Abstract
We combined the interstitial sampling method of microdialysis with the natural tracer qualities (i.e. non-recyclability) of the amino acid 3-methylhistidine (3MH) to uniquely study in vivo degradation of the two most abundant skeletal muscle proteins, myosin and actin. Interstitial 3MH concentration was measured before and for 24 h following a single bout of resistance exercise in eight young (27 ± 2 years) and eight old (75 ± 4 years) men. The exercise bout consisted of four exercises (3 sets of 8 repetitions at 80% one-repetition maximum (1RM) per exercise) emphasizing the quadriceps. Interstitial 3MH concentration was calculated using the internal reference method from microdialysate samples that were obtained from two microdialysis probes placed in the vastus lateralis. Resting interstitial 3MH concentration was 44% higher (P < 0.05) in the old (6.16 ± 0.56 nmol ml−1) as compared with the young (4.28 ± 0.27 nmol ml−1). Interstitial 3MH was not different (P > 0.05) from preexercise at any time point within the 24 h following exercise in both the young and the old. Leg arteriovenous exchange measurements in a separate group of young subjects also showed no increase in 3MH release during the 4 h following a resistance exercise bout compared with a non-exercised control leg (control leg: −28 ± 6, exercise leg: −28 ± 11 nmol min−1). These results suggest that myosin and actin proteolysis are not increased in the first 24 h following a standard bout of resistance exercise, and this response is not altered with ageing. The higher interstitial 3MH concentration in the old suggests an increased proteolysis of the two main contractile proteins in the rested and fasted state, which is consistent with a decrease in muscle mass with ageing. Microdialysis is an appropriate methodology for use in ageing individuals and is compatible with high-intensity resistance exercise.
Skeletal muscle proteolysis in humans has been relatively understudied, primarily due to methodological limitations. As a result, little is known about the role of muscle protein degradation in the loss of muscle mass that occurs with ageing. The primary methodology used to address this question in humans has been the appearance of the unique amino acid 3-methylhistidine (3MH) in the urine (Uauy et al. 1978; Young & Munro, 1978; Fielding et al. 1991; Welle et al. 1995; Hasten et al. 2000). 3MH is unique because it cannot be reutilized for protein synthesis (Young et al. 1972; Long et al. 1975; Young & Munro, 1978), it is a component of the two main skeletal muscle contractile proteins, myosin and actin (Bilmazes et al. 1978; Young & Munro, 1978), and is primarily found in skeletal muscle (Haverberg et al. 1975; Young & Munro, 1978). Therefore, appearance of 3MH in the urine provides a measure of whole body myosin and actin proteolysis (Young & Munro, 1978). However, this method has been strongly criticized because non-skeletal muscle sources with high protein turnover rates, relative to skeletal muscle, could disproportionately contribute to urinary 3MH levels (Rennie & Millward, 1983). Total urinary 3MH excretion does not allow for the study of specific muscles due to the nature of the whole body estimate that urinary measurements provide. To circumvent these issues, arteriovenous differences of 3MH across a limb have been successfully employed (Rennie et al. 1984; Rifai et al. 1993; Sjolin et al. 1989), but to our knowledge have not been used to study muscle proteolysis in ageing individuals. More recently, three stable isotope methodologies have been introduced to measure mixed muscle proteolysis, a tracee release method (Zhang et al. 1996; Phillips et al. 1997), an arteriovenous balance method (Wolfe, 1992; Volpi et al. 2001), and a three-pool method (Biolo et al. 1995a), of which the latter two have provided conflicting results regarding resting proteolysis in ageing individuals (Volpi et al. 1999, 2000, 2001).
Microdialysis is an established methodology that has been used over the last decade to study the interstitial space of skeletal muscle under a wide range of experimental conditions (Lonnroth et al. 1987; Henriksson, 1999; Langberg et al. 1999; Hickner, 2000). In this investigation, we sought to couple the muscle-specific nature of the microdialysis methodology with the unique qualities of 3MH to address the issue of skeletal muscle proteolysis with ageing.
Given that resistance exercise increases muscle mass and function in the young and elderly, we were also interested in the proteolytic response to a single bout of resistance exercise. A single bout of resistance exercise in young men and women has been shown to increase mixed muscle proteolysis for up to 24 h postexercise (Biolo et al. 1995b, 1999; Phillips et al. 1997, 1999). Thus, an increase in muscle proteolysis is apparently a necessary component to the metabolic response following resistance exercise. It would be of interest to know if myosin and actin were a component of the postresistance exercise increase in mixed muscle proteolysis, and if this response was altered with ageing.
In a group of older and younger men we examined skeletal muscle proteolysis by measuring the skeletal muscle interstitial concentration of 3MH at rest and for 24 h after a single bout of resistance exercise. We hypothesized that at rest skeletal muscle 3MH concentration would be higher in older as compared with younger individuals. We also hypothesized that the interstitial 3MH concentration following a single bout of resistance exercise would be increased from preexercise in both the young and old, but this response would be blunted in the older relative to the younger individuals. Femoral arteriovenous 3MH differences were also measured following resistance exercise in a separate group of young individuals, which support the findings from the microdialysis measurements.
