Abstract
Cell line models of colonic electrolyte transport have been extensively used despite lacking some of the characteristics of native tissue. While native colonic crypts absorb or secrete NaCl, immortalized cell lines only retain the secretory phenotype. In the present study we have characterized functionally and molecularly, vectorial fluid and electrolyte transport in the morphologically differentiated human colonic cell line LIM1863. LIM1863 cells form morphologically differentiated organoids resembling native human colonic crypts, which secrete fluid and electrolytes across the apical membrane into a centrally located lumen. Net fluid secretion was evaluated by means of morphometric measurement of lumens formed in LIM organoids in response to known secretagogues. Pharmacological profiling of the channels and transporters involved in fluid and electrolyte transport showed that net fluid transport requires Cl− uptake across the basolateral membrane through a Na+–K+–2Cl− cotransporter (NKCC1) and its subsequent exit across an apical cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel. Similar to the native colon, net Cl− secretion in the LIM1863 cell line is activated by cAMP-mediated agonists. Carbachol, a Ca2+-mediated agonist, does not induce net Cl− secretion but modulates the cAMP-activated response. Expression of chloride channels (CFTR and the Ca2+-dependent Cl− channel, ClCa1), potassium channels (KCNN4 and KCNQ1), epithelial Na+ channel (ENaC) α, β and γ subunits and ion transporters (NKCC1; anion exchanger, AE2; Na+/H+ exchangers, NHE1–3) was detected by RT-PCR and Western blot in the case of ENaC. Based on this evidence we propose that LIM1863 cells provide a unique model for studying CFTR-dependent Cl− secretion in a morphologically differentiated human colonic crypt cell line that also expresses ENaC.
The colon is most noted for its absorptive role in removing salt and water from intestinal fluid prior to defecation (Halm & Frizzell, 1991; Greger et al. 2000; Thiagarajah et al. 2001) in addition to producing a basal level of fluid secretion (Halm & Frizzell, 1991; Greger et al. 2000). NaCl secretion in colonic epithelial cells occurs as a result of co-ordinated ion transport mechanisms at the basolateral and apical membranes of the secretory cell (Halm & Frizzell, 1991). Cl− enters the cell via one or more of the following cotransporters: the bumetanide-sensitive Na+–K+–2Cl− cotransporter (NKCC1), the Na+/H+ (NHE), Cl−/HCO3− (AE) exchange system, or the Na+–Cl− cotransporter (Kunzelmann & Mall, 2002). The energy favourable for Cl− entry is provided by the Na+ gradient, set up by the action of the basolaterally located Na+–K+-ATPase and maintained by recycling of K+ via basolateral channels. Cl− is accumulated within the cell above its electrochemical gradient and its subsequent exit occurs through apically located Cl− channels (Halm & Frizzell, 1991). On the other hand, Na+ absorption typically occurs via apical electroneutral (NHE and AE) or rheogenic (epithelial Na+ channel, ENaC) systems (Halm & Frizzell, 1991; Greger, 2000).
Various human colonic cells lines have been used as models for investigating colonic Cl− secretion, including HT-29 (Anderson & Welsh, 1991; Zweibaum et al. 1991), T84 (Dharmsathaphorn & Pandol, 1986; Anderson & Welsh, 1991; Zweibaum et al. 1991; Merlin et al. 1998), Caco-2 (Anderson & Welsh, 1991; Zweibaum et al. 1991) and more recently NCM460 (Sahi et al. 1998). It is now well established that colonic crypts, classically associated solely with the secretory phenotype, show both NaCl absorption and secretion (Singh et al. 1995; Greger et al. 2000). However, none of the above-mentioned cell lines show the typical crypt-like structure or express ENaC.
In that respect, the LIM1863 colonic cell line used in this study is a unique model that retains a morphologically differentiated phenotype consisting of cells arranged around a central lumen forming spherical or crypt-like organoids (Whitehead et al. 1987). These organoids contain goblet, columnar and caveolated cells (Hayward & Whitehead, 1992), and express mucins and various brush border enzymes (Whitehead et al. 1987). In this study, we have characterized the secretory response of the LIM1863 cell line, a colonic cell model that preserves crypt-like morphology and ENaC expression, typical features of fully differentiated colonic crypts.
Methods
Cell culture
LIM1863 cells were grown in suspension in RPMI 1640 medium (1 × glutamine) (Gibco-BRL, Invitrogen S.A., Spain) supplemented with 5% fetal bovine serum (Australian approved, Gibco-BRL), 1% penicillin–streptomycin (Gibco-BRL), 2 mm l-glutamine, 1 μg ml−1 hydrocortisone, 0.01 mm ml−1 α-monothioglycerol and 1 μg ml−1 insulin (Gibco-BRL). The cells were maintained at 37°C in a humidified 5% CO2, 95% O2 atmosphere and were split (1 : 12) twice weekly when confluent. The cylindrical crypt-like structures were passaged by disaggregation into spherical organoids by vigorous pipetting. Cells used were from passage 34–60.
Morphometric measurement
A similar technique has been used previously (Lock & Valverde, 2000). A drop of spherical LIM1863 organoids was placed on cellagen-coated (ICN Pharmaceuticals, Barcelona, Spain) Petri dishes, and allowed to adhere for 20 min. Subsequently, the organoids were bathed in Iso (Isotonic) Hanks (Table 1) for 15 min prior to initiating the experiment. The solution was replaced with similarly composed solution (controls), or alternatively with solution containing the secretagogue(s) to be tested, at the start of the experiment (time = 0). In experiments involving the use of blockers, the blockers were added to the organoids 3 min prior to time = 0 and also during solution change at time = 0. Organoids were recorded using a CCD camera (Sony) connected to an inverted microscope (Nikon TMS). The digital images were then analysed using NIH 1.58 Image analysis software to establish the initial area of each organoid, and the area of the lumen as it appeared throughout the experiment. In organoids that produced lumens, normalization was obtained by dividing the lumen area by the organoid radius (at time = 0).
