Abstract
The vanilloid receptor TRPV1 (formerly VR1) has been implicated in the activation of nociceptive sensory nerves by capsaicin, noxious heat, protons, bradykinin, cannabinoids such as anandamide, and certain metabolites of arachidonic acid. Using TRPV1 knockout mouse (TRPV1−/−) we address the question of whether TRPV1 is obligatory for action potential discharge in vagal C-fibre terminals evoked by capsaicin, anandamide, acid and bradykinin. The response of a defined subtype of the vagal afferent bronchopulmonary C-fibres (conduction velocity < 0.7 ms−1) to the putative TRPV1 activators was studied in vitro in the mouse isolated/perfused lung–nerve preparation. Capsaicin (1 μm) evoked action potential discharge of ∼90% (28/31) of C-fibres in the TRPV1+/+ mice, but failed to activate bronchopulmonary C-fibres in TRPV1−/− animals (n = 10). Anandamide (3–100 μm) induced concentration-dependent activation of capsaicin-sensitive TRPV1+/+ C-fibres with a threshold of 3–10 μm, but failed to evoke substantive discharge in TRPV1−/− C-fibres. In the TRPV1+/+ mice, the B2 receptor-mediated activation by bradykinin (1 μm) was restricted to the capsaicin-sensitive C-fibres. Bradykinin was effective in evoking B2 receptor-mediated action potential discharge in TRPV1−/− C-fibres, but the response was significantly (P < 0.05) less persistent than in TRPV1+/+ C-fibres. Exposing the tissue to acid (pH = 5) excited both TRPV1+/+ and TRPV1−/− C-fibres. We conclude that TRPV1 is obligatory for vagal C-fibre activation by capsaicin and anandamide. By contrast, whereas TRPV1 may have a modulatory role in bradykinin and acid-induced activation of bronchopulmonary C-fibres, it is not required for action potential discharge evoked by these stimuli.
The vanilloid receptor, TRPV1 (formerly VR1), is a ligand-gated ion channel that is expressed by a large percentage of mammalian nociceptive C-fibre nerves (Caterina & Julius, 2001). TRPV1 is required for activation of sensory nerves by vanilloids such as capsaicin. In addition to its role as a vanilloid receptor, TRPV1 may also contribute to activation of sensory nerves by a range of disparate stimuli including noxious heat, acid and certain lipid mediators exemplified by anandamide and 12- and 15-lipoxygenase products of arachidonic acid (Caterina et al. 1997; Tominaga et al. 1998; Zygmunt et al. 1999; Hwang et al. 2000; Mazzone & Canning, 2002; Trevisani et al. 2002). These lipid mediators are thought to gate the channel by directly binding to the vanilloid binding site on the receptor. Stimulation of G-protein coupled receptors such as the bradykinin B2 receptor may also lead to TRPV1 activation indirectly by undefined signalling pathways that may include lipid second messengers and/or release from phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2)-mediated inhibition (Chuang et al. 2001; Shin et al. 2002; Carr et al. 2003). Collectively, these findings support the hypothesis that TRPV1 is a central regulator of nociceptor activity.
The contention that TRPV1 is involved in the action potential discharge of C-fibre terminals to various stimuli is largely based on pharmacological antagonism with capsazepine and iodo-resinoferatoxin. One or both of these antagonists have been shown to inhibit action potential discharge evoked by heat, acid, bradykinin, cannabinoids, and lipoxygenase products of arachidonic acid (Caterina et al. 1997; Tominaga et al. 1998; Zygmunt et al. 1999; Wahl et al. 2001; Shin et al. 2002; Undem & Kollarik, 2002; Carr et al. 2003). A limitation in these studies is that although the antagonists are selective, at concentrations required to block TRPV1 they can have other non-specific effects (Amann & Maggi, 1991; Docherty et al. 1997; Cibulsky & Sather, 1999; Liu & Simon, 2000). In addition, when either of these competitive antagonists inhibit the response to a stimulus it is difficult to ascertain if TRPV1 is necessary for the response or if it merely contributes to the final effect.
In the present study we took advantage of the TRPV1 knockout mice to address the hypothesis that TRPV1 is obligatory for the activation of bronchopulmonary C-fibre terminals by putative endogenous TRPV1 agonists.