Methods
Subjects
Eight young (27 ± 2 years, 178 ± 5 cm, 79 ± 10 kg, 20 ± 7% body fat; mean ± s.d.) and eight old (75 ± 4 years*, 178 ± 7 cm, 91 ± 11 kg*, 29 ± 10% body fat; *P < 0.05 versus young) men were included in this investigation following a physical examination, which included blood and urine analyses, and an electrocardiogram (older subjects). Subjects were excluded if they had any acute or chronic illness, cardiac, pulmonary, liver, or kidney abnormalities, uncontrolled hypertension, insulin- or non-insulin-dependent diabetes, abnormal blood or urine chemistries, arthritis, a history of neuromuscular problems, or if they smoked tobacco. It was our intent to include sedentary healthy older and younger individuals; therefore, we excluded individuals that were completing any formal exercise programs or physical activity outside of their activities of daily living within the previous 6 months. Body composition was determined using whole-body air-displacement plethysmography (Life Measurement Instruments, Concord, CA, USA). All procedures, risks and benefits associated with the experimental testing were explained to the subjects prior to signing a consent form adhering to the guidelines of the Institutional Review Board of the University of Arkansas for Medical Sciences. This study was conducted in accordance with the Declaration of Helsinki.
Experimental design
Each subject completed the protocol over a period of 4.5 days, which entailed 3 days of diet and activity control, two overnight stays, and a 1.5-day microdialysis and exercise study of skeletal muscle 3MH. Specific details of the study design and methods are listed below.
Diet and activity control
Prior to day 1 of the protocol, each subject was counselled by the General Clinical Research Center (GCRC) dietitian to maintain their normal dietary habits (i.e. general food choices and times of eating), to eat an adequate amount of protein (1.0–1.2 g per kg body weight) and calories (estimated from Harris–Benedict (Harris & Benedict, 1919) times an activity factor of 1.5), and to ensure their diet was meat-free for the duration of the study. These dietary controls were instilled to ensure no subject consumed an abnormally high or low amount of protein or calories prior to the metabolic measurements on days 4 and 5 and to eliminate the influence of exogenous 3MH on the measurement of endogenous 3MH. Subjects were also asked to refrain from completing any activity outside of their normal daily activities during the study. On day 3, subjects were provided with their evening meal, which consisted of 50% of their estimated daily calories (56% carbohydrate, 13% protein and 30% fat). On day 4, subjects were provided with 100% of their estimated calories, 50% from Ensure Plus (Ross, Columbus, OH, USA) (53% carbohydrate, 15% protein, and 32% fat) during hour 7 of the microdialysis protocol (see Microdialysis and exercise bout section) and the other 50% provided as their evening meal (identical to the evening meal on day 3). No food was provided prior to or during the morning measurements on day 5. This feeding regimen standardized the morning postabsorptive metabolic measurements on days 4 and 5 (∼12 h fast each day) and controlled the composition, amount, and timing of feeding during the microdialysis studies.
Microdialysis and exercise bout
Prior to the evening meal on the third day of diet and activity control, two CMA 60 microdialysis probes (30 mm, 20 kDa cutoff, CMA Microdialysis, Solna, Sweden) (Rosdahl et al. 1993) were placed in the vastus lateralis of the dominant leg. The microdialysis probes were perfused with sterile water at 10 μl min−1 for 5 min before and immediately after probe insertion with a calibrated microinfusion pump (Harvard PHD 2000 MD, Natick, MA, USA). The probes were then disconnected from the perfusion pump and left in place with the inlet and outlet tubing capped, which was secured to the leg for the overnight stay. The timing of the probe insertions (∼12 h before the preexercise measurements) was to ensure that the preexercise measurements would not be influenced by the probe insertion (Langberg et al. 1999). The following morning each subject underwent the measurement of resting skeletal muscle interstitial 3MH levels and blood flow, estimated with the ethanol technique (Hickner et al. 1991, 1992, 1994, 1995). The microdialysis probes were perfused with a sterile Ringer solution supplemented with 37 g l−1 dextran-70 (70 kDa, Sigma, St Louis, MO, USA), a small amount of d-[3H]glucose to calculate the recovery over the microdialysis probe membranes (see Microdialysis probe recovery section), and 10 mm ethanol to estimate local blood flow. Dextran was used to assure there was no net fluid transport across the microdialysis membrane (i.e. perfusate volume equalled dialysate volume) (Hamrin et al. 2002). All dialysate samples were collected in 30 min aliquots in sealed microvials (CMA Microdialysis, Solna, Sweden), that were weighed on a precision microbalance (Cahn 35, Orion Research, Beverly, MA, USA) before and after each collection period to determine actual dialysate volume. At the start of the resting measurements, each probe was reconnected to the perfusion pump and perfused for 5 min at 10 μl min−1 (flush) and then at 2.0 μl min−1 for 2.5 h while the subjects rested quietly. The first 30 min was discarded and the next two 30 min collections from both probes were used to measure dialysate 3MH and ethanol concentrations, and probe recovery (see 3MH, d-[3H]glucose, and ethanol determination and Microdialysis probe recovery sections).
Following the resting measurements, subjects completed a unilateral exercise bout that emphasized the quadriceps muscles of the dominant leg and lasted about 1 h. Prior to the resistance exercise bout, each subject completed light stretching and a 10 min warm-up on a cycle ergometer. The exercise bout consisted of four exercises, two on a leg extension machine and two on a leg press machine. One-repetition maximum (1RM) measurements were completed for each exercise prior to completing that exercise, followed by 5 min rest. Each exercise consisted of three sets of eight repetitions at 80% of the individual's 1RM, with 2 min rest between sets and 5 min rest between exercises.