Table 1.
Composition of physiological solutions used
| NaCl | KCl | CaCl2 | MgCl2 | Hepes | Glucose | Sodium gluconate | Calcium gluconate | Potassium gluconate | Magnesium gluconate | NMDG-Cl | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iso Hanks | 140 | 2.5 | 1.2 | 0.5 | 10 | 5 | — | — | — | — | — |
| Cl− free | — | — | — | — | 10 | 5 | 140 | 10 | 2.5 | 0.5 | — |
| K+ free | 140 | — | 1.2 | 0.5 | 10 | 5 | — | — | — | — | — |
| Na+ free | — | 2.5 | 1.2 | 0.5 | 10 | 5 | — | — | — | — | 140 |
All quantities are given in millimolar. Solutions were fixed to pH 7.4 with Tris, and osmolarity of 300 mosmol kg−1 using d-mannitol
RNA extraction and RT-PCR
RNA was isolated from LIM1863 cells using the Nucleospin RNA II kit (Macherey-Nagel, Germany), according to the manufacturer's instructions. Total RNA was reverse-transcribed to cDNA. After 2 min denaturation at 90°C, the RNA was incubated for 1 h at 35°C in 20 μl reverse transcriptase mix containing: 1 × RT buffer (Promega, Madison, WI, USA), 2 μm oligodT12 primer, 125 μm pooled dNTPs and 200 U of MMLV-reverse transcriptase (Promega). Heating at 95°C for 5 min terminated the reaction. The RT product was amplified by PCR using the following profile: 4 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at the optimal annealing temperature for each primer (as indicated in Table 2), and 1.5 min at 72°C, concluding with 3 min at 72°C.
Table 2. The amplified genes, the sequences of sense and antisense primers, expected fragment size and annealing temperatures (Ta) used to investigate the expression of various ion channels, cotransporters and exchangers at the level of mRNA in the LIM1863 cell line.
| Gene | Sense primer | Antisense primer | Size (bp) | Ta (°C) |
|---|---|---|---|---|
| hGADPH | TGACATCAAGAAGGTGGTG | GATGGTACATGACAAGGTGC | 418 | 55 |
| hCFTR | GACTCTCCTTTTGGATACC | CTGGTACTAAGGACAGCCT | 186 | 55 |
| hNHE1 | CCTTCACCTCCCGATTTAC | ATAGGCGATGATGAACTGGT | 413 | 57 |
| hNHE2 | ACACAGGGCTTCCACTTCAACC | TTCCGATGCCCTCCAGACCA | 427 | 55 |
| hNHE3 | GAGGTCCATGTCAACGAGGTC | ACAGCAGATGCCACAGAAGGT | 354 | 57 |
| hAE2 | TGACTGCCCCAGAAAAGAG | ATCCCCGATAACTATGGAGAG | 416 | 55 |
| hNKCC1 | ACGACGGCTGGAAAAGGAACC | TGGCAAAGGCGAAGATTAGACC | 387 | 55 |
| hKCNQ1 | CTTTGCCATCTCCTTCTTTG | AGTGTTGGGCTCTTCCTTAC | 411 | 55 |
| hENaC α | GCCTGCTTTTCGGAGAGT | CCACCCCTGATGAGTATGTC | 429 | 57 |
| hENaC β | ACTTCCCCGCCGTCACCA | GTTGCAGGGCTCAGCTCCGAATAG | 500 | 70 |
| hENaC γ | GGCCCTGAAGTCCCTGTATGG | TCGGGTGGTGAAAAAGCGTGAA | 467 | 60 |
| hClCa1 | CGATGGGGAGTATTTGACGAGT | GTGGGATTTGGTGGCTGTGT | 404 | 55 |
| hClCa4 | GATTTCAGCCGAACAGCATC | GTCATCAGGATTTGCTTGAGG | 438 | 55 |
| hKCNN4 | GTGCGTGCAGGATTTAG | GAAGCGGACTTGATTGAGAG | 200 | 55 |
Primers used in this study were designed from the various gene sequences using DNAstar (Table 2). The housekeeper gene GAPDH was amplified during each reaction as a positive control, and also negative controls in which the RT protocol was carried out in the absence of MMLV-RT were performed for each of the primers. PCR products were analysed on a 2% agarose gel containing a final concentration of 0.5 μg ml−1 ethidium bromide. Where appropriate, subsequent sequencing of the bands confirmed their identity.
Membrane preparations of LIM1863 cells
Plasma membrane proteins were prepared using a previously published protocol (Graeser & Neubig, 1992; Fernández-Fernández et al. 2002). Briefly, the cells were washed with PBS (Ca2+- and Mg2+-free), incubated in PBS (Ca2+- and Mg2+-free) containing 0.1 mm EGTA for 10 min at 4°C, and centrifuged at 800 g for 5 min 4°C. The pellet was then resuspended in (mm): 137 NaCl, 5.6 dextrose, 1 EGTA, and 5 Hepes, pH 7.4 and centrifuged at 800 g for 5 min at 4°C. The pellet was then homogenized in (mm): 5 Tris-HCl, 5 MgCl2 and 1 EGTA, pH 7.5. The homogenate was centrifuged at 1000 g for 5 min, and the supernatant was centrifuged at 100 000 g for 1 h to obtain a crude membrane preparation. Membranes were resuspended in 50 mm Tris-HCl, 5 mm MgCl2 and 1 mm EGTA at pH 7.6 supplemented with 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, and 10 μg ml−1 phenylmethylsulphonyl fluoride and stored at −80°C. Protein content was determined using the Lowry method (Bio-Rad).
