Abstract
Previous work on the whole neurohypophysis has shown that hypotonic conditions increase release of taurine from neurohypophysial astrocytes (pituicytes). The present work confirms that taurine is present in cultured pituicytes, and that its specific release increases in response to a hypotonic shock. We next show that vasopressin (VP) and oxytocin (OT) also specifically release taurine from pituicytes. With an EC50 of ∼2 nm, VP is much more potent than OT, and the effects of both hormones are blocked by SR 49059, a V1a receptor antagonist. This pharmacological profile matches the one for VP- and OT-evoked calcium signals in pituicytes, consistent with the fact that VP-induced taurine efflux is blocked by BAPTA-AM. However, BAPTA-AM also blocks the taurine efflux induced by a 270 mosmol l−1 challenge, which per se does not evoke any calcium signal, suggesting a permissive role for calcium in this case. Nevertheless, the fact that structurally unrelated calcium-mobilizing agents and ionomycin are able to induce taurine efflux suggests that calcium may also play a signalling role in this event. It is widely accepted that in hypotonic conditions taurine exits cells through anionic channels. Antagonism by the chloride channel inhibitors 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS) and 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) suggests the same pathway for VP-induced taurine efflux, which is also blocked in hypertonic conditions (330 mosmol l−1). Moreover, it is likely that the osmosensitivity of the taurine channel is up-regulated by calcium. These results, together with our in situ experiments showing stimulation of taurine release by endogenous VP, strengthen the concept of a glial control of neurohormone output.
Several decades ago, Leveque & Small (1959) hypothesized that pituicytes, the specialized astrocytes of the neurohypophysis, may play a role in the regulation of neurohypophysial hormone output. Working at the ultrastructural level, Krsulovic & Brückner (1969) and Wittkowski & Brinkmann (1974) showed that pituicytes undergo morphological changes during dehydration, a physiological state that requires an increased output of vasopressin (VP). Tweedle & Hatton (1982) reported similar phenomena with respect to parturition and lactation, which correspond to an increased output of oxytocin (OT). In light of numerous observations that seem to involve glial shape changes in the control of neurohormone output, Hatton (1988, 1999) proposed a model accounting for the interactions between pituicytes and neurohypophysial terminals and capillaries. In this scheme, increased demand of VP or OT would induce pituicytes to retract both from between terminals, and from between the latter and the basal lamina of the perivascular space. This in turn would increase hormone availability by, respectively, increasing terminal excitability and facilitating hormone release into the blood. Various signals might be involved in pituicyte shape changes, including catecholamines (Hatton, 1988, 1999) and ATP, which is present in neurosecretory vesicles and therefore is coreleased with neurohypophysial hormones (Gratzl et al. 1980; Zimmermann, 1994; Sperlágh et al. 1999). Indeed, our in vitro experiments have shown that ATP induces pituicyte stellation via its metabolism to adenosine (Rosso et al. 2002a), probably owing to the presence of ectoATPases on these cells (Thirion et al. 1996). From the postulate that such structural changes facilitate hormone release (Hatton, 1988, 1999), it would appear that the action of ATP and adenosine constitutes a self-stimulating feedback loop, for which a stop signal should exist if vesicular exhaustion is to be prevented. We found that such a signal could be VP itself because this neurohormone is capable of reversing pituicyte stellation at physiological concentrations (Rosso et al. 2002b). We now provide evidence for another means for VP and OT to limit their own output, that is through stimulation of pituicytes to release the inhibitory amino acid taurine.
Taurine functions as a ubiquitous osmolyte in the regulation of cell volume, but its role in the neurohypophysis appears to be different (reviewed by Hussy, 2002). Taurine has been shown to inhibit VP release by activating strychnine-sensitive glycine receptors located on neurohypophysial terminals (Hussy et al. 2001). The physiological significance of this result was highlighted by the finding that experimental hypotonic shocks increase taurine levels in the isolated neurohypophysis, which is consistent with the need to keep VP levels low in these conditions. As it was demonstrated that endogenous neurohypophysial taurine originates in pituicytes (Pow, 1993; Miyata et al. 1997; Pow et al. 2002), we felt that showing similar data with cultured pituicytes would provide a means to validate our in vitro model. We herein confirm that cultured pituicytes indeed contain endogenous taurine, and that their release of [3H]taurine increases sharply in response to hypotonicity. In addition, we show that VP and OT also enhance taurine release, which establishes the distinct possibility that neurohypophysial hormones and pituicytes engage in a short-loop negative feedback.
Methods
Cell preparation
Pituicyte explant cultures were prepared as previously described (Rosso et al. 2002a) from adult Wistar rats (150–200 g) anaesthetized with CO2 and decapitated in accordance with French/European ethical guidelines. For taurine efflux and calcium imaging experiments, we used 35-mm plastic dishes. For immunofluorescence, 18-mm glass coverslips coated with 0.05 mg ml−1 collagen were inserted in the same plastic dishes prior to plating. Cells were used after 10–11 days in vitro.
Immunofluorescence
Taurine was labelled with a polyclonal antibody provided to us by Dr David Pow, University of Queensland, Brisbane, Australia (Pow, 1993). Pituicytes were fixed with 3% paraformaldehyde + 1% glutaraldehyde at 37°C, washed three times in PBS, and left for 15 min in 1% NaBH4. After three more rinses in PBS, they were permeabilized in PBS + 0.02% Triton X-100 (5 min), and again rinsed three times in PBS. Cells were then saturated by 15 min in PBS + 5% serum. Primary antitaurine antibody was diluted 1/2000 and applied for 4 h in a wet chamber at room temperature (RT). After rinsing in PBS, pituicytes were incubated (1.5 h; RT) with goat antirabbit secondary antibody coupled to fluorescein isothiocyanate (FITC; Nordic/tebu-bio, Le Perray en Yvelines, France; 1/100 dilution). After a final rinse, cells were mounted on microscope slides in the presence of Mowiol. Digital images were acquired with a Delta Vision system (Applied Precision, Issaquah, WA, USA) coupled to an IX-70 Olympus microscope.
[3H]Amino acid efflux
Prior to experiments, pituicytes were switched to Locke's medium containing (mm) 120 NaCl, 4 KCl, 1 KH2PO4, 2 CaCl2, 2 MgCl2, 10 Hepes, 10 glucose and 30 mannitol (pH 7.4; 300 mosmol l−1 by freezing point), and supplemented with 0.025% fetal calf serum to control morphology (Rosso et al. 2002b). Each dish of cells contained 800 μl of this solution to which 2 μCi [3H]taurine or [3H]glutamate was added. Cells were left to incubate at 37°C for 90 min. Before experiments, the dishes were rinsed five times with 1 ml Locke's solution without amino acid. The medium was collected every 5 min, and replaced with fresh medium with or without test drugs at the appropriate time. [3H]Taurine and [3H]glutamate release were estimated by scintillation counting. Results are expressed as efflux rate normalized to baseline (averaged over the first 5–7 samples, i.e. 25–35 min). Efflux rate corresponds to the ratio of c.p.m. released at a given time point to releasable c.p.m., which was calculated after cell lysis at the end of each experiment. Based on the mean of two control experiments performed in triplicate for each amino acid, basal efflux was 308 ± 78 c.p.m. per 5 min for taurine and 447 ± 103 c.p.m. per 5 min for glutamate.