After the exercise training bout each subject, while resting quietly, underwent 10 h of postexercise measurements, of which the 0.5–5, 7.5–8, and 9–10 h samples were used for 3MH, d-[3H]glucose, and ethanol measurements. During this time, the microdialysis probes were perfused as before. Following the postexercise collection period the microdialysis probes were flushed with sterile water at 10 μl min−1 for 5 min and then disconnected and secured for the overnight stay as before. The following morning, subjects underwent 2.5 h of resting measurements, with the 0.5–1.5 h of collections being used for 23–24 h postexercise measurements. Both microdialysis probes were reconnected, flushed, and perfused in the same manner as the previous morning.
Collections from 1 h segments before (1.5–2.5 h), and after exercise (5–6, 8–9, and 24–25 h) were used for other unrelated measurements and these data are not presented here.
Microdialysis probe recovery
Microdialysis probe recovery for 3MH was determined by the internal reference technique (Scheller & Kolb, 1991) by adding 0.2 μCi ml−1 of d-[3H]glucose (Amersham Pharmacia Biotech, Piscataway, NJ, USA) to the perfusate, since glucose is similar in size (180 Da) to 3MH (169 Da). In vivo recovery was calculated using the following formula: (Perfusatedpm – Dialysatedpm)/Perfusatedpm.
3MH, ethanol, and d-[3H]glucose determination
The microvials for each 30 min collection were immediately stored at 4°C, until an aliquot was removed the day of the experiments for ethanol and d-[3H]glucose analysis, and the remaining volume was stored at −80°C until assayed for 3MH.
The concentration of 3MH in the dialysate samples was determined by high-performance liquid chromatography (250 mm × 4.6 mm C-18 reverse phase column, flow rate 1.5 ml min−1) and fluorometric detection (model 474, Waters, Milford, MA, USA) as previously described (Wassner et al. 1980; Fetterer & Allen, 2000), with slight modification for sample volumes. Derivatization was completed by placing 25 μl of dialysate or standard (M19930, Pfaltz and Bauer, Waterbury, CT, USA), 65 μl of borate buffer (0.4 m boric acid, adjusted to pH 12.2 with NaOH), 65 μl of fluorescamine reagent (160 mg fluorescamine in 100 ml acetonitrile) in a 0.6 ml microcentrifuge tube, which was then vortexed and allowed to stand at room temperature for 5 min. Ten microlitres of concentrated perchloric acid was added to the tube, the tube was then capped and heated at 80°C for 1 h. After cooling to room temperature, samples were neutralized with 25 μl of 0.5 m MOPS in 3 m NaOH. Samples were injected with an autosampler (model 717plus, Waters) maintained at 4°C. The mobile phase was 25% acetonitrile and 75% 10 mm Na2HPO4 adjusted to pH 7.5 with phosphoric acid. Peaks were monitored at 365 nm (excitation) and 460 nm (emission) and integrated with chromatography software (Millennium, ver. 3.2, Waters). Interstitial 3MH concentration for each sample was determined by accounting for probe recovery for that sample. Calculated interstitial 3MH concentrations from both probes from a subject for each time point were averaged to represent that time point.
Ethanol concentration in each perfusate and dialysate sample was measured according to the method originally described by Bernt & Gutmann (1974) and modified by Hickner et al. (1994, 1995). Briefly, 2 μl of sample or standard was added to 1.0 ml of glycine–hydrazine buffer (74.6 mm sodium pyrophosphate, 22.0 mm glycine, 60.0 mm hydrazine sulphate and 0.54 mm βNAD, pH 8.9). Before and 60 min after the addition of 10 μl of enzyme (1.7 mg alcohol dehydrogenase per ml of water), each sample or standard was read on a fluorometer (Turner Quantech, Barnstead Thermolyne, Dubuque, IA, USA), with the excitation and emission wavelengths set at 360 and 415 nm, respectively. After determination of actual perfusate and dialysate concentrations (mm) from the standard curve, ethanol outflow to inflow ratio (o/i) was calculated for each probe at each time point. The calculated o/i from both probes for a subject at each time point were averaged to represent that time point.
DPM of d-[3H]glucose was measured from 10 μl of each perfusate and dialysate sample placed in 3 ml of scintillation fluid and 250 μl of water in a 7 ml scintillation vial on a Beckman LS 6500 scintillation counter (Beckman Coulter, Inc., Fullerton, CA, USA).
Arteriovenous 3MH Measurements
Arteriovenous 3MH exchange across the legs was measured in a separate group of young males (n = 3; 23 ± 2 years, 181 ± 3 cm, 70 ± 9 kg; mean ± s.d.) to compare with the findings of the microdialysis measurements from the young and old. All procedures, risks and benefits associated with the experimental testing were explained to the subjects prior to obtaining consent adhering to the guidelines of the Ethics Committee of Copenhagen and were in accordance with the Declaration of Helsinki. Subjects for this component of the study were recreationally active and were asked to follow the same diet and activity restrictions as outlined for the microdialysis study with the young and old.