Western blot analysis
LIM1863 membrane proteins (60 μg) were resolved by SDS-PAGE (12%; 100 V for 1.5 h) and electrotransferred to nitrocellulose membrane (100 V for 2 h). Non-specific binding was prevented by blocking the membranes with TTBS (100 mm Tris-HCl, 150 mm NaCl, and 0.1% Tween 20, pH 7.5) containing 5% non-fat dry milk overnight at 4°C. Membranes were then incubated with the primary antibodies, rabbit polyclonal antibodies raised against the α, β or γ subunit of ENaC (Duc et al. 1994; Trujillo et al. 2002) at a dilution of 1 : 5000, for 2 h at room temperature. Subsequently the membranes were washed with TTBS containing 5% non-fat dry milk five times for 10 min each time, and then incubated with donkey antirabbit horseradish peroxidase (HRP)-conjugated secondary antibody (1 : 2,000, Amersham Pharmacia Biotech) for 1 h. The membranes were then washed thoroughly and the HRP-conjugated secondary antibody was detected using the SuperSignal West Pico Chemiluminescent Substrate (Pierce) and autoradiographed on Amersham Hyperfilm ECL.
Intracellular Ca2+ ([Ca2+]i) measurements
Cells were incubated in Iso Hanks solution containing 2 μm fura-2 AM (Molecular Probes, Leiden, the Netherlands) for 30 min at room temperature. The cells were then washed thoroughly (30 min) before initiating the experiment. Video microscopic measurements of [Ca2+]i were obtained using an Olympus IX70 inverted microscope (Hamburg, Germany) with a 40 × oil-immersion objective (Olympus, Hamburg, Germany). A Polychrome IV monochromator (Till Photonics, Martinsried, Germany) supplied the excitation light (340 nm and 380 nm), which was directed towards the cells in the field of view by a 505DR dichromatic mirror (Omega Optical, Brattleboro, VT, USA). Fluorescence images were collected by a digital CCD camera (Hamamatsu Photonics, Japan), following their passage through a 535DF emission filter (Omega Optical), using the AquaCosmos software programme (Hamamatsu Photonics, Japan). 340/380 nm ratio images were computed every 5 s. See Lindqvist et al. (1998) for a detailed description of the technique.
Statistics
Results are expressed as means ± s.e.m. of n observations. ANOVA analysis followed by Bonferroni post hoc test was used for multiple comparison of the statistical significance of the differences in the lumen area under different conditions.
Chemicals
All chemicals were purchased from Sigma Chemical Co. (Poole, Dorset, UK), unless otherwise stated.
Results
Ion transport mechanisms in LIM1863 cells
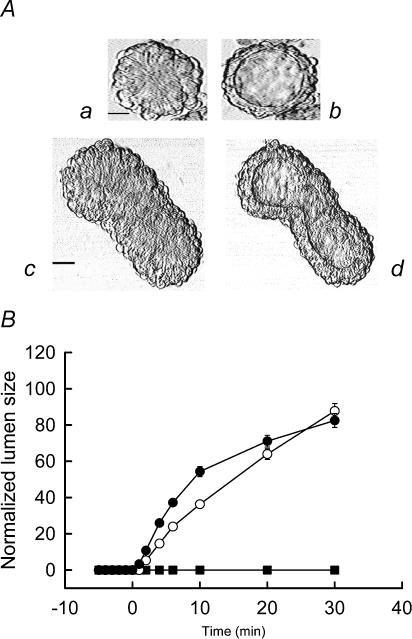
The evaluation of the LIM1863 cell line as a valid model for studying intestinal NaCl secretion was initially addressed by analysing the formation of lumens in response to known secretagogues. Lumens in unstimulated LIM1863 organoids could not be detected by conventional phase contrast microscopy (Fig. 1Aa and c). However, LIM1863 organoids produced a visible lumen (Fig. 1Ab and d) within 2 min following stimulation with 10 μm forskolin (an activator of adenylyl cyclase), which could be quantified throughout the experiment using phase contrast microscopy (Fig. 1B). For quantification purposes, only spherical organoids (like that shown in Fig. 1Aa and b) were utilized for the functional assessment of LIM1863 cells. Formation of a lumen did not occur under control conditions (Fig. 1B, ▪). The response to 1 μm (Fig. 1B, ○) or 10 μm (Fig. 1B, •) forskolin showed similar values 30 min after the addition of the agonist, although the higher concentration increased the rate of lumen formation. The presence of a sizeable lumen in response to a cAMP-elevating agonist is consistent with the vectorial transport of electrolytes and osmotically obliged fluid. Therefore, we used RT-PCR (Figs 2 and 3) and ion substitution experiments (Fig. 5A) to identify the putative transport systems associated with colonic electrolyte transport. Amplicons for Cl− channels (CFTR and Ca2+-dependent Cl− channel (ClCa1 but not ClCa4)), K+ channels (KCNQ1 and KCNN4), epithelial Na+ channel (ENaC) α, β and γ subunits and ion transporters (NKCC1; anion exchanger, AE2; Na+/H+ exchangers, NHE1–3) were identified. The presence of α, β and γ ENaC subunits was further demonstrated by Western blot (Fig. 4). Of the different transport systems detected in LIM1863 cells the expression of ENaC is particularly noteworthy, as to the best of our knowledge, this is the first description of a human colonic cell line expressing ENaC.
Figure 1. Electrolyte secretion in LIM1863 cells.
