Abstract
Motoneurones (MNs) are particularly affected by the inhibition of mitochondrial metabolism, which has been linked to their selective vulnerability during pathophysiological states like hypoxia and amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disorder. To elucidate underlying events, we used sodium cyanide (CN) as a pharmacological inhibitor of complex IV of the mitochondrial respiratory chain (‘chemical hypoxia’) and investigated the cellular response in vulnerable and resistant neurone types. Bath application of 2 mm CN activated TTX-insensitive Na+ conductances in vulnerable hypoglossal MNs, which depolarized these MNs by 10.2 ± 1.1 mV and increased their action potential activity. This response was mimicked by sodium azide (2 mm) and largely prevented by preincubation with the antioxidants ascorbic acid (1 mm) and Trolox (750 μm), indicating an involvement of reactive oxygen species (ROS) in the activation mechanism. CN also elevated cytosolic [Ca2+] levels through (i) Ca2+ release from mitochondria-controlled stores, (ii) significant retardation of cytosolic Ca2+ clearance rates, even when cytosolic ATP levels were held constant during whole-cell recording, and (iii) secondary Ca2+ influx during elevated firing rates. Blocking mitochondrial ATP production additionally raised cytosolic Ca2+ levels and prolonged recovery of Ca2+ transients with a delay of 5–6 min. Comparative studies on hypoglossal MNs, facial MNs and dorsal vagal neurones suggested that CN responses were dominated by the activation of K+ conductances in resistant neurones, thus reducing excitability during mitochondrial inhibition. In summary, our observations therefore support a model where selective MN vulnerability results from a synergistic accumulation of risk factors, including low cytosolic Ca2+ buffering, strong mitochondrial impact on [Ca2+]i, and a mitochondria-controlled increase in electrical excitability during metabolic disturbances.
Motoneurones (MNs) are particularly vulnerable to inhibition of mitochondrial metabolism, which occurs during cellular hypoxia. This observation is based on several in vivo and in vitro studies, where MNs have been shown to be more sensitive to short periods of oxygen deprivation than other cell types such as cortical neurones (O'Reilly et al. 1995; Pierrefiche et al. 1997; Telgkamp & Ramirez, 1999). The exceptional vulnerability of MNs to mitochondrial inhibition was also demonstrated for chemical disruption of the respiratory chain. Cultured MNs from spinal cord degenerated significantly faster when exposed to mitochondrial inhibitors such as malonate or sodium azide compared to neurones in the dorsal horn (Kaal et al. 2000). Also in vivo, MN vulnerability could be demonstrated, in particular, after inhibition of the respiratory chain by dietary cyanide (Tylleskar et al. 1992; Soto-Blanco et al. 2002).
There are several observations linking inhibition of mitochondrial metabolism in MNs to the neurodegenerative disorder amyotrophic lateral sclerosis (ALS), where selective MN degeneration leads to death usually within 5 years (Beal, 2000; Rowland & Shneider, 2001). Chronic inhibition of the respiratory chain with sodium azide or malonate is an accepted in vitro model for ALS based on the selective pattern of neuronal degeneration in spinal cord cultures (Kaal et al. 2000). Also, in a familial form of ALS, dysfunction of oxidative phosphorylation induced by a mutated Cu/Zn super oxide dismutase (SOD1) is thought to be causally involved in MN degeneration (Jung et al. 2002; Mattiazzi et al. 2002; Menzies et al. 2002). Finally, hypoxia and the resulting decline in mitochondrial metabolism have been proposed as causative or modifying factors in ALS. This is based on the observation that impaired vascular endothelial growth factor (VEGF) synthesis due to hypoxia selectively damages MNs (Oosthuyse et al. 2001; Lambrechts et al. 2003).
However, little is known about the underlying mechanisms rendering motoneurones more vulnerable to mitochondrial impairment than other cell types. To elucidate underlying events, we investigated the consequences of mitochondrial inhibition on neuronal excitability and [Ca2+]i in selectively vulnerable and resistant brainstem neurones. The experiments were performed on hypoglossal MNs, facial MNs and dorsal vagal neurones in brainstem slice preparations from mice, where mitochondrial inhibition was induced by bath application of sodium cyanide. By this, our study identifies mechanisms that potentially account for the sensitivity of motoneurones following mitochondrial inhibition.
Methods
Preparation of acute mouse brain slices
Brain stem slices were obtained from young (1–5 days) NMRI mice. Animal experiments were carried out in accordance with the guidelines of the Ethics Committee of the University of Göttingen. Animals were decapitated, brains were removed and subsequently cooled in artificial cerebrospinal fluid (aCSF, mm: 118 NaCl, 3 KCl, 1 MgCl2, 25 NaHCO3, 1 NaH2PO4, 1.5 CaCl2, 30 glucose; pH 7.4; 325 mosmol l−1) at 4°C. Transverse slices of the brainstem were cut with a thickness of ∼200 μm using a vibroslicer (Leika VT 1000S) according to a method previously described (Ladewig & Keller, 2000). To ensure maximum oxygen supply, aCSF was continuously bubbled with carbogen (95% O2, 5% CO2). After slicing, slices were maintained at 30°C for 15 min and then allowed to cool down to room temperature (RT, 20–23°C). All experiments were carried out at RT.
Electrophysiological recordings
In patch clamp experiments, suitable MNs were selected by their intact overall shape, their ability to fire action potentials in drug-free solution and the occurrence of spontaneous synaptic activity. The intracellular pipette solution contained (mm) 140 KCl (alternatively 120 CsCl and 20 TEA), 10 Hepes, 2 MgCl2, 4 Na2-ATP, 0.4 Na-GTP (adjusted to pH 7.3 with KOH or CsOH). Patch pipettes were pulled from borosilicate glass tubes (KIMAX-51, Kimble Products, USA). When filled with intracellular solution, they had resistances of 1.8–3.5 MΩ. Voltage clamp and current clamp recordings were performed with an EPC-9 patch clamp amplifier (HEKA Electronics, Lambrecht, Germany). Membrane seals displayed resistances >1 GΩ. After seal rupture, series resistance (Rs, usually 6–15 MΩ) was continuously monitored and cells displaying Rs higher than 20 MΩ were excluded from analysis. Voltage and current pulse generation and data acquisition were performed with a Macintosh computer running Pulse software (HEKA Electronics). An oscillographic recorder (OR1400, Yokogawa, Herrsching, Germany) and a Macintosh computer running Acquire software (Bruxton Corporation, Seattle, WA, USA) were additionally used for data acquisition. Unless stated otherwise, whole-cell currents were recorded with sampling frequencies of 4–10 kHz and filtered (Bessel filter, 2.9 kHz) before analysis.
