Abstract
The peptide cholecystokinin (CCK) is abundant in the rat nucleus accumbens (NAc). Although it is colocalized with dopamine (DA) in afferent terminals in this region, neurochemical and behavioural reports are equally divided as to whether CCK enhances or diminishes DA's actions in this nucleus. To better understand the role of this peptide in the physiology of the NAc, we examined the effects of CCK on excitatory synaptic transmission and tested whether these are dependent on DA and/or other neuromodulators. Using whole-cell recording in rat forebrain slices containing the NAc, we show that sulphated CCK octapeptide (CCK-8S), the endogenously active neuropeptide, consistently depolarized cells and depressed evoked excitatory postsynaptic currents (EPSCs) in the rostral NAc. It caused a reversible, dose-dependent decrease in evoked EPSC amplitude that was accompanied by an increase in the decay constant of the EPSC but with no apparent change in paired pulse ratio. It was mimicked by unsulphated CCK-8 (CCK-8US), a CCKB receptor-selective agonist, and blocked by LY225910, a CCKB receptor-selective antagonist. Both CCK-8S and CCK-8US induced an inward current with a reversal potential around −90 mV that was accompanied by an increase in input resistance and action potential firing. The CCK-8S-induced EPSC depression was slightly reduced in the presence of SCH23390 but not in the presence of sulpiride or 8-cyclopentyltheophylline. By contrast, it was completely blocked by CGP55845, a potent GABAB receptor-selective antagonist. These results indicate that CCK excites NAc cells directly while depressing evoked EPSCs indirectly, mainly through the release of GABA.
The NAc is a forebrain structure that has been implicated in complex behaviours and in the pathophysiology of psychiatric disorders (Koob & Bloom, 1988; Koob & Swerdlow, 1988). This nucleus is composed mainly of medium spiny GABAergic neurones (>90%; O'Donnell & Grace, 1993; Pennartz et al. 1994). These cells are the projection neurones of the nucleus and produce a strong lateral inhibition through extensive axon collateral connections with neighbouring cells.
The NAc is believed to serve as an interface where emotional events of limbic origin are converted into behavioural motor output. These behaviours are mediated by mesolimbic DA; a well recognized and extensively studied transmitter system with cell bodies located predominantly in the ventral tegmental area (VTA) (Koob & Bloom, 1988; Kuhar et al. 1991; Pennartz et al. 1994). CCK is abundant in this nucleus and has been reported to affect these DA-mediated behaviours (Crawley, 1988; Vaccarino, 1994). It is colocalized with DA arising from the VTA (Hokfelt et al. 1980) and substantia nigra (Lanca et al. 1998). A non-dopaminergic source from cortical areas has also been reported (Zaborszky et al. 1985; Seroogy & Fallon, 1989). CCK's innervations in NAc appear to follow a rostro-caudal pattern such that some subregions receive inputs from predominantly one source with minor contribution from other sources. In this regard, its inputs to the rostral NAc are from extra-mesencephalic regions (e.g. prefrontal cortex and amygdala; Gilles et al. 1983; Studler et al. 1985; Fallon & Seroogy, 1985; Zaborszky et al. 1985; Crawley, 1991) and mesencephalic structures (substantia nigra and the VTA; Lanca et al. 1998) while the caudal subregion receives its inputs primarily from the VTA (Zahm & Borg, 1992; Deutch, 1993; Zahm & Heimer, 1993; Lanca et al. 1998).
In addition to its presence in the NAc, both CCK receptors, CCKA and CCKB are also present (Carlberg et al. 1992) and there is evidence to suggest that these receptors also follow a rostro-caudal distribution (Crawley, 1992; Vaccarino, 1994; Mercer et al. 2000). CCKB receptors are predominantly localized on the somatodendrites and axons of the intrinsic GABAergic neurones (Berresford et al. 1987; Mercer et al. 2000). CCKA receptors on the other hand are present predominantly on dopaminergic (DAergic) afferent terminals in the NAC, since chemical lesioning of DAergic neurones results in marked reduction in the binding of a CCKA receptor ligand (Graham et al. 1991).
Pharmacological, neurochemical (White & Wang, 1984; Voigt et al. 1986; Marshall et al. 1991; Ferraro et al. 1996; Reum et al. 1997) and behavioural (De Witte et al. 1987; Dauge et al. 1989; Vaccarino & Rankin, 1989; Crawley, 1992) evidence indicate that CCK interacts with DA in the NAc to affect its function. However, most of the evidence shows that it either enhances or diminishes the DAergic system depending on whether it is applied to the rostral or caudal ends of the nucleus. Different receptors are reported to mediate these opposing effects (Voigt et al. 1986; De Witte et al. 1987; Marshall et al. 1991; Crawley, 1992; Reum et al. 1997).
In addition to the above CCK–DA interactions, CCK is also reported to increase or decrease the release of GABA in the NAc depending on the receptor subtype that is activated (Ferraro et al. 1996; Lanza & Makovec, 2000). In this nucleus, GABA is predominantly from axon collaterals arising from neighbouring projection cells (O'Donnell & Grace, 1993; Pennartz et al. 1994), as well as possible GABAergic interneurones (Meredith, 1999).
How this peptide interacts with all these transmitters to produce behavioural modifications is not clear. While the neurochemical and behavioural effects of CCK have been extensively studied, its cellular and synaptic effects are not well characterized. Electrophysiologically, DA is reported to act either directly on D1-like dopamine receptors located on glutamatergic terminals to decrease synaptic transmission (Harvey & Lacey, 1996; Nicola & Malenka, 1997) or indirectly on these same receptors located on medium spiny neurones to cause the generation of adenosine, which then acts on presynaptic glutamatergic terminals to decrease EPSCs (Harvey & Lacey, 1997; Buckby & Lacey, 2001; Kombian et al. 2003a). How CCK may influence these effects of DA is not known. The only evidence for any such interaction, to our knowledge, is from studies showing that iontophoretic application of CCK excited NAc cells in a dose-dependent manner (Wang et al. 1985; Wang, 1988). This excitation was similar to that produced by glutamate and could be reversed by DA. To better understand how CCK-induced neurochemical alterations in the NAc might translate into changes in cellular communication, we tested the effects of exogenous CCK on evoked excitatory postsynaptic currents recorded in NAc neurones. Furthermore, we examined the role that DA and GABA may play on any CCK effects.
Methods
All the rats used in this study were obtained from the Kuwait University Animal Resource Centre. Canadian Council on Animal Care and Kuwait University Health Science Center guidelines on the humane handling of animals were followed throughout this study and the minimum number of animals necessary to produce the required results was used.
Slice preparation
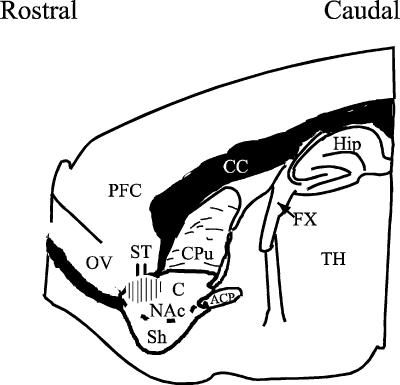
Parasagittal forebrain slices containing the NAc and the cortex were generated using previously published techniques (Kombian & Malenka, 1994; see Fig. 1). Briefly, male Sprague-Dawley rats (75−150 g, ∼3–5 weeks old) were anaesthetized with halothane and decapitated. The brain was quickly removed from the cranium and placed in ice-cold (4°C) artificial cerebrospinal fluid (ACSF) that was bubbled with 95% O2 and 5% CO2. The composition of the ACSF was (in mm): 126 NaCl; 2.5 KCl; 1.2 NaH2PO4; 1.2 MgCl2; 2.4 CaCl2; 18 NaHCO3; 11 glucose, producing a solution with osmolarity of between 310 and 320 mosmol l−1. The slices (350 μm thick) were cut in the ice-cold ACSF using OTS-4000 (Electron Microscopy Sciences, Pennsylvania, USA) or VT 1000S (Leica Microsystems, Wetzlar, Germany) tissue slicers. Slices were incubated in ACSF (bubbled continuously with 95% O2 and 5% CO2) at room temperature and allowed to recover for at least 1 h before experimentation.
Figure 1. A drawing of a parasagittal slice of the rat brain showing the placement of the stimulating electrode (ST) and the region in which the recordings were performed (hatched area).
