Abstract
The β2-adrenoceptor agonist (β2-agonist) fenoterol has potent anabolic effects on rat skeletal muscle. We conducted an extensive dose–response study to determine the most efficacious dose of fenoterol for increasing skeletal muscle mass in adult rats and used this dose in testing the hypothesis that fenoterol may have therapeutic potential for ameliorating age-related muscle wasting and weakness. We used adult (16-month-old) rats that had completed their growth and development, and old (28-month-old) rats that exhibited characteristic muscle wasting and weakness, and treated them daily with either fenoterol (1.4 mg kg−1, i.p), or saline vehicle, for 4 weeks. Following treatment, functional characteristics of fast-twitch extensor digitorum longus (EDL) and predominantly slow-twitch soleus muscles of the hindlimb were assessed in vitro. Untreated old rats exhibited a loss of skeletal muscle mass and a decrease in force-producing capacity, in both fast and slow muscles, compared with adult rats(P < 0.05). However, there was no age-associated decrease in skeletal muscle β-adrenoceptor density, nor was the muscle response to chronic β-agonist stimulation reduced with age. Thus, muscle mass and force-producing capacity of EDL and soleus muscles from old rats treated with fenoterol was equivalent to, or greater than, untreated adult rats. The increase in mass and strength was attributed to a non-selective increase in the cross-sectional area of all muscle fibre types, in both the EDL and soleus. Fenoterol treatment caused a small increase in fatiguability due to a decrease in oxidative metabolism in both EDL and soleus muscles, with some cardiac hypertrophy. Further studies are needed to fully separate the desirable effects on skeletal muscle and the undesirable effects on the heart. Nevertheless, our results demonstrate that fenoterol is a powerful anabolic agent that can restore muscle mass and strength in old rats, and provide preliminary evidence of therapeutic potential for age-related muscle wasting and weakness.
Ageing is associated with a progressive loss of skeletal muscle mass (sarcopenia) and a subsequent decline in muscle strength (Brooks & Faulkner, 1994; Larsson & Ramamurthy, 2000; Morley et al. 2001). Over the past two decades much research has focused on the underlying mechanisms of age-related effects on skeletal muscle, responsible for the gradual loss of functional independence amongst the elderly. Progressive muscle fibre denervation, a loss of motor units, and potential motor unit remodelling have been implicated, but since the slowing of contraction occurs before significant muscle wasting, intrinsic changes to skeletal muscle fibres, including excitation–contraction coupling, cannot be ruled out (Faulkner et al. 1995; Larsson, 1995; Larsson & Ansved, 1995; Roos et al. 1997; Plant & Lynch, 2002).
Developing therapeutic interventions to prevent or reverse the age-related decline in function is of increasing importance for two reasons. First, many elderly people require the use of all their muscle strength to complete simple tasks such as rising from a chair, and any further impairment in muscle function (such as that following extended bed rest after surgery) can result in a loss of functional independence (Larsson & Ramamurthy, 2000). Secondly, as the proportion of the elderly within the population continues to escalate worldwide, so does the associated socio-economic impact of age-related frailty and weakness, placing an increasing burden on the healthcare system (Larsson & Ramamurthy, 2000). There is a profound need for strategies that can ameliorate the effects of ageing on muscle structure and function, and thus restore functional independence (Lynch, 2002).
Associated with normal ageing is a decrease in the circulating levels of anabolic hormones, including, but not limited to: growth hormone (GH), insulin, insulin-like growth factor I (IGF-I), and testosterone (Janssens & Vanderschueren, 2000). These hormonal changes are thought to be responsible, at least in part, for the age-related loss of muscle mass and strength. Numerous clinical studies have tried increasing the circulating levels of one or more of these hormones, with the aim of increasing muscle mass and strength. However, to date, hormone replacement therapy has produced mixed success in humans (Janssens & Vanderschueren, 2000). Studies reporting on the effects of testosterone and testosterone precursor supplementation on muscle mass and strength have produced equivocal results in elderly human subjects (Morley et al. 1993; Sih et al. 1997; Snyder et al. 1999). Whereas testosterone has been shown to increase muscle strength in hypogonadal elderly men, testosterone administration to elderly men with normal testosterone levels was associated with an increased risk of polycythemia (Morley et al. 1993; Drinka et al. 1995). Furthermore, testosterone treatment for elderly women may be inappropriate due to the masculinizing effects of androgens.
Several studies have postulated that GH and (or) IGF-I administration may prevent the muscle wasting associated with ageing. To date, GH supplementation has been shown to alter body composition by decreasing body fat mass and increasing lean body mass in elderly men. However, GH did not augment muscle strength or produce muscle hypertrophy (Lange et al. 2002). The administration or up-regulation of IGF-I has proven more promising in a number of animal models of pathologies where muscle wasting is indicated (Barton-Davis et al. 1998; Gregorevic et al. 2002), but there is concern that elevated levels of IGF-I may be implicated in tumour formation (Adams, 2000). Furthermore, both GH and IGF-I are expensive therapies that must be given daily. These findings suggest that hormone replacement alone has limited therapeutic efficacy for treating sarcopenia in the frail elderly. In the absence of successful hormone replacement therapies, muscle anabolic agents have been used in an attempt to treat sarcopenia.
Although traditionally administered locally (by inhalation) at low doses for bronchodilatation in the treatment of asthma, when given at higher doses, systemically, β2-adrenoceptor agonists (β2-agonists, such as the most widely described, clenbuterol) have potent anabolic effects in healthy muscle (Emery et al. 1984). Their experimental use in the treatment for muscle wasting conditions has also yielded promising results (Maltin et al. 1993; Sneddon et al. 2000). Carter et al. (1991), administered clenbuterol at a dose of 1.5 mg kg−1 day−1 subcutaneously for 22 days to 3, 12 and 23-month-old Fischer 344 (F344) rats. The clenbuterol-induced increase in muscle mass was equivalent in 23- and 12-month-old rats (Carter et al. 1991), supporting the hypothesis that β2-agonists might be effective for ameliorating sarcopenia. However, when administered at a much lower dose (10 μg kg−1 day−1), clenbuterol caused only a modest attenuation in the loss of muscle mass and strength associated with hindlimb suspension in the slow-twitch soleus muscle, and did not affect muscle mass or force of the fast-twitch plantaris muscle, in old (38 month) F344 × Brown Norway F1 rats (Chen & Alway, 2000). Therefore, the use of a more potent β2-agonist, and (or) a higher dose, may be necessary for treating sarcopenia.
We recently reported that at an equimolar dose to clenbuterol, fenoterol has a 10–15% greater anabolic effect on rat fast- (EDL) and slow- (soleus) twitch skeletal muscle (Ryall et al. 2002). Therefore, we chose to investigate whether fenoterol may have therapeutic potential for treating sarcopenia. To this end, we conducted an extensive dose–response examination in adult rats to determine the dose of fenoterol that would produce a maximal increase in the mass of EDL and soleus muscles. We hypothesized that at this dose, fenoterol treatment would attenuate the age-related decline in skeletal muscle mass and strength in old F344 rats, specifically by causing hypertrophy of muscle fibres.
Methods
Animals
All experiments were approved by the Animal Experimentation Ethics Committee of The University of Melbourne, and were conducted in accordance with the guidelines for the care and use of experimental animals as outlined by the National Health and Medical Research Council of Australia.
