Abstract
Hippocampal CA1 inhibitory interneurones control the excitability and synchronization of pyramidal cells, and participate in hippocampal synaptic plasticity. Pairing theta-burst stimulation (TBS) with postsynaptic depolarization, we induced long-term potentiation (LTP) of putative single-fibre excitatory postsynaptic currents (EPSCs) in stratum oriens/alveus (O/A) interneurones of mouse hippocampal slices. LTP induction was absent in metabotropic glutamate receptor 1 (mGluR1) knockout mice, was correlated with the postsynaptic presence of mGluR1a, and required a postsynaptic Ca2+ rise. Changes in paired-pulse facilitation and coefficient of variation indicated that LTP expression involved presynaptic mechanisms. LTP was synapse specific, occurring selectively at synapses modulated by presynaptic group II, but not group III, mGluRs. Furthermore, the TBS protocol applied in O/A induced a long-term increase of polysynaptic inhibitory responses in CA1 pyramidal cells, that was absent in mGluR1 knockout mice. These results uncover the mechanisms of a novel form of interneurone synaptic plasticity that can adaptively regulate inhibition of hippocampal pyramidal cells.
Long-term potentiation (LTP) has been extensively studied at glutamatergic synapses onto principal cells of the hippocampus, but recent evidence indicates that it is also present at excitatory synapses on interneurones (Alle et al. 2001; Perez et al. 2001). The divergent projections of hippocampal interneurones allow them to control the excitability and synchronization of pyramidal cells (Buzsáki & Chrobak, 1995; Whittington et al. 1995; Ylinen et al. 1995; Freund & Buzsáki, 1996). Modelling studies have suggested that a hebbian type of plasticity at excitatory synapses onto interneurones is important for the synchronous activity of neuronal populations, as well as for learning and recall in the hippocampus (Grunze et al. 1996; Bibbig et al. 2001). However, clear evidence of plasticity at excitatory synapses on interneurones remains scarce (McMahon & Kauer, 1997; Cowan et al. 1998; Laezza et al. 1999; Alle et al. 2001; Perez et al. 2001; Ross & Soltesz, 2001), and the mechanisms involved are still largely unknown (McMahon & Kauer, 1997; Tóth & McBain, 1998, 2000; Laezza et al. 1999; Tóth et al. 2000; Alle et al. 2001; Perez et al. 2001; Ross & Soltesz, 2001).
In the CA1 region of the hippocampus, LTP in interneurones is cell-type specific. It is observed in interneurones of stratum oriens/alveus (O/A) but absent in those near the border of stratum radiatum and lacunosum-moleculare (Ouardouz & Lacaille, 1995; Perez et al. 2001). The use of a minimal stimulation protocol (Stevens & Wang, 1994; Raastad, 1995) in combination with a hebbian induction protocol demonstrated that LTP occurs directly at excitatory synapses of O/A interneurones, and does not result from passive propagation of LTP from pyramidal cells (Perez et al. 2001). Furthermore, this LTP is independent of N-methyl-d-aspartate receptors (NMDARs) but blocked by an antagonist of the metabotropic glutamate receptor 1a (mGluR1a) (Perez et al. 2001). Therefore, this cell-type specific and mGluR1a-dependent LTP in interneurones appears to be mediated by mechanisms different from those of the LTP at Schaffer collateral synapses on CA1 pyramidal cells (Bliss & Collingridge, 1993; Nicoll & Malenka, 1995).
The aim of the present study was to characterize the mechanisms of LTP at excitatory synapses of mouse O/A interneurones. Specifically, we examined (i) the mechanisms of induction and expression, (ii) the role of mGluR1 using immunocytochemical and knockout approaches, (iii) the selective sensitivity of synapses undergoing LTP to presynaptic modulation by mGluRs agonists, and (iv) the impact of LTP in interneurones on pyramidal cell inhibition. We report that LTP in mouse O/A interneurones is mGluR1 dependent, involves postsynaptic induction and presynaptic expression mechanisms, is specific to pharmacologically distinct synapses, and can regulate the inhibition of pyramidal cells.
Methods
Hippocampal slices
All experiments were done in accordance with the University of Montreal animal care guidelines. Coronal hippocampal slices (300 μm thick) were obtained (Ouardouz & Lacaille, 1995; Perez et al. 2001) from 18- to 37-day-old C57BL/6 (Charles River, Montreal) and mGluR1 (+/+, +/–, –/–) (Conquet et al. 1994) mice that had been anaesthetized with halothane and decapitated. Control littermates (+/+, +/–) and mGluR1 (–/–) mice were distinguished based on their phenotype (Conquet et al. 1994). Slices were maintained in oxygenated artificial cerebrospinal fluid (ACSF) containing (mm): 124 NaCl, 5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, and 10 dextrose (pH 7.3–7.4, 300 mosmol l−1). Prior to recordings, CA1 and CA3 regions were isolated by a cut. Individual slices were transferred to a recording chamber perfused with oxygenated ACSF (2–3 ml min−1) at room temperature (20–22°C). O/A interneurones and pyramidal cells were visually identified and selected for whole cell recordings using an upright microscope (Zeiss Axioskop, Germany) equipped with a long-range water immersion objective (×40, Nomarski optics) and infrared video camera.