Fluorescence measurements
For fluorescence measurements, a modified version of the CCD camera system (TILL Photonics, Planegg, Germany) was employed as previously described (Ladewig & Keller, 2000). Briefly, a computer-controlled monochromator based on a galvanometric scanner (Polychrome II, TILL Photonics) was connected to an upright microscope (Axioskop, Fa. Zeiss, Göttingen, Germany) via quartz fibre optics and a minimum number of optical components for maximum fluorescence excitation (objective Achroplan W × 63, 0.9 W). A 12 bit CCD camera (PCO, Germany) was employed to monitor fluorescence changes in defined ‘regions of interest’ (ROIs) using a PC running TILLvisION 4.0 software (TILL Photonics, Martinsried, Germany); binning was set to 4 × 4, exposure time was 30–80 ms, sampling rate varied between 3 and 13 Hz.
Changes in the metabolic state of motoneurones were assessed by changes in the NADH autofluorescence excited at 360 nm (Kovacs et al. 2002). Increase in NADH autofluorescence indicates accumulation of NADH. Changes in mitochondrial membrane potential (ΔΨ) were monitored using rhodamine 123 (rhod123) introduced into the electrode solution at 10 μg ml−1 according to a previously described method (Schuchmann et al. 2000). Because of its positive charge rhod123, accumulates in mitochondria, where its fluorescence is quenched. Upon depolarization of ΔΨ, rhod123 is released from mitochondria and fluorescence increases. The clear differences in excitation spectra allowed for measurement of changes in autofluorescence and ΔΨ simultaneously. For this purpose, alternate excitation was done at 360 nm (ΔΨ) and 475 nm (rhod123), and a dichroic mirror with mid-reflection at 510 nm was used. Changes in cytosolic [Ca2+] ([Ca2+]i) were monitored using fura-2 (Kd∼0.2 μm) introduced into the pipette solution at 100 μm. Fura-2 was alternately excited at 356 nm and 385 nm, emitted light was directed to a dichroic mirror with mid-reflection at 425 nm and filtered by a band-pass filter (505–530 nm). Background fluorescence, which primarily represented mitochondrial autofluorescence, was measured in a region next to the fura-2-filled cell for both excitation wavelengths and subtracted from each image before calculating the ratio. Fluorescence intensities of fura-2 were converted into Ca2+ concentrations according to Grynkiewicz et al. (Grynkiewicz et al. 1985), assuming Kd to be in the range of 224–240 nm for hypoglossal MNs (Lips & Keller, 1999; Ladewig & Keller, 2000). The fluorescence ratios Rmin and Rmax were determined ‘in vivo’ by patch clamping neurones with intracellular solutions containing either no Ca2+ and 10 mm BAPTA (Rmin) or 10 mm Ca2+ (Rmax). Autofluorescence and rhod123 fluorescence intensities are given in relative values, F/F0, where F0 is the baseline fluorescence before stimulus or drug application. Further analysis of fluorescence recordings was performed off-line with Pulsefit (Heka) and IGOR (Wavemetrics, Lake Oswego, OR, USA) software.
Materials
Fura-2 pentapotassium salt was purchased from Molecular Probes (Leiden, Netherlands), tetrodotoxin citrate (TTX), 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX) and D-(–)-2-amino-5-phosphonopentanoic acid (AP-5) were from Tocris (Bristol, UK). All other substances were from Sigma-Aldrich. 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), rhodamine 123 and carbonyl cyanide 4-trifluoro-methoxyphenylhydrazone (FCCP) were dissolved in ethanol as stocks of 250 mm, 10 mg ml−1 and 10 mm, respectively. Cyclopiazonic acid (CPA) and oligomycin were dissolved in DMSO (250 mm and 10 mg ml−1), sodium cyanide (CN), sodium azide and ascorbic acid were dissolved as × 1000 stock in water. The CN stock solution was kept on ice and diluted to the final concentration immediately before the experiment.
Statistical analysis
If not indicated otherwise, values in the text are given as mean ± standard error of the mean (s.e.m.); error bars in figures also represent s.e.m. The significance after pharmacological intervention was calculated using Student's t test. The significance of linear correlation coefficients (r0) was determined by calculation of the probability P(|r|≥r0) according to Taylor (1982).
Results
Impact of CN on electrical properties of hypoglossal MNs
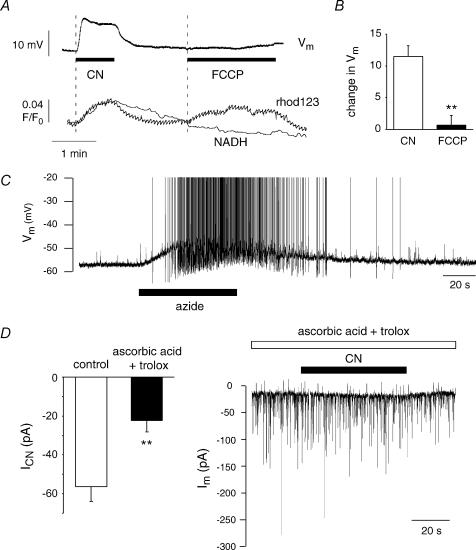
To investigate the impact of mitochondrial inhibition, patch clamp experiments were performed on vulnerable hypoglossal motoneurones (MNs) and mitochondrial function was disturbed by bath application of 1–2 mm sodium cyanide (CN), which inhibits complex IV (cytochrome c oxidase) of the electron transport chain. This protocol was chosen for two reasons: (i) it is considered to be a valid model for hypoxia (‘chemical hypoxia’), and (ii) in ALS, a notable decrease in complex IV activity has been observed (Menzies et al. 2002; Wiedemann et al. 2002). Additionally, CN action has been described as quick and reversible (Nowicky & Duchen, 1998; Kawai et al. 1999; Müller et al. 2002).
In current clamp mode, hypoglossal MNs displayed a resting membrane potential (Vm) of –62.1 ± 1 mV (n= 12). Approximately 30% of the cells showed spontaneous spike discharge with a mean discharge frequency of 0.27 ± 0.19 Hz when checked within 1 min before drug application. Upon addition of 2 mm CN, hypoglossal MNs depolarized by 10.2 ± 1.1 mV (n= 9) within 15 s (Fig. 1A) and mean discharge frequencies increased to 0.46 ± 0.2 Hz (P < 0.01). CN also increased synaptic activity as seen in Fig. 4A. After washout of CN, potential changes reversed within 1 min, whereas synaptic and action potential activity returned to baseline levels within 3 min. To investigate the relative contribution of synaptic activity and firing rates, we performed CN applications in the presence of synaptic blockers (10 μm CNQX, 100 μm APV, 10 μm bicuculline and 10 μm strychnine) and TTX (Fig. 1B and C). When postsynaptic receptors were blocked, hypoglossal MNs displayed a mean resting Vm of –63.3 ± 1.6 mV (n= 12) and a CN-induced depolarization of 13.8 ± 3 mV, n= 11, P > 0.5, which was comparable to control responses.
Figure 1. Sodium cyanide (CN) depolarizes hypoglossal motoneurones (MNs) and enhances neuronal activity.
