Abstract
Systemic or intraventricular administration of cannabinoids causes analgesic effects, but relatively little is known for their cellular mechanism. Using brainstem slices with the mandibular nerve attached, we examined the effect of cannabinoids on glutamatergic transmission in superficial trigeminal caudal nucleus of juvenile rats. The exogenous cannabinoid receptor agonist WIN 55,212-2 (WIN), as well as the endogenous agonist anandamide, hyperpolarized trigeminal caudal neurones and depressed the amplitude of excitatory postsynaptic potentials (EPSPs) or currents (EPSCs) monosynaptically evoked by stimulating mandibular nerves in a concentration-dependent manner. The inhibitory action of WIN was blocked or fully reversed by the CB1 receptor antagonist SR 141716A. WIN had no effect on the amplitude of miniature excitatory postsynaptic currents (mEPSCs) recorded in the presence of tetrodotoxin or cadmium. The inhibitory effect of WIN on EPSCs was greater for those with longer synaptic latency, suggesting that cannabinoids have a stronger effect on C-fibre EPSPs than on Aδ-fibre EPSPs. Ba2+ (100 μm) blocked the hyperpolarizing effect of cannabinoids, but did not affect their inhibitory effect on EPSPs. The N-type Ca2+ channel blocker ω-conotoxin GVIA (ω-CgTX) occluded the WIN-mediated presynaptic inhibition, whereas the P/Q-type Ca2+ channel blocker ω-agatoxin TK (ω-Aga) had no effect. These results suggest that cannabinoids preferentially activate CB1 receptors at the nerve terminal of small-diameter primary afferent fibres. Upon activation, CB1 receptors may selectively inhibit presynaptic N-type Ca2+ channels and exocytotic release machinery, thereby attenuating the transmitter release at the trigeminal nociceptive synapses.
The trigeminal nucleus receives somatic afferents from the orofacial regions (e.g. tooth pulp, oral mucous and facial skin), and the superficial caudal layer of the substantia gelatinosa (SG) is thought to be a principal relay station for nociceptive input (Dubner & Bennett, 1983; Sessle, 1987). As in the spinal cord, nociceptive information in the superficial trigeminal caudal nucleus is conveyed by fine myelinated Aβ- and Aδ-fibres and unmyelinated C-fibres in the brainstem (Hu et al. 1981; Sessle, 1987). The primary afferent synaptic transmission is mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors (Chen & Huang, 1990; Grudt & Williams, 1994; Onodera et al. 2000). In this trigeminal caudal subnucleus, activity-dependent neuronal hyperexcitability such as wind-up phenomena (Hamba, 1998, 1992, Onodera et al. 2000) and long-term potentiation (LTP; Hamba et al. 2000) have been observed exclusively for C-fibre input. Thus, this area seems essentially involved in the processing and modulation of pain sensations. Slice preparations with the peripheral nerve attached have been used for studying nociceptive transmission. These include the horizontal spinal cord slice with the dorsal root attached (Grudt & Williams, 1994; Travagli, 1996; Jennings et al. 2001), and the parasagittal brainstem slice with the mandibular nerve attached (Hamba, 1998; Hamba & Onimaru, 1998; Hamba et al. 2000; Onodera et al. 2000).
Cannabinoids, the active components of Cannabis sativa (marijuana) and their endogenous counterparts, have a wide range of effects on the CNS, including loss of concentration, impairment of memory, enhancement of sensory perception, and mild euphoria (Dewey, 1986; Ameri, 1999). Several behavioural studies suggest that cannabinoids dampen the pain evoked by a variety of stimuli (Martin et al. 1993, 1998, Calignano et al. 1998; Smith et al. 1998; Stragman et al. 1998; Stragman & Walker, 1999; Rice, 2001). Cannabinoids consistently inhibit the responses of spinal dorsal horn neurones and ventral posterolateral thalamic neurones to noxious stimuli (Hohmann et al. 1995, 1999, Martin et al. 1996). In the spinal cord, cannabinoids presynaptically attenuate glutamatergic EPSCs in the substantia gelatinosa (Morisset & Urban, 2001). In contrast, in the medullary dorsal horn, cannabinoids have no effect on glutamatergic EPSCs evoked by stimulation of the rostral trigeminal tract, whereas they attenuate inhibitory postsynaptic currents (Jennings et al. 2001). It has been proposed that cannabinoids may play a hyperalgesic role at the level of the medullar dorsal horn. Here, we re-examined the effects of cannabinoids on the primary afferent glutamatergic transmission in the trigeminal caudal nucleus evoked by mandibular nerve stimulation in brainstem slices of juvenile rats. Our results demonstrate that cannabinoids inhibit the primary afferent glutamatergic transmission, most likely by inhibiting presynaptic N-type Ca2+ channels.
Methods
Slice preparation
All experiments were performed according to the guidelines laid down by the Institutional Animal Care and Use Committee of National Cheng Kung University. A parasagittal brainstem slice preparation including the trigeminal caudal nucleus with mandibular nerve trunks attached was prepared from 10- to 14-day-old male Sprague-Dawley rats after decapitation under halothane anaesthesia. All dissecting procedures were performed as previously described (Hamba, 1998; Hamba & Onimaru, 1998) in an oxygenated (95% O2–5% CO2) artificial cerebrospinal fluid (ACSF) of the following composition (mm): NaCl 117, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaHCO3 25, NaH2PO4 1.2, glucose 11, equilibrated with 95% O2–5% CO2 (pH 7.4). In brief, after the brainstem was isolated together with a mandibular nerve trunk, Pontamin Sky-Blue dye markings were made at two points on the dorsal surface of the brainstem along the medial border of the trigeminal spinal tract nucleus, one at the height of obex and the other 2 mm caudal to the obex at 0.8 mm and 0.4 mm from the lateral margin of the brainstem. The brainstem was then parasagittally cut with a surgical knife along the line passing through the points, then sectioned transversely at 2 mm caudal to the obex. Because the trigeminal caudalis contains thick, laminated structures of substantia gelatinosa (SG) in the medial border area (Sugimoto et al. 1997), the cutting line was made perpendicularly to align SG neurones across a wide range of the cut surface. The slice attached to a mandibular nerve trunk was submerged in a recording chamber where it was immobilized with a platinum grid with the cut surface up (see Fig. 1A of Onodera et al. 2000). The recording chamber was continuously superfused at 3–4 ml min−1 with the ACSF equilibrated with 95% O2 and 5% CO2. To block inhibitory transmission, strychnine sulphate (0.5 μm) and bicuculline methiodide (10 μm) were routinely included in the superfusate. All experiments were carried out at 32.0 ± 0.5°C. In some experiments, Ca2+ concentration in ACSF was reduced to 0.8 mm and Mg2+ concentration was increased to 2.9 mm.
