Abstract
In Bacillus subtilis, an export-import pathway regulates production of the Phr pentapeptide inhibitors of Rap proteins. Processing of the Phr precursor proteins into the active pentapeptide form is a key event in the initiation of sporulation and competence development. The PhrA (ARNQT) and PhrE (SRNVT) peptides inhibit the RapA and RapE phosphatases, respectively, whose activity is directed toward the Spo0F∼P intermediate response regulator of the sporulation phosphorelay. The PhrC (ERGMT) peptide inhibits the RapC protein acting on the ComA response regulator for competence with regard to DNA transformation. The structural organization of PhrA, PhrE, and PhrC suggested a role for type I signal peptidases in the processing of the Phr preinhibitor, encoded by the phr genes, into the proinhibitor form. The proinhibitor was then postulated to be cleaved to the active pentapeptide inhibitor by an additional enzyme. In this report, we provide evidence that Phr preinhibitor proteins are subject to only one processing event at the peptide bond on the amino-terminal end of the pentapeptide. This processing event is most likely independent of type I signal peptidase activity. In vivo and in vitro analyses indicate that none of the five signal peptidases of B. subtilis (SipS, SipT, SipU, SipV, and SipW) are indispensable for Phr processing. However, we show that SipV and SipT have a previously undescribed role in sporulation, competence, and cell growth.
The phosphorelay signal transduction system for sporulation initiation in Bacillus subtilis is the premier example of a complex regulatory pathway governing a physiological developmental process of a relatively simple organism (16). In the phosphorelay, the positive inputs brought about by multiple protein histidine kinases (KinA to KinE) (19, 22, 24, 57) in response to a variety of metabolic, environmental, and cell cycle signals are counteracted by numerous negative regulatory mechanisms activated by another set of signals (3, 35, 43, 63). These negative signals generally interpret physiological conditions antithetical to sporulation, and thus they reset the system toward vegetative growth.
Protein phosphatases acting on the response regulator components of the phosphorelay play a major role in negatively regulating the sporulation system. The level of phosphorylation of the intermediate response regulator Spo0F is affected by the RapA, RapB, and RapE members of the Rap family of proteins (18, 39). The Spo0A∼P response regulator and major transcription factor for sporulation initiation is dephosphorylated by the three members of the Spo0E family of phosphatases, namely Spo0E, YisI, and YnzD (29, 37). The activities of Rap and Spo0E phosphatases are highly specific toward the corresponding target proteins, Spo0F∼P and Spo0A∼P, respectively.
Transcription of the genes encoding the Rap or Spo0E proteins is under control of physiological conditions such as competence for DNA transformation (RapA and RapE), vegetative growth (RapB, YisI, and YnzD), and transition phase (Spo0E) that are alternative to sporulation (18, 27, 29, 39, 41). Both expression and activation of the Rap or Spo0E phosphatases are entry points for signals to inhibit the initiation of the sporulation process. Rap phosphatase activity is directly subject to the inhibitory effect of a pentapeptide generated from the complex export-import processing pathway of the Phr proteins.
The inhibitor of RapA is the PhrA pentapeptide, NH2-ARNQT-COOH, which is generated from the carboxy-terminal end of the 44-amino-acid product of the phrA gene. The deduced amino acid sequence of this preprotein precursor is suggestive of a protein subject to export through the SecA-dependent system because of the presence of a typical Sec type amino-terminal signal peptide (10, 53). Additionally, a putative type I signal peptidase (type I SPase) cleavage site was identified between the amino-terminal hydrophobic signal peptide and the carboxy-terminal hydrophilic 19 amino acid residues (34, 42). These observations suggested the hypothesis that the production of the carboxy-terminal pentapeptide inhibitor was the result of two processing events: the first by means of type I SPase during or after the export of the 44-amino-acid preprotein that would result in a 19-amino-acid peptide proinhibitor and the second carried out by an unknown enzyme on the proinhibitor to generate the pentapeptide that is then reimported by the oligopeptide permease system (15, 40, 45).
A similar processing pathway was proposed for the production of the PhrC pentapeptide inhibitor, NH2-ERGMT-COOH, from the 40-amino-acid product of the phrC gene (23, 25, 38). For the production of the PhrE pentapeptide inhibitor, NH2-SRNVT-COOH, from the 44-amino-acid precursor encoded by the phrE gene, an additional processing event was proposed to occur at the C end of the threonine residue because of the position of the active inhibitor 9 amino acids in from the carboxy-terminal end (18).
Type I SPases are integral components of the SecA-dependent secretory machinery and function by removing signal peptides from secretory preproteins during or shortly after translocation. This event is required for the release of the mature proteins from the membrane (10). Five type I SPase-encoding genes have been identified on the B. subtilis chromosome (sipS, sipT, sipU, sipV, and sipW) (54, 55). Like that in Escherichia coli, Staphylococcus aureus, and Saccharomyces cerevisiae (2, 9, 11, 12), SPase activity is required for cell viability in B. subtilis as the presence of either SipS or SipT is essential (55). B. subtilis SPases were shown to have largely overlapping substrate specificities, with the only example of distinct substrate specificity being the processing of the TasA and YqxM preproteins by SipW (51, 55, 56).
In this study, we analyzed the role of type I SPases in Phr peptide processing and identified the sites of cleavage that are essential for the production of the active PhrA, PhrC, and PhrE pentapeptides. Additionally, we observed that the SipT and SipV SPases are essential for the initiation of the sporulation process in B. subtilis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. subtilis strains used in this study are shown in Table 1. Strains JH11842 (phrA::aphIII) and JH11969 (phrE::aphIII) were constructed by transforming competent cells of strain JH642 with linearized plasmids pMP9257 and pSK48, respectively. Transformants were selected on the basis of kanamycin resistance and screened for sporulation deficiency. Strains JH22097 (sipT::cat) and JH22098 (sipV::aphIII) were also constructed by double crossover integration of a linearized plasmid carrying a chromosomal fragment with the chloramphenicol resistance cassette in the sipT coding gene and the kanamycin resistance cassette in the sipV gene, respectively.
TABLE 1.
B. subtilis strains used in this study
| Straina | Relevant genotype |
|---|---|
| JH642 | Wild type |
| JH12834 | rapA::Tn917 erm |
| JH11435 | spo0F (Y13S) |
| JH11842 | phrA::aphIII |
| JH11969 | phrE::aphIII |
| JH22097 | sipT::cat |
| JH22098 | sipV::aphIII |
| JH22108 | sipT::cat sipV::aphIII |
| JH11783 | sipT::cat sipV::aphIII rapA::Tn917 erm |
| JH11784 | sipT::cat sipV::aphIII spo0F (Y13S) |
| JH12981 | amyE::(rapA′-lacZ aphIII) |
| JH23059 | phrC (T35P) cat amyE::(rapA′-lacZ aphIII) |
| JH23060 | phrC (T35A) cat amyE::(rapA′-lacZ aphIII) |
All strains are derivatives of JH642 and thus carry the trpC2 and phe-1 auxotrophic markers.
Cells were grown in Schaeffer's sporulation medium (46) or Penassay broth (Difco antibiotic medium 3) with the appropriate antibiotic at the following concentrations: chloramphenicol, 5 μg/ml; kanamycin, 2 μg/ml; and erythromycin, 1 μg/ml. The efficiency of sporulation was assayed by growing cells in 5 ml of Schaeffer's sporulation medium for 24 h. Serial dilutions were then plated onto Schaeffer's agar plates before and after treatment with CHCl3 in order to obtain viable cell counts and spore counts.
E. coli DH5α and TB1 (Stratagene) were used for plasmid construction and propagation. Luria-Bertani (LB) medium was used for growth of E. coli cultures and was supplemented with the appropriate antibiotic at the following concentrations: chloramphenicol, 7 μg/ml; ampicillin, 100 μg/ml; and kanamycin, 20 μg/ml. Growth and β-galactosidase assays were carried out as previously described (13). Activity was expressed in Miller units (26).
Plasmid construction.
