Abstract
G-protein-coupled receptor signalling has been suggested to be voltage dependent in a number of cell types; however, the limits of sensitivity of this potentially important phenomenon are unknown. Using the non-excitable rat megakaryocyte as a model system, we now show that P2Y receptor-evoked Ca2+ mobilization is controlled by membrane voltage in a graded and bipolar manner without evidence for a discrete threshold potential. Throughout the range of potentials studied, the peak increase in intracellular Ca2+ concentration ([Ca2+]i) in response to depolarization was always larger than the maximal reduction in [Ca2+]i following an equivalent amplitude hyperpolarization. Significant [Ca2+]i increases were observed in response to small amplitude (<5 mV, 5 s duration) or short duration (25 ms, 135 mV) depolarizations. Individual cardiac action potential waveforms were also able to repeatedly potentiate P2Y receptor-evoked Ca2+ release and the response to trains of normally paced stimuli fused to generate prolonged [Ca2+]i increases. Furthermore, elevation of the temperature to physiological levels (36°C) resulted in a more sustained depolarization-evoked Ca2+ increase compared with more transient or oscillatory responses at 20–24°C. The ability of signalling via a G-protein-coupled receptor to be potentiated by action potential waveforms and small amplitude depolarizations has broad implications in excitable and non-excitable tissues.
G-protein-coupled receptors (GPCRs) are the largest family of cell surface receptors. They are responsible for transducing external stimuli into cellular activity and represent the principal targets for therapeutic intervention, particularly in the cardiovascular system (Rockman et al. 2002). A variety of evidence now supports the concept that a signalling via number of GPCRs can be controlled by changes in the cell membrane potential. For example, Ca2+ release stimulated by either muscarinic receptors in guinea-pig coronary artery smooth muscle or P2Y receptors in rat megakaryocytes is potentiated by depolarization and inhibited by hyperpolarization (Ganitkevich & Isenberg, 1993; Mahaut-Smith et al. 1999; Mason et al. 2000). The underlying mechanism is unknown, but can be explained by voltage control of IP3 production, as suggested by Itoh et al. (1992) during activation of adrenergic receptors in rabbit mesenteric artery smooth muscle. An alternative explanation, as discussed in detail elsewhere (Mahaut-Smith et al. 1999; Mason & Mahaut-Smith, 2001), is that voltage-dependent configurational coupling between proteins in the surface and endoplasmic reticular membranes can modify IP3-dependent Ca2+ release.
In the megakaryocyte and coronary or mesenteric artery smooth muscle, preactivation of a GPCR was required to observe voltage control of Ca2+ release or IP3 generation (Itoh et al. 1992; Ganitkevich & Isenberg, 1993; Mahaut-Smith et al. 1999). However, there are also examples where constitutive voltage control of IP3-dependent Ca2+ release has been described, including skeletal muscle (Vergara et al. 1985; Araya et al. 2003), smooth muscle (Best & Bolton, 1986; Suzuki & Hirst, 1999; Van Helden et al. 2000) and the giant algae Chara (Wacke & Thiel, 2001). GPCRs can generate responses in the absence of agonists (Seifert & Wenzel-Seifert, 2002), and therefore regulation of GPCR activity may also be responsible for the apparent intrinsic voltage control of IP3-dependent Ca2+ release in these tissues. It is also worth noting that absorption of light by rhodopsin, the most widely studied GPCR, generates a charge displacement comparable with the gating currents of voltage-dependent ion channels (Cone, 1967; Sullivan & Shukla, 1999). Thus, transmembrane voltage may directly control GPCR activation and thereby regulate other downstream targets of this class of receptor such as adenylate cyclase and ion channels (Dascal, 2001). Indeed, the activation of G-protein-activated inwardly rectifying K+ channels by M2 muscarinic receptors expressed in Xenopus oocytes has recently been suggested to be voltage-dependent (Ben Chaim et al. 2003).
The extent to which Ca2+ signalling through GPCRs can be directly controlled by the membrane potential is unknown. Using the non-excitable rat megakaryocyte as a model system, we now show that voltage control of P2Y receptor-evoked Ca2+ release is graded, without evidence for a threshold potential, such that this signalling pathway can be controlled by small amplitude and short duration fluctuations of membrane voltage. The marked voltage sensitivity of signalling via this receptor allows cardiac action potential waveforms to significantly potentiate ADP-evoked Ca2+ mobilization. Therefore, the direct regulation of GPCR signalling by the cell potential should be more widely considered.
Methods
Cell isolation
Male adult (>150 g) Wistar rats were killed by exposure to a rising concentration of CO2 followed by cervical dislocation, in accordance with UK Home Office guidelines. Marrow cells were isolated from femoral and tibial bones as previously described (Mahaut-Smith et al. 1999) in standard external saline (see below) containing 0.32 U ml−1 type VII apyrase (Sigma-Aldrich, Poole, UK). Apyrase was present during the preparation and storage of cells, but omitted during experiments. Megakaryocytes were distinguished on the basis of their large size and recordings were made 2–12 h after marrow removal.