Methods
The experiments were approved by the Johns Hopkins Animal Care and Use Committee. Male TRPV1−/− strain B6.129S4-TRPV1tm1Jul mice and male control TRPV1+/+ strain C57BL/6 J mice were used (The Jackson Laboratory Bar Harbor, ME, USA). The method for the extracellular recording from the cell bodies of mouse vagal afferent nerve fibres projecting to the lung has been described in detail previously (Kollarik et al. 2003). Briefly, mice were killed by CO2 inhalation and exsanguination. The blood from the pulmonary circulation was washed out by in situ perfusion with Krebs bicarbonate buffer (KBS, composed of: NaCl, 118 mm; KCl, 5.4 mm; NaH2PO4, 1.0 mm; MgSO4, 1.2 mm; CaCl2, 1.9 mm; NaHCO3, 25.0 mm; dextrose, 11.1 mm, indomethacin, 3 μm, gassed with 95% O2–5% CO2, pH = 7.4) through the right heart chamber. The airways and lungs with intact extrinsic vagal innervation (including jugular–nodose ganglia complexes, JNC) were dissected and the tissue was pinned in a small Sylgard-lined Perspex chamber. The JNCs, along with the rostralmost vagi were pulled through a small hole into an adjacent chamber for extracellular recording. A piece of PE60 tubing was inserted into the trachea and connected to the infusion pump for continuous perfusion with KBS (35°C, 2 ml min−1) of the lungs. Short cuts (< 1 mm deep, 6–10 per lobe) were made on the lung surface to allow perfusing KBS to exit the tissue. The perfusion pressure was recorded by pressure transducer attached to the TA240S chart recorder (Gould, Valley View, OH, USA). The tissue and recording chambers were separately superfused with KBS (35°C, 4 ml min−1).
Extracellular recordings were performed using an aluminosilicate glass microelectrode (pulled with Flaming-Brown micropipette puller, Sutter Instrument Company, Novato, CA, USA) and filled with 3 m sodium chloride (electrode resistance ∼2 MΩ). The electrode was placed into an electrode holder connected directly to a headstage (A-M Systems, Everett, WA, USA). A return electrode of silver–silver chloride wire and an earthed silver–silver chloride pellet were placed in the perfusion fluid of the recording chamber. The recorded signal was amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cut-off, 0.3 kHz; high cut-off, 1 kHz) and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR, USA) and the TA240S chart recorder. The data were stored and analysed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz, PHOCIS, Baltimore, MD, USA) and further processed using Microsoft Excel 98. The recording microelectrode was initially manipulated into the right JNC. If the attempt to identify a bronchopulmonary afferent was unsuccessful in the right JNC, a subsequent attempt was made in the left JNC. A receptive field was identified in the lung tissue using a concentric stimulation electrode (100 V, 0.5 ms, 1 Hz). The electrode was sequentially positioned at different places on the surface of the lung lobes and the activity recorded from the JNC was observed. When electrical stimulation of the tissue evoked action potentials, the tissue was probed with a mechanical probe (Von Frey hair, 60–1800 mN). A mechanosensitive receptive field was identified when the mechanical stimulus evoked a burst of action potentials. All the afferent nerve fibres included in this study could be stimulated by a punctuate mechanical stimulus to a discrete region of the lung parenchyma. Conduction velocity was calculated by dividing the distance along the nerve pathway by the time between the shock artifact and the action potential evoked by electrical stimulation of the mechanosensitive receptive field. In most experiments one fibre per animal was recorded but occasionally two consecutive fibres were recorded in an individual preparation and care was taken to avoid desensitization due to repeated agonist administration.
The response of lung afferent nerve fibres to the following agonists was tested: adenosine 5′-triphosphate (ATP, 30 μm), capsaicin (1 μm), bradykinin (1 μm), [des-Arg9]-bradykinin (1 μm). The agonists were administered to the lung by adding a 1 ml bolus of KBS containing appropriate agonist to the tracheal perfusion (2 ml min−1) followed by at least 10 min of washout with KBS. In rare instances when no response could be obtained to any chemical stimulus used, the rate of perfusion was doubled (4 ml min−1) to increase perfusion pressure and increase perfusion of possibly underperfused areas. Under these circumstances, the agonists were administered in double the volume (2 ml) to maintain a constant transit time (30 s) of the agonist in the tissue. Acid challenge was performed by continuous perfusion with a phosphate buffer containing NaCl, 137 mm; KCl, 5.4 mm; NaH2PO4, 6.0 mm; Na2HPO2, 0.15 mm; MgSO4, 1.2 mm; CaCl2, 1.9 mm; dextrose, 11.1 mm, indomethacin, 3 μm, gassed with 95% O2–5% CO2, pH = 5.0) In all nerve fibres studied the access of the drugs to the receptive field was confirmed by activation by at least one agonist used. To address the effect of the bradykinin B2 receptor antagonist HOE 140 on the bradykinin response, the tissue was perfused with KBS containing 1 μm HOE 140 for 15 min prior to bradykinin (1 μm) administration.
