Abstract
Retinal ganglion cells exhibit fast and slow inhibitory synaptic glycine currents that can be selectively inhibited by strychnine and 5,7-dichlorokynurenic acid (DCKA), respectively. In this study we examined whether strychnine and DCKA selectivity correlated with the subunit composition of the glycine receptor. Homomeric α1, α2 or α2* glycine subunits were in vitro expressed in human embryonic kidney cells (HEK 293). In cells expressing the α1 subunit, responses to 200 μm glycine were blocked by 1 μm strychnine but not by 500 μm DCKA. In cells expressing the α2 subunit, both 1 μm strychnine and 500 μm DCKA were effective antagonists of 200 μm glycine. In cells expressing α2* subunits, which are much less glycine-sensitive, 10 mm glycine was inhibited by 500 μm DCKA but not by 1 μm strychnine. A single amino acid mutation in the α1 subunit (R196G), converted this subunit from DCKA-insensitive to DCKA-sensitive. In conclusion, the comparative effectiveness of strychnine and DCKA can be used to distinguish between the α1, α2 and α2* receptor responses. Furthermore, a single amino acid near the glycine receptor's putative agonist binding site may account for differences in DCKA sensitivity amongst the α subunits.
The superfamily of ligand-gated ionotropic receptors, which includes the acetylcholine, GABA, and glycine receptors, are made up of multiple and variable subunits. Subunit composition and stoichiometry determine many properties of these receptors such as their affinity, kinetics and ion selectivity. The impact of glycine subunit composition is evident in heterologous expression systems while in vivo studies have documented developmental changes in subunit composition.
The rodent spinal cord has been used as a model system for studies of the glycine receptor, leading to the conclusion that subunit composition is an important developmental switch. The receptor in the prenatal spinal cord is a pentamer of α subunits, while the glycine receptor in the adult is composed of α and β subunits in a 3 : 2 stoichiometry. During development there is a switch from α2 subunit predominance in the fetal spinal cord to α1 in the adult (Becker et al. 1988; Langosch et al. 1988; Akagi et al. 1991, 1994; Takahashi et al. 1992). However, various glycine subunits have been shown to have uneven regional distributions in the adult central nervous system (Malosio et al. 1991; Betz, 1991). This implies that, in addition to their significance in development, the permutations permitted by the expression of multiple subunits can add dimensions to the information processing capacity of the adult nervous system. In this context, anatomical studies have demonstrated that α1, α2, and α3 as well as beta subunits are expressed in retinal ganglion cells of the adult rat (Greferath et al. 1994). Thus, glycine receptors on a single neurone might produce different responses based on their subunit composition.
This is intriguing because we recently observed that retinal ganglion cells manifest two kinetically distinct glycine currents that serve as low and high pass filters of information into retinal ganglion cells (Han et al. 1997). One glycine current is characterized by fast onset and desensitization and is blocked by nanomolar concentrations of strychnine. The other current has a slow onset and very slow desensitization. It is less sensitive to strychnine but is selectively inhibited by 5,7-dichlorokynurenic acid (DCKA).
These responses may relate to subunit composition. This possibility was explored by correlating the pharmacology of in vitro expressed glycine receptor subunits with the native glycine responses in retinal ganglion cells. Multiple GlyR alpha isoforms have been cloned from rat, mouse and human. Of particular interest is the unique α2* clone, isolated from newborn rat spinal cord (Kuhse et al. 1990), which has unusually low strychnine sensitivity. Since the DCKA-sensitive glycine current in ganglion cells was relatively strychnine-insensitive, we determined if DCKA and strychnine sensitivity could be related to subunit composition. We found that α1, α2, and α2* each has a unique profile of strychnine/DCKA sensitivity.
While the α2* isoform has low strychnine sensitivity, another neonatal isoform, α2 is very sensitive to strychnine inhibition (Akagi et al. 1991). These two isoforms differ in one key amino acid residue. Switching glutamate-167 residue in α2* to the corresponding glycine in α2 changes the subunit from strychnine-insensitive to strychnine-sensitive (Kuhse et al. 1990). In this paper, we report a correspondingly important amino acid that appears to account, at least in part, for the DCKA sensitivity of alpha subunits.
Methods
Subcloning and site direct mutagenesis
The cDNAs encoding the α1 and α2 subunits of the glycine receptor were gifts from Dr Akagi of The Tokyo Metropolitan Institute of Medical Science. They were subcloned from pSPT 19 and pBluescript SK(–) vectors separately into pcDNA3 mammalian vector. The α2 cDNA was mutated at glycine-167 to replicate the strychnine insensitivity of the α2* subunit. This mutated subunit is referred to as α2* in Results. All mutations were made using a QuikChange site-directed mutagenesis kit (Stratagene, CA, USA). All mutations were sequence-confirmed before further experimentation.