Electrophysiological recordings
The whole cell recording solution for O/A interneurones contained (mm): 135 CsMeSO3, 5 NaCl, 1 MgCl2, 10 Hepes, 10 phosphocreatine, 2 ATP-Tris, 0.4 GTP-Tris and 0.1% biocytin (pH 7.2–7.3, 290 mosmol l−1). In some experiments, BAPTA-tetracesium salt (10 mm) was included in the solution. For CA1 pyramidal cells, the recording solution contained (mm): 130 potassium gluconate, 5 NaCl, 2 MgCl2, 10 Hepes, 0.5 EGTA, 10 phosphocreatine, 2 ATP-Tris, 2 QX-314 Cl− and 0.1% biocytin (pH 7.2–7.3, 280 mosmol l−1). Voltage-clamp recordings were made using an Axopatch 1D (Axon Instruments, Foster City, CA, USA). Data were low-pass filtered at 1 kHz, digitized at 10 or 20 kHz, and acquired using pCLAMP (versions 7 or 9). Interneurones were held at −60 mV whereas pyramidal cells were held near subthreshold membrane potential (−51.8 ± 0.6 mV, n= 16). Recordings were accepted if holding current and series resistance were stable. Series resistance was assessed regularly and was 17.6 ± 0.5 MΩ for interneurones (n= 71) and 18.2 ± 1.2 MΩ for pyramidal cells (n= 16).
Stimulation procedures
Synaptic responses were evoked in interneurones using constant current pulses (50 μs) delivered through a bipolar θ-glass electrode filled with ACSF and positioned in O/A within 100 μm lateral from the recorded cell somata. Putative single-fibre EPSCs were evoked at 0.5 Hz using minimal stimulation (Stevens & Wang, 1994; Raastad, 1995), as previously described (Perez et al. 2001). In some experiments, paired stimulations (50 ms interval) were given during the entire recording session. LTP was induced by three episodes (given at 30 s intervals) of theta-burst stimulation (TBS) of afferents (five bursts each consisting of four pulses at 100 Hz with a 250 ms interburst interval) paired with postsynaptic depolarization (five depolarizing steps to −20 mV, 60 ms long). Polysynaptic responses were evoked in CA1 pyramidal cells using constant current pulses (50 μs) delivered through a bipolar concentric platinum/iridium electrode positioned in O/A. The stimulus intensity was adjusted so that control inhibitory postsynaptic currents (IPSCs) were ∼50% of maximal amplitude. Responses were evoked at 0.05 Hz. Long-term changes in IPSC amplitude were examined 30 min after three episodes of TBS (given at 30 s intervals). Long-term changes in E/IPSCs were examined in one cell per slice, and the different experimental conditions were interleaved.
Pharmacology
EPSC recordings in interneurones were done in the presence of bicuculline (10 μm, Sigma, Oakville, Ontario, Canada) and elevated concentrations of Ca2+ and Mg2+ (4 mm each) (Perez et al. 2001). When indicated (2S,2′R,3′R)-2-(2′3-dicarboxycyclopropyl) glycine (DCG-IV, 1 μm) and l-(+)-2-amino-4-phosphonobutyric acid (l-AP4, 100 μm) (Tocris, MO, USA) were also added to the superfusion medium. Compound E/IPSCs were recorded in pyramidal cells in the presence of (+/−)-2-amino-5-phosphonovalerate (AP-5, 50 μm) and (2S)-3-[[(1S)-1-(3,4-dicholorophenyl)ethyl]amino-2-hydroxypropyl] (phenylmethyl)phosphinic acid (CGP 55845; 1 μm; Tocris).
Measurements and statistical analysis
About 20% of recordings (n= 84) met our criteria for interneurone morphology (see below), minimal stimulation, and stability of recordings and were included in the analysis of EPSCs. Responses were analysed off-line using Clampfit (versions 6, 8 or 9; Axon Instruments). E/IPSC amplitude was measured as the peak amplitude minus the average of the baseline points preceding the stimulation artefact. Long-term changes were taken as a significant change (Student's t test) in the amplitude of responses, 30 min postpairing or TBS. Amplitude of average EPSCs (including failures) and EPSCs (excluding failures), failure rate (number of failures as percentage of total number of stimulations) and paired-pulse ratio (PPR; second average EPSC/first average EPSC) were calculated for 4 min bins. The coefficient of variation (CV−2) was calculated according to the equation: CV =σ/M, where M is the mean EPSC amplitude, and σ is the standard deviation of the responses (Malinow & Tsien, 1991). The amplitude of the IPSCs was calculated for 10 min bins. Group comparisons were done using Student's paired t tests, Wilcoxon signed rank test or Student's t tests. The level of significance was set at P < 0.05 and data were expressed as mean ±s.e.m.
Biocytin labelling
In all experiments, biocytin labelling was used to verify the non-pyramidal nature of O/A cells. Following recordings, slices were fixed overnight in 4% paraformaldehyde in 0.1 m phosphate buffer (PB, pH 7.4). Slices were washed in PB, treated with 0.3% H2O2 (30 min) and incubated in avidin–biotin complex (Elite ABC kit; 1: 200, Vector Laboratories; 24 h). The reaction product was visualized using 0.05% 3′3-diaminobenzidine (DAB, Sigma), 0.2% nickel sulphate (Sigma), 0.1 m imidazole and 0.0015% H2O2 in Tris buffer (TB, 0.05 m, pH 7.6). This post hoc morphological identification confirmed that recordings were made from both horizontal and vertical subtypes of interneurones (Perez et al. 2001) (data not shown).
Biocytin labelling and mGluR1a immunofluorescence
Whole (300 μm thick) or resectioned (60 μm thick) slices were placed in 0.3% H2O2, blocked in 10% normal goat serum and incubated in rabbit anti-mGluR1a (1: 500, Diasorin, MI, USA; 24–48 h). Sections were then incubated in goat anti-rabbit IgGs conjugated to Alexa Fluor 488 (1: 200, 1.5 h, Jackson Immunoresearch Laboratories) to reveal mGluR1a labelling, and in rhodamine avidin D (1: 200, Vector Laboratories) to reveal biocytin labelling. Sections were mounted in Prolong Antifade kit (Molecular Probes) and observed with a confocal microscope using appropriate filters.