Determination of optimal dose of fenoterol
Male 3-month-old F344 rats (n=48 rats, body mass: 270 g) obtained from the Animal Resource Centre (Canning Vale, Western Australia) were allocated into either control or one of five fenoterol-treated groups. Treated rats received either 0.025, 0.25, 0.5, 1.0, or 2.0 mg kg−1 fenoterol (in saline) every day for 4 weeks via a once daily intraperitoneal injection, whereas (untreated) control rats received an identical volume of saline only. For all animals, body mass was measured daily throughout the study.
Following 4 weeks of treatment, rats were anaesthetized with sodium pentobarbitone (Nembutal, Rhone Merieux, Pinkenba, QLD, Australia: 60 mg kg−1, i.p.), with supplemental doses administered to maintain an adequate depth of anaesthesia, such that there was no response to tactile stimulation. The EDL (fast-twitch) and the soleus (slow-twitch) muscles were surgically excised from both hindlimbs, blotted on filter paper, trimmed of their tendons, and weighed on an analytical balance. The rats were killed by opening of the thoracic cavity and immediate cardiac excision. The heart was blotted on filter paper, trimmed of large vessels, and weighed. All tissues were frozen immediately in thawing isopentane and then stored at –80°C for later biochemical analyses.
Protein concentration was determined in the EDL and soleus muscles using the Bradford assay. This was to determine whether the hypertrophy observed following fenoterol treatment was due to protein accretion and not simply increases in water content.
Analysis of the dose–response data enabled the determination of the optimal dose of fenoterol to produce maximal increases in EDL and soleus muscle mass. This dose was then used for studying the efficacy of fenoterol for treating age-related muscle wasting and weakness.
Efficacy of fenoterol for treating age-related muscle wasting and weakness
Adult (16-month-old, n=18) and old (28-month-old, n=18) male F344 rats (460–500 g) obtained from Harlan Sprague-Dawley (Indianapolis, IN, USA), were allocated at random to a control or fenoterol-treated group. All rats were housed in a pathogen-free environment in standard cages, and were provided with a standard laboratory diet (rat chow) and water sufficient for them to eat and drink ad libitum. In some previous studies where F344 rats have been used as a model for human sarcopenia, the animals were still growing and did not show an age-related loss of muscle mass and strength (Carter et al. 1991; Carter & Lynch, 1994; Smith et al. 2002). Therefore, we chose to compare rats at two ages. Adult 16-month-old rats are weight stable, have completed all growth and development, and (most importantly) do not exhibit muscle wasting or weakness (Larkin et al. 1996). In contrast, after the age of 24 months, F344 rats exhibit significant muscle atrophy and a lower force-producing capacity, characteristic of age-related muscle wasting and weakness (Kadhiresan et al. 1996). Fenoterol-treated rats received 1.4 mg kg−1 of fenoterol (Sigma-Aldrich Castle Hill, NSW, Australia) administered via intraperitoneal injections in 0.5 ml of isotonic saline every day for 4 weeks. Control rats received a daily injection of 0.5 ml saline vehicle.
Preparation of muscle tissue
At the completion of the 4 week treatment period rats were anaesthetized with sodium pentobarbitone (Nembutal, Rhone Merieux: 60 mg kg−1, i.p.), with supplemental doses administered to maintain an adequate depth of anaesthesia, such that there was no response to tactile stimulation. The EDL and the soleus muscles from the left hindlimb were surgically exposed and a length of silk suture (3/0, Pearsalls Suture, Somerset, UK) was tied to the proximal and distal tendons of each muscle. The nerve and blood supply to each muscle was severed just prior to excision to ensure the optimum condition of muscles. The excised muscle was blotted once on filter paper and placed immediately into a custom-built plexiglass chamber filled with Krebs solution (composition (mm); NaCl 1.37, NaHCO3 24, D-glucose 11, KCl 5, CaCl2 2, NaH2PO4.H2O 1, MgSO4.7H2O 0.487, D-tubocurarine chloride 0.293) oxygenated with 95% O2 and 5% CO2 (BOC Gases, Preston, Victoria, Australia) and thermostatically maintained at 25°C, in accordance with the conditions optimal for maintaining the viability of the muscles in vitro for the duration of the experiment (Segal & Faulkner, 1985; Ryall et al. 2002). Once all contractile measurements had been completed, the deeply anaesthetized rats were killed by rapid surgical excision of the heart, which was trimmed of all connective tissue, blotted once on filter paper and weighed.
Muscle function
Isometric contractile properties of the muscles were assessed in vitro according to the optimal methods that we have described in detail previously (Gregorevic et al. 2002; Ryall et al. 2002). Briefly, the distal tendon of the muscle was tied to a fixed pin and the proximal tendon to the lever arm of a dual mode servomotor (Aurora Scientific, Aurora, Ontario, Canada) in the organ bath. Platinum plate electrodes flanked the muscle either side to deliver supramaximal square wave pulses (0.2 ms duration) that were amplified (EP500B, Audio Assemblers, Campbellfield, Victoria, Australia) to maintain a current intensity sufficient to produce a maximum isometric tetanic contraction (Po), with a stimulation duration of 350 ms for EDL and 1200 ms for soleus muscles. The servomotor and stimulation operations were controlled by custom-written software applications (D.R. Stom Software Solutions, Ann Arbor, MI, USA) using LabView software (National Instruments, Austin, TX, USA) to drive a personal computer with onboard controller (PCI-MIO-16XE-10, National Instruments, USA) interfaced with the servomotor control/feedback hardware (Aurora Scientific, Canada). Optimum muscle length (Lo) was determined from maximum isometric twitch force (Pt), and optimum fibre length (Lf) was determined by multiplying Lo by previously determined ratios of fibre length to muscle length: 0.44 for EDL, and 0.71 for soleus muscle (Brooks & Faulkner, 1988; Ryall et al. 2002).
The value for Po was determined from the plateau of a frequency–force curve, employing a range of stimuli between 10 and 150 Hz for EDL muscles, and 5–120 Hz for soleus muscles, with 2 min rest between stimuli. After determination of Po, each muscle was subjected to a 4 min stimulation protocol to induce muscle fatigue. During the fatigue protocol, Po was determined once every 5 s for 4 min, and then determined once at 5, 10 and 15 min after the completion of the fatigue protocol to assess recovery.
At the conclusion of the contractile measurements, the muscle was trimmed of connective tissue, blotted once on filter paper and weighed on an analytical balance. Specific force (sPo; kN m−2) was calculated for each muscle according to the well-accepted procedures that account for cross-sectional area, determined after dividing muscle mass by the product of Lf and 1.06 mg mm−3, the density of mammalian skeletal muscle (Mendez & Keys, 1960). Muscles were frozen at Lo in thawing isopentane for later histological, histochemical and biochemical examination, and radioligand binding assays.