Site-directed mutagenesis on the phrA gene was carried out with a derivative of the pJM105A vector (pBluescript KS+ carrying the cat gene; Stratagene) (33) containing a 728-bp BglII-BamHI fragment from position 1,315,805 to 1,316,533 in the B. subtilis genome (http://genolist.Pasteur.fr). This fragment contains the 3′-end half of the rapA gene and the entire phrA gene (fragment 1 in Fig. 1A). Transformation of B. subtilis competent cells and selection for chloramphenicol resistance result in a single crossover integration event that may place the mutation in the gene copy that will be transcribed or in the duplicated copy lacking the promoter sequence. The correctness of the integration events was checked by amplifying a diagnostic fragment via PCR and subjecting the product to sequence analysis.
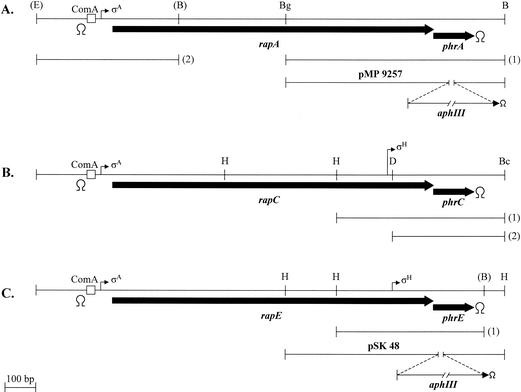
FIG. 1.
Schematic representation of the rapA-phrA (A), rapC-phrC (B), and rapE-phrE (C) loci. The thick arrows indicate the extents of the open reading frames. Thin arrows indicate the positions of the transcriptional promoters present in each locus with the corresponding sigma factors. The positions of the consensus sequences for binding of the ComA response regulator are indicated by boxes. Relevant restriction sites are indicated in parentheses if they were generated through a PCR amplification reaction and without parentheses if they exist on the B. subtilis chromosome. Restriction site symbols: E, EcoRI; B, BamHI; Bg, BglII; H, HindIII; D, DraI; Bc, BclI. The lengths of the fragments used in this study for site-directed mutagenesis are shown by the lines labeled with a number in parentheses. Transcription terminators are indicated by the symbol Ω.
Since some of the phrA deletion mutations were also mutating the end of the rapA gene corresponding to the carboxy terminus of the gene product in such a way that an inactive RapA protein would be generated upon integration by a single crossover, a different integration strategy was employed for testing some of the constructs. The 728-bp fragment carrying the wild-type or the mutated phrA gene (fragment 1 in Fig. 1A) was fused to a 500-bp fragment containing the promoter region and the 5′ end of the rapA gene (fragment 2 in Fig. 1A). The fusion results in an in-frame deletion of the rapA gene, thus generating an operon expressing a RapA deletion protein (lacking 135 central amino acids) and the PhrA protein. These constructs were cloned into plasmid pJM116 (33) which, upon linearization with PstI and transformation of B. subtilis competent cells, integrates into the chromosome at the amyE gene and confers chloramphenicol resistance.
Site-directed mutagenesis on the phrC gene was carried out by using a derivative of pJM105A containing a 550-bp HindIII-BclI fragment spanning from position 429,240 to position 429,790 in the B. subtilis genome (fragment 1 in Fig. 1B). The plasmids containing the desired mutations and wild-type sequence were then digested with DraI and HindIII in order to separate the phrC coding sequence from its SigH-dependent promoter. The resulting 370-bp fragment was cloned into the integrative vector pJM103 (33) digested with SmaI and HindIII (fragment 2 in Fig. 1B). These plasmids were also used for transformation of B. subtilis competent cells, followed by selection for chloramphenicol resistance, and the correctness of the recombination event was tested by diagnostic PCR and sequencing analysis of the amplification product.
Site-directed mutagenesis on the phrE gene was carried out by using a derivative of pJM105A carrying a 527-bp fragment spanning from position 2,659,215 to position 2,659,742 in the B. subtilis genome (fragment 1 in Fig. 1C). The fragments containing the desired mutations were then recovered as HindIII-BamHI fragments and transferred to the pJM116 vector in order to integrate them at the amyE locus and test their complementation activities in trans.
Every construct was fully sequenced in order to ensure the absence of unwanted mutations.
Protein expression.
The pMAL system (New England Biolabs) was used to construct plasmids pMal-SipS and pMal-SipV. The sipS and sipV genes were amplified from B. subtilis JH642 chromosomal DNA by using oligonucleotides that modified the coding sequences to carry a KpnI site at the 5′ end and a BamHI site downstream of the stop codon at the 3′ end. After restriction digestion, the amplified fragments were cloned into the KpnI-BamHI sites of pMALc2E, which resulted in placement of the sip genes into the same translational reading frame as the malE gene. These constructions resulted in the replacement of the initial methionine residue encoded by the sip genes with a proline residue and the incorporation of a valine residue between the enterokinase cleavage site of the vector and the replacement proline.
A PCR-amplified fragment containing codons 37 to 193 of the sipT gene was cloned into the NdeI-BamHI sites of the pET28a expression vector (Novagen), thus creating a fusion at the 5′ end with six histidine codons. The plasmid was named pET28-SipT.
For the construction of pET20-PhrA-AmyE, the phrA coding sequence was amplified from the chromosome of strain JH642 by using oligonucleotides that introduced an NdeI site at the 5′ end and XhoI at the 3′ end that replaced the stop codon. The fragment was cloned into the pET20 vector (Novagen). Oligonucleotides were also designed to amplify the DNA fragment from JH642 chromosomal DNA encoding the mature part of the AmyE protein (codons 34 to 660). The oligonucleotides modified the coding sequence to carry 5′ and 3′ XhoI sites so that the fragment could be cloned into the XhoI site of plasmid pET20-phrA, thus generating the fusion. The cloning strategy resulted in the insertion of six base pairs, CTCGAG, between the phrA and amyE coding sequences. The correctness of the cloning was checked by restriction analysis and nucleotide sequencing.
Plasmid pETON2 (kindly provided by Ross Dalbey) was used for the expression of the pro-OmpA-nuclease A (PONA) substrate (52) modified to contain a six-His extension at the C-terminal end (4). The purification of PONA was carried out as described by Chatterjee et al. and Carlos et al. (4, 5).
The synthetic 44-amino-acid PhrA protein was obtained from United Biochemical Research, Inc. (Seattle, Wash.). The peptide was resuspended in 100% dimethyl sulfoxide (DMSO).
Protein purification.
The fusion proteins MalE-SipS and MalE-SipV were purified by using the conditions previously described by Carlos et al. for MalE-SipS (4) with the following modifications: E. coli TB1 cells carrying the pMal-Sip vectors were grown in LB medium containing 100 μg of ampicillin/ml and 0.2% glucose. Additionally, a 55% ammonium sulfate cut was performed on the cell lysate before loading it onto the amylose resin affinity column (New England Biolabs).
The purification of His-SipT was based on a method previously described by van Roosmalen et al. (60). E. coli BL21 (DE3) cells (Novagen) carrying plasmid pET28-SipT were picked from a fresh transformation plate and used to inoculate 2 liters of LB medium containing kanamycin (30 μg/ml). Cells were grown at 37°C and 220 rpm until an optical density at 600 nm of ∼0.6 was reached, at which point IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM final concentration) was added. The cells were grown for a further 2 h before they were harvested by centrifugation and stored at −20°C. The cell pellets were thawed on ice in cold lysis buffer (50 mM Tris-HCl [pH 8.0], 300 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 5 mM imidazole) and then passed through a French pressure cell three times. Cellular debris was removed from the extract by ultracentrifugation at 157,000 × g, and the resulting cell lysate was applied to a precharged nickel affinity column (Novagen). The resin was washed with lysis buffer containing 5 and 20 mM imidazole, and the His-SipT protein was eluted from the column by using an imidazole gradient (50 to 200 mM) made in lysis buffer. Fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and pure fractions were pooled and dialyzed (50 mM HEPES [pH 8], 50 mM NaCl, 0.1 mM EDTA, 1 mM dithiothreitol). The protein was concentrated, and 20% glycerol (final concentration) was added before the protein was stored at −80°C.