A, phase contrast imaging of LIM1863 cells, which exist as spherical (a) or crypt-like organoids (c). Forskolin (10 μm, 30 min), induced the formation of a visible fluid-filled lumen in both the spherical (b) and crypt-like (d) organoids. Scale bar =25μm. B, time course of forskolin-induced chloride secretion (1 μm (○, n = 24) and 10 μm (•, n = 95)). Control conditions (Iso Hanks) produced no lumen (▪, n = 22)
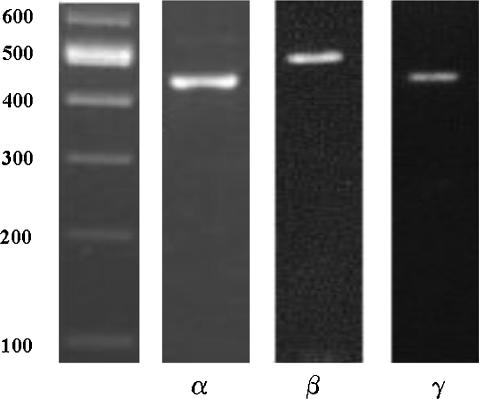
Figure 2. RT-PCR analysis of ion channels, exchangers and cotransporters present in the LIM1863 cell line.
A, mRNA transcripts (Table 2) detected by gel electrophoresis. B, negative control.
Figure 3. RT-PCR analysis of α, β and γ subunits of ENaC.
Amplicons of the expected size were obtained for the three subunits. See Table 2 for details of the primers and experimental conditions used.
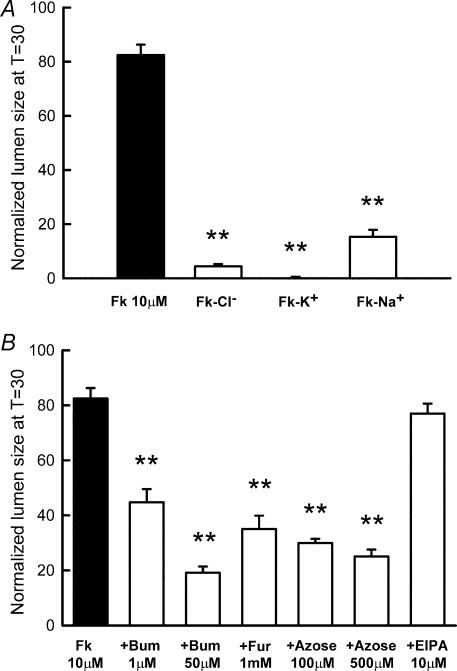
Figure 5. Ion transport pathways involved in LIM 1863 chloride secretion.
A, ion-substitution experiments. Bars represent the forskolin-mediated (10 μm) normalized lumen size 30 min following exposure to the secretagogue under control conditions (Fk, n = 95), or alternatively in the absence of extracellular Cl− (Fk-Cl−, n = 19), K+ (Fk-K+, n = 41) or Na+ (Fk-Na+, n = 22). B, forskolin-induced response in the presence of specific blockers of NKCC1 (bumetanide (1 μm, n = 25 and 50 μm, n = 8), furosemide (1 mm, n = 17) and azosemide (100 μm, n = 33 and 500 μm, n = 10)) and NHE (EIPA, 10 μm, n = 42). P < 0.001 for all conditions except EIPA (P > 0.05) when compared to control (Fk 10 μm, n = 95). **P < 0.001 compared to controls..
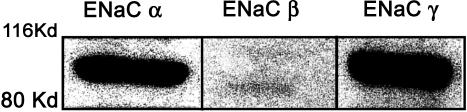
Figure 4. Western blot of membrane preparations of LIM1863 cells (20 μg lane−1).
Bands of the expected molecular mass ∼90 kDa (for all three subunits) were recognized by rabbit polyclonal antibodies raised against the α, β or γ subunit of ENaC, respectively.
Also of interest was the identification of splice variants of the KCNQ1 channel (Fig. 2). The strongest, upper band is the normal transcript and the lowest band corresponds to a transcript lacking exon 8 (confirmed by sequence analysis), a splice variant that has not been previously reported. At the moment, no information regarding its functional significance is available and further work is necessary to characterize it and the intermediate band.
Stimulation of LIM organoids with 10 μm forskolin in the absence of extracellular Cl− or Na+ resulted in a marked reduction in lumen size (Fig. 5A), and in the case of K+-free solutions, a virtual absence of lumen formation. These results on the one hand indicated that lumen formation is due to net electrolyte secretion and on the other hand are suggestive of the involvement of the triple cotransporter NKCC1 in Cl− uptake through the basolateral membrane. The role of the NKCC1 cotransporter in the secretory response of LIM organoids was further evaluated by using the so-called loop diuretics of the furosemide type (Schlatter et al. 1983), and tetrazolate-type diuretics, such as azosemide (Ecke et al. 1996) and bumetanide (Schlatter et al. 1983), all of which are potent inhibitors of colonic secretion (Fig. 5B). The participation of the Cl−/HCO3− and Na+/H+ exchange systems in electrolyte uptake was also evaluated. 5-(N-Ethyl-N-isopropyl) amiloride (EIPA), at a concentration that specifically blocks the Na+/H+ exchanger (10 μm) did not inhibit the forskolin-mediated secretion (Fig. 5B).