The design of this component of the study and the timeline of the sampling were set to include arterial and venous 3MH measurements during the initial 4 h after a resistance exercise bout. The resistance exercise bout was identical to that used in the microdialysis study of the young and old, with regards to emphasizing the quadriceps muscles, 1RM measurements, duration of the rest periods, exercise intensity, sets and repetitions for each exercise, and the fact the exercise was completed with one leg (while the other leg served as a control for the arteriovenous measurements). However, the exercise bout included three exercises (as compared with four), all completed on a leg extension device.
Subjects reported to the lab in the morning and measurements were made following an overnight fast. No food was provided prior to or during the measurements on this day. Indwelling catheters were placed in both femoral veins and one femoral artery in the inguinal region of the legs. Catheters were kept patent with a saline infusion. Arterial and venous blood samples were collected every 30 min following exercise starting at 45 min and ending at 3 h and 45 min postexercise. Blood samples for 3MH determination were collected in EDTA tubes, centrifuged at 2000 g and 4°C for 10 min, and the plasma was stored at −80°C until analysis. Separate blood samples were taken at each time point for the measurement of haematocrit. The arterial and venous plasma samples were deproteinized with concentrated perchloric acid and analysed for 3MH concentration as described in the 3MH, ethanol, and d-[3H]glucose determination section.
Leg blood flow was determined using an ultrasound Doppler machine (SONOLINE Sienna, Siemens, Japan) equipped with a linear array transducer (7.5L40, Siemens) probe (42 mm length) by measuring femoral arterial diameter and mean blood velocity (Radegran, 1999). All of the bilateral measurements were made distal to the inguinal ligament and catheter insertions. Plasma flow was calculated as the blood flow multiplied by 1 – haematocrit. Net release or uptake of 3MH was calculated as the femoral arteriovenous difference in the plasma concentration of 3MH multiplied by the plasma flow (Andersson et al. 1987). We chose plasma flow and plasma concentration to calculate the exchange across each leg; however, whole blood flow and concentration could also be used since the concentration of 3MH in red blood cells is similar to that of plasma (Andersson et al. 1987). For each leg, the average of each 30-min measurement was taken to represent the average net release of 3MH during the 4 h postexercise period.
Statistics
Subject characteristics were compared with Student's paired t test. A two-way (age and time) analysis of variance (ANOVA) with repeated measures on time was used to compare the interstitial 3MH levels, ethanol o/i, microdialysis vial weights, and the in vivo probe recovery between the young and old before and after exercise. A paired t test was used to compare the average arteriovenous 3MH release from the control and exercise leg from the separate study of young subjects. When necessary, Tukey's post hoc test was completed to determine the location of differences over time. Significance was accepted at P < 0.05. Except where noted, data are presented as means ± s.e.m.
Results
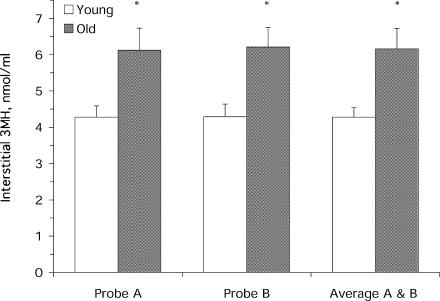
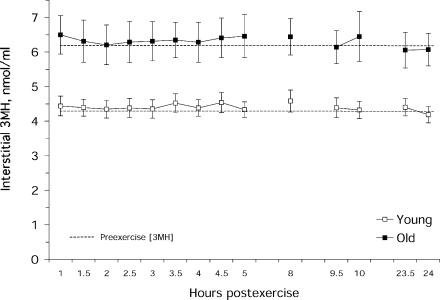
Resting interstitial 3MH levels were 44% higher (P < 0.05) in the old versus young (Fig. 1). The coefficient of variation for the two measurements of interstitial 3MH made at rest from the same probe was 8 ± 2% for the young and 5 ± 1% for the old. Interstitial 3MH concentration after exercise was unchanged (P > 0.05) from rest in both the young and old (Fig. 2). The coefficient of variation between the two probes within each subject was similar between the young and old, and similar across all time points before and after exercise (young: 7 ± 1%, old: 6 ± 0.3%).
Figure 1. Resting interstitial 3MH concentration in the young and old individuals.
Values for probe A and B are shown to illustrate the similarity between probes within each subject (see Results). *P < 0.05 from young.
Figure 2. Interstitial 3MH concentration following exercise in the young and old individuals.
There were no differences (P > 0.05) from preexercise in either the young or old individuals. Hours represent the 30 min collection prior to that time.
Ethanol o/i at rest and at all time points after exercise was similar (P > 0.05) between the young and old (Table 1). Ethanol o/i was significantly lower (P < 0.05) than preexercise at 0.5–1.0, and 1.0–1.5 h postexercise, and was also significantly lower (P < 0.05) than preexercise the first measurement the morning after the exercise bout (23.0–23.5 postexercise) (Table 1).
Table 1.