Abbreviations: Nac, nucleus accumbens (Sh, shell; C, core); ACP, anterior commissure, posterior; CC, corpus collosum; Cpu, caudate-putamen; Fx, fornix; OV, olfactory ventricle; TH, thalamus; PFC, prefrontal cortex; Hip, hippocampus. (Adapted from Paxinos & Watson, 1998.)
Electrophysiological recordings
One brain slice was trimmed and transferred into a 500 μl capacity recording chamber and perfused submerged at a flow rate of 2–3 ml min−1 with ACSF that was bubbled continuously with 95% O2 and 5% CO2 at a temperature of 28–31°C. ‘Blind patch’ recordings were made from the rostral NAc (see Fig. 1) in the conventional whole-cell mode using glass electrodes with tip resistance of 4.0–8.0 MΩ. The internal recording solution had the following composition (in mm): 135 K-gluconate, 8 NaCl, 0.2 EGTA, 10 Hepes, 2 Mg-ATP, 0.2 GTP; pH and osmolarity adjusted to 7.3 (with KOH) and 270–280 mosmol l−1, respectively. Bipolar tungsten stimulating electrodes were positioned at the prefrontal cortex–accumbens border to evoke synaptic responses. Recordings were made using Axopatch 1D amplifiers (Axon Instruments Inc., Foster City, CA, USA) in either voltage or current clamp modes. Cells were voltage clamped at −80 mV (holding potential, Vh) and input (Rinput) and access (Ra) resistances of all cells were determined and monitored regularly throughout each experiment by applying a 20 mV hyperpolarizing pulse for 75 ms. Rinput was calculated from the steady-state current obtained during the pulse. The decay constant (τ) of the capacitance transient was taken as a measure of Ra. All cells reported in this study had Ra values of 10–30 MΩ. Data from cells that showed >15% changes in Ra during the experiment were excluded from further analysis.
All synaptic responses were recorded as inward currents at Vh of −80 mV. In control, the evoked synaptic response was a mixture of GABAA- and non-NMDA glutamate-receptor-mediated responses. Glutamate-induced, non-NMDA receptor-mediated pure EPSCs were isolated by applying 50 μm of picrotoxin, a GABAA receptor–chloride channel blocker. At this holding potential and in the presence of picrotoxin, the response was entirely non-NMDA receptor mediated, as it could be completely blocked by 6-7-dinitroquinoxaline-2,3-dione (DNQX; 10 μm). All cells had a graded evoked EPSC response to increasing stimulation intensity (ranging from 0.25 to 3.0 mA) and an intensity giving 50–60% of the maximum evoked synaptic response was used to evoke test responses. Steady-state current–voltage (I–V) curves were produced by an initial step change in the holding potential from −80 to 120 mV. The membrane potential was then slowly changed in a ramp fashion from −120 to −40 mV (in ∼18 s) before returning to the holding potential. The current produced in response to this ramped membrane potential was recorded to produce the I–V curve.
All data were acquired using pCLAMP Software (Clampex 7 or 8; Axon Instruments Inc.) at a sampling rate of 6.6 kHz and filtered at 1 kHz and stored for off-line analysis. Each stored trace was an average of two successive synaptic responses elicited at 10 s intervals. Hard copy chart records were also captured on Gould chart recorders (TA 240, Gould Instruments System Inc., Valley View, OH, USA) in some experiments.
Data analysis
EPSC amplitudes were measured from baseline to peak and taken as the excitatory synaptic strength at the chosen stimulus intensity. Responses were normalized by taking the mean of the last three or four responses prior to drug application and dividing the rest of the responses by this mean. These normalized values were then used for average plots. For these plots, all cells receiving the same treatment were aligned at the first minute of drug application and averaged over the entire period. All values are stated as mean ± standard error. One-way ANOVA and post hoc tests, as indicated in the Results section, were used to compare different values or treatments using SigmaStat® (Jandel Scientific Software, San Rafael, CA, USA). Significance was taken at the level of P≤ 0.05. Graphs were plotted using SigmaPlot® (Jandel Scientific Software, San Rafael, CA, USA), GraphPad Prism® (GraphPad Software Inc, San Diego, CA, USA) and CorelDraw® (Corel Corporation, Ottawa, ON, Canada).
Drug preparation and sources
All drugs were prepared and were bath perfused at final concentrations indicated by dissolving aliquots of stock in the ACSF. SCH23390 and sulpiride were prepared daily and used within 24 h. Most routine laboratory chemicals as well as, 8-cyclopentyltheophylline (8-CPT), were from Sigma-Aldrich Chemie Gmbh (Steinheim, Germany). 6-7-Dinitroquinoxaline-2,3-dione (DNQX), SCH23390, sulpiride, proglumide were obtained from RBI (Natick, MA, USA); sulphated CCK octapeptide (CCK-8S), unsulphated CCK octapeptide (CCK-8US), LY225910, and CGP55845 were from Tocris (Bristol, UK).
Results
Recording of EPSCs in rostral accumbens cells
The results reported in this study were obtained from recordings in 78 cells located in the rostral pole of the core region of the NAc (Fig. 1). All cells were recorded very close to the stimulating electrode that was placed at the cortico-accumbens junction to activate prefrontal cortical excitatory afferents into this region (Fig. 1). By virtue of their relative abundance in the NAc, most of these recorded cells are likely to be medium spiny GABAergic neurones. These cells had passive and active membrane properties similar to those previously reported (Kombian & Malenka, 1994; Kombian et al. 2003a). All cells in this region had evoked responses composed of both glutamate- and GABA-mediated components. In the presence of picrotoxin 50 (μm) and at Vh of −80 mV, all of the evoked inward currents were glutamate-mediated excitatory postsynaptic currents (EPSCs) as they were completely blocked by DNQX (10 μm; data not shown but see Kombian et al. 2003a,b).
Effects of CCK on evoked EPSCs and NAc cells
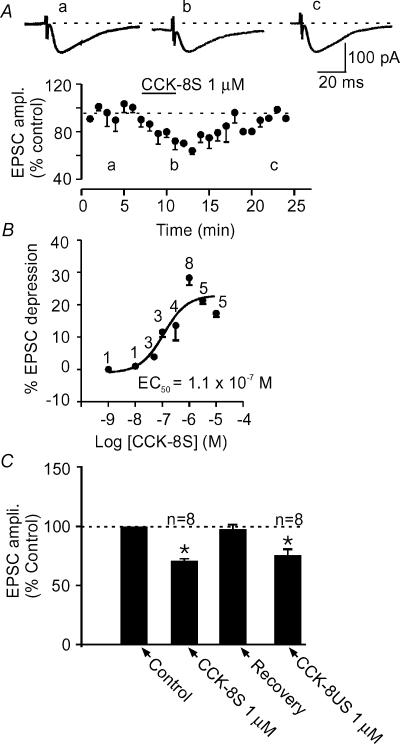
Bath application of CCK-8S for 5–6 min in the presence of picrotoxin (50 μm) consistently caused a decrease in the amplitude of evoked EPSCs in 41 out of 44 (>90%) cells tested with CCK-8S alone. The onset of action was between 1 and 2 min with a peak effect in about 5–6 min (Fig. 2A). This CCK-8S-induced EPSC depression was concentration-dependent, with a maximum synaptic depression observed at 1 μm (−28.8 ± 1.6%, n= 8) and a calculated EC50 of 0.11 μm (Fig. 2B). This effect of CCK-8S tended to decrease in magnitude at concentrations higher than 1 μm. It was reversible, showing a recovery of 93.2 ± 7.3% after 10–15 min of washing out CCK-8S (Fig. 2A). Similar magnitudes of depression were obtained by repeated application of CCK-8S (1 μm) to the same cell following the washout period (−34.1 ± 11.1% in the first application compared to −32.9 ± 6.7% in the repeat application; n= 3 cells; P >0.05, paired t test). In order to obtain a robust response that could be subjected to pharmacological characterization, 1 μm CCK-8S was employed for the rest of this study.
Figure 2. CCK inhibits evoked EPSCs in the NAc.