Identification of the secretory ion channels in LIM1863 cells
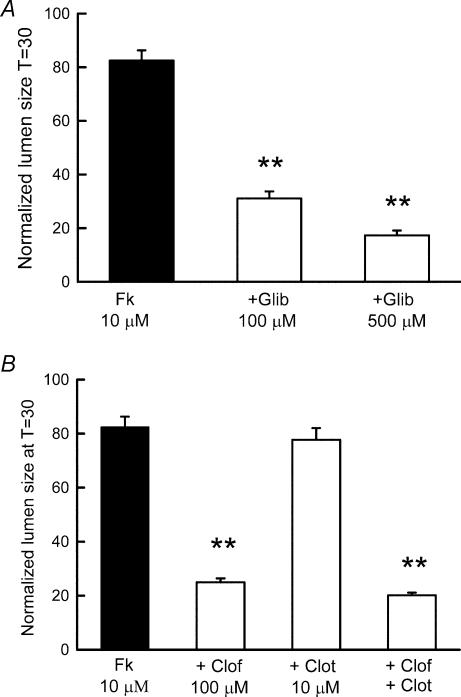
As shown in Fig. 2, bands for both CFTR and a Ca2+-dependent Cl− channel (ClCa1) were detected in LIM cells by RT-PCR. Both channels have been implicated in the apical exit of Cl− in different secretory epithelia. However, in the case of the colon, the involvement of ClCa channels in Cl− exit appears to be relevant only in unpolarized cells (Anderson & Welsh, 1991). In LIM1863 organoids, the use of the membrane permeant CFTR Cl− channel inhibitor glibenclamide (Sheppard & Welsh, 1992; Schultz et al. 1999) induced a dose-dependent inhibition of the forskolin-mediated secretory response (Fig. 6A).
Figure 6. Ion channels involved in LIM 1863 chloride secretion.
A, pharmacological identification of secretory Cl− channels in LIM1863 cells. Fk-mediated Cl− secretion (measured at 30 min) in the presence of glibenclamide (100 μm, n = 21 and 500 μm, n = 21). Control (Fk 10 μm, n = 95). B, pharmacological identification of K+ channels in LIM1863 cells. Fk-mediated Cl− secretion (measured at 30 min) in the presence of clofilium (100 μm, n = 19) and clotrimazole (10 μm, n = 27) or both blockers (Clof + Clot, n = 18). Control as above. **P < 0.001 compared to controls.
Two K+ channels have been implicated in K+ recycling and maintenance of the driving force for Cl− exit in the colon: a clotrimazole-sensitive Ca2+-dependent channel of intermediate conductance (IK, also known as KCNN4; Greger, 2000; Warth et al. 1999; Devor et al. 1997) and a 293B- and clofilium-sensitive KvLQT1 channel (also known as KCNQ1; Warth et al. 1996; MacVinish et al. 1998; Greger, 2000). The expression of both potassium channels in LIM1863 cells was detected by RT-PCR (Fig. 2). Clofilium (100 μm), unlike clotrimazole (10 μm), markedly reduced the forskolin-induced secretion (Fig. 6B). The inhibitory effect of the simultaneous addition of clotrimazole and clofilium did not differ from that observed in the presence of clofilium alone.
Ca2+-mediated responses in LIM1863 organoids
Chloride secretion is under the control of different secretagogues, many of which induce increases in the intracellular Ca2+ levels. A typical secretagogue involved in the generation of Ca2+ signals and the modulation of Cl− secretion in the colon is the muscarinic agonist carbachol (O'Malley et al. 1995; Lindqvist et al. 1998). The mechanisms by which carbachol modulates Cl− secretion have been extensively debated during recent years. Does carbachol activate Ca2+-dependent Cl− channels in the apical membrane or does it require the participation of CFTR to elicit its effect? This question was addressed in our cell model (Fig. 7).
Figure 7. Ca2+-mediated Cl− secretion in LIM1863 cells.
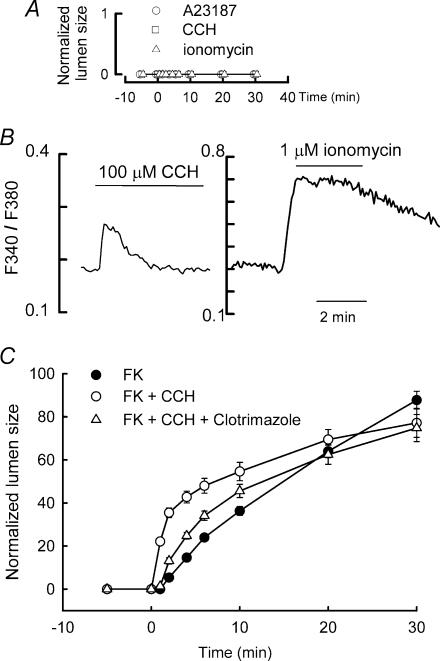
A, time course of lumen formation in response to carbachol (100 μm; □, n = 31) and the ionophores ionomycin (1 μm; ▵, n = 15) and A23187 (5 μm; ○, n = 5); B, representative intracellular Ca2+ ([Ca2+]i) measurements in response to carbachol (100 μm) and ionomycin (1 μm) obtained in cells loaded with fura-2 and represented as F340/F380 ratio images. C, carbachol (100 μm; n = 9, ○) transiently potentiated the Fk-induced secretion (1 μm; n = 24, •) without affecting the response at 30 min. The transient, carbachol-induced potentiation was inhibited by the KCNN4 K+ channel blocker clotrimazole (100 μm; n = 15, ▵).
Exposure of LIM1863 organoids to 100 μm carbachol failed to elicit a measurable lumen (Fig. 7A, □). Similarly, the addition of two calcium ionophores, ionomycin (▵) and A23187 (○), did not trigger any secretory response. To investigate the presence of functional muscarinic receptors in LIM1863 cells, the intracellular Ca2+ response to carbachol was monitored in cells loaded with the Ca2+-sensitive dye, fura-2 (Fig. 7B). Carbachol produced a small transient increase in intracellular Ca2+ levels while ionomycin produced a larger and more sustained increase.
In order to evaluate whether carbachol was able to modulate the vectorial transport induced by forskolin, we carried out experiments in which both forskolin and carbachol were superfused conjointly (Fig. 7C, ○). The presence of both compounds resulted in a marked acceleration of the response in the initial stages of lumen formation, without affecting the maximum response. The effect of carbachol on the forskolin-induced secretion was reverted by clotrimazole (Fig. 7C, ▵) suggestive of the participation of Ca2+-dependent K+ channels in the response to carbachol.