In situ[3H]taurine efflux
Whole isolated neurohypophyses were incubated for 2 h in Locke's medium supplemented with 500 nm[3H]taurine at 35°C, rinsed three times, placed in perfusion chambers (250 μl, one neurohypophysis per chamber) at 35°C, and perfused at a rate of 250 μl min−1 with oxygenated Locke's solution. After 20 min rest, perfusate was collected every 5 min with a sample collector (Gilson FC204, Villiers-le-Bel, France). Basal release of taurine in isotonic medium was fitted with a monoexponential function, and data were normalized to this fit to express release as a percentage of basal release (Deleuze et al. 1998, 2000). Three chambers were systematically used as control, and the effects of the drugs were always compared with the controls of the same set of experiments. Experiments were realized on at least two different preparations.
Calcium imaging
Intracellular Ca2+ ([Ca2+]i) was measured with the ratiometric, membrane-permeant, fluorescent probe Fura-2 AM. Briefly, pituicyte cultures were incubated for 45 min at 37°C in the presence of 5 μm Fura-2 AM + 0.01% pluronic acid. During experiments, culture dishes were continuously superfused with Locke's solution (3 ml min−1). These experiments were performed on the stage of an inverted microscope (Zeiss ICM 405) equipped with a xenon lamp and a rotating filter set allowing 350/380 nm excitation. Axon Imaging Workbench 2.2 software was used to drive the filter wheel, acquire fluorescence images and process data. For any given experiment, fluorescence signals were averaged from 10 to 20 cells defined as ‘regions of interest’. Free [Ca2+]i was estimated from a calibration procedure using a ‘zero-Ca’ solution (3 mm EGTA + 2 μm ionomycin) and a Ca2+-saturated solution (3 mm CaCl2+ 2 μm ionomycin). The F350/F380 ratio was converted to free [Ca2+]i using a standard equation (Grynkiewicz et al. 1985). Drugs were applied locally via a miniperfusion system.
Drugs
[3H]Taurine and [3H]glutamate were purchased from Amersham Pharmacia Biotech (Orsay, France); SR49059 was provided by C. Serradeil-Le Gal (Sanofi-Synthelabo, Toulouse, France); Y-27632 and Fura-2 AM were, respectively, purchased from Calbiochem, Meudon, and TEF Laboratories/Euromedex, Mundolsheim, France. All other drugs are from Sigma.
Data analysis
Data for taurine efflux are presented as means ± s.e.m. The number of observations, n, refers to the number of culture dishes (or neurohypophyses) counted. For any given condition, 2–3 dishes per culture (i.e. rat) were used. Except where noted, test experiments were assigned their own controls (same day of experiment and same culture), and these controls were pooled to present general results (Figs 2A and 3A). Test versus control comparisons were performed with Student's two-tail unpaired t tests.
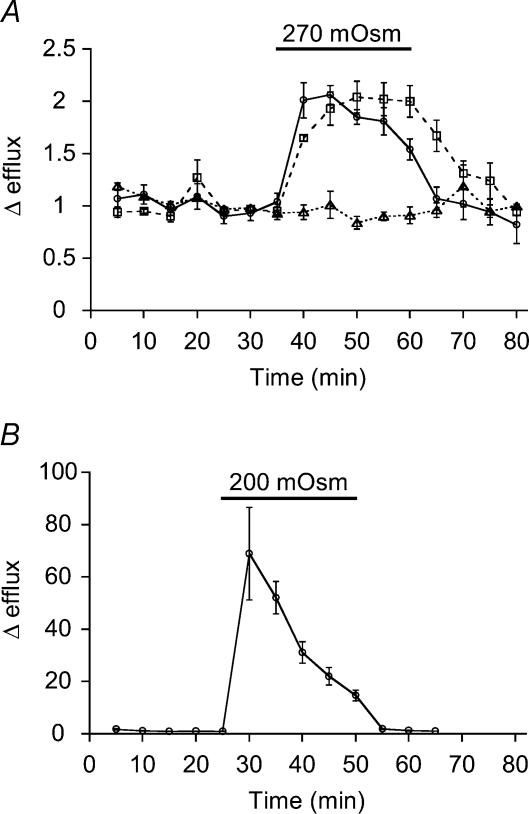
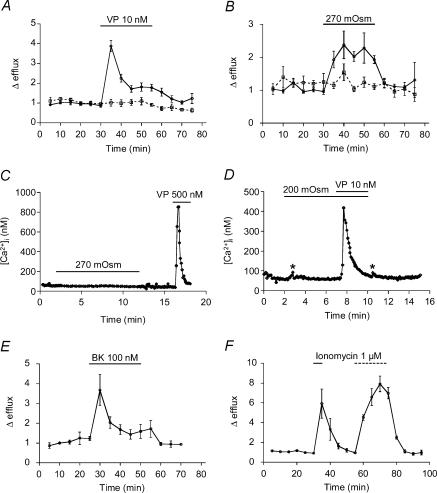
Figure 2. Hypotonicity-induced [3H]taurine efflux from rat pituicytes.
A, taurine efflux induced by a 270 mosmol l−1 hypotonic shock applied (as indicated by the bar) to non-stellate pituicytes (continuous line; n = 12) or stellate pituicytes (dashed line; n = 6). The dotted line shows glutamate efflux, which is not affected by the 270 mosmol l−1 medium (n = 4). The latter consisted of Locke's medium without mannitol (see Methods). B, efflux induced by a 200 mosmol l−1 shock applied to non-stellate pituicytes (n = 4). The 200 mosmol l−1 medium was prepared by diluting the 270 mosmol l−1 solution with water. In this and subsequent figures, efflux is a unitless ratio normalized to baseline (see Methods).