A, membrane potential (Vm) recorded from a patch-clamped hypoglossal MN in acute mouse brainstem slice. Bath application of CN (2 mm) quickly induced MN depolarization and enhanced synaptic activity, leading to spontaneous action potential generation. Wash-out of CN repolarized membrane potential and reduced synaptic activity over 1–2 min; finally action potential activity stopped. B and C, the depolarizing response of hypoglossal MNs to CN persists in the presence of TTX (0.5 μm) and/or during blockade of postsynaptic receptors with (μm) 10 CNQX, 100 APV, 10 bicuculline (bic) and 10 strychnine (stry), indicating that membrane depolarization was mediated by changes in MN membrane properties and not by changes in synaptic transmission or glutamate release.
Figure 4. Effect of CN on [Ca2+]i in hypoglossal MNs.
A, B, simultaneous recording of membrane potential (Vm) and [Ca2+]i in a patch-clamped hypoglossal MN. Action potential (AP) activity was blocked with 0.5 μm TTX. Bath application of CN (2 mm) first depolarized Vm and then increased resting [Ca2+]i with a delay of ∼18 s both in the soma and primary dendrites. After wash-out of CN, [Ca2+]i slowly returned to baseline C, fura-2 filled MN. Rectangles indicate areas of recording (A, B). D, because ICN depolarized MNs by 10.2 ± 1.1 mV and increased AP firing (see Fig. 1A), the relations between Vm, AP discharge and increase in [Ca2+]i were investigated in experiments where AP firing was elicited by increasing current injection over a defined time interval (5 s) and corresponding [Ca2+] changes were assessed (n= 9). The AP frequency/Vm relation (left) could be approximated by a sigmoid curve; [Ca2+]i changes in response to AP firing were well described by a linear function (right). The plots indicate that the MN resting membrane potential is a critical determinant for ICN-induced increases in AP firing rates and subsequent Ca2+ influx, and (ii) ICN-induced depolarizations can account for basal Ca2+ elevations of ∼100 nm, provided that the resting membrane potential of cells is in the appropriate voltage range. (A–D, intracellular solution contained 100 μm fura-2.)
When 0.5 μm TTX was added alone (Fig. 1B) or in addition to synaptic blockers, CN-induced depolarizations were again comparable to control (11.3 ± 1.7 mV, n= 7; 13.3 ± 2 mV, n= 6; P > 0.5; Fig. 1C), indicating that they primarily resulted from intrinsic membrane conductances.
CN-induced inward currents (ICN)
Membrane conductances in motoneurones were further investigated in voltage clamp mode, where bath application of 2 mm CN activated an inward current ICN=–51 ± 9 pA (n= 8, holding potential –60 mV). Its magnitude was relatively constant in a voltage range of –80 to –40 mV as revealed by voltage ramp protocols (20 mV/100 ms, not shown). To identify the underlying charge carrier, ICN was investigated under different ionic and pharmacological conditions (Fig. 2A). At first we studied the potential contribution of persistent, TTX-sensitive Na+ channels which have recently been described in hypoglossal MNs (Powers & Binder, 2003). In agreement with the current clamp data (compare Fig. 1B), TTX did not significantly alter ICN (–56 ± 13 pA, n= 7, P > 0.05). A contribution of K+ conductances was probed by blocking K+ currents with tetraethylammonium chloride (TEA, 20 mm internal and 10 mm external) and replacing K+ by caesium in the pipette, without any significant effect (ICN=–62 ± 15 pA, n= 10, P > 0.05). Blockade of Ca2+ currents by the non-selective Ca2+ channel blocker CdCl2 (200 μm) also did not significantly change ICN (–46 ± 8 pA, n= 4, P > 0.05). The observation that Ca2+ was not the main charge carrier was additionally confirmed by experiments in current clamp mode, where removal of Ca2+ from the extracellular solution (Ca2+-free aCSF containing 1 mm EGTA; n= 3) did not influence CN-induced depolarizations (Fig. 2B).
Figure 2. The CN-induced inward current (ICN) is carried by Na+ influx.
The magnitude of the inward current induced by 2 mm CN (ICN) in hypoglossal MNs held at –60 or –70 mV was compared under different experimental conditions (A and C). A, no significant difference (n.s.) in the amplitude of ICN was found when TTX was present in the aCSF compared to the control condition (control, –51 ± 9 pA, n= 8; TTX, –56 ± 13 pA, n= 7). Blockade of K+ currents with TEA and replacement of intracellular K+ by Cs+ did not change the magnitude of ICN either (TTX, TEA, CsCl, –62 ± 15 pA, n= 10). Similarly, additional wash-in of Cd2+ to block Ca2+ conductances did not change ICN amplitude (TTX, TEA, CsCl, CdCl2, –46 ± 8 pA, n= 4). B, recording of MN membrane potential (Vm) in the presence of TTX. CN depolarized Vm even when Ca2+ was completely removed from the extracellular solution (containing 1 mm EGTA), further indicating that Ca2+ was not the charge carrier of ICN. C, reducing the extracellular Na+ concentration from 144 to 26 mm decreased ICN to 40 ± 13% of the control value (ICN: control, 45 ± 9 pA; low Na+, 18 ± 9; P < 0.05, n= 4); re-addition of Na+ increased ICN back to 76 ± 10%, identifying Na+ as the main charge carrier of ICN at the given membrane potential. (A and B, intracellular solution contained 100 μm fura-2.)
Interestingly, ICN reversed around 50 mV after blockade of K+, Ca2+ and TTX-sensitive Na+ channels. Since the reversal potential for chloride (Cl−) was chosen ∼0 mV, TTX-insensitive Na+ conductances were identified as potential charge carriers. To evaluate this, we changed the extracellular Na+ concentration from 144 mm to 26 mm (replacement of NaCl by choline chloride), which reduced ICN to 40 ± 13% of control (ICN: control, 45 ± 9 pA; low Na+, 18 ± 9 pA, P < 0.05, n= 4; Fig. 2C). Re-addition of Na+ to the perfusion solution substantially increased ICN (76 ± 10% of control). Taken together, these experiments identify TTX-insensitive Na+ conductances as prominent charge carriers.
Activation profile of ICN
An interesting question is related to the cellular mechanisms that mediate the fast onset of ICN. As the primary cellular target of CN is the mitochondrial respiratory chain, CN application is thought to block complex IV, collapse the potential gradient (ΔΨ) across the inner mitochondrial membrane and thus lead to the accumulation of physiological electron donors NADH and FADH2. We monitored the dynamic changes of these parameters parallel to Vm in patch-clamped hypoglossal MNs by using rhod123 as an indicator of ΔΨ and by the autofluorescence of NADH (Fig. 3A). Addition of 2 mm CN to the bathing solution increased rhod123 fluorescence as well as NADH autofluorescence with a delay after onset of depolarization of 3 ± 2.2 s (rhod123, n= 6) and 6.3 ± 1.5 s (NADH, n= 8). To test whether the onset of ICN was dependent on the depolarization of ΔΨ, we added the mitochondrial uncoupler p-trifluoromethoxy-phenylhydrazone (FCCP; 1 μm). FCCP shunts the proton gradient over the inner mitochondrial membrane, thus depolarizing mitochondria while respiratory chain activity continues. As expected, FCCP increased rhod123 fluorescence, but left NADH-autofluorescence essentially unaffected (Fig. 3A). FCCP also failed to induce changes in Vm (Vm change during FCCP: 1 ± 2 mV, n= 6, P < 0.01, Fig. 3B) and did not induce inward currents in voltage clamp mode (–60 mV; n= 3), indicating that activation of ICN was independent of ΔΨ depolarization.