A, bath application of CCK-8S, the endogenously active analogue of CCK, caused a reversible depression of evoked EPSC amplitude. An average time–effect plot generated from eight cells that received 1 μm of CCK-8S for the duration indicated by the length of the line. Upper panel shows representative EPSC traces taken from the times indicated by letters in the time–effect plot. B, dose–response curve of CCK's effect on evoked EPSC amplitude obtained by applying different concentrations of CCK-8S. The calculated EC50 is 0.11 μm. The number above each point indicates the number of cells that received the corresponding concentration. C, a bar graph summarizing the effect of CCK-8S on evoked EPSC in comparison with CCK-8US. In this and in all other figures, asterisks indicate a significant difference compared to control at P < 0.05.
Similar to the action of CCK-8S (the endogenous peptide), the unsulphated peptide, CCK-8US at 1 μm concentration also caused a depression of the evoked EPSC amplitude. This depression (−24.3 ± 4.9%; n= 8, Fig. 2C) was similar in magnitude to that produced by CCK-8S (P > 0.05, unpaired t test). For reasons not clear to us, the effect of CCK-8US was not as consistent as that of CCK-8S, as four other cells did not respond to CCK-8US with a decrease in EPSC amplitude. In addition, the effect of CCK-8US was not reversible in our hands as cells depolarized to an extent that action potentials were riding on EPSCs and cells tended not to recover from the bigger depolarization. This occurred despite the fact that the synaptic depression at this concentration was comparable to that induced with 1 μm CCK-8S.
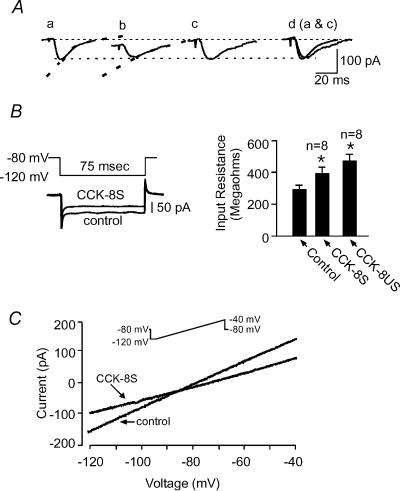
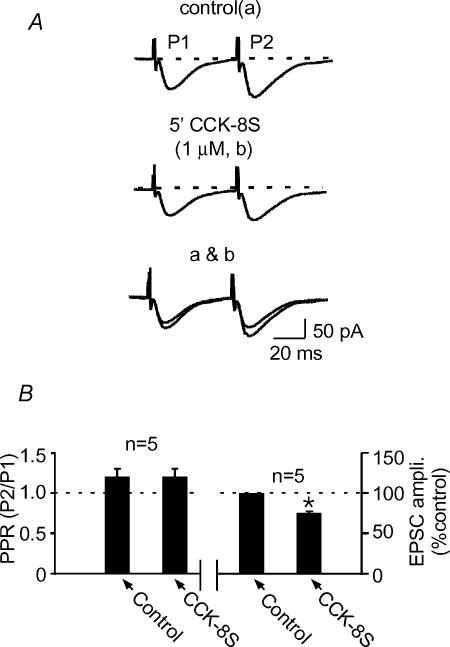
In addition to the synaptic depression, both CCK-8S and CCK-8US induced an inward current in ∼90% of cells tested (19 out of 21 cells). CCK-8S induced an inward current that ranged from 5 to 30 pA (24.2 ± 9.2 pA; Fig. 3A) that recovered upon washout. CCK-8US, on the other hand, induced inward currents ranging from 10 to 50 pA (n= 8/10 cells), most of which did not recover as cells fired action potentials and were lost in the process. The inward current was accompanied by an increase in the input resistance of the cells (295.0 ± 24.6 MΩ in control and 393.90 ± 38.6 MΩ in CCK-8S; n= 8; and 474.0 ± 39.4 MΩ in CCK-8US; n= 8; P < 0.05, one-way ANOVA; Fig. 3B). The steady-state I–V curve in the presence of CCK-8S intersected the curve produced in control at relatively negative potentials (−85 to −95 mV; Fig. 3C), producing an average estimated reversal potential (Erev) of −94.6 ± 3.0 mV (n= 4). In addition to these direct postsynaptic effects, CCK-8S also altered the kinetics of the evoked EPSC, causing a slowing in the decay rate resulting in an increase in the decay constant (τ) of evoked EPSCs in 6 out of 8 cells (τ= 16.3 ± 1.8 ms versus 28.5 ± 1.0 ms in the presence of CCK-8S 1 μm; n= 6; P < 0.05, Fig. 3A). One of the other two cells showed a decrease in τ(while the other showed no change. In contrast to these postsynaptic actions of CCK-8S, we did not detect a change in the paired pulse ratio (PPR), a test often used to test for presynaptic actions of drugs (Manabe et al. 1993). The PPR was 1.2 ± 0.1 in control and 1.2 ± 0.1 in the presence of CCK-8S (n= 5, P > 0.05; paired t test; Fig. 4). These latter results suggest that CCK-8S does not act directly to cause a decrease in glutamate release.
Figure 3. CCK-8S causes postsynaptic changes in NAc cells.
A, bath application of both CCK-8S and CCK-8US in most cells recorded in this region induced an inward current. Sample EPSCs recorded in control (a) and in the presence of 1 μm CCK-8S (b). Note the inward shift in the holding current in trace b. Dashed line on each trace is the exponential fit to the decay of the response. Trace c is trace b scaled to the amplitude of trace a, and d shows the scaled trace b superimposed on trace a. B, left panel: instantaneous steady-state current responses to 20 mV negative voltage steps (above) from the holding potential to measure input resistance (Rinput). Note that the inward current induced by CCK-8S was offset to superimpose this trace on the control trace. B, right panel: a bar graph summarizing the changes in Rinput induced by CCK-8S and CCK-8US, calculated from the traces on the left. C, steady-state current–voltage (I–V) relationships obtained from the same cell as in A in control and at the peak of the CCK-8S-induced synaptic depression. Inset shows the ramp protocol used to generate these curves.
Figure 4. CCK-8S does not cause a detectable change in paired pulse ratio.
A, sample traces of a pair of synaptic responses evoked at 50 ms interval in control (upper) and at the peak of CCK-8S-induced synaptic depression (middle) and both traces superimposed (bottom). B, summary bar graph showing that, despite the synaptic depression (right panel), the ratio between the second trace (P2) and the first trace (P1; [P2/P1]; paired pulse ratio) under both conditions remained unchanged (left panel).