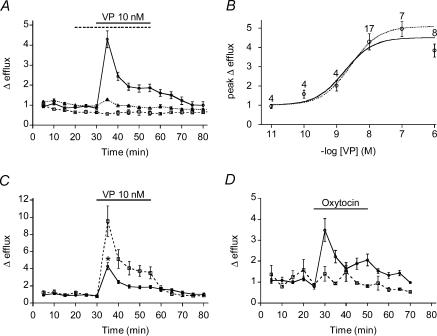
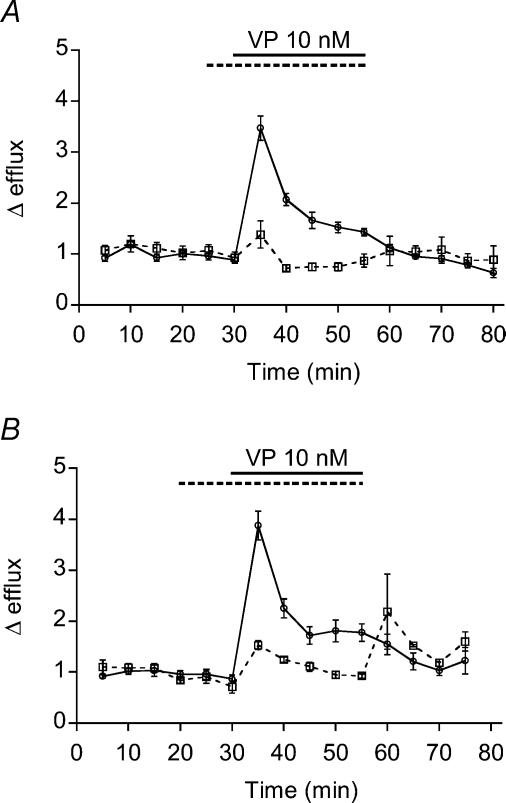
Figure 3. Hormone-induced [3H]taurine efflux from rat pituicytes.
A, taurine efflux induced by 10 nm VP in the absence (continuous line; n = 17) or in the presence (dashed line; n = 5) of 100 nm SR 49059. The dotted line shows glutamate efflux, which is barely affected by 10 nm VP (n = 5). B, dose–response relationships for the peak of taurine efflux versus VP concentration. Data were fitted with a logistic equation of the form 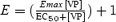 where E and Emax represent the peak of relative efflux and its maximum, and EC50 is the VP concentration that elicits 50% of Emax. The number of observations is indicated above data points. The dotted curve was fitted by excluding the 1 μm data point. C, efflux induced by 10 nm VP in 300 mosmol l−1 medium (continuous line; same data as control in A) or in 270 mosmol l−1 medium (dashed line; n = 5). >*P = 5 × 10−4. D, efflux induced by 500 nm (continuous line; n = 6) or 10 nm (dashed line; n = 3) OT.
where E and Emax represent the peak of relative efflux and its maximum, and EC50 is the VP concentration that elicits 50% of Emax. The number of observations is indicated above data points. The dotted curve was fitted by excluding the 1 μm data point. C, efflux induced by 10 nm VP in 300 mosmol l−1 medium (continuous line; same data as control in A) or in 270 mosmol l−1 medium (dashed line; n = 5). >*P = 5 × 10−4. D, efflux induced by 500 nm (continuous line; n = 6) or 10 nm (dashed line; n = 3) OT.
Results
Detection of taurine in cultured pituicytes
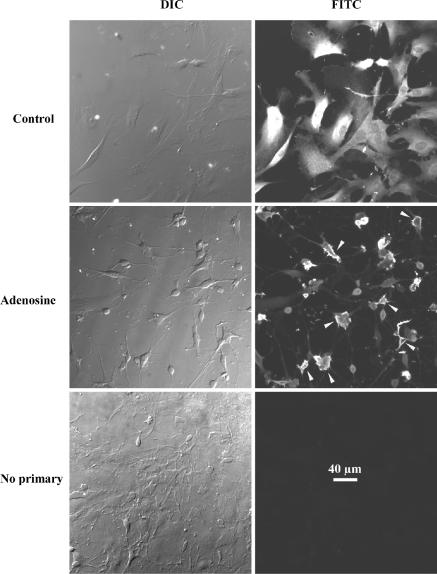
We first confirmed that pituicytes constitute the likely source for the taurine released in the hypoosmotic neurohypophysis by demonstrating the presence of the amino acid in these cells, as well as its increased release in response to a hypotonic shock. Using immunohistochemistry of pituitary sections, Miyata et al. (1997) and Pow et al. (2002) previously inferred selective labelling of pituicytes by antitaurine antibodies. Obviating the heterogeneity of whole-tissue sections, we now confirm that pituicytes indeed contain taurine. This can be seen in Fig. 1, which shows both flat and stellate pituicytes labelled with antitaurine antibody. Neither unspecific adsorption of the secondary antibody nor cell autofluorescence can account for this result since virtually no fluorescence was detected in the absence of primary antibody (lower right panel). Note the subcellular relocalization of taurine when pituicytes become stellate; in flat cells taurine labelling appears diffuse and homogeneous throughout the cell soma (upper right), whereas it displays a submembrane pattern in most stellate cells (middle right).
Figure 1. Fluorescence labelling of cultured rat pituicytes with antitaurine antibody.
Left panels are differential interference contrast pictures; right panels are the corresponding FITC pictures showing antitaurine labelling. Upper panels show a standard pituicyte culture (1 h in 0.025% serum) with mostly flat or elongated cells. Middle panels show another culture to which 10 μm adenosine was added to induce pituicyte stellation (Rosso et al. 2002a). Most cells have shrunken and roundish somas with numerous processes. Arrowheads point to cells in which taurine displays clear submembrane localization. Bottom panels show a mixed culture (with both flat and stellate pituicytes) in which primary but not secondary antibody was omitted. All pictures were taken with a 20 × objective lens.
Hypotonic release of taurine from pituicytes
Next, we found that a mild hypotonic shock (270 mosmol l−1) increases taurine efflux from pituicytes to about twice basal levels (Fig. 2A). Given that a 270 mosmol l−1 shock doubles basal taurine efflux in the whole neurohypophysis (Hussy et al. 2001), our results provide a further argument in favour of an exclusive release of taurine from pituicytes in this tissue. As these cells are morphologically highly plastic, we wondered whether their ability to release taurine might be influenced by their morphology. Induction of pituicyte stellation by 10 μm Y-27632 (Rosso et al. 2002a), a p160Rho kinase inhibitor, had no effect on the magnitude of taurine efflux, although a lag in the response and slightly different kinetics seemed to occur (Fig. 2A). To rule out the possibility of unspecific release of amino acids, we tested the effect of a 270 mosmol l−1 medium on [3H]glutamate efflux. No increase in glutamate release over basal was induced by the osmotic stimulus (Fig. 2A), indicating that the effect of a hypotonic shock on pituicytes is specific for taurine, at least versus glutamate, as previously observed (Miyata et al. 1997). Though probably not of strict physiological relevance, we also investigated the effect of a 200 mosmol l−1 shock on taurine efflux. Consistent with the exponential relationship between hypotonicity and taurine release (Hussy et al. 2001), a 200 mosmol l−1 solution elicited a massive efflux of taurine from pituicytes (Fig. 2B).