Solutions
The standard external saline contained (mm): 145 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 10 d-glucose, titrated to pH 7.35 with NaOH. For Ca2+-free saline, CaCl2 was replaced by an equal concentration of MgCl2, and where stated 0.5 mm Na2EGTA also included. For Na+-free saline, NaCl was replaced by N-methyl-d-glucamine (NMDG) and the pH adjusted to 7.35 with HCl. The pipette saline contained (mm): 150 KCl, 2 MgCl2, 0.1 EGTA, 0.05 Na2GTP, 0.05 K5fura-2, 10 Hepes, adjusted to pH 7.2 with KOH. K5fura-2 was purchased from Molecular Probes (Eugene, OR, USA). ADP (Sigma, UK) was treated by incubation with hexokinase and glucose to remove contaminating levels of ATP as previously described (Mahaut-Smith et al. 2000) and was applied to the cells via gravity-driven bath superfusion.
Electrophysiology
Conventional whole-cell patch clamp recordings were carried out in voltage clamp mode using an Axopatch 200B amplifier (Axon Instruments, CA, USA). pCLAMP and a Digidata interface (Axon Instruments) were used to deliver either voltage steps or action potential waveforms (APWs) derived from Oxsoft Heart 4.8 (Noble, 1999). To study the dependence of the P2Y receptor-evoked Ca2+ response on voltage pulse amplitude and duration, depolarizing and hyperpolarizing steps of increasing amplitude or duration were applied over two overlapping ranges. For increasing duration, the ranges were 25–300 ms in 25 ms increments and 100–1500 ms in 100 ms increments, with a holding potential of –85 mV and a depolarization of 135 mV. For increasing amplitude, the ranges were 1–5 mV in 1 mV increments and 5–75 mV in 5 mV increments, with a holding potential of –75 mV and a duration of 5 s. Due to desensitization of responses following large amplitude depolarizations, the maximum Ca2+ increase was also assessed in individual cells during the application of repeated steps of 40, 60 and 80 mV amplitudes from –75 mV. Action potentials were scaled to give a resting membrane potential of –85 mV with a maximum overshoot of +46 and +42 mV for ventricular and atrial APWs, respectively, as previously described (Lu et al. 2001). Recordings were made at room temperature (20–24°C), or at 36°C by regulation of the chamber temperature as previously described (Lu et al. 2001).
Fluorescence measurements
A Cairn spectrophotometer system (Cairn Research Ltd, Kent, UK) coupled to a Nikon Diaphot inverted microscope (Nikon, Japan) was used to measure fura-2 fluorescence as described in detail elsewhere (Mahaut-Smith, 1998). Fura-2 fluorescence signals were sampled at 60 Hz, averaged to give a final acquisition rate of 15 Hz and exported for analysis within Microcal Origin (Microcal Software Inc., Northampton, MA, USA). For presentation, some traces were filtered in Origin using 3–5 point averaging. Calibration constants Rmin and Rmax were measured extracellularly and a calibration kit (Molecular Probes) was used to derive a Kd for fura-2 at room temperature (258 nm). For experiments at 36°C, the Kd was corrected as previously described (Shuttleworth & Thompson, 1991), yielding a value of 216 nm. After application of a viscosity correction factor (0.85) to Rmin and Rmax (Poenie, 1990), background-corrected 340 nm/380 nm fluorescence ratios were converted to [Ca2+]i as previously described (Grynkiewicz et al. 1985; Mahaut-Smith et al. 1999). Data are expressed as the means ±s.e.m. with statistical difference assessed using Student's unpaired t test.
Results
Relationship between depolarization amplitude and [Ca2+]i increase
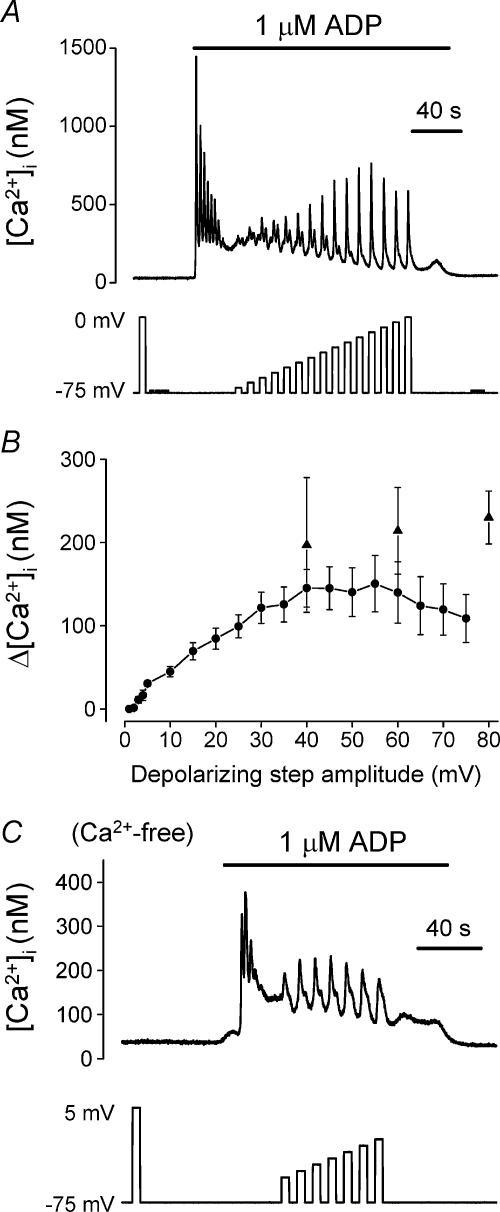
In the non-excitable rat megakaryocyte, depolarization from a holding potential of –75 mV has no effect on [Ca2+]i in unstimulated cells, yet can repeatedly generate single or multiple spikes of Ca2+ increase during exposure to the P2Y receptor agonist ADP (see Fig. 1a and Mahaut-Smith et al. 1999). Application of increasing amplitude depolarizing steps of 5 s duration showed that the peak [Ca2+]i response is graded with pulse magnitude up to approximately 40 mV (Fig. 1A and b, circles). The response tended to desensitize during repeated large amplitude depolarizations (≥40 mV; not shown), which could partially account for the reduced response at larger potential steps in the series (Fig. 1b, circles). Therefore, we also measured the maximal [Ca2+]i increase in individual cells during repeated application of either 40, 60 or 80 mV amplitude, 5 s duration, depolarizing steps from –75 mV, applied at the same frequency as in Fig. 1a (Fig. 1b, triangles). Although a large variation between cells was observed, these data suggest that the response increases with depolarization magnitude up to at least 80 mV. Previous studies have shown that this response to depolarization results predominantly from Ca2+ release, supplemented by concomitant store-dependent Ca2+ influx (Mahaut-Smith et al. 1999). Figure 1C shows an example of a response in Ca2+-free medium, demonstrating that Ca2+ release could be repeatedly stimulated by a series of increasing amplitude depolarizations. However, run-down of store content in Ca2+-free medium complicated the detailed study of the response under these conditions.