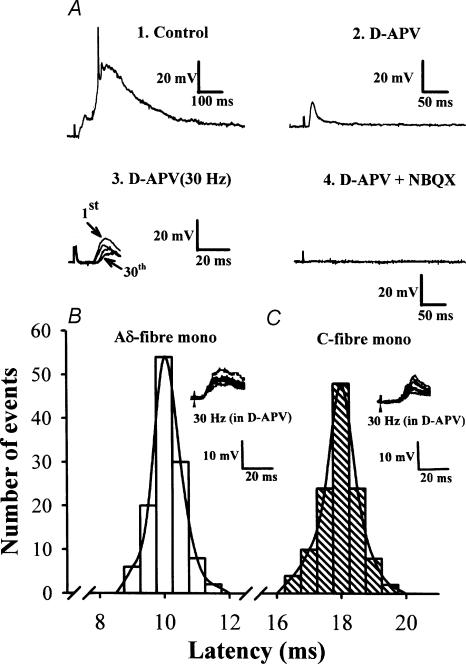
Figure 1. EPSPs recorded from trigeminal caudal neurones in response to mandibular nerve stimulation.
A, EPSPs evoked in the trigeminal caudal neurones by stimulating the mandibular nerve at 0.033 Hz (1, 2, and 4) and at 30 Hz (3) in the presence of strychnine sulphate (0.5 μm) and bicuculline methiodide (10 μm). Perfusing the slices with NMDA receptor antagonist d-APV (50 μm) largely attenuated slow EPSP components (2) and further addition of the non-NMDA receptor antagonist NBQX (20 μm) completely abolished the synaptic response (4). Note that the short-latency fast EPSP component recorded in the presence of d-APV (5 traces superimposed) persisted up to 30 Hz with a relatively stable latency with no failures (3). The resting membrane potential of this cell was –67 mV. B and C, monosynaptic nature of EPSPs remaining after high-frequency repetitive stimulation in the presence of d-APV (50 μm). The latency histograms of EPSPs evoked at 30 Hz in two different trigeminal neurones are shown. From the stable latency and absence of failure during 30 Hz stimulation, we deduced that these afferent inputs are monosynaptically connected. Inset in B, representative monosynaptic Aδ-fibre EPSPs (6 traces superimposed) in one neurone had a low threshold (4 V; 0.3 ms) and short latency (10.6 ms). Inset in C, representative monosynaptic C-fibre EPSPs (6 traces superimposed) in one neurone had a high threshold (11 V; 0.3 ms) and long latency (18.6 ms). The latencies (120 responses each in B and C) were fitted with a Gaussian distribution.
Electrophysiological recordings
The SG was identified as a translucent band in the superficial layer of the brainstem 50–200 μm from the dorsal margin when viewed under a dissecting microscope with transmitted illumination. Conventional electrophysiological techniques were used for intracellular recordings from the SG neurones (Huang & Hsu, 1999) using single glass microelectrodes, filled with 4 m potassium acetate, having resistances ranging from 90 to 120 MΩ. Microelectrodes were pulled from microfibre-containing glass capillary tubings (o.d., 1.0 mm) on a Brown-Flaming electrode puller (Sutter Instruments). Electrical signals were recorded using an Axoclamp-2B amplifier (Axon Instruments), filtered at 1 kHz and sampled at 10 kHz. Data were analysed on- or off-line using pCLAMP software (Version 7.0, Axon Instruments) with an Intel Pentium-based computer. The input resistance of neurones was measured from the slope of the voltage–current relationship obtained by hyperpolarizing current pulses of various amplitudes passed through recording microelectrodes. A bridge circuit was used to record membrane potential while current injection was applied through the recording microelectrodes. Only neurones with a resting membrane potential more negative than −60 mV and that produced an action potential amplitude greater than 70 mV were adopted for data analysis. The basic characteristics of the trigeminal caudal neurones were essentially the same as those previously reported (Onodera et al. 2000) with a resting membrane potential (RMP) of −67.3 ± 2.7 mV (n= 81), and an input resistance (IR) of 93.8 ± 2.7 MΩ (n= 81). To evoke EPSPs, the mandibular nerve was stimulated every 30 s with a square pulse (0.3 ms in duration) delivered through a bipolar tungsten electrode. Classification of synaptic responses into Aδ- and C-fibre was based on a combination of response threshold and response latency. Response threshold was measured with depolarizing pulses of graded intensity (1–15 V). Aδ- and C-fibre EPSPs were classified as monosynaptic if the response latency remained stable upon high-frequency (30 Hz) repetitive stimulation. Polysynaptic responses evoked in trigeminal caudal neurones could be largely blocked by d-APV (50 μm) as previously described (Onodera et al. 2000).
Whole-cell patch-clamp recordings of mEPSCs were made from brainstem trigeminal caudal neurones visually identified in slices with an upright microscope at room temperature (24–26°C) (Edwards et al. 1989; Onodera et al. 2000). The microscope (Olympus BX50WI; Olympus Optical, Tokyo, Japan) was equipped with a water-immersion × 40 objective and a Nomarski condenser combined with infrared videomicroscopy. Patch pipettes were pulled from borosilicate capillary tubing (Harvard Apparatus, UK) and heat-polished. The pipette resistance was typically 4–5 MΩ. The composition of intracellular solution was (mm): potassium gluconate 110, KCl 30, Hepes 10, MgCl2 1, EGTA 5, MgATP 2, Na3GTP 0.3. Whole-cell recordings were made using a patch-clamp amplifier (Axopatch 200B; Axon Instruments). Tight-seal (> 2 GΩ) was assured before rupturing the patch membrane. Electrical signals were low-pass filtered at 1 kHz and digitized at 4–10 kHz using a Digidata 1320 interface, and data acquired on-line were analysed off-line using pCLAMP software (Version 8.0; Axon Instruments) on an Intel Pentium-based computer. Only data recorded with series resistance <20 MΩ were used for analysis. EPSCs were recorded under voltage clamp at a holding potential of −70 mV in the presence of strychnine sulphate (0.5 μm) and bicuculline methiodide (10 μm) throughout this study. Miniature EPSCs were recorded in the presence of tetrodotoxin (TTX, 1 μm) and were analysed off-line using Mini Analysis software (Synaptosoft, Leonia, NJ, USA). mEPSCs were abolished by NBQX (20 μm) plus d-APV (50 μm), indicating that these are glutamatergic events. The software detects events having amplitudes exceeding a threshold set just above the baseline noise of the recording (3 pA). All events were subjected to visual inspection for excluding artifacts. Background current noise was estimated from the baseline with no clear event and was subtracted from signals for analysis. The mEPSC frequency was calculated by dividing the total number of detected events by the total time sampled. Periods of 6 min were analysed for WIN treatment. Amplitude histograms were binned in 1 pA intervals.