Ethanol outflow to inflow ratio
| Rest | 0.5–1 | 1–1.5 | 1.5–2 | 2–2.5 | 2.5–3 | 3–3.5 | 3.5–4 | 4–4.5 | 4.5–5 | 7.5–8 | 9–9.5 | 9.5–10 | 23–23.5 | 23.5–24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young | 0.140 | 0.094 | 0.102 | 0.122 | 0.134 | 0.120 | 0.142 | 0.110 | 0.136 | 0.117 | 0.115 | 0.122 | 0.153 | 0.097 | 0.127 |
| ± 0.017 | ± 0.01* | ± 0.02* | ± 0.021 | ± 0.026 | ± 0.015 | ± 0.017 | ± 0.021 | ± 0.019 | ± 0.014 | ± 0.011 | ± 0.025 | ± 0.020 | ± 0.017* | ± 0.027 | |
| Old | 0.149 | 0.107 | 0.121 | 0.127 | 0.131 | 0.149 | 0.137 | 0.127 | 0.118 | 0.127 | 0.123 | 0.117 | 0.131 | 0.108 | 0.110 |
| ± 0.020 | ± 0.019 | ± 0.023 | ± 0.029 | ± 0.038 | ± 0.031 | ± 0.026 | ± 0.024 | ± 0.027 | ± 0.024 | ± 0.023 | ± 0.023 | ± 0.020 | ± 0.026 | ± 0.026 |
Values are means ± s.e.m. Average for each 30 min time point is the average of all eight subjects in each group with each subject represented by the average percentage recovery obtained from their two probes.
P < 0.05 compared with resting values for young and old combined.
Dialysate weights were similar to the expected weight of 62.22 mg (assuming a perfusate density of 1.037 g ml−1 and an expected dialysate volume of 60.00 μl) for all of the collection periods (data not shown). Dialysate weights were also similar between young and old over all the collection times, and averaged 62.73 ± 0.21 and 61.26 ± 0.35 mg, respectively. At rest and following exercise, the probe recovery of 3MH was not different (P > 0.05) between the young and old. However, probe recovery did increase (P < 0.05) in both groups postexercise as compared with preexercise (Table 2).
Table 2.
Microdialysis probe recovery
| Rest | 0.5–1 | 1–1.5 | 1.5–2 | 2–2.5 | 2.5–3 | 3–3.5 | 3.5–4 | 4–4.5 | 4.5–5 | 7.5–8 | 9–9.5 | 9.5–10 | 23–23.5 | 23.5–24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young | 47 ± 2* | 56 ± 1 | 55 ± 1 | 56 ± 1 | 57 ± 1 | 55 ± 1 | 54 ± 2 | 55 ± 1 | 54 ± 1 | 55 ± 1 | 55 ± 1 | 58 ± 2 | 56 ± 1 | 55 ± 2 | 57 ± 1 |
| Old | 48 ± 2* | 56 ± 3 | 57 ± 2 | 57 ± 3 | 58 ± 3 | 56 ± 3 | 56 ± 3 | 57 ± 3 | 57 ± 3 | 57 ± 3 | 54 ± 2 | 54 ± 3 | 53 ± 2 | 61 ± 2 | 61 ± 3 |
Values are means ± s.e.m. Data are presented as percentages. Average for each 30 min time point is the average of all eight subjects in each group with each subject represented by the average percent recovery obtained from their two probes. Resting values were significantly lower (P < 0.05) than all other time points for both young and old.
The average arteriovenous 3MH exchange across the leg in the group of three young men was similar (P > 0.05) between the control and exercise legs during the 4 h following the resistance exercise bout (control: −28 ± 6, exercise: −28 ± 11 nmol min−1).
Discussion
There are several novel findings from this study. First, it appears that proteolysis of myosin and actin, the two most abundant contractile proteins, is increased with ageing, which may contribute to the loss of muscle mass that occurs with ageing. Second, proteolysis of myosin and actin does not appear to increase within the first 24 h following a single bout of high intensity resistance exercise in young men, and this response does not seem to be altered in 71- to 83-year-old men. Third, microdialysis is compatible with the high dynamic forces associated with high-intensity resistance exercise. Fourth, microdialysis is an appropriate methodology for use in the study of skeletal muscle in older individuals at rest and following exercise.
Comparing the current study to the relatively few studies of ageing and resting skeletal muscle proteolysis (Uauy et al. 1978; Fielding et al. 1991; Welle et al. 1995; Volpi et al. 1999, 2000, 2001; Hasten et al. 2000) is difficult due to conflicting results obtained from different methods within the same individuals (Volpi et al. 2001), different proteins studied (i.e. mixed muscle protein versus myosin and actin), and the aforementioned methodological limitations (Rennie & Millward, 1983). However, regardless of methodology, a general observation regarding the magnitude of difference reported between young and old individuals provides some insight. Previous studies report a range from no change to a 25% increase in skeletal muscle proteolysis with ageing (Uauy et al. 1978; Fielding et al. 1991; Welle et al. 1995; Volpi et al. 1999, 2000, 2001; Hasten et al. 2000). The increase in muscle proteolysis with ageing from the current investigation was greater (+44%) than any of the previous studies, and is likely to be explained by the direct sampling of the muscle that the microdialysis methodology provides.