The response to capsaicin and ATP is presented as the number of action potentials (mean ± s.e.m.) and the peak frequency (Hz, mean ±s.e.m.) in the 30 s interval following the onset of the response. The response to bradykinin and acid is presented as the total number (mean ±s.e.m.) and the peak frequency (impulses (10 s bin)−1, mean ± s.e.m.) of action potential discharge recorded in the 120 s interval after the onset of the response. A non-paired t test was used to quantitatively compare the responses. The χ2 test was used to compare the proportions of the fibres responsive to a particular agonist.
The following chemicals were dissolved in the distilled water (stock concentrations in parnetheses): bradykinin (1 mm), [des-Arg9]-bradykinin (10 mm), HOE 140 (10 mm). Indomethacin (30 mm) and capsaicin (10 mm) were dissolved in ethanol. The stock solutions were stored at −20°C (indomethacin stock was stored at 4°C) and diluted to their final concentrations in KBS on the day of use. Adenosine 5′-triphosphate (ATP) was diluted in KBS to its final concentration on the day of use. All drugs were purchased from Sigma-Aldrich (St Louis, MO, USA) except for bradykinin (Peninsula Laboratories, Belmont, CA, USA) and HOE 140 (provided by Aventis, Germany).
Results
Capsaicin
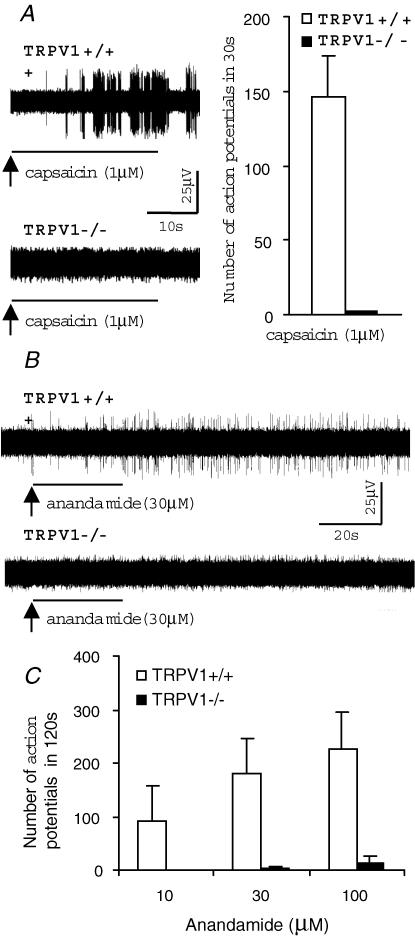
We have previously shown that, based on compound potential analysis, C-fibres in the mouse vagus nerve conduct action potentials at velocities in the approximate range of 0.3–1.5 ms−1 (Kollarik et al. 2003). However, the capsaicin sensitivity of individual afferent fibres in mouse lungs was limited to C-fibres with conduction velocities < 0.7 ms−1 (Kollarik et al. 2003). In the present study the role of TRPV1 in the activation of bronchopulmonary C-fibres was therefore investigated in C-fibres with conduction velocities < 0.7 ms−1. In the TRPV1+/+ mice, capsaicin (1 μm) evoked robust activation in 90% (28/31 fibres) of tested bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 (Fig. 1A). As expected, capsaicin (1 μm) did not induce action potential discharge in any of the tested bronchopulmonary C-fibres in TRPV1−/− animals (n = 10, Fig. 1A).
Figure 1. TRPV1 is obligatory for the activation of the vagal bronchopulmonary C-fibres by capsaicin and anandamide.
Extracellular recordings were made from vagal jugular–nodose ganglion complex neurones with defined receptive fields in the lungs of an in vitro isolated–perfused mouse lung–nerve preparation. The agonists were delivered in a continuous tracheal perfusion for 30 s followed by a 10 min washout period. A, representative traces of the capsaicin-evoked action potential discharge of the vagal bronchopulmonary C-fibres.
