Abstract
The electroneutral Na+-dependent HCO3− transporter NBCn1 is strongly expressed in the basolateral membrane of rat medullary thick ascending limb cells (mTAL) and is up-regulated during NH4+-induced metabolic acidosis. Here we used in vitro perfusion and BCECF video-imaging of mTAL tubules to investigate functional localization and regulation of Na+-dependent HCO3− influx during NH4+-induced metabolic acidosis. Tubule acidification was induced by removing luminal Na+ (ΔpHi: 0.88 ± 0.11 pH units, n= 10). Subsequently the basolateral perfusion solution was changed to CO2/HCO3− buffer with and without Na+. Basolateral Na+–H+ exchange function was inhibited with amiloride. Na+-dependent HCO3− influx was determined by calculating initial base flux of Na+-mediated re-alkalinization. In untreated animals base flux was 8.4 ± 0.9 pmol min−1 mm−1. A 2.4-fold increase of base flux to 21.8 ± 3.2 pmol min−1 mm−1 was measured in NH4+-treated animals (11 days, n= 11). Na+-dependent re-alkalinization was significantly larger when compared to control animals (0.38 ± 0.03 versus 0.22 ± 0.02 pH units, n= 10). In addition, Na+-dependent HCO3− influx was of similar magnitude in chloride-free medium and also up-regulated after NH4+ loading. Na+-dependent HCO3− influx was not inhibited by 400 μm DIDS. A strong up-regulation of NBCn1 staining was confirmed in immunolabelling experiments. RT-PCR analysis revealed no evidence for the Na+-dependent HCO3− transporter NBC4 or the two Na+-dependent CI−/HCO3− exchangers NCBE and NDCBE. These data strongly indicate that rat mTAL tubules functionally express basolateral DIDS-insensitive NBCn1. Function and protein are strongly up-regulated during NH4+-induced metabolic acidosis. We suggest that NBCn1-mediated basolateral HCO3− influx is important for basolateral NH3 exit and thus NH4+ excretion by means of setting pHi to a more alkaline value.
The thick ascending limb of Henle's loop (TAL) serves a number of important transport functions. On the one hand it is critically involved in NaCl absorption, generation of high interstitial osmolality and thus the countercurrent system of urine concentration (Greger, 1985). In addition it is involved in the regulation of acid–base homeostasis. Up to 15% of HCO3− is absorbed in TAL, all of which appears dependent on the presence of the luminal Na+–H+ exchanger NHE3 (Good et al. 1984; Good, 1985). Subsequently mTAL is involved in the excretion of NH4+. NH4+ entering the loop of Henle is able to travel transcellularly and uses a ‘medullary short cut’ to eventually appear in the collecting duct (Good, 1994).
Recently an electroneutral Na+-dependent HCO3− cotransporter (NBCn1) was cloned (Choi et al. 2000) and immunolabelling demonstrated its presence in basolateral membrane domains of thick ascending limb in rat kidney outer medulla (Vorum et al. 2000). The labelling was more pronounced in the inner stripe of the outer medulla (ISOM), somewhat less in the outer stripe of the outer medulla and absent in the cortex (Vorum et al. 2000). An electroneutral Na+-dependent HCO3− transporter with a proposed stoichiometry of 1 Na+ and 1 HCO3− will function as a HCO3− importer (Choi et al. 2000). Indeed, basolateral Na+-dependent HCO3− influx was identified recently in isolated perfused rat medullary thick ascending limbs (Bourgeois et al. 2002). Apparently, this is in conflict with the need for basolateral HCO3− extrusion during HCO3− absorption (Good et al. 1984; Good, 1985) As such, the functional significance of a basolateral Na+-dependent HCO3− importer remains undefined. A subsequent study from our group identified that NBCn1 protein is strongly up-regulated in a rat model of chronic metabolic acidosis induced by either NH4+ feeding or inclusion of NH4+ into the drinking water (Kwon et al. 2002). Thus we have speculated that NBCn1 may play a significant role in the excretion of NH4+ (Kwon et al. 2002). NH4+ that has entered the mTAL cell via the furosemide (frusemide)-sensitive NKCC2 transporter or the ROMK channel will dissociate into NH4+ and NH3. The generated NH3 will leave the cell via non-ionic diffusion preferentially over the basolateral membrane and finally is transported into the acidic compartment of the collecting duct. The remaining proton may either be transported directly via a Na+–H+ antiporter and/or could be buffered by import of HCO3−. The generation of CO2 would subsequently allow for the recycling of HCO3− over the basolateral membrane. In this hypothesis the extrusion of a proton/import of a HCO3− ion serves to set pHi to more alkaline values, a state where, for example, NH3 extrusion but also NaCl absorption is less restrained. Thus the purpose of this study was to functionally localize Na+-dependent HCO3− uptake in the intact, isolated perfused mTAL tubules. Furthermore it was intended to investigate if basolateral Na+-dependent HCO3− uptake is functionally up-regulated in rats treated with added NH4+ in their drinking water, a well-known condition for up-regulated NBCn1 expression (Kwon et al. 2002).
Methods
Tubule perfusion
All handling and use of the animals complied with Danish animal welfare regulations. Experiments were carried out using 4- to 6-week-old female Wistar rats weighing 70–80 g. The animals were divided into two age-matched groups. The control group had free access to food (standard rat laboratory diet, Altromin, Lage, Germany) and tap water. Also in the experimental group the animals had free access to food and water. Their drinking water contained 0.196 m ammonium chloride as previously described (Kwon et al. 2002). Animals were killed after 8.0 ± 1.4 days (n= 11) by decapitation, and the left or right kidney was removed rapidly, placed in ice-cold Ringer solution (see below) and subsequently sliced as previously described (Wright et al. 1990). Kidney slices were transferred into a dissection chamber continuously cooled at 4°C and gassed with carbogen (5% CO2–95% O2). Medullary thick ascending limbs (mTAL) were dissected from the inner stripe of the outer medulla using ultrafine watchmaker forceps. The kidney tubules were transferred into a specialized perfusion chamber mounted on an inverted microscope. Isolated tubules were perfused using a system of concentric glass pipettes previously used and developed by R. F. Greger and W. Hampel (Greger & Hampel, 1981).
Digital video imaging
The set-up consisted of an inverted microscope (Axiovert 100 TV, Zeiss, Jena, Germany) with a ×63 objective (C-Apochromat ×63, 1.2 water, Zeiss, Jena, Germany), a monochromator (Polychrome IV, Till Photonics, Planegg, Germany) and a digital camera (MicroMax, 5 MHz, Prinction Instruments, NJ, USA). Image acquisition and data analysis were performed with the software package Metamorph/Metafluor (Universal Imaging, West Chester, PA, USA). Freshly dissected mTAL were mounted into the perfusion system (Greger & Hampel, 1981). Measurement of pHi was performed with the 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF). Tubules were incubated in 20 μm basolateral BCECF/AM for 20 min at room temperature in control solution (No. 1, see Table 1). As a measure of pHi the fluorescence emission ratio at 488 nm/436 nm excitation was used and the recording speed was 1–2 Hz. Care was taken to reduce cellular damage induced by excitation light (Weiner & Hamm, 1989). This was accomplished by using neutral grey filters in the excitation light path and a 4-fold binning function of the imaging system. In each experiment the fluorescence signal was recorded from the entire tubule. The average length of the perfused tubule was around 300 μm. During the dye-loading period the tubule was continuously perfused from the luminal side with solution 1. The experiment was started 5–10 min after washout of extracellular dye and after a stable fluorescence ratio was reached. Depending on the viability of the individual tubule, stable BCECF 488 nm/436 nm ratios could be measured for 45 min or more.