Figure 3. Investigating the mechanism underlying activation of ICN.
A, simultaneous monitoring of MN membrane potential (Vm), NADH autofluorescence and mitochondrial potential (ΔΨ) using rhod123. Bath application of CN (2 mm) depolarized MNs and increases autofluorescence as well as rhod123 fluorescence, indicating accumulation of the electron donor NADH and depolarization of ΔΨ. Addition of 2 μm FCCP depolarized ΔΨ but did not change Vm or NADH autofluorescence, indicating that depolarization of ΔΨ is not sufficient to induce MN depolarization. B, the mean change in Vm induced by CN was 12 ± 2 mV, whereas FCCP changed Vm by only 1 ± 2 mV (n= 6, P < 0.01). C, the depolarization of Vm by the complex IV inhibitor sodium azide (2 mm) was similar to the effect of CN. D, pre-incubation with the antioxidants and free radical scavengers ascorbic acid (1 mm) and Trolox (750 μm) reduced the mean amplitude of ICN to 40%(−22 ± 6 pA, n= 8) of the value that had been found under control conditions (P < 0.01), indicating that a change in the redox state of proteins, possibly due to increased formation of reactive oxygen species (ROS), is involved in the activation of ICN.
In a subsequent series of experiments we investigated whether activation of ICN could be reproduced by other substances that interfere with the mitochondrial respiratory chain. Whereas the complex I inhibitor rotenone (25–50 μm, n= 3) failed to alter Vm within 5 min, the complex IV inhibitor, sodium azide (2 mm) depolarized the plasma membrane by 7.1 ± 1 mV, which was similar to the action of CN (n= 9; Fig. 3C). These findings suggested that the mechanism that mediated the activation of ICN was located downstream of complex IV.
Next, the possibility was considered that inhibition of complex IV may increase the formation of superoxide () by the transfer of electrons to oxygen at the semiubiquinone site of the respiratory chain. Superoxide and related reactive oxygen species (ROS) may act as signalling molecules by shifting redox pairs to the oxidized state (Lopez-Barneo et al. 2001). We tested the potential involvement of ROS in mediating ICN by the ability of the antioxidants and ROS scavengers ascorbic acid and Trolox to interfere with the induction of ICN. Brain slices were preincubated with 1 mm ascorbic acid and 750 μm Trolox, a derivative of α-tocopherol (vitamin E), for 30–50 min, and the drugs were also present during the following CN exposure (2 mm for 50–60 s). The drug concentrations used were shown to be effective in previous studies in vitro (Vergun et al. 2001; MacGregor et al. 2003). Pre-incubation with ascorbic acid and Trolox reduced the mean amplitude of ICN to 40% (–22 ± 6 pA, n= 8) of the value that had been found under control conditions (P < 0.01; Fig. 3D). In two of the eight cells tested, ICN was totally abolished. Taken together, the experiments suggested that an increase in the formation of ROS following complex IV inhibition was involved in the activation of ICN.
CN increases resting [Ca2+]i in hypoglossal MNs
Next, the influence of mitochondrial inhibition on cytosolic [Ca2+] in hypoglossal MNs was investigated. In current clamp, short (45–60 s) applications of CN increased basal [Ca2+]i by two different mechanisms. First, a small increase in [Ca2+]i independently of action potential (AP) generation or synaptic activity was observed (Fig. 4A, Fig. 4B, Fig. 4C). This augmentation, most likely mediated by mitochondria-controlled Ca2+ release from intracellular stores (see below), started 19 ± 3 s (n= 7) after wash-in of CN and displayed amplitudes ranging from 10 to 50 nm, which decreased upon repetitive CN exposure. A second source of CN-dependent Ca2+ elevation was apparent when ICN-mediated depolarizations evoked a series of APs as exemplified in Fig. 1. In this case, activation of voltage-dependent Ca2+ channels elevated cytosolic [Ca2+]i levels as previously investigated in great detail (Lips & Keller, 1999). To evaluate the impact of ICN in a more systematic way, we performed a series of depolarizations in current clamp mode (5 s) and recorded AP firing rates and corresponding changes in somatic [Ca2+](n= 9). As indicated in Fig. 4D (left), AP firing was strongly dependent on Vm and characteristic depolarizations of 10.2 ± 1.1 mV mediated by ICN corresponded to increases in AP firing rates from 4 Hz to 16 Hz in the voltage interval –50 to –40 mV (dashed lines in Fig. 4D, left). Correspondingly, these firing rates were associated with [Ca2+]i elevations of ∼100 nm as illustrated in Fig. 4D (right, correlation coefficient 0.86). In summary, these observations illustrate that (i) the MN resting membrane potential is a critical determinant for ICN-induced increases in AP firing rates and subsequent Ca2+ influx, and (ii) ICN-induced depolarizations can account for basal Ca2+ elevations of 100 nm, provided that the resting membrane potential of the cells is in the appropriate voltage range.
CN releases Ca2+ from mitochondria-controlled stores
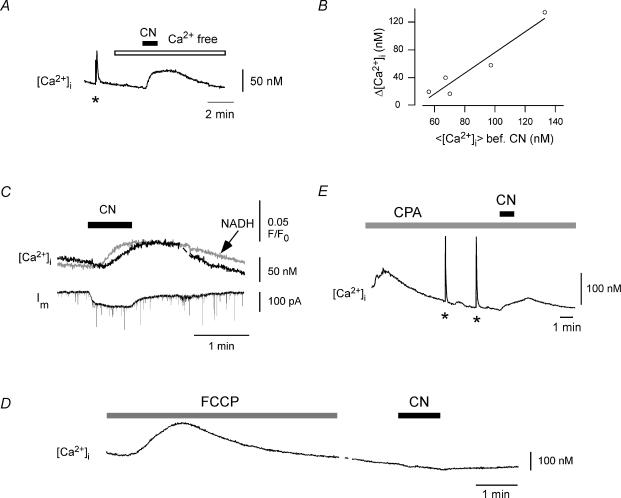
In subsequent experiments, we investigated the action potential-independent increase in [Ca2+] in more detail. When HMs were held in voltage clamp (–60 or –70 mV) in the presence of 0.5 μm TTX, CN (2 mm for 45–70 s) produced a delayed rise in [Ca2+]i of 36 ± 8 nm(n= 10) that was comparable to the [Ca2+]i elevation observed under current clamp. Removal of Ca2+ from the extracellular solution did not prevent the increase in [Ca2+] (n= 6, Fig. 5A), indicating that Ca2+ was released from intracellular stores. As shown in Fig. 5B, the amount of releasable Ca2+ depended on the average [Ca2+]i before CN addition with a statistically significant correlation coefficient r0= 0.96 (probability P(|r|≥r0) < 0.05, see also Methods). The average [Ca2+]i before CN addition was assessed within a time interval of 5 min from five different cells, where variations in average [Ca2+]i (<[Ca2+]i >) were given by differential resting Ca2+ levels in the whole-cell configuration and a variable number (0–3) of depolarizations to 0 mV (500 ms) within the indicated time interval. Taken together, these experiments suggested that the CN-sensitive store takes up Ca2+ during [Ca2+]i elevations and releases it during subsequent CN action.