Expression of glycine receptors in HEK 293 cells
One day before transfection, HEK 293 cells were plated out on glass coverslips in culture dishes. The culture medium was Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum. Plasmid DNA containing the cDNA encoding the glycine receptor subunits was added to subconfluent cell layers using the calcium phosphate transfection technique (Ausubel et al. 1992) or FuGENE-6 (Roche Inc). Plasmid pGREEN LANTERN-1 (Gibco, Grand Island, NY, USA), containing green fluorescent protein (GFP), was cotransfected as an expression marker. A total of 3.5 μg DNA, with the ratio 3 : 1 (GlyR:GFP), was used per 35 mm culture dish. The medium was changed about 20 h after the start of transfection. Cells were incubated for another 24 h before electrophysiological experiments. Plasmid pGREEN LANTERN-1 was used as a positive control for monitoring expression. The fluorescent cells were used to study the properties of GlyRs; non-fluorescing cells were used as a non-transfected control. Normal HEK 293 cells were unaffected by glycine. The cells with green fluorescence after transfection generated glycine responses (except for one mutant, as mentioned in Results).
Electrophysiological recordings
Patch clamp recordings were made 1–3 days after transfection. The whole-cell voltage clamp mode was used. During recording, HEK 293 cells were bathed at room temperature with Dulbecco's phosphate-buffered saline (PBS, Gibco) containing (mm): 137 NaCl, 2.7 KCl, 0.9 CaCl2, 0.5 MgCl2, 1.5 KH2PO4 and 6.6 Na2HPO4, pH 7.3. Glycine and DCKA (RBI, Natick, MA, USA) were dissolved in PBS and were applied with a local superfusion system (DAD-12, ALA Scientific Instrument, Long Island, NY, USA). The recording pipettes contained (mm): 140 potassium gluconate or KCl, 5.4 NaCl, 2.0 MgCl2, 1.0 CaCl2, 11 EGTA, buffered with 10 mm Hepes and adjusted to pH 7.4 with KOH. With this internal solution, electrodes had resistances of 5–10 MΩ. Data were recorded with an Axopatch-2B amplifier in combination with a Pentium computer and pCLAMP software (Axon Instruments, Union City, CA, USA). Cumulative data are expressed as means ±s.e.m. Two particular issues arose in electrophysiological recordings. One was that the currents were sometimes large, which could lead to voltage clamp errors due to currents through the access resistance. This was addressed by resistance compensation of up to 80% and by sometimes clamping cells at potentials to reduce the chloride driving force to 20 mV instead of 60 mV. Consequently, the estimated access resistance error did not exceed 10 mV and was generally much less. Secondly, there are a number of studies showing that the glycine EC50 measurements change with the amount of receptor expression. Legendre et al. (2002) has shown that the increased receptor expression results in faster desensitization, compressing the dose–response curve and making it appear that the EC50 is reduced. The experiments described in this paper were done in two stages, the first using calcium phosphate for transfection, the second using FuGENE 6 (Roche, Inc.). Experiments in the second stage exhibited larger whole-cell currents and lower EC50 values. When using the calcium phosphate transfection technique the glycine-induced currents were generally below 1 nA and rarely exceeded 2 nA in α1 or α2 homo-oligomeric receptors. But when using FuGENE-6 transfection the glycine-induced currents averaged slightly above 2 nA and sometimes approached 10 nA (with a 60 mV chloride driving force) in α1 or α2 homo-oligomeric receptors. This reflects the correlation between receptor expression and EC50 measurements. However, as seen in Results, there was not much variation in dose–response measurements within each transfection protocol. Qualitatively the results were the same with either protocol. But quantitative comparisons between different drugs were only made within the experimental group using the same transfection protocol.
Data analysis
The peak currents produced by different concentrations of glycine were plotted and fitted to the Hill equation:
where I is the peak current elicited by a given glycine concentration, Imax is the maximum whole-cell current amplitude produced by a saturating glycine concentration, [glycine] is the glycine concentration, EC50 is the glycine concentration eliciting a current half of Imax, and n is the Hill coefficient.
Dose–inhibition curves of DCKA were constructed for glycine (200 μm for α1 and α2; 10 mm for α2*)-activated currents obtained in the absence (I) and presence (IDCKA) of various concentrations of DCKA, and fitted with the equation:
where IDCKA/I is the fraction of the glycine current produced in the presence of DCKA (IDCKA) compared to the current produced in the same concentration of glycine but in the absence of DCKA (I), [DCKA] is the DCKA concentration, IC50 is the DCKA concentration that inhibited half of the glycine current, and n is the Hill coefficient.