Table 1.
Exerimental solutions
| Component | No. 1: Hepes | No. 2: Hepes Na+ free HCO3− free | No. 3: HCO3− Na+ free | No. 4: HCO3− Cl− free | No. 5: HCO3− Cl− free | No. 6: HCO3− Na+ free | No. 7: Hepes Cl− free, Na+ free |
|---|---|---|---|---|---|---|---|
| NaCl | 145 | — | 118 | — | — | — | — |
| NMDG | — | 145 | — | 120 | — | 119 | 145 |
| KH2PO4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.23 | 0.4 |
| K2HPO4 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 0.95 | 1.6 |
| Glucose | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Hepes | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| MgCl2 | 1 | 1 | 1 | 1 | — | — | — |
| MgSO4 | — | — | — | — | — | 6 | — |
| Ca gluconate | 1.3 | 1.3 | 1.3 | 1.3 | 8 | 4 | 4 |
| EDTA | — | — | — | — | — | 0.026 | — |
| NaHCO3− | — | — | 24 | — | 24 | — | — |
| HCl | — | 130 | — | 100 | — | — | — |
| Choline-HCO3− | — | — | — | 24 | — | 24 | — |
| Na gluconate | — | — | — | — | 121 | — | — |
| Gluconic acid | — | — | — | — | — | 121 | 145 |
All solutions had a pH of 7.40 prior to experiments. All solutions were gassed with carbogen (5% CO2–95 %O2) except for solutions 1 and 7.
BCECF signals were calibrated using the high K+–nigericin method at the end of the experiment (Thomas et al. 1979). The following calibration solution previously used by others for the same tubule segment (Watts et al. 1994) was used and contained (mm): KCl 95, NaH2PO4 0.4, Na2HPO4 1.6, glucose 5, MgCl2 1, calcium gluconate 1.3, Hepes 25, N-methyl-d-glucamine (NMDG) 20 and NaCl 15.
Measurement of intracellular buffering capacity
Quantification of HCO3− transport requires the knowledge of intracellular buffering capacity βi. We therefore measured the intracellular buffering capacity in normal and NH4+-treated animals using a similar protocol to that described by D. Good (Watts et al. 1994). To minimize the effect of HCO3/CO2 buffering and to block Na+-dependent pHi regulatory mechanisms Na+-free Hepes-containing solution (No. 1) was used on both sides of the tubule. In addition 5 mm Ba2+, 100 μm furosemide and 1 mm ouabain were added to the bath. Subsequent addition of 2.5 mm trimethylamine to the bath rapidly increased mTAL pHi by 0.26 ± 0.03 units in normal and 0.19 ± 0.02 units in NH4+-treated rats. βi was calculated as Δ[HB+]/ΔpHi, where ΔpHi is the increase in pHi resulting from weak base addition and Δ[HB+] is the change in intracellular trimethylammonium concentration, calculated from its pKa (9.8 at 37°C) and assuming that the concentration of trimethylamine base is equal in intracellular and extracellular fluids at steady state. pHi values in the experiments for measuring βi corresponded closely to pHi values in the experiments in which Na+-dependent HCO3− influx was quantified (pHi 6.4). In normal rat tubules βi was calculated to be 98 ± 16 mm (n= 5), which was not significantly different from 114 ± 12 mm (n= 8) found in tubules from NH4+-treated animals.
Calculation of base flux
Base flux (Jbase) (in pmol min−1 mm−1) was calculated using the following equation: Jbase=ΔpHi/Δt×βtotal×V, where ΔpHi/Δt is the initial rate of pHi change (in pH units min−1), βtotal is the total cellular buffering capacity (in mm (pH unit)−1 l−1), and V is the cell volume (in nl) per 1 mm of tubule length. pHi changes were determined by a linear fit of the pHi rise during the first 10–20 s after re-addition of Na+. To account for the increased buffering capacity in CO2/HCO3−-containing solutions βtotal was calculated as βi+ 2.3 ×[HCO3−]i, where [HCO3−]i is the intracellular HCO3− concentration (Roos & Boron, 1981). [HCO3−]i was calculated as follows: [HCO3]i= 0.03 PCO2× 10(pHi-6.1). In carbogen-gassed solutions, PCO2 was assumed to be 37 mmHg. V (0.32 nl mm−1) was calculated by measuring the dimensions of the tubule (volume = (radius of the tubule)2×π× length) with subtraction of the volume of the tubule lumen. A positive (Jbase) value indicates a net base influx.
Solutions and chemicals
All experimental solutions not mentioned directly in the text are listed in Table 1. BCECF was obtained from Molecular Probes (Eugene, OR, USA). All other chemicals were of the highest grade of purity available and were obtained from Sigma-Aldrich Denmark (Vallensbæk Strand) and Merck (Darmstadt, Germany).
Immunohistochemistry
Kidneys from six NH4Cl-loaded rats and six control rats were fixed by retrograde perfusion via the aorta with 4% paraformaldehyde in 0.1 m cacodylate buffer (pH 7.4) and postfixed for 30 min in the same fixative. Kidney slices containing all kidney zones were dehydrated and embedded in paraffin. The paraffin embedded tissues were cut at 2 μm on a rotary microtome (Leica, Germany). The sections were dewaxed in xylene followed by rehydration to 99% and 96% ethanol. At this point, the sections were incubated in 0.3% H2O2 in methanol to block endogenous peroxidase activity. After a rinse in 96% ethanol, the sections were rehydrated using 70% ethanol and finally water. To reveal antigens, sections were placed in 1 mm Tris buffer (pH 9.0) supplemented with 0.5 mm EGTA (3,6-di-oxa-octa-methylene-di-nitrilo-tetra-acetic acid) and heated in a microwave oven for 10 min. Non-specific binding of immunoglobulin was prevented by incubating the sections in 50 mm NH4Cl for 30 min followed by blocking in PBS supplemented with 1% BSA, 0.05% saponin and 0.2% gelatine. Sections were incubated overnight at 4°C with NBCn1 antibodies diluted in 10 mm PBS (pH 7.4) containing 0.1% Triton X-100 and 0.1% BSA. Subsequently, the sections were incubated with horseradish peroxidase-linked goat anti-rabbit secondary antibodies (P448, DAKO Glostrup, Denmark). The labelling was visualized by the diaminobenzidine (DAB) technique and the sections counterstained using Mayer's haematoxylin.