Figure 5. CN releases Ca2+ from mitochondria-controlled stores.
Monitoring of [Ca2+]i using fura-2 (100 μm) in voltage-clamped hypoglossal MNs (holding potential –60 or –70 mV). A, (pre-)incubation with nominally Ca2+-free aCSF(open bar) did not prevent CN-induced [Ca2+]i elevation, indicating that Ca2+ was released from intracellular stores (n= 6). The asterisk marks depolarization to +10 mV (500 ms) inducing a somatic Ca2+ transient. B, the magnitude of CN-induced Ca2+ release reflects the previous history of intracellular Ca2+ activity, which was determined by the average [Ca2+]i (< [Ca2+]i >) within 5 min before CN addition (n= 5, correlation coefficient, r0= 0.96; P < 0.05, see Methods). Variations in < [Ca2+]i > were given by differential resting Ca2+ levels in the whole-cell configuration and a variable number (0–3) of depolarizations to 0 mV (500 ms) within the indicated time interval. C, simultaneous monitoring of membrane current (Im), [Ca2+]i and mitochondrial metabolism (NADH autofluorescence). Application of CN (2 mm) raises [Ca2+]i with kinetics that closely follow the changes in mitochondrial metabolism, suggesting that Ca2+ was released from mitochondria-controlled stores. D, FCCP (2 μm) was added to destroy ΔΨ and to deplete mitochondrial Ca2+ content. Subsequent addition of CN did not change [Ca2+]i, confirming that the CN-induced Ca2+ release was dependent on ΔΨ. E, slices were incubated with cyclopiazonic acid (CPA) to inhibit the ER Ca2+-ATPase and to deplete ER Ca2+ content (CPA incubation started ∼25 s before onset of imaging). The asterisks mark voltage-induced Ca2+ transients, which were applied to fill mitochondrial stores. Subsequent CN application raised [Ca2+]i to a level similar to that without CPA, indicating that the CN-sensitive Ca2+ store was most likely represented by the mitochondria and not the ER.
We then monitored Im and [Ca2+]i of the patch-clamped cell together with NADH autofluorescence of neighbouring MNs (Fig. 5C). A series of experiments indicated that changes in NADH autofluorescence during CN exposure are extremely synchronous among MNs in the same area so that neighbouring MNs can be considered as representative of the patch-clamped cell regarding this particular parameter. Recording of [Ca2+]i and NADH fluorescence in the same cell was not possible due to the overlapping spectra of fura-2 and NADH. This experiment revealed that the CN-induced release of Ca2+ followed the dynamics of mitochondrial metabolism very closely: the rise in [Ca2+]i followed the rise in NADH fluorescence with a constant temporal interval of 4 ± 1 s (n= 12). As CN-induced accumulation of NADH is paralleled by the depolarization of ΔΨ (Fig. 3A), the experiment suggested that the release of Ca2+ by CN was dependent on mitochondrial metabolism and ΔΨ. This assumption was further confirmed by experiments where preincubation with the mitochondrial uncoupler carbonyl cyanide 4-trifluoro-methoxyphenylhydrazone (FCCP, 2 μm), which destroys ΔΨ and depletes mitochondrial Ca2+ content, prevented the [Ca2+]i elevation during subsequent CN action (n= 2, Fig. 5D).
However, in previous work we have shown that both mitochondria and endoplasmic reticulum (ER) act as important Ca2+ buffers in hypoglossal MNs and that release from both stores is closely linked to ΔΨ (Bergmann & Keller, 2002; Ladewig et al. 2003). The observed CN-induced Ca2+ release could therefore originate from both stores. To test for the contribution of the ER, we inhibited the ER Ca2+-ATPase with 50 μm CPA for at least 5 min, which released Ca2+ from the ER due to leakage of the ER membrane (Fig. 5E). Control experiments showed that caffeine, which usually produces large Ca2+ elevations in hypoglossal MNs (Ladewig et al. 2003), did not invoke a rise in Ca2+, when CPA incubation preceded its action, indicating that CPA largely depleted the ER Ca2+ content (not shown). When CN was then added after CPA preincubation, it still produced an increase in [Ca2+]i of 26 ± 5 nm(n= 6). Since the amount of Ca2+ released depended on preceding cytosolic Ca2+ activity (Fig. 5B), it was difficult to compare this value with the control condition. However, these observations strongly suggest that the CN-sensitive store, from which Ca2+ may be eventually released, is represented by the mitochondria.
Impact of CN and ATP depletion on activity-dependent Ca2+ elevations
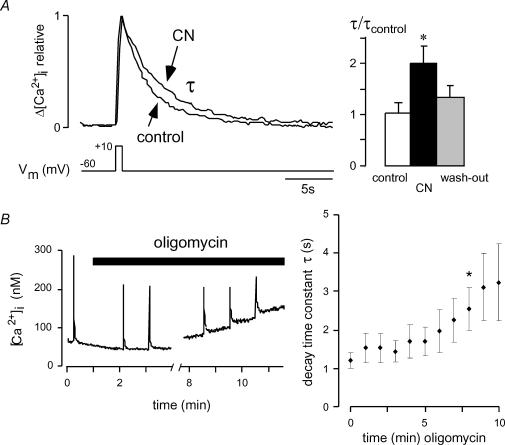
As we have shown previously, hypoglossal MNs display repetitive elevations in [Ca2+]i, which are linked to rhythmic respiratory activity and mainly result from the opening of voltage-activated Ca2+ channels (Lips & Keller, 1999; Ladewig & Keller, 2000). A fast clearance of these repetitive elevations in [Ca2+]i is essential to maintain a low resting [Ca2+]i, particularly considering the low Ca2+-buffering capacity of hypoglossal MNs. In previous work we have established that mitochondrial Ca2+ uptake importantly contributes to rapid clearance of cytosolic Ca2+ transients (Bergmann & Keller, 2002). Because mitochondrial Ca2+ uptake is dependent on the potential gradient over the inner mitochondrial membrane, depolarization of ΔΨ as observed during CN action (Fig. 3A) would predict a disturbance in cytosolic Ca2+ clearance. To mimic the physiological situation in our set of experiments, Ca2+ transients were repetitively elicited by short (200–500 ms) depolarizations to +10 mV from a holding potential of –60 mV elevating [Ca2+]i to 200–500 nm from a resting level of ∼80 nm. Clearance of Ca2+ transients was assessed by determining the recovery time constant tau (τ) after fitting with a single-exponential function. As expected, incubation with 1–2 mm CN for 1–4 min markedly prolonged the recovery of somatic Ca2+ transients to 1.96 ± 0.35 times control (n= 6, P < 0.03; Fig. 6A). After wash-out of CN, the fast clearance of Ca2+ transients was largely restored.