The inhibition produced by DCKA at two concentrations of glycine was fit to the equation of the Schild plot:
|
|
Results
Functional expression of GlyR subunits in HEK 293 cells
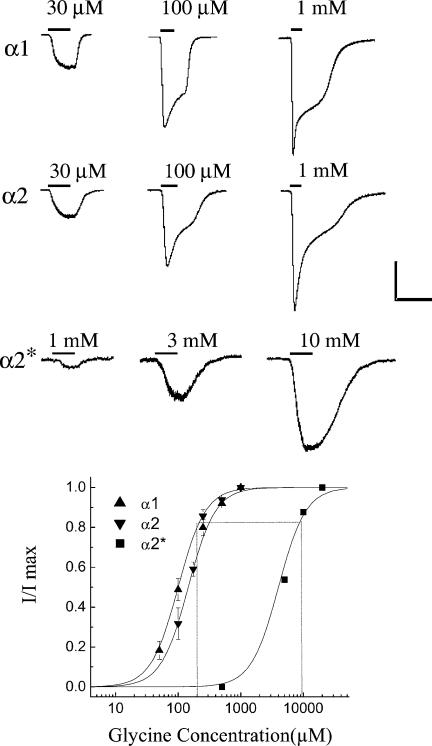
The native glycine receptor is a pentamer composed of two homologous glycosylated membrane polypeptides, α (48 kDa) and β (58 kDa) (Betz et al. 1987), but the α subunit alone can form a functional glycine receptor in vitro (Hoch et al. 1989). Changes in homomeric receptor complexes are more easily interpreted, especially in mutagenesis experiments. Thus homomeric expression was used in this study. The clones we were interested in were GlyR α1, α2 and α2* because of the variation in their sensitivity to the classic glycine receptor antagonist, strychnine (Becker et al. 1988; Kuhse et al. 1990). HEK 293 cells expressing the α1 or α2 subunit produced large currents in response to 100–200 μm glycine application, while HEK 293 cells expressing α2* subunits required 5–10 mm glycine and produced smaller whole-cell currents (Fig. 1). The glycine dose–response relationship was determined for cells expressing each of the subunits using the calcium phosphate transfection technique (see Methods). The EC50 at the α1 subunit was 108 ± 9 μm(n= 8), at the α2 receptor it was 143 ± 45 μm(n= 9), and at the α2* it was 4.8 ± 0.2 mm(n= 5) (Fig. 1). It was previously reported that the α2* is less sensitive to glycine than the other subunits (Kuhse et al. 1990). Because of this difference in agonist potency, 200 μm glycine was used to stimulate α1 and α2, while 10 mm glycine was used to activate the α2* clone. Based on the dose–response relationships shown in Fig. 1, these glycine concentrations were of similar potency, activating approximately 80% of the maximum current (vertical lines in Fig. 1). We confirmed that the glycine currents were due to chloride flux by stepping the cell to various potentials and then applying glycine. The response to glycine reversed close to the calculated chloride reversal potential in HEK 293 cells expressing the α1, α2 or α2* subunit. Untransfected cells did not produce a current in response to glycine.
Figure 1. Glycine responses in HEK 293 cells expressing homo-oligomers of α1, α2, or α2* subunits.
Upper panel, typical currents from receptors formed from each subunit are shown at several glycine concentrations. The scale bar is 500 pA for α1 and α2 and 50 pA for α2*. The time scale is 10 s. Cells were clamped, using the KCl electrode filling solution, at –20 mV for α1 and α2, and at –60 mV for α2*. This provided a chloride driving force of ∼20 mV and ∼60 mV, respectively. Recordings are from cells transfected using FuGENE-6. The EC50 at the α1 subunit was 60.6 ± 3.1 μm(n= 5), at the α2 receptor it was 62.2 ± 0.9 μm(n= 8), and at the α2* it was about 3.1 ± 0.2 mm(n= 8). In these examples drugs were applied by a rapid puff but wash out was by bath exchange, which slowed recovery especially at high glycine concentrations. Lower panel, dose–response curves for receptors formed from each subunit, using the calcium phosphate transfection technique which produced smaller currents and higher EC50 values than when using FuGENE-6. The vertical lines show that 200 μm glycine produced approximately 80% of the peak response in α1 and α2 subunit expression, while 10 mm glycine produced a similar relative response in cells expressing α2* subunits. Curves were fitted to the data using the Hill equation as described in Methods.