The previously described antibodies against NBCn1 (Vorum et al. 2000) and NBCe1 (anti-rkNBC1-CT15) (Maunsbach et al. 2000) were used in this study.
RT-PCR from microdissected mTAL segments
Using a commercially available kit (Trizol reagent, Invitrogen Life Technology, USA) total RNA was isolated from 10 microdissected mTAL segments (approx. 2 mm of renal tubule). Isolated total RNA was transcribed into cDNA using the RETROscript. Kit (Ambion, TX, USA). The primers shown in Table 2, derived from cloned rat or human sequences, were used to identify NBCn1, NBC4, NCBE, NDCBE, NKCC2 and BTR1 transcripts. Primers derived from human cDNA were aligned with the corresponding rat genomic sequences (http://www.ensembl.org). Thus the human and rat sequences were identical for the chosen regions of interest.
Table 2.
Primers used to identify NBCn1, NBC4, NCBE, NDCBE, NKCC2 and BTR1 transcripts
| Transporter | Primer (5′–3′) | Gene accession number | |
|---|---|---|---|
| NBCn1* | F | GACTCCATAAGGGAGAATGTTCGA | AF080106 (rat) |
| A-cassette | R | TCACCACTTTTACTACTGTCCAGG | |
| NBCn1** | F | CAAGCTCATGGATCTGTGC | AF080106 (rat) |
| B-cassette | R | ACTCACAGGCTTTTCAGGG | |
| NBC4 | F | ATGGAGAGCTTCCTGGGCAC | AF243499 (human) |
| R | CTCAGCAGAGACCAGTCCAG | ||
| NCBE | F | GCAGGTCAGGTTGTTTCTCCTC | NM_022058 (human) |
| R | TCTTCCTCTTCTCCTGGGAAGG | ||
| NDCBE | F | GCTCAAGAAAGGCTGTGGCTAC | NM_004858 (human) |
| R | ACGCCTTAATGACCCAGAGCAG | ||
| BTR1 | F | CACCTGCTGTCAGATACCATCC | AF336127 (human) |
| R | TGGTGAGCAGCTGTCTCTGATG | ||
| NKCC2 | F | CAGTGGTGCCAGTCGTTTCC | NM_019134 (rat) |
| R | TGGTGTTGTGGCCAAAGGTT |
These primers were designed to be able to recognize if NBCn1 transcripts contain exon 7 (with 369 bp) and the so-called ‘A-cassette’ (42 bp) (Choi et al. 2000).
These primers were designed to be able to recognize if NBCn1 transcripts contain the so-called ‘B-cassette’ (108 bp) (Choi et al. 2000). F, forward; R, reverse primers.
Statistics
The data shown are either original traces or mean values ±s.e.m. (n), where n refers to the number of experiments. Paired or unpaired t tests were used to compare mean values within or between experimental series. A P value of <0.05 was taken to indicate statistical significance.
Results
Immunolabelling of NBCn1 in normal and NH4+-treated young rats
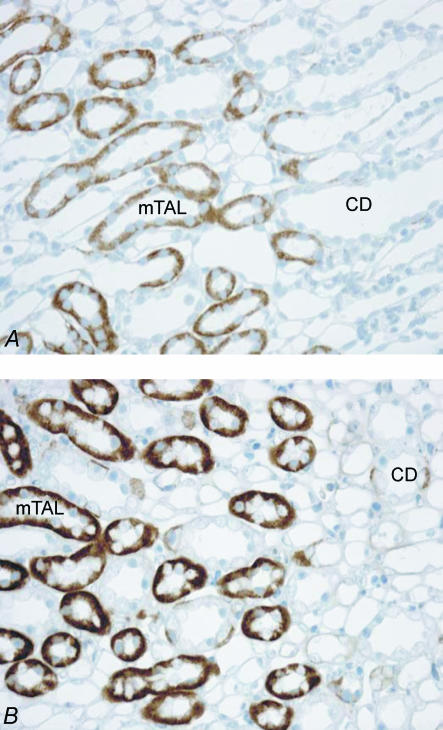
The preceding study identified a strong up-regulation of NBCn1 protein in ‘older’ rats (mean body weight 250 g) treated with NH4+ (Kwon et al. 2002). Since successful microdissection of intact single isolated mTAL tubules is critically dependent on the age of the animal, it was necessary to choose significantly younger rats for this study. Tubule dissection in young rats weighing between 70 and 80 g renders sufficient access to single nephron segments, whereas this it is profoundly more difficult in older animals. We thus repeated the initial immunolabelling experiments in young rats (weighing 70–80 g). Labelling of NBCn1 was compared in untreated and treated young rats (70–80 g). Treated rats received NH4+ in their drinking water for 8 days (see Methods). The previously measured NH4+-induced metabolic acidosis (Kwon et al. 2002) was confirmed by measuring urine pH which was 7.04 ± 0.18 units in normal and 5.9 ± 0.08 units in NH4+-treated animals (n= 6). As apparent from Fig. 1 there was a very strong up-regulation of NBCn1 labelling in the inner stripe of the outer medulla (ISOM) confirming the observation in adult rats (Kwon et al. 2002).
Figure 1. Immunohistochemical localization of the electroneutral Na+-dependent HCO3− transporter NBCn1 in control and NH4Cl-loaded rats.
NBCn1 was localized to the basolateral plasma membrane domains of mTAL segments in the inner stripe of the outer medulla and to the basolateral membrane of intercalated type A cells in collecting ducts. In comparison to controls (A), NH4Cl-loaded rats (B) showed a marked increase of NBCn1 labelling. mTAL, medullary thick ascending limb; CD, collecting duct.