In TRPV1+/+ mice, capsaicin (1 μm) selectively activated vagal bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 (28/31), so conduction velocity was used as a marker of the capsaicin-sensitive C-fibre phenotype. In TRPV1−/− animals, capsaicin (1 μm) failed to activate vagal bronchopulmonary C-fibres, including C-fibres with conduction velocities < 0.7 ms−1(n = 8). Inset: total numbers (mean ±s.e.m.) of action potentials counted during the 30 s period after the onset of the response to capsaicin (1 μm) in TRPV1+/+ and TRPV1−/− animals. B, representative traces of the anandamide (30 μm)-induced response of vagal bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 in TRPV1+/+ and TRPV1−/− animals. C, total numbers (mean ± s.e.m.) of action potentials evoked by anandamide during the 120s period after the onset of the response. In TRPV1+/+ mice, anandamide induced activation in a concentration-dependent manner with a threshold of ∼3–10 μm. In contrast, anandamide evoked only a trivial response in TRPV1−/− mice at a concentration of 100 μm.
Anandamide
Anandamide is an endogenous lipid mediator that activates the metabotropic cannabinoid receptors (CB1) and, at much higher concentrations, TRPV1 (Zygmunt et al. 1999). Pharmacological studies suggest that the activation of peripheral sensory neurones by anandamide is mediated by TRPV1 and does not involve the CB1 receptor. We tested the hypothesis that TRPV1 is obligatory for anandamide-evoked action potential discharge in mouse bronchopulmonary C-fibres. In TRPV1+/+ mice, anandamide (10–100 μm) evoked action potential discharge in 6/6 C-fibres with conduction velocities < 0.7 ms−1 (n = 6, Fig. 1B and C). Relatively large concentrations of anandamide were required to evoke action potential discharge (Fig. 1C). The threshold for anandamide-induced activation was approximately 3–10 μm. Two C-fibres from TRPV1+/+ mouse with the conduction velocities in the C-fibre range, but > 0.7 ms−1 did not respond to either anandamide (100 μm) or capsaicin. In TRPV1−/− mice, anandamide (10–30 μm) failed to evoke activation of C-fibres with conduction velocities < 0.7 ms−1 (Fig. 1B–C). A trivial but noticeable effect of anandamide was observed at a concentration of 100 μm (Fig. 1C).
Bradykinin
Bradykinin acts on the metabotropic bradykinin B2 receptor to activate sensory nerves, but the ionic mechanisms coupling B2 receptor activation to membrane depolarization are not known. It has been postulated that B2 receptor-mediated activation of sensory nerves involves TRPV1. We have previously shown that, similar to capsaicin, bradykinin selectively activates mouse bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 and that every bradykinin-sensitive C-fibre was also sensitive to capsaicin (Kollarik et al. 2003). In TRPV1−/− animals, bradykinin (1 μm) induced action potential discharge in 10 of 11 tested bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 (Fig. 2). The total number of action potentials counted over a period of 120 s after the onset of the response to bradykinin in TRPV1−/− animals was 93 ± 21. The selective bradykinin B1 receptor agonist [des-Arg9]-bradykinin (1 μm) failed to activate 5 of 7 bradykinin-sensitive C-fibres and evoked only a trivial activation (11 and 12 action potentials) in the remaining two C-fibres. These data confirm that the response to bradykinin in TRPV1−/− mice is mediated by the bradykinin B2 receptor and are consistent with our previous observation in TRPV1+/+ animals (Kollarik et al. 2003).
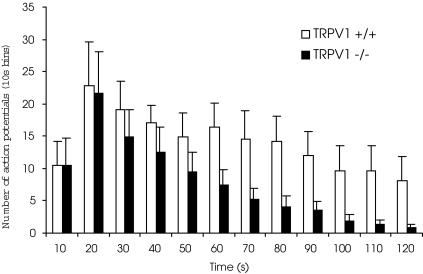
Figure 2. TRPV1 is not obligatory for activation of vagal bronchopulmonary C-fibres by bradykinin but may exert a modulatory effect.
Time course of bradykinin (1 μm)-evoked action potential discharge of vagal bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 in TRPV1+/+(n = 7) and TRPV1−/−(n = 10) animals. Although the peak frequencies (10 s bin) of the bradykinin-evoked action potential discharge was similar, the bradykinin response was less persistent in TRPV1−/− mice compared to TRPV1+/+ mice (the time required to elicit 50% of the total number of action potentials was 24 ± 5 s versus 56 ± 6 s, respectively; P < 0.01). Bradykinin activated 19 of 27 and 10 of 11 bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 in TRPV1+/+ and TRPV1−/− animals, respectively.
There was no difference in the peak frequency (per 10 s bin) of the bradykinin-evoked action potential discharge between TRPV1+/+ and TRPV1−/− mice (Fig. 2). However, as shown in Fig. 2, the response to bradykinin was significantly less persistent in the TRPV1−/− mice. The activation half-time (the time required to elicit 50% of the total number of action potentials) was significantly higher in TRPV1+/+ mice compared to TRPV1−/− mice (56 ± 6 s versus 24 ± 5 s; P < 0.01).