Figure 6. Impact of CN and ATP depletion on activity-dependent Ca2+ transients.
Monitoring of [Ca2+]i in patch-clamped hypoglossal MNs using fura-2 (100 μm), where elevations in [Ca2+]i (100–400 nm above baseline) were elicited by short depolarizations to +10 mV from a holding potential of –60 or –70 mV. A, somatic Ca2+ transients normalized to the same amplitude before and during 1–4 min of CN incubation (1 mm) are superimposed. Note that CN prolongs the recovery time constant τ of Ca2+ transients to 1.96 ± 0.35 times control (n= 6; P < 0.03). Wash-out of CN largely restores fast Ca2+ clearance. Because cells were dialysed with 4 mm ATP, the effect is attributable to disturbed mitochondrial Ca2+ uptake and not to ATP depletion. B, hypoglossal MNs were dialysed with ATP-free intracellular solution and 10 μg ml−1 oligomycin was added as indicated to block mitochondrial ATP production. While usually no change in recovery time constants of Ca2+ transients (τ) or resting [Ca2+]i was noticed within the first 5 min, longer incubation times progressively prolonged τ (*P < 0.05 compared to time point 0). After a mean incubation time of 6.4 ± 1.2 min, resting [Ca2+]i steadily increased.
In these experiments, we could rule out ATP deficiency as a causative factor for prolongation of recovery times, since millimolar ATP concentrations were continuously supplied via the patch pipette. However, a decrease in cellular ATP levels is certainly expected during persistent inhibition of mitochondrial electron transport during in vivo conditions of hypoxia and may affect [Ca2+]i in addition. To test the impact of ATP depletion on the regulation of [Ca2+]i and the recovery of activity-dependent Ca2+ transients, [Ca2+]i was monitored in cells dialysed with ATP-free intracellular solution and Ca2+ transients were elicited every 60 s. Oligomycin (10 μg ml−1) was then added to block mitochondrial ATP production. In contrast to CN, oligomycin does not decrease ΔΨ, which was consistent with a lacking effect on the NADH fluorescence (not shown). As illustrated in Fig. 6B, wash-in of oligomycin left basal Ca2+ levels, as well as recovery times of Ca2+ transients, unaffected for several minutes, before the recovery times became progressively prolonged and, after a mean incubation time of 6.4 ± 1.2 min, resting [Ca2+]i steadily increased (n= 6).
Differential response of vulnerable and resistant neurones to CN
In order to test whether the increased neuronal excitability of hypoglossal MNs during CN action was a feature attributable to MNs or if this might represent a general cellular response in our preparation, we performed complementary recordings from vulnerable facial (VII) MNs and neurones of the nucleus dorsalis vagus (X), which is located directly dorsal to the hypoglossal nucleus and which is typically tolerant to hypoxia and resistant to damage in ALS.
In current clamp mode, facial MNs displayed a resting Vm of –61.7 ± 1.7 mV (n= 7). Similar to hypoglossal MNs, addition of CN reversibly depolarized facial MNs by 7 ± 1 mV (n= 7; Fig. 7A). In contrast, vagal neurones showed a resting membrane potential around –41 mV and displayed a tonic spike discharge at frequencies of 3–4 Hz (Fig. 7B). CN hyperpolarized vagal neurones by 7.5 ± 0.9 mV (n= 4) and reduced action potential firing, which was consistent with previous studies showing that CN activates hyperpolarizing ATP-sensitive K+ channels (Kulik et al. 2002; Müller et al. 2002). However, it was possible that the activation of large K+ conductances covers the activation of smaller inward currents, therefore we checked for the activation of CN-induced inward currents, when K+ was replaced by caesium in the pipette solution and K+ channels were additionally blocked with 10 mm TEA in the bath solution. Vagal neurones were held between –40 and –50 mV and 2 mm CN was applied for 1–3 min. This hardly affected membrane currents (maximum observed inward current: 6 pA) indicating that vagal neurones do not possess CN-sensitive sodium conductances as observed in hypoglossal MNs.
Figure 7. Differential response of vulnerable and resistant neurones to CN.
A, membrane potential (Vm) recorded from a patch-clamped MN in the facial nucleus (VII). Bath application of CN (2 mm) depolarized facial MNs by 7 ± 1 mV (n= 7) with repolarization after wash-out of CN. B, Vm recorded from a patch-clamped neurone in the nucleus dorsalis vagus (X). Addition of CN (2 mm) reversibly hyperpolarized the vagal neurone by 7.5 ± 0.9 mV (n= 4) and reduced spontaneous spike discharge. C, comparison of the CN-induced Vm change in vulnerable MNs (hypoglossal and facial) and resistant vagal neurones. Intracellular solution contained 100 μm fura-2 (A and B).
Discussion
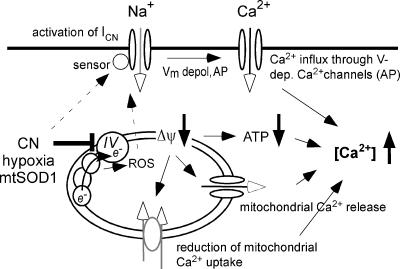
A prime objective of the present investigation was to elucidate molecular mechanisms underlying motoneurone (MN) vulnerability during the inhibition of mitochondrial metabolism, which typically occurs during cellular hypoxia and in motoneurone disease such as amyotrophic lateral sclerosis (ALS). We investigated the impact of a disturbed respiratory chain on membrane currents and [Ca2+]i using cyanide (CN), a known inhibitor of cytochrome c oxidase (complex IV). A summary of the mechanisms identified is given in Fig. 8. First, CN activated a TTX-insensitive Na+ current (ICN), which depolarized the resting membrane potential (Vm) of MNs by ∼10 mV and increased action potential activity. This observation was in good agreement with previous studies implicating Na+ influx in hypoxia-induced depolarization of MNs (Haddad & Jiang, 1993; Le Corronc et al. 1999) and underlined the use of CN as a model for hypoxia.
Figure 8. Events following inhibition of mitochondrial metabolism in vulnerable hypoglossal MNs.