RT-PCR analysis
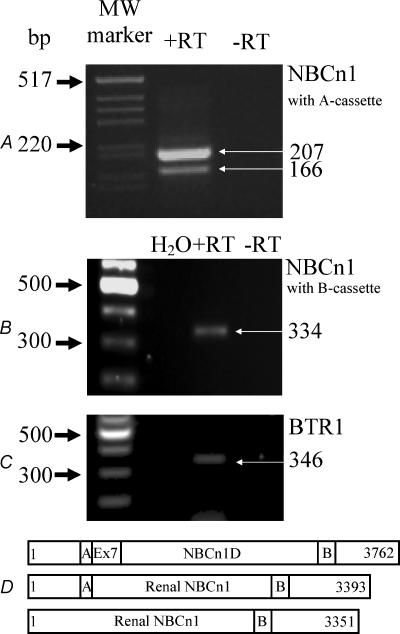
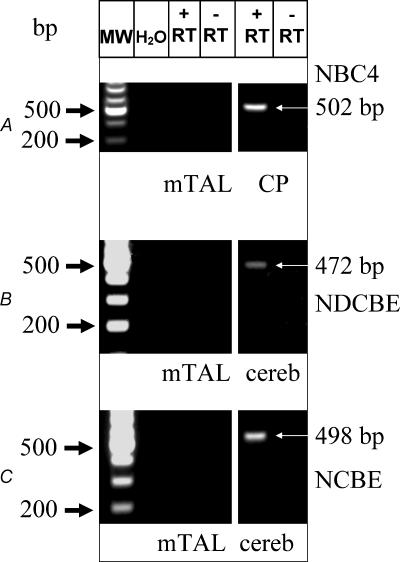
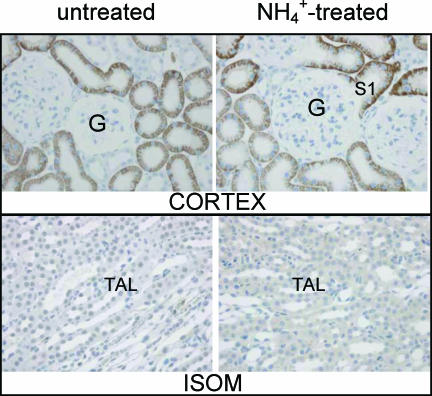
NBCn1 as isolated from a smooth muscle library was shown to represent three different variants (NBCn1B, C or D) (Choi et al. 2000). The longest variant, NBCn1D, is composed of 1254 amino acid (AA) residues (3762 bp) (Fig. 2D). The two shorter variants lack either a so-called ‘A-cassette’ (14 AAs, NBCn1C) or the ‘B-cassette’ (36 AAs, NBCn1B). Close similarity was found to a human clone isolated from skeletal muscle. The authors assigned the name NBC3 to this isoform (Pushkin et al. 1999) (accession no. NM-003615). In a subsequent publication, the same group also identified a human kidney isoform of skeletal NBC3 termed NBC2b (accession no. AF089726). This isoform is lacking a segment of 369 bp corresponding to the information encoded in exon 7. Similar results (Fig. 2A) are presented here for the rat transcript of NBCn1. One set of primers was designed to recognize if the expected transcript contains exon 7 (bp 964–1332 in NBC1nD, gene accession number AF080106) and the ‘A-cassette’. Our amplified cDNA from isolated mTAL tubules was near to 207 bp in size, as expected for a message without exon 7 but containing the ‘A-cassette’. RT-PCR results with the same primers from heart or smooth muscle revealed a band size of close to 576 bp, indicating the expression of an exon 7-containing NBCn1 variant in those tissues (data not shown). Interestingly, an additional band of smaller size, close to 160 bp, was also amplified from the mTAL tubules. This is consistent with our interpretation that renal tubules also express a splice variant which does not contain the ‘A-cassette’. The expected size of the amplified message without the ‘A-cassette’ is 166 bp. This and all the other reported PCR products generated with the different sets of primers were sequenced and identity with NBCn1 or other transporter messages confirmed. A second set of primers was designed to investigate if the mTAL NBCn1 contains the so-called ‘B-cassette’ (Table 2). As shown in Fig. 2B we found a single band of 334 bp indicating the presence of the ‘B-cassette’. In summary, our single mTAL tubule RT-PCR approach identified that the predominant variant contains the ‘A-’ and the ‘B-cassette’ (Fig. 2D). Furthermore, an RT-PCR approach using single isolated mTAL tubules was used to investigate if specific mRNA transcripts for other known Na+-dependent HCO3− transporters were expressed in rat mTAL. As quality control, single tubules were tested in parallel for the presence of NKCC2. Two independent tubule isolation procedures showed identical results. No evidence was found for the expression of NBC4, NCBE or NDCBE in isolated mTAL segments (see Fig. 3A, Fig. 3B, Fig. 3C) (See different primers and gene accession nos in Table 2.) Positive control RT-PCR amplicons for NBC4 were found in choroid plexus (502 bp), for NCBE in cerebellum (498 bp) and for NDCBE in cerebellum (472 bp) (Fig. 3A–C). Clear results were obtained for the expression of the as yet uncharacterized BTR1 in isolated mTAL tubules, a novel member of the SLC4A solute carrier gene family (Fig. 2C). Despite the fact that the electrogenic Na+-dependent HCO3− transporter (NBCe1) is known to be expressed only in kidney cortex (Maunsbach et al. 2000), we used immunohistochemistry to investigate if NBCe1 could be expressed in rat mTAL during NH4+-induced metabolic acidosis. The results are shown in Fig. 4. It can be clearly seen that NBCe1 is exclusively expressed in the proximal tubule and that no up-regulation occurs during metabolic acidosis. No NBCe1 labelling was observed in the inner stripe of outer medulla.
Figure 2. RT-PCR characterization of NBCn1 message extracted from 10 single mTAL tubule segments.
Two different primer sets were used (Table 2) to investigate which splice variant is expressed in rat mTAL. The longer transcript in A (207 bp) reflects a message which does not contain the information of exon 7 but that of the so-called ‘A-cassette’. The shorter amplicon in A does not contain the A-cassette. The second primer set (B, Table 2) identified the presence of the so-called ‘B-cassette’ in renal NBCn1. In D the full length NBCn1 and the two different renal splice variants are depicted schematically. Also seen in Fig. 3C are data showing the specific message for BTR1 in single isolated mTAL tubules.
Figure 3. RT-PCR analysis of specific message for NBC4 (A), NDCBE (B) and NCBE (C) from 10 single mTAL tubule segments.
No evidence was found for the expression of any of the three cotransporters. Positive controls for NBC4 were obtained from liver and for NDCBE and NCBE from cerebellum.
Figure 4. Immunohistochemical localization of NBCe1 in control and NH4Cl-loaded rats.
NBCe1 was localized to the basolateral plasma membrane domains of proximal tubules in the renal cortex (upper panel). No up-regulation of NBCe1 staining was observed in NH4Cl-loaded rats. No immunolabelling of NBCe1 was seen in the same rats in the thick ascending limb in the inner stripe of the outer medulla (ISOM) or other nephron segments (lower panel). TAL, medullary thick ascending limb; G, glomerulus; S1, first segment of proximal tubule.
Thus our results strongly indicate that other members of the family of Na+-coupled HCO3− transporters are not expressed in mTAL.
Subsequently we set out to investigate basolateral Na+-dependent HCO3− import in pHi measurements of isolated perfused mTAL segments.
Calibration of pHi
An original trace of a single calibration experiment is shown in Fig. 5. The right panel depicts the cumulative curve of 17 experiments. Calibrations were performed at the end of the experiments. In Hepes-buffered solution (No. 1) the resting pHi was 7.25 ± 0.04 units. In HCO3/CO2-containing buffer (solution No. 3) the resting pHi was 7.1 ± 0.03 units (n= 9). No difference in resting pHi was measured between normal and treated animals in the absence or presence of HCO3−.
Figure 5. pHi calibration of BCECF signal in isolated mTAL segments using the high K+–nigericin method.