CCK-induced cellular and synaptic effects are through CCKB receptors
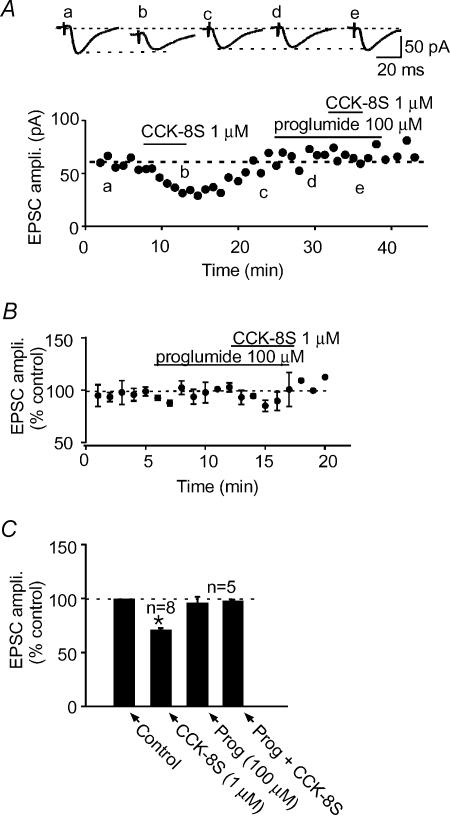
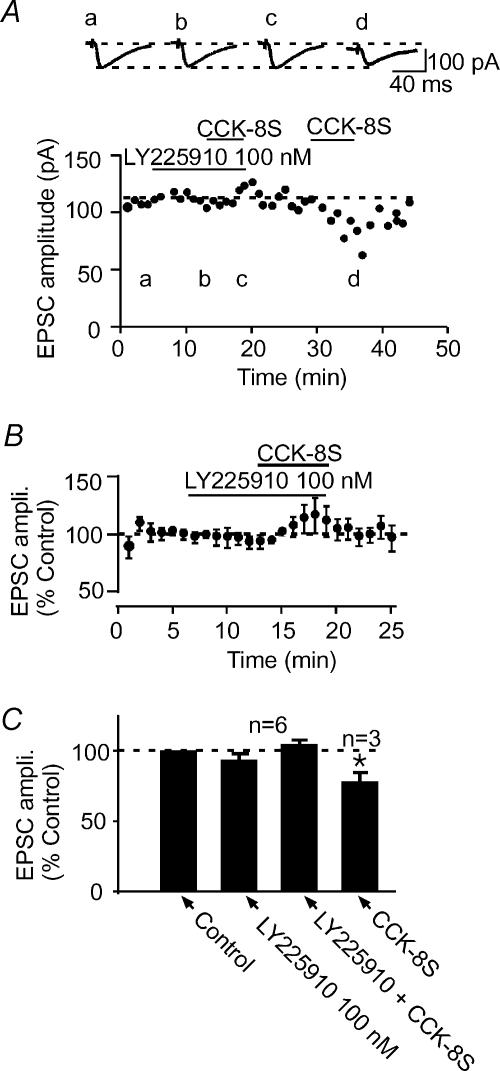
To verify that CCK-8S produced the above effects by activating CCK receptors present in the NAc (Innis & Snyder, 1980; Van Dijk et al. 1984; Gaudreau et al. 1985; Moran et al. 1986; Berresford et al. 1987; Mercer et al. 2000), we pretreated slices with proglumide (100 μm), a non-selective CCK receptor antagonist for 5–8 min and tested the effects of 1 μm CCK-8S. Cells, such as the one shown in Fig. 5A, that responded to CCK-8S with a robust synaptic depression no longer responded to CCK-8S in the presence of proglumide (−3.7 ± 1.7%, n= 5, P > 0.05 compared to control; Fig. 5). In addition, the postsynaptic current induced by CCK-8S was also blocked. Proglumide by itself did not produce significant changes in the postsynaptic holding current or synaptic response (−8.5 ± 13.2%, n= 5; P > 0.05 compared to control; paired t test; Fig. 5B and C). This effect of proglumide, combined with the fact that CCK-8US is known to selectively activate CCKB receptors (Innis & Snyder, 1980; Gaudreau et al. 1985), mimicking the postsynaptic and synaptic effects of the endogenous peptide CCK-8S (Figs. 2 and 3), suggests that CCKB receptors mediate these effects of the endogenous peptide. To verify this, we used a potent selective CCKB receptor antagonist, LY225910 (Yu et al. 1991), in an attempt to block the CCK effect. Bath application of 100 nm LY225910 for 5–6 min caused no change in the holding current and evoked EPSC amplitude (−8.4 ± 4.3%, n= 6; P > 0.05, Fig. 6). When CCK-8S was subsequently applied in the presence of LY225910, it neither caused the predicted decrease in EPSC amplitude (5.5 ± 4.6%, n= 6, P > 0.05, unpaired t test, Fig. 6) nor induced the inward current. In three of these cells, following 10–15 min washout of LY225910 and CCK-8S, subsequent re-application of CCK-8S alone induced an inward current (Fig. 6A, insert) as well as causing a decrease in the evoked EPSC amplitude (−22.1 ± 6.5%; P < 0.05 compared to control; paired t test; Fig. 6A and C). These results indicate that CCK-8S produces both the postsynaptic inward current and excitatory synaptic depressant effects by activating CCKB receptors.
Figure 5. CCK receptor antagonist blocks CCK-induced EPSC depression.
A, in a representative cell, CCK-8S (1 μm) causes a reversible depression of the evoked EPSC amplitude (a–c). Following washout of CCK-8S and pretreatment of the same cell with proglumide (Prog. 100 μm; d), 1 μm CCK-8S no longer had an effect on the evoked EPSC amplitude (e). Above this graph are sample EPSC traces taken from the times indicated by letters in the time–effect plot. B, normalized average time–effect plot obtained from five cells that were pretreated with Prog (100 μm), a-non-selective CCK receptor antagonist, prior to CCK-8S application. C, a bar graph summarizing the effects of 1 μm CCK-8S alone (from Fig. 2), and in the presence of Prog (100 μm).
Figure 6. CCKB receptor selective antagonist blocks CCK-induced EPSC depression.
A, in a representative cell, pretreatment of the slice with LY225910 (100 nm; b), a selective CCKB receptor antagonist, blocked the ability of 1 μm CCK-8S to depress the evoked EPSC amplitude (c). Following washout of LY225910 and CCK-8S, subsequent application of CCK-8S alone caused a robust depression of the evoked EPSC amplitude (d). Above this graph are sample EPSC traces taken from the times indicated by the letters in the time–effect plot. B, normalized average time–effect plot obtained from six cells that were pretreated with 100 nm LY225910 prior to CCK-8S. C, a bar graph summarizing the effects of 1 μm CCK-8S in the presence of LY225910 (100 nm) and alone (n= 3) following washout of the antagonist.
Dopamine, adenosine and CCK-induced EPSC depression
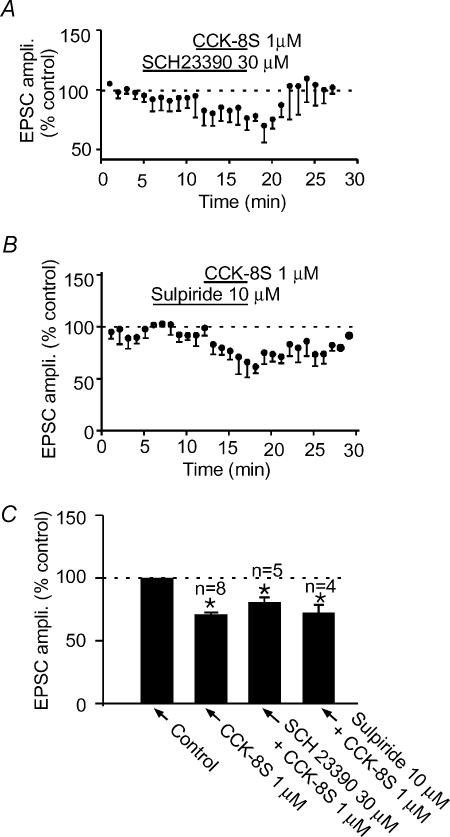
Several lines of evidence indicate that CCK interacts with DA in the NAc to modulate the function of this nucleus (Voigt et al. 1986; De Witte et al. 1987; Dauge et al. 1989; Vaccarino & Rankin, 1989; Marshall et al. 1991; Crawley, 1992; Ferraro et al. 1996; Reum et al. 1997). In particular, neurochemical evidence indicates that CCK-8S increases DA outflow in the rostral NAc, the region in which the current studies were conducted, although another group observed the exact opposite (Marshall et al. 1991). Because, DA has been shown to depress EPSCs in this region via D1-like receptors (Pennartz et al. 1992; Nicola et al. 1996; Harvey & Lacey, 1996), we tested to see if CCK-8S produced the observed depression by utilizing DA as an intermediate. When cells were pretreated with 30 μm SCH23390, a DA D1-like receptor antagonist that has been shown to completely block DA's synaptic effects in this nucleus (Harvey & Lacey, 1996; Nicola et al. 1996), CCK-8S subsequently still produced a significant depression of −17.0 ± 6.5% (n= 5; P < 0.05 compared to control; paired t test; Fig. 7A and C). This level of depression was, however, less than that produced in the absence of SCH23390 (−28.8 ± 1.6%; P < 0.05 compared to above depression, unpaired t test) obtained using the same batch of CCK-8S. This suggests that DA, acting on D1-like receptors, may contribute to the depressant effect of CCK-8S on evoked EPSCs. On the other hand, bath application of sulpiride (10 μm), a DA D2-like receptor antagonist predictably had no effect on the evoked EPSC. When CCK-8S (1 μm) was applied in the presence of sulpiride, it still produced a depression in evoked EPSC amplitude (−27.5 ± 9.6%, n= 4,P > 0.05 compared to the effect of the same batch of CCK-8S applied alone, unpaired t test; Fig. 7B and C).
Figure 7. Dopamine receptor antagonists do not completely block CCK-8S-induced synaptic depression.
Normalized average time–effect plots showing that pretreatment of slices with 30 μm SCH23390 (A), 10 μm sulpiride (B), D1-like and D2-like dopamine receptor antagonists, respectively, do not completely block the ability of CCK-8S to depress the EPSC amplitude. C, summary bar graph showing the inability of sulpiride (n= 4) and SCH23390 (n= 5) to completely block the CCK-induced EPSC depression.