Acid
Two well-established mechanisms for the activation of sensory nerves by acid are TRPV1 and acid-sensing ion channels (ASICs). The TRPV1-mediated response to acid (pH = 5) is sustained while most of the ASIC-type receptors mediate brief transient responses to pH = 5 (typically < 3 s) (Waldmann et al. 1997; Tominaga et al. 1998). It has been demonstrated that the activation of C-fibres innervating guinea pig trachea by acid (pH = 5) could be substantially inhibited but not abolished by large concentrations of the TRPV1 antagonists capsazepine (10 μm) and iodoresinoferatoxin (1 μm) (Fox et al. 1995; Kollarik & Undem, 2002). Here we address the question of whether TRPV1 is obligatory for the activation of mouse bronchopulmonary C-fibres by acid (pH = 5).
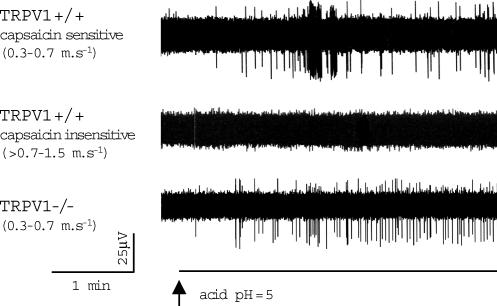
In TRPV1+/+ mice, acid challenge with the phosphate buffer (pH = 5) evoked activation in 5 of 6 bronchopulmonary C-fibres with the conduction velocities < 0.7 ms−1 (Fig. 3, upper trace). The number of action potentials recorded during the 2 min period following the onset of action potential discharge was 97 ± 48 (peak frequency 48 ± 16 per 10 s bin, n = 4). The one acid-insensitive C-fibre with a conduction velocity < 0.7 ms−1 was also capsaicin insensitive. The acid evoked no activation in the capsaicin-insensitive bronchopulmonary C-fibres with conduction velocities > 0.7 ms−1 in TRPV1+/+ animals (n = 4; Fig. 3, middle trace).
Figure 3. TRPV1 is not obligatory for activation of vagal bronchopulmonary C-fibres by acid.
Representative traces of the action potential discharge of vagal bronchopulmonary C-fibres evoked by acid (pH = 5.0). In TRPV1+/+ mice, acid activated 5 of 5 capsaicin-sensitive vagal bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 (upper trace), but failed to activate capsaicin-insensitive vagal bronchopulmonary C-fibres with conduction velocities > 0.7–1.5 ms−1(middle trace, n = 4). Lower trace, in TRPV1−/− mice, acid evoked activation in 4 of 4 vagal bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1. The peak frequency of acid-evoked action potential discharge was significantly smaller in TRPV1−/−(n = 4) compared to TRPV1+/+(n = 4) animals (48 ± 16 versus 8 ± 2 action potentials per 10 s bin, respectively; P < 0.05).
The correlation between acid sensitivity and capsaicin sensitivity in TRPV1+/+ mice suggests a major role for TRPV1 in the acid response. However, in TRPV1−/− animals, acid (pH = 5) induced activation of all tested C-fibres with conduction velocities < 0.7 ms−1(n = 4) (Fig. 3, lower trace). The number of action potentials recorded during the 2 min period following the onset of action potential discharge was 49 ± 13. The peak frequency of discharge (8 ± 2 action potentials per 10 s bin) was less than that observed in TRPV1+/+ mice (P < 0.05). Similar to TRPV1+/+ animals, acid did not activate 2 of 2 bronchopulmonary C-fibres with conduction velocities > 0.7 ms−1 in TRPV1−/− mice.
ATP
To address the possibility that the excitability of mouse bronchopulmonary C-fibres is non-specifically affected in TRPV1−/− mice, we tested the response of the C-fibres to ATP. In TRPV1+/+ mice, ATP evokes action potential discharge in both capsaicin-sensitive and capsaicin-insensitive bronchopulmonary C-fibres and this response is mimicked by the P2X selective agonist α, β-methylene ATP (Kollarik et al. 2003). The proportion of bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 that were sensitive to ATP (30 μm) was similar in TRPV1+/+ (35 of 40) and TRPV1−/− (7 of 8) animals (P > 0.1, χ2 test). The response of bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 to ATP (30 μm) did not quantitatively differ between TRPV1+/+(n = 6) and TRPV1−/−(n = 12) mice (number of action potentials in the first 30 s of the response was 91 ± 21 versus 115 ± 22, respectively, P > 0.1; the peak frequency was 11 ± 4 Hz versus 12 ± 2 Hz, respectively, P > 0.1). ATP-evoked activation of C-fibres with conduction velocities > 0.7–1.5 ms−1 was also qualitatively and quantitatively similar in TRPV1+/+ and TRPV1−/− animals (data not shown).