Left panel, orignial BCECF fluorescence recording. Right panel, summary of 17 calibration experiments; resting pH of normal rats in Hepes buffer was 7.25
Na+-dependent recovery from acid load in HCO3−-containing buffer
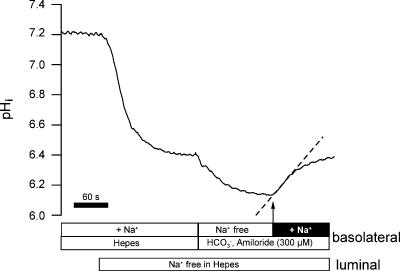
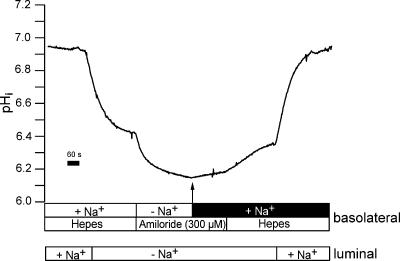
To evaluate NBCn1 function we measured basolateral Na+-dependent import of HCO3− in isolated perfused mTAL segments. The following protocol as depicted in Fig. 6 was applied. It is well established in this nephron segment that removal of luminal Na+ induces a large intracellular acidification (Sun et al. 1992; Watts et al. 1994). This pHi acidification reflects largely the activity of luminal Na+–H+ (NHE3) exchange activity (Good & Watts, 1996). We therefore chose to acid-load mTAL segments by removing luminal Na+. This also served the important purpose of excluding any effect of luminal NHE3 action when analysing Na+-dependent recovery from acid load. As shown, removal of luminal Na+ strongly acidified pHi by 0.88 ± 0.11 units (n= 10). In NH4+-treated animals this pHi decrease amounted to 0.64 ± 0.08 units (n= 11). Subsequently the perfusion solution on the basolateral side was changed to solution No. 4 (see Table 1) containing CO2/HCO3− and amiloride (300 μm) but no Na+. This led to a further stable acidification by 0.16 ± 0.05 pH units in control rats (n= 10) and 0.42 ± 0.07 pH units in NH4+-treated rats (n= 11). After 3 min, basolateral Na+ was added in the continuous presence of basolateral amiloride (300 or 600 μm). Amiloride was used to minimize any effect of the basolateral NHE antiporter-mediated H+ efflux. Na+ addition in the presence of amiloride led to an instantaneous increase of pHi. To establish the requirement for HCO3− in this re-alkalinization, the same experimental protocol was used in HCO3−-free Hepes-buffered solution (solution No. 1). An original experiment is shown in Fig. 7. The addition of Na+ in the presence of 300 μm basolateral amiloride induced a very small re-alkalinization (0.017 ± 0.01 pH units min−1, n= 6). In another series of experiments we tested 600 μm basolateral amiloride. Without amiloride the re-alkalinization was 0.26 ± 0.05 pH units min−1 and was nearly completely (94.2%) inhibited to 0.015 ± 0.14 pH units min−1 (n= 6). Thus no apparent difference was observed when using either 300 or 600 μm amiloride. To minimize any eventual effects of the luminal acid–base transport molecules (NHE3 and H+-ATPase), these experiments were conducted in the absence of luminal Na+ and in the presence of luminal amiloride (1 mm) and omeprazole (100 μm). Therefore the large recovery from acid load shown in Fig. 6 has an absolute requirement for HCO3− and thus reflects basolateral Na+-dependent import of HCO3−. The recovery rate from acid load was calculated from the initial (linear) increase of pHi. The data of the first 10–20 s were fitted to a linear function and expressed pHi change/time. Subsequently we investigated if Na+-dependent HCO3− influx is also functional at less acidic pHi values. To impose a less dramatic acidification, two different protocols were applied and are shown in Fig. 8. After changing from a Hepes-containing buffer to a Na+-free solution containing CO2/HCO3− with 600 μm amiloride, the mTALs acidified from 7.41 ± 0.02 to 7.08 ± 0.11 pH units. Subsequent addition of basolateral Na+ induced a rapid alkalinization (0.26 ± 0.07 pH units min−1, n= 9). Subsequently luminal NHE3 was inhibited with amiloride (1 mm). In basolateral Na+-free solution this led to a further acidification to 6.8 ± 0.02 units. The addition of Na+ under these conditions again induced a rapid re-alkalinization (0.32 ± 0.13 pH units min−1, n= 4). Therefore, Na+-dependent HCO3− influx is functional at all tested pHi values (pHi 6.49, 6.80 and 7.08).
Figure 6. Original experiment showing the protocol used to acidify mTAL segments.
See main text for detailed description. mTAL tubules were acidified by removal of luminal Na+. Shifting basolateral Hepes buffer to CO2/HCO3− buffer in the absence of basolateral Na+ mediated a further acidification. In the presence of basolateral amiloride re-addition of basolateral Na+ induced a rapid re-alkalinization. The dashed line indicates the initial rate of re-alkalinization.
Figure 7. Absence of basolateral Na+-dependent re-alkalinization in Hepes buffer.
Original experiment of the same type as in Fig. 4 but in HCO3−-free Hepes-containing solution. mTAL tubules were acidified by removal of luminal Na+. Removal of basolateral Na+ and addition of amiloride (300 μm) induced a further acidification. In the presence of basolateral amiloride, re-addition of basolateral Na+ induced no significant re-alkalinization.
Figure 8. Na+-dependent HCO3− influx is present at different pHi values.
mTAL tubules were acidified by luminal amiloride (1 mm) or in addition further acidified by basolateral amiloride (600 μm). This original trace shows a decrease of pHi to 7.2 or 7.05, respectively. In both parts of the experiment addition of basolateral Na+ induced a rapid and reversible re-alkalinization.
Up-regulation of Na+-dependent HCO3− influx in NH4+-treated animals
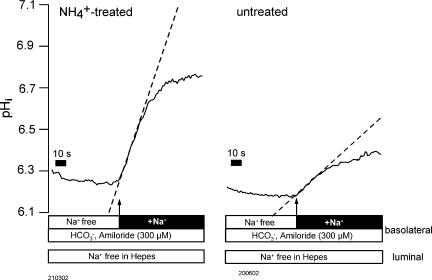
Initially we tested whether Na+–H+ exchange activity was altered in control versus NH4+-treated rats. Interestingly no difference was observed. In control rats the Na+-dependent pHi recovery rate in Hepes-buffered solutions was 0.26 ± 0.05 versus 0.27 ± 0.04 pH units min−1 in rats treated for 7.4 ± 0.7 days with NH4+ (n= 9). These results are supported by immunolabelling results showing no change of expression of the NHE1 protein in the basolateral membrane of normal versus NH4+-treated animals (data not shown, S. Frische). The most important purpose of this study was to investigate if up-regulation of NBCn1 protein, seen in immunolabelling experiments and Western blotting (Kwon et al. 2002), correlates with an increase of basolateral Na+-dependent HCO3− influx. Two single experiments in NH4+-treated and untreated animals are depicted for comparison in Fig. 9. Isolated tubules were acidified as described and shown in Fig. 6. Two significant differences between normal and NH4+-treated rats could be observed. (1) The initial rate of recovery (indicated by the dashed line) was significantly increased in NH4+-treated animals by a factor of 2.4. Recovery rates were compared at identical pHi values. In normal rats the initial recovery rate was 0.24 ± 0.0309 pH units min−1 (n= 10) as compared to 0.64 ± 0.09 pH units min−1 (n= 11) in NH4+-treated animals. (2) From Fig. 9 it is also apparent that the new resting pHi value after re-addition of Na+ is significantly higher in NH4+-treated animals. In NH4+-treated animals the Na+-dependent increase to a new stable pHi value amounted to 0.38 ± 0.03 pH units (n= 11) as compared to 0.22 ± 0.02 pH units (n= 10) in untreated animals. Calculating initial Jbase after re-addition of Na+ (see Methods) revealed 8.4 ± 0.9 pmol min−1 mm−1 in normal and 21.8 ± 3.2 pmol min−1 mm−1 in rats treated with NH4+ and thus a 2.6-fold increase of HCO3− flux. Taken together these data indicate a strong functional up-regulation of Na+-dependent HCO3− influx in NH4+-treated animals.