Our previous studies in this nucleus also reported that adenosine, acting on A1 receptors, was a mediator of EPSC depression induced by substance P, another peptide that is present in the NAc (Kombian et al. 2003a). To determine if adenosine participated in mediating the CCK-8S-induced EPSC depression, we tested whether CCK-8S still depressed the EPSC in the presence of 1 μm 8-CPT, an adenosine A1 receptor antagonist, at a concentration previously shown to block adenosine effects in this nucleus (Kombian et al. 2003b). In three cells, 1 μm 8-CPT caused a predictable increase in EPSC amplitude (15.1 ± 3.8%). When CCK-8S was subsequently applied at the peak of this 8-CPT effect, it still caused a depression in the EPSC amplitude (−13.7 ± 2.8%). Because these experiments were performed using a different batch of CCK-8S, we did control experiments to verify that this batch was equipotent with previous batches. Using this batch of CCK-8S, 1 μm caused an EPSC depression of −13.5 ± 1.9% (n= 3) compared to a depression of −28.8 ± 1.6% (n= 8) produced by 1 μm of other batches (P < 0.05; unpaired t test). This level of depression was not significantly different (P > 0.05; unpaired t test) from the depression produced in the presence of 8-CPT. Taken together, these results indicate that DA but not adenosine plays a role, albeit minor, in the CCK-8S-induced decrease in EPSC in this nucleus.
GABA mediates CCK-8S-induced depression of evoked EPSCs
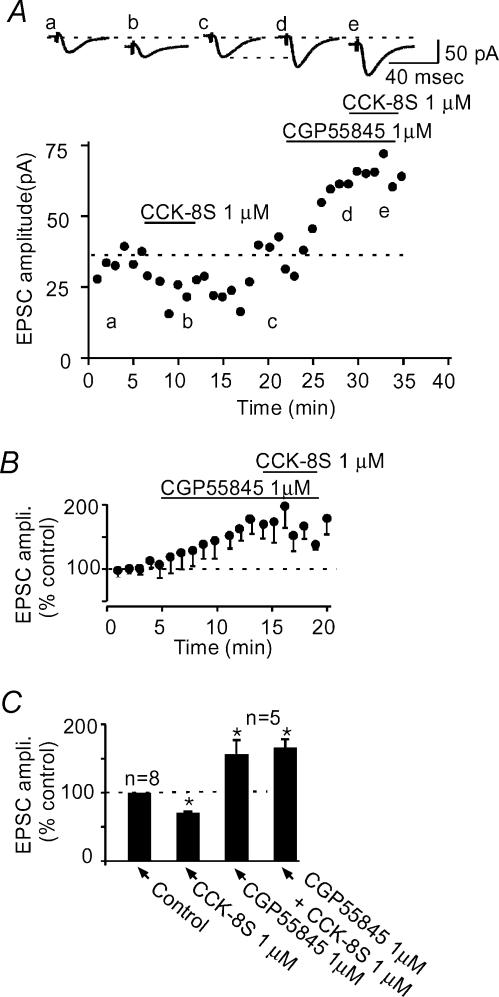
Because CCK has been reported to increase the release of GABA in this subregion of the NAc (Lanza & Makovec, 2000), and GABA is known to depress EPSCs in this nucleus through GABAB receptors (Uchimura & North, 1991), it is possible that GABA, acting on GABAB receptors mediated the CCK-induced decrease in EPSC amplitude. To examine if GABA does indeed play a role in the CCK-8S-induced synaptic depression, we blocked GABAB receptors using CGP55845, a potent GABAB receptor antagonist (Davies et al. 1993; Lacey & Curtis, 1994). CGP55845 (1 μm) by itself caused an enhancement in the evoked EPSC amplitude (56.7 ± 20.2%; n= 5; P < 0.05 compared to control, paired t test, Fig. 8), indicating a tonic action of GABA in depressing excitatory transmission in these cells. At the peak of the CGP55845 effect, CCK-8S was applied and it no longer depressed the evoked EPSC amplitude (7.0 ± 8.1%; n= 5; P > 0.05 compared to control, paired t test, Fig. 8). However, in all five cells tested, CCK-8S still caused an inward current (26.2 ± 8.7 pA; n= 5; P > 0.05 compared to the CCK-8S-induced current in control, unpaired t test, Fig. 8A), suggesting that the synaptic depressant effect and the postsynaptic excitation (inward current) are produced by different mechanisms. Furthermore, in four additional cells, when the paired pulse protocol was applied in the presence of CGP55845 (1 μm), this compound caused the expected increase in the evoked EPSC amplitude (58.7 ± 38.5%), which was accompanied by a predictable decrease in paired pulse facilitation (PPF) (1.8 ± 0.2 in control versus 1.2 ± 0.3 in CGP55845; P < 0.05; paired t test), suggesting that the GABAB receptors responsible for synaptic regulation in this nucleus are located on presynaptic glutamate terminals (see Fig. 9). Taken together, these results indicate that CCK-8S directly excites the medium spiny GABAergic neurones of the NAc to release GABA, which then acts on GABAB receptors located on glutamate terminals to decrease glutamate release and consequently depress the evoked EPSC.
Figure 8. A GABAB receptor antagonist completely blocks CCK-8S-induced synaptic depression.
A, a time–effect plot of a representative cell showing that CCK-8S reversibly depresses evoked EPSC (a–c). In the same cell, following washout of CCK-8S, application of CGP55845 (1 μm) causes an enhancement in the evoked EPSC amplitude (d). At the peak of this effect, CCK-8S no longer causes a depression in the EPSC amplitude (e). Above this graph are sample synaptic responses taken from the times indicated by the letters in the time–effect plot. Note that traces b and e, both taken in the presence of CCK-8S are associated with an inward current (displacement from dashed line). B, normalized average plot (n= 5 cells) showing the effect of CGP55845 on EPSC amplitude and subsequent lack of effect of CCK-8S in the presence of CGP55845. C, summary bar graph comparing the effect of CCK-8S in control (from Fig. 2) and in the presence of 1 μm CGP55845.
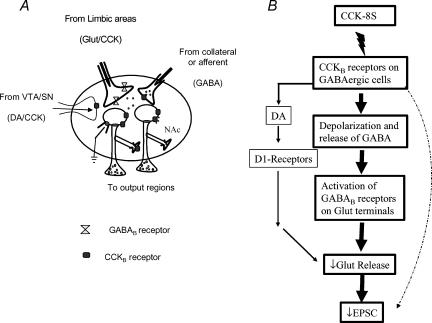
Figure 9. Schematic diagrams showing the synaptic organization of the NAc and the proposed mechanisms of action of CCK to decrease evoked EPSCs in the NAc.
A, CCKB receptors are present mainly on intrinsic projection neurones which form extensive axon collateral networks exerting a powerful inhibitory effect on to neighbouring cells. The NAc receives excitatory inputs from limbic areas, e.g. prefrontal cortex, amygdala and hippocampus which have GABAB receptors on their terminals. It also receives a dopaminergic input from the ventral tegmental area. CCK is colocalized with glutamate and DA in these afferent terminals. B, a schematic showing the possible mechanisms of action of CCK for depressing EPSC and the likely contributions of the different mechanisms to the CCK effect. The thickness of the arrows represents the postulated contributions of each pathway to the overall effect.
Discussion
The results obtained from this study show that CCK activates CCKB receptors in the rostral pole of the NAc to excite cells located in this region. This excitation leads to an increase in extracellular GABA and possibly DA. These latter transmitters then activate GABAB and D1-like receptors, respectively, to cause or contribute to the CCK-induced excitatory synaptic depression in this nucleus.
CCKB receptors mediate CCK's cellular and synaptic effects
Both CCKA and CCKB receptors are present in the NAc (Carlberg et al. 1992; Mercer et al. 2000) and, as such, either one or both receptors may be activated by CCK to produce the above effects. The endogenously active CCK receptor ligand, CCK-8S caused a synaptic depression that was mimicked by CCK-8US, a ligand that binds preferentially only to CCKB receptors (Innis & Snyder, 1980; Gaudreau et al. 1985) and blocked by a selective CCKB receptor antagonist LY 225910 (Yu et al. 1991), indicating that CCK depresses excitatory synaptic transmission by activating CCKB receptors. The effect of CCK-8S on the EPSC amplitude peaked at a concentration of about 1 μm and tended to decline thereafter. This can arise from a possible activation of CCKA receptors that may have effects opposite to those of CCKB receptors or may be due to the desensitization of the CCKB receptors at higher concentrations (Burdakov & Galione, 2000).