Hippocampal mGluR1a immunolabelling
Mice were deeply anaesthetized with sodium pentobarbital and perfused with 4% paraformaldehyde in 0.1 m PB. The brain was dissected, postfixed and placed in 30% sucrose (12 h at 4°C). Coronal sections (30–35 μm thick) were cut on a freezing microtome (Leica SM 2000R, Germany) and processed as for double labelling with rabbit anti-mGluR1a (1: 1200, 24 h). Sections were subsequently incubated in biotinylated goat anti-rabbit IgGs (1: 200, 2 h) and in avidin–biotin complex (1: 200, 2 h). The reaction product was visualized using DAB and nickel sulphate.
Results
LTP in O/A interneurones
Minimal stimulation combined with a pairing induction protocol during whole cell recording (Perez et al. 2001) was used to induce LTP directly at excitatory synapses of O/A interneurones in the CA1 region of mouse hippocampal slices (Fig. 1A–C). In C57BL/6 mice, the amplitude of average EPSCs (including failures) was significantly increased to 203 ± 31% of control following the pairing of TBS with postsynaptic depolarization, but was unchanged in untetanized (neither TBS nor postsynaptic depolarization) interneurones (94 ± 19% of control). TBS or postsynaptic depolarization alone did not produce LTP (n= 3 each, data not shown). These results indicate that the pairing of TBS and postsynaptic depolarization is necessary to produce LTP at excitatory synapses of O/A interneurones in mouse hippocampus, extending previous findings obtained in rats (Perez et al. 2001).
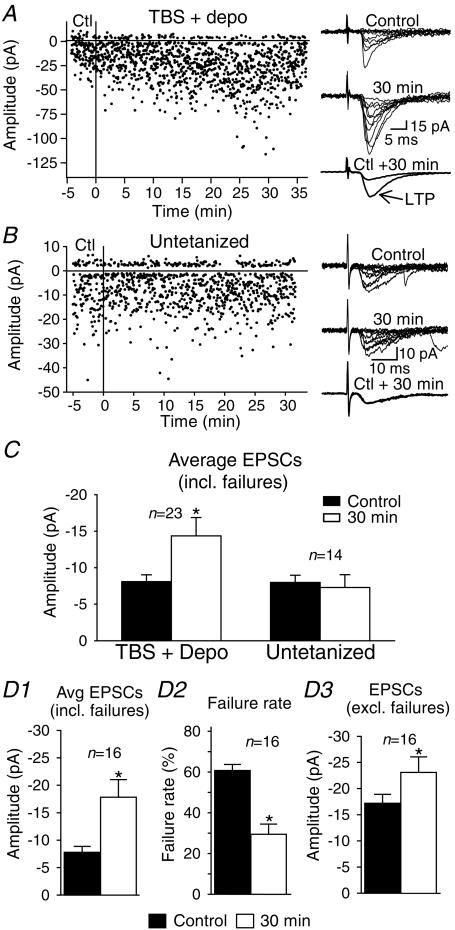
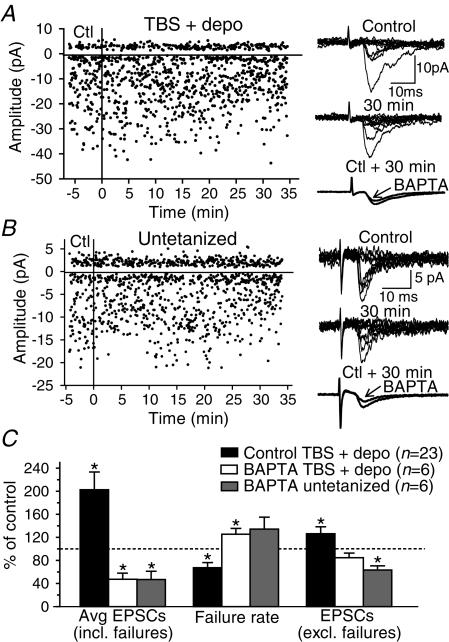
Figure 1. Mechanisms of long-term potentiation expression in stratum oriens/alveus interneurones.
A, graph of EPSC amplitude from a representative interneurone showing long-term potentiation (LTP) after the pairing of theta-burst stimulation (TBS) of afferents with postsynaptic depolarization (TBS + depo) delivered at time = 0 (vertical line). Ten consecutive sample traces are shown to the right for the control (top) and the 30 min period (middle). Superimposed average EPSCs (including failures; n= 128) demonstrate the increase of responses at 30 min postpairing (bottom right). In this cell, LTP results from both an increase in EPSC amplitude (potency) and a decrease in failure rate. B, graph from an untetanized interneurone indicating that test stimuli had no effect on EPSC amplitude. Consecutive traces and superimposed average EPSCs, at right, illustrate the absence of LTP in the untetanized interneurone. C, bar graph for all cells tested, showing the significant increase in amplitude of average EPSCs in tetanized interneurones and the stability of average EPSCs in the absence of tetanization. D, summary bar graphs for interneurones with LTP of average EPSCs (D1), showing that this change was accompanied by a significant decrease in failure rate (D2) and increase in amplitude (D3) of EPSCs. (*P < 0.05.)
Mechanisms of LTP expression
LTP can result from an increase in EPSC amplitude, a decrease in transmission failure, or both. We examined changes in these parameters in cells expressing LTP (n= 16/23, Fig. 1D). For this group of interneurones, a significant increase in amplitude of average EPSCs (including failures) to 257 ± 36% of control was associated with a decrease in failure rate to 47 ± 7% of control and an increase in EPSC amplitude (excluding failures; also called potency) to 144 ± 15% of control. The combined reduction of failure rate and increase of potency was observed in 62.5% of cells expressing LTP, whereas 25.0% displayed solely a significant decrease in failure rate, and 12.5% showed only a significant increase in potency. These data indicate that LTP in O/A interneurones usually arises from changes in both failure rate and EPSC amplitude, but in some instances each change may occur independently.