Figure 9. Comparison of Na+-dependent re-alkalinization in normal and NH4+-treated animals.
The protocol was as described in Fig. 4. Left panel: NH4+-treated animals. Right panel: untreated animals. The dashed lines indicate the initial rate of re-alkalinization. In NH4+-treated animals a significantly faster rate of re-alkalinization is obvious. In addition a significantly higher steady state pHi after Na+-dependent HCO3− influx is reached in NH4+-treated animals.
Cl− independence of Na+-dependent HCO3− influx in normal and NH4+-treated animals
The family of Na+-dependent HCO3− transporters also encompasses Cl−-dependent transporters (NDCBE: Na+-dependent Cl−–HCO3− exchangers). We therefore repeated the experiments described in Fig. 9 in the absence of luminal and basolateral Cl−. The same protocol was used as described for the experiments shown in Figs 6 and 9. Two original traces are shown in Fig. 10 in normal and NH4+-treated tubules. Cl− was removed bilaterally 17.8 ± 3.2 min (n= 5) before re-addition of basolateral Na+. Clearly it is seen that addition of Na+ induces a rapid alkalinization and that this alkalinization is strongly up-regulated in NH4+-treated rats. Table 3 summarizes all results. In chloride-free conditions a 3-fold up-regulation of Na+-dependent base influx was measured in NH4+-treated rats. Also in these experiments the Na+-dependent increase to a new stable pHi value was significantly larger in NH4+-treated rats (0.47 ± 0.04 pH units, n= 5) as compared to untreated animals (0.23 ± 0.05 pH units, n= 5). Table 3 shows that in both experimental series the up-regulation on Na+-dependent HCO3− influx in NH4+-treated animals was of similar magnitude. These experiments clearly indicate that Na+-dependent HCO3− influx occurs independently of Cl− and thus argues for NBCn1 as the relevant transporter.
Figure 10. Comparison of Na+-dependent re-alkalinization in normal and NH4+-treated animals under Cl−-free conditions.
The protocol was as described in Fig. 4. Left panel: NH4+-treated animals. Right panel: untreated animals. The dashed lines indicate the initial rate of re-alkalinization. Also after removal of bilateral Cl− in NH4+-treated animals a significantly faster rate of re-alkalinization is obvious. In addition a significantly higher steady state pHi after Na+-dependent HCO3− influx is reached in NH4+-treated animals.
Table 3. Summary of pHi recovery rate and base flux data in normal and NH4+-treated rats.
| Untreated | NH4+ treated | ||
|---|---|---|---|
| With chloride (n= 11) | pHi recovery (pH units min−1) | 0.24 ± 0.03 | 0.64 ± 0.09 |
| Base flux (pmol min−1 mm−1) | 8.4 ± 0.9 | 21.8 ± 3.2 | |
| Without chloride (n= 5) | pHi recovery (pH units min−1) | 0.28 ± 0.04 | 0.72 ± 0.25 |
| Base flux (pmol min−1 mm−1) | 8.7 ± 1.36 | 26.1 ± 9.3 |
DIDS-insensitivity of basolateral Na+-dependent HCO3− influx
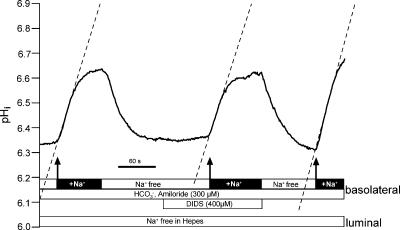
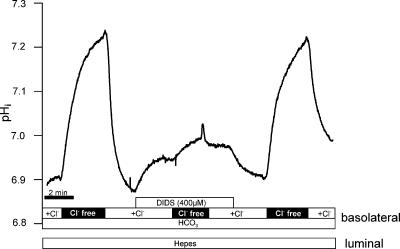
To further characterize Na+-dependent HCO3− influx we subsequently investigated its possible DIDS sensitivity. The experiments were conducted with the same protocol as those described in Fig. 6 and performed using animals treated with NH4+ in their drinking water for 1 week. An original experiment is shown in Fig. 11. Acid loading was again performed by removing luminal Na+. Basolateral amiloride (300 μm) was present during the entire relevant experimental period. In CO2/HCO3−-containing buffer, rapid alkalinizations are visible whenever Na+ was re-added. These Na+-dependent rapid alkalinizations represent HCO3− influx. Removal of Na+ resulted in a return to the acidic pre-control pHi value of near 6.3. During the second addition of Na+, DIDS (400 μm) was present (including the indicated preincubation period of 60–90 s). As shown in Fig. 11 this had no effect on Na+-dependent HCO3− influx. Similar results were observed in 10 experiments (pH recovery rate before DIDS: 0.63 ± 0.11, during DIDS 0.68 ± 0.16 and after washout of DIDS 0.67 ± 0.17 pH units min−1). These results indicate that basolateral Na+-dependent HCO3− influx is not blocked by DIDS. DIDS-mediated inhibition of another HCO3− transporter located in the basolateral membrane of mTAL could, however, be shown. Medullary TAL segments also express a basolateral Cl−–HCO3− antiporter, which is known to be DIDS inhibitable. Functional activity of the Cl−–HCO3− antiporter can be made visible by removing basolateral Cl−. This is known to induce rapid, reversible alkalinizations (Sun, 1998). As shown in Fig. 12, alkalinization mediated by Cl− removal was almost completely inhibited with 400 μm DIDS (n= 3). These experiments strengthen the results indicating that Na+-dependent HCO3− influx in rat mTAL is not blocked by DIDS.
Figure 11. No effect of DIDS on Na+-dependent re-alkalinization in an NH4+-treated animal.
Figure 12. Effect of DIDS on alkalinization induced by removal of extracellular Cl−.
Note that DIDS had a prompt and rapid alkalinizing effect on resting pHi. The subsequent Cl−-removal-induced alkalinization was almost completely inhibited with 400 μm DIDS. The DIDS inhibition was reversible.