Histology and histochemistry
A portion of each frozen muscle sample was cryosectioned transversely through the midbelly region on a cryostat microtome at −20°C (CTI cryostat, IEC, Needham Heights, MA, USA). Serial sections of each muscle (8 μm thick) were placed onto uncoated glass microscope slides, with each slide holding one EDL and one soleus section. The sections were stained with haematoxylin and eosin (H&E) to determine muscle fibre cross-sectional area and general muscle architecture, Van Gieson's stain to assess collagen location, reacted for myosin ATPase (mATPase) activity for determination of muscle fibre-type proportions, and reacted for succinate dehydrogenase (SDH) activity for determination of oxidative enzyme activity. Measurement of SDH activity was based upon a modified version of the method described by Blanco et al. (1988). Briefly, sections were incubated (composition (mm): phosphate buffer 100 (pH 7.4); succinic acid 50; ethylenediaminetetraacetic acid 5; nitroblue tetrazolium 1.5; 1-methoxyphenzine methosulphate 1; sodium azide 0.75) for exactly 4 min at 25°C, to produce a coloured nitroblue-diformozan precipitate indicator, that represented increasing SDH activity within the muscle fibres in the sections. The reaction was terminated by multiple rinses in distilled water, and then the sections were air-dried and coverslips applied for quantitative examination (Gregorevic et al. 2002).
Myosin ATPase reactivity was determined according to the methods described by Hämäläinen & Pette (1993). This technique was used to correlate cross-sectional area measurements with fibre type, since β2-agonists affect fast and slow isoforms differently (Ryall et al. 2002). Serial sections were preincubated at pH 4.3 and 4.55, to identify four specific muscle fibre types (type I, IIa, IIb and IId/x). After the procedure, sections were dehydrated in alcohol and coverslips applied. Muscle fibres were classified according to their mATPase activity by interactive determination of fibre histochemical reaction intensity (Hämäläinen & Pette, 1993).
Collagen localization was determined by fixing frozen sections in 1 : 3 acetic acid: alcohol for 10 min and then placing them in Van Gieson's stain (0.05 mm 1% acid fuchsin, 16.5 mm saturated picric acid). Sections were then rinsed in ethanol and coverslips applied.
Images of all sections were acquired using a digital imaging camera (Spot model 1.3.0, Diagnostic Instruments, Sterling Heights, MI, USA) attached to an upright microscope (BX-51, Olympus, Tokyo, Japan). An identical field of view was acquired from the H&E stained sections, and the SDH- and the myosin ATPase-reacted sections (∼325 000 μm2). A larger field of view (∼750 000 μm2) was chosen from the Van Gieson's stained sections. Image files were analysed using an analytical imaging station (AIS, v6.0, Imaging Research, Ontario, Canada) in a double-blind manner. The mean cross-sectional area (CSA) of individual muscle fibres was calculated by interactive determination of the circumference of no less than 120 adjacent fibres from the centre of every muscle section.
β-Adrenoceptor density
The remaining portions of muscle samples in each treatment group were combined to ensure adequate protein content for β2-adrenoceptor density measurements (EDL or soleus muscles from two rats per sample). The EDL and soleus samples were each placed in 2 ml of ice-cold buffer A (mm: Tris (pH 7.0) 50, sucrose 250, EGTA 1; pH 7.4 at 4°C) and homogenized (Polytron PT 2100, Kinematica AG, Luzernerstrasse, Switzerland) separately for 30 s. Cell membrane fragments were prepared by multiple centrifugation steps at 4°C, which we have described in detail previously (Sillence & Matthews, 1994; Sillence et al. 2000; Ryall et al. 2002).
Radioligand binding assays utilized the methodology of Sillence et al. (1993). Frozen cell membrane pellets were thawed, resuspended in buffer and then vortexed for 30 s. Protein concentration was determined by the Bradford protein assay (Bio-Rad, Richmond, Virginia, USA), with bovine serum albumin standards. The membrane suspension was used at an assay concentration of 0.15 mg ml−1, since previous studies had shown that binding of the radioligand to β2-adrenoceptor sites was linear over the protein concentration range of 0.05–0.3 mg ml−1 (Sillence et al. 1993).
Due to the limited quantity of membrane protein obtained from these small muscles, only single point saturation assays were performed. Cell membrane suspension (400 μl) was incubated with 50 μl [125I]iodocyanopindolol (135 ρM; ICYP, the radioligand), and 50 μl of either buffer B (mm: Tris (pH 7.7) 50, MgCl2 10, NaCl 150; pH 7.4 at 37°C; to determine the total counts of ICYP bound to β2-adrenoceptors), or DL-propranolol (2 μm; a non-selective β-adrenoceptor antagonist that determines non-specific binding of ICYP to the membrane) in polyethylene tubes (12 mm × 75 mm). Assays were initiated with the addition of cell membranes, and tubes were incubated for 90 min in a shaking water bath at 37°C (130 cycles min−1). Separation of bound ligand from free ligand was achieved by filtering the contents of each tube through Whatman GF-C glass fibre filter papers (Whatman, Maidstone, UK) with 21 ml of ice-cold buffer B using a cell harvester (Brandel M-48R cell harvester, Biomedical Research and Development Laboratories, Gaithersburg, MD, USA). Radioactivity on the filters was determined in a gamma counter (1470 Wizardautomatic gamma counter, Wallac OY, Turku, Finland) at a counting efficiency of 78%. Results were obtained as γ-radiation counts per minute (c.p.m.) for all tubes, and then converted into β-adrenoceptor density (fmol of ICYP bound per milligram of protein) as described by Sillence et al. (1993). Previous experiments have shown that rat muscle contains a predominant population of β2-adrenoceptors, with β1-adrenoceptors usually undetectable by this technique (Sillence et al. 1993). Hence all adrenoceptors measured were designated as the β2-subtype.
Statistical analyses
Individual variables were compared between groups using a two-way analysis of variance, and Fisher's LSD post hoc multiple comparison procedure where significance was detected. Significance was set at P < 0.05. All values are expressed as mean ±s.e.m. unless specified otherwise.
Results
Investigation of fenoterol dose–response relationship
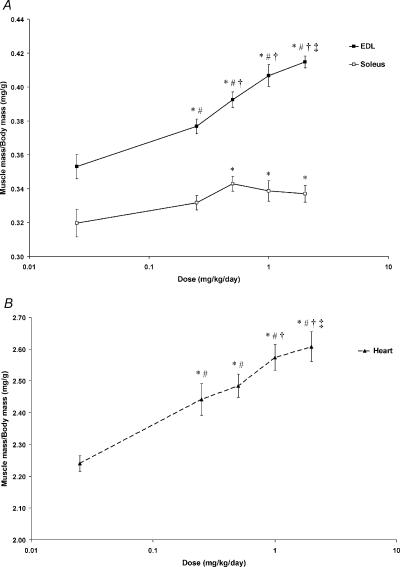
Body mass and absolute EDL, soleus and heart mass in young rats following 4 weeks of administration of fenoterol at different doses is presented in Table 1. Body mass was increased following daily fenoterol treatment at 0.025, 0.25 and 2.0 mg kg−1, but not at 0.5 and 1.0 mg kg−1. On the other hand, EDL and soleus muscle mass was increased following 4 weeks of administration of fenoterol at all doses tested. The largest increase in EDL muscle mass occurred at a dose of 2.0 mg kg−1 day−1, whereas soleus muscle mass was similar at fenoterol doses of 0.25 mg kg−1 day−1 and greater. Similarly, heart mass was greatest at fenoterol doses of 0.25 mg kg−1 day−1 or higher.
Table 1.