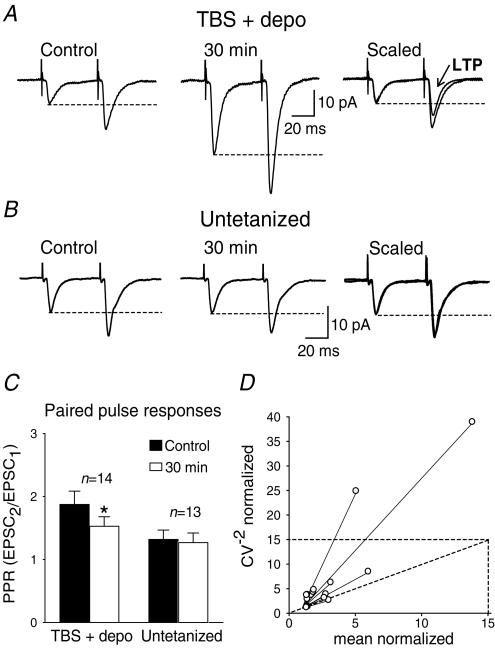
The decrease in failure rate, observed occasionally with no change in EPSC amplitude, suggests the implication of presynaptic mechanisms (Stevens & Wang, 1994). The involvement of presynaptic mechanisms in LTP expression was investigated using paired-pulse stimulation (Fig. 2). In control conditions, average EPSCs (including failures) elicited by the second pulse were facilitated relative to the responses to the first pulse (Ali & Thomson, 1998). The magnitude of paired-pulse facilitation was decreased following the induction of LTP (1.87 ± 0.21 in control versus 1.53 ± 0.15 at 30 min postpairing), whereas it was stable in untetanized interneurones (1.32 ± 0.14 in control versus 1.26 ± 0.15 at 30 min). To examine further the involvement of presynaptic mechanisms, we compared the coefficient of variation (CV−2) of EPSCs before and after LTP (Malinow & Tsien, 1991; Alle et al. 2001). The plot of the CV−2 against the average EPSC amplitude, both normalized to their control values, indicated that the changes in CV−2 were consistent with presynaptic changes (Fig. 2D). These changes in paired-pulse facilitation and coefficient of variation suggest that presynaptic mechanisms are involved in the expression of LTP in O/A interneurones.
Figure 2. Change in paired-pulse facilitation and coefficient of variation during LTP.
A, average EPSCs (including failures) evoked by paired-pulse stimulation during the control (left) and 30 min postpairing (middle) periods in an interneurone showing LTP. At right, traces are scaled to the amplitude of the first responses in control. The dashed lines indicate that the amplitude of the second average EPSC is facilitated relative to the first response. However, paired-pulse facilitation was reduced after LTP. B, paired-pulse facilitation was unchanged in an untetanized cell. C, bar graph of the paired-pulse ratio for all cells tested, showing the significant decrease in paired-pulse facilitation in interneurones with LTP, but not in untetanized interneurones. D, plot of the coefficient of variation (CV−2) against average EPSC amplitude, both normalized to their control values. The location of data points in relation to the identity line is indicative of presynaptic changes during LTP. (*P < 0.05.)
mGluR1 in LTP induction
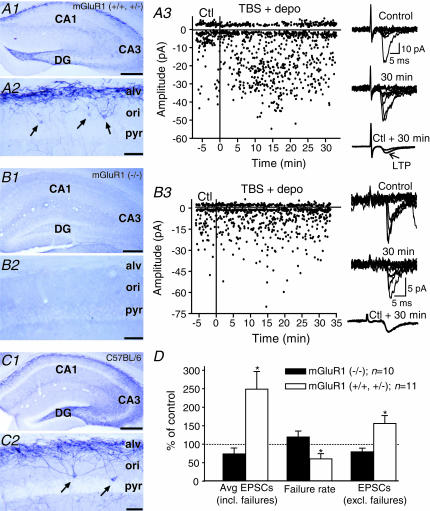
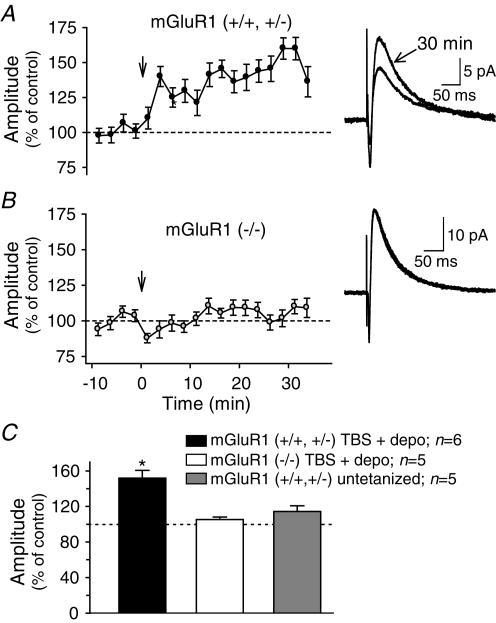
To determine precisely the role of mGluR1 in LTP (Perez et al. 2001), we combined mGluR1a immunocytochemical labelling with the analysis of LTP in normal and transgenic mice with mGluR1 knockout (Fig. 3). In the CA1 region of C57BL/6 mice (Fig. 3C), mGluR1a-immunopositive interneurone somata and dendritic processes were mostly found in stratum oriens and in the alveus as previously reported (Baude et al. 1993). Immunolabelled fine processes were more sparsely observed in stratum radiatum and lacunosum-moleculare. A similar mGluR1a immunocytochemical distribution was found in control littermates (+/+ or +/–) of mGluR1 knockout mice (Fig. 3a). Following the pairing protocol, LTP was observed in 73% of O/A interneurones from mGluR1 control littermates (n= 8/11). For these cells, the potentiation of average EPSCs (249 ± 48% of control, n= 11) was associated with a decrease in failure rate (60 ± 13% of control) and an increase in EPSC amplitude (excluding failures, 156 ± 21% of control) (Fig. 3D). These changes were not different from those in C57BL/6 mice (see Fig. 1) suggesting similar LTP mechanisms in O/A interneurones from control littermates of transgenic mice. In contrast, in mGluR1 (–/–) transgenic mice, mGluR1a-immunolabelling was absent from the hippocampus (Fig. 3b) and, during whole cell recording, the pairing protocol failed to induce LTP in 90%(n= 9/10) of O/A interneurones. In these cells, the amplitude of EPSCs and the failure rates were unchanged postpairing. These results demonstrate the absence of both mGluR1a and LTP in O/A interneurones of mGluR1 knockout mice, and clearly indicate that mGluR1a are necessary for LTP in these cells.