Discussion
This study serves to increase our understanding of the recently identified electroneutral Na+-dependent HCO3− transporter NBCn1 localized in rat inner stripe mTAL segments (Choi et al. 2000). A preceding immunolabelling study indicated the localization of NBCn1 in the basolateral membrane of mTAL segments (Vorum et al. 2000). Thus, the initial purpose was to identify Na+-dependent HCO3− influx across the basolateral membrane of mTAL segments. This required the use of the isolated perfused kidney tubule technique to achieve sufficient control over the different luminal and basolateral acid–base transporters known in this segment. Our results identify Na+-dependent HCO3− influx across the basolateral membrane of mTAL segments. We propose that this HCO3− influx is mediated via the NBCn1 protein. To investigate this further we studied Na+-dependent HCO3− influx in normal and NH4+-treated animals. The addition of NH4+ to the drinking water is a well-established method of producing a model of chronic metabolic acidosis which has previously been shown to induce a strong up-regulation of NBCn1 protein in mTAL (Kwon et al. 2002). This up-regulation is confirmed in this study. We hypothesized that protein up-regulation should be reflected in the up-regulation of Na+-dependent HCO3− influx. Indeed, our experiments show a strong and significant up-regulation of Na+-dependent HCO3− influx in rats treated with NH4+. Therefore, the close correlation between up-regulation of protein and function strengthens the proposal that NBCn1 mediates Na+-dependent HCO3− influx in rat mTAL. Importantly, a near to identical Na+-dependent HCO3− influx in control rats was observed in the absence of Cl−. Likewise in NH4+-treated rats Cl− depletion left the strong up-regluation of Na+-dependent HCO3− influx unchanged (Fig. 10 and Table 3). This strongly indicates that Na+-dependent HCO3− influx in rat mTAL does not occur via the closely related Na+-dependent Cl−–HCO3− exchangers.
DIDS is a widely used inhibitor of anion and HCO3− transport (Cabantchik & Greger, 1992). Our study presents evidence that the basolateral Na+-dependent HCO3− influx is not blocked by DIDS. This is in close agreement with the initial cloning paper showing a very low DIDS sensitivity (Choi et al. 2000) Some DIDS sensitivity was, however, observed in a preceding study in which the entire kidney slice was used to measure pHiin situ in mTAL segments (Kwon et al. 2002). Currently we do not believe that our recently shown DIDS sensitivity of Na+-dependent recovery from acid load in whole kidney slices reflects NBCn1 function alone (Kwon et al. 2002). The observed DIDS insensitivity supports the interpretation that Na+-dependent HCO3− influx is mediated via NBCn1.
Noteworthy is a recent study which investigated a number of basolateral acid–base transporters in isolated perfused rat mTAL segments. The authors reported Na+-dependent HCO3− influx over the basolateral membrane and similarly found no effects of DIDS (Bourgeois et al. 2002). In addition they performed their experiments in Cl−-free medium, indicating that Na+-dependent HCO3− transport occurs independently of Cl−. Thus this study is in close agreement with our results presented here.
Is NBCn1 the only basolateral Na+-dependent HCO3− importer in mTAL?
A recent publication indicates that in addition to NBCn1 yet another variant of Na+-dependent HCO3− transporters, namely NBC4, could be present in rat mTAL segments as based on mRNA data (Xu et al. 2002). However, our RT-PCR results did not detect NBC4 message in isolated mTAL tubules. Since the rat genome has become available in 2003 we found that the primers used by Xu et al. to identify NBC4 in rat kidney do not recognize any relevant sequence of the rat NBC4 gene (SLC4A5). Furthermore a BLAST search revealed that the forward primer (ATGGTTGACCGATCCTTG) used by Xu et al. matches mouse NBC3. The reverse primer (GCTGGCTCTTAATAATGATGGC) identified 30 different sequences of diverse origin and not related to NBC sequences. We currently do not believe that rat mTAL express NBC4 and think that the above-mentioned study is faulty. In addition our RT-PCR did not show the expression of other known members of the Na+-coupled HCO3− transporters NCBE (Wang et al. 2000; Choi et al. 2002) and NDCBE (Grichtchenko et al. 2000). Immunolabelling studies furthermore strengthened the findings that electrogenic NBCe1 is only expressed in the proximal tubules of the renal cortex and that no NH4+-induced expression occurs in mTAL segments. Our screen also included the functionally still uncharacterized BTR1 protein which, based on a homology analysis, was suggested to be a HCO3− transporter (Parker et al. 2001). As shown we could identify BTR1 message in isolated rat mTAL tubule; however, its significance is currently obscure.
As mentioned above the rat NBCn1 protein is a close homologue of the human NBC3 protein (Pushkin et al. 1999). Recently an NBC3 knock-out mouse was generated with the somewhat surprising phenotype of developing blindness and deafness (Bok et al. 2003). An apparent renal phenotype was not observed. This mouse will enable us to definitively investigate if in mTAL the NBCn1/NBC3 protein is the only Na+-dependent HCO3− transporter. Nonetheless our compiled data strongly suggest that NBCn1 is probably the only protein involved in basolateral Na+-dependent HCO3− uptake and is strongly up-regulated during metabolic acidosis. Our results however, do not explicitly rule out that other unforeseen candidates may contribute to this function.
Functionally basolateral Na+–H+ exchange (NHE1, NHE4) and Na+-dependent HCO3− uptake could serve the same purpose, namely to extrude acid equivalents from the cytosol. Na+-dependent recovery rates in the presence and absence of CO2/HCO3−-containing buffer allow the quantification of the contribution of the two mechanisms involved. NBC-mediated recovery in normal rats was 0.24 ± 0.03 pH units min−1. In comparison NHE-mediated recovery from acid amounted to 0.26 ± 0.05 pH units min−1. Interestingly, we found no up-regulation of NHE-mediated recovery from acid load in NH4+-treated rats. Confirmatory results were presented on the levels of the protein and mRNA for NHE1, where no up-regulation was observed in metabolic acidosis (Laghmani et al. 2001). In addition, our own immunolabelling results confirm that NHE1 is not up-regulated in NH4+-treated animals (authors' unpublished observations).
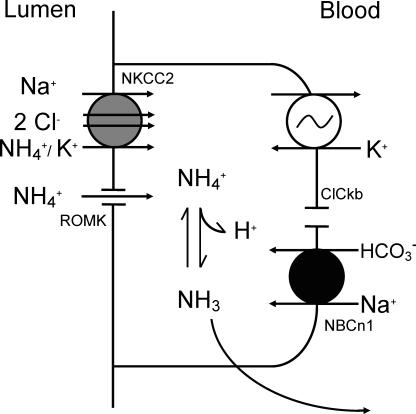
An integrated functional role for NBCn1 in mTAL may become apparent when we consider the major functions of mTAL. As summarized in the Introduction mTAL is critically involved in NaCl, Mg2+, Ca2+, HCO3− absorption and NH4+ excretion. Considering the absorptive functions of mTAL, no direct involvement may intuitively be reasonable as basolateral Na+ influx may appear counterproductive and HCO3− absorption requires a basolateral HCO3− exit step and not the contrary. However, the function of NH4+ excretion may indeed profit from a basolateral HCO3− importing mechanism. The functional concept is schematically shown in Fig. 13 and has already been mentioned in some detail in the Introduction. Metabolic acidosis is associated with increased proximal tubular formation of NH4+ and subsequently its handling along the tubulus (Curthoys & Gstraunthaler, 2001). It is suggested that basolateral NBCn1 serves to maintain medullary transcellular NH4+ shuttling by maintaining a favourable mTAL pHi. Luminal entry of NH4+ via the NKCC2 transporter and the ROMK channel is well established and results in a large and continuous acid load of the mTAL cell (Good, 1994). The nature of this significant NH4+-induced acid load is complex, including more than the uptake of a proton via NH4+ (Good, 1994). An acidic pHi of the mTAL cell will reduce the diffusable fraction of NH3 (pKb 9.3). On the contrary a more alkaline pHi should significantly increase the free diffusable NH3 and thus will allow basolateral NH3 exit and further trapping of NH3/NH4+ in the acidic collecting duct fluid. It remains to be measured more directly, however, whether functional NBCn1 is significantly required for unrestrained NH4+ excretion.