Discussion
The large intestine transports electrolytes and fluid in both absorptive and secretory directions. These two epithelial functions have been classically assigned to different cell types. While the crypt cells were thought to be responsible for the secretory response (Welsh et al. 1982), their differentiation into surface cells was associated with the gain of an absorptive function. This paradigm has been recently challenged by studies showing the presence of absorptive fluid and electrolyte transport in crypt cells as they travel from the base to the surface (Greger et al. 2000; Pedley & Naftalin, 1993). The vast majority of studies addressing the cellular and molecular mechanisms controlling electrolyte and fluid transport have been performed on tissue obtained from laboratory animals and normal, undifferentiated or carcinoma-derived colonic cell lines. However, all the existing cell line models used to study the transport properties of the colon lack two essential properties of the native tissue: morphological differentiation and the expression of ENaC channels. In this study, we have characterized the electrolyte transport in a human colonic cell line that preserves both the morphological and functional characteristics of the native tissue.
LIM1863 organoids produce visible lumens, which are attributable to net fluid and electrolyte transport, when targeted with cAMP-mediated agonists, but not Ca2+-dependent agonists. The pharmacological study of the lumen formation in response to forskolin was consistent with the activation of a glibenclamide-sensitive CFTR channel (Schultz et al. 1999).
Carbachol did not trigger secretion in LIM1863 organoids, despite the transient increase in intracellular Ca2+ levels (Lindqvist et al. 1998; Klaren et al. 2001; this study), in agreement with previous reports showing that cholinergic ion secretion in the native human colon, unlike the respiratory epithelium (Anderson & Welsh, 1991; Anderson et al. 1992) requires the opening of the apical CFTR channel (Mall et al. 1998). Similar conclusions were drawn from experiments carried out on the intestine of cystic fibrosis (CF) patients (Berschneider et al. 1988; Mall et al. 2000) or CF animal models (Grubb & Boucher, 1999; Klaren et al. 2001), where in the absence of functional CFTR channels, no Ca2+-mediated net secretion occurred. These observations have been interpreted in terms of the cAMP levels; when cellular levels of cAMP are high enough to open CFTR channels, even in the absence of a cAMP-mediated secretagogue, secretion would occur (Mall et al. 1998). In accordance with this view, although carbachol did not trigger secretion on its own, it transiently potentiated forskolin-mediated Cl− secretion (Fig. 7C).
The lack of Ca2+-induced Cl− secretion in LIM organoids also poses another interesting question: is there a functional Ca2+-activated Cl− channel in differentiated colonic crypts? Ca2+-activated Cl− channels have been identified in secretory epithelia (Anderson et al. 1992; Fuller & Benos, 2000), including airway epithelia (Wagner et al. 1991; Grubb et al. 1994), exocrine glands (Gray et al. 1994) and undifferentiated colonic cell lines (Morris & Frizzell, 1993a, b). However, polarization of colonic cells, unlike airway cells, results in the disappearance of the Ca2+-activated Cl− channel from the apical membrane (discussed by Anderson et al. 1992). We detected an amplicon for hClCa1 but not hClCa4 by RT-PCR, yet no Ca2+-dependent net Cl− secretion occurred (discussed above), suggesting a potential basolateral location for hClCa1.
Potassium channels are also required for effective electrolyte transport in epithelia (Dawson & Richards, 1990; Schultz et al. 1998; Cotton, 2000). Among the different K+ channels identified in secretory epithelia, KCNQ1 and KCNN4 channels appear to be pivotal players in colonic Cl− secretion. Both channels were detected in LIM1863 cells by RT-PCR. Forskolin-induced Cl− secretion was markedly reduced by clofilium, an inhibitor of colonic KCNQ1 channels (Loussouarn et al. 1997; MacVinish et al. 2001), but not by clotrimazole, a blocker of KCNN4 (Devor et al. 1997). On the other hand, clotrimazole abolished the carbachol-mediated transient potentiation of the forskolin response.
The molecular and pharmacological characterization of the basolateral transporters required for Cl− uptake suggested the involvement of NKCC1, as the secretory response was reduced by furosemide, azosemide and bumetanide, all of which block this transporter while EIPA, an inhibitor of the NHE, was ineffective in reducing Cl− secretion.
Finally, the expression of ENaC was evaluated in LIM1863 cells. ENaC is involved in Na+ absorption in different epithelia (Kunzelmann & Mall, 2002; Alvarez de la Rosa et al. 2000). Native colonic crypt cells express ENaC in their apical membrane as they differentiate from base to more superficial cells (Kunzelmann & Mall, 2002). The expression of ENaC has not been previously reported for any of the existing colonic cell models. Therefore, it was of great relevance to investigate whether the morphologically differentiated LIM1863 cells expressed ENaC. RT-PCR and Western blot analysis revealed the presence of the α, β and γ ENaC subunits. Such a pattern has been reported for distal rat colon (Greig et al. 2002) but not proximal colon. At present we do not know whether the presence of all three subunits in the LIM1863 organoids is due to their differentiation in culture towards a more distal phenotype or if native human proximal colon normally express the three subunits. In conclusion, the presence of ENaC in LIM1863 combined with their crypt-like architecture make them an excellent model for the study of CFTR-mediated Cl− secretion and ENaC-mediated Na+ absorption, and the interaction between both channel proteins.
Acknowledgments
LIM1863 cells were a gift from Dr R. Whitehead (Ludwig Institute for Cancer Research, Royal Melbourne Hospital, Melbourne, Australia), Azosemide was a gift from Dr J. Leipziger (Albert-Ludwigs-University, Freiburg, Germany) and anti-ENaC antibodies were a gift from C. Canessa (Yale University School of Medicine, New Haven, USA). We greatly acknowledge the help of Ms M. I. Bahamonde with membrane preparations and immunolocalization studies, and Dr A. Mallabiabarrena for assistance with confocal microscopy. This work was supported by the Spanish Ministry of Science and Technology, Distinció de la Generalitat de Catalunya per a la Promoció de la Recerca Universitaria, Human Frontiers Science Program and the King's Medical Research Trust (KMRT).