For the purification of PhrA-AmyE-His6 (PAH), E. coli expression strain BLR (DE3)/pLysS (Novagen) was transformed with plasmid pET20-PhrA-AmyE. Transformants were scraped from the plate and used to inoculate 4 liters of LB medium supplemented with ampicillin. The cultures were grown at 37°C until an optical density of ∼0.6 was reached, and IPTG (1 mM final concentration) was added. Growth was allowed to continue for a further 2 h before the cells were harvested by centrifugation and stored at −80°C. PAH was purified from the cells by using the “denaturing purification of insoluble proteins” protocol outlined in the QiaExpressionist handbook supplied with Qiagen's Ni-NTA (nitrilotriacetic acid) Superflow resin. Steps involving buffer B and buffer F were eliminated from the purification process. Fractions were analyzed by SDS-PAGE, and the purest fractions were pooled. Refolding was carried out in buffer containing 20 mM Tris-HCl (pH 8) and 0.5% Triton X-100 while guanidine concentrations were reduced from 6 to 1 M. As guanidine was decreased from 1.0 to 0 M, the concentration of Triton X-100 in the buffer was increased to 2%. Residual unfolded protein was eliminated by centrifugation, and clear supernatant was stored at −20°C.
Cleavage reactions.
PONA, PhrA, or PAH was incubated in the presence of SipS, SipT, or SipV for the times indicated in the figures. The reactions were carried out at 37°C in buffer containing 50 mM Tris-HCl (pH 8), 10 mM CaCl2, 1% Triton X-100, and 10% DMSO. When the reaction was carried out at pH 10, CAPS buffer was substituted for Tris-HCl buffer. When the reaction was carried out in the presence of the lipids, either phosphatidylethanolamine (Sigma), cardiolipin (Sigma), or lipid extract prepared from B. subtilis (1) was added. When the B. subtilis lipid extract was used, 10 μl was dried onto the bottom of the reaction tube and then DMSO and PONA, PhrA, or PAH were added for 10 min at room temperature before the remaining components were added. The cleavage reaction was stopped by the addition of sample buffer followed by freezing of the mixture in a dry ice ethanol bath. The reaction products were analyzed by SDS-PAGE by using Tris-HCl-Tricine buffer at pH 8.45 and acrylamide at 10 or 18% in the separating gel, 10% in the spacer gel, and 4% in the stacking gel as described by Schägger and von Jagow (47).
RESULTS
PhrA cleavage site.
Previous studies suggested that the 44-amino-acid product of the phrA gene was an exported protein because of the presence of a typical amino-terminal signal peptide spanning from residues 1 to 25 (Fig. 2). The peptide bond between the alanine residue at position 25 and the glycine at position 26 (encompassed by amino acid sequence VHA-GE, in which the hyphen represents the bond) was defined by the SignalP prediction server (www.cbs.dtu.dk/service/SignalP) (28) as the putative site of cleavage by gram-positive type I SPases. We examined the relevance of this prediction with site-directed mutagenesis by replacing Ala25 with a proline residue. Because the residue in position −3 (relative to the type I SPase cleavage site) is known to be part of the enzyme recognition site, the valine at position 23 was also mutated to proline. Proline was chosen because it is known from results of previous studies to inhibit SPase cleavage of some substrates (14, 20). Leucine was chosen because it is one of the most common residues at position −2 (20).
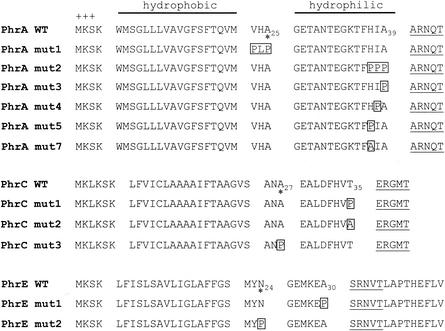
FIG. 2.
Phr peptide cleavage sites. The amino acid sequences of the wild-type (WT) PhrA, PhrC, and PhrE peptides are shown with those of their mutant derivatives generated by site-directed mutagenesis. The positions of residues relevant to this study are indicated by subscript numbers. The mutated residues are boxed. The active pentapeptide inhibitors are underlined. The domain organization of Phr proteins includes the positively charged amino-terminal residues (+++) and the hydrophobic and the hydrophilic regions. Asterisks indicate the putative SPase cleavage sites as identified by the program SignalP (28).
The plasmid carrying the phrA gene expressing the mutated PhrA protein, PhrA-mut1 (Fig. 1 and 2), was introduced into the B. subtilis strain JH11842 carrying a deletion of the wild-type phrA gene (plasmid pMP9257) (Fig. 1). The sporulation phenotypes of the transformants obtained were analyzed on Schaeffer's agar plates. Strain JH11842 is sporulation deficient due to the lack of PhrA peptide and, as a consequence, deregulation of RapA phosphatase activity. Expression of the PhrA-mut1 protein in JH11842 restored a sporulation-proficient phenotype, indicating that the VHA-PLP replacement did not affect the processing pathway that generates the PhrA pentapeptide (Table 2).
TABLE 2.
Efficiency of sporulation of B. subtilis strains carrying mutations in the PhrA cleavage site
| Straina | Relevant genotype | Viable cell count ml−1 | Spore count ml−1 | Percentagec |
|---|---|---|---|---|
| JH642 | Wild type | 2.2 × 108 | 1.1 × 108 | 50 |
| JH11842 | phrA::aphIII | 2.4 × 108 | 1.2 × 107 | 5 |
| JH23043b | phrA wild type | 2.4 × 108 | 1.3 × 108 | 54.1 |
| JH23044b | phrA mutant 1 | 1.6 × 108 | 7.7 × 107 | 48.1 |
| JH23045b | phrA mutant 2 | 2.6 × 108 | 8.3 × 106 | 3.2 |
| JH23046b | phrA mutant 3 | 2.6 × 108 | 7.7 × 106 | 2.9 |
| JH23047b | phrA mutant 4 | 2.1 × 108 | 1.2 × 108 | 57.1 |
| JH23048b | phrA mutant 5 | 3.1 × 108 | 1.3 × 108 | 41.9 |
| JH23038b | phrA mutant 7 | 3.9 × 108 | 1.5 × 108 | 38.5 |
Strains were grown in Schaeffer's sporulation medium for 24 h. The data reported are representative of results from two independent experiments.
Genotype data for these strains are in reference to the phrA gene carried by the integrated vector. The amino acid sequence of each mutant PhrA protein is reported in Fig. 2.
The percentage is calculated as the ratio between spore count and viable cell count.
We then analyzed the amino acid sequence of the PhrA-mut1 protein and realized that a possible SPase cleavage site could be identified between the alanine residues at positions 39 and 40 (HIA-AR). Thus, the HIA residues at positions 37, 38, and 39, respectively, were mutated into proline residues, singly and in combination. The triple mutation resulting in HIA-PPP (PhrA-mut2) and the single mutation A39P (PhrA-mut3) did not restore a sporulation-proficient phenotype upon expression in the phrA mutant strain JH11842. On the contrary, the I38P and H37P mutations (resulting in PhrA-mut4 and PhrA-mut5, respectively) (Fig. 2) induced sporulation of the phrA mutant strain (Table 2). These results indicate that the alanine residue at position 39 is critical for the correct processing to generate the terminal pentapeptide inhibitor. Since the sporulation efficiency of the strain carrying the PhrA-mut5 mutant protein (H37P) was slightly lower than that of the wild-type strain, we further analyzed any possible role in processing for the histidine at position 37 (position −3 from the cleavage site). This residue was replaced by alanine (PhrA-mut7) (Fig. 2), and sporulation assays were carried out on the strain expressing this mutant PhrA protein. The results consistently indicated a reduction in sporulation efficiency compared to that of the wild-type strain (Table 2), suggesting a possible specificity requirement for the histidine residue at position 37.
PhrC cleavage site.