Selected morphometric parameters following 4 weeks of daily fenoterol administration at different doses
| Fenoterol (mg kg−1) | ||||||
|---|---|---|---|---|---|---|
| Control (untreated) | 0.025 | 0.25 | 0.5 | 1.0 | 2.0 | |
| Final BM (g) | 298 ± 4 | 314 ± 4* | 320 ± 5* | 308 ± 3 | 304 ± 5 | 313 ± 4* |
| EDL mass (mg) | 104 ± 1 | 111 ± 1* | 120 ± 2*# | 121 ± 1*# | 123 ± 1*# | 130 ± 2*#†‡ |
| Soleus mass (mg) | 94 ± 1 | 100 ± 2* | 106 ± 2*# | 106 ± 1*# | 103 ± 1* | 105 ± 2*# |
| Heart mass (mg) | 661 ± 12 | 699 ± 13 | 787 ± 20*# | 765 ± 13*# | 781 ± 12*# | 815 ± 16*#‡ |
BM, body mass; P < 0.05 greater than control;
P < 0.05 greater than 0.025 mg kg−1 day−1;
P < 0.05 greater than 0.25 mg kg−1 day−1;
P < 0.05 greater than 0.5 mg kg−1 day−1;
P < 0.05 greater than 1.0 mg kg−1 day−1.
The dose–response relationship of fenoterol for producing increases in skeletal muscle mass (expressed relative to body mass) is shown in Fig. 1. There was no increase in EDL or soleus muscle mass (relative to body mass) following daily treatment at 0.025 mg kg−1 day−1. In the EDL muscle there was a progressive increase in muscle mass following treatment at increasing doses (0.25, 0.5 and 1.0 mg kg−1 day−1). For the soleus muscle, relative mass increased progressively for increasing doses of fenoterol (0.25 and 0.5 mg kg−1 day−1) but did not increase further after doses of 1.0 and 2.0 mg kg−1 day−1 (Fig. 1A). EDL and soleus mass (expressed relative to body mass) was similar following administration at 1.0 and 2.0 mg kg−1day−1.
Figure 1. Dose–response relations for the EDL and soleus muscles (A), and the heart (B).
Fenoterol did not alter muscle mass (relative to body mass) at the lowest dose of 0.025 mg kg−1 day−1. Note log scale on abscissa. *P < 0.05 greater than control; #P < 0.05 greater than 0.025 mg kg−1 day−1; †P < 0.05 greater than 0.25 mg kg−1 day−1; ‡P < 0.05 greater than 0.5 mg kg−1 day−1.
Fenoterol treatment at 0.025 mg kg−1 day−1 did not affect heart mass (expressed relative to body mass) but at 0.25, 0.5 and 1.0 mg kg−1 day−1, relative heart mass increased in a dose-dependent manner. However, there was no further increase in relative heart mass following treatment at 2.0 mg kg−1 day−1 (Fig. 1B).
EDL and soleus muscle protein concentration was unchanged after 4 weeks of fenoterol treatment (i.e. not different from saline-treated control rats; data not shown). This indicated that the observed increase in mass was due entirely to fenoterol-mediated increases in protein accretion.
Based on these findings it was appropriate that the dose of fenoterol used for investigating its efficacy for ameliorating age-related muscle wasting and weakness, was one that produced a maximal increase in both EDL and soleus muscle mass. This dose was between 1.0 and 2.0 mg kg−1 day−1. Since in our previous investigations we used a dose of 2.8 mg kg−1 day−1, selecting a working dose half that value (1.4 mg kg−1 day−1) would ensure significant increases in both EDL and soleus muscle mass.
Morphometric properties
In untreated rats, ageing was associated with a decrease in body mass (10%, P < 0.05), reflected by a proportional decrease in mass of the EDL (11%) and soleus muscles (12%), so that the ratio of muscle mass to body mass was unchanged. As well as being lighter, there was a decrease in fibre CSA in EDL (4%) and soleus muscles (5%) from old rats, compared with adult control rats (P < 0.05). However, there was no apparent effect on collagen levels in EDL or soleus muscles, nor on heart mass (Table 2).
Table 2.
Selected morphometric parameters of adult and old rats following treatment with fenoterol or saline
| Adult (16 months) | Old (28 months) | |||
|---|---|---|---|---|
| Control | Fenoterol | Control | Fenoterol | |
| Initial body mass (g) | 483 ± 6 | 482 ± 9 | 454 ± 14 | 477 ± 12 |
| Final body mass (g) | 488 ± 5 | 497 ± 6 | 440 ± 17† | 482 ± 12* |
| EDL mass (mg) | 163 ± 5 | 214 ± 10* | 145 ± 5† | 182 ± 4*‡ |
| EDL mass/body mass (mg g−1) | 0.34 ± 0.01 | 0.43 ± 0.02** | 0.28 ± 0.01 | 0.39 ± 0.01** |
| EDL fibre CSA (μm2) | 1525 ± 19 | 2163 ± 51** | 1463 ± 24†† | 2059 ± 39*‡*‡ |
| EDL collagen (%) | 7.2 ± 0.6 | 5.3 ± 0.4 | 5.3 ± 1.4 | 5.5 ± 0.4 |
| Soleus mass (mg) | 148 ± 3 | 169 ± 6** | 130 ± 3†† | 148 ± 3** |
| Soleus mass/body mass (mg g−1) | 0.31 ± 0.00 | 0.34 ± 0.01** | 0.29 ± 0.01 | 0.31 ± 0.01 |
| Soleus fibre CSA (μm2) | 1946 ± 19 | 2463 ± 27** | 1839 ± 18†† | 2204 ± 24*‡*‡ |
| Soleus collagen (%) | 6.4 ± 0.9 | 5.7 ± 1.0 | 3.8 ± 0.9 | 5.0 ± 0.6 |
| Heart mass (mg) | 1284 ± 22 | 1587 ± 35** | 1361 ± 50 | 1719 ± 71*‡ |
| Heart mass/body mass (mg g−1) | 2.63 ± 0.03 | 3.19 ± 0.05** | 3.11 ± 0.09†‡ | 3.54 ± 0.10* ‡*‡ |
CSA, cross-sectional area; P < 0.05 treated versus age-matched control;
P < 0.05 old control versus adult control;
P < 0.05 old treated versus adult control.
In adult rats, fenoterol treatment did not alter body mass significantly, but caused an increase in the mass of EDL (31%) and soleus muscles (14%), with a concomitant increase in fibre CSA (42 and 27% for EDL and soleus, respectively, P < 0.05). Fenoterol did not affect collagen deposition, but increased heart mass in adult rats, both on an absolute basis (24%), and as a proportion of body mass (21%, Table 2).
In old rats, fenoterol caused similar anabolic effects to those seen in adult rats, so that the catabolic effects caused by ageing were counteracted. Body mass was increased by 10% following treatment, so that treated old rats were not significantly lighter than untreated adult rats. Similarly, soleus muscle mass was restored to that seen in untreated adult rats, whereas EDL muscle mass was greater than that seen in untreated adult rats. Compared with the modest effect of ageing on fibre CSA, the effects of fenoterol treatment were marked in old rats, resulting in an increase in CSA of 41% and 20% for EDL and soleus muscles, respectively (P < 0.01). As seen in adult rats, old rats treated with fenoterol showed no change in muscle collagen, but significant cardiac hypertrophy on an absolute (26%) and proportional (13%) basis (Table 2).