Drug application
All drugs were applied by manually switching the superfusates. Drugs were diluted from stock solutions just before application. Stock solutions of WIN 55,212-2, SR 141716A and SR 144528 were dissolved in dimethylsulfoxide (DMSO) and stored at −20°C. The final DMSO concentration in the superfusate was less than 0.1%. At this concentration, DMSO had no effect on EPSPs. WIN 55,212-2, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide (NBQX), nimodipine, TTX and anandamide were purchased from Tocris Cookson (Bristol, UK); bicuculline methiodide, strychnine sulphate, CdCl2 and Potamine Sky-Blue were from Sigma (St Louis, MO, USA); ω-CgTX and ω-Aga were from Alomone (Jerusalem, Israel). SR 141716A and SR 144528 were gifts from Sanofi Recherche (Montpellier, France).
Statistical analysis
The significance of the difference between the means was calculated by a paired or unpaired Student's t test. Statistical significance was set at P < 0.05. Kolmogorov-Smirnov tests were used to compare the cumulative histograms of the amplitude and frequency of mEPSCs between control slices and experimental slices after drug applications. Distributions were considered different at P < 0.01.
Results
The primary afferent excitatory postsynaptic responses
After a stable intracellular recording was established from the trigeminal caudal neurones, EPSPs were evoked by stimulating mandibular nerves at 0.033 Hz. In the majority of caudal neurones, when the stimulus intensity was gradually increased, a small and brief EPSP component first appeared, followed by larger and slower components, often accompanied by action potentials at stronger stimuli (Fig. 1A). These indicate that the primary afferent fibres having different conduction velocities innervate the trigeminal caudal neurones through monosynaptic and polysynaptic pathways. As reported previously (Onodera et al. 2000), the NMDA receptor blocker d-APV (50 μm) reduced the amplitude and duration of compound EPSPs, leaving monosynaptic EPSPs, which persisted during high-frequency (30 Hz) stimulation with a stable synaptic latency (Fig. 1A). Thus, NMDA receptors substantially contribute to polysynaptic transmission at this synapse of juvenile rats. Simultaneous application of d-APV and the AMPA/kainate receptor antagonist NBQX (20 μm) completely abolished EPSPs, confirming that the primary afferent EPSPs are predominantly mediated by ionotropic glutamate receptors (Onodera et al. 2000). To obtain monosynaptic responses in isolation from polysynaptic components, d-APV (50 μm) was routinely included in the superfusate. During 30 Hz stimulation, 81 of 102 trigeminal caudal neurones showed stable monosynaptic responses; the synaptic response became undetectable in the remaining 21 neurones. The action of the cannabinoids was tested only for these monosynaptic EPSPs.
As previously reported (Onodera et al. 2000), monosynaptic EPSPs recorded from the trigeminal caudal neurones by mandibular nerve stimulation can be classified into two categories based on the criteria of threshold and synaptic latency; the Aδ-fibre EPSPs have a low threshold and short latency, and the C-fibre EPSPs have a high threshold and long latency. Figure 1B and C illustrate two examples of EPSPs, one with a relatively low threshold and short latency (B), and the other with a high threshold and long latency (C). Both EPSPs displayed no failure and a stable latency when stimulated at 30 Hz (Figs 1B and C). Under the present experimental conditions, one group of EPSPs had a threshold of less than 5 V (1–5 V; 0.3 ms in pulse duration) and another group had a threshold higher than 10 V (10–15 V, 0.3 ms). Assuming 1 ms for synaptic delay and 7 mm for the approximate distance between the stimulating and recording electrodes, the calculated conduction velocity of the low-threshold EPSPs ranged from 0.5 to 0.8 m s−1, whereas that of the high-threshold EPSPs ranged from 0.3 to 0.4 m s−1. These conduction velocities correspond to Aδ-afferent fibres and C-afferent fibres, respectively, in animals of this age (Fulton, 1987; Hamba, 1998).
Cannabinoid receptor activation decreases monosynaptic glutamatergic transmission
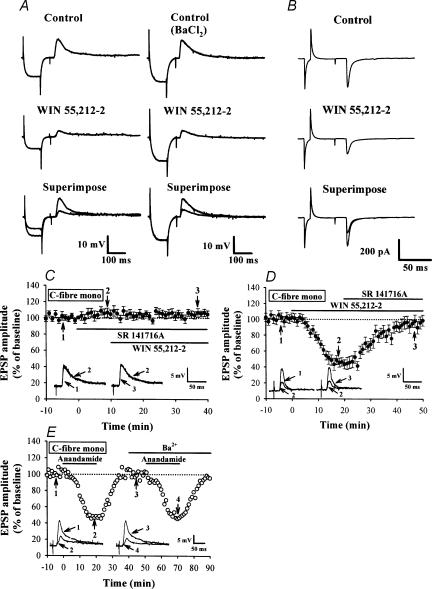
We examined whether cannabinoids modulate primary afferent glutamatergic transmission in SG by first applying the selective CB1 receptor agonist WIN 55,212-2 (Compton et al. 1992). As shown in Fig. 2A and B, bath application of WIN (5 μm, for 20 min) markedly decreased the amplitude of monosynaptic EPSPs and EPSCs. WIN reduced the amplitude of EPSCs in all five cells tested on average by 43.9 ± 4.2%. The inhibitory effect of WIN was slow in onset: it reached maximal inhibition 15–20 min after application (Figs 2D and 3A and B) and was sustained for at least 20 min after washout (Figs 3A and B). The CB1 receptor antagonist SR 141716A (5 μm) reversed the inhibitory effect of WIN on EPSPs (Fig. 3D). SR 141716A alone elicited a slight but significant increase in the amplitude of EPSPs (Fig. 2C and 8.6 ± 4.3%, n= 5; P > 0.05), suggesting that CB1 receptors may be tonically activated by endogenous cannabinoids. The CB2 receptor antagonist SR 144528 had no effect on the inhibitory effect of WIN on EPSPs (data not shown). These results suggest that the WIN-mediated synaptic inhibition of primary afferent EPSPs is mediated by CB1 receptors. WIN also hyperpolarized SG neurones on average by 7.8 ± 2.2 mV (from −68.4 ± 2.3 to −76.2 ± 2.2 mV, n= 14, P < 0.05; paired Student's t test), associated with a decrease in input resistance by 31.4 ± 3.6 MΩ (from 92.8 ± 3.1 to 61.3 ± 3.4 MΩ, n= 14, P < 0.05; paired Student's t test). Thus, these results are in contrast with a previous report that WIN affected neither glutamatergic EPSCs nor postsynaptic cells in brainstem dorsal horn (Jennings et al. 2001).