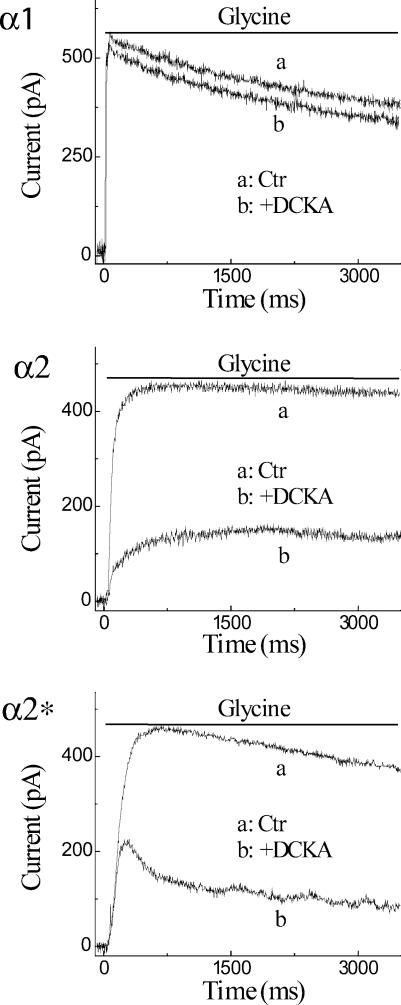
In amphibian retinal ganglion cells the IC50 of strychnine is 37 nm (against 250 μm glycine) for the fast glycine current while the slow glycine current is incompletely blocked by strychnine with an IC50 of 1 μm (Han et al. 1997). Similarly, differing IC50 values have been reported in Xenopus oocytes expressing homomeric glycine receptor alpha subunits. Homomeric α1 subunits have a strychnine IC50 of 16 nm in the presence of 290 μm glycine; α2 subunits have an IC50 of 18 nm against 310 μm glycine; α2* subunits have an IC50 of 18 μm against 12 mm glycine (Grenningloh et al. 1990; Kuhse et al. 1990). All glycine concentrations in these inhibition experiments were the EC50 values at the respective receptors. We tested the strychnine sensitivity in our expression system by comparing the response of transfected HEK 293 cells to glycine alone (Fig. 2, trace a in each panel) or after pretreatment and in the continued presence of 1 μm strychnine (trace b). In cells expressing α1 or α2 subunits, strychnine blocked the response to 200 μm glycine. But in cells expressing α2* subunits, the current produced by 10 mm glycine was only partially reduced.
Figure 2. Strychnine sensitivity of GlyR α1, α2, and α2*.
In HEK 293 cells transfected with one of the subunits, glycine was applied alone (a) or after pretreatment and in the presence of 1 μm strychnine (b). The glycine concentration was 200 μm in cells expressing α1 or α2 subunits and was 10 mm in cells expressing α2* subunits. The bar at the top of each panel indicates the timing of glycine application. Cells were voltage clamped to 0 mV using the potassium gluconate filling solution, providing a chloride driving force of ∼50 mV.
DCKA sensitivity of GlyR α1, α2 and α2*
Since we found that those retinal glycine responses that were relatively insensitive to strychnine were selectively suppressed by DCKA, we tested the ability of DCKA to suppress glycine responses in HEK 293 cells expressing the α1, α2 or α2* homo-oligomers. Glycine alone elicited an outward current (Fig. 3, curve a in each panel). Then cells were pretreated with 500 μm DCKA and glycine was reapplied in the continued presence of DCKA. The glycine-induced current was blocked by DCKA in HEK 293 cells transfected with GlyR α2 or α2* but not cells transfected with GlyR α1. Figure 4 shows the inhibitory dose–response curves for DCKA inhibition of receptors expressed by each of the three subunits. These were obtained using the fixed concentrations of glycine (200 μm for α1 and α2; 10 mm for α2*) and increasing concentrations of DCKA. The DCKA IC50 value for α2 was 188 ± 13 μm(n= 6) and for α2* it was 243 ± 61 μm(n= 10). DCKA was an ineffective antagonist, at concentrations up to 1 mm, at α1 homo-oligomeric glycine receptors (n= 5). Comparing Figs 2–4 discloses that sensitivity to strychnine and DCKA can distinguish between the three alpha subunits. The α2 subunit is sensitive to both antagonists, the α1 is inhibited selectively by strychnine, and the α2* selectively by DCKA.
Figure 3. DCKA sensitivity of GlyR α1, α2 and α2*.
HEK 293 cells were transfected with cDNA of the different alpha subunits. In each case, the cells were tested by applying glycine alone (curve a in each panel), or in the presence of 500 μm DCKA (curve b). The glycine concentration was 200 μm in cells expressing α1 or α2 subunits and was 10 mm in cells expressing α2* subunits. Cells were voltage clamped to 0 mV using the potassium gluconate filling solution, providing a chloride driving force of ∼50 mV.
Figure 4. DCKA inhibition dose–response curves.
Glycine was applied alone or in the presence of various concentrations of DCKA. The amplitude of the glycine currents (normalized to the glycine current in the absence of DCKA) are plotted against DCKA concentration. The glycine concentration was 200 μm in HEK cells expressing α1 or α2 subunits and was 10 mm in cells expressing α2* subunits. Inhibition curves were fitted as described in Methods.