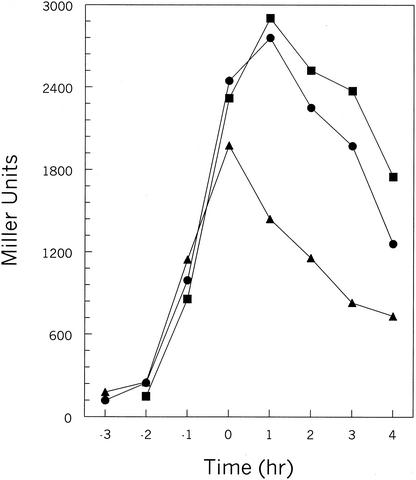
The RapC-PhrC pair of proteins is known to affect the transcription of competence-controlled genes through the activity of the ComA response regulator (25, 50). Analysis of the 40-amino-acid product of the phrC gene by the SignalP program identified a putative SPase cleavage site between positions 27 and 28 (ANA-EA) located 8 amino acids upstream of the terminal pentapeptide inhibitor (Fig. 2). Replacement of the alanine at position 27 with a proline residue did not affect the transcription of the ComA-dependent rapA promoter (data not shown). On the contrary, changing the threonine residue at position 35 affected the level of transcription of rapA as the T35P mutation resulted in a decrease of β-galactosidase activity from the rapA promoter-lacZ fusion construct (Fig. 3). The threonine at position 35 was also changed to an alanine residue in order to test whether a small residue, often found to precede active Phr peptides, would increase the efficiency of processing. The T35A change gave rise to a slight but reproducible increase of rapA transcription (Fig. 3). These results are consistent with the presence of an essential cleavage site at the peptide bond between the T and E residues at positions 35 and 36, respectively (HVT-ER). Notably, the mutations generated did not affect the initial rates of β-galactosidase production (before T0, i.e., before the transition from vegetative phase to sporulation) but rather the timing of promoter turnoff. This may suggest that PhrC does not affect the initial development of competence but only the amount of time for which competence lasts.
FIG. 3.
Transcription analysis of the rapA-lacZ fusion in phrC mutants. β-galactosidase assays were carried out in Schaeffer's sporulation medium, and β-galactosidase activity was time point analyzed at hourly intervals before and after the transition phase (T0). Strains: JH12981 (wild type), •; JH23059 (phrC mutant 1 [T35P]), ▴; JH23060 (phrC mutant 2 [T35A]), ▪.
PhrE cleavage site.
The 44-amino-acid sequence of the PhrE peptide was also analyzed with the SignalP program, which identified a putative SPase cleavage site between residues 24 and 25 (MYN-GE) (Fig. 2). We replaced the N24 residue with proline and analyzed the sporulation phenotype resulting from the expression of this mutant protein in B. subtilis. Strain JH11969 is sporulation deficient because of the deletion of the phrE gene. Introduction of the clone expressing the PhrE-mut2 mutant peptide restored sporulation, indicating that the N24P substitution did not affect the processing pathway leading to the production of the pentapeptide. In view of the results obtained with the mutagenesis of phrA and phrC, we mutated to proline the alanine residue at position 30 that immediately precedes the pentapeptide. Strain JH11969 transformed with the resulting PhrE-mut1 clone (A30P) remained sporulation deficient, indicating that the alanine preceding the SRNVT sequence of the pentapeptide is essential for the processing (KEA-SR) (Table 3).
TABLE 3.
Efficiency of sporulation of B. subtilis strains carrying mutations in the phrE genea
| Strain | Relevant genotype | Viable cell count ml−1 | Spore count ml−1 | Percentageb |
|---|---|---|---|---|
| JH642 | Wild type | 3.4 × 108 | 1.1 × 108 | 32.3 |
| JH11969 | ΔphrE | 6.9 × 108 | 1.2 × 108 | 17.4 |
| JH11970 | ΔphrE amyE::phrE wild type | 4.0 × 108 | 1.3 × 108 | 32.5 |
| JH11971 | ΔphrE amyE::phrE mutant 1 | 6.5 × 108 | 1.0 × 108 | 15.4 |
| JH23037 | ΔphrE amyE::phrE mutant 2 | 4.5 × 108 | 1.9 × 108 | 42.2 |
Data are representative of results from two independent experiments.
The percentage is calculated as the ratio between spore count and viable cell count.
Analysis of PhrA domains.
Since the results indicated that the predicted signal peptide cleavage site was not required for Phr peptide processing, we wondered whether other structural features typical of secreted proteins may be dispensable as well. The requirement for the hydrophobic domain or the hydrophilic region preceding the active pentapeptide inhibitor was analyzed by site-directed mutagenesis. Deletions in the phrA gene were generated that resulted in mutant genes encoding Phr proteins lacking either the amino-terminal positively charged and hydrophobic region (strain JH23007 phrAΔ21),the central hydrophilic domain (strain JH23054 phrAΔ14), or both (strain JH11847 phrAΔ38) (Fig. 4). Expression of the deleted proteins in the phrA deletion strain JH11842 resulted in all cases in a partial sporulation-deficient phenotype compared to that of the strain expressing the wild-type PhrA protein (Table 4).
FIG. 4.
Domain requirement for the processing of PhrA. The wild-type (WT) sequence of the 44-amino-acid PhrA peptide is shown with the deletion mutant proteins and the His tag extension mutant protein (PhrA-His). The A-to-P change in the PhrAΔ14-6 protein is indicated by a box. The active pentapeptide inhibitors are underlined. The domain organization is indicated as described in the legend to Fig. 2. The positions of two relevant residues are given by subscript numbers for reference. +++ indicates positively charged amino-terminal residues.
TABLE 4.
Efficiency of sporulation of phrA deletion and insertion strainsa
| Strainb | Relevant genotype | Viable cell count ml−1 | Spore count ml−1 | Percentagef |
|---|---|---|---|---|
| JH642 | Wild type | 3.1 × 108 | 1.6 × 108 | 51.6 |
| JH11842 | ΔphrA | 2.8 × 108 | 1.2 × 107 | 4.3 |
| JH23053c | phrA (wild type) | 2.0 × 108 | 1.1 × 108 | 55.0 |
| JH23054c | phrAΔ14 | 3.1 × 108 | 9.8 × 107 | 31.6 |
| JH23055c | phrAΔ14-6 | 4.5 × 108 | 1.3 × 108 | 28.9 |
| JH23056c | phrA-(His codon)6 | 6.4 × 107 | 1.3 × 107 | 20.3 |
| JH23008d,e | ΔphrA amyE::phrA (wild type) | 9.4 × 107 | 5.7 × 107 | 60.6 |
| JH11831d | amyE::phrAΔ38 | 2.4 × 108 | 1.4 × 108 | 58.3 |
| JH11847d,e | ΔphrA amyE::phrAΔ38 | 2.3 × 108 | 5.8 × 107 | 25.2 |
| JH23007d,e | ΔphrA amyE::phrAΔ21 | 3.9 × 108 | 1.3 × 108 | 33.3 |
Data are representative of results from three independent experiments.
Strains were grown for 24 h at 37°C in Schaeffer's sporulation medium.
The phrA construct is integrated isotopically by single crossover.
The phrA construct is integrated ectopically (amyE) by double crossover.
Strains are derivatives of JH11842.
The percentage is calculated as the ratio between spore count and viable cell count.
The requirement for the C-terminal location of the pentapeptide inhibitor was tested by constructing a phrA gene encoding the 44-amino-acid protein with a six-His tag extension at the carboxy-terminal end (Fig. 4). Expression of this protein in the phrA mutant strain JH11842 gave rise to a partial sporulation-deficient phenotype, as the efficiency of spore formation was approximately 50% of that observed in the strain expressing the wild-type PhrA protein (Table 4).
The sporulation efficiencies observed in the strains expressing domain deletion PhrA mutant proteins ranged between 35 and 55% of that in the wild-type strain, indicating that some active pentapeptide was produced, presumably through alternative pathways. The efficiency of pentapeptide inhibition was, however, lower than the one reached by the wild-type strain. This finding suggests that the use of an optimized processing pathway in the wild-type strain must result in the maximal inhibition of the RapA phosphatase. It should be pointed out that the PhrAΔ14 mutant protein is still recognized by the SignalP program as having a strong SPase cleavage site at the VHA-AR site while the PhrAΔ14-6 protein is not recognized as having a strong site because of the VHA-to-VHP change. Yet, the expression of both proteins in the phrA mutant strain results in comparable levels of sporulation. This result suggests that the VHA sequence is unlikely to be a SPase-cleavable site.