CN (similar to azide, hypoxia and SOD1) inhibits complex IV (cytochrome c oxidase) of the respiratory chain. This increases the formation of reactive oxygen species (ROS), which potentially activate a depolarizing Na+ current (ICN). Alternatively, the Na+ current is activated by a direct redox mechanism. The Na+ influx enhances neuronal excitability and promotes Ca2+ influx during elevated firing rates (action potentials, AP). Inhibition of the respiratory chain furthermore decreases the mitochondrial potential gradient (ΔΨ), leading to reduced Ca2+ uptake into the mitochondrial matrix and release of Ca2+ that was taken up during the preceding activity. Mitochondrial inhibition additionally decreases cellular ATP levels, and this further enhances accumulation of intracellular Ca2+.
Activation of CN-induced Na+ influx
In subsequent experiments we identified mechanisms that potentially mediated the activation of ICN. Most importantly, the experiments indicated that a redox mechanism was involved in the activation of ICN, because the antioxidants and free radical scavengers ascorbic acid and Trolox largely prevented it. The change in the redox state was possibly induced by increased levels of reactive oxygen species (ROS), which originate at the respiratory chain when complex IV activity is inhibited (Lopez-Barneo et al. 2001). However, the involvement of ROS is challenged by the fact that the activation of ICN significantly preceded the increase in NADH fluorescence which indirectly monitors the build-up of electrons at the respiratory chain. Although a very localized production of ROS that was not represented by the global NADH signal could account for the observed discrepancy, alternative models for ICN activation should be considered. For example, a molecule other than complex IV could serve as an oxygen sensor capable of responding to a drop in oxygen levels, CN and azide. In this case, the redox state of a thiol-rich molecule as part of the channel itself or of a regulatory protein could serve as a sensor. Hammarström & Gage (1998, 2000) made a similar suggestion for oxygen- and CN-sensing Na+ channels in hippocampal neurones. Taken together, despite the plausible involvement of ROS in activation of ICN, more experiments are necessary to determine the exact signalling pathway by which ICN is mediated in MNs.
Mitochondrial inhibition increases cytosolic Ca2+ load
In the second part of the study, we identified several mechanisms, by which mitochondrial inhibition with CN increased the cytosolic Ca2+ load of vulnerable hypoglossal MNs. Attributable to dissipation of the mitochondrial potential gradient we observed (i) release of Ca2+ from mitochondria-controlled stores, and (ii) a notable retardation of cytosolic Ca2+ clearance rates within 1–3 min of mitochondrial depolarization. Additionally, voltage-dependent Ca2+ influx occurred during elevated firing rates. Since millimolar ATP concentrations were continuously supplied via the patch pipette, these effects were clearly independent of a drop in cytosolic ATP levels. On the other hand, a decrease in cellular ATP concentration, which was induced by oligomycin application while respiratory chain activity continued, prolonged the recovery times of Ca2+ transients and progressively built up basal [Ca2+]i with a delay of 5–6 min. These observations clearly indicate that early cytosolic Ca2+ disturbance during mitochondrial inhibition does not arise from energy depletion but rather from insufficient mitochondrial Ca2+ buffering and changes in the neuronal excitability of MNs. Nevertheless, when cytosolic ATP levels drop below a certain critical value, [Ca2+]i regulation is even more severely impaired.
Selective vulnerability of motoneurones
The reason why MN populations are preferentially injured by mitochondrial inhibition is still incompletely understood, and a variety of explanations have been proposed. MNs are large, highly active cells with exceptional energy requirements, a fact that exposes them to elevated risks during mitochondrial impairment and a subsequent drop in ATP levels. They also display a remarkably low cytosolic Ca2+-buffering capacity (KS) (Alexianu et al. 1994; Lips & Keller, 1998; Palecek et al. 1999), which renders them particularly sensitive to disturbances in cytosolic Ca2+ levels. Since our data suggest such a disturbance as a consequence of impaired mitochondrial function, the limited ability to buffer increased cytosolic Ca2+ loads may well contribute to the observed vulnerability. Our study furthermore indicates that vulnerable MNs are characterized by membrane properties which promote a profound depolarization during inhibition of complex IV. In contrast, neurones in the nucleus dorsalis vagus, which are tolerant to hypoxia and also resistant to degeneration in ALS, hyperpolarize under the same conditions. Hyperpolarization in vagal but also in hippocampal neurones in response to mitochondrial inhibition has been attributed to the activation of ATP-sensitive and Ca2+-dependent K+ channels (Koyama et al. 1999; Englund et al. 2001; Müller et al. 2002). Although the activation of ATP-dependent K+ channels during mitochondrial inhibition has also been observed in hypoglossal MNs (Jiang & Haddad, 1991), its activation is apparently not sufficient to counteract the depolarization induced by Na+ influx. Taken together, these observations therefore suggest that selective MN vulnerability most likely results from a synergistic accumulation of risk factors, including low Ca2+ buffering, strong mitochondrial control of [Ca2+]i and a weak protection against hypoxia-related changes in neuronal excitability.
Potential relevance of the findings to amyotrophic lateral sclerosis-associated motoneurone degeneration
Inhibition of the respiratory chain and mitochondrial dysfunction has been linked to many aspects of motoneurone pathophysiology, including the pronounced vulnerability of motoneurones to hypoxia and their selective degeneration in amyotrophic lateral sclerosis (ALS). Although we used very young animals in our study and investigated cellular changes in response to CN over a time range of minutes whereas ALS-related motoneurone degeneration occurs over months, the experimental protocol of mitochondrial inhibition may still have interesting implications for ALS-related motoneurone pathologies. For example, increased cytosolic Ca2+ loads resulting from mitochondrial inhibition are paralleled by observations in cell lines expressing mutant SOD1, which show increased basal Ca2+ loads (Carri et al. 1997; Kruman et al. 1999). Moreover, our findings of depolarizing Na+ currents during complex IV inhibition resemble increased Na+ currents and enhanced neuronal excitability in mutant SOD1 mouse spinal MNs (Kuo et al. 2002, 2003). Accordingly, the potential link between motoneurone responses to mitochondrial inhibition and their selective vulnerability during ALS-related motoneurone disease will be an interesting area for future investigation.
Acknowledgments
We thank Drs M. Müller, E. Neher and D. W. Richter for valuable discussion, and D. Crzan and C. Bartje for technical assistance. Financial support was from SFB 406, DFG-Center CMPB, DFG Ke 403/12-2 and Ke 403/14-1 and European Graduiertenkolleg ‘Neuroplasticity, from molecules to systems’.