Site-directed mutagensis of α1 and α2*
Sequence comparisons between GlyRs α1, α2 and α2* indicate that they are highly homologous. Each subunit is composed of a large extracellular glycosylated N-terminal domain, followed by four hydrophobic, putative transmembrane regions that form the ion channel. Site-directed mutagenesis has led to the suggestion that the ligand binding sites of the GlyR are composed of discontinuous extracellular regions of the N-terminal domain, involving three loops created by two disulphide bonds (Rajendra et al. 1995). When comparing these putative binding regions of the three glycine alpha subunits, there are pronounced differences at three residues: G160/A175/R196 in α1, the corresponding G167/P182/G203 in α2, and E167/P182/G203 in α2* (Fig. 5, the three sites enclosed in boxes). In the DCKA-insensitive α1 and the DCKA-sensitive α2 and α2*, each of these residues either incorporates or replaces a glycine or proline. Both of these amino acids are known to place kinks in an amino acid chain. Since DCKA is a much larger molecule than glycine, steric hindrance might be a determinant of DCKA action. If true, then a bend in the amino acid chain could be critical to DCKA antagonism. Therefore, the influence of each of the three amino acid residues on the receptor's sensitivity to DCKA was evaluated. In each case, the amino acid in one alpha subunit was compared to the homologous amino acid in another subunit.
Figure 5. Alignment of the extracellular, N-terminal domains of GlyR α1, α2 and α2*.
The amino acid sequence of α1 is shown and compared to α2 and α2*. The differences in amino acid sequence are marked and the amino acid sites subject to site-directed mutagenesis are enclosed in boxes.
The first of these sites (glycine-160 in α1, glycine-167 in α2, glutamate-167 in α2*) is critical for strychnine sensitivity (Kuhse et al. 1990) but does not appear to affect DCKA sensitivity. This conclusion is based on experiments indicating that α1 is not DCKA-sensitive and that α2 is, even though they both have a glycine at this site. Furthermore, α2* is DCKA-sensitive though it has a glutamate at this site.
The second site (alanine-175 in α1, proline-182 in α2 and α2*) had a slight effect on the efficacy of DCKA, but was not critical to its action. Thus, when the alanine in the α1 subunit was replaced with proline (A175P), these mutated α1 homo-oligomers were still insensitive to DCKA (Fig. 6, left panel) while the glycine EC50 was not significantly different from the wild-type α1 subunit receptor. Interestingly, the reverse mutation, in which the proline in α2* was replaced by alanine found at this position in α1 subunits, resulted in an α2*(P182A) GlyR with reduced DCKA sensitivity (Fig. 6, right panel). The DCKA IC50 shifted from 243 ± 61 μm(n= 10) for wild-type α2* to 724 ± 52 μm(n= 6) after the α2*(P182A) mutation. The glycine EC50 remained essentially unchanged. This indicates that this proline, although not a critical determinant of DCKA action, may nevertheless be a factor in the activity of this antagonist at the different alpha subunits.
Figure 6. DCKA sensitivity of GlyR α1 and α2 mutations.
Glycine was applied in the presence of various concentrations of DCKA. The response to glycine at each DCKA concentration was normalized to the response to glycine alone. Left panel, glycine (200 μm) was applied to HEK 293 cells expressing homo-oligomers of the wild-type α1 (▪) subunit or two mutations of this subunit (A175P (▴) and R196G (▾)). Mutation R196G in GlyR α1 resulted in DCKA sensitivity with an IC50= 448 ± 180 μm. Right panel, comparison the response to 200 μm glycine in the presence of various DCKA concentrations to HEK 293 cells expressing wild-type GlyR α2 (▪) or α2* (•) homo-oligomers or the P182A mutant (▴) of the α2* subunit. The glycine responses of α2 homo-oligomer were inhibited by DCKA with an IC50= 188 ± 13 μm(n= 6), while α2* had an IC50= 243 ± 61 μm(n= 10). On the other hand, α2* mutation P182A reduced DCKA sensitivity to an IC50= 724 ± 53 μm(n= 6). Inhibition curves were derived as described in Methods.
However, the third site is critical for the action of DCKA. If the arginine in position 196 of α1 was converted to the glycine that is normally found at the corresponding location in α2 and α2*, then the α1(R196G) subunit became susceptible to DCKA inhibition (Fig. 6, left panel). The mutated α1(R196G) homo-oligomer was still activated by comparatively low concentrations of glycine and was blocked by strychnine, indicating that the mutation had a specific effect on DCKA activity.