Discussion
Electrophysiological patch-clamp recording studies on the cell bodies of TRPV1-expressing cells have provided unequivocal evidence that TRPV1 can be gated not only by vanilloids such as capsaicin, but also by protons, heat, stimulation of certain G-protein-coupled receptors, ethanol, cannabinoids and lipoxygenase products of arachidonic acid (Tominaga et al. 1998; Zygmunt et al. 1999; Hwang et al. 2000; Shin et al. 2002; Trevisani et al. 2002). Action potential discharge of sensory nerve terminals begins with the formation of a membrane depolarization referred to as the generator potential. If the generator potential is of sufficient magnitude when it reaches the so-called active zone of the terminal, action potential discharge is evoked. It follows therefore in TRPV1-expressing sensory nerves that ions flowing through TRPV1 may be responsible for the generator potential that is induced by a wide variety of stimuli. This hypothesis is supported by studies using antagonists of TRPV1. Pharmacological antagonism of TRPV1 has been shown to inhibit action potential discharge evoked by each of the aforementioned stimuli (Fox et al. 1995; Hwang et al. 2000; Lin & Lee, 2002; Trevisani et al. 2002). The pharmacological approach has two limitations. First, at the concentrations required to inhibit TRPV1, the antagonists may have non-specific inhibitory effects (Amann & Maggi, 1991; Docherty et al. 1997; Cibulsky & Sather, 1999; Liu & Simon, 2000). Second, often the TRPV1 antagonist inhibits but does not abolish the response to a given stimuli, leaving the question open as to whether TRPV1 is obligatory for the response (and the action of the antagonist was surmounted by the stimulus), or whether TRPV1 plays only a contributory role in the overall stimulus-induced response. In the present study we used TRPV1−/− mice to address the hypothesis that TRPV1 is obligatory for action potential discharge evoked in vagal C-fibre terminals by anandamide, acid and bradykinin. We conclude that whereas TRPV1 is required for action potential discharge of C-fibre terminals evoked by capsaicin and anandamide, it plays a contributory role in the response evoked by acid or bradykinin.
The TRPV1 gene disruption does not seem to influence TRPV1-independent properties of the sensory neurones. The original study reports that the TRPV1 gene knock-out does not affect the histochemical phenotype, sensitivity to non-TRPV1 stimuli such as ATP as well as electrophysiological properties (resting membrane potential and voltage-gated sodium currents) of primary sensory neurones (Caterina et al. 2000). Consistent with these observations we found that in TRPV1−/− mice the sensitivity of the bronchopulmonary C-fibre terminals to ATP was not affected in our more complex system. We also noted, that the mechanical sensitivity of the bronchopulmonary C-fibres did not qualitatively or quantitatively differ between TRPV1+/+ and TRPV1−/− animals (data not shown).
The bronchopulmonary C-fibres in mice conduct action potentials at velocities in the 0.3–1.5 ms−1 range and can be subclassified into two distinct phenotypes based on conduction velocity and chemical sensitivity (Kollarik et al. 2003). The C-fibres of one phenotype conduct action potentials in the > 0.7–1.5 ms−1 range and are insensitive to capsaicin. The other C-fibre phenotype has a conduction velocity < 0.7 ms−1 and responds vigorously to capsaicin. Based on this information we used C-fibre conduction velocities < 0.7 ms−1 as a marker of the capsaicin-sensitive C-fibre phenotype in TRPV1−/− animals. Consistent with an obligatory role of TRPV1 in the response to capsaicin, capsaicin failed to activate bronchopulmonary C-fibres with the conduction velocities < 0.7 ms−1 in TRPV1−/− mice.