Figure 2. Cannabinoid receptor agonists reduce monosynaptic EPSPs and EPSCs evoked by mandibular nerve stimulation.
A, sample traces showing synaptically evoked EPSPs from cells recorded before and 20 min after application of WIN (5 μm) in the absence (left) or presence of Ba2+ (100 μm) (right). The EPSP was preceded by a transient hyperpolarizing current pulse (0.1 nA, 100 ms) passed through the recording microelectrode to measure input resistance (IR) of postsynaptic neurones. Note that bath application of WIN decreased the amplitude of EPSPs, which was accompanied by a substantial decrease in membrane IR. In the presence of Ba2+, WIN was still able to depress EPSP amplitude without affecting membrane IR. The average resting membrane potential (RMP) and IR of 12 neurones tested were −66.9 ± 2.7 mV and 105.4 ± 3.5 MΩ, respectively, before WIN application, and −68.9 ± 2.5 mV and 99.6 ± 3.2 MΩ after WIN application. B, sample traces showing the effect of WIN (5 μm) on monosynaptic EPSCs. The neurone was voltage clamped at a holding potential of −70 mV, and EPSCs were evoked at 30 s intervals in the presence of strychnine sulphate (0.5 μm), bicuculline methiodide (10 μm) and d-APV (50 μm). Traces are averaged from 6 responses. C, summary time plot of C-fibre EPSPs (n= 5) showing a potentiating effect of SR 141716A (5 μm) on the EPSP amplitude and its blocking effect on the WIN-induced inhibition of EPSPs. D, summary time plot of C-fibre EPSPs (n= 4) showing that SR 141716A (5 μm) reversed the WIN-induced inhibition of EPSPs. E, effect of external Ba2+ on the anandamide-induced inhibition of EPSPs. Ba2+ (100 μm) had no effect on the inhibitory effect of anandamide (30 μm) on monosynaptic C-fibre EPSPs. Superimposed EPSPs were taken at the times indicated. Similar results were also observed in other three cells. Bars indicate the period of drug application.
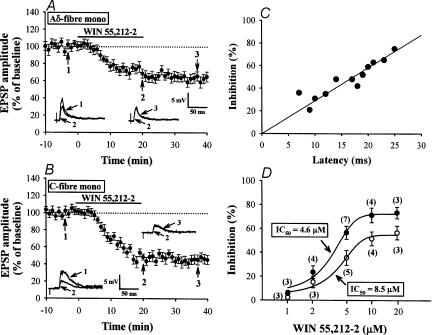
Figure 3. Stronger inhibition by WIN of C-fibre EPSPs than Aδ-fibre EPSPs.
A, averaged time course of the inhibitory effect of WIN (5 μm) on monosynaptic Aδ-fibre EPSPs (n= 5). B, averaged time course showing the effect of WIN on monosynaptic C-fibre EPSPs (n= 7). Insets show superimposed EPSPs taken at the indicated times (1–3). C, comparison between the magnitude of the WIN-induced EPSP inhibition (ordinate, 20 min after WIN application) and the synaptic latency of EPSPs (abscissa). A regression line was drawn using the least squares method (r= 0.89). D, dose–response curves for depression of Aδ- (◯) and C-fibre EPSPs (•) by WIN. The numbers in parentheses indicate the number of slices tested.
Similar to WIN, the endogenous cannabinoid anandamide (30 μm) attenuated the EPSP amplitude by 51.4 ± 4.9% (n= 4; Fig. 2E) and hyperpolarized SG neurones by 7.4 ± 2.3 mV (n= 4, data not shown). The inhibitory effect of anandamide on EPSPs was also slow in onset, but completely recovered after washout (Fig. 2E).
SR 141716A (5 μm) also abolished the membrane hyperpolarization and a decrease in the input resistance of SG neurones caused by WIN (5 μm) (n= 4), indicating that the WIN-induced changes of postsynaptic neuronal excitability were also CB1 receptor mediated. In the presence of Ba2+ (100 μm), which is known to block inward rectifying potassium conductance (Hagiwara et al. 1978), WIN (5 μm) still clearly decreased the amplitude of monosynaptic EPSPs (Fig. 2A). The magnitude of inhibition of EPSPs by WIN in the presence of Ba2+ (47.6 ± 5.8%, n= 12) was similar to that without Ba2+(51.9 ± 5.3%, n= 14) (P > 0.05; unpaired Student's t test). Similarly, Ba2+ had no effect on the inhibitory action of anandamide (30 μm) on the amplitude of EPSPs (Fig. 2E). These results suggest that the Ba2+-sensitive inwardly rectifying potassium conductance is not involved in the inhibitory effect of cannabinoids on EPSPs. In the following experiments, Ba2+ (100 μm) was routinely included in the superfusate.