Figure 3. Absence of mGluR1a and LTP in mGluR1 knockout mice.
A1, digital images showing dense mGluR1a-immunolabelling in stratum oriens/alveus (O/A) of the CA1 region in mGluR1 control littermates (+/+ or +/–). A2, higher power image illustrating mGluR1a-immunopositive somata (arrows) and dendritic processes in O/A. A3, graph of EPSC amplitude and sample traces (right) from a representative interneurone of a mGluR1 control littermate showing LTP after the pairing protocol. B1–B2, similar low (B1) and high (B2) power digital images showing the absence of mGluR1a-immunolabelling in mGluR1 (–/–) mice. B3, electrophysiological results from a representative interneurone of a mGluR1 (–/–) mouse indicating the absence of LTP after the pairing protocol. C1–C2, images illustrating that the hippocampal mGluR1a-immunolabelling in C57BL/6 mice was similar to that of mGluR1 littermates (A1–A2). D, bar graphs indicating the presence of LTP (increased average EPSCs, decreased failures and increased EPSCs) in mGluR1 control littermates, and the absence of LTP in mGluR1 (–/–) mice. Scale bars: 300 μm for A1, B1 and C1; 50 μm for A2, B2 and C2. Abbreviations: ori, stratum oriens; alv, alveus; pyr, stratum pyramidale; DG, dentate gyrus. (*P < 0.05.)
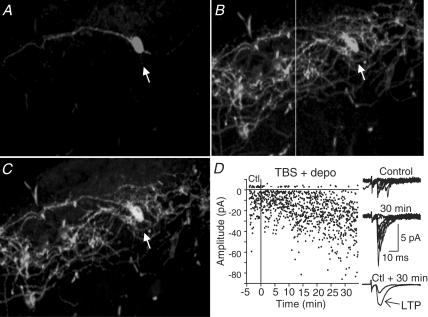
To characterize further the relation between mGluR1a and LTP, we examined the presence of mGluR1a in individual interneurones showing LTP by combining whole cell recordings, biocytin cell labelling and mGluR1a immunofluorescence (Fig. 4). Eighty-three percent of biocytin-filled cells that showed LTP were also mGluR1a-immunopositive (n= 5/6). One interneurone that showed LTP was mGluR1a-immunonegative. Another interneurone which did not show LTP was mGluR1a-immunopositive. These double-labelling experiments demonstrate that the occurrence of LTP is associated with the presence of mGluR1a in the same cells. This immunocytochemical evidence, together with the absence of LTP in mGluR1 knockout mice, clearly indicate that the induction of LTP requires postsynaptic activation of mGluR1a in O/A interneurones.
Figure 4. Presence of mGluR1a in cells with LTP.
A, confocal image of a biocytin-labelled interneurone (arrow) that showed LTP (data in D). B, immunocytochemical labelling for mGluR1a in the same tissue section. C, confocal images are merged to show mGluR1a-immunolabelling of the biocytin-filled interneurone. D, electrophysiological results showing LTP in the biocytin-filled and mGluR1a-positive interneurone illustrated in A–C.
Postsynaptic Ca2+ in LTP induction
Since mGluR1 is linked to intracellular Ca2+, the role of postsynaptic Ca2+ in LTP was explored by including the Ca2+ chelator BAPTA (10 mm) in the whole cell recording solution (Fig. 5). During recordings with BAPTA, the pairing protocol did not produce LTP. At 30 min postpairing, the amplitude of average EPSCs (including failures) was not increased but instead significantly decreased to 47 ± 8% of control. A similar decrease in EPSC amplitude was observed in untetanized interneurones recorded with solution containing BAPTA. Group comparisons showed that BAPTA prevented the long-term increase in amplitude of EPSCs and the decrease in failure rate induced by the pairing protocol in control recordings (Fig. 5). Similar experiments using a lower concentration of BAPTA (5 mm) did not block the induction of LTP; in 5 of 6 cells tested with 5 mm BAPTA, EPSC amplitude increased by 206 ± 31% at 30 min postpairing. These data demonstrate that the induction of LTP requires a postsynaptic increase in intracellular Ca2+ levels in interneurones.
Figure 5. LTP induction requires a postsynaptic Ca2+ rise.
A, graph of EPSC amplitude, sample traces and superimposed average EPSCs (including failures) showing the absence of LTP in an interneurone receiving the pairing protocol with the calcium-chelator BAPTA in the recording electrode. With time, a decrease in EPSC amplitude was noted. B, results from a cell that did not receive the pairing protocol, illustrating a similar decrease in EPSC amplitude caused by BAPTA. C, summary bar graphs for all cells tested with BAPTA (with or without the pairing protocol) and for cells given the pairing protocol with control recording solution (data from TBS + depo group shown in Fig. 1). BAPTA prevented the increase in EPSC amplitude and the decrease in failure rate induced by the pairing protocol in control solution. The decrease in amplitude of average EPSCs was similar in cells with BAPTA that received or did not receive the pairing protocol. (*P < 0.05.)