Anandamide evoked activation of the capsaicin-sensitive TRPV1+/+ mouse bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 in a concentration-dependent manner (Fig. 1C). Although a rigorous pharmacological characterization of the anandamide effect was not carried out, anandamide was markedly less potent than capsaicin in our system, with a threshold concentration for anandamide-induced activation of 3–10 μm. These data are consistent with the reports on the low relative potency and efficacy of anadamide compared to capsaicin in rat and guinea pig bronchopulmonary C-fibres (Tucker et al. 2001; Lin & Lee, 2002; Undem & Kollarik, 2002). The apparent potency of anandamide could be reduced by its non-TRPV1-mediated actions or due to its degradation. However, in similar guinea pig airway sensory nerve and mouse trigeminal neurone systems, CB1 receptor antagonists did not affect the anandamide response (Tucker et al. 2001; Roberts et al. 2002; Undem & Kollarik, 2002), and inhibition of the principal enzyme of anandamide degradation did not enhance anandamide effects on guinea pig airway sensory nerves (Tucker et al. 2001). The rate of anandamide diffusion across the neuronal membrane to an intracellular vanilloid binding site may also affect its apparent potency (Di Marzo et al. 1994).
In TRPV1−/− animals, anandamide (10–30 μm) failed to activate bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1. A small number of action potentials were evoked at the largest concentration of anandamide studied (100 μm) in 3 of 5 TRPV1−/− C-fibres. The mechanism of this activation is not known, but given its low magnitude, it is unlikely that it contributes significantly to anandamide-evoked excitation in TRPV1+/+ animals. However, because of the low potency of anandamide in some systems, large concentrations (> 30 μm) are often used (Tucker et al. 2001; Lin & Lee, 2002). In these instances potential non-TRPV1-mediated contributions to sensory nerve activation should be considered.
The bradykinin B2 receptor is one of a few G-protein-coupled receptors capable of evoking action potential discharge in vagal C-fibres of numerous species (Undem & Carr, 2001). The ion channels mediating the bradykinin-induced generator potential and action potential discharge are unknown. Evidence indicates that TRPV1 may be one of the ion channels gated in response to B2 receptor activation. In the rat, the TRPV1 antagonist capsazepine largely inhibited bradykinin-induced action potential discharge of C-fibres in a skin–nerve preparation as well as the bradykinin-evoked currents in DRG neurones (Shin et al. 2002). Likewise, we have reported a similar effect of capsazepine and ruthenium red on guinea pig vagal airway C-fibres (Carr et al. 2003). A mechanism for B2-mediated TRPV1 activation via the generation of 12-lipoxygenase products such as 12-hydroperoxy-eicosatetraenoic acid (12-HpETE) has been proposed, since the inhibitors of this pathway reduced bradykinin-evoked activation of rat somatosensory neurones as well as guinea pig airway sensory nerves (Shin et al. 2002; Carr et al. 2003). Alternatively, bradykinin B2 receptor activation may release TRPV1 from phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) inhibition (Chuang et al. 2001). The question addressed in the present study was whether TRPV1 is obligatory or merely contributory for bradykinin-induced action potential discharge in vagal C-fibre terminals. The results unequivocally demonstrate that bradykinin can effectively evoke action potential discharge in vagal afferent C-fibre terminals in the absence of TRPV1. Bradykinin activated 10 of 11 bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1 in TRPV1−/− mice. As was seen in TRPV1+/+ animals, the response to bradykinin was not mimicked by the bradykinin B1-selective agonist [des-Arg9]-bradykinin. In one TRPV1−/− bradykinin-sensitive C-fibre, pretreatment with the selective bradykinin B2 receptor antagonist HOE 140 (1 μm, 15 min) abolished the response to subsequent bradykinin (1 μm) application. Moreover, in the mouse, bradykinin is highly selective for B2 receptors (Li et al. 1998). These data indicate that, as in TRPV1+/+ mice, the response to bradykinin in TRPV1 −/− mice is mediated by B2 receptors.
We also found that the action potential discharge in response to bradykinin was significantly less persistent in C-fibres of TRPV1−/− mice (Fig. 2). This finding suggests that in TRPV1+/+ mice TRPV1 contributes to the overall bradykinin response. The simplest hypothesis reconciling our results with previous reports is that TRPV1 is one of multiple ion channels responsible for the bradykinin-evoked generator potentials in C-fibre terminals. The molecular mechanisms of the non-TRPV1-mediated component of bradykinin-induced C-fibre activation remain to be determined.
