
To determine if BopE possesses GEF activity, we employed fluorescence spectrometry using mGDP-loaded Rac1 (Fig. 4A) or Cdc42 (Fig. 4B) as a substrate (8). mGDP is a GDP derivative which is popular for kinetic studies because the fluorescence intensity of the mant moiety changes dramatically upon binding to GTPases. mGDP bound to Cdc42 has a fourfold-higher fluorescence intensity than unbound mGDP (20, 27, 28). Generally, the presence of the fluorophore has little effect on the kinetic parameters of G nucleotide release or GTP hydrolysis (7, 18, 19, 20, 32).
FIG. 4.
BopE acts as a GEF for the RhoGTPases Rac1 and Cdc42. The multiple turnover kinetics of guanine nucleotide exchange by BopE was analyzed by measuring the release of mantGDP from 10 μM Rac1-191·mantGDP (A) or Cdc42 Hs1-192·mantGDP (B) in the presence of 1 mM GDP and 25 nM or 250 nM BopE using fluorescence spectrometry (excitation wavelength, 366 nm; emission wavelength, 440 nm; step size, 1; band-pass, 4) at 20°C in a buffer containing 40 mM HEPES-NaOH (pH 7.4), 100 mM NaCl, and 5 mM MgCl2. Spontaneous dissociation of the RhoGTPase·mantGDP complex in the assay buffer or in assay buffer supplemented with 10 mM EDTA was measured as the control.
BopE78-261 was expressed and purified as described above and mGDP-Rac1 or mGDP-Cdc42 was prepared as described previously (8, 9). In the assay buffer (no EDTA or BopE) mGDP dissociation from Rac1 was very slow. In contrast, fast dissociation of the mGDP-Rac1 complex was observed in the presence of 25 nM BopE and even faster in the presence of 250 nM BopE (Fig. 4A) (kobs = 0.48 s−1). Similar observations were made using mGDP-Cdc42 as a substrate (Fig. 4B). These data demonstrate that BopE is an efficient GEF for Cdc42 and Rac1. The observed G-nucleotide exchange rates are lower than those observed with SopE from Salmonella serovar Typhimurium (8) but range in the same order of magnitude.
Taken together our observations suggest that B. pseudomallei enters epithelial cells by a mechanism dependent at least in part upon the Bsa type III protein secretion apparatus and one of its secreted proteins, BopE. It is likely that BopE is translocated into the host cell cytosol, where it may promote membrane ruffling by acting as a GEF for Cdc42 and Rac1. In Salmonella several Inv/Spa/Prg-secreted proteins (SopE, SopE2, and SopB) act in concert to promote bacterial uptake by nonphagocytic cells (38, 39), and Salmonella invasion probably evolved through the acquisition of new sequence elements (22). Given that a B. pseudomallei bipD mutant was impaired in invasion of HeLa cells to a greater extent than a bopE mutant, it is likely that other Bsa-secreted proteins may be involved in bacterial uptake. We are investigating the role of other putative type III secreted proteins in the host-cell interactions of B. pseudomallei.
REFERENCES
- 1.Attree, O., and I. Attree. 2001. A second type III secretion system in Burkholderia pseudomallei: who is the real culprit? Microbiology 147:3197-3199. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi, C. S., V. P. Singh, M. W. Wood, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2000. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol. 82:2341-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brett, P. J., and D. E. Woods. 2000. Pathogenesis of and immunity to melioidosis. Acta Trop. 74:201-210. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul, W., Y. Suputtamongkol, D. A. B. Dance, A. Rajchanuvong, J. Pattaraarechachai, and N. J. White. 1993. Relapse in melioidosis: incidence and risk factors. J. Infect. Dis. 168:1181-1185. [PubMed] [Google Scholar]
- 5.Cornelis, G. R., and F. van Gijsegem. 2000. Assembly and function of type III secretion systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 6.Dance, D. A. 2000. Melioidosis is an emerging global problem. Acta Trop. 74:115-119. [DOI] [PubMed] [Google Scholar]
- 7.Franken, S. M., A. J. Scheidig, U. Krengel, H. Rensland, A. Lautwein, M. Geyer, K. Scheffzek, R. S. Goody, H. R. Kalbitzer, E. F. Pai, et al. 1993. Three-dimensional structures and properties of a transforming and a nontransforming glycine-12 mutant of p21H-ras. Biochemistry 32:8411-8420. [DOI] [PubMed] [Google Scholar]
- 8.Friebel, A., H. Ilchmann, M. Aepfelbacher, K. Ehrbar, W. Machleidt, and W.-D. Hardt. 2001. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 276:34035-34040. [DOI] [PubMed] [Google Scholar]
- 9.Friebel, A., and W.-D. Hardt. 2000. Purification and biochemical activity of Salmonella exchange factor SopE. Methods Enzymol. 325:82-91. [DOI] [PubMed] [Google Scholar]
- 10.Galán, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell. Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 11.Galyov, E. E., M. W. Wood, R. Rosqvist, P. B. Mullan, P. R. Watson, S. Hedges, and T. S. Wallis. 1997. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 25:903-912. [DOI] [PubMed] [Google Scholar]
- 12.Hardt, W.-D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galán. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 13.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaniga, K., D. Trollinger, and J. E. Galán. 1995. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J. Bacteriol. 177:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kespichayawattana, W., S. Rattanachetkul, T. Wanun, P. Utaisincharoen, and S. Sirisinha. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism of cell-to-cell spreading. Infect. Immun. 68:5377-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519-529. [DOI] [PubMed] [Google Scholar]