Muscle fibre-type proportions
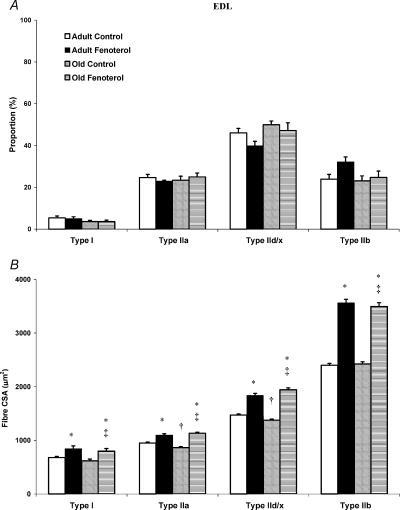
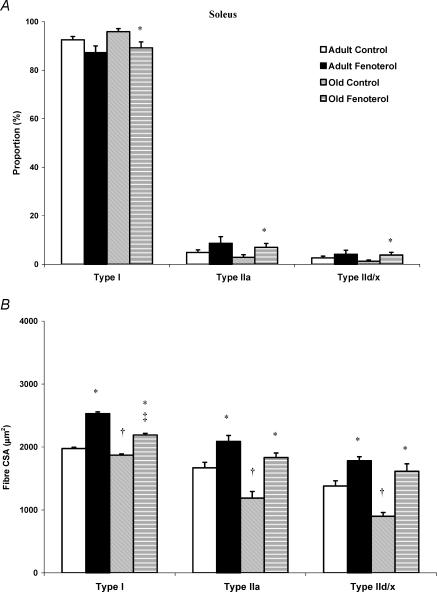
Ageing did not alter the proportion of different fibres in EDL muscle, but decreased the CSA of type IIa and IId/x fibres by 9 and 7%, respectively (P < 0.05, Fig. 2). Similarly, there was no effect of ageing on fibre-type proportions in soleus muscle, whereas the CSA of types I, IIa and IId/x fibres was decreased by 5, 29 and 35%, respectively (Fig. 3).
Figure 2. Fibre-type proportions (A) and corresponding cross-sectional area (CSA; B) in the EDL muscle of control and fenoterol-treated adult and old rats.
Whilst fenoterol had no effect on fibre type, fibre CSA was greatly increased following treatment. *P < 0.05 treatedversus age-matched control, †P < 0.05 old control versus adult control, ‡P < 0.05 old treated versus adult control.
Figure 3. Fibre-type proportions (A) and corresponding cross-sectional area (CSA; B) in the soleus muscle of control and fenoterol-treated adult and old rats.
As with the EDL, fenoterol had little effect on fibre-type proportions in the soleus muscle, but greatly increased fibre CSA. *P < 0.05 treated versus age-matched control, †P < 0.05 old control versus adult control, ‡P < 0.05 old treated versus adult control.
Fenoterol did not alter fibre proportions in the EDL muscle at either age. However, in soleus muscles, fenoterol caused a decrease in the proportion of type I fibres, with a concomitant increase in type IIa and IId/x fibres (P < 0.05). The pattern of response appeared to be similar in adult rats, but apparent differences did not reach statistical significance.
With respect to fibre CSA, the same pattern of response to fenoterol was seen in adult and old rats, with an increase in fibre CSA in all fibre types, in both muscles. The net result was that any decrease in fibre CSA caused by ageing rats was completely counteracted by fenoterol, consistent with the effects observed on muscle mass (Figs 2 and 3).
Biochemical analysis of relative oxidative capacity
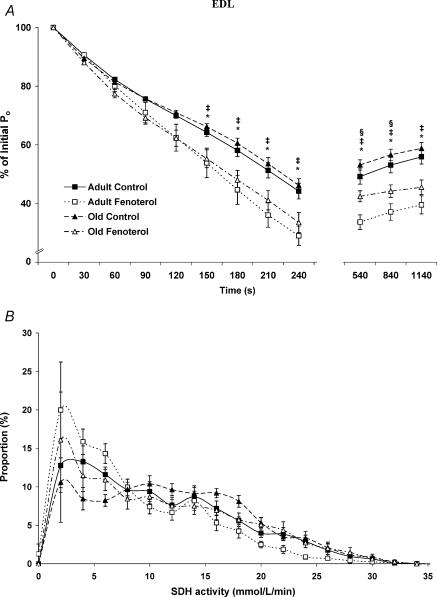
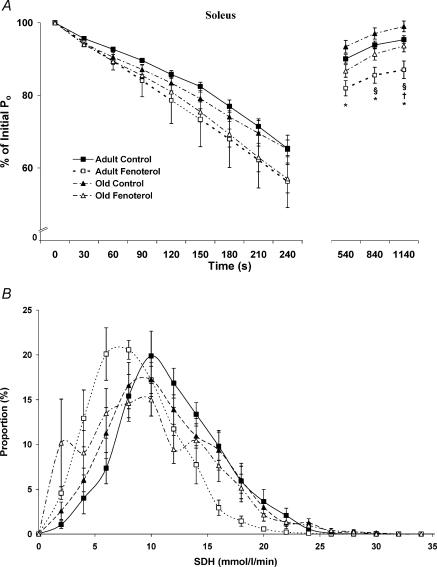
Quantitative histochemical methods were used to determine SDH activity (as an estimate of relative oxidative capacity) of individual fibres in the EDL and soleus muscles. A mean value of 9.4 ± 0.1 mmol fumarate (l of tissue)−1 min−1 for the EDL of adult control rats was obtained, which was increased by 18% with age (11.1 ± 0.1 mmol l−1 min−1 for old controls, P < 0.05). In contrast, fenoterol treatment decreased SDH activity of EDL muscles by 21 and 13% in adult and old rats, respectively (7.4 ± 0.1 and 9.7 ± 0.1 mmol l−1 min−1, P < 0.05). In the soleus muscles of adult rats, a mean value of 11.1 ± 0.1 mmol l−1 min−1 was obtained, but was lower in old rats (10.4 ± 0.1 mmol l−1 min−1, P < 0.05), and was decreased further by fenoterol treatment at both ages (7.6 ± 0.1 and 9.1 ± 0.1 mmol l−1 min−1 for adult and old rats, respectively).
Both the EDL and soleus muscles of adult treated rats contained the greatest proportion of fibres with low SDH activity (< 5 mmol l−1 min−1). The EDL muscles of old control rats contained the least number of fibres with low SDH activity, whilst those from old treated rats contained an increased number of fibres with low SDH activity, such that the proportion of these fibres was not different from EDL muscles of adult control rats (Fig. 4). Soleus muscles from adult and old control rats contained the lowest proportion of fibres with low SDH activity, whilst those from old treated rats exhibited a moderate proportion of fibres with low SDH activity (Fig. 5).
Figure 4. Fatigue and recovery (A), and SDH activity (B) for the EDL muscle from control and fenoterol-treated adult and old rats.
The reduced Po in fatigue and recovery of fenoterol-treated rats was associated with a decrease in the muscle SDH activity. *P < 0.05 treated versus age-matched control, †P < 0.05 old control versus adult control, §P < 0.05 old treated versus adult treated.
Figure 5. Fatigue and recovery (A), and SDH activity (B) for the soleus muscle from control and fenoterol-treated adult and old rats.
As with EDL, the reduced Po in the recovery of fenoterol-treated rats was associated with a decrease in the muscle SDH activity. *P < 0.05 treated versus age-matched control, †P < 0.05 old control versus adult control, §P < 0.05 old treated versus adult treated.