Figure 1. Effect of pulse amplitude on the depolarization-evoked [Ca2+]i increase during stimulation of P2Y receptors.
[Ca2+]i responses of rat megakaryocytes to ADP (1 μm, horizontal bar) and step depolarizations from a holding potential of –75 mV. The effect of depolarization during ADP application was assessed after the agonist-evoked increase had settled to a raised plateau level. A, effect of increasing the amplitude of the depolarizing step in 5 mV increments up to 75 mV. B, relationship between average peak [Ca2+]i response and depolarizing pulse amplitude (mean ±s.e.m.). Data plotted as circles were from two series of increasing amplitude voltage steps; up to 75 mV in 5 mV increments (as shown in A; 18 cells) and up to 5 mV in 1 mV increments (9 cells). Data plotted as triangles represent the average (5–9 cells) maximal [Ca2+]i increase during application of repeated 5 s duration pulses, of either 40, 60 or 80 mV amplitude, at the same frequency as in A. C, example of an experiment demonstrating that depolarization still potentiated ADP-evoked responses in a graded manner in Ca2+-free saline.
Depolarization potentiated the ADP-evoked Ca2+ response in all megakaryocytes (120/120 cells in this study), although the magnitude of the response displayed marked single cell heterogeneity. A detectable [Ca2+]i increase was observed in 100% of cells (27/27) following depolarizations of 5 mV, in 56% of cells (5/9) with steps of 3 mV, and in only a single cell (1/9; 11%) following a 2 mV step. This variability in absolute sensitivity most likely reflects the extent to which responses can be detected above the background noise as the cells with the largest Ca2+ increases were the ones that showed responses following depolarizations of only 2–3 mV. Overall, therefore, these data suggest that the phenomenon displays no discrete threshold for activation by depolarization.
Dependence on duration of the depolarizing step
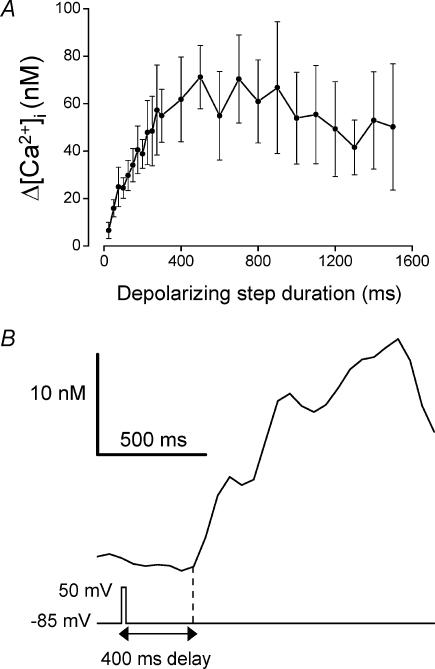
The duration of a fixed-amplitude (–85 to +50 mV) depolarization was also a major factor in determining the magnitude of the Ca2+ increase during stimulation of P2Y receptors (Fig. 2a; data from 20 cells). The largest peak [Ca2+]i increases were observed when the pulse length was ≥≈500 ms, and gradually decreased as the duration was reduced. As described above for the sensitivity limit for pulse amplitude, the shortest duration required for a detectable response varied with the extent to which the cell displayed the voltage-dependent Ca2+ release phenomenon. [Ca2+]i increases were detected following pulses as brief as 25 ms in the cells which displayed the most robust responses (5/11 cells, see for example Fig. 2b). Interestingly, the delay from depolarization to the first detectable Ca2+ increase did not vary with the duration of the step. The delay from the start of a 25 ms duration step was 440 ±70 ms (n= 5), which was not significantly different (P= 0.62) from the delay when the pulse duration was 300 ms (480 ± 50 ms, n= 7). Thus, for the shorter pulses, a pronounced interval was observed between the end of a voltage step and the initial Ca2+ increase (Fig. 2b).