Using either the tracee release method or the three-pool method, mixed muscle proteolysis has been shown to increase on average 31–61% in the first 3–4 h (Biolo et al. 1995b, 1999; Phillips et al. 1997, 1999), and remain increased through 24 h (Phillips et al. 1997) following a single bout of resistance training in young individuals. Data from the current study suggest that myosin and actin proteolysis are not a significant component of the mixed muscle proteolysis response to a standard bout of resistance exercise in the young, and this response is maintained in ageing skeletal muscle. The measurements of arteriovenous 3MH exchange across the leg in the young subjects provide additional support to the findings from the microdialysis measurements. The average net release of 3MH from the control leg (and resistance exercise leg) over the 4 h following the resistance exercise bout are comparable to resting levels previously reported for young individuals (Andersson et al. 1987), and show that myosin and actin proteolysis are not increased during this time frame following resistance exercise. However, moderate long-term aerobic exercise has been reported to decrease skeletal muscle myosin and actin proteolysis, as shown by a decrease in intramuscular free 3MH in the muscle (Rennie et al. 1981). The suggestion that proteolysis of certain muscle protein fractions may respond differently to various stimuli is also supported by the study of Svanberg et al. (1996). These authors have shown, with arteriovenous measurements across the arm and leg, that an infusion of amino acids has no influence on myosin and actin proteolysis, while proteolysis of other proteins is responsive to an amino acid provision.
Our microdialysis measurements of myosin and actin proteolysis within and between individuals are based on a few assumptions. First, the contribution of exogenous 3MH to the interstitial measurements is negligible. This has been addressed by eliminating 3MH in the diet of the subjects for 3 days prior to and during the 2 days of the microdialysis experiments (Bilmazes et al. 1978; Young & Munro, 1978; Lukaski et al. 1981). Second, the amount of methylated myosin and actin is consistent among all subjects. Bilmazes et al. (1978) have shown that the amount of 3MH in several different skeletal muscles from men and women age 19–74 years is equivalent. Further, we have shown that the amount of myosin and actin per unit wet weight of muscle is similar between young and old men and women (Trappe et al. 2003). Therefore, we feel it is appropriate to assume the amount of 3MH per unit myosin and actin and per unit muscle of our subjects is not different. Third, the amount of muscle sampled by the microdialysis probe is equivalent between subjects. Rosdahl et al. (1993) have calculated the amount of tissue sampled with the microdialysis probes used in the current study to be ∼100 mg. Given that our measurements are taken from the average of two probes, we essentially sampled 200 mg of muscle during our measurements, which is greater than is typically used for most methodologies that incorporate human muscle biopsy measurements. If this assumption is violated, it is likely to be violated in a direction that supports an even larger difference in resting proteolysis. That is, the older individuals have less muscle protein per unit of muscle tissue sampled (i.e. per microdialysis probe) (Trappe et al. 2003), which would suggest they are effectively producing more 3MH per unit muscle. Fourth, circulating levels of 3MH are either not different among individuals and/or the circulating levels do not contribute to the interstitial levels that we measured. We did not measure arterial 3MH levels in each subject during the course of the experiment. However, at any given rate of myosin and actin proteolysis the amount of circulating 3MH is directly related to the amount of muscle mass of the individual (Lukaski et al. 1981; Uauy et al. 1978). Although we did not measure muscle mass in our subjects, it is reasonable to assume that muscle mass was lower in the older men as compared with the younger men. Thus, any contribution that circulating 3MH may have made to the interstitial levels would have suppressed the difference that we found between the young and old, suggesting an even larger difference in proteolysis between the young and old. Fifth, the transmembrane transport of 3MH is similar between young and old. Human skeletal muscle transmembrane 3MH transport has not been directly measured in this or any other investigation to our knowledge. However, the few studies which have investigated general amino acid transport in skeletal muscle in young and old men and women at rest in the fasted and fed state suggest that it is not influenced by age (Volpi et al. 1999, 2000, 2001). Finally, it should be noted that these assumptions primarily relate to comparisons between individuals and not necessarily within the same individual for repeated measurements.
Because blood flow near the microdialysis probe could influence the measurements of 3MH and our estimates of proteolysis, we chose to monitor local changes in blood flow with the ethanol technique (Hickner et al. 1991, 1992, 1994, 1995). This method utilizes the ratio of the concentration of ethanol in the outflow (dialysate) to inflow (perfusate) (i.e. o/i) as an indicator of local blood flow. A decrease in the o/i reflects an increase in blood flow and an increase in the o/i reflects a decrease in blood flow in the region sampled by the microdialysis probe. Our measurements of interstitial 3MH could have been influenced by differences and/or changes in blood flow; however, our comparisons of 3MH concentrations between the young and old were probably not confounded by differences in blood flow as we did not show any differences in blood flow (i.e. o/i) between the young and old at any time point. We did see a transient increase in blood flow following the resistance exercise bout, as would be expected based on data from previous studies of limb blood flow following resistance or high intensity exercise (Biolo et al. 1995b, 1999; Hussain et al. 1996). Thus, there is a possibility that 3MH was released from the muscle at an increased rate while blood flow was increased (i.e. in the 1.5 h following the exercise); however, the results of the leg arteriovenous 3MH measurements in the young individuals do not support this contention.
The current investigation studied only males and it is unclear if the same results for resting and postexercise proteolysis would be seen in young and old females. However, studies that have used females as a portion of their study population suggest that the young and old males and females may respond similarly, with respect to skeletal muscle protein turnover at rest and after exercise (Phillips et al. 1997, 1999; Welle & Thornton, 1998; Tipton et al. 1999; Hasten et al. 2000; Balagopal et al. 2001). Results from equivalent groups of women with a statistically relevant number of subjects are warranted.