Neurohypophysial hormone-induced release of taurine from pituicytes
In view of the tight neuroglial relationships existing within the neurohypophysis, further emphasized by the inhibitory effect of taurine on VP output (Hussy, 2002), we tested for a possible reciprocal effect of VP on taurine efflux. We found that 10 nm VP elicits a ∼4-fold increase in taurine efflux from non-stellate pituicytes. This effect was completely blocked by 100 nm SR 49059, an antagonist of VP V1a receptors (Fig. 3A). Conversely, SR 49059 did not affect the taurine efflux induced by a 270 mosmol l−1 hypotonic shock (mean peak Δefflux = 2.8; n = 2), indicating that the hypotonic effect is not mediated by VP released from pituicytes, which may contain the hormone (Pu et al. 1995). In contrast to its effect on taurine efflux, VP only elicited a faint increase of glutamate release from pituicytes (Fig. 3A).
Next, we performed a dose–response experiment for the peak of taurine efflux versus VP concentration (Fig. 3B). Some efflux was observed at a concentration as low as 0.1 nm, and a plateau was reached around 100 nm. We found that 1 μm yields a smaller effect than 100 nm VP, which is unlikely to be related to mere variability as three separate experiments confirmed that trend. (We therefore present the data with two curves, one that fits all points and yields an EC50 of 1.7 nm, and one for which the 1 μm point was omitted and that yields an EC50 of 2.6 nm.) When we combined a near saturating concentration of VP (10 nm) with a 270 mosmol l−1 solution, taurine efflux was much larger than with VP alone (Fig. 3C), suggesting different mechanisms of action for the two stimuli. Since OT is released from neurohypophysial terminals, and has weak vasopressor and antidiuretic activities (Manning et al. 1981), we also tested its effect on taurine efflux from pituicytes. We found that 500 nm OT releases an amount of taurine not significantly different from that released by 10 nm VP (P = 0.31), whereas 10 nm OT had little effect, if any (Fig. 3D). Furthermore, SR 49059 (100 nm) completely blocked 500 nm OT-evoked taurine efflux (mean peak Δefflux = 1.1; n = 2), revealing that OT exerts its effect through weak agonist action on V1a receptors. These data are consistent with those we recently obtained on intracellular Ca2+ () signalling by VP and OT in pituicytes (Rosso et al. 2002b), suggesting that a Ca2+ signal might be involved in hormone-evoked taurine efflux.
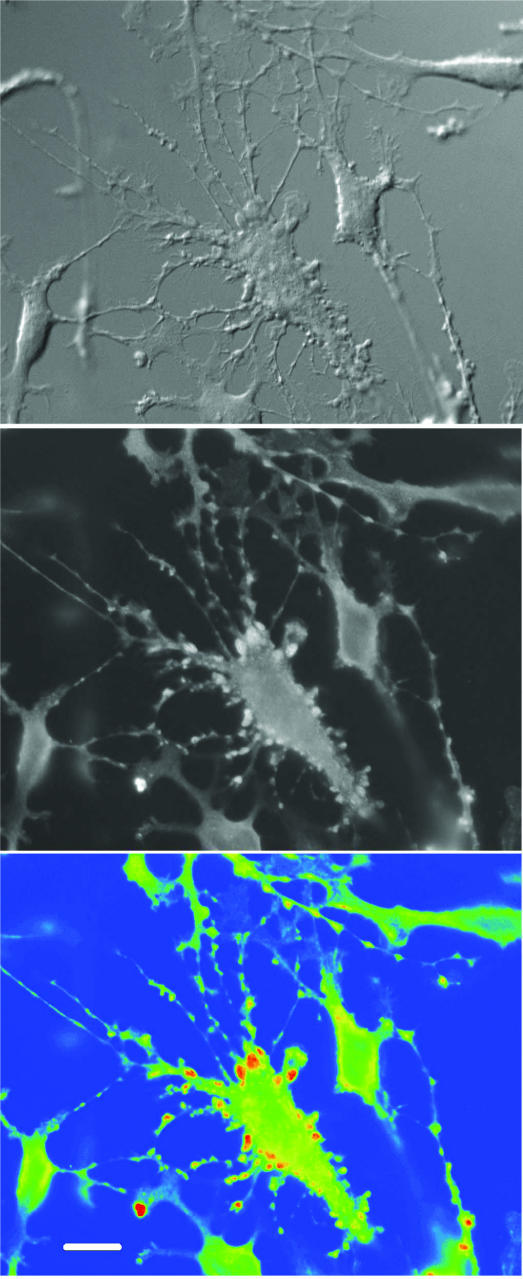
As seen for hypotonic stimulation, the magnitude of VP-induced efflux appeared independent of the morphological state of pituicytes, as stellate cells responded to VP to the same extent as non-stellate pituicytes (mean peak Δefflux = 5.1 ± 1.4, n = 6, and 5.4 ± 0.9, n = 5, respectively; P = 0.54). The fact that VP reverses pituicyte stellation cannot account for this result because the peak of evoked taurine efflux occurs within 5 min after the onset of VP application (Fig. 3A), whereas stellation reversal takes at least 20 min (Rosso et al. 2002b). However, we systematically observed a peculiar phenomenon occurring within 0.5–2 min after VP application onto stellate pituicytes; at this time, both soma and processes started emitting many protrusions intensely stained for taurine (Fig. 4). Thereafter, pituicytes reverted to their flat shape, and taurine staining resumed its diffuse pattern.
Figure 4. Selective concentration of taurine in pericellular protrusions.
Upper panel shows a differential interference contrast picture of stellate pituicytes 1 min after 10 nm VP application. The centre cell displays intense reorganization with formation of pericellular protrusions. This asynchronous process is not observed in all pituicytes simultaneously. Middle panel shows corresponding FITC fluorescence revealing taurine labelling. Lower panel shows pseudo-colour conversion of FITC fluorescence displaying low-to-high (blue-to-red) intensity. Pericellular protrusions selectively light up in red. Pictures were taken with a 40 × objective lens. Calibration = 16 μm.