Figure 2. Effect of pulse length on the depolarization-evoked [Ca2+]i increase during stimulation of P2Y receptors.
Megakaryocytes were held at –85 mV and stepped to +50 mV for increasing durations (25–1500 ms) during the plateau phase of the 1 μm ADP-evoked Ca2+ response. A, relationship between average depolarization-evoked Ca2+ increase and pulse length. Data were obtained from two series of voltage protocols; 25–300 ms in 25 ms increments at a rate of 0.25 Hz (10 cells) and 100–1500 ms with 100 ms increments at a rate of 0.1 Hz (12 cells). B, [Ca2+]i response to a 25 ms duration depolarization showing the characteristic long delay from the voltage step to initial Ca2+ increase. The delay from the start of the depolarization to the initial Ca2+ increase (vertical dashed line) was 400 ms.
Relative role of hyperpolarization-induced inhibition of the P2Y receptor response
In both the megakaryocyte and coronary artery smooth muscle, hyperpolarizations are generally inhibitory to the agonist-evoked Ca2+ increases via P2Y and mACh receptors, respectively (Ganitkevich & Isenberg, 1993; Mason et al. 2000). We also tested the effect of varying the amplitude and duration of the hyperpolarizing step on the ADP-stimulated Ca2+ response once a raised plateau level had been achieved. The peak reduction in Ca2+ during a 10 s hyperpolarization from –45 mV was graded with the pulse amplitude (Fig. 3a) with no apparent threshold potential. The amplitude of the Ca2+ decrease to a fixed-amplitude hyperpolarization from –45 mV to –100 mV also increased in a graded manner with the pulse duration (n= 19; not shown). Although the effects of hyperpolarization on [Ca2+]i during P2Y receptor activation were essentially the opposite of those of depolarization, the hyperpolarization-evoked decreases were always smaller in magnitude compared to the depolarization-evoked Ca2+ increases (see Fig. 3A and b). Consequently, in the experiment of Fig. 3a, the Ca2+ increases following repolarization to –45 mV were significantly larger than the hyperpolarization-induced decreases (Fig. 3b). Thus, although the voltage control of ADP-evoked Ca2+ release in the megakaryocyte is bipolar in nature, the potentiation by depolarization dominates over hyperpolarization-mediated inhibition within the physiological range of membrane potentials.
Figure 3. Hyperpolarization-evoked [Ca2+]i decreases during exposure to ADP.
Megakaryocytes were held at –45 mV and stepped to different hyperpolarized potentials for 10 s duration during the plateau phase of the ADP-evoked [Ca2+]i increase. A, average peak change in [Ca2+]i against amplitude of the hyperpolarizing voltage step. The values are means ±s.e.m. from 13 cells. Hyperpolarizations to potentials of –50 mV to –100 mV were applied in 5 mV increments (see inset) with an interpulse interval of either 10 or 20 s. B, [Ca2+]i response to a 10 s duration voltage step to –100 mV, illustrating the typical larger magnitude of the depolarization-evoked Ca2+ increase upon repolarization to –45 mV compared with the initial hyperpolarization-induced Ca2+ decrease.
Influence of single and multiple cardiac action potential waveforms on ADP-evoked Ca2+ responses
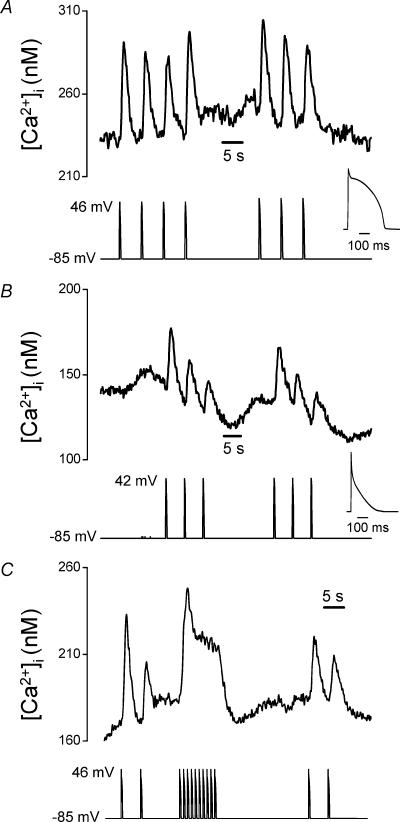
The concept that small or brief changes in the membrane potential can modify GPCR-induced Ca2+ signals has important implications for many cells, particularly in excitable tissues where larger changes of membrane potential regularly occur. Since the Ca2+ response increases with the period of depolarization up to about 500 ms, the phenomenon may have particular relevance in cardiac myocytes which display long duration action potentials and possess GPCRs and functional IP3 receptors (Gorza et al. 1993; Lipp et al. 2000; Rockman et al. 2002). Ion channels can have markedly different responses to APWs compared with simple step depolarizations (McCobb & Beam, 1991; Lu et al. 2001), therefore we examined the effect of APWs on [Ca2+]i during P2Y receptor activation in the megakaryocyte. Individual ventricular (Fig. 4a) and atrial (Fig. 4b) action potential waveforms repeatedly elevated [Ca2+]i during exposure to 1 μm ADP. Neither waveform had an effect on [Ca2+]i under resting conditions (not shown, n= 23). The peak Ca2+ increase in response to a single action potential varied between cells, as predicted from the marked heterogeneity of the response, and was 159 ± 30 nm(n= 13) for ventricular and 47 ± 11 nm(n= 10) for atrial APWs. This Ca2+-mobilizing effect of action potential waveforms was due primarily to the modulation of Ca2+ release, since it was present in Ca2+-free, 0.5 mm EGTA (n= 6, not shown) and Na+-free (n= 4, not shown) saline solutions, as previously demonstrated for the response to step depolarizations (Mahaut-Smith et al. 1999). The APWs used in the experiment of Fig. 4A and b were from normally paced hearts, but applied at a frequency of 0.2 Hz, therefore we also examined the effect of ventricular APWs at their normal frequency of 1 Hz. At this faster rate, the responses to individual action potentials fused to generate an elevated plateau level of Ca2+ (Fig. 4C, n= 10).