Preferential inhibition of C-fibre EPSPs by cannabinoids
We next examined whether the inhibitory effects of WIN on EPSPs conveyed by Aδ- and C-fibres are different. WIN attenuated the amplitude of both monosynaptic Aδ- and C-fibre EPSPs (Fig. 3A and B). The magnitude of WIN-evoked inhibition was significantly greater for C-fibre EPSPs (56.5 ± 5.5%; n= 7, Fig. 3B) than for Aδ-fibre EPSPs (35.5 ± 6.2%; n= 5, Fig. 3A) (P < 0.05; unpaired Student's t test). Moreover, there was a positive correlation between this magnitude and the latency of the EPSPs (Fig. 3C; correlation coefficient r= 0.89). Comparison of the dose–response curves for the inhibitory effects of WIN clearly indicates that the effect of WIN on C-fibre EPSPs (IC50= 4.6 μm) is stronger than that on A-fibre EPSPs (IC50= 8.5 μm) (Fig. 3D). These results suggest that cannabinoids preferentially inhibit trigeminal primary afferent synaptic transmission mediated by fibres of small diameter.
Presynaptic site of the inhibitory effect of WIN
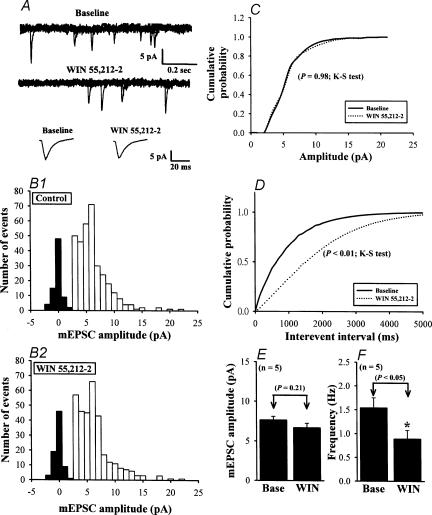
To determine whether the site of the inhibitory effect of WIN on EPSPs is presynaptic or postsynaptic, we examined the effects of WIN on miniature EPSCs (mEPSCs) recorded at a holding potential of −70 mV and in the presence of TTX (1 μm) (Fig. 4A). The amplitude histogram showed no significant effect of WIN on the amplitude of mEPSCs (Fig. 4B). The mean amplitude of mEPSCs was 6.68 ± 0.56 pA in the presence of WIN (5 μm) and 7.68 ± 0.46 pA before WIN application (n= 5; P > 0.05; paired Student's t test) (Fig. 4E). The cumulative probability plots also indicated no significant difference in the mEPSC amplitude (Figs 4C, P= 0.98 in Kolmogorov-Smirnov test). These data indicate that CB1 receptor activation does not alter the postsynaptic sensitivity to glutamate, suggesting that the site of the inhibitory action of WIN on EPSPs is presynaptic. WIN also had no effect on the holding current, but it did decrease the mEPSC frequency, on average by 42.3 ± 7.3% (from 1.54 ± 0.21 to 0.89 ± 0.18 Hz; P < 0.05, n= 5; paired Student's t test) (Fig. 4F), with a significant difference in cumulative interevent interval distributions (Figs 4D, P < 0.01; Kolmogorov-Smirnov test).
Figure 4. Effect of WIN on the mEPSCs.
A, sample traces of mEPSCs before (Baseline: in the presence of 1 μm TTX to block Na+ channels) and during application of 5 μm WIN. Lower traces are averaged mEPSCs of 35 events each before and after WIN application, demonstrating the lack of effect of WIN on the amplitude and kinetics of mEPSCs. B, amplitude histograms of mEPSCs before (B1) and during WIN application (B2) obtained from 323 and 312 mEPSC events, respectively. C, cumulative probability plots of the mEPSC amplitude before (dotted line) and during (continuous line) application of WIN. No changes occurred in the distribution during WIN application (P= 0.98; Kolmogorov-Smirnov test). D, cumulative interevent interval distribution, illustrating a significant increase in the interevent interval (i.e. decreased frequency; P < 0.01; Kolmogorov-Smirnov test) during WIN application. Data in A–D were obtained from the same neurone. E and F, summary of the effect of 5 μm WIN on the average amplitude (E) and frequency (F) of mEPSCs (n= 5). Data are presented as means ±s.e.m. Asterisk indicates P < 0.05 compared with control (Base). Holding potential was −70 mV.
To examine whether the inhibitory action of WIN on the mEPSC frequency is caused by a reduction of Ca2+ influx (Bao et al. 1998), we tested the effect of WIN on the mEPSC frequency in the presence of cadmium (Cd2+, 100 μm). At this concentration Cd2+ blocks high-voltage-activated (HVA) Ca2+ channels (Fox et al. 1987). Cd2+ (100 μm) affected neither the frequency nor amplitude of mEPSCs (data not shown). In the presence of Cd2+, WIN (5 μm) still decreased the mEPSC frequency (supplementary Fig. S1), to a similar extent as in its absence (Fig. 4). WIN decreased the mEPSC frequency on average by 41.6 ± 7.5% (from 1.63 ± 0.19 to 0.95 ± 0.18 Hz; P < 0.05, n= 5; paired Student's t test), with a significant difference in cumulative interevent interval distributions (P < 0.01; Kolmogorov-Smirnov test). These findings indicate that WIN-mediated inhibition of the mEPSC frequency is not caused by presynaptic Ca2+ influx through HVA Ca2+ channels.