Because this experiment indicated that glycine-196 could induce DCKA sensitivity in α1 subunits, we also performed the reverse experiment to determine if removing this glycine from α2* (at position 203) would remove DCKA inhibition. Unfortunately, when we replaced the glycine in the α2* with arginine normally found in α1(G203R), we were unable to observe a glycine response, even at a glycine concentration of 20 mm. This mutation either disrupted expression or the receptor was not functional.
DCKA is a competitive antagonist
Since DCKA inhibits glycine at the α1(R196G) GlyR, we compared the potency and mechanism of action at this mutated receptor with the α2 GlyR, which is also DCKA-sensitive. As opposed to the experiments described above which used calcium phosphate transfection, the following series of experiments were performed on HEK 293 cells transfected using FuGENE 6. This resulted in higher levels of receptor expression and lower values for glycine EC50 (see Methods). Under these conditions the glycine EC50 at the α2 GlyR was 62 ± 0.89 μm(n= 8). Pretreatment with 250 μm or 500 μm DCKA shifted the glycine dose–response relationship to the right (Fig. 7). The EC50 of glycine was 95 ± 5.86 μm in the presence of 250 μm DCKA (n= 5), and it was 128 ± 5.62 μm in the presence of 500 μm DCKA (n= 6). DCKA inhibition did not produce a statistically significant change in the Hill coefficient (1.49 ± 0.03 without DCKA, 1.37 ± 0.10 in 250 μm DCKA, and 1.43 ± 0.08 in 500 μm DCKA).
Figure 7. DCKA is a competitive antagonist at α2 and α1(R196G) glycine receptors.
The top two panels show glycine dose–response curves in control Ringer solution and in the presence of 250 μm or 500 μm DCKA for α2 and α1(R196G) homo-oligomers. Cells were clamped at –20 mV, providing a chloride driving force of ∼20 mV. Data were fitted as described in Methods. The lower panel is a Schild plot (log scale in both axes) of DCKA antagonism at the α2 and α1(R196G) GlyRs
Similar experiments on the mutated α1 GlyR (R196G) revealed that the glycine EC50 was 56 ± 2.14 μm(n= 8). This is slightly less than the EC50 for either wild-type α1 or α2 GlyR. Therefore, the DCKA inhibition at the mutated receptor was not due to a reduced responsiveness to glycine. DCKA still appeared to be a competitive antagonist. The EC50 was 73 ± 0.83 μm in the presence of 250 μm DCKA (n= 6) and was 90 ± 4.10 μm in the presence of 500 μm DCKA (n= 6). The Hill coefficient was not altered significantly (1.61 ± 0.07 in control, 1.72 ± 0.03 in 250 μm DCKA, and 1.72 ± 0.11 in 500 μm DCKA).
DCKA was a competitive antagonist at both the α2 GlyR and at the mutated α1(R196G). This suggests that the R196G mutation alters the α1 GlyR so that it behaves like the α2 GlyR. Schild plots of these two subunits gave slopes very close to 1, indicative of a competitive mechanism of inhibition. The plots indicated that the Kd of DCKA was 467 μm at the α2 GlyR and 802 μm at the α1(R196G) GlyR. Thus, DCKA is a weak competitive antagonist at a subset of glycine receptors.
Discussion
Pharmacology of glycine subunits
There have been only a few efforts to distinguish subtypes of the native glycine receptor. One potentially valuable method is to define the pharmacology of glycine subunits and use this to probe native receptors. An example of this approach is the evaluation of cyanotriphenylborate (CTB), a glycine receptor antagonist that was found to block α1 homo-oligomers and α1/β hetero-oligomers (IC50= 2–3 μm) but was less effective at α2 homo-oligomers (IC50 > 20 μm) and had intermediate effectiveness at α2/β hetero-oligomers (IC50= 7.5 μm) (Rundstrom et al. 1994). Glycine receptors in rat retinal rod bipolar cells contain α1 subunits and CTB blocks their glycine responses (Enz & Bormann, 1995).
We followed this approach in reverse. DCKA selectively suppressed one type of glycine response in retinal ganglion cells. This response could be distinguished from a second glycine response based on its kinetics (Han et al. 1997), effects of phosphorylation (Han & Slaughter, 1998), and zinc inhibition (Han & Wu, 1999). All these factors suggest that DCKA is inhibiting one subtype of glycine receptor. The current study provides additional evidence that DCKA may distinguish a subtype of glycine receptor. The relative effectiveness of strychnine and DCKA can also be used to distinguish between α1, α2 and α2* subunits.