Figure 4. Cardiac action potential waveforms (APWs) repeatedly stimulate Ca2+ mobilization by potentiation of P2Y receptor signalling.
[Ca2+]i responses in the presence of 1 μm ADP to repeated ventricular (A) and atrial (B) APWs applied at 0.2 Hz. The insets show the shape of the normally paced waveforms on an expanded timescale. C, [Ca2+]i responses to ventricular APWs, applied at both 0.2 Hz and 1 Hz.
Effect of an elevation in temperature to physiological levels
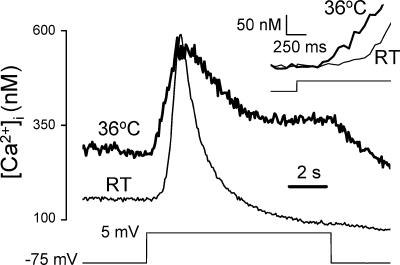
Since all experiments to date on the voltage control of megakaryocyte P2Y-mediated Ca2+ signals have been conducted at room temperature (RT; 20–24°C) (Mahaut-Smith et al. 1999; Mason et al. 2000; Mason & Mahaut-Smith, 2001; Thomas et al. 2001), we also examined the response at physiological temperatures. In the presence of 1 μm ADP, typical Ca2+ increases at RT and 36°C in response to 80 mV, 10 s steps from a holding potential of –75 mV are shown in Fig. 5. The higher temperature resulted in a raised plateau level during ADP stimulation and had two main effects on the depolarization-evoked Ca2+ increase. Firstly, the response was converted from a transient to a more sustained response, with a significant increase in the integral above the plateau level (36°C versus RT: 1047 ± 178 versus 586 ± 105 nm s, respectively, P < 0.05, n= 11), despite a reduction of the peak increase (36°C versus RT: 204 ± 62 versus 316 ± 31 nm, respectively, P < 0.01, n= 11). Secondly, the time delay from depolarization to the first detectable increase was reduced (36°C versus RT: 0.3 ± 0.04 versus 0.6 ± 0.06 s, respectively, P < 0.01, n= 11) (see Fig. 5 inset, in which the predepolarization [Ca2+]i values have been normalized for the two temperatures). Thus, increasing the experimental temperature to physiological levels accelerates the response to voltage and prolongs the Ca2+-mobilizing effects of depolarization during ADP stimulation.
Figure 5. Comparison of depolarization-evoked responses at room temperature and physiological temperatures.
[Ca2+]i response to a depolarizing voltage step of 80 mV and 10 s duration in the presence of 1 μm ADP at room temperature (RT) and 36°C. Data are from two different megakaryocytes, representative of a total of 11 cells at 36°C and 8 cells at room temperature (RT, 20–24°C). The inset shows the onset of the responses on a faster timescale. To allow comparison of the kinetics at the two temperatures, the Ca2+ recording at 36°C was offset to start from the same level as at RT.
Discussion
In this study we address the extent to which signalling via GPCRs, a major class of transmembrane proteins not normally considered to be voltage dependent, can be controlled by changes in the cell membrane potential. We selected P2Y receptors in the rat megakaryocyte as a model system due to the absence of ryanodine receptors and voltage-gated Ca2+ and Na+ channels in this typical non-excitable cell (Uneyama et al. 1993; Somasundaram & Mahaut-Smith, 1994; Mahaut-Smith et al. 1999; Mason & Mahaut-Smith, 2001). In addition, P2Y receptors are expressed in a range of tissues, including the cardiovascular and nervous systems (Kunapuli & Daniel, 1998; Moore et al. 2000), and so understanding the regulation of P2Y receptors has broad biomedical implications.