Presynaptic N-type VACCs may mediate WIN-induced presynaptic inhibition
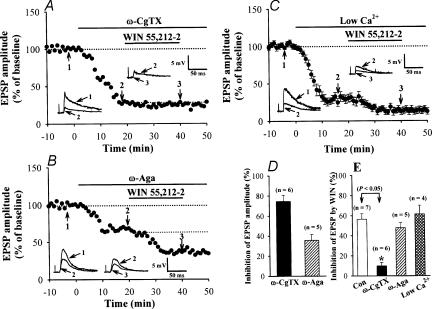
WIN presynaptically depresses glutamatergic transmission in the presence of Ba2+, suggesting that G-protein-coupled inwardly rectifying potassium channels (GIRK) are not involved in the effect, as in the case of baclofen at the calyx of Held (Takahashi et al. 1998). Given that WIN-induced inhibition of EPSCs can be blocked by the N-type Ca2+ channel blocker ω-CgTX at corticostriatal synapses (Huang et al. 2001), we examined whether this is also the case at the primary afferent synapse. ω-CgTX (1 μm) irreversibly attenuated the EPSP amplitude by 74.5 ± 6.3%(n= 6; P < 0.05). In the presence of ω-CgTX, the inhibitory effect of WIN was much less (9.8 ± 3.6%, n= 6) than control (P < 0.05, Figs 5A and E), with ω-CgTX attenuating the inhibitory effect of WIN by 82.7 ± 6.3%. We next determined whether the P/Q-type Ca2+ channel blocker ω-Aga affects the inhibitory effect of WIN. ω-Aga (200 nm) attenuated the amplitude of EPSPs by 35.7 ± 5.7% (Figs 5B and D, n= 5; P < 0.05). In contrast to ω-CgTX, however, ω-Aga did not significantly affect the inhibitory effect of WIN on EPSPs (Fig. 5E). In the presence of ω-Aga-TK, WIN inhibited EPSPs by 47.8 ± 5.2%(n= 5), similar to control (P > 0.05). It might be argued that the effect of ω-CgTX is secondary to the small amplitude of EPSPs remaining after ω-CgTX application. To examine this possibility directly, we reduced the Ca2+/Mg2+ ratio in ACSF (from 2.5/1.2 to 0.8/2.9). This reduced the amplitude of EPSP to 31.5 ± 5.6%(n= 4), comparable in magnitude to the reduction by ω-CgTX (Fig. 5D). Under these conditions, WIN clearly inhibited EPSPs, on average by 61.5 ± 8.7% (n= 4; Fig. 5C), which was similar to the inhibition produced by WIN in normal ACSF (Fig. 5E). These results indicate that WIN-induced presynaptic inhibition of evoked primary afferent glutamatergic transmission may be mediated by selective inhibition of presynaptic N-type Ca2+ channels.
Figure 5. Involvement of presynaptic N-type VACCs in WIN-induced synaptic inhibition.
A, a typical experiment in which the slice was perfused with 1 μmω-CgTX, which blocked ∼74% of the monosynaptic C-fibre EPSPs. In the presence of ω-CgTX, WIN (5 μm) no longer attenuated EPSPs. B, a typical experiment in which the slice was perfused with 200 nmω-Aga-TK, which blocked ∼34% of the monosynaptic C-fibre EPSPs. The fraction of EPSPs insensitive to ω-Aga was still sensitive to WIN. C, average time course (n= 4) showing the WIN-induced depression of the monosynaptic C-fibre EPSPs in a low Ca2+ solution. The standard ACSF was replaced by a low-Ca2+ solution containing 0.8 mm Ca2+ and 2.9 mm Mg2+. The superimposed EPSPs in the insets in each graph illustrate respective recordings from example experiments taken at the times indicated by the numbers (1–3). Horizontal bars indicate the period of application of drugs and low-Ca2+ solution. D, the magnitude of EPSP attenuation by Ca2+ channel blockers. E, summary of WIN (5 μm)-induced synaptic inhibition, in the presence of Ca2+ channel blockers or in a low-Ca2+ solution. Data are presented as means ±s.e.m. Asterisk indicates P < 0.05 in Student's unpaired t test.
Discussion
Inhibitory effect of cannabinoids on glutamatergic excitatory synaptic transmission
Activation of CB1 receptors can inhibit glutamatergic transmission at various central synapses in the hippocampus (Sullivan, 1999; Hoffman & Lupica, 2000), the substantia nigra pars compacta (Chan & Yung, 1999), the nucleus accumbens (Robbe et al. 2001), the dorsal striatum (Huang et al. 2001; Gerdeman & Lovinger, 2001), the periaqueductal grey (Vaughan et al. 1999), the cerebellum (Lèvènés et al. 1998), the prefrontal cortex (Auclair et al. 2000), and the spinal cord dorsal horn (Morisset & Urban, 2001; Luo et al. 2002a). However, in the medullary dorsal horn, cannabinoids have no effect on excitatory transmission, whereas they do decrease the amplitude of inhibitory postsynaptic transmission, which has led to the hypothesis that cannabinoids play a hyperalgesic role at the medullar dorsal horn (Jennings et al. 2001). In the present study, however, we have demonstrated that cannabinoids presynaptically inhibit primary afferent glutamatergic transmission. Our results also suggest that the inhibitory effect of cannabinoids is stronger on C-fibre EPSPs than Aδ-fibre EPSPs. Thus, cannabinoids probably also exert analgesic effects at the medullary level. The exact reason for the discrepancy between the findings of Jennings et al. (2001) and our present study is unclear. Because WIN decreased the EPSC amplitude in whole-cell recording to a similar extent as in intracellular recording (P > 0.05), the difference cannot be due to recording methods. The WIN concentration in their study was 3 μm, whereas it was 5 μm in most of our experiments. In our study, WIN at 3 μm inhibited Aδ-fibre EPSPs by only 15% (Fig. 4B). In addition, in their study responses were evoked by stimulation of the rostral trigeminal nucleus in transverse slices, whereas ours were evoked by the mandibular nerve attached to the parasaggital brainstem slice.
Preferential inhibition of cannabinoids on C-fibre-mediated transmission
Nociceptive information is carried from the periphery to the CNS through fine myelinated Aδ-fibres and unmyelinated C-fibres to the superficial laminae of the spinal cord and the spinal trigeminal caudal nucleus in the medulla where they make exclusively excitatory synaptic contacts with second-order neurones (Light & Perl, 1979; Sugiura et al. 1986). A growing body of studies has provided evidence for presynaptic CB1 receptors on primary afferent terminals and postsynaptic CB1-like immunoreactive neurones in both the spinal and medullary superficial dorsal horn (Hohmann & Herkenham, 1998; Tsou et al. 1998). Using in situ hybridization and immunohistochemistry methods, Price et al. (2003) have recently showed that CB1 receptor mRNA is localized to neurones of the maxillary and mandibular branches of the trigeminal ganglion in the adult rat. Our present study revealed that C-fibre EPSPs are more sensitive to WIN than Aδ-fibre EPSPs. This observation is consistent with reports indicating that cannabinoids predominantly inhibit C-fibre-driven nociceptive responses following repetitive C-fibre stimulation in rat spinal dorsal horn neurones, and have a weaker effect on Aδ-fibre evoked responses (Stragman & Walker, 1999; Drew et al. 2000). Like WIN, the GABAB receptor agonist baclofen (Ataka et al. 2000) and the opioid receptor-like1 (ORL1) receptor ligand nociceptin (Luo et al. 2002b) are reported to inhibit C-fibre-mediated transmission more strongly than Aδ-fibre-mediated transmission. The preferential inhibition by cannabinoids of C-fibre EPSPs may arise from the higher density of CB1 receptors in the terminals of C-fibres relative to Aδ-fibres. It is also possible that Aδ- and C-fibre terminals express distinct splice variants of CB1 receptors, having different sensitivities to cannabinoids (Shire et al. 1995; Rinaldi-Carmona et al. 1996). Given that Aδ-fibres are responsible for mediating fast sharp pain and that C-fibres mediate slow dull pain (Basbaum & Jessell, 2000), cannabinoids may inhibit dull pain more effectively than sharp pain.