Large concentrations of capsazepine have been found to nearly abolish the C-fibre response to acid application in isolated guinea pig airway preparations (Fox et al. 1995; Kollarik & Undem, 2002). This led to the hypothesis that TRPV1 is the major ion channel required for acid-induced activation of airway C-fibres. We noted, however, that the airway C-fibre response to acid has transient and sustained components, and the latter are inhibited, but not abolished, by selective TRPV1 antagonism (Kollarik & Undem, 2002). We concluded that acid activates airway C-fibres by TRPV1-dependent and -independent pathways. The present findings that acid evoked sustained activation in TRPV1−/− mouse bronchopulmonary C-fibres prove that ion channels other than TRPV1 can lead to generator potentials in vagal C-fibres in response to decreases in tissue pH. The mechanism(s) of the TRPV1-independent response to acid in the bronchopulmonary C-fibres is not known. That acid challenge failed to stimulate bronchopulmonary afferent fibres with conduction velocities in the 0.7–1.5 ms−1 range indicates that the stimulus was not a non-specific effect on some ubiquitous proteins or ion channels. Numerous acid-sensitive ion channels may contribute to this response, including TRPV4 (Suzuki et al. 2003), various channels of the ASIC family (Kellenberger & Schild, 2002), and certain types of voltage-gated potassium channels (O'Connell et al. 2002).
The response of vagal C-fibres in TRPV1−/− mice to acid is inconsistent with findings in the somatosensory system. In the skin–nerve preparation, acid stimulates about 50% of cutaneous C-fibres in TRPV1+/+ mice. In contrast, acid-sensitive cutaneous C-fibres were nearly non-existent in TRPV1−/− animals (Caterina et al. 2000). The somatosensory C-fibres supplying the skin may have different properties compared to vagal visceral C-fibres. The reduction in the overall number of acid-sensitive neurones in the dorsal root ganglia of TRPV1−/− mice was less severe than that observed in the innervated skin preparation. The percentage of DRG neurones, irrespective of phenotype, that responded to acid was 38% in TRPV1+/+versus 7% in TRPV1−/− mice. It is tempting to speculate that perhaps the 7% of DRG neurones that retained acid sensitivity in TRPV1−/− mice innervated visceral structures.
Even though acid and bradykinin evoked action potential discharge in TRPV1−/− C-fibres, we found a concordance between the acid and capsaicin sensitivity of the bronchopulmonary C-fibres in the TRPV1+/+ mouse. Thus the capsaicin-sensitive C-fibres with conduction velocities < 0.7 ms−1 (5/5) showed a response to acid while the capsaicin-insensitive C-fibres with conduction velocities in the range 0.7–1.5 ms−1 (4/4) were not activated by acid. This observation was paralleled in TRPV1−/− mice where acid activated 4 of 4 tested bronchopulmonary C-fibres with conduction velocities < 0.7 ms−1, which correspond to the capsaicin-sensitive phenotype in TRPV1+/+ animals, and did not activate 2 of 2 bronchopulmonary C-fibres with conduction velocities > 0.7 ms−1+, which correspond to the capsaicin-insensitive phenotype in TRPV1+/+ mice. These data support our hypothesis that mouse vagal bronchopulmonary C-fibres (with conduction velocities of 0.3–1.5 ms−1) can be subdivided into at least two phenotypes (Kollarik & Undem, 2002). This hypothesis can now be based not only on TRPV1 responsiveness, but also on TRPV1-independent responses to bradykinin and acid.
References
- Amann R, Maggi CA. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–856. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304:1275–1279. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns (4,5),P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Cibulsky SM, Sather WA. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J Pharmacol Exp Ther. 1999;289:1447–1453. [PubMed] [Google Scholar]
- DiMarzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience. 1995;67:741–752. doi: 10.1016/0306-4522(95)00115-y. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and – insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Vaali K, Paakkari I, Vapaatalo H. Involvement of bradykinin B1 and B2 receptors in relaxation of mouse isolated trachea. Br J Pharmacol. 1998;123:1337–1342. doi: 10.1038/sj.bjp.0701741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Lee LY. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J Physiol. 2002;539:947–955. doi: 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin, acid and heat-evoked currents in rat trigeminal ganglion neurons: relationship to functional VR1 receptors. Physiol Behav. 2000;69:363–378. doi: 10.1016/s0031-9384(00)00209-2. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2002;283:R86–R98. doi: 10.1152/ajpregu.00007.2002. [DOI] [PubMed] [Google Scholar]
- O'Connell AD, Morton MJ, Hunter M. Two-pore domain K+ channels-molecular sensors. Biochim Biophys Acta. 2002;1566:152–161. doi: 10.1016/s0005-2736(02)00597-7. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br J Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Tucker RC, Kagaya M, Page CP, Spina D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br J Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Carr MJ. Pharmacology of airway afferent nerve activity. Respir Res. 2001;2:234–244. doi: 10.1186/rr62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Kollarik M. Characterization of the vanilloid receptor 1 antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J Pharmacol Exp Ther. 2002;303:716–722. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]
- Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]