Are SPases involved in Phr processing?
The structural organization of the Phr peptides is suggestive of a SecA-dependent mechanism of protein export with type I SPases as putative enzymes involved in removing the signal peptides from the preproteins. Five type I SPases have been identified in B. subtilis, encoded by the chromosomal genes sipS, sipT, sipU, sipV, and sipW (53). We examined the possibility that these enzymes were involved in Phr processing by generating single and multiple sip mutations in the wild-type strain JH642 and analyzing the sporulation phenotypes of the mutants. As previously reported, the deletion of each sip gene singly had no detectable effect on cell growth or viability (54, 55). Furthermore, no effect on the efficiency of sporulation was observed (55) (Table 5 and our unpublished data). Given the redundant role that SPases seem to have in B. subtilis, we generated multiple sip gene deletions in various combinations. We confirmed that the only mutant that cannot be generated was the one lacking both sipS and sipT (55). Additionally, we identified a new phenotype associated with the sipV sipT double mutant: the strain containing this double deletion, JH22108, was severely affected in its ability to sporulate (Table 5) and failed to induce the transcription of Spo0A at the end of vegetative phase (6) (data not shown). The sporulation defect was not affected by the addition of the sipU and sipW deletions (data not shown). Strain JH22108 was also affected in cell viability, as indicated by the lower cell counts obtained in sporulation assays (Table 5), and it was not competent for DNA transformation (data not shown).
TABLE 5.
Efficiency of sporulation of B. subtilis strains carrying sipV and sipT mutationsa
| Strain | Relevant genotype | Viable cell count ml−1 | Spore count ml−1 | Percentageb |
|---|---|---|---|---|
| JH642 | Wild type | 2.9 × 108 | 1.3 × 108 | 44.8 |
| JH22097 | sipT | 3.0 × 108 | 1.5 × 108 | 50.0 |
| JH22098 | sipV | 2.4 × 108 | 1.1 × 108 | 45.8 |
| JH22108 | sipT sipV | 2.5 × 107 | 9.0 × 105 | 3.6 |
| JH11783 | sipT sipV rapA | 1.1 × 107 | 2.5 × 105 | 2.3 |
| JH11784 | sipT sipV spo0F (Y13S) | 4.6 × 107 | 9.0 × 105 | 1.9 |
| JH11435 | spo0F (Y13S) | 3.1 × 108 | 1.7 × 108 | 54.8 |
| JH12834 | rapA | 2.1 × 108 | 1.2 × 108 | 57.1 |
Data are representative of results from two independent experiments. Cells were grown for 24 h in Schaeffer's sporulation medium.
Calculated as the ratio between the number of spores and the viable cell count.
The possibility that the sporulation deficiency of strain JH22108 was the result of an inability to process the product of the phrA gene was tested. The constructs generating the deletion of the sipV and sipT genes were introduced by transformation of the strains carrying either the deletion of the rapA gene (JH11783) or the spo0F mutant that results in the Spo0F Y13S protein resistant to Rap-dependent dephosphorylation (JH11784) (39). The rationale for this approach was that sporulation in these two strains is independent from the processing of the PhrA peptide. Thus, these mutations should suppress the sporulation-deficient phenotype of the sipT sipV double mutant if this was indeed resulting from the lack of processing of the inhibitor pentapeptide. As shown in Table 5, the sporulation-deficient phenotype of strain JH22108 was not suppressed by the two mutations tested. These results suggested that the sporulation defect of a sipT sipV double mutant was not due to the lack of processing of Phr peptides involved in sporulation alone but may be the result of a more serious defect that affects sporulation as well as growth and competence.
SipS, SipT, and SipV do not process PhrA in vitro.
A biochemical approach was undertaken to determine whether type I SPases had a role in PhrA processing. The SipS, SipT, and SipV proteins were overexpressed and purified from E. coli strains as described in Materials and Methods. These enzymes were chosen because they are the major SPases in B. subtilis (SipS and SipT) (59) or because they are involved in some aspect of sporulation (SipT and SipV), as described previously. Active SipS and SipV were obtained as full-length fusions with the maltose binding protein (MBP). A similar fusion was generated between the MBP and the full-length SipT. This protein, however, was proteolytically cleaved during either cell growth or purification. The site of proteolysis was determined to be within the SipT sequence based on the sizes of the products obtained (data not shown). Self-cleavage of B. subtilis SPases overproduced in E. coli was previously reported (60). As an alternative strategy, a stable and active SipT enzyme was constructed and purified with an amino-terminal His tag extension that replaced the transmembrane region.
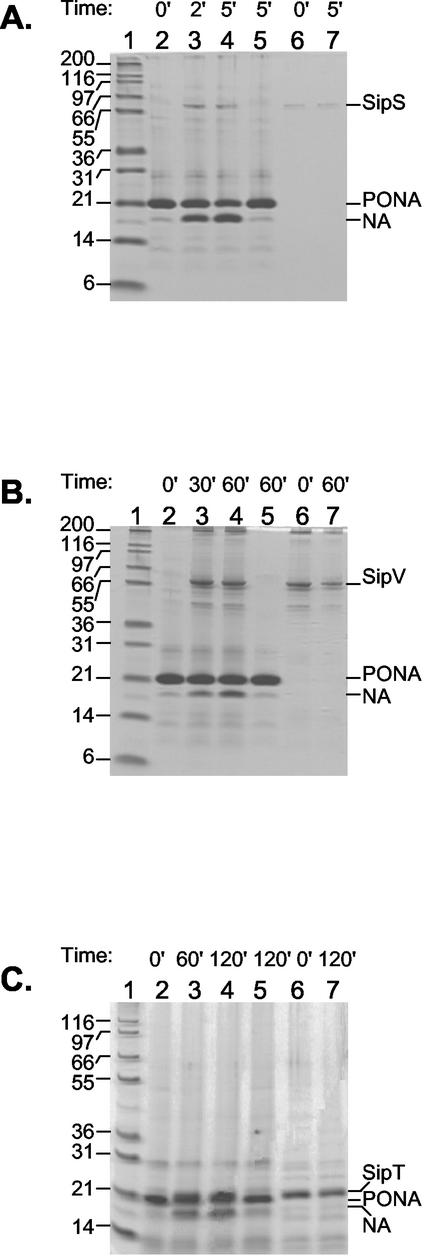
The activity of the purified proteins was tested in vitro as their ability to process the PONA fusion protein originally described by Takahara et al. (52). The purified SipS protein was able to process purified PONA substrate in vitro as shown in Fig. 5A and as previously shown by Carlos et al. (4). The purified SipV and SipT proteins also exhibited enzymatic activity toward the PONA substrate, although with different efficiencies (Fig. 5B and C). The results indicated that the SipS enzyme was the most active in our assay conditions as it processed more than 50% of the substrate in 5 min with a 1:224 molar ratio between enzyme and substrate (Fig. 5A). Processing of approximately 50% of the substrate was also obtained with SipT, although with 120 min of incubation time and a 1:10 molar ratio between enzyme and substrate (Fig. 5C). The SipV protein was the least active, with, at the maximum efficiency, approximately 30% of PONA processed in 60 min of incubation with a 1:5 molar ratio between enzyme and substrate (Fig. 5B). The efficiencies of processing by the three enzymes were not increased by the addition of lipids or detergents (data not shown). A similar observation was previously reported with regard to the activity of a truncated B. subtilis SipS protein (60).
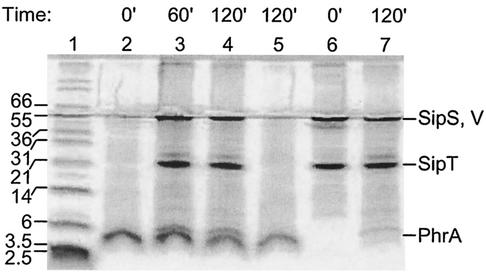
FIG. 5.