Previous work has shown that depolarization amplifies, whereas hyperpolarization inhibits, ADP-evoked release of Ca2+ from IP3-dependent intracellular stores in the megakaryocyte (Mahaut-Smith et al. 1999; Mason et al. 2000; Mason & Mahaut-Smith, 2001). We now show that the voltage control of P2Y receptor-evoked Ca2+ release in the megakaryocyte displays no threshold potential, such that depolarizations of only a few millivolts can potentiate the ADP-evoked Ca2+ increase. The phenomenon was also sufficiently sensitive for depolarizing steps of only 25 ms duration over the voltage range observed for action potentials (–85 to +50 mV) to generate significant Ca2+ increases. Hyperpolarizations inhibited the ADP-evoked Ca2+ increase in a graded manner, also without evidence for a threshold voltage or minimum duration. This further indicates that the underlying mechanism is bipolar in nature. However, regardless of the pulse length or amplitude, depolarization-mediated increases always dominated over the hyperpolarization-induced inhibition for square wave pulses. It was also interesting that during a 5 s duration depolarization or hyperpolarization at room temperature, the evoked [Ca2+]i change was transient and eventually came to rest at a level similar to the prestimulus plateau (although altered driving forces for Ca2+ entry most likely account for shifts in the steady state levels particularly at room temperature; see for example Figs 3b and 5). This suggests that the mechanism predominantly responds to changes in potential rather than steady state transmembrane potential levels. From these properties, it would be predicted that repeated cardiac action potentials would potentiate Ca2+ release during P2Y stimulation and indeed this was observed for both atrial and ventricular waveforms (see Fig. 4b). It is unclear at present why the response to depolarization is more sustained at 36°C compared to room temperature (Fig. 5); however, this would tend to enhance the potentiation of ADP-evoked responses by action potentials at physiological temperatures. Interestingly, due to the irreducible delay in the depolarization-evoked Ca2+ response described above, the Ca2+ increase commenced after completion of the atrial APW and towards the end of the ventricular APW. Together with the fact that the underlying mechanism appears to respond to changes in potential rather than steady state levels (see above), this resulted in a prolonged plateau of Ca2+ increase following multiple APWs at their normal frequency of 1 Hz (Noble, 1984). GPCRs, including P2Y purinoceptors, and functional IP3 receptors (IP3Rs), are present in the heart where this mechanism could have important physiological consequences (Lipp et al. 2000; Vassort, 2003). Furthermore, during pathophysiological states such as heart failure, the levels of IP3R expression are known to increase (Go et al. 1995) and ventricular APWs are prolonged (Tomaselli et al. 1994). For neuronal tissue, this could represent an important means whereby changes in membrane potential, can modify postsynaptic signalling via GPCRs (see Dascal, 2001, for review). In non-excitable tissues, significant fluctuations in membrane potential are often associated with agonist stimulation and could alter the resultant Ca2+ signals via the mechanism we describe here. Indeed we have previously shown that the shifts in membrane potential associated with repeated activation of Ca2+-dependent K+ channels can induce small [Ca2+]i oscillations due to repeated release from intracellular stores in the rat megakaryocyte (Mason et al. 2000).
The megakaryocyte is frequently used as a model for signalling in its product, the anuclear platelet. An increasing number of studies now suggest that most platelet receptors are expressed on the surface of the mature megakaryocyte and signal via identical pathways (Ikeda et al. 1992; Briddon et al. 1999; Vial et al. 2002). Platelets possess three P2 receptors; one ionotropic (P2X1) and two GPCRs (P2Y1 and P2Y12) (Gachet, 2001; Kunapuli et al. 2003). In the present study, we selectively activated P2Y over P2X1 receptors using hexokinase-purified ADP (Mahaut-Smith et al. 2000). P2Y1 receptors signal primarily via Gαq and phospholipase-Cβ to release Ca2+ from internal stores, whereas P2Y12 receptors are coupled to Gαi, resulting in an inhibition of adenylate cyclase and activation of PI3-kinase (Gachet, 2001; Kunapuli et al. 2003). P2Y1 and P2Y12 receptor-specific knock-outs have confirmed that the ADP-evoked Ca2+ response in the platelet is mediated primarily via P2Y1 receptors (Leon et al. 1999; Fabre et al. 1999; Foster et al. 2001). Thus, P2Y1 receptors coupled to Gαq and phospholipase-Cβ most likely represent the ADP receptor pathway controlled by membrane potential in the megakaryocyte.
To date, Ca2+ signalling via four different GPCRs has been suggested to be directly controlled by the membrane potential: mACh receptors in coronary artery smooth muscle and heterologously expressed in Xenopus oocytes, adrenergic receptors in mesenteric artery smooth muscle, and P2Y1 and thromboxane A2 receptors in rat megakaryocytes (Itoh et al. 1992; Ganitkevich & Isenberg, 1993; Mahaut-Smith et al. 1999; Mason & Mahaut-Smith, 2001; Ben Chaim et al. 2003). In each case, preactivation of the receptor was required to detect voltage modulation of intracellular Ca2+ release (or in the case of adrenergic receptors, IP3 generation). All four receptors are members of the rhodopsin/β2 adrenergic-like family (family A) of GPCRs (Gether, 2000). Therefore, it is particularly interesting that the activation of rhodopsin generates a conformation-dependent charge movement similar to the gating currents of voltage-dependent ion channels (Cone, 1967; Sullivan & Shukla, 1999). Any event that generates movement of charges within the membrane would be expected to be sensitive to the transmembrane potential and therefore the activation of rhodopsin and related GPCRs could be a voltage-dependent process. Indeed, Ben Chaim et al. (2003) have recently proposed that M2 muscarinic receptors expressed in