Differential regulation by presynaptic mGluRs
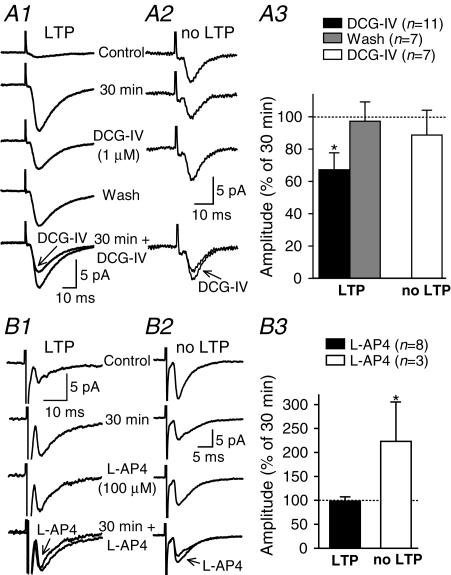
Since we found that LTP is observed in approximately 70–75% of O/A cells tested in control conditions, we postulated that synapses displaying LTP could be pharmacologically distinct from those not showing LTP. Therefore, we examined the modulation of both groups of synaptic responses by agonists of group II (DCG-IV) and group III (l-AP4) mGluRs. In cells that displayed LTP, DCG-IV (1 μm) reversibly reduced the amplitude of average EPSCs (including failures) by 33 ± 10% (Fig. 6a). In contrast, in O/A cells that did not show LTP, DCG-IV had no significant effect on the amplitude of average EPSCs (Fig. 6a), suggesting that group II mGluRs may selectively depress synapses showing LTP in O/A interneurones. Conversely, l-AP4 (100 μm) did not affect the amplitude of average EPSCs (including failures) in cells showing LTP, but produced a significant increase of 123 ± 80% in O/A cells not displaying LTP (Fig. 6b), suggesting that synapses that do not show LTP are specifically modulated by group III mGluRs. This differential sensitivity does not result from changes induced by the tetanic stimulation, since naive synapses were also sensitive to the agonists. In 6 of 7 naive synapses tested, DCG-IV (1 μm) significantly reduced the amplitude of average EPSCs (including failures) by 78 ± 10%. The one naive synapse that did not respond to DCG-IV was sensitive instead to l-AP4 (100 μm), which caused an increase in response to 134% of its initial value. Thus, our results indicate that synapses with and without LTP can be distinguished by their differential sensitivity to agonists of group II and III mGluRs, respectively. This differential pharmacological profile of synaptic responses with or without LTP implies that LTP may be synapse specific in O/A interneurones.
Figure 6. Synapse specificity of LTP: differential sensitivity to groups II and III mGluR agonists.
A, the group II mGluR agonist DCG-IV selectively decreased the amplitude of synaptic responses showing LTP. Average EPSCs (including failures; n= 128) from control, 30 min postpairing, in 1 μm DCG-IV and after washout from representative cells showing (A1) or not showing (A2) LTP. A3, bar graph for all interneurones showing a differential effect of DCG-IV on average EPSCs from cells with and without LTP. B, selective facilitation of synaptic responses not showing LTP by the group III mGluR agonist l-AP4. Average traces (including failures; n= 128) from control, 30 min postpairing and in 100 μml-AP4 from representative cells showing (B1) or not showing (B2) LTP. B3, bar graph for all interneurones showing a significant increase in amplitude of average EPSCs by l-AP4 only for synaptic responses without LTP. (*P < 0.05.)
We also examined whether properties of short-term plasticity differed in synapses with different pharmacological sensitivity. The PPF ratio did not differ significantly in synapses sensitive to DCG-IV versusl-AP4 (P= 0.63, n= 7 and 5, respectively); nor did it differ, when measured during the initial control period, between synapses that subsequently showed or did not show LTP (P= 0.95, n= 23 and 9, respectively). Hence the PPF ratio did not predict whether a particular synapse would be sensitive to DCG-IV or l-AP4, nor whether it would show LTP or not.
mGluR1-dependent potentiation of inhibition
To assess the functional impact of LTP at excitatory synapses in O/A interneurones on the hippocampal network, we examined the effect of the TBS protocol on polysynaptic inhibition of CA1 pyramidal cells in control littermates and mGluR1 transgenic mice (Fig. 7). To prevent both NMDA-dependent plasticity and direct modulation of inhibitory synapses onto pyramidal cells whole cell recordings were obtained in the presence of the NMDAR antagonist AP-5 and the GABAB receptor antagonist CGP 55845, and GTP was omitted from the recording solution. In these conditions, a single shock evoked a fast non-NMDA EPSC followed by a GABAA IPSC in CA1 pyramidal cells (Fig. 7). TBS applied in stratum O/A produced a significant long-term increase in the amplitude of IPSCs in pyramidal cells (152 ± 9% of control at 30 min post-TBS) in control littermates of mGluR1 transgenic mice. EPSC amplitude was unchanged (105 ± 34% of control). In contrast, in pyramidal cells of mGluR1 (–/–) mice, no significant change in IPSC amplitude was seen following TBS (101 ± 2% of control). The long-term increase in IPSC amplitude was also absent in untetanized cells (114 ± 6% of control; Fig. 7C) from mGluR1 control littermates. These data indicate that mGluR1-dependent LTP at excitatory synapses of O/A interneurones can regulate the polysynaptic inhibition of pyramidal cells.
Figure 7. mGluR1-dependent potentiation of polysynaptic inhibition by theta-burst stimulation.
A, graph for all pyramidal cells tested from mGluR1 control littermates showing a long-term increase in IPSC amplitude following theta-burst stimulation (TBS; arrow). Superimposed average traces (right panel; n= 21) in a representative cell show the increase in IPSC at 30 min post-TBS. B, results for pyramidal cells from mGluR1 (–/–) transgenic mice illustrating the absence of a long-term increase in IPSC amplitude following TBS in these animals. C, summary bar graph for all groups showing the significant long-term increase in IPSC amplitude (30 min post-TBS) in cells from mGluR1 control littermates, but not in pyramidal cells from mGluR1 (–/–) mice nor in untetanized cells of mGluR1 control littermates. (*P < 0.05.)