In addition to these synaptic effects, CCK also induced an inward current (depolarization) in most cells in this region resulting in excitation and firing of action potentials. Furthermore, it changed the kinetics of the recorded non-NMDA receptor-mediated current, which could contribute to the decrease in amplitude of the recorded synaptic current. The mechanism by which CCK produces this effect on the non-NMDA receptor kinetics is yet to be determined but could involve changes in either the desensitization rate (Vyklicky et al. 1991) or channel conductance or both. These postsynaptic effects were direct, through the CCKB receptors, as they were blocked by CCKB, but not GABAB or DA receptor antagonists. Thus, while the postsynaptic (cellular) effects of CCK-8S were produced directly through the activation of CCKB receptors, the synaptic depressant effect is more complicated, involving (a) a possible direct postsynaptic action of CCK on CCKB receptors and (b) an indirect action through GABA, and possibly DA.
Postsynaptic actions of CCK in NAc cells
The inward current induced by both CCK-8S and CCK-8US in NAc neurones resulted in their excitation as they fired action potentials that were superimposed on the evoked EPSC. This is in agreement with previous in vivo reports that showed that CCK excited NAc cells leading to increased single unit activity (Wang et al. 1985). This inward current was accompanied by an increase in the input resistance (Rinput) recorded around the resting potentials of these cells. This increase in Rinput suggests that a resting current was closed to produce the inward current. As these cells rest at very negative potentials (around −80 mV), the only currents that are active at such potentials are those carried by potassium. Furthermore, the very negative estimated reversal potential of the CCK-8S-induced current suggests that it is a potassium current. Thus, CCK-8S closes one or more potassium currents to depolarize these cells. This action of CCK-8S is via CCKB receptors which have been shown immunohistochemically to be present on somatodendrites of NAc cells (Mercer et al. 2000). This action of CCK contrasts with its action in the arcuate nucleus where it does not induce any current but instead potentiates A-currents to slow down the firing of these cells (Burdakov & Ashcroft, 2002). Interestingly, this effect was also produced through the activation of postsynaptic CCKB receptors, the same receptors that we observed here to cause the closure of this as-yet-uncharacterized potassium channel(s). It is important to know the nature of this potassium current as we strive to understand the actions of this peptide in the NAc and the CNS in general. In addition to the above, CCK-8S also slowed down the kinetics of the non-NMDA glutamate receptor-mediated EPSC. The increase in the decay constant (τ) suggests that CCK directly interacts with this channel to either decrease desensitization (Vyklicky et al. 1991) or affect other channel kinetics and this may be responsible, at least in part, for the decrease in the EPSC amplitude.
GABA, and to a lesser extent DA, mediates CCK-induced synaptic depression
The reported co-localization and interaction of CCK with DA in the NAc to influence several behaviours (Vaccarino & Koob, 1984; De Witte et al. 1987; Vaccarino & Rankin, 1989; Crawley, 1992) suggests that, at least, some of CCK's effects on cellular and synaptic responses and conductances in this nucleus may be mediated through DA. Indeed, while we found that CCK's effect on EPSC was slightly attenuated by SCH23390, a DA D1-like receptor antagonist that has been shown to block DA synaptic effects in this nucleus (Pennartz et al. 1992; Harvey & Lacey, 1996; Nicola et al. 1996), this blocking effect of SCH23390 was incomplete as CCK still caused a statistically significant depression of the evoked EPSC, albeit less than in control. This may mean that CCK does not rely strongly on DA to mediate its synaptic effects, or it may also reflect the well-documented opposing actions of CCK on DA release (Voigt et al. 1986; Lane et al. 1986; Ruggeri et al. 1987; Vickroy & Bianchi, 1989) whereby the opposing actions of CCKA and CCKB receptor activation can lead to a minimal change in DA release (Hamilton et al. 1984; Marshall et al. 1991). While it is possible for CCK to modulate the release of DA in vivo to affect DA-dependent behaviours, the finding here suggests that the main action of CCK in modulating these behaviours in the NAc (Vaccarino & Koob, 1984; De Witte et al. 1987; Vaccarino & Rankin, 1989; Crawley, 1992) may be direct, by influencing the response of NAc cells to DA rather than through the release or blockade of DA release. Even if the release of DA plays an important role in CCK actions, the level of activation of CCKA and CCKB receptors would have to be fine tuned to offset the balance in favour of one or the other. How this fine-tuning may be attained in vivo remains to be determined but the differential distribution of CCK-containing terminals and receptors in the different subregions of the NAc may allow for selective regional release and activation of only one type of receptor.
Further to this DA–CCK interaction, CCK has also been reported to increase the release of GABA in the rostral NAc (Lanza & Makovec, 2000). The depolarization and the resultant action potential firing observed above would cause an increase in the release of GABA from terminals of axon collaterals. The released GABA can act on appropriate receptors, usually GABAB receptors, to cause depression of excitatory synaptic transmission (Uchimura & North, 1991). These GABAB receptors are located on presynaptic glutamate terminals and their activation leads to a decrease in glutamate release and hence a decrease in the EPSC amplitude. This presynaptic locus of action of GABA to depress the EPSC is inferred from the observation that CGP55845 caused an increase in the evoked EPSC amplitude, an effect that was accompanied by a change (decrease) in paired pulse facilitation; a mainly presynaptic phenomenon (Manabe et al. 1993; Zucker, 1989). Our finding that CGP55845, the GABAB receptor antagonist, completely blocked the CCK-8S-induced synaptic effects, but not the inward current, indicates that CCK mainly employs GABA to mediate synaptic depression in this nucleus. This is in agreement with a recent report by Lanza & Makovec (2000) that CCK, in contrast to its opposing effect on DA release, causes only an increase in the release of GABA in the rostral NAc (but see Ferraro et al. 1996).
An intriguing finding in this study was that, despite the reported possible contribution of DA to the CCK-induced synaptic depression, blockade of GABAB receptors produced a complete block of the CCK effect, suggesting that this is the main mechanism responsible for the CCK-induced synaptic depression. This may happen if GABA's effect possibly overwhelms the contributions of direct postsynaptic and DAergic mechanisms to the CCK-induced synaptic depression. Alternatively, it may also indicate that DA produces some of its effect in the NAc indirectly through the release of GABA. This possibility needs to be further examined as it may reveal yet another novel mechanism by which DA produces synaptic depression in this nucleus (see Harvey & Lacey, 1997). Another intriguing finding was that, although both GABA and DA are widely known, and are reported in this nucleus to depress synaptic transmission by presynaptic mechanisms (Uchimura & North, 1991; Harvey & Lacey, 1996), we did not see a change in PPR, a paradigm often used to test for the presence of presynaptic action of substances in synaptic physiology (Zucker, 1989; Manabe et al. 1993; Kombian et al. 2003a). Our inability to detect a change in PPR in this case may be due to the reported inability of PPR to detect presynaptic effects when changes in synaptic responses are not greater than 60% of the initial response (Manabe et al. 1993). It may also be a consequence of the combined pre- and postsynaptic actions of CCK that mask any possible changes in PPR.
This study thus reveals that the effect of CCK in the NAc is to excite the projection medium spiny GABAergic neurones directly through the activation of CCKB receptors which are present on these cells. This excitation results in the release of GABA which is the main mediator of CCK-induced synaptic depression (Fig. 9). The minimal effect of DA observed here may reflect the well-documented opposing actions of CCK on DA release in this nucleus (see Introduction). The physiological significance of directly exciting NAc cells while depressing glutamate-mediated excitation is not yet clear to us. The obvious benefit of such a dual action would be to prevent or reduce excessive excitation of NAc neurones, especially if the direct postsynaptic excitation precedes excitatory synaptic depression (see Fig. 9B). This may be the case, as the inward current was usually observed first and peaked before the peak of the synaptic depression. The CCK-induced inhibition of excitatory synaptic transmission may help to ensure that afferent (cortical) control of NAc output is minimized while local intra-accumbal control is optimized (Fig. 9A). Since CCK itself does not appear to have a direct presynaptic effect on glutamate release (see Lanza & Makovec, 2000), its ability to select and dampen certain excitatory inputs would be limited except through intermediate modulators such as GABA.