Xenopus oocytes are voltage dependent and that the voltage sensor lies in the region of the receptor which interacts with the heterotrimeric G-protein. Another possibility to explain the voltage control of P2Y receptors and other GPCRs is that ligand binding within its active site is influenced by changes in the transmembrane potential. Activation of NMDA receptors by depolarization is a well-established example whereby modulation of binding by an external ion (in this case Mg2+) accounts for the voltage sensitivity of a transmembrane receptor. Site-directed mutagenesis studies of ligand-binding sites within the P2Y1 receptor indicate that two out of the three amino acids with the most significant effect on receptor activation are positively charged arginines (Jiang et al. 1997). These presumably reflect binding to the negatively charged phosphate moieties of ADP and thus represent potential interactions that could be modified by the transmembrane voltage. Although ligand–receptor interaction or conformational state of the receptor are the main candidates for the voltage-dependent processes during P2Y receptor signalling, the activity of either the heterotrimeric G-protein or phospholipase-Cβ cannot at present be excluded. For example in neurones, depolarization modulates G-protein inhibition of Ca2+ channels by displacing βγ subunits from their target (Golard & Siegelbaum, 1993; Zamponi & Snutch, 1998). However, compared to the voltage-dependent Ca2+ release phenomenon in the megakaryocyte, this effect requires larger amplitude depolarizations and reduces rather than potentiates the action of the GPCR on its target. A further mechanism that can explain the phenomenon in the megakaryocyte is one of configurational coupling, as proposed between transient receptor potential (TRP) channels and IP3 receptors on the endoplasmic reticulum (Kiselyov et al. 1998). However, one would expect this to involve very little delay and we observe a delay of ≥ 400 ms between depolarization and the initial Ca2+ increase in the megakaryocyte (Fig. 2b). This delay is comparable with the time required for ADP-dependent generation of IP3 and Ca2+ release in the megakaryocyte (Somasundaram & Mahaut-Smith, 1994; Vial et al. 2002). Together with the reduced delay at higher temperatures (Fig. 5), these data indicate the involvement of a metabolic step rather than one that depends purely upon a configurational coupling between plasma and internal store proteins. The modulation of P2Y1 receptor-evoked IP3 production by the membrane potential, as opposed to configurational coupling, is further supported by the indistinguishable characteristics of ADP- and depolarization-evoked waves in the megakaryocyte (Thomas et al. 2001). In summary, most evidence points towards voltage control of P2Y receptor binding or its conformation, leading to altered IP3 production and subsequent Ca2+ release. Production of IP3 by phospholipase-Cβ is just one example of a vast number of different signalling events that are controlled by GPCRs. Other common GPCR-regulated pathways include adenylate cyclase, and thus cAMP production, and direct effects on ion channels (Dascal, 2001). Such signalling cascades may also be under the control of membrane voltage, as described for Ca2+ release in our studies.
A further key question is why P2Y receptor-evoked Ca2+ responses in the megakaryocyte are so markedly potentiated by depolarization and inhibited by hyperpolarization. In some non-excitable cell types, such as mast cells and lymphocytes (Penner et al. 1988; Lewis & Cahalan, 1989), the main influence of membrane potential during agonist stimulation results from alterations of driving force on Ca2+ influx and therefore the effects on [Ca2+]i are the opposite of those observed in the megakaryocyte. Thus, the voltage-dependent mechanism may not be present, or as dominant, during agonist-evoked Ca2+ signalling in these other cells. In coronary artery smooth muscle, where depolarization potentiates Ca2+ release during stimulation of mACh receptors (Ganitkevich & Isenberg, 1993), larger amplitude voltage steps are required compared to during activation of P2Y receptors in the megakaryocyte. This may again be due to a difference in receptor type or ligand–receptor interaction. Another possibility is that the membrane invagination system of the megakaryocyte, which functions to provide additional membrane for platelet formation, amplifies an innate voltage dependence to P2Y receptor signals. Recent specific capacitance measurements have shown that these invaginations increase the amount of surface-connected membrane by 4- to 14-fold (Mahaut-Smith et al. 2003). This is equivalent to the specific capacitance of mammalian skeletal muscle, where the t-tubular system increases the amount of surface-connected membrane. Membrane invaginations may enhance voltage-dependent Ca2+ release via P2Y receptors by increasing the ratio of surface membrane (and thus receptor number) to cytoplasmic volume. In this scenario, the phenomenon may be more important in cells with a high specific capacitance such as skeletal muscle and certain types of cardiac muscle. In addition, cell compartmentalization can increase the ratio of surface membrane to cytoplasmic volume, for example in dendritic spines. These foci of neuronal integration express functional IP3 receptors (Miyata et al. 2000) and represent one structure where modulation of GPCRs by ionotropic receptors or action potentials would have clear physiological relevance.
In conclusion, we have demonstrated that Ca2+ signals evoked via P2Y1 receptors can be altered by changes in membrane potential of only a few millivolts or of only short duration, including action potential waveforms. This marked sensitivity of a GPCR of the rhodopsin family to transmembrane voltage has important implications for the regulation of GPCR signalling in all cell types.
Acknowledgments
This work was supported by the British Heart Foundation (PG/2000108 and BS/10) and the Medical Research Council (G9901465). We thank Jon Holdich for expert technical assistance.