Taurine efflux and intracellular Ca2+
The similar pharmacological profile of VP and OT for taurine efflux and signalling prompted us to investigate whether the two processes are linked. Incubating cells for 1 h with 10 μm BAPTA-AM completely blocked VP-evoked taurine efflux (Fig. 5A), indicating a requirement for Ca2+ in the effect of VP. We could not assess the involvement of extracellular Ca2+ in this process because incubating cells in a Ca2+-free medium (+ 3 mm EGTA) led to a slowly increasing baseline drift, probably due to cell damage and non-physiological taurine efflux (Mongin et al. 1999). We then investigated whether BAPTA-AM would also affect hypotonicity-evoked taurine efflux, which we found virtually eliminated by the chelator (Fig. 5B). This prompted the hypothesis that hypotonicity might also elicit a Ca2+ signal in pituicytes. We thus resorted to Fura-2 fluorescence experiments to investigate whether the 270 mosmol l−1 medium would affect basal [Ca2+]i, which was clearly ruled out (n = 4; Fig. 5C). Similarly, a challenge with a 200 mosmol l−1 solution did not elicit any signal in three experiments (Fig. 5D), and only elicited a faint signal in two experiments (mean increase in [Ca2+]i= 30 nm). A likely explanation for these results is that a resting concentration of constitutes a permissive factor for hypotonicity-evoked taurine efflux, as indeed proposed by others (Mongin et al. 1999). Therefore, the inhibitory effect of BAPTA-AM on VP-induced taurine efflux could also be regarded as resulting from a decrease in resting Ca2+ concentration, raising the possibility that the Ca2+ signal and taurine efflux elicited by VP are unrelated mechanisms. To determine whether an increase in [Ca2+]i constitutes the mechanism for VP-induced taurine efflux, we studied the effects of other well-characterized -mobilizing tools in pituicytes, such as ATP-γ-S (Troadec et al. 1999) and bradykinin (Rosso et al. 2002a). Indeed, we found that both agents are capable of evoking taurine efflux. While 100 μm ATP-γ-S elicited a modest effect (mean peak Δefflux = 1.9 ± 0.2, n = 4), the potency of bradykinin was comparable to that of VP (Fig. 5E). Interestingly, in our hands ATP-γ-S (100 μm) seems capable of evoking only a small Ca2+ signal (100 and 250 nm; n = 2), whereas bradykinin evokes a signal (several hundred nanomolar; Rosso et al. 2002a) comparable to that of VP (Fig. 5C and D). Although this suggests that Ca2+ is the relevant messenger for receptor-mediated taurine efflux, it has to be mentioned that VP, ATP-γ-S and bradykinin all activate the phospholipase C (PLC) pathway. Since PLC activation can elicit effects in astrocytes – such as ATP release – independent of Ca2+ (Haydon, 2001), this might also be the case for taurine release. To test this idea, we sought to increase [Ca2+]i directly and independently of PLC to see if this would trigger taurine efflux. This was accomplished with the Ca2+ ionophore ionomycin, which indeed also induced substantial taurine efflux (Fig. 5F). Upon subsequent challenge with a 270 mosmol l−1 medium containing ionomycin, the effects of both stimuli appeared to be additive (Fig. 5F). Thus, whereas Ca2+ appears as a mere permissive factor in hypotonic release of taurine, it may also constitute a full signal by itself.
Figure 1. Role of calcium in hypotonic and hormone-induced [3H]taurine efflux from rat pituicytes.
A, efflux induced by 10 nm VP in control conditions (continuous line; n = 5) or after BAPTA-AM incubation (dashed line; n = 6). B, efflux induced by a hypotonic shock applied in control conditions (continuous line; n = 7) or after BAPTA-AM incubation (dashed line; n = 8). C, lack of effect of a 270 mosmol l−1 medium on pituicyte [Ca2+]i. A subsequent response to VP is shown for reference. Results depict the average of 12 cells and are representative of 4 similar experiments. D, lack of effect of a 200 mosmol l−1 medium on pituicyte [Ca2+]i. A subsequent response to VP during the hypotonic shock is shown for reference. Results depict the average of 12 cells and are representative of 3 other experiments. The 200 mosmol l−1 medium was prepared by omitting mannitol and 35 mm NaCl from Locke's solution. The KD of Fura-2 for Ca2+ was adjusted according to Grynkiewicz et al. (1985) to compensate for the decreased ionic strength (artifacts marked by asterisks). E, taurine efflux induced by 100 nm bradykinin (n = 3). F, efflux induced by 1 μm ionomycin in 300 mosmol l−1 (continuous bar) and in 270 mosmol l−1 (dashed bar) medium (n = 4). Ionomycin was first applied for a short duration (5 min) to avoid cell toxicity.
To investigate whether calmodulin is the downstream effector of this signal, we tested the effect of the calmodulin inhibitor N-(6-aminohexyl)-5-chloro-1-naphtalene sulphonamide (W-7; 50 μm) on VP- and hypotonicity-induced taurine release. Whereas the amplitude of VP-induced responses was not affected (mean peak Δefflux = 4.4 ± 0.7, n = 3), the decay typically seen with VP responses (e.g. Fig. 3A) disappeared in W-7. On the other hand, the response to a 270 mosmol l−1 medium was substantially potentiated by W-7 (mean peak Δefflux = 7.3 ± 0.9, n = 3). A possible interpretation of this result is that W-7 attenuates the Ca2+-buffering capacity of calmodulin, making more Ca2+ available to enhance taurine release. This view is consistent with our finding that W-7 induces a sustained Ca2+ signal in pituicytes (not shown). At any rate, these data suggest that calmodulin in not a downstream effector of the Ca2+ signal.
Pharmacological and physiological blockade of taurine efflux
There is a general consensus that in hypotonic conditions taurine exits cells through volume-sensitive anion channels (Strange et al. 1996). This is based on several criteria including antagonism by Cl− channel blockers, which inhibit taurine efflux in the whole neurohypophysis (Hussy et al. 2001). Bearing out the same mechanism for VP-evoked taurine efflux, we found that the latter is blocked by 1 mm 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS; Fig. 6A) or 0.1 mm 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB; n = 5; not shown). Finally, we investigated the effect of hypertonicity on VP-mediated taurine efflux. Consistent with previous observations that hypertonicity decreases basal taurine efflux in the neurohypophysis (Hussy et al. 2001), we observed a trend towards a slight decrease during the first 10 min following substitution of a mildly hypertonic medium (330 mosmol l−1). Note also that there was a rebound increase in taurine efflux upon return to an isoosmotic medium (Fig. 6B). On the other hand, the hypertonic condition largely blocked VP-mediated taurine efflux (Fig. 6B).
Figure 6. Modulation of VP-induced [3H]taurine efflux from rat pituicytes.
A, efflux induced by 10 nm VP in the absence (continuous line; n = 7) or in the presence (dashed line; n = 6) of 1 mm DIDS. B, efflux induced by 10 nm VP in 300 mosmol l−1 medium (continuous line; same data as control in Fig. 2B) or in 330 mosmol l−1 medium preapplied for 10 min (dashed line; n = 3). Hypertonic solution was prepared by adding 30 mm mannitol to Locke's medium.