Cellular mechanisms underlying the inhibitory effect of cannabinoids
In the presence of Ba2+, WIN inhibited EPSPs without affecting the input resistance or membrane potential of SG neurones, but had no effect on the mEPSC amplitude. The inhibitory effect of WIN on EPSPs was abolished by SR 141716A. These results suggest that WIN activates CB1 receptors in the presynaptic terminal, thereby inhibiting release of transmitter glutamate. In the absence of Ba2+, WIN decreased input resistance and hyperpolarized SG cells, suggesting that it also acts on postsynaptic CB receptors, thereby activating GIRK. Because the magnitude of inhibition of EPSPs in the presence and absence of Ba2+ was similar, GIRK in the presynaptic terminal is unlikely to be involved in the inhibitory effect of the cannabinoids. In our present study, ω-CgTX blocked the presynaptic inhibitory effect of WIN whereas ω-Aga had no effect. These results indicate that N-type Ca2+ channels may mediate the inhibitory effect of WIN. However, our results do not rule out the possibility that CB1 receptors are selectively expressed in the nerve terminals that express N-type Ca2+ channels, whereas the nerve terminals expressing P/Q-type Ca2+ channels do not express CB1 receptors. At the striatal glutamatergic synapse, ω-CgTX also abolishes the presynaptic inhibitory effect of WIN on EPSPs (Huang et al. 2001). In cultured rat hippocampal neurones, however, cannabinoids inhibit both N- and P/Q-type VACCs (Shen & Thayer, 1998; Sullivan, 1999; Twitchell et al. 1997). Thus, the apparent selective linkage between the CB1 receptor and N-type Ca2+ channels may not be general. For example, CB1 receptor may be expressed closer to N-type Ca2+ channels than to P/Q type channels in the nerve terminal. However, it is also possible that a given type of CB1 receptor splice variant might selectively couple with N-type Ca2+ channels.
Various subtypes of VACCs are involved in neurotransmitter release (Takahashi & Momiyama, 1993). Upon activation of presynaptic CB1 receptors by cannabinoids, the βγ subunit of G-protein Go may inhibit Ca2+ channels via a membrane-delimited mechanism, as in the case of presynaptic GABAB receptor at the calyx of Held (Kajikawa et al. 2001). By analogy to the calyx of Held, another G-protein, Gi, may presumably be missing in the primary afferent terminals and may therefore be unable to couple with GIRK.
In our present study, WIN also decreased the frequency of mEPSCs. Given that Cd2+ had no effect on the inhibitory action of WIN on mEPSC frequency, WIN-induced inhibition of spontaneous transmitter release cannot be caused by inhibition of VACCs. In addition to their inhibitory effect on N-type Ca2+ channels, cannabinoids may directly inhibit the exocytotic machinery responsible for spontaneous transmitter release. It is also possible that cannabinoids reduce the intracellular Ca2+ concentration in the nerve terminal, for example by closing resting Ca2+ conductance and/or by stimulating the Ca2+ sequestration mechanism. Thus these effects may make a minor contribution to the WIN-induced EPSP inhibition observed after blocking N-type Ca2+ channels with ω-CgTX (Fig. 5E).
In conclusion, the present results provide a cellular basis for the antinociceptive action of cannabinoids through a presynaptic mechanism in the trigeminal caudal neurones and support a physiological role for cannabinoids as important negative modulators of pain transmission to the superficial layer of trigeminal caudal subnucleus. It has previously been reported that the antinociceptive effects of cannabinoids and morphine in an animal model of neuropathic pain are mediated through different pathways (Mao et al. 2000). Thus, cannabinoids may be useful for the management of currently intractable, debilitating clinical pathological pain syndromes, many of which are resistant to conventional opioid analgesics.
Acknowledgments
We are grateful to Drs K. Onodera, T. Tsujimoto and T. Yamauchi for skilful technical assistance and this work was financially supported by research grant (NSC91-2320-B-006–079) from the National Science Council of Taiwan, R.O.C. to K.-S. H and a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to T.T.