Glycine receptor structure
The binding pocket of the glycine receptor is thought to be formed by three loops made from the two disulphide bonds in the extracellular, N-terminal domain of the alpha subunit (Fig. 5). A discontinuous binding sequence formed by amino acids in the three loops may be a common property of binding pockets in this family of receptors, shared by the nicotinic acetylcholine and the GABAA receptors (Grenningloh et al. 1987; Devillers-Thiery et al. 1993; Rajendra et al. 1995). Two discontinuous regions in these loops on the α1 subunit are known to be involved in agonist and antagonist binding. The F-G-Y region in the second loop is essential for strychnine binding. For example, altering the glycine (G167) in α2 to a glutamate (E167), found in α2*, converts the receptor from strychnine- sensitive to -insensitive. The third loop region is also important for both strychnine and glycine binding. This loop is predicted to form a β sheet with a β turn produced by glycine (G205). Furthermore, the region around glycine-205 (T-G-X-F/Y) is conserved in the α and β subunits of the glycine receptor and in the α1, β1, and ρ subunits of the GABA receptor. In the glycine receptor, residues 200–204 form part of the putative β sheet that may be terminated by a β turn at the invariant residue glycine-205. Mutations of lysine-200, tyrosine-202 and threonine-204 reduced agonist binding, while lysine-200 and tyrosine-202 mutations eliminated strychnine binding. The opposite side of the putative β-sheet does not appear to be involved in ligand binding (Vandenberg et al. 1992a b; Rajendra et al. 1995). Here we show that a mutation of arginine-196 in α1 to glycine induced DCKA sensitivity in α1 homo-oligomer receptors. Arginine-196 is just outside the postulated third loop (near the putative glycine site) and this mutation would be predicted to cause a bend in the protein structure. The fact that α2 and α2* subunits contain a glycine at this position and are sensitive to DCKA, coupled with the observation that a single mutation to incorporate a glycine at this site makes the α1 subunit DCKA-sensitive, could indicate that a repositioning of the third loop is a determinant of DCKA inhibition. Unfortunately, the structure of the glycine receptor has not been determined so the link between mutational analysis and receptor binding sites is speculative.
The glycine binding site on the inhibitory and NMDA receptors
Glycine can bind to two different transmitter receptor proteins: the inhibitory glycine receptor and the NMDA subtype of the glutamate receptor. DCKA is a potent blocker of the glycine site of the NMDA receptor. Primary structure reveals little homology between the two proteins. The l-form of glycine analogues acts as an agonist at the inhibitory glycine receptor while d-amino acids such as d-serine are preferred agonists at the NMDA receptor. But there are suggestions that the pharmacophores of the two proteins share significant similarities. For example, Kuryatov et al. (1994) point out a F-x-Y motif is important for agonist and antagonist action near the putative glycine binding site in both receptors. Also, closely related quinolinic acid derivatives act as antagonists at the two receptors. Schmieden et al. (1996) suggest that both receptors form hydrogen bonds with the amino nitrogen moiety in position 1 and the hydroxyl group at position 4 of quinolinic acid and form charge–charge interactions with the carboxyl group of quinolinic acid. Quinolinic acid derivatives with a carboxyl group at the 3 position are selective antagonists to GlyR α1 and inactive at the NMDA receptor. DCKA, which is a quinolinic acid derivative with the carboxylic acid in the 2 position, does not affect the α1 receptor but blocks GlyR α2 and α2* (as well as the glycine binding site on the NMDA receptor). It may indicate that a key difference between the α1 and the α2 subunit is the position of the portion of the protein engaged in this charge–charge interaction with the carboxyl group of quinolinic acids.
Comparisons of glycine responses of retinal neurones and glycine subunits
Experiments on retinal ganglion cells have revealed two, temporally distinct glycine currents (Han et al. 1997). Synaptic glycinergic inputs to ganglion cells consist of a fast, strychnine-sensitive IPSP and a slow, relatively strychnine-insensitive IPSP. Exogenous glycine application elicited a current that could be separated into fast and slow components based on their selective inhibition by 1 μm strychnine and 500 μm DCKA. The present study demonstrates that there is a correlation between the pharmacology of the native glycine responses and that of individual glycine receptor α subunits. The α1 subunit, like the fast native glycine current, is strychnine-sensitive and DCKA-insensitive. In contrast, like the slow native glycine current, the α2* subunit is less sensitive to strychnine and suppressed by DCKA. The α2 subunit is inhibited by both strychnine and DCKA. Thus, the relative sensitivity to strychnine and DCKA can be used to distinguish between three α subunits of the glycine receptor as well as glycine currents in ganglion cells. DCKA may be a useful tool in analysing inhibitory glycinergic synapses.
But there are also many differences between the properties of each alpha subunit and that of the native retinal glycine receptors. The α2 subunit is inhibited by DCKA, but unlike the retinal current it is also sensitive to strychnine. The α2* is very insensitive to glycine, but the DCKA-sensitive current in ganglion cells has the same glycine affinity as the strychnine-sensitive glycine current (Han et al. 1997). Nor did the clones show the distinct differences in desensitization that were observed in native receptors. It is possible that combinations of alpha subunits or the addition of beta subunits are required to explain the properties of the native glycine receptors. Temporal differences may depend on the presence of beta subunits in the native glycine receptor, similar to the influence of γ2 subunits in the GABA receptor (Dominguez-Perrot et al. 1996).