Modulation of taurine release in situ
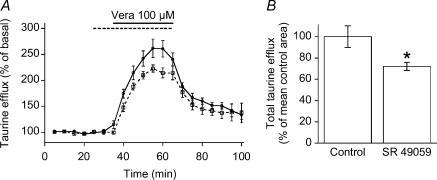
To investigate the physiological relevance of our data, we designed an in situ experiment aimed at evoking endogenous neurohypophysial hormone release in a whole neurohypophysis preparation. This in turn should stimulate [3H]taurine efflux if our in vitro results indeed have any physiological relevance. To evoke neurohypophysial hormone release we chose to use the Na+ channel activator veratridine rather than KCl, which per se releases taurine (Mongin et al. 1999). Moreover, veratridine has been shown to powerfully activate VP and OT release in the whole neurohypophysis (Nordmann & Dyball, 1978). We included 3 μm strychnine and an antipeptidase cocktail (see Rosso et al. 2002a) in the perfusion medium. The purpose for using strychnine was to shunt taurine feedback on to secretory terminals, which might dampen further hormone release, whereas the antipeptidase cocktail was meant to protect endogenous hormone. Veratridine (100 μm) increased taurine efflux up to 160% above baseline and SR 49059 significantly antagonized this effect (Fig. 7A), demonstrating that part of the evoked taurine efflux is due to endogenously released hormone activating V1a receptors. As the degree of antagonism was not different at 100 nm(n = 3)versus 1 μm SR 49059 (n = 5), we pooled the data obtained at both concentrations. SR 49059 blocked 30% of the total amount of taurine release integrated over time (Fig. 7B). As 1 μm SR 49059 was not more potent than 100 nm, we do not believe that this partial antagonism is due to poor penetration of the drug into the tissue. Rather, partial antagonism can be expected for the reasons discussed below.
Figure 7. [3H]Taurine efflux in situ.
A, taurine efflux induced in whole neurohypophyses by 100 μm veratridine in the absence (continuous line; n = 7) or in the presence (dashed line; n = 8) of 0.1–1 μm SR 49059. B, effect of SR 49059 on total taurine efflux integrated over time, and normalized to mean control area under the curve. *P < 0.05.
Discussion
The output of neurohypophysial hormones appears to be regulated in a complex manner if one takes into account the GABAergic, dopaminergic, glutamatergic, purinergic and peptidergic receptors expressed on secretory axon terminals of the neurohypophysis (see Sperlágh et al. 1999). Our results strengthen the novel concept of a local control of neurohypophysial hormone release mediated by glial secretions (see Hussy, 2002), and provide two pieces of information regarding this putative ‘intrinsic’ regulation. First we have shown selective hypoosmotic release of taurine from a purified pituicyte population, and second we have demonstrated that taurine efflux can also be induced or enhanced by VP, pointing to an important physiological regulatory loop involved in the control of neurohormone secretion.
Hypotonic and VP stimulation of taurine efflux
The pituicyte origin of taurine release in the neurohypophysis was previously demonstrated from the observation of a selective concentration of taurine in these cells and its selective release in response to physiological hypotonicity (Pow, 1993; Miyata et al. 1997; Pow et al. 2002). We now confirm that pituicytes contribute a major, and possibly the sole source for the taurine released by a hypotonic shock in the neurohypophysis (Miyata et al. 1997; Hussy et al. 2001). This is analogous to the situation in the supraoptic nucleus whence many neurohypophysial axons originate, and in which hypotonicity releases taurine specifically from glial cells (Deleuze et al. 1998). Taurine released by pituicytes sensing a hypotonic stimulus can then act upon neurohypophysial terminals to inhibit VP release (Hussy et al. 2001). This should provide an efficient mechanism for a local control of VP release, contributing to a decreased VP secretion and increased diuresis in hypoosmotic conditions.
The second important result of our study is that VP and OT are able to induce pituicytes to selectively release taurine via V1a receptors, whose activation increases pituicyte [Ca2+]i (Hatton et al. 1992). Our data thus provide evidence for a further physiological role of these receptors in pituicytes in addition to their demonstrated role in morphological plasticity (Rosso et al. 2002b). As there is good evidence that VP and OT are released locally from swellings present along the magnocellular axons invading the neurohypophysis (Morris et al. 1988; Nordmann et al. 1988), these hormones could act locally on pituicytes to release taurine as a negative feedback mechanism to perhaps contribute to termination of hormone secretion or prevention of secretory vesicle depletion. Interestingly, such a process does not seem to be present in the supraoptic nucleus, where neither VP nor OT induce any release of taurine from astrocytes (N. Hussy, personal observation; see Hussy et al. 2000).
Cytoskeletal aspects of taurine efflux
Our immunolabelling experiments provide further interesting detail as to the subcellular localization of taurine and its dynamic regulation in various conditions. Taurine staining appeared uniform in flat, unstimulated pituicytes, and clearly displayed a submembrane pattern after induction of stellation. The physiological consequence is not obvious, however, since the magnitude of the hypotonicity-evoked release of taurine did not depend on the morphological state of the cells. As pituicyte stellation parallels major actin/tubulin reorganization (Rosso et al. 2002a), a direct participation of the cytoskeleton in taurine efflux, if at all substantiated, would probably involve other elements than those recruited during stellation. Absence of blockade of hypotonicity-induced taurine efflux from astrocytes by the Rho kinase inhibitor Y-27632, which inhibits actin stress fibres, was previously reported in the supraoptic nucleus (Deleuze et al. 2000). Still, it is worth noting that Y-27632 seemed to introduce a lag in the osmosensitive response (Fig. 2A), which is consistent with the delay observed by Nilius et al. (1999) in volume-regulated anion channel (VRAC) activation following application of this drug. At variance with our data, however, these authors also found substantial reduction of VRAC current by Y-27632. Similar to the hypotonic shock, VP released the same peak amount of taurine from stellate versus non-stellate cells. However, following VP application onto stellate pituicytes we observed a striking phenomenon consisting of a rapid appearance of pericellular protrusions densely stained for taurine. It is clear that much work needs to be done to unravel the molecular nature and physiological relevance of these phenomena.