References
- Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu Rev Physiol. 2000;62:573–594. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol. 1992;263:L1–L14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Welsh MJ. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991;88:6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider HM, Knowles MR, Azizkhan RG, Boucher RC, Tobey NA, Orlando RC, Powell DW. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988;2:2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Cotton CU. Basolateral potassium channels and epithelial ion transport. Am J Respir Cell Mol Biol. 2000;23:270–272. doi: 10.1165/ajrcmb.23.3.f198. [DOI] [PubMed] [Google Scholar]
- Dawson DC, Richards NW. Basolateral K+ conductance: role in regulation of NaCl absorption and secretion. Am J Physiol. 1990;259:C181–C195. doi: 10.1152/ajpcell.1990.259.2.C181. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Gerlach AC, Frizzell RA, Bridges RJ. Inhibition of intestinal Cl− secretion by clotrimazole: direct effect on basolateral membrane K+ channels. Am J Physiol. 1997;273:C531–C540. doi: 10.1152/ajpcell.1997.273.2.C531. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986;77:348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol. 1994;127:1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecke D, Bleich M, Greger R. Crypt base cells show forskolin-induced Cl− secretion but no cation inward conductance. Pflugers Arch. 1996;431:427–434. doi: 10.1007/BF02207282. [DOI] [PubMed] [Google Scholar]
- Fernández-Fernández JM, Nobles M, Currid A, Vázquez E, Valverde MA. Maxi K+ channel mediates the regulatory volume decrease response in a human bronchial epithelial cell line. Am J Physiol Cell Physiol. 2002;283:C1705–C1714. doi: 10.1152/ajpcell.00245.2002. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Benos DJ. Ca2+-activated Cl− channels: a newly emerging anion transport family. News Physiol Sci. 2000;15:165–171. [PubMed] [Google Scholar]
- Graeser D, Neubig RR. Methods for the study of receptor/G-protein interaction. In: Milligan G, editor. Signal Transduction: A Practical Approach. Oxford: Oxford University Press; 1992. pp. 1–29. [Google Scholar]
- Gray MA, Winpenny JP, Porteous DJ, Dorin JR, Argent BE. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol. 1994;266:C213–C221. doi: 10.1152/ajpcell.1994.266.1.C213. [DOI] [PubMed] [Google Scholar]
- Greger R. Role of CFTR in the colon. Annu Rev Physiol. 2000;62:467–491. doi: 10.1146/annurev.physiol.62.1.467. [DOI] [PubMed] [Google Scholar]
- Greger R, Bleich M, Leipziger J, Ecke D, Mall M, Kunzelmann K. Regulation of ion transport in colonic crypts. News Physiol Sci. 2000;12:62–66. [Google Scholar]
- Greig ER, Baker EH, Mathialahan T, Boot-Handford RP, Sandle GI. Segmental variability of ENaC subunit expression in rat colon during dietary sodium depletion. Pflugers Arch. 2002;444:476–483. doi: 10.1007/s00424-002-0828-7. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca2+-mediated Cl− secretion in nasal epithelia of CF mice. Am J Physiol. 1994;266:C1478–C1483. doi: 10.1152/ajpcell.1994.266.5.C1478. [DOI] [PubMed] [Google Scholar]
- Halm DR, Frizzell RA. Ion tranport across the large intestine. In: Field M, Frizzell RA, editors. Handbook of Physiology, section 6, The Gastrointestinal System, Intestinal Absorption and Secretion. IV. Bethesda: American Physiological Society; 1991. pp. 257–273. [Google Scholar]
- Hayward IP, Whitehead RH. Patterns of growth and differentiation in the colon carcinoma cell line LIM 1863. Int J Cancer. 1992;50:752–759. doi: 10.1002/ijc.2910500515. [DOI] [PubMed] [Google Scholar]
- Klaren PH, Hardcastle J, Evans S, Colledge WH, Evans MJ, Taylor CJ, Hardcastle PT, White SJ. Acetylcholine induces cytosolic Ca2+ mobilization in isolated distal colonic crypts from normal and cystic fibrosis mice. J Pharm Pharmacol. 2001;53:371–377. doi: 10.1211/0022357011775424. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- Lindqvist SM, Sharp P, Johnson IT, Satoh Y, Williams MR. Acetylcholine-induced calcium signaling along the rat colonic crypt axis. Gastroenterology. 1998;115:1131–1143. doi: 10.1016/s0016-5085(98)70084-8. [DOI] [PubMed] [Google Scholar]
- Lock H, Valverde MA. Contribution of the IsK (MinK) potassium channel subunit to regulatory volume decrease in murine tracheal epithelial cells. J Biol Chem. 2000;275:34849–34852. doi: 10.1074/jbc.C000633200. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Charpentier F, Mohammad-Panah R, Kunzelmann K, Baro I, Escande D. KvLQT1 potassium channel but not IsK is the molecular target for trans-6-cyano-4-(N-ethylsulfonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane. Mol Pharmacol. 1997;52:1131–1136. doi: 10.1124/mol.52.6.1131. [DOI] [PubMed] [Google Scholar]
- MacVinish LJ, Guo Y, Dixon AK, Murrell-Lagnado RD, Cuthbert AW. Xe991 reveals differences in K+ channels regulating chloride secretion in murine airway and colonic epithelium. Mol Pharmacol. 2001;60:753–760. [PubMed] [Google Scholar]