Figure 13. Schematic cell model of transcellular NH4+ transport in medullary thick ascending limb.
NBCn1 is suggested to be important for buffering the acid load imposed on the cell by uptake of luminal NH4+ via the NKCC2 transporter and ROMK channels.
In summary this study strongly suggests that basolateral Na+-dependent HCO3− uptake occurs via NBCn1. This Na+-dependent HCO3− influx occurs independently of Cl−. In isolated perfused mTAL segments NBCn1 was found to be DIDS insensitive. Chronic metabolic acidosis induced a strong up-regulation of NBCn1 protein and function. We suggest that NBCn1-mediated HCO3− uptake is an important event in transcellular transport of NH4+.
Acknowledgments
We thank Susie Mogensen, Edith Bjørn Møller, Kirsten Skaarup, Ann-Charlotte Andersen, Ida Maria Jalk and Inger Merete Poulsen for their expert technical assistance. Support for this study was provided by the Danish Medical Research Council and the Water and Salt Research Center at the University of Aarhus which is established and supported by the Danish National Research Foundation (Danmarks Grundforkningsfond).
References
- Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, Van Sligtenhorst I, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu W, Kurtz I. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet. 2003;34:313–319. doi: 10.1038/ng1176. [DOI] [PubMed] [Google Scholar]
- Bourgeois S, Masse S, Paillard M, Houillier P. Basolateral membrane Cl−-, Na+-, and K+-coupled base transport mechanisms in rat MTALH. Am J Physiol Renal Physiol. 2002;282:F655–F668. doi: 10.1152/ajprenal.00220.2000. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992;262:C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Choi I, Rojas JD, Kobayashi C, Boron W. Functional characterization of ‘NCBE’, a Na/HCO3 cotransporter. FASEB J. 2002;16:A796. [Google Scholar]
- Curthoys NP, Gstraunthaler G. Mechanism of increased renal gene expression during metabolic acidosis. Am J Physiol Renal Physiol. 2001;281:F381–F390. doi: 10.1152/ajprenal.2001.281.3.F381. [DOI] [PubMed] [Google Scholar]
- Good DW. Sodium-dependent bicarbonate absorption by cortical thick ascending limb of rat kidney. Am J Physiol. 1985;248:F821–F829. doi: 10.1152/ajprenal.1985.248.6.F821. [DOI] [PubMed] [Google Scholar]
- Good DW. Ammonium transport by the thick ascending limb of Henle's loop. Annu Rev Physiol. 1994;56:623–647. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- Good DW, Knepper MA, Burg MB. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol. 1984;247:F35–F44. doi: 10.1152/ajprenal.1984.247.1.F35. [DOI] [PubMed] [Google Scholar]
- Good DW, Watts BA., III Functional roles of apical membrane Na+/H+ exchange in rat medullary thick ascending limb. Am J Physiol. 1996;270:F691–F699. doi: 10.1152/ajprenal.1996.270.4.F691. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985;65:760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Greger R, Hampel W. A modified system for in vitro perfusion of isolated renal tubules. Pflugers Arch. 1981;389:175–176. doi: 10.1007/BF00582110. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Romero MF, Boron WF. Extracellular HCO3− dependence of electrogenic Na/HCO3 cotransporters cloned from salamander and rat kidney. J Gen Physiol. 2000;115:533–546. doi: 10.1085/jgp.115.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon TH, Fulton C, Wang W, Kurtz I, Frokiaer J, Aalkjaer C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na-HCO cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol. 2002;282:F341–F351. doi: 10.1152/ajprenal.00104.2001. [DOI] [PubMed] [Google Scholar]
- Laghmani K, Richer C, Borensztein P, Paillard M, Froissart M. Expression of rat thick limb Na/H exchangers in potassium depletion and chronic metabolic acidosis. Kidney Int. 2001;60:1386–1396. doi: 10.1046/j.1523-1755.2001.00942.x. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjaer C. Immunoelectron microscopic localization of the electrogenic Na/HCO3 cotransporter in rat and ambystoma kidney. J Am Soc Nephrol. 2000;11:2179–2189. doi: 10.1681/ASN.V11122179. [DOI] [PubMed] [Google Scholar]
- Parker MD, Ourmozdi EP, Tanner MJ. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun. 2001;282:1103–1109. doi: 10.1006/bbrc.2001.4692. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sun A. Expression of Cl−/HCO3− exchanger in the basolateral membrane of mouse medullary thick ascending limb. Am J Physiol. 1998;274:F358–F364. doi: 10.1152/ajprenal.1998.274.2.F358. [DOI] [PubMed] [Google Scholar]
- Sun AM, Kikeri D, Hebert SC. Vasopressin regulates apical and basolateral Na+-H+ antiporters in mouse medullary thick ascending limbs. Am J Physiol. 1992;262:F241–F247. doi: 10.1152/ajprenal.1992.262.2.F241. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, Boron W, Maunsbach AB, Nielsen S, Aalkjaer C. Immunolocalization of electroneutral Na-HCO3− cotransporter in rat kidney. Am J Physiol Renal Physiol. 2000;279:F901–F909. doi: 10.1152/ajprenal.2000.279.5.F901. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger – Cloning, tissue distribution, and functional characterization. J Biol Chem. 2000;275:35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Watts BA, III, Good DW. Apical membrane Na+/H+ exchange in rat medullary thick ascending limb. pH-dependence and inhibition by hyperosmolality. J Biol Chem. 1994;269:20250–20255. [PubMed] [Google Scholar]
- Weiner ID, Hamm LL. Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. Am J Physiol. 1989;256:F957–F964. doi: 10.1152/ajprenal.1989.256.5.F957. [DOI] [PubMed] [Google Scholar]
- Wright PA, Burg MB, Knepper MA. Microdissection of kidney tubule segments. Meth Enzymol. 1990;191:226–231. doi: 10.1016/0076-6879(90)91015-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang Z, Barone S, Petrovic M, Amlal H, Conforti L, Petrovic S, Soleimani M. The expression of Na+-HCO3− cotransporter NBC4 in rat kidney and characterization of a novel NBC4 variant. Am J Physiol Renal Physiol. 2002;284:F41–F50. doi: 10.1152/ajprenal.00055.2002. [DOI] [PubMed] [Google Scholar]