- 18.Lenzen, C., R. H. Cool, and A. Wittinghofer. 1995. Analysis of intrinsic and CDC25-stimulated guanine nucleotide exchange of p21ras-nucleotide complexes by fluorescence measurements. Methods Enzymol. 255:95-109. [DOI] [PubMed] [Google Scholar]
- 19.Lenzen, C., R. H. Cool, H. Prinz, J. Kuhlmann, and A. Wittinghofer. 1998. Kinetic analysis by fluorescence of the interaction between Ras and the catalytic domain of the guanine nucleotide exchange factor Cdc25Mm. Biochemistry 37:7420-7430. [DOI] [PubMed] [Google Scholar]
- 20.Leonard, D. A., T. Evans, M. Hart, R. A. Cerione, and D. Manor. 1994. Investigation of the GTP-binding/GTPase cycle of Cdc42Hs using fluorescence spectroscopy. Biochemistry 33:12323-12328. [DOI] [PubMed] [Google Scholar]
- 21.Mays, E. E., and E. A. Ricketts. 1975. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six years following initial geographic exposure. Chest 68:261-263. [DOI] [PubMed] [Google Scholar]
- 22.Mirold, S., K. Ehrbar, A. Weissmuller, R. Prager, H. Tschape, H. Russmann, and W.-D. Hardt. 2001. Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1 (SPI1), SPI5, and sopE2. J. Bacteriol. 183:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee, K., S. Parashuraman, M. Raje, and A. Mukhopadhyay. 2001. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J. Biol. Chem. 276:23607-23615. [DOI] [PubMed] [Google Scholar]
- 24.Norris, F. A., M. P. Wilson, T. S. Wallis, E. E. Galyov, and P. W. Majerus. 1998. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphatase. Proc. Natl. Acad. Sci. USA 95:14057-14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruksachartvuthi, S., N. Aswapokee, and K. Thankerngpol. 1990. Survival of Pseudomonas pseudomallei in human phagocytes. J. Med. Microbiol. 31:109-114. [DOI] [PubMed] [Google Scholar]
- 26.Rainbow, L., C. A. Hart, and C. Winstanley. 2002. Distribution of type III secretion gene clusters in Burkholderia pseudomallei, B. thailandensis and B. mallei. J. Med. Microbiol. 51:374-384. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph, M. G., C. Weise, S. Mirold, B. Hillenbrand, B. Bader, A. Wittinghofer, and W.-D. Hardt. 1999. Biochemical analysis of SopE from Salmonella typhimurium, a highly efficient guanosine nucleotide exchange factor for RhoGTPases. J. Biol. Chem. 274:30501-30509. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph, M. G., P. Bayer, A. Abo, J. Kuhlmann, I. R. Vetter, and A. Wittinghofer. 1998. The Cdc42/Rac interactive binding region motif of the Wiskott Aldrich syndrome protein (WASP) is necessary but not sufficient for tight binding to Cdc42 and structure formation. J. Biol. Chem. 273:18067-18076. [DOI] [PubMed] [Google Scholar]
- 29.Sansonetti, P. J. 2001. Rupture, invasion and inflammatory destruction of the intestinal barrier by Shigella, making sense of prokaryote-eukaryote cross-talks. FEMS Microbiol. Rev. 25:3-14. [DOI] [PubMed] [Google Scholar]
- 30.Stender, S., A. Friebel, S. Linder, M. Rohde, S. Mirold, and W.-D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 31.Stevens, M. P., M. W. Wood, L. A. Taylor, P. Monaghan, P. Hawes, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2002. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behavior of the pathogen. Mol. Microbiol. 46:649-659. [DOI] [PubMed] [Google Scholar]
- 32.Tan, Y. C., H. Wu, W. N. Wang, Y. Zheng, and Z. X. Wang. 2002. Characterization of the interactions between the small GTPase RhoA and its guanine nucleotide exchange factors. Anal. Biochem. 310:156-162. [DOI] [PubMed] [Google Scholar]
- 33.Wallis, T. S., and E. E. Galyov. 2000. Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36:997-1005. [DOI] [PubMed] [Google Scholar]
- 34.Wood, M. W., M. A. Jones, P. R. Watson, A. M. Siber, B. A. McCormick, S. Hedges, R. Rosqvist, T. S. Wallis, and E. E. Galyov. 2000. The secreted effector protein of Salmonella dublin, SopA, is translocated into eukaryotic cells and influences the induction of enteritis. Cell. Microbiol. 2:293-303. [DOI] [PubMed] [Google Scholar]
- 35.Wood, M. W., R. Rosqvist, P. B. Mullan, M. H. Edwards, and E. E. Galyov. 1996. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a Sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol. 22:327-338. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, S., R. A. Kingsley, R. L. Santos, H. Andrews-Polymenis, M. Raffatellu, J. Figueiredo, J. Nunes, R. M. Tsolis, L. G. Adams, and A. J. Baumler. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, S., R. L. Santos, R. M. Tsolis, S. Mirold, W.-D. Hardt, L. G. Adams, and A. J. Baumler. 2002. Phage mediated horizontal transfer of the sopE1 gene increases enteropathogenicity of Salmonella enterica serotype Typhimurium for calves. FEMS Microbiol. Lett. 217:243-247. [DOI] [PubMed] [Google Scholar]
- 38.Zhou, D., and J. Galán. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3:1293-1298. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, D., L. M. Chen, L. Hernandez, S. B. Shears, and J. E. Galán. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248-259. [DOI] [PubMed] [Google Scholar]