References
- Araya R, Liberona JL, Cardenas JC, Riveros N, Estrada M, Powell JA, Carrasco MA, Jaimovich E. Dihydropyridine receptors as voltage sensors for a depolarization-evoked, IP3R-mediated, slow calcium signal in skeletal muscle cells. J Gen Physiol. 2003;121:3–16. doi: 10.1085/jgp.20028671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278:22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- Best L, Bolton TB. Depolarisation of guinea-pig visceral smooth muscle causes hydrolysis of inositol phospholipids. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:78–82. doi: 10.1007/BF00569664. [DOI] [PubMed] [Google Scholar]
- Briddon SJ, Melford SK, Turner M, Tybulewicz V, Watson SP. Collagen mediates changes in intracellular calcium in primary mouse megakaryocytes through syk-dependent and – independent pathways. Blood. 1999;93:3847–3855. [PubMed] [Google Scholar]
- Cone RA. Early receptor potential: photoreversible charge displacement in rhodopsin. Science. 1967;155:1128–1131. doi: 10.1126/science.155.3766.1128. [DOI] [PubMed] [Google Scholar]
- Dascal N. Ion-channel regulation by G proteins. Trends Endocrinol Metabolism. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJJ, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost. 2001;86:222–232. [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golard A, Siegelbaum SA. Kinetic basis for the voltage-dependent inhibition of N-type calcium current by somatostatin and norepinephrine in chick sympathetic neurons. J Neurosci. 1993;13:3884–3894. doi: 10.1523/JNEUROSCI.13-09-03884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L, Schiaffino S, Volpe P. Inositol 1,4,5-trisphosphate receptor in heart: evidence for its concentration in Purkinje myocytes of the conduction system. J Cell Biol. 1993;121:345–353. doi: 10.1083/jcb.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Ikeda M, Kurokawa K, Maruyama Y. Cyclic nucleotide-dependent regulation of agonist-induced calcium increases in mouse megakaryocytes. J Physiol. 1992;447:711–728. doi: 10.1113/jphysiol.1992.sp019025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Seki N, Suzuki S, Ito S, Kajikuri J, Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Guo D, Lee BX, Van Rhee AM, Kim YC, Nicholas RA, Schachter JB, Harden TK, Jacobson KA. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol. 1997;52:499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli SP, Dorsam RT, Kim S, Quinton TM. Platelet purinergic receptors. Curr Opin Pharmacol. 2003;3:175–180. doi: 10.1016/s1471-4892(03)00007-9. [DOI] [PubMed] [Google Scholar]
- Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regulation. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Lu Y, Mahaut-Smith MP, Varghese A, Huang CL, Kemp PR, Vandenberg JI. Effects of premature stimulation on HERG K+ channels. J Physiol. 2001;537:843–851. doi: 10.1111/j.1469-7793.2001.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith MP. An infra-red-transmitting aperture controller for use in single-cell fluorescence photometry. J Microscopy. 1998;191:60–66. doi: 10.1046/j.1365-2818.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Ennion SJ, Rolf MG, Evans RJ. ADP is not an agonist at P2X1 receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br J Pharmacol. 2000;131:108–114. doi: 10.1038/sj.bjp.0703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Hussain JF, Mason MJ. Depolarization-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte. J Physiol. 1999;515:385–390. doi: 10.1111/j.1469-7793.1999.385ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Thomas D, Higham AB, Usher-Smith JA, Hussain JF, Martinez-Pinna J, Skepper JN, Mason MJ. Properties of the demarcation membrane system in living rat megakaryocytes. Biophys J. 2003;84:2646–2654. doi: 10.1016/S0006-3495(03)75070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Hussain JF, Mahaut-Smith MP. A novel role for membrane potential in the modulation of intracellular Ca2+ oscillations in rat megakaryocytes. J Physiol. 2000;524:437–446. doi: 10.1111/j.1469-7793.2000.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Mahaut-Smith MP. Voltage-dependent Ca2+ release in megakaryocytes requires functional IP3 receptors. J Physiol. 2001;533:175–183. doi: 10.1111/j.1469-7793.2001.0175b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb DP, Beam KG. Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron. 1991;7:119–127. doi: 10.1016/0896-6273(91)90080-j. [DOI] [PubMed] [Google Scholar]
- Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000;421:374–384. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Oxsoft Heart Program Manual. Oxford: Oxsoft Ltd; 1999. [Google Scholar]
- Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990;11:85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Effect of temperature on receptor-activated changes in [Ca2+]i and their determination using fluorescent probes. J Biol Chem. 1991;266:1410–1414. [PubMed] [Google Scholar]
- Somasundaram B, Mahaut-Smith MP. Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. J Physiol. 1994;480:225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Shukla P. Time-resolved rhodopsin activation currents in a unicellular expression system. Biophys J. 1999;77:1333–1357. doi: 10.1016/S0006-3495(99)76983-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Hirst GDS. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Mason MJ, Mahaut-Smith MP. Depolarization-evoked Ca2+ waves in the non-excitable rat megakaryocyte. J Physiol. 2001;537:371–378. doi: 10.1111/j.1469-7793.2001.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, Kass D, Feldman AM, Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- Uneyama H, Uneyama C, Akaike N. Intracellular mechanisms of cytoplasmic Ca2+ oscillation in rat megakaryocyte. J Biol Chem. 1993;268:168–174. [PubMed] [Google Scholar]
- Van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2003;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Vergara J, Tsien RY, Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985;82:6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Rolf MG, Mahaut-Smith MP, Evans RJ. A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Br J Pharmacol. 2002;135:363–372. doi: 10.1038/sj.bjp.0704486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacke M, Thiel G. Electrically triggered all-or-none Ca2+-liberation during action potential in the giant alga Chara. J Gen Physiol. 2001;118:11–22. doi: 10.1085/jgp.118.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gβ subunit. Proc Natl Acad Sci U S A. 1998;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]