Acknowledgments
This work was supported by NEI grant EY14960.
References
- Akagi H, Hirai K, Hishinuma F. Cloning of a glycine receptor subtype expressed in rat brain and spinal cord during a specific period of neuronal development. FEBS Lett. 1991;281:160–166. doi: 10.1016/0014-5793(91)80383-e. [DOI] [PubMed] [Google Scholar]
- Akagi H, Majima T, Uchiyama M. Function and modulation of the cloned glycine receptor channels expressed in Xenopus oocytes. Jap J Physiol. 1994;44:S91–S96. [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. New York: John Wiley & Sons; 1992. [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991;14:458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Betz H, Schmitt B, Becker CM, Grenningloh G, Rienitz A, Hermans-Borgmeyer I, Zopf D, Schloss P, Sawruk E, Gundelfinger E. Structure and biology of central nervous system neurotransmitter receptors. Biochem Soc Trans. 1987;15:107–108. doi: 10.1042/bst0150107. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A, Galzi JL, Eisele JL, Bertrand S, Bertrand D, Changeux JP. Functional architecture of the nicotinic acetylcholine receptor: a prototype of ligand-gated ion channels. J Memb Biol. 1993;136:97–112. doi: 10.1007/BF02505755. [DOI] [PubMed] [Google Scholar]
- Dominguez-Perrot C, Feltz P, Poulter MO. Recombinant GABAA receptor desensitization: the role of the gamma 2 subunit and its physiological significance. J Physiol. 1996;497:145–159. doi: 10.1113/jphysiol.1996.sp021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Bormann J. Expression of glycine receptor subunits and gephyrin in single bipolar cells of the rat retina. Vis Neurosci. 1995;12:501–507. doi: 10.1017/s0952523800008403. [DOI] [PubMed] [Google Scholar]
- Greferath U, Brandstätter JH, Wässle H, Kirsch J, Kuhse J, Grünert U. Differential expression of glycine receptor subunits in the retina of the rat: a study using immunohistochemistry and in situ hybridization. Vis Neurosci. 1994;11:721–729. doi: 10.1017/s0952523800003023. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen MB, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker C-M, Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990;9:771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Slaughter MM. Protein kinases modulate two glycine currents in salamander retinal ganglion cells. J Physiol. 1998;508:681–690. doi: 10.1111/j.1469-7793.1998.681bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wu SM. Modulation of glycine receptors in retinal ganglion cells by zinc. Proc Natl Acad Sci U S A. 1999;96:3234–3238. doi: 10.1073/pnas.96.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang J, Slaughter MM. Partition of transient and sustained inhibitory glycinergic input to retinal ganglion cells. J Neurosci. 1997;17:3392–3400. doi: 10.1523/JNEUROSCI.17-10-03392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch W, Betz H, Becker CM. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989;3:339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Schmieden V, Betz H. A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990;5:867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Muller E, Badiu CI, Meier J, Vannier C, Triller A. Desensitization of homomeric α1 glycine receptor increases with receptor density. Mol Pharm. 2002;62:817–827. doi: 10.1124/mol.62.4.817. [DOI] [PubMed] [Google Scholar]
- Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendra S, Vandenberg RJ, Pierce KD, Cunningham AM, French PW, Barry PH, Schofield PR. The unique extracellular disulfide loop of the glycine receptor is a principal ligand binding element. EMBO J. 1995;14:2987–2998. doi: 10.1002/j.1460-2075.1995.tb07301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundstrom N, Schmieden V, Betz H, Bormann J, Langosch D. Cyanotriphenylborate: subtype-specific blocker of glycine receptor chloride channels. Proc Natl Acad Sci U S A. 1994;91:8950–8954. doi: 10.1073/pnas.91.19.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V, Jezequel S, Betz H. Novel antagonists of the inhibitory glycine receptor derived from quinolinic acid compounds. Mol Pharmacol. 1996;50:1200–1206. [PubMed] [Google Scholar]
- Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, French CR, Barry PH, Shine J, Schofield PR. Antagonism of ligand-gated ion channel receptors: two domains of the glycine receptor alpha subunit form the strychnine-binding site. Proc Natl Acad Sci U S A. 1992b;89:1765–1769. doi: 10.1073/pnas.89.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg RJ, Handford CA, Schofield PR. Distinct agonist- and antagonist-binding sites on the glycine receptor. Neuron. 1992a;9:491–496. doi: 10.1016/0896-6273(92)90186-h. [DOI] [PubMed] [Google Scholar]