Contractile properties
Ageing did not alter the twitch force (Pt) in either EDL or soleus muscle. However, the time to peak twitch tension (TPT) and half-relaxation time (½RT) were measured to obtain an estimate of the speed of muscle contraction and relaxation, and both these variables were increased with ageing in EDL muscles, with an increase in ½RT in soleus muscle (P < 0.05, Table 3). More importantly, ageing decreased the maximum absolute force (Po) of both EDL and soleus muscles (12%), with EDL muscles also showing a 7% decline in specific force (sPo), highlighting the reduced functional capacity with age.
Table 3.
Isometric contractile measurements of adult and old rat EDL and soleus muscles, following treatment with fenoterol or saline
| Adult (16 months) | Old (28 months) | ||||
|---|---|---|---|---|---|
| Control | Fenoterol | Control | Fenoterol | ||
| EDL | Pt(mN) | 1052 ± 36 | 1368 ± 49* | 1067 ± 37 | 1356 ± 43*‡ |
| TPT (ms) | 30 ± 1 | 30 ± 1 | 33 ± 1† | 31 ± 1*‡ | |
| RT (ms) | 24 ± 1 | 23 ± 1 | 29 ± 2† | 25 ± 1* | |
| Po (mN) | 3324 ± 65 | 4131 ± 113* | 2911 ± 84† | 3560 ± 98* | |
| sPo (kN m−2) | 309 ± 10 | 310 ± 6 | 286 ± 7† | 280 ± 6‡ | |
| Soleus | Pt (mN) | 339 ± 15 | 414 ± 23* | 345 ± 19 | 438 ± 18*‡ |
| TPT (ms) | 83 ± 2 | 78 ± 2 | 90 ± 4 | 96 ± 5‡ | |
| RT (ms) | 124 ± 3 | 122 ± 7 | 158 ± 10† | 159 ± 9‡ | |
| Po (mN) | 1811 ± 57 | 2107 ± 79* | 1597 ± 33† | 1931 ± 49* | |
| sPo (kN m−2) | 266 ± 4 | 269 ± 4 | 252 ± 6 | 261 ± 7 | |
Pt, twitch force; TPT, time to peak twitch tension; ½RT, twitch half-relaxation time; Po, maximum force; sPo, specific force;
P < 0.05 treated versus age-matched control;
p < 0.05 old control versus adult control;
p < 0.05 old treated versus adult control.
At both ages, and in both EDL and soleus muscles, fenoterol treatment increased Pt beyond the level seen in control rats (P < 0.05). TPT was reduced significantly by fenoterol in EDL muscles of old rats, while ½RT was restored to the faster rate seen in adult control rats. For soleus muscle, TPT and ½RT were not affected by fenoterol treatment (Table 3).
Fenoterol increased Po in both EDL and soleus muscles at both ages, such that Po in fenoterol-treated old rats was as high as in untreated adults. This increase in force appeared to be associated without any increase in sPo when Po was adjusted for CSA.
Muscle fatigue and recovery
Ageing had no significant effect on the development of fatigue in either muscle, and only a slight effect of improving the rate of recovery in soleus muscles. After the 4 min fatigue protocol, the Po of EDL muscles from adult and old control rats was reduced to 44 and 46% of initial values, whereas with fenoterol these values were reduced to 29 and 35% of initial values, respectively (Fig. 4), indicating a greater susceptibility to fatigue after β-agonist treatment. After 5 min of recovery, the Po of EDL muscles from adult and old control rats was restored to 49 and 53% of initial Po values, respectively. After 10 and 15 min of recovery the Po values were as follows: adult control, 53 and 56%; old control, 57 and 59% of initial values. Fenoterol-treated adult and old rats exhibited a reduced recovery of Po following fatigue, compared with age-matched control rats (Fig. 4).
After the 4 min fatigue protocol, Po of the soleus muscles was not different between treatment and age groups. The Po was restored close to initial values during recovery, with the highest Po observed in old control rats and the lowest Po in adult treated rats (Fig. 5).
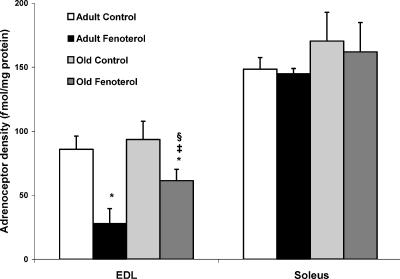
β-Adrenoceptor density
At both ages, β-adrenoceptor density was greatest in soleus muscles (adult, 149 ± 9; old, 170 ± 23 fmol (mg protein)−1), and was not affected by fenoterol treatment. In EDL muscles (adult, 86 ± 10; old, 96 ± 14 fmol (mg protein)−1), β−adrenoceptor density did not change with age but decreased markedly with fenoterol treatment (Fig. 6), indicating β2-agonist-induced down-regulation. After 4 weeks of treatment the β-adrenoceptor density of EDL muscles from old rats was 117% greater than in adult treated rats, but 29% less than in adult controls.
Figure 6. β-Adrenoceptor density in EDL and soleus muscles of control and fenoterol-treated adult and old rats.
Following treatment, down-regulation occurred in EDL but not soleus muscle, and was greater in adult than old rats. *P < 0.05 treated versus age-matched control, ‡P < 0.05 old treated versus adult control, §P < 0.05 old treated versus adult treated.
Discussion
The most important findings of this study were that skeletal muscles from old rats were as responsive to anabolic treatment as adult rats, and that 4 weeks of fenoterol treatment can counteract the atrophy and weakness associated with sarcopenia. Our dose–response investigation determined that the most efficacious dose of fenoterol for increasing fast and slow skeletal muscle mass was between 1.0 and 2.0 mg kg−1 day−1. We found that the mass and force-producing capacity of fast- and slow-twitch skeletal muscles from old rats treated for 4 weeks with fenoterol (1.4 mg kg−1 day−1) was equal or greater than that of adult rats that exhibited no signs of sarcopenia, providing support for the hypothesis that fenoterol can reverse the muscle atrophy and weakness concomitant with ageing.
Both fast- and slow-twitch skeletal muscles from old rats exhibited the characteristics of sarcopenia: a loss of muscle mass and strength. All fibre types exhibited atrophy in the EDL muscles of old rats, the greatest atrophy observed in type IIa and IId/x fibres, which is consistent with previous findings (Brooks & Faulkner, 1994; Larsson & Ramamurthy, 2000). However, the loss of mass and strength cannot be attributed solely to muscle fibre atrophy, as there was also a decrease in sPo in EDL muscles, when fibre CSA was taken into account. Thus, the loss in mass and strength is a likely result of both fibre loss and atrophy, as well as other intrinsic factors (Brooks & Faulkner, 1994; Larsson & Ansved, 1995; Plant & Lynch, 2002).
The increase in muscle mass and strength after fenoterol treatment was explained directly by the increase in fibre CSA. Although sPo values were higher in both adult and old animals than has been reported previously (Lynch et al. 2001), the deficit in sPo observed in old rats, persisted in muscles of treated aged rats, despite the significant improvement in absolute force-producing capacity. From a physiological perspective and applying these findings to a clinical situation, an improvement in absolute force has greater implications for carrying out the tasks of daily living than changes in force per muscle cross-sectional area. The reduced sPo of the EDL muscles from old rats was not associated with increased collagen localization within the muscle, and may be explained by the presence of other non-contractile material such as fat and other connective tissue.