Functionally, the NAc is thought to filter out competing afferent excitatory signals allowing only appropriate ones through. This enables animals to focus on only certain tasks at any particular time. If released CCK excites NAc projection neurones indiscriminately, then this ability of the NAc would be lost. This would lead to an inability to concentrate or focus on appropriate or relevant tasks and behaviours while ignoring irrelevant ones, a characteristic seen in schizophrenics. In this regard, it has been reported that latent inhibition in rats, an animal model that is relevant to schizophrenia, is modulated by CCK receptor antagonists (Feifel & Swerdlow, 1997; Gracey et al. 2000, 2002) suggesting that CCK over-activity may be involved in the pathophysiology of schizophrenia and other psychiatric disorders (Tachikawa et al. 2001; Hattori et al. 2001; Wang et al. 2002; De Wied & Sigling, 2002). Indeed, CCK receptor antagonists are being developed and evaluated as antipsychotic agents (Feifel & Swerdlow, 1997; Feifel et al. 1999). The selective action of such drugs in the NAc in altering synaptic and cellular excitability induced by CCK may serve as the basis for their therapeutic action.
Acknowledgments
Grant number KFAS-98-07-09 from the Kuwait Foundation for the Advancement of Science to S.B.K. supported this work.
References
- Berresford IJM, Hall MD, Clark CR, Hill RG, Hughes J, Sirinathsinghji DJ. Striatal lesions and transplants demonstrate that CCK receptors are localized on intrinsic striatal neurons: a quantitative autoradiographic study. Neuropeptides. 1987;10:109–136. doi: 10.1016/0143-4179(87)90014-x. [DOI] [PubMed] [Google Scholar]
- Buckby LE, Lacey MG. Depression of excitatory cortico-nucleus accumbens synaptic transmission in rat brain slices by dopamine, but not adenosine, depends upon intracortical mechanisms. Exp Brain Res. 2001;141:560–566. doi: 10.1007/s00221-001-0927-2. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Ashcroft FM. Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-Type potassium channels. J Neurosci. 2002;22:6380–6387. doi: 10.1523/JNEUROSCI.22-15-06380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Galione A. Two neuropeptides recruit different messenger pathways to evoke Ca2+ signals in the same cell. Curr Biol. 2000;10:993–996. doi: 10.1016/s0960-9822(00)00649-7. [DOI] [PubMed] [Google Scholar]
- Carlberg M, Gundlach AL, Mercer LD, Beart PM. Autoradiographic localization of cholecystokinin A and B receptors in rat brain using [125I]d-Tyr25 (Nle28,31) -CCK 25–33S. Eur J Neurosci. 1992;4:563–573. doi: 10.1111/j.1460-9568.1992.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Modulation of mesolimbic dopaminergic behaviors by cholecystokinin. Ann N Y Acad Sci. 1988;537:380–396. doi: 10.1111/j.1749-6632.1988.tb42121.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Cholecystokinin–dopamine interactions. Trends Pharmacol Sci. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Subtype-selective cholecystokinin receptor antagonists block cholecystokinin modulation of dopamine-mediated behaviors in the rat mesolimbic pathway. J Neurosci. 1992;12:3380–3391. doi: 10.1523/JNEUROSCI.12-09-03380.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauge V, Steimes P, Derrien M, Beau N, Roques BP, Feger J. CCK8 effects on motivational and emotional states of rats involve CCKA receptors of the postero-median part of the nucleus accumbens. Pharmacol Biochem Behav. 1989;34:157–163. doi: 10.1016/0091-3057(89)90367-5. [DOI] [PubMed] [Google Scholar]
- Davies CH, Pozza MF, Collingridge GL. CGP 55845A: a potent antagonist of GABAB receptors in the CA1 region of rat hippocampus. Neuropharmacology. 1993;32:1071–1073. doi: 10.1016/0028-3908(93)90073-c. [DOI] [PubMed] [Google Scholar]
- De Wied D, Sigling HO. Neuropeptides involved in the pathophysiology of schizophrenia and major depression. Neurotox Res. 2002;4:453–468. doi: 10.1080/10298420290031432. [DOI] [PubMed] [Google Scholar]
- De Witte P, Heidbreder C, Roques BP, Vanderhaeghen JJ. Opposite effects of cholecystokinin octapeptide (CCK8) and tetrapeptide (CCK4) after injection into the caudal part of the nucleus accumbens or into its rostral part and the cerebral ventricles. Neurochem Int. 1987;10:473–479. doi: 10.1016/0197-0186(87)90074-x. [DOI] [PubMed] [Google Scholar]
- Deutch AY. Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: implications for schizophrenia and Parkinson's disease. J Neural Transm General Sect. 1993;91:197–221. doi: 10.1007/BF01245232. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Seroogy KB. The distribution and some connections of cholecystokinin neurons in the rat brain. Ann N Y Acad Sci. 1985;448:121–132. doi: 10.1111/j.1749-6632.1985.tb29912.x. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza T, Robeck S. Antipsychotic potential of CCK-based treatments: an assessment using the prepulse inhibition model of psychosis. Neuropsychopharmacology. 1999;20:141–149. doi: 10.1016/S0893-133X(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Feifel D, Swerdlow NR. The modulation of sensorimotor gating deficits by mesolimbic cholecystokinin. Neurosci Lett. 1997;229:5–8. doi: 10.1016/s0304-3940(97)00409-6. [DOI] [PubMed] [Google Scholar]
- Ferraro L, O'Connor WT, Li XM, Rimondini R, Beani L, Ungerstedt U, Fuxe K, Tanganelli S. Evidence for a differential cholecystokinin-B and -A receptor regulation of GABA release in the rat nucleus accumbens mediated via dopaminergic and cholinergic mechanisms. Neuroscience. 1996;73:941–950. doi: 10.1016/0306-4522(96)00098-x. [DOI] [PubMed] [Google Scholar]
- Gaudreau P, St Pierre S, Pert CB, Quirion R. Cholecystokinin receptors in mammalian brain. A comparative characterization and visualization. Ann N Y Acad Sci. 1985;448:198–219. doi: 10.1111/j.1749-6632.1985.tb29919.x. [DOI] [PubMed] [Google Scholar]
- Gilles C, Lotstra F, Vanderhaeghen JJ. CCK nerve terminals in the rat striatal and limbic areas originate partly in the brain stem and partly in telencephalic structures. Life Sc. 1983;32:1683–1690. doi: 10.1016/0024-3205(83)90829-9. [DOI] [PubMed] [Google Scholar]
- Gracey DJ, Bell R, King DJ. PD-135,158, a cholecystokinin (B) antagonist, enhances latent inhibition in the rat. Pharmacol Biochem Behav. 2000;65:459–463. doi: 10.1016/s0091-3057(99)00227-0. [DOI] [PubMed] [Google Scholar]
- Gracey DJ, Bell R, King DJ. Differential effects of the CCKA receptor ligands PD-140,548 and A-71623 on latent inhibition in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:497–504. doi: 10.1016/s0278-5846(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Graham WC, Hill DR, Woodruff GN, Sambrook MA, Crossman AR. Reduction of [123I]Bolton Hunter CCK8 and [3H]MK-329 (devazepide) binding to CCK receptors in the substantia nigra/VTA complex and its forebrain projection areas following MPTP-induced hemi-parkinsonism in the monkey. Neurosci Lett. 1991;131:129–134. doi: 10.1016/0304-3940(91)90353-u. [DOI] [PubMed] [Google Scholar]
- Hamilton M, Sheehan MJ, de Belleroche J, Herberg LJ. The cholecystokinin analogue, caerulein, does not modulate dopamine release or dopamine-induced locomotor activity in the nucleus accumbens of rat. Neurosci Lett. 1984;44:77–82. doi: 10.1016/0304-3940(84)90224-6. [DOI] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. Endogenous and exogenous dopamine depress EPSCs in rat nucleus accumbens in vitro via D1 receptors activation. J Physiol. 1996;492:143–154. doi: 10.1113/jphysiol.1996.sp021296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori E, Yamada K, Toyota T, Yoshitsugu K, Toru M, Shibuya H, Yoshikawa T. Association studies of the CT repeat polymorphism in the 5′ upstream region of the cholecystokinin B receptor gene with panic disorder and schizophrenia in Japanese subjects. Am J Med Genet. 2001;105:779–782. doi: 10.1002/ajmg.10043. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Lundberg JM, Schultzberg M, Johansson O, Skirboll L, Anggard A, Fredholm B, Hamberger B, Pernow B, Rehfeld J, Goldstein M. Cellular localization of peptides in neural structures. Proc R Soc Lond B Biol Sci. 1980;210:63–77. doi: 10.1098/rspb.1980.0119. [DOI] [PubMed] [Google Scholar]