The basis for studying the skeletal muscle proteolytic response at rest and after activity is to better understand the role of protein metabolism in the development of frailty and reduced functional independence in the elderly. Interpretation of the current findings is difficult in the context of total muscle mass changes with ageing and the conflicting reports that the synthesis rates of skeletal muscle are either decreased (Welle et al. 1993; Yarasheski et al. 1993, 1999; Balagopal et al. 1997), not changed (Volpi et al. 2001), or increased (Volpi et al. 2001) with ageing. Regardless, the results from the current investigation are consistent with a decrease in muscle mass loss with ageing.
In conclusion, our results suggest that the main muscle proteins, myosin and actin, which also are the main proteins involved in the production of muscular force, are degraded at a higher rate in older as compared with younger individuals in the resting and postabsorptive state. This difference in resting proteolysis may contribute to the loss of muscle mass with ageing. It also appears that myosin and actin are not degraded at a higher rate than resting in either young or old men within 24 h of a single bout of resistance exercise that is known to increase skeletal muscle mass and function when performed chronically. Studies of interventions, such as exercise training or nutritional supplementation, which may reduce the increased resting proteolysis of myosin and actin in the old are warranted. Finally, microdialysis appears to be compatible with high-intensity resistance exercise and is appropriate for use in skeletal muscle of older individuals.
Acknowledgments
The authors would like to thank the subjects for their participation, James Fluckey, Ph.D and Ray Fetterer, Ph.D., and Chad Carroll, M.S. for their assistance with the 3MH analysis, William Evans, Ph.D. for general laboratory support, and the medical and technical staff at the Bispebjerg Hospital for assistance with the arteriovenous measurements. This work was supported by NIH grants K01 AG00831 (T.T.) and M01 RR14288, the Gatorade Sports Science Institute (R.W.), the Arkansas Space Grant Consortium (T.T.), and the University of Arkansas for Medical Sciences Graduate School (R.W.). Robert Hickner received salary support during this project from the American Heart Association (99602384) and NIH (AG18407) and (AG19209).
References
- Andersson E, Hakanson E, Larsson J, Martensson J. Rapid and sensitive method for the determination of arterial-venous differences and leg efflux of 3-methylhistidine using ion-pair high-performance liquid chromatography and post-column fluorescence derivatization. J Chromatogr. 1987;414:174–179. doi: 10.1016/0378-4347(87)80037-3. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280:E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- Bernt E, Gutmann I. Ethanol: Determination with alcohol dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods in Enzymatic Analysis. Weinheim, Germany: Verlag Chemie GmbH; 1974. pp. 1499–1505. [Google Scholar]
- Bilmazes C, Uauy R, Haverberg LN, Munro HN, Young VR. Muscle protein breakdown rates in humans based on Ntau-methylhistidine (3-methylhistidine) content of mixed proteins in skeletal muscle and urinary output of Ntau-methylhistidine. Metabolism. 1978;27:525–530. doi: 10.1016/0026-0495(78)90018-5. [DOI] [PubMed] [Google Scholar]
- Biolo G, Fleming D, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995a;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995b;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- Fetterer RH, Allen PC. Eimeria acervulina infection elevates plasma and muscle 3- methylhistidine levels in chickens. J Parasitol. 2000;86:783–791. doi: 10.1645/0022-3395(2000)086[0783:EAIEPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Meredith CA, O'Reilly KP, Frontera WR, Cannon JG, Evans WJ. Enhanced protein breakdown following eccentric exercise in young and old men. J Appl Physiol. 1991;71:674–679. doi: 10.1152/jappl.1991.71.2.674. [DOI] [PubMed] [Google Scholar]
- Hamrin K, Rosdahl H, Ungerstedt U, Henriksson J. Microdialysis in human skeletal muscle: effects of adding a colloid to the perfusate. J Appl Physiol. 2002;92:385–393. doi: 10.1152/jappl.2002.92.1.385. [DOI] [PubMed] [Google Scholar]
- Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institution of Washington; 1919. [Google Scholar]
- Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Haverberg LN, Omstedt PT, Munro HN, Young VR. Ntau-methylhistidine content of mixed proteins in various rat tissues. Biochim Biophys Acta. 1975;405:67–71. doi: 10.1016/0005-2795(75)90315-3. [DOI] [PubMed] [Google Scholar]
- Henriksson J. Microdialysis of skeletal muscle at rest. Proc Nutr Soc. 1999;58:919–923. doi: 10.1017/s0029665199001226. [DOI] [PubMed] [Google Scholar]
- Hickner RC. Applications of microdialysis in studies of exercise. Exerc Sport Sci Rev. 2000;28:117–122. [PubMed] [Google Scholar]
- Hickner RC, Bone D, Ungerstedt U, Jorfeldt L, Henriksson J. Muscle blood flow during intermittent exercise: comparison of the microdialysis ethanol technique and 133Xe clearance. Clin Sci. 1994;86:15–25. doi: 10.1042/cs0860015. [DOI] [PubMed] [Google Scholar]
- Hickner RC, Ekelund U, Mellander S, Ungerstedt U, Henriksson J. Muscle blood flow in cats: comparison of microdialysis ethanol technique with direct measurement. J Appl Physiol. 1995;79:638–647. doi: 10.1152/jappl.1995.79.2.638. [DOI] [PubMed] [Google Scholar]