Supplementary Material
The online version of this paper can be found at: DOI: 10.1113/jphysiol.2003.056986 and contains material entitled: Effect of WIN on the mEPSCs in the presence of cadmium. This material can also be found at http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp105/tjp105sm.htm
References
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. doi: 10.1016/S0304-3959(00)00255-4. [DOI] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crépel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Bao JP, Li JJ, Perl ER. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J Neurosci. 1998;18:8740–8750. doi: 10.1523/JNEUROSCI.18-21-08740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Jessell TM. The perception of pain. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4. Columbus: McGraw-Hill Companies; 2000. pp. 472–491. [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Chan PKY, Yung WH. Inhibitory postsynaptic currents of rat substantia nigra pars reticulata neurons: role of GABA receptors and GABA uptake. Brain Res. 1999;838:18–26. doi: 10.1016/s0006-8993(99)01654-6. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LYM. Ionic currents in retrogradely labeled trigeminothalamic neurons in slices of rat medulla. Neurosci Lett. 1990;110:66–71. doi: 10.1016/0304-3940(90)90788-b. [DOI] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- Drew LJ, Harris J, Millins PJ, Kendall DA, Chapman V. Activation of spinal cannabinoid 1 receptors inhibits C-fibre driven hyperexcitable neuronal responses and increases [35S]GTPγS binding in the dorsal horn of the spinal cord of noninflamed and inflamed rats. Eur J Neurosci. 2000;12:2079–2086. doi: 10.1046/j.1460-9568.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Ann Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton BP. Postnatal changes in conduction velocity and soma action potential paramaters of rat dorsal root ganglion neurones. Neurosci Lett. 1997;73:125–130. doi: 10.1016/0304-3940(87)90005-x. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Williams JT. Mu-opioid agonists inhibit spinal trigeminal substantia gelatinosa neurons in guinea pig and rat. J Neurosci. 1994;14:1646–1654. doi: 10.1523/JNEUROSCI.14-03-01646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamba M. Stimulation-induced responses of the trigeminal caudal neurons in the brainstem preparation isolated from newborn rats. Brain Res. 1998;785:66–74. doi: 10.1016/s0006-8993(97)01382-6. [DOI] [PubMed] [Google Scholar]
- Hamba M, Hisamitsu H, Muro M. Wind-up of tooth pulp-evoked responses and its suppression in rat trigeminal caudal neurons. Brain Res Bull. 1992;29:883–889. doi: 10.1016/0361-9230(92)90160-y. [DOI] [PubMed] [Google Scholar]
- Hamba M, Onimaru H. Newborn rat brainstem preparation with the trigeminal nerve attached for pain study. Brain Res Protoc. 1998;3:7–13. doi: 10.1016/s1385-299x(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Hamba M, Onodera K, Takahashi T. Long-term potentiation of primary afferent neurotransmission at trigeminal synapses of juvenile rats. Eur J Neurosci. 2000;12:1128–1134. doi: 10.1046/j.1460-9568.2000.01028.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Regulation of cannabinoid and μ-opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci Lett. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Martin WJ, Tsou K, Walker JM. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212–2. Life Sci. 1995;56:2111–2118. doi: 10.1016/0024-3205(95)00196-d. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Tsou K, Walker JM. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. J Neurophysiol. 1999;81:575–583. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- Hu JW, Dostrovsky JO, Sessle BJ. Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). I. Responses to oral-facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. J Neurophysiol. 1981;45:173–192. doi: 10.1152/jn.1981.45.2.173. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Protein tyrosine kinase is required for the induction of long-term potentiation in the rat hippocampus. J Physiol. 1999;520:783–796. doi: 10.1111/j.1469-7793.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534:805–812. doi: 10.1111/j.1469-7793.2001.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y, Saitoh N, Takahashi T. GTP-binding protein βγ subunits mediate presynaptic calcium current inhibition by GABAB receptor. Proc Natl Acad Sci U S A. 2001;98:8054–8058. doi: 10.1073/pnas.141031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lèvènés C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Perl ER. Re-examination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Com Neurol. 1979;186:117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Luo C, Kumamoto E, Furue H, Chen J, Yoshimura M. Anandamide inhibits excitatory transmission to rat substantia gelatinosa neurons in a manner different from that of capsaicin. Neurosci Lett. 2002a;321:17–20. doi: 10.1016/s0304-3940(01)02471-5. [DOI] [PubMed] [Google Scholar]
- Luo C, Kumamoto E, Furue H, Chen J, Yoshimura M. Nociceptin inhibits excitatory but not inhibitory transmission to substantia gelatinosa neurones of adult rat spinal cord. Neuroscience. 2002b;109:349–358. doi: 10.1016/s0306-4522(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci Lett. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Hohmann AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J Neurosci. 1996;16:6601–6611. doi: 10.1523/JNEUROSCI.16-20-06601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Lai NK, Patrick SL, Tsou K, Walker JM. Antinociceptive actions of cannabinoids following intraventricular administration in rats. Brain Res. 1993;629:300–304. doi: 10.1016/0006-8993(93)91334-o. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Tsou K, Walker JM. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci Lett. 1998;242:33–36. doi: 10.1016/s0304-3940(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol. 2001;86:40–48. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- Onodera K, Hamba M, Takahashi T. Primary afferent synaptic responses recorded from trigeminal caudal neurons in a mandibular nerve-brainstem preparation of neonatal rats. J Physiol. 2000;524:503–512. doi: 10.1111/j.1469-7793.2000.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 2003;120:155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ASC. Cannabinoids and pain. Curr Opin Invest Drugs. 2001;2:399–414. [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Calandra B, Shire D, Bouaboula M, Oustric D, Barth F, Casellas P, Ferrara P, Le Fur G. Characterization of two cloned human CB1 cannabinoid receptor isoforms. J Pharmacol Exp Ther. 1996;278:871–878. [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ. The neurobiology of facial and dental pain: present knowledge, future directions. J Dent Res. 1987;66:962–981. doi: 10.1177/00220345870660052201. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer SA. The cannabinoid agonist WIN55212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le Fur G, Caput D, Ferrara P. An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- Smith FL, Cichewicz D, Martin ZL, Welch SP. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;60:559–566. doi: 10.1016/s0091-3057(98)00012-4. [DOI] [PubMed] [Google Scholar]
- Stragman NM, Patrick SL, Hohmann AG, Tsou K, Walker JM. Evidence for a role of endogenous cannabinoids in the modulation of acute and tonic pain sensitivity. Brain Res. 1998;813:323–328. doi: 10.1016/s0006-8993(98)01031-2. [DOI] [PubMed] [Google Scholar]
- Stragman NM, Walker JM. Cannabinoid WIN 55,212–2 inhibits the activity-dependent facilitation of spinal nociceptive responses. J Neurophysiol. 1999;82:472–477. doi: 10.1152/jn.1999.82.1.472. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Fujiyoshi Y, He YF, Xiao C, Ichikawa H. Trigeminal primary projection to the rat brain stem sensory trigeminal nuclear complex and surrounding structures revealed by anterograde transport of cholera toxin B subunit-conjugated and Bandeiraea simplicifolia isolectin B4-conjugated horseradish peroxidase. Neurosci Res. 1997;28:361–371. doi: 10.1016/s0168-0102(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Mechanisms of cannabinoid receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-Protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Travagli RA. Muscarine receptor activation in the substantia gelatinosa of the spinal trigeminal nucleus of the guinea pig. J Neurophysiol. 1996;76:3817–3822. doi: 10.1152/jn.1996.76.6.3817. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be found at: DOI: 10.1113/jphysiol.2003.056986 and contains material entitled: Effect of WIN on the mEPSCs in the presence of cadmium. This material can also be found at http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp105/tjp105sm.htm