- Innis RB, Snyder SH. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci USA. 1980;77:6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Ananthalakshmi KVV, Parvathy SS, Matowe WC. Substance P depresses excitatory synaptic transmission in the nucleus accumbens through dopaminergic and purinergic mechanisms. J Neurophysiol. 2003a;89:728–738. doi: 10.1152/jn.00854.2002. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Ananthalakshmi KVV, Parvathy SS, Matowe WC. Dopamine and adenosine mediate substance P-induced depression of evoked IPSCs in the rat nucleus accumbens in vitro. Eur J Neurosci. 2003b;18:303–311. doi: 10.1046/j.1460-9568.2003.02753.x. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Malenka RC. Simultaneous LTP of non-NMDA and LTD of NMDA receptor-mediated responses in the nucleus accumbens. Nature. 1994;368:242–246. doi: 10.1038/368242a0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system. Ann N Y Acad Sci. 1988;357:216–227. doi: 10.1111/j.1749-6632.1988.tb42108.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Lacey G, Curtis DR. Phosphinic acid derivatives as baclofen agonists and antagonists in the mammalian spinal cord: an in vivo study. Exp Brain Res. 1994;101:59–72. doi: 10.1007/BF00243217. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ. Cholecystokinergic innervation of nucleus accumbens subregions. Peptides. 1998;19:859–868. doi: 10.1016/s0196-9781(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Lane RF, Blaha CD, Phillips AG. In vivo electrochemical analysis of cholecystokinin-induced inhibition of dopamine release in the nucleus accumbens. Brain Res. 1986;397:200–204. doi: 10.1016/0006-8993(86)91388-0. [DOI] [PubMed] [Google Scholar]
- Lanza M, Makovec F. Cholecystokinin (CCK) increases GABA release in the rat rostral nucleus accumbens via CCK (B) receptors located on glutamatergic interneurons. Naunyn-Schmiedeberg's Arch Pharmacol. 2000;361:33–38. doi: 10.1007/s002109900161. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Barnes S, Hughes J, Woodruff GN, Hunter JC. Cholecystokinin modulates the release of dopamine from the anterior and posterior nucleus accumbens by two different mechanisms. J Neurochem. 1991;56:917–922. doi: 10.1111/j.1471-4159.1991.tb02009.x. [DOI] [PubMed] [Google Scholar]
- Mercer LD, Le VQ, Nunan J, Jones NM, Beart PM. Direct visualization of cholecystokinin subtype2 receptors in rat central nervous system using anti-peptide antibodies. Neurosci Lett. 2000;293:167–170. doi: 10.1016/s0304-3940(00)01504-4. [DOI] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. New York: Academic Press; 1998. [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes daSilva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Reum T, Schafer U, Marsden CA, Fink H, Morgenstern R. Cholecystokinin increases extracellular dopamine overflow in the anterior nucleus accumbens via CCK (B) receptors in the VTA assessed by in vivo voltammetry. Neuropeptides. 1997;31:82–88. doi: 10.1016/s0143-4179(97)90025-1. [DOI] [PubMed] [Google Scholar]
- Ruggeri M, Ungerstedt U, Agnati L, Mutt V, Harfstrand A, Fuxe K. Effects of cholecystokinin peptides and neurotensin on dopamine release and metabolism in the rostral and caudal part of the nucleus accumbens using intracerebral dialysis in the anaesthetized rat. Neurochem Int. 1987;10:509–520. doi: 10.1016/0197-0186(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Fallon JH. Forebrain projections from cholecystokininlike-immunoreactive neurons in the rat midbrain. J Comp Neurol. 1989;279:415–435. doi: 10.1002/cne.902790307. [DOI] [PubMed] [Google Scholar]
- Studler JM, Reibaud M, Tramu G, Blanc G, Glowinski J, Tassin JP. Distinct properties of cholecystokinin-8 and mixed dopamine- cholecystokinin-8 neurons innervating the nucleus accumbens. Ann N Y Acad Sci. 1985;448:306–314. doi: 10.1111/j.1749-6632.1985.tb29926.x. [DOI] [PubMed] [Google Scholar]
- Tachikawa H, Harada S, Kawanishi Y, Okubo T, Suzuki T. Linked polymorphisms (-333G>T and -286A>G) in the promoter region of the CCK-A receptor gene may be associated with schizophrenia. Psychiatry Res. 2001;103:147–155. doi: 10.1016/s0165-1781(01)00276-1. [DOI] [PubMed] [Google Scholar]
- Uchimura N, North RA. Baclofen and adenosine inhibit synaptic potentials mediated by gamma-aminobutyric acid and glutamate release in rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258:663–668. [PubMed] [Google Scholar]
- Vaccarino FJ. Nucleus accumbens dopamine–CCK interactions in psychostimulant reward and related behaviors. Neurosci Biobehav Rev. 1994;18:207–214. doi: 10.1016/0149-7634(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ, Koob GF. Microinjections of nanogram amounts of sulfated cholecystokinin octapeptide into the rat nucleus accumbens attenuates brain stimulation reward. Neurosci Lett. 1984;52:61–66. doi: 10.1016/0304-3940(84)90351-3. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ, Rankin J. Nucleus accumbens cholecystokinin (CCK) can either attenuate or potentiate amphetamine-induced locomotor activity: evidence for rostral-caudal differences in accumbens CCK function. Behav Neurosci. 1989;103:831–836. [PubMed] [Google Scholar]
- Van Dijk A, Richards JG, Trzeciak A, Gillessen D, Mohler H. Cholecystokinin receptors: biochemical demonstration and autoradiographical localization in rat brain and pancreas using [3H] cholecystokinin-8 as radioligand. J Neurosci. 1984;4:1021–1033. doi: 10.1523/JNEUROSCI.04-04-01021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickroy TW, Bianchi BR. Pharmacological and mechanistic studies of cholecystokinin-facilitated [3H]dopamine efflux from rat nucleus accumbens. Neuropeptides. 1989;13:43–50. doi: 10.1016/0143-4179(89)90020-6. [DOI] [PubMed] [Google Scholar]
- Voigt M, Wang RY, Westfall TC. Cholecystokinin octapeptides alter the release of endogenous dopamine from the rat nucleus accumbens in vitro. J Pharmacol Exp Ther. 1986;237:147–153. [PubMed] [Google Scholar]
- Vyklicky L, Patneau DK, Jr, Mayer ML. Modulation of excitatory synaptic transmission by drugs that reduce desensitization of AMPA/kainite receptors. Neuron. 1991;7:971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Wang RY. Cholecystokinin, dopamine, and schizophrenia: recent progress and current problems. Ann N Y Acad Sci. 1988;537:362–379. doi: 10.1111/j.1749-6632.1988.tb42120.x. [DOI] [PubMed] [Google Scholar]
- Wang RY, White FJ, Voigt MM. Interactions of cholecystokinin and dopamine in the nucleus accumbens. Ann N Y Acad Sci. 1985;448:352–360. doi: 10.1111/j.1749-6632.1985.tb29930.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wassink T, Andreasen NC, Crowe RR. Possible association of a cholecystokinin promoter variant to schizophrenia. Am J Med Genet. 2002;114:479–482. doi: 10.1002/ajmg.10408. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Interactions of cholecystokinin octapeptide and dopamine on nucleus accumbens neurons. Brain Res. 1984;300:161–166. doi: 10.1016/0006-8993(84)91352-0. [DOI] [PubMed] [Google Scholar]
- Yu MJ, Thrasher KJ, McCowan JR, Mason NR, Mendelsohn LG. Quinazolinone cholecystokinin-B receptor ligands. J Med Chem. 1991;34:1505–1508. doi: 10.1021/jm00108a040. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14:427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Borg JS. On the significance of the sub-territories in the ‘accumbens’ part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]