Discussion
Our results indicate that a hebbian form of LTP was present at excitatory synapses of O/A interneurones in mouse CA1 hippocampus. The induction of this LTP depended on the activation of postsynaptic mGluR1a and a rise in postsynaptic Ca2+ levels. The expression of LTP involved presynaptic mechanisms and was associated with a decrease in transmission failure rate and an increase in EPSC amplitude. Moreover, this LTP was synapse specific, selectively occurring at synapses that can be modulated by presynaptic group II, but not by group III, mGluRs. Finally, a similar stimulation protocol (TBS) given in O/A induced a long-term increase in polysynaptic inhibition of CA1 pyramidal cells, which was absent in mGluR1 knockout mice, suggesting that mGluR1-dependent LTP in interneurones can adaptively upregulate pyramidal cell inhibition.
LTP induction
The experimental protocol used allowed us to characterize synaptic plasticity directly at interneurone synapses, confirming in mice the requirement of pairing synaptic stimulation and postsynaptic depolarization to induce LTP in O/A interneurones (Perez et al. 2001). Moreover, the absence of LTP in interneurones of mGluR1 (–/–) mice, combined with the correlated presence of postsynaptic mGluR1a and LTP in individual cells, provide clear evidence that the activation of postsynaptic mGluR1a during the pairing protocol is necessary for LTP induction. These data are consistent with previous pharmacological evidence that LTP in O/A interneurones was blocked by a mGluR1a specific antagonist (Perez et al. 2001). Overall, these data suggest that the high level of expression of mGluR1a in O/A interneurones (Baude et al. 1993; Freund & Buzsáki, 1996; Luján et al. 1996; Shigemoto et al. 1997) likely explains the cell-type specific presence of LTP in CA1 interneurones (Perez et al. 2001).
The signalling cascade involved in the induction of mGluR1-dependent LTP required a postsynaptic intracellular Ca2+ rise, since BAPTA prevented LTP. A similar Ca2+ dependence was previously reported for tetanus-induced LTP of population EPSCs in O/A interneurones (Ouardouz & Lacaille, 1995). Activation of group I/II mGluRs in O/A interneurones has been linked to increases in intracellular Ca2+ via the coupling of Ca2+ influx through voltage-dependent Ca2+ channels and release from ryanodine-sensitive intracellular stores (Woodhall et al. 1999; Gee et al. 2001). Together these findings suggest that the hebbian feature of LTP induction in O/A interneurones arises from the requirement for coincident activation of mGluR1a and voltage-dependent Ca2+ channels to trigger the postsynaptic Ca2+ signalling cascade. This novel induction mechanism is different from those involved in long-term increases at other hippocampal interneurone synapses which are dependent on either postsynaptic activation of protein kinase C (Alle et al. 2001), or postsynaptic excitability changes linked to Na+, K+-ATPase (Ross & Soltesz, 2001). In addition, since we observed that buffering postsynaptic Ca2+ with 10 mm BAPTA resulted in a decrease in EPSC amplitude over time in untetanized cells, basal synaptic transmission might also be regulated by postsynaptic Ca2+ in O/A interneurones.
Presynaptic expression
LTP of average EPSCs was predominantly associated with an increase in EPSC amplitude (potency) and a decrease in transmission failures. In some cells, however, a decrease in transmission failures was solely involved. Coupled to the observations that the paired-pulse ratio was decreased and that the coefficient of variation (CV−2) increased during LTP, these results suggest that presynaptic mechanisms contribute to LTP expression (Malinow & Tsien, 1991; Manabe et al. 1993; Sokolov et al. 1998; Alle et al. 2001). Our results do not rule out, however, that some postsynaptic changes, such as the activation of silent synapses (Isaac et al. 1995; Liao et al. 1995), might also contribute to LTP expression. Notwithstanding this, LTP in O/A interneurones thus appears to involve postsynaptic induction and presynaptic expression mechanisms. A similar pattern of induction/expression mechanisms has been reported for LTP at mossy fibre–interneurone cell synapses (Alle et al. 2001), suggesting that these may represent common features of LTP at interneurone synapses. Such properties are in contrast to those of NMDA-dependent LTP at the Schaffer collateral–CA1 pyramidal cell synapses which implicate postsynaptic induction and expression mechanisms (Malenka & Nicoll, 1999). These different properties of LTP in interneurones provide further evidence that long-term plasticity is a genuine property of some interneurone synapses, and not simply due to passive propagation of plasticity from projection cells (McBain et al. 1999).
Synapse-specific plasticity
The presence/absence of LTP in O/A interneurones was associated with a differential sensitivity of synaptic responses to group II and III mGluR agonists. The group II mGluR agonist DCG-IV decreased the amplitude of EPSCs that showed LTP, and conversely the group III mGluR agonist l-AP4 increased the amplitude of EPSCs that did not show LTP. Consistent with our results, immunohistochemical labelling of group II mGluRs (mGluR2 and 3) has been reported mainly in unmyelinated axons in CA1 alveus (Petralia et al. 1996), and that of group III mGluRs predominantly on axon terminals in stratum oriens (Shigemoto et al. 1997). Although the activation of presynaptic group III mGluRs (mGluR4, 6, 7 and 8) results in the inhibition of glutamate release in many cell types (Manzoni et al. 1995; Macek et al. 1996), consistent with our observations, the activation of mGluR4a by l-AP4 was recently reported to increase glutamatergic transmission in entorhinal cortex (Evans et al. 2001). Our results showing a differential regulation by presynaptic group II mGluRs of synapses capable of undergoing LTP and by presynaptic group III mGluRs of synapses that do not show LTP, suggest that LTP may be a property that is synapse specific in O/A interneurones. Hence, LTP may be both cell-type (Perez et al. 2001) and synapse specific in CA1 interneurones. The synapse-specific presynaptic depression by group II mGluRs is analogous to the pathway-specific group II mGluR modulation of mossy fibre, but not CA3 recurrent collateral, synapses onto CA3 interneurones (Tóth & McBain, 1998) and pyramidal cells (Kamiya et al. 1996). Interestingly, this pathway-specific regulation by mGluRs is also associated with a pathway-specific long-term depression in CA3 interneurones (Tóth et al. 2000). It is possible that the synapse specificity of LTP in O/A interneurones is similarly due to different afferent pathways onto these cells; however, this issue remains to be clarified by further experiments.