Processing of the PONA substrate with the six-His tag into the nuclease A (NA) mature protein by the B. subtilis SipS, SipV, and SipT SPases. The reactions were carried out at pH 8.0 as described in Materials and Methods, and reaction products were analyzed on SDS-10% PAGE in Tris-HCl-Tricine buffer, pH 8.45. The reaction timesare expressed in minutes. The sizes of the molecular weight markers (lane 1) are indicated. (A) PONA (18 μM) was incubated alone (lanes 2 and 5) or with SipS (0.13 μM; lanes 3 and 4) for the times indicated. SipS alone is shown in lanes 6 and 7. Shown are the results for reaction mixtures containing phosphatidylethanolamine, cardiolipin, and lipid extracts. (B) PONA (18 μM) was incubated alone (lanes 2 and 5) or with SipV (3 μM; lanes 3 and 4) for the times indicated. SipV alone is shown in lanes 6 and 7. Shown are the results for reaction mixtures containing lipids, as in panel A. (C) PONA (14 μM) was incubated alone (lanes 2 and 5) or with SipT (1.4 μM; lanes 3 and 4) for the times indicated. SipT alone is shown in lanes 6 and 7. Shown are the results for reaction mixtures containing only the B. subtilis lipid extract.
The optimized conditions for Sip peptidase activity on PONA were applied to test the ability of the Sip proteins to process the PhrA peptide. A synthetic 44-amino-acid PhrA protein was incubated with each individual Sip protein or with all three proteins, and the reaction products were analyzed by SDS-PAGE and Western blotting with anti-Phr antibody (17). As shown by a representative gel in Fig. 6, we never observed any processing of PhrA by any of the SPases. As an additional control, mass spectrometry of the reaction products was undertaken but pentapeptide product was never detected (data not shown).
FIG. 6.
Analysis of PhrA processing by SPases in vitro. Samples were analyzed by SDS-PAGE electrophoresis using 18% acrylamide in Tris-HCl-Tricine buffer, pH 8.45. A synthetic PhrA peptide (44 amino acids; 57 μM) was incubated with SipS (0.57 μM), SipT (5.7 μM), and SipV (5.7 μM) singly (data not shown) or in combination in reaction buffer at pH 10.0. Lane 1, molecular size markers; lanes 2 and 5, PhrA peptide alone; lanes 3 and 4, PhrA peptide and SipS, SipT, and SipV; lanes 6 and 7, SipS, SipT, and SipV.
With the aim of improving detection of a processing product, we constructed a PhrA-AmyE hybrid protein (see Materials and Methods) by fusing the full-length 44-amino-acid PhrA peptide to the mature AmyE protein of B. subtilis. The resulting protein was overexpressed and purified from E. coli and tested for processing by SipS, SipT, and SipV. This approach showed no processing of the hybrid protein at the expected site, i.e., the HIA-AR site of the PhrA peptide. We observed only some partial processing of the PhrA-AmyE protein by the SipT peptidase. However, by the size of the products obtained, we estimated that this processing occurred within the sequence of the AmyE protein (data not shown).
These results strongly suggest that the processing of the PhrA peptide at the HIA-AR site is most likely independent of the activity of the SipS, SipV, and SipT SPases and, by extrapolation from the genetic results, independent of all type I SPase activities so far identified in B. subtilis.
DISCUSSION
The export-import pathway followed by the Phr precursor proteins for the production of the pentapeptide inhibitors was originally defined as a timing device (42). As such, it was hypothesized that the activity of the enzymes involved in the processing of the peptides could be temporally controlled and thus determine the appropriate timing for sporulation initiation in response to physiological or environmental signals. The structural organization of Phr proteins is typical of SecA-dependent secretion proteins, with a signal peptide suggestive of type I SPase involvement in the cleavage process. Notably, the transcription of the genes encoding two of the five type I SPases of B. subtilis, SipS and SipT, is temporally controlled (55). Thus, we hypothesized a processing pathway for the PhrA peptide involving SPase cleavage at the VHA-GE site (Fig. 2) that would generate a 19-amino-acid preprotein only at the appropriate time. Further processing of the preprotein by an unknown enzyme at the HIA-AR site would liberate the active pentapeptide inhibitor. The second processing event may also be subject to timing control.
In this study, we have shown that the putative VHA-GE cleavage site of the PhrA protein (between residues 27 and 28) can be mutated without affecting the production of the terminal pentapeptide. On the contrary, processing of the PhrA protein was prevented by replacing the alanine preceding the pentapeptide with a proline, i.e., changing the HIA-AR site (between residues 39 and 40) to HIP-AR. This indicated that the production of the terminal pentapeptide inhibitor is most likely to depend on a single processing event occurring between residues 39 and 40. Whether this processing is carried out by type I SPases was tested by in vivo and in vitro approaches.
The in vivo approach consisted of analyzing the sporulation phenotypes of type I SPase mutant strains. None of the single or multiple sip mutants analyzed pointed to a direct or specific role for any of the five type I SPases in Phr processing. A genetic analysis alone is subject to the counter argument that, since the presence of either SipS or SipT is essential for viability, one enzyme was always present in our analysis to carry out the processing event and thus, given their redundancy, we were not able to detect any processing effect. This rationale would also imply that no specific type I SPase is required for Phr processing; thus SipS and SipT, and perhaps others, could be interchangeable and equally efficient for this task. However, the in vitro analysis of B. subtilis SPase activity on PhrA did not provide any evidence of processing by SipS, SipT, or SipV in any of the conditions tested. Processing was not detected at the VHA-GE site or at the HIA-AR site of PhrA. The conditions tested included the use of a synthetic 44-amino-acid PhrA peptide as well as a PhrA-AmyE fusion protein that could have facilitated the detection of a mature product. Testing conditions also included the use of detergents or various lipids that were shown to improve SPase efficiency in some instances (32, 58). These results, together with the in vivo mutagenesis analysis on the Phr processing sites, seem to point to the conclusion that type I SPases are dispensable for Phr processing.
Support for this conclusion comes from the analysis of the amino acid residues present at position −3 with respect to the cleavage site. This position is critical for SPase activity (14, 20, 49). It is interesting that the −3 positions relative to the cleavage sites of the PhrA and PhrC peptides are occupied by a histidine and that another positively charged residue, lysine, occupies the same position in the PhrE peptide. Substitution of lysine at the −3 position of the alkaline phosphatase preprotein, as well as histidine and proline, prevented processing in vivo by the E. coli type I SPase (20). Statistical studies of sequences surrounding SPase cleavage sites led to the formulation of the Ala-X-Ala rule defining the preferred residue (i.e., Ala) at the −1 and −3 positions relative to the cleavage site as a critical determinant for enzyme recognition and cleavage. The requirement for the Ala-X-Ala sequence was explained by the crystal structure of the E. coli type I SPase in the presence and absence of an inhibitor or substrate (30, 31). A shallow and hydrophobic substrate-binding pocket in the E. coli enzyme is in fact consistent with substrate specificity for small and neutral residues at the −1 and −3 positions. This is in agreement with the current understanding that the proline residue is not allowed in the −3-to-+1 regions of substrates for bacterial SPases (44, 48, 61, 62). This rule was at the basis of our choice to substitute proline to assess cleavage site function. This approach showed that the alanine residue in the HIA sequence preceding the terminal pentapeptide is necessary for peptide processing while the replacement of the histidine residue with proline did not affect the processing of the PhrA precursor. Thus, the HIA, HVT, and KEA sequences that precede the PhrA, PhrC, and PhrE pentapeptides, respectively, are unlikely to be SPase cleavage sites. It should also be noted that none of the 151 known cleavage sites from gram-negative bacteria analyzed by Karamyshev et al. (20) or the 169 predicted secretory signal peptides of B. subtilis (53) had a histidine or lysine residue at position −3.
The Ala-X-Ala rule was also at the basis of our approach that included mutating the histidine residue at position −3 of PhrA into alanine (HIA→AIA, PhrA-mut7), thus generating a prototypical type I SPase recognition sequence. The strain carrying this mutation, however, did not show any improvement in PhrA processing since its sporulation frequency did not increase compared to that of the wild-type strain (Table 2). This may suggest that the mutation did not give rise to a type I SPase-cleavable site because, in such a case, we would have expected an increase in peptide processing and thus some phenotypes of precocious sporulation or a higher rate of sporulation. On the contrary, this strain consistently seemed to have a slight sporulation defect, indicating that some requirement at position −3 may exist in order to maximize the processing of the pentapeptide.