Mechanism of VP-induced taurine efflux
The molecular mechanism of taurine efflux appears to involve a ‘VRAC-like’ channel capable of sensing a drop in external osmolarity, as proposed earlier (Strange et al. 1996; Hussy et al. 2001). Our novel finding is that, independent of a change in osmotic pressure, this channel can be activated by a Ca2+ signal such as that produced by V1a receptor activation. It is generally admitted that VRAC activation does not depend on a rise in [Ca2+]i (Pasantes-Morales & Schousboe, 1997). Indeed, the osmosensitive efflux of taurine from pituicytes does not seem to require a Ca2+ signal since hypoosmotic challenges induce taurine release without eliciting any rise in [Ca2+]i (Figs 5C.D). However, chelating with BAPTA eliminates taurine release, in agreement with the proposed idea that resting constitutes a permissive factor for swelling-evoked taurine efflux (Szucs et al. 1996; Mongin et al. 1999; Li et al. 2002). By contrast, activation of taurine efflux by VP appears to result from an increased [Ca2+]i. Not only is efflux blocked by intracellular BAPTA, but the EC50 of VP-activated taurine efflux (∼2 nm) is almost identical to that of VP-induced peak mobilization (∼4 nm) in pituicytes (Rosso et al. 2002b). Moreover, taurine efflux can also be evoked by other Ca2+-mobilizing agents such as bradykinin and ATP-γ-S, as well as by a direct elevation of [Ca2+]i by ionomycin. It is interesting to note that a comparable situation was found in a study of rabbit DC1 (kidney tubule) cells in which hypotonic activation of VRAC current was mimicked by adenosine acting via a Ca2+ signal (Rubera et al. 2001). Also, modulation of amino acid release through volume-activated channels has been shown in cultured astrocytes, where ATP activation of P2Y receptors greatly potentiates hypotonicity-induced aspartate efflux (Mongin & Kimelberg, 2002). Presently, at least two mechanisms can be proposed to account for the induction of taurine efflux from pituicytes. For instance, it is possible that either an osmotic stimulus or a Ca2+ signal can lead to activation of a common anionic channel through separate intracellular pathways. In turn, an interesting and more likely alternative is that changes in [Ca2+]i shift the osmosensitivity of the channel responsible for taurine efflux, consistent with a similar recent proposal by Cardin et al. (2003). Because this channel is already active in isoosmotic conditions, any increase in [Ca2+]i should theoretically be able to directly enhance its activity. In fact, this unifying hypothesis accounts for our principal observations, namely (1) the activation of taurine efflux by VP, bradykinin, ATP-γ-S and ionomycin, (2) the inhibition of VP-induced efflux in hypertonic conditions, and (3) the permissive effect of on swelling-activated efflux. To the extent that taurine conductance is measurable (Li et al. 2002), or that Cl− may provide an acceptable substitute for taurine (Junankar & Kirk, 2000), these issues could be resolved through patch-clamp experiments, which can provide both high time resolution and strict control of Ca2+ concentration on both sides of the cell membrane.
Physiological relevance of neurohypophysial hormone-induced taurine release
Our in situ experiments on whole neurohypophyses show that endogenous VP or OT does in fact stimulate taurine release. This is borne out by the fact that a selective antagonist of V1a receptors, SR 49059, partially blocked veratridine-induced taurine release. Veratridine was shown to evoke VP and OT secretion in a whole neurohypophysis preparation (Nordmann & Dyball, 1978), and thus there is little doubt that this was also the case in our experiments. The view that the taurine released by endogenous hormone comes from pituicytes is supported by previous data demonstrating that neurohypophysial taurine originates in pituicytes (Miyata et al. 1997). Moreover, the specific expression of the taurine transporter in these glial cells (Pow et al. 2002) further argues for the selective uptake of [3H]taurine in pituicytes. Lastly, the specific astrocytic localization of taurine in the supraoptic nucleus (Decavel & Hatton, 1995) has been firmly correlated with the exclusive uptake and release of [3H]taurine by glial cells (Deleuze et al. 1998). Only 30% of the veratridine-induced release of taurine is inhibited by SR 49059. Therefore, veratridine also induces taurine release via other mechanisms than VP and OT secretion. These could include secretion of other peptides or transmitters either coreleased with the neurohormones, as is the case of ATP (Gratzl et al. 1980; Zimmermann, 1994; Sperlágh et al. 1999), or released by other neuronal terminals present in the neurohypophysis (see Hussy, 2002). This mechanism seems plausible since any substance that will trigger a rise in pituicyte intracellular Ca2+ should also enhance taurine release. In addition, intense axonal activity induced by veratridine could have resulted in the accumulation of extracellular K+. The uptake of K+ by pituicytes is accompanied by swelling, which would elicit VP-independent release of taurine from glial cells (Mongin et al. 1999; Hussy et al. 2000). If all or some of these factors combined, this could explain that V1a receptor antagonism only blocked 30% of veratridine-induced taurine efflux.
Conclusions
Although the mystery remains as to the precise molecular mechanism that releases taurine from pituicytes, our results bring about an important contribution to the understanding of neurohypophysial control of hormone release. If we consider the sole case of VP, for which physiological concentrations are involved, the concerted action of hypotonic conditions together with VP and ATP corelease (Gratzl et al. 1980; Zimmermann, 1994; Sperlágh et al. 1999) might provide a powerful negative feedback that would contribute to limit excess hormone release. Thus, the decreased osmolarity brought about by antidiuresis subsequent to VP output would act in synergy with the hormone to release taurine from pituicytes. Taurine in turn would act in a paracrine fashion to inhibit vasopressinergic terminal activity via activation of glycine receptors (Hussy et al. 2001).
Acknowledgments
We thank Cédric Matthews, UMR 6543, UNSA, for his help with the microscopy work.
References
- Cardin V, Lezama R, Torres-Marquez ME, Pasantes-Morales H. Potentiation of the osmosensitive taurine release and cell Volume regulation by cytosolic Ca2+ rise in cultured cerebellar astrocytes. Glia. 2003;44:119–128. doi: 10.1002/glia.10271. [DOI] [PubMed] [Google Scholar]
- Decavel C, Hatton GI. Taurine immunoreactivity in the rat supraoptic nucleus: prominent localization in glial cells. J Comp Neurol. 1995;354:13–26. doi: 10.1002/cne.903540103. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol. 1998;507:463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Moos FC, Hussy N. Tyrosine phosphorylation modulates the osmosensitivity of volume-dependent taurine efflux from glial cells in the rat supraoptic nucleus. J Physiol. 2000;523:291–299. doi: 10.1111/j.1469-7793.2000.t01-2-00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzl M, Torp-Pedersen C, Daertt D, Treiman M, Thorn NA. Isolation and characterization of secretory vesicles from bovine neurohypophyses. Hoppe-Seylers Z Physiol Chem. 1980;361:1615–1628. doi: 10.1515/bchm2.1980.361.2.1615. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hatton GI. Pituicytes, glia and control of terminal secretion. J Exp Biol. 1988;139:67–79. doi: 10.1242/jeb.139.1.67. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Astroglial modulation of neurotransmitter/peptide release from the neurohypophysis: present status. J Chem Neuroanat. 1999;16:203–222. doi: 10.1016/s0891-0618(98)00067-2. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Bicknell RJ, Hoyland J, Bunting R, Mason WT. Arginine vasopressin mobilises intracellular calcium via V1-receptor activation in astrocytes (pituicytes) cultured from adult rat neural lobes. Brain Res. 1992;588:75–83. doi: 10.1016/0006-8993(92)91346-g. [DOI] [PubMed] [Google Scholar]