- MacVinish LJ, Hickman ME, Mufti DA, Durrington HJ, Cuthbert AW. Importance of basolateral K+ conductance in maintaining Cl− secretion in murine nasal and colonic epithelia. J Physiol. 1998;510:237–247. doi: 10.1111/j.1469-7793.1998.237bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Bleich M, Schurlein M, Kuhr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am J Physiol. 1998;275:G1274–G1281. doi: 10.1152/ajpgi.1998.275.6.G1274. [DOI] [PubMed] [Google Scholar]
- Mall M, Wissner A, Seydewitz HH, Kuhr J, Brandis M, Greger R, Kunzelmann K. Defective cholinergic Cl− secretion and detection of K+ secretion in rectal biopsies from cystic fibrosis patients. Am J Physiol Gastrointest Liver Physiol. 2000;278:G617–G624. doi: 10.1152/ajpgi.2000.278.4.G617. [DOI] [PubMed] [Google Scholar]
- Merlin D, Jiang L, Strohmeier GR, Nusrat A, Alper SL, Lencer WI, Madara JL. Distinct Ca2+- and cAMP-dependent anion conductances in the apical membrane of polarized T84 cells. Am J Physiol. 1998;275:C484–C495. doi: 10.1152/ajpcell.1998.275.2.C484. [DOI] [PubMed] [Google Scholar]
- Morris AP, Frizzell RA. Ca2+-dependent Cl− channels in undifferentiated human colonic cells (HT-29). I. Single-channel properties. Am J Physiol. 1993a;264:C968–C976. doi: 10.1152/ajpcell.1993.264.4.C968. [DOI] [PubMed] [Google Scholar]
- Morris AP, Frizzell RA. Ca2+-dependent Cl− channels in undifferentiated human colonic cells (HT-29). II. Regulation and rundown. Am J Physiol. 1993b;264:C977–C985. doi: 10.1152/ajpcell.1993.264.4.C977. [DOI] [PubMed] [Google Scholar]
- O'Malley KE, Farrell CB, O'Boyle KM, Baird AW. Cholinergic activation of Cl− secretion in rat colonic epithelia. Eur J Pharmacol. 1995;275:83–89. doi: 10.1016/0014-2999(94)00758-y. [DOI] [PubMed] [Google Scholar]
- Pedley KC, Naftalin RJ. Evidence from fluorescence microscopy and comparative studies that rat, ovine and bovine colonic crypts are absorptive. J Physiol. 1993;460:525–547. doi: 10.1113/jphysiol.1993.sp019485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahi J, Nataraja SG, Layden TJ, Goldstein JL, Moyer MP, Rao MC. Cl− transport in an immortalized human epithelial cell line (NCM460) derived from the normal transverse colon. Am J Physiol. 1998;275:C1048–C1057. doi: 10.1152/ajpcell.1998.275.4.C1048. [DOI] [PubMed] [Google Scholar]
- Schlatter E, Greger R, Weidtke C. Effect of ‘high ceiling’ diuretics on active salt transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Correlation of chemical structure and inhibitory potency. Pflugers Arch. 1983;396:210–217. doi: 10.1007/BF00587857. [DOI] [PubMed] [Google Scholar]
- Schultz SG, Dubinsky WP, Lapointe JY. Volume regulation and ‘cross-talk’ in sodium-absorbing epithelial cells. Contrib Nephrol. 1998;123:205–219. doi: 10.1159/000059914. [DOI] [PubMed] [Google Scholar]
- Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol Rev. 1999;79:S109–S144. doi: 10.1152/physrev.1999.79.1.S109. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J General Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Binder HJ, Boron WF, Geibel JP. Fluid absorption in isolated perfused colonic crypts. J Clin Invest. 1995;96:2373–2379. doi: 10.1172/JCI118294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah JR, Jayaraman S, Naftalin RJ, Verkman AS. In vivo fluorescence measurement of Na+ concentration in the pericryptal space of mouse descending colon. Am J Physiol Cell Physiol. 2001;281:C1898–C1903. doi: 10.1152/ajpcell.2001.281.6.C1898. [DOI] [PubMed] [Google Scholar]
- Trujillo E, Alvarez de la Rosa D, Mobasheri A, Gonzalez T, Canessa CM, Martin-Vasallo P. Sodium transport systems in human chondrocytes. II. Expression of ENaC, Na+/K+/2Cl− cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol. 2002;14:1023–1031. doi: 10.14670/HH-14.1023. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Cozens AL, Schulman H, Gruenert DC, Stryer L, Gardner P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature. 1991;349:793–796. doi: 10.1038/349793a0. [DOI] [PubMed] [Google Scholar]
- Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, Greger R. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflugers Arch. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- Warth R, Riedemann N, Bleich M, Van Driessche W, Busch AE, Greger R. The cAMP-regulated and 293B-inhibited K+ conductance of rat colonic crypt base cells. Pflugers Arch. 1996;432:81–88. doi: 10.1007/s004240050108. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Smith PL, Fromm M, Frizzell RA. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982;218:1219–1221. doi: 10.1126/science.6293054. [DOI] [PubMed] [Google Scholar]
- Whitehead RH, Jones JK, Gabriel A, Lukies RE. A new colon carcinoma cell line (LIM1863) that grows as organoids with spontaneous differentiation into crypt-like structures in vitro. Cancer Res. 1987;47:2683–2689. [PubMed] [Google Scholar]
- Zweibaum A, Laburthe M, Grasset E, Louvard D. Use of cultured cell lines in studies of intestinal cell differentiation and function. In: Field M, Frizzell RA, editors. Handbook of Physiology, section 61, The Gastrointestinal System, Intestinal Absorption and Secretion. IV. Bethesda: American Physiological Society; 1991. pp. 223–256. [Google Scholar]