Although none of the B. subtilis type I SPases seem to be directly involved in the processing of Phr peptides, this study has revealed that a SipV-SipT double deficiency has a strong effect on the cell's ability to sporulate (Table 5) and develop competence for genetic transformation and motility (unpublished observation). The molecular basis for this phenotype is not understood at this time, but it should be pointed out that in B. amyloliquefaciens a sporulation-deficient phenotype was observed upon disruption of the sipT gene encoding the equivalent of the B. subtilis SipT enzyme. In B. amyloliquefaciens, deletion of the sipV orthologue resulted in impaired growth, inhibited cell autolysis, and reduced motility (7). This could be interpreted to mean that while in B. subtilis SipV and SipT have developed overlapping specificity, in B. amyloliquefaciens the two enzymes have maintained distinct substrates. The orthologues of some of these substrates may actually be substrates of the SipV-SipT enzymes of B. subtilis due to the somehow similar phenotypes of the corresponding mutant strains. Overlapping specificities of SipV and SipT of B. subtilis do not seem to include the PhrA peptide: the results of the in vivo and in vitro analyses that we report here indeed suggest that the sporulation defect of the sipV sipT double mutant cannot be rescued by bypassing the requirement of PhrA processing. Additionally, purified active proteins did not process the PhrA 44-amino-acid peptide in vitro. Although caution is suggested when in vitro results with SPases are interpreted (10), the fact that our purified MBP-SipS, MBP-SipV, and His tag-SipT proteins were active in processing an unnatural substrate such as PONA should be a good indication that a direct target would have been processed as well in our assay conditions. This belief is strengthened by the notion that all in vitro studies to date are consistent with bacterial type I SPases' not requiring additional proteins or cofactors for their catalytic activity (10).
If SPases are not involved in processing the Phr peptides, how does this cleavage event occur? Is there one specific enzyme that processes all the Phr peptides? Is there a specific enzyme for the processing of each peptide? Are there overlapping activities from multiple enzymes directed toward all the Phr proteins? The existence of a specific enzyme for the processing of each Phr peptide seems unlikely: a mutation in the gene encoding the specific PhrA-processing enzyme would result in a sporulation-deficient phenotype identical to the phenotype of a phrA mutant. Such a mutant strain has never been identified, despite the numerous screenings carried out in our laboratory and others. It is possible, however, that such an enzyme has an additional function that is essential for viability, making it undetectable by conventional gene inactivation screening methods. Furthermore, the direct inactivation of approximately 100 genes encoding enzymes with protease activity in B. subtilis did not lead to the identification of any mutant defective in PhrA processing (unpublished data). These results support the belief that the processing is not dependent upon one specific protease. However, since few genes were found to be essential for viability, it is possible that PhrA may be processed by the product of one of these genes (our unpublished observations) (21).
Alternatively, multiple enzymes with overlapping activities may be involved in the processing of one or all Phr proteins in a specific manner.
Independently of the identity of the Phr-processing enzyme(s), it seems that its activity may require an Ala residue or at least a small noncharged residue, such as Thr, at the amino end of the cleavable peptide bond (position −1). In addition to the known active PhrA, PhrC, and PhrE peptides, the PhrF active pentapeptide (QRGMI) is also preceded by an Ala residue (EVA-QR) (36; our unpublished results). The nature of the first residue following the cleavable bond, on the contrary, may not necessarily be governed by a strong rule since nonpolar (Ala), polar noncharged (Ser or Gln), and polar and negatively charged (Glu) residues are equally found in that position.
A specific processing seems to be required in order to produce the highest level of pentapeptide that can then be internalized and thus counteract the activity of the corresponding Rap protein. Alternative and/or nonspecific processing pathways may exist, either inside or outside the cell membrane, as our analysis of the role of the PhrA domains suggested (Table 4): any modification of the structural organization of PhrA partially affected the cell's ability to produce active pentapeptide. Since there is a strict requirement for peptide length and sequence (23, 34) and a stoichiometry close to unity characterizes the Phr-Rap interaction (8), we interpret the results shown in Table 4 to mean that perhaps the activity of nonspecific endopeptidases, aminopeptidases, and/or carboxypeptidases results in the accumulation of sufficient active PhrA pentapeptide to partially inhibit RapA activity. The export-import pathway can then be seen as a mechanism evolutionarily developed to maximize the production of pentapeptides and as a consequence their regulatory role in the cell physiology.
Acknowledgments
This research was supported, in part, by Public Health Service grant GM55594 from the National Institute of General Medical Sciences, National Institutes of Health. The Stein Beneficial Trust supported, in part, oligonucleotide synthesis and DNA sequencing.
Footnotes
This is publication 15076-MEM from the Scripps Research Institute.
REFERENCES
- 1.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 2.Bohni, P. C., R. J. Deshaies, and R. W. Schekman. 1988. SEC11 is required for signal peptide processing and yeast cell growth. J. Cell Biol. 106:1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkholder, W. F., I. Kurtser, and A. D. Grossman. 2001. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell 104:269-279. [DOI] [PubMed] [Google Scholar]
- 4.Carlos, J. L., M. Paetzel, G. Brubaker, A. Karla, C. M. Ashwell, M. O. Lively, G. Cao, P. Bullinger, and R. E. Dalbey. 2000. The role of the membrane-spanning domain of type I signal peptidases in substrate cleavage site selection. J. Biol. Chem. 275:38813-38822. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee, S., D. Suciu, R. E. Dalbey, P. C. Kahn, and M. Inouye. 1995. Determination of Km and kcat for signal peptidase I using a full length secretory precursor, pro-OmpA-nuclease A. J. Mol. Biol. 245:311-314. [DOI] [PubMed] [Google Scholar]
- 6.Chibazakura, T., F. Kawamura, and H. Takahashi. 1991. Differential regulation of spo0A transcription in Bacillus subtilis: glucose represses promoter switching at the initiation of sporulation. J. Bacteriol. 173:2625-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, H. H., V. Hoang, P. Kreutzmann, B. Hofemeister, M. Melzer, and J. Hofemeister. 2002. Identification and properties of type I-signal peptidases of Bacillus amyloliquefaciens. Eur. J. Biochem. 269:458-469. [DOI] [PubMed] [Google Scholar]
- 8.Core, L. J., S. Ishikawa, and M. Perego. 2001. A free terminal carboxylate group is required for PhrA pentapeptide inhibition of RapA phosphatase. Peptides 22:1549-1553. [DOI] [PubMed] [Google Scholar]
- 9.Cregg, K. M., I. Wilding, and M. T. Black. 1996. Molecular cloning and expression of the spsB gene encoding an essential type I signal peptidase from Staphylococcus aureus. J. Bacteriol. 178:5712-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalbey, R. E., M. O. Lively, S. Bron, and J. M. van Dijl. 1997. The chemistry and enzymology of the type I signal peptidases. Protein Sci. 6:1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalbey, R. E., and W. Wickner. 1985. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J. Biol. Chem. 260:15925-15931. [PubMed] [Google Scholar]
- 12.Date, T. 1983. Demonstration by a novel genetic technique that leader peptidase is an essential enzyme of Escherichia coli. J. Bacteriol. 154:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari, E., S. M. H. Howard, and J. A. Hoch. 1986. Effect of stage 0 mutations on subtilisin expression. J. Bacteriol. 166:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fikes, J. D., G. A. Barkocy-Gallagher, D. G. Klapper, and P. J. Bassford, Jr. 1990. Maturation of Escherichia coli maltose-binding protein by signal peptidase I in vivo. J. Biol. Chem. 265:3417-3423. [PubMed] [Google Scholar]