The loss of muscle mass and strength that occurred with ageing was associated with a slowing of the time course of contraction. Slowing of movement is a serious problem in the elderly, since it increases the susceptibility to fall-related injuries (Larsson & Ramamurthy, 2000). We also demonstrated that the slowing of contraction in EDL and soleus muscles from old rats could not be attributed to an increase in the proportion of slow type I muscle fibres. Rather, as we have demonstrated previously, impaired excitation–contraction coupling and decreased function of calcium release from the sarcoplasmic reticulum, also contribute to the age-related slowing of contraction (Plant & Lynch, 2002). In old rats, fenoterol treatment was associated with a faster isometric twitch response in the EDL muscle. Such an adaptation might be advantageous for the elderly since the ability to perform more rapid movements might help in avoidance behaviours that prevent falls and subsequent injury.
The dose of fenoterol used in this study (1.4 mg kg−1 day−1) was half that used previously (Ryall et al. 2002), but the increases in muscle mass and strength were similar in magnitude. Even at this lower dose, the mass of the EDL muscles from fenoterol-treated old rats far exceeded that of adult (untreated) rats, indicating the potential for fenoterol to actually reverse the muscle wasting associated with ageing. Thus, a dose capable of restoring mass and strength to muscles in the elderly could be within the estimated safe limits for humans (Carter & Lynch, 1994). Even a modest (∼10%) increase in strength could dramatically improve the quality of life for the elderly. We have shown that even at a low dose of 0.025 mg kg−1 day−1, fenoterol increased absolute mass of the EDL and soleus muscles in adult rats (by ∼5–10%). These findings support the notion that for the elderly it would be possible to produce a desirable increase in skeletal muscle mass even at a low dose of this β-agonist. Further examination of the dose–response relationship of fenoterol and muscle mass in old rats is warranted in order to understand the therapeutic utility of fenoterol.
The EDL muscles from both adult and old rats treated with fenoterol were more prone to fatigue, and did not recover from fatigue as well as age-matched controls. In contrast, ageing had no effect on fatigue development and only a minor effect on recovery in soleus muscles. The greater susceptibility to fatigue and impaired recovery in fenoterol-treated rats could be attributed to the reduced oxidative capacity of these muscles, evidenced by the greater proportion of fibres with low SDH activity in both the EDL and soleus muscles of adult treated rats. Whilst such effects of fenoterol are not beneficial, in practice the advantages of a greatly improved force-producing capacity and increased speed of contraction in treated old rats are likely to outweigh the slight increase in fatigue following repeated maximal contractions, in terms of clinical significance. However, further studies are needed to fully separate the potentially desirable and undesirable effects of fenoterol on skeletal muscle.
Previous studies have found an age-associated decrease in β-adrenoceptor sensitivity and density in the heart (Lakatta & Sollott, 2002), which has been attributed to down-regulation and impaired coupling of β-adrenoceptors to adenyl cyclase (Xiao et al. 1998). In contrast, β−adrenoceptor density in both fast and slow skeletal muscle is not reduced with ageing (Larkin et al. 1996; Farrar et al. 1997), as confirmed in the present study. The present results also show that the responsiveness of skeletal muscle β-adrenoceptors to chronic β-agonist treatment is not reduced in old compared with adult rats. Furthermore, our findings indicate that β-adrenoceptors in EDL muscles are more susceptible to down-regulation than β-adrenoceptors in soleus muscles, during β-agonist administration. However, the greater extent of adrenoceptor down-regulation in the EDL muscle was not associated with a reduced physiological response, as evidenced by the equivalent increases in EDL and soleus muscle mass. The differential β-adrenoceptor response might be explained by a greater level of desensitization in the soleus muscle with chronic β2-agonist treatment. Agonist-dependent desensitization is initiated by phosphorylation of the receptor by receptor kinases, which become a target for arrestin proteins, inhibiting further G-protein coupling (Claing et al. 2002). We have also demonstrated that the adrenoceptor population in the EDL muscles of old rats is less susceptible to down-regulation than in EDL muscles of adult rats. The higher adrenoceptor density in the EDL muscles of old rats was not associated with a greater increase in mass or force-producing capacity, confirming previous reports that relative adrenoceptor density does not correlate with agonist-induced increases in muscle mass and function (Ryall et al. 2002). Further investigation is warranted into the differences in adrenergic signalling between fast- and slow-twitch skeletal muscles, and the effect of ageing.
Associated with high-dose administration of a β2-agonist in adult rats is cardiac hypertrophy (Duncan et al. 2000). We have shown an equivalent increase in heart mass in adult and old rats following fenoterol treatment. Whilst the increases in skeletal muscle mass and strength of old rats could be of clear benefit to an elderly person, the associated increase in heart mass remains a limiting factor for immediate clinical application. When given in high doses to animals, other β2-agonists, such as clenbuterol, have been shown to increase resting heart rate for the first few days of administration. This tachycardia is largely preventable in cattle with coadministration of a β1-adrenoceptor antagonist (CGP20712A), which would be unlikely to counteract the beneficial effects of β-agonist administration on skeletal muscle (Hoey et al. 1995). We have shown that at a low dose of 0.025 mg kg−1 day−1, fenoterol did not increase heart mass significantly in adult rats (P > 0.05) but there was still evidence of a hypertrophic effect, with cardiac size being increased by ∼5%. Our dose–response data indicate that at the lowest dose (0.025 mg kg−1 day−1), cardiac hypertrophy was only one-fifth that observed following 1.4 mg kg−1 day−1, with a greatly reduced effect on cardiac mass. Further studies that examine whether low dose treatment with such powerful β-agonists have similar effects in old rats are warranted. Whether low dose β-agonist treatment affects cardiac function deleteriously also deserves further attention, including whether any tachycardia can be prevented effectively with a β1-adrenoceptor antagonist.
Further investigation into the β-agonist-stimulated pathways leading to cardiac hypertrophy and the effects of chronic β2-agonist administration on cardiac function is essential for the continued development of this approach for tackling sarcopenia.
The limitations of this study were that following our extensive dose–response examination of the effects of fenoterol on skeletal and cardiac muscle in adult rats, we only tested the effects of a single dose of fenoterol on the skeletal muscles of old rats. Additional dose–response studies are required to fully understand the mechanisms of fenoterol's actions on skeletal and cardiac muscle, to identify potential advantages of fenoterol over other β−agonists, such as clenbuterol, and to separate the effects of these β-agonists on skeletal and cardiac muscle. Taken together, our results demonstrate that the skeletal muscle response of adult and old rats following fenoterol treatment (at a single dose of 1.4 mg kg−1 day−1) is similar, and that 4 weeks of treatment can ameliorate the age-associated loss of skeletal muscle mass and function. These exciting preliminary findings indicate that β-agonists have a definite application for treating sarcopenia, but further research is needed to separate their desirable effects on skeletal muscle from any undesirable effects on the heart, so as to optimize their therapeutic potential.
Acknowledgments
This work was supported by research grants from the Muscular Dystrophy Association (USA), the Rebecca L. cooper Medical Research Foundation, and The University of Melbourne J.G.R. was supported by a postgraduate scholarship from the National Heart Foundation of Australia.