Functional implications
Previous work has indicated that O/A interneurones are involved in recurrent inhibition of pyramidal cells (Lacaille et al. 1987; Blasco-Ibanez & Freund, 1995). Modelling studies have postulated that the long-term increase in synaptic efficacy at excitatory synapses of inhibitory interneurones, and a consequent enhancement of inhibition of pyramidal cells, play a critical role in information processing and synchronization of pyramidal cells (Grunze et al. 1996; Bibbig et al. 2001). Our findings that the TBS protocol produces a long-lasting increase in polysynaptic IPSC amplitude in pyramidal cells, that is absent in mGluR1 (–/–) mice, clearly indicate that mGluR1-dependent LTP at O/A interneurone excitatory synapses may adaptively regulate pyramidal cell inhibition. Thus, the synapse-specific, mGluR1-dependent and hebbian LTP described here provides a biological substrate for the interneurone plasticity postulated in modelling studies (Grunze et al. 1996; Bibbig et al. 2001). This novel form of interneurone plasticity may therefore be critical for adaptively adjusting the excitability and synchrony of CA1 pyramidal cells.
Acknowledgments
This research was supported by grants from the Canadian Institutes for Health Research (J.-C.L), the Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (J.-C.L.), the Fonds de la Recherche en Santé du Québec (J.-C.L. and V.L.) and the Human Frontier Science Program Organization (J.-C.L.) J.-C.L. is the holder of a Canada Research Chair in Cellular and Molecular Neurophysiology. A.C. and S.R. were supported by postdoctoral fellowships from the Centre de Recherche en Sciences Neurologiques and the Savoy Foundation. The authors thank Dr Patrice Congar for helpful comments on the manuscript.
References
- Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. J Physiol. 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci U S A. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at the perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bibbig A, Faulkner HJ, Whittington MA, Traub RD. Self-organized synaptic plasticity contributes to the shaping of γ and β oscillations In Vitro. J Neurosci. 2001;21:9053–9067. doi: 10.1523/JNEUROSCI.21-22-09053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur J Neurosci. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Condé F, Collingridge GL, Crépel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Cowan AI, Stricker C, Reece LJ, Redman SJ. Long-term plasticity at excitatory synapses on aspinous interneurons in area CA1 lacks synaptic specificity. J Neurophysiol. 1998;79:13–20. doi: 10.1152/jn.1998.79.1.13. [DOI] [PubMed] [Google Scholar]
- Evans DI, Jones RS, Woodhall G. Differential actions of PKA and PKC in the regulation of glutamate release by group III mGluRs in the entorhinal cortex. J Neurophysiol. 2001;85:571–579. doi: 10.1152/jn.2001.85.2.571. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gee CE, Woodhall G, Lacaille J-C. Synaptically activated calcium responses in dendrites of hippocampal oriens-alveus interneurons. J Neurophysiol. 2001;85:1603–1613. doi: 10.1152/jn.2001.85.4.1603. [DOI] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fiber synapses. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J-C, Muller A, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: Joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Luján R, Nusser Z, Roberts JDB, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends Neurosci. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- Macek TA, Winder DG, Gereau RW, Ladd CO, Conn PJ. Differential involvement of group II and group III mGluRs as autoreceptors at lateral and medial perforant path synapses. J Neurosci. 1996;76:3798–3806. doi: 10.1152/jn.1996.76.6.3798. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation: a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Tsien RW. Long-term potentiation: postsynaptic activation of Ca2+-dependent protein kinases with subsequent presynaptic enhancement. Prog Brain Res. 1991;89:271–289. doi: 10.1016/s0079-6123(08)61728-8. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired-pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Castillo PE, Nicoll RA. Pharmacology of metabotropic glutamate receptors at the mossy fiber synapses of the guinea pig hippocampus. Neuropharmacol. 1995;34:965–971. doi: 10.1016/0028-3908(95)00060-j. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Lacaille J-C. Mechanisms of selective long-term potentiation of excitatory synapses in stratum oriens/alveus interneurons of rat hippocampal slices. J Neurophysiol. 1995;73:810–819. doi: 10.1152/jn.1995.73.2.810. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille J-C. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang Y-X, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Raastad M. Extracellular activation of unitary excitatory synapses between hippocampal CA3 and CA1 pyramidal cells. Eur J Neurosci. 1995;7:1882–1888. doi: 10.1111/j.1460-9568.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Ross ST, Soltesz I. Long-term plasticity in interneurons of the dentate gyrus. Proc Natl Acad Sci U S A. 2001;98:8874–8879. doi: 10.1073/pnas.141042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov MV, Rossokhin AV, Behnisch T, Reymann KG, Voronin LL. Interaction between paired-pulse facilitation and long-term potentiation of minimal excitatory postsynaptic potentials in rat hippocampal slices: a patch-clamp study. Neuroscience. 1998;85:1–13. doi: 10.1016/s0306-4522(97)00592-7. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Tóth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nature Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Tóth K, McBain CJ. Target-specific expression of pre- and postsynaptic mechanisms. J Physiol. 2000;525:41–51. doi: 10.1111/j.1469-7793.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Gee CE, Robitaille R, Lacaille J-C. Membrane potential and intracellular Ca2+ oscillations activated by mGluRs in hippocampal stratum oriens/alveus interneurons. J Neurophysiol. 1999;81:371–382. doi: 10.1152/jn.1999.81.1.371. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sík A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]