- Hickner RC, Rosdahl H, Borg I, Ungerstedt U, Jorfeldt L, Henriksson J. Ethanol may be used with the microdialysis technique to monitor blood flow changes in skeletal muscle: dialysate glucose concentration is blood-flow-dependent. Acta Physiol Scand. 1991;143:355–356. doi: 10.1111/j.1748-1716.1991.tb09243.x. [DOI] [PubMed] [Google Scholar]
- Hickner RC, Rosdahl H, Borg I, Ungerstedt U, Jorfeldt L, Henriksson J. The ethanol technique of monitoring local blood flow changes in rat skeletal muscle: implications for microdialysis. Acta Physiol Scand. 1992;146:87–97. doi: 10.1111/j.1748-1716.1992.tb09396.x. [DOI] [PubMed] [Google Scholar]
- Hussain ST, Smith RE, Medbak S, Wood RF, Whipp BJ. Haemodynamic and metabolic responses of the lower limb after high intensity exercise in humans. Exp Physiol. 1996;81:173–187. doi: 10.1113/expphysiol.1996.sp003923. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjaer M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J Physiol. 1999;515:919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CL, Haverberg LN, Young VR, Kinney JM, Munro HN, Geiger JW. Metabolism of 3-methylhistidine in man. Metabolism. 1975;24:929–935. doi: 10.1016/0026-0495(75)90084-0. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Lukaski HC, Mendez J, Buskirk ER, Cohn SH. Relationship between endogenous 3-methylhistidine excretion and body composition. Am J Physiol. 1981;240:E302–E307. doi: 10.1152/ajpendo.1981.240.3.E302. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 1999;276:E118–E124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- Radegran G. Limb and skeletal muscle blood flow measurements at rest and during exercise in human subjects. Proc Nutr Soc. 1999;58:887–898. doi: 10.1017/s0029665199001196. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Bennegard K, Eden E, Emery PW, Lundholm K. Urinary excretion and efflux from the leg of 3-methylhistidine before and after major surgical operation. Metabolism. 1984;33:250–256. doi: 10.1016/0026-0495(84)90046-5. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Krywawych S, Davies CT, Halliday D, Waterlow JC, Millward DJ. Effect of exercise on protein turnover in man. Clin Sci. 1981;61:627–639. doi: 10.1042/cs0610627. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Millward DJ. 3-Methylhistidine excretion and the urinary 3-methylhistidine/creatinine ratio are poor indicators of skeletal muscle protein breakdown. Clin Sci. 1983;65:217–225. doi: 10.1042/cs0650217. [DOI] [PubMed] [Google Scholar]
- Rifai Z, Kingston WJ, McCraith B, Moxley RT., 3rd Forearm 3-methylhistidine efflux in myotonic dystrophy. Ann Neurol. 1993;34:682–686. doi: 10.1002/ana.410340510. [DOI] [PubMed] [Google Scholar]
- Rosdahl H, Ungerstedt U, Jorfeldt L, Henriksson J. Interstitial glucose and lactate balance in human skeletal muscle and adipose tissue studied by microdialysis. J Physiol. 1993;471:637–657. doi: 10.1113/jphysiol.1993.sp019920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Meth. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Sjolin J, Stjernstrom H, Henneberg S, Andersson E, Martensson J, Friman G, Larsson J. Splanchnic and peripheral release of 3-methylhistidine in relation to its urinary excretion in human infection. Metabolism. 1989;38:23–29. doi: 10.1016/0026-0495(89)90175-3. [DOI] [PubMed] [Google Scholar]
- Svanberg E, Moller-Loswick AC, Matthews DE, Korner U, Andersson M, Lundholm K. Effects of amino acids on synthesis and degradation of skeletal muscle proteins in humans. Am J Physiol. 1996;271:E718–E724. doi: 10.1152/ajpendo.1996.271.4.E718. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy R, Winterer JC, Bilmazes C, Haverberg LN, Scrimshaw NS, Munro HN, Young VR. The changing pattern of whole body protein metabolism in aging humans. J Gerontol. 1978;33:663–671. doi: 10.1093/geronj/33.5.663. [DOI] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. J Am Med Assoc. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassner SJ, Schlitzer JL, Li JB. A rapid, sensitive method for the determination of 3-methylhistidine levels in urine and plasma using high-pressure liquid chromatography. Anal Biochem. 1980;104:284–289. doi: 10.1016/0003-2697(80)90076-7. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol. 1998;274:E677–E683. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol. 1993;264:E693–E698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol. 1995;268:E422–E427. doi: 10.1152/ajpendo.1995.268.3.E422. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. Selection of tracer infusion and sampling sites. In: Wolfe RR, editor. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. pp. 167–188. [Google Scholar]
- Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men > / = 76 yr old. Am J Physiol. 1999;277:E118–E125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- Young VR, Alexis SD, Baliga BS, Munro HN, Muecke W. Metabolism of administered 3-methylhistidine. Lack of muscle transfer ribonucleic acid charging and quantitative excretion as 3- methylhistidine and its N-acetyl derivative. J Biol Chem. 1972;247:3592–3600. [PubMed] [Google Scholar]
- Young VR, Munro HN. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978;37:2291–2300. [PubMed] [Google Scholar]
- Zhang X-J, Chinkes DL, Sakuri Y, Wolfe RR. An isotopic method for measurement of muscle protein fractional breakdown rate in vivo. Am J Physiol. 1996;270:E759–E767. doi: 10.1152/ajpendo.1996.270.5.E759. [DOI] [PubMed] [Google Scholar]