References
- Adams GR. Insulin-like growth factor in muscle growth and its potential abuse by athletes. Br J Sports Med. 2000;34:412–413. doi: 10.1136/bjsm.34.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco CE, Sieck GC, Edgerton VR. Quantitative histochemical determination of succinic dehydrogenase activity in skeletal muscle fibres. Histochem J. 1988;20:230–243. doi: 10.1007/BF01747468. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exercise. 1994;26:432–439. [PubMed] [Google Scholar]
- Carter WJ, Dang AQ, Faas FH, Lynch ME. Effects of clenbuterol on skeletal muscle mass, body composition, and recovery from surgical stress in senescent rats. Metab Clin Exp. 1991;40:855–860. doi: 10.1016/0026-0495(91)90015-o. [DOI] [PubMed] [Google Scholar]
- Carter WJ, Lynch ME. Comparison of the effects of salbutamol and clenbuterol on skeletal muscle mass and carcass composition in senescent rats. Metab Clin Exp. 1994;43:1119–1125. doi: 10.1016/0026-0495(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Chen KD, Alway SE. A physiological level of clenbuterol does not prevent atrophy or loss of force in skeletal muscle of old rats. J Appl Physiol. 2000;89:606–612. doi: 10.1152/jappl.2000.89.2.606. [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Drinka PJ, Jochen AL, Cuisinier M, Bloom R, Rudman I, Rudman D. Polycythemia as a complication of testosterone replacement therapy in nursing home men with low testosterone levels. J Am Geriatr Soc. 1995;43:899–901. doi: 10.1111/j.1532-5415.1995.tb05534.x. [DOI] [PubMed] [Google Scholar]
- Duncan ND, Williams DA, Lynch GS. Deleterious effects of chronic clenbuterol treatment on endurance and sprint exercise performance in rats. Clin Sci. 2000;98:339–347. [PubMed] [Google Scholar]
- Emery PW, Rothwell NJ, Stock MJ, Winter PD. Chronic effects of beta 2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci Rep. 1984;4:83–91. doi: 10.1007/BF01120827. [DOI] [PubMed] [Google Scholar]
- Farrar RP, Monnin KA, Fordyce DE, Walters TJ. Uncoupling of changes in skeletal muscle beta-adrenergic receptor density and aerobic capacity during the aging process. Aging Clin Exp Res. 1997;9:153–158. doi: 10.1007/BF03340141. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50:124–129. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol. 2002;161:2263–2272. doi: 10.1016/S0002-9440(10)64502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem. 1993;41:733–743. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- Hoey AJ, Matthews ML, Badran TW, Pegg GG, Sillence MN. Cardiovascular effects of clenbuterol are α2-adrenoceptor-mediated in steers. J Anim Sci. 1995;73:1754–1765. doi: 10.2527/1995.7361754x. [DOI] [PubMed] [Google Scholar]
- Janssens H, Vanderschueren DM. Endocrinological aspects of aging in men: is hormone replacement of benefit. Eur J Obstet Gynecol Reprod Biol. 2000;92:7–12. doi: 10.1016/s0301-2115(00)00420-6. [DOI] [PubMed] [Google Scholar]
- Kadhiresan VA, Hassett CA, Faulkner JA. Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol. 1996;493:543–552. doi: 10.1113/jphysiol.1996.sp021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:699–721. doi: 10.1016/s1095-6433(02)00124-1. [DOI] [PubMed] [Google Scholar]
- Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, Juul A, Bulow J, Kjaer M. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- Larkin LM, Halter JB, Supiano MA. Effect of aging on rat skeletal muscle beta-AR function in male Fischer 344 × brown Norway rats. Am J Physiol. 1996;270:R462–R468. doi: 10.1152/ajpregu.1996.270.2.R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. Motor units: Remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–96. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- Larsson L, Ramamurthy B. Aging-related changes in skeletal muscle. Mechanisms and interventions. Drugs Aging. 2000;17:303–316. doi: 10.2165/00002512-200017040-00006. [DOI] [PubMed] [Google Scholar]
- Lynch GS. Novel therapies for sarcopenia: ameliorating age-related changes in skeletal muscle. Expert Opin Ther Pat. 2002;12:11–27. [Google Scholar]
- Lynch GS, Hinkle RT, Brooks SV, Chamberlain JS, Faulkner JA. Force and power output of fast and slow skeletal muscles from old dystrophic mdx mice. J Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltin CA, Delday MI, Watson JS, Heys SD, Nevison IM, Ritchie IK, Gibson PH. Clenbuterol, a beta-adrenoceptor agonist, increases relative muscle strength in orthopaedic patients. Clin Sci. 1993;84:651–654. doi: 10.1042/cs0840651. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Laboratory Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- Morley JE, Perry HM, 3rd, Kaiser FE, Kraenzle D, Jensen J, Houston K, Mattammal M, Perry HM., Jr Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41:149–152. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Plant DR, Lynch GS. Excitation–contraction coupling and sarcoplasmic reticulum function in mechanically skinned fibres from fast skeletal muscles of aged mice. J Physiol. 2002;543:169–176. doi: 10.1113/jphysiol.2002.022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. doi: 10.1002/(sici)1097-4598(199706)20:6<679::aid-mus4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Gregorevic P, Plant DR, Sillence MN, Lynch GS. β2-Agonist fenoterol has greater effects on contractile function of rat skeletal muscles than clenbuterol. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1386–R1394. doi: 10.1152/ajpregu.00324.2002. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985;248:C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- Sillence MN, Matthews ML. Classical and atypical binding sites for beta-adrenoceptor ligands and activation of adenylyl cyclase in bovine skeletal muscle and adipose tissue membranes. Br J Pharmacol. 1994;111:866–872. doi: 10.1111/j.1476-5381.1994.tb14818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence MN, Matthews ML, Badran TW, Pegg GG. Effects of clenbuterol on growth in underfed cattle. Aust J Agric Res. 2000;51:401–406. [Google Scholar]
- Sillence MN, Moore NG, Pegg GG, Lindsay DB. Ligand binding properties of putative beta-3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br J Pharmacol. 1993;109:1157–1163. doi: 10.1111/j.1476-5381.1993.tb13743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WN, Dirks A, Sugiura T, Muller S, Scarpace P, Powers SK. Alteration of contractile force and mass in the senescent diaphragm with beta2-agonist treatment. J Appl Physiol. 2002;92:941–948. doi: 10.1152/japplphysiol.00576.2001. [DOI] [PubMed] [Google Scholar]
- Sneddon AA, Delday MI, Maltin CA. Amelioration of denervation-induced atrophy by clenbuterol is associated with increased PKC-alpha activity. Am J Physiol Endocrinol Metab. 2000;279:E188–E195. doi: 10.1152/ajpendo.2000.279.1.E188. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, Staley J, Santanna J, Kapoor SC, Attie MF, Haddad JG, Jr, Strom BL. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Tomhave ED, Wang DJ, Ji X, Boluyt MO, Cheng H, Lakatta EG, Koch WJ. Age-associated reductions in cardiac beta1- and beta2-adrenergic responses without changes in inhibitory G proteins or receptor kinases. J Clin Invest. 1998;101:1273–1282. doi: 10.1172/JCI1335. [DOI] [PMC free article] [PubMed] [Google Scholar]