- 15.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 16.Hoch, J. A. 1998. Initiation of bacterial development. Curr. Opin. Microbiol. 1:170-174. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa, S., L. J. Core, and M. Perego. 2002. Biochemical characterization of aspartyl phosphatase phosphatase interaction with a phosphorylated response regulator and its inhibition by a pentapeptide. J. Biol. Chem. 277:20483-20489. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, M., R. Grau, and M. Perego. 2000. Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis. J. Bacteriol. 182:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, M., W. Shao, M. Perego, and J. A. Hoch. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 38:535-542. [DOI] [PubMed] [Google Scholar]
- 20.Karamyshev, A. L., Z. N. Karamysheva, A. V. Kajava, V. N. Ksenzenko, and M. A. Nesmeyanova. 1998. Processing of Escherichia coli alkaline phosphatase: role of the primary structure of the signal peptide cleavage region. J. Mol. Biol. 277:859-870. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Debarbouille, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M.-F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauel, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. O. M. Ohanan, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. Seegers, J. Sekiguchi, A. Sekowska, S. J. Seror, M. Simon, P. Stragier, R. Struder, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, H. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi, K., K. Shoji, T. Shimizu, K. Nakano, T. Sato, and Y. Kobayaski. 1995. Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. J. Bacteriol. 177:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazazzera, B. A., J. M. Solomon, and A. D. Grossman. 1997. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell 89:917-925. [DOI] [PubMed] [Google Scholar]
- 24.LeDeaux, J. R., and A. D. Grossman. 1995. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J. Bacteriol. 177:166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnuson, R., J. Solomon, and A. D. Grossman. 1994. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell 77:207-216. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Mueller, J. P., G. Bukusoglu, and A. L. Sonenshein. 1992. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J. Bacteriol. 174:4361-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 29.Ohlsen, K. L., J. K. Grimsley, and J. A. Hoch. 1994. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc. Natl. Acad. Sci. USA 91:1756-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paetzel, M., R. E. Dalbey, and N. C. Strynadka. 1998. Crystal structure of a bacterial signal peptidase in complex with a beta-lactam inhibitor. Nature 396:186-190. [DOI] [PubMed] [Google Scholar]
- 31.Paetzel, M., R. E. Dalbey, and N. C. Strynadka. 2002. Crystal structure of a bacterial signal peptidase apo-enzyme. Implications for signal peptide binding and the SER-LYS dyad mechanism. J. Biol. Chem. 277:9512-9519. [DOI] [PubMed] [Google Scholar]
- 32.Peng, S.-B., L. Wang, J. Moomaw, R. B. Peery, P.-M. Sun, R. B. Johnson, J. Lu, P. Treadway, P. L. Skatrud, and Q. M. Wang. 2001. Biochemical characterization of signal peptidase I from gram-positive Streptococcus pneumoniae. J. Bacteriol. 183:621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perego, M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615-624. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 34.Perego, M. 1997. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc. Natl. Acad. Sci. USA 94:8612-8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perego, M. 1998. Kinase-phosphatase competition regulates Bacillus subtilis development. Trends Microbiol. 6:366-370. [DOI] [PubMed] [Google Scholar]
- 36.Perego, M. 1999. Self-signaling by Phr peptides modulates Bacillus subtilis development, p. 243-258. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 37.Perego, M. 2001. A new family of aspartyl-phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Mol. Microbiol. 42:133-144. [DOI] [PubMed] [Google Scholar]
- 38.Perego, M., P. Glaser, and J. A. Hoch. 1996. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol. Microbiol. 19:1151-1157. [DOI] [PubMed] [Google Scholar]
- 39.Perego, M., C. G. Hanstein, K. M. Welsh, T. Djavakhishvili, P. Glaser, and J. A. Hoch. 1994. Multiple protein aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in Bacillus subtilis. Cell 79:1047-1055. [DOI] [PubMed] [Google Scholar]
- 40.Perego, M., C. F. Higgins, S. R. Pearce, M. P. Gallagher, and J. A. Hoch. 1991. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol. Microbiol. 5:173-185. [DOI] [PubMed] [Google Scholar]
- 41.Perego, M., and J. A. Hoch. 1991. Negative regulation of Bacillus subtilis sporulation by the spo0E gene product. J. Bacteriol. 173:2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perego, M., and J. A. Hoch. 1996. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:1549-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perego, M., and J. A. Hoch. 2002. Two-component systems, phosphorelays, and regulation of their activities by phosphatases, p. 473-481. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 44.Perlman, D., and H. O. Halvorson. 1983. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J. Mol. Biol. 167:391-409. [DOI] [PubMed] [Google Scholar]
- 45.Rudner, D. Z., J. R. Ladeaux, K. Breton, and A. D. Grossman. 1991. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J. Bacteriol. 173:1388-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaeffer, P., J. Millet, and J. P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 48.Sharkov, N. A., and D. Cai. 2002. Discovery of substrate for type I signal peptidase SpsB from Staphylococcus aureus. J. Biol. Chem. 277:5796-5803. [DOI] [PubMed] [Google Scholar]
- 49.Shen, L. M., J.-I. Lee, S. Cheng, H. Jutte, A. Kuhn, and R. E. Dalbey. 1991. Use of site-directed mutagenesis to define the limits of sequence variation tolerated for processing of the M13 procoat protein by the Escherichia coli leader peptidase. Biochemistry 30:11775-11781. [DOI] [PubMed] [Google Scholar]
- 50.Solomon, J. M., B. A. Lazazzera, and A. D. Grossman. 1996. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 10:2014-2024. [DOI] [PubMed] [Google Scholar]
- 51.Stöver, A. G., and A. Driks. 1999. Control of synthesis and secretion of the Bacillus subtilis protein YqxM. J. Bacteriol. 181:7065-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahara, M., D. W. Hibler, P. J. Barr, J. A. Gerlt, and M. Inouye. 1985. The ompA signal peptide directed secretion of Staphylococcal nuclease A by Escherichia coli. J. Biol. Chem. 260:2670-2674. [PubMed] [Google Scholar]
- 53.Tjalsma, H., A. Bolhuis, J. D. H. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tjalsma, H., A. Bolhuis, M. L. van Roosmalen, T. Wiegert, W. Schumann, C. P. Broekhuizen, W. J. Quax, G. Venema, S. Bron, and J. M. van Dijl. 1998. Functional analysis of the secretory precursor processing machinery of Bacillus subtilis: identification of a eubacterial homolog of archaeal and eukaryotic signal peptidases. Genes Dev. 12:2318-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tjalsma, H., M. A. Noback, S. Bron, G. Venema, K. Yamane, and J. M. van Dijl. 1997. Bacillus subtilis contains four closely related type I signal peptidases with overlapping substrate specificities. J. Biol. Chem. 272:25983-25992. [DOI] [PubMed] [Google Scholar]
- 56.Tjalsma, H., A. G. Stöver, A. Driks, G. Venema, S. Bron, and J. M. van Dijl. 2000. Conserved serine and histidine residues are critical for activity of the ER-type signal peptidase SipW of Bacillus subtilis. J. Biol. Chem. 275:25102-25108. [DOI] [PubMed] [Google Scholar]
- 57.Trach, K. A., and J. A. Hoch. 1993. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway. Mol. Microbiol. 8:69-79. [DOI] [PubMed] [Google Scholar]
- 58.Tschantz, W. R., M. Paetzel, G. Cao, D. Suciu, M. Inouye, and R. E. Dalbey. 1995. Characterization of a soluble, catalytically active form of Escherichia coli leader peptidase: requirement of detergent or phospholipid for optimal activity. Biochemistry 34:3935-3941. [DOI] [PubMed] [Google Scholar]
- 59.van Roosmalen, M. L., J. D. Jongbloed, J. Y. Dubois, G. Venema, S. Bron, and J. M. van Dijl. 2001. Distinction between major and minor Bacillus signal peptidases based on phylogenetic and structural criteria. J. Biol. Chem. 276:25230-25235. [DOI] [PubMed] [Google Scholar]
- 60.van Roosmalen, M. L., J. D. H. Jongbloed, A. Kuipers, G. Venema, S. Bron, and J. M. van Dijl. 2000. A truncated soluble Bacillus signal peptidase produced in Escherichia coli is subject to self-cleavage at its active site. J. Bacteriol. 182:5765-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Heijne, G. 1983. Patterns of amino acids near signal-sequence cleavage sites. Eur. J. Biochem. 133:17-21. [DOI] [PubMed] [Google Scholar]
- 62.von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14:4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, L., R. Grau, M. Perego, and J. A. Hoch. 1997. A novel histidine kinase inhibitor regulating development in Bacillus subtilis. Genes Dev. 11:2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]