- Haydon Glia: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hussy N. Glial cells in the hypothalamo-neurohypophysial system: key elements of the regulation of neuronal electrical and secretory activity. Prog Brain Res. 2002;139:95–112. doi: 10.1016/s0079-6123(02)39010-1. [DOI] [PubMed] [Google Scholar]
- Hussy N, Bres V, Rochette M, Duvoid A, Alonso G, Dayanithi G, Moos FC. Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. J Neurosci. 2001;21:7110–7116. doi: 10.1523/JNEUROSCI.21-18-07110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Desarménien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Junankar PR, Kirk K. Organic osmolyte channels: a comparative view. Cell Physiol Biochem. 2000;10:355–360. doi: 10.1159/000016368. [DOI] [PubMed] [Google Scholar]
- Krsulovic J, Brückner G. Morphological characteristics of pituicytes in different functional stages. Light- and electronmicroscopy of the neurohypophysis of the albino rat. Z Zellforsch Mikrosk Anat. 1969;99:210–220. doi: 10.1007/BF00342222. [DOI] [PubMed] [Google Scholar]
- Leveque TF, Small M. The relationship of the pituicyte to the posterior lobe hormones. Endocrinology. 1959;65:909–915. doi: 10.1210/endo-65-6-909. [DOI] [PubMed] [Google Scholar]
- Li G, Liu Y, Olson JE. Calcium/calmodulin-modulated chloride and taurine conductances in cultured rat astrocytes. Brain Res. 2002;925:1–8. doi: 10.1016/s0006-8993(01)03235-8. [DOI] [PubMed] [Google Scholar]
- Manning M, Grzonka Z, Sawyer WH. Synthesis of posterior pituitary hormones and hormone analogues. In: Beardwell C, Robertson GL, editors. The Pituitary. London: Butterworths; 1981. pp. 265–296. [Google Scholar]
- Miyata S, Matsushima O, Hatton GI. Taurine in rat posterior pituitary: localization in astrocytes and selective release by hypoosmotic stimulation. J Comp Neurol. 1997;381:513–523. [PubMed] [Google Scholar]
- Mongin AA, Cai Z, Kimelberg HK. Volume-dependent taurine release from cultured astrocytes requires permissive [Ca2+]i and calmodulin. Am J Physiol. 1999;277:C823–C832. doi: 10.1152/ajpcell.1999.277.4.C823. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol. 2002;283:C569–C578. doi: 10.1152/ajpcell.00438.2001. [DOI] [PubMed] [Google Scholar]
- Morris JF, Pow DV, Shaw FD. Release of neuropeptides from magnocellular neurons: does anatomical compartmentalization have a functional significance. In: Pickering BT, Wakerley JB, Summerlee AJS, editors. Neurosecretion: Cellular Aspects of the Production and Release of Neuropeptides. New York: Plenum; 1988. pp. 113–122. [Google Scholar]
- Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J Physiol. 1999;516:67–74. doi: 10.1111/j.1469-7793.1999.067aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann JJ, Dayanithi G, Cazalis M, Kretz-Zaepfel M, Colin D. Isolated neurohypophysial nerve endings. A promising tool to study the mechanisms of stimulus-secretion coupling. In: Pickering BT, Wakerley JB, Summerlee AJS, editors. Neurosecretion: Cellular Aspects of the Production and Release of Neuropeptides. New York: Plenum; 1988. pp. 147–156. [Google Scholar]
- Nordmann JJ, Dyball RE. Effects of veratridine on Ca fluxes and the release of oxytocin and vasopressin from the isolated rat neurohypophysis. J General Physiol. 1978;72:297–304. doi: 10.1085/jgp.72.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasantes-Morales H, Schousboe A. Role of taurine in osmoregulation in brain cells: Mechanisms and functional implications. Amino Acids. 1997;12:281–292. [Google Scholar]
- Pow DV. Immunocytochemistry of amino-acids in the rodent pituitary using extremely specific, very high titre antisera. J Neuroendocrinol. 1993;5:349–356. doi: 10.1111/j.1365-2826.1993.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Pow DV, Sullivan R, Reye P, Hermanussen S. Localization of taurine transporters, taurine, and 3H taurine accumulation in the rat retina, pituitary, and brain. Glia. 2002;37:153–168. doi: 10.1002/glia.10026. [DOI] [PubMed] [Google Scholar]
- Pu LP, Van Leeuwen FW, Tracer HL, Sonnemans MA, Loh YP. Localization of vasopressin mRNA and immunoreactivity in pituicytes of pituitary stalk-transected rats after osmotic stimulation. Proc Natl Acad Sci U S A. 1995;92:10653–10657. doi: 10.1073/pnas.92.23.10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso L, Peteri-Brunbäck B, Vouret-Craviari V, Deroanne C, Troadec J-D, Thirion S, Van Obberghen-Schilling E, Mienville J-M. RhoA inhibition is a key step in pituicyte stellation induced by A1-type adenosine receptor activation. Glia. 2002a;38:351–362. doi: 10.1002/glia.10072. [DOI] [PubMed] [Google Scholar]
- Rosso L, Peteri-Brunbäck B, Vouret-Craviari V, Deroanne C, Van Obberghen-Schilling E, Mienville J-M. Vasopressin and oxytocin reverse adenosine-induced pituicyte stellation via calcium-dependent activation of Cdc42. Eur J Neurosci. 2002b;16:2324–2332. doi: 10.1046/j.1460-9568.2002.02401.x. [DOI] [PubMed] [Google Scholar]
- Rubera I, Barrière H, Tauc M, Bidet M, Verheecke-Mauze C, Poujeol C, Cuiller B, Poujeol P. Extracellular adenosine modulates a volume-sensitive-like chloride conductance in immortalized rabbit DC1 cells. Am J Physiol. 2001;280:F126–F145. doi: 10.1152/ajprenal.2001.280.1.F126. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Mergl Z, Jurányi Z, Vizi ES, Makara GB. Local regulation of vasopressin and oxytocin secretion by extracellular ATP in the isolated posterior lobe of the rat hypophysis. J Endocrinol. 1999;160:343–350. doi: 10.1677/joe.0.1600343. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Szucs G, Heinke S, Droogmans G, Nilius B. Activation of the volume-sensitive chloride current in vascular endothelial cells requires a permissive intracellular Ca2+ concentration. Pflugers Arch. 1996;431:467–469. doi: 10.1007/BF02207289. [DOI] [PubMed] [Google Scholar]
- Thirion S, Troadec J-D, Nicaise G. Cytochemical localization of ecto-ATPases in rat neurohypophysis. J Histochem Cytochem. 1996;44:103–111. doi: 10.1177/44.2.8609366. [DOI] [PubMed] [Google Scholar]
- Troadec J-D, Thirion S, Petturiti D, Bohn MT, Poujeol P. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes) Pflugers Arch. 1999;437:745–753. doi: 10.1007/s004240050841. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Magnocellular neuropeptidergic terminals in neurohypophysis: rapid glial release of enclosed axons during parturition. Brain Res Bull. 1982;8:205–209. doi: 10.1016/0361-9230(82)90047-8. [DOI] [PubMed] [Google Scholar]
- Wittkowski W, Brinkmann H. Changes of extent of neuro-vascular contacts and number of neuro-glial synaptoid contacts in the pituitary posterior lobe of dehydrated rats. Anat Embryol. 1974;146:157–165. doi: 10.1007/BF00315592. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]