References
- Alexianu ME, Ho BK, Mohamed AH, La Bella V, Smith RG, Appel SH. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria and the pathogenesis of ALS. Brain. 2000;123:1291–1292. doi: 10.1093/brain/123.7.1291. [DOI] [PubMed] [Google Scholar]
- Bergmann F, Keller BU. Mitochondrial control of calcium signaling in motoneurons that are particularly vulnerable in amyotrophic lateral sclerosis (ALS) Pflugers Arch. 2002;443:S322. [Google Scholar]
- Carri MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G. Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Lett. 1997;414:365–368. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- Englund M, Hyllienmark L, Brismar T. Chemical hypoxia in hippocampal pyramidal cells affects membrane potential differentially depending on resting potential. Neuroscience. 2001;106:89–94. doi: 10.1016/s0306-4522(01)00259-7. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haddad GG, Jiang C. Mechanisms of anoxia-induced depolarization in brainstem neurons: in vitro current and voltage clamp studies in the adult rat. Brain Res. 1993;625:261–268. doi: 10.1016/0006-8993(93)91067-3. [DOI] [PubMed] [Google Scholar]
- Hammarström AK, Gage PW. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J Physiol. 1998;510:735–741. doi: 10.1111/j.1469-7793.1998.735bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström AK, Gage PW. Oxygen-sensing persistent sodium channels in rat hippocampus. J Physiol. 2000;529:107–118. doi: 10.1111/j.1469-7793.2000.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Haddad GG. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol. 1991;66:103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- Jung C, Higgins CM, Xu Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J Neurochem. 2002;83:535–545. doi: 10.1046/j.1471-4159.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- Kaal EC, Vlug AS, Versleijen MW, Kuilman M, Joosten EA, Bar PR. Chronic mitochondrial inhibition induces selective motoneuron death in vitro: a new model for amyotrophic lateral sclerosis. J Neurochem. 2000;74:1158–1165. doi: 10.1046/j.1471-4159.2000.741158.x. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Qi J, Comer AM, Gibbons H, Win J, Lipski J. Effects of cyanide and hypoxia on membrane currents in neurones acutely dissociated from the rostral ventrolateral medulla of the rat. Brain Res. 1999;830:246–257. doi: 10.1016/s0006-8993(99)01397-9. [DOI] [PubMed] [Google Scholar]
- Kovacs R, Schuchmann S, Gabriel S, Kann O, Kardos J, Heinemann U. Free radical-mediated cell damage after experimental status epilepticus in hippocampal slice cultures. J Neurophysiol. 2002;88:2909–2918. doi: 10.1152/jn.00149.2002. [DOI] [PubMed] [Google Scholar]
- Koyama S, Jin YH, Akaike N. ATP-sensitive and Ca2+-activated K+ channel activities in the rat locus coeruleus neurons during metabolic inhibition. Brain Res. 1999;828:189–192. doi: 10.1016/s0006-8993(99)01303-7. [DOI] [PubMed] [Google Scholar]
- Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- Kulik A, Brockhaus J, Pedarzani P, Ballanyi K. Chemical anoxia activates ATP-sensitive and blocks Ca2+-dependent K+ channels in rat dorsal vagal neurons in situ. Neuroscience. 2002;110:541–554. doi: 10.1016/s0306-4522(01)00468-7. [DOI] [PubMed] [Google Scholar]
- Kuo J, Fu R, Siddique T, Heckman CJ. Persistent inward currents from SOD1 transgenic mouse spinal cultures. Abstr Soc Neurosci. 2002:789.7. [Google Scholar]
- Kuo JJ, Schonewille M, Siddique T, Schults AN, Fu R, Bar PR, Anelli R, Heckman CJ, Kroese AB. Hyperexcitability of cultured spinal motoneurons from presymptomatic ALS mice. J Neurophysiol. 2003 doi: 10.1152/jn.00665.2003. epub ahead of print, 1 October 2003. [DOI] [PubMed] [Google Scholar]
- Ladewig T, Keller BU. Simultaneous patch-clamp recording and calcium imaging in a rhythmically active neuronal network in the brainstem slice preparation from mouse. Pflugers Arch. 2000;440:322–332. doi: 10.1007/s004240000277. [DOI] [PubMed] [Google Scholar]
- Ladewig T, Kloppenburg P, Lalley PM, Zipfel WR, Webb WW, Keller BU. Spatial profiles of store-dependent calcium release in motoneurones of the nucleus hypoglossus from newborn mouse. J Physiol. 2003;547:775–787. doi: 10.1113/jphysiol.2002.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, Wyns S, Thijs V, Andersson J, Van Marion I, Al-Chalabi A, Bornes S, Musson R, Hansen V, Beckman L, Adolfsson R, Pall HS, Prats H, Vermeire S, Rutgeerts P, Katayama S, Awata T, Leigh N, Lang-Lazdunski L, Dewerchin M, Shaw C, Moons L, Vlietinck R, Morrison KE, Robberecht W, Van Broeckhoven C, Collen D, Andersen PM, Carmeliet P. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Le Corronc H, Hue B, Pitman RM. Ionic mechanisms underlying depolarizing responses of an identified insect motor neuron to short periods of hypoxia. J Neurophysiol. 1999;81:307–318. doi: 10.1152/jn.1999.81.1.307. [DOI] [PubMed] [Google Scholar]
- Lips MB, Keller BU. Endogenous calcium buffering in motoneurones of the nucleus hypoglossus from mouse. J Physiol. 1998;511:105–117. doi: 10.1111/j.1469-7793.1998.105bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips MB, Keller BU. Activity-related calcium dynamics in motoneurons of the nucleus hypoglossus from mouse. J Neurophysiol. 1999;82:2936–2946. doi: 10.1152/jn.1999.82.6.2936. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- MacGregor DG, Avshalumov MV, Rice ME. Brain edema induced by in vitro ischemia: causal factors and neuroprotection. J Neurochem. 2003;85:1402–1411. doi: 10.1046/j.1471-4159.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;227:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Cookson MR, Taylor RW, Turnbull DM, Chrzanowska-Lightowlers ZM, Dong L, Figlewicz DA, Shaw PJ. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain. 2002;125:1522–1533. doi: 10.1093/brain/awf167. [DOI] [PubMed] [Google Scholar]
- Müller M, Brockhaus J, Ballanyi K. ATP-independent anoxic activation of ATP-sensitive K+ channels in dorsal vagal neurons of juvenile mice in situ. Neuroscience. 2002;109:313–328. doi: 10.1016/s0306-4522(01)00498-5. [DOI] [PubMed] [Google Scholar]
- Nowicky AV, Duchen MR. Changes in [Cai and membrane currents during impaired mitochondrial metabolism in dissociated rat hippocampal neurons. J Physiol. 1998;507:131–145. doi: 10.1111/j.1469-7793.1998.131bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- O'Reilly JP, Jiang C, Haddad GG. Major differences in response to graded hypoxia between hypoglossal and neocortical neurons. Brain Res. 1995;683:179–186. doi: 10.1016/0006-8993(95)00373-x. [DOI] [PubMed] [Google Scholar]
- Palecek J, Lips MB, Keller BU. Calcium dynamics and buffering in motoneurones of the mouse spinal cord. J Physiol. 1999;520:485–502. doi: 10.1111/j.1469-7793.1999.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW, Spyer KM. Hypoxic response of hypoglossal motoneurones in the in vivo cat. J Physiol. 1997;505:785–795. doi: 10.1111/j.1469-7793.1997.785ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–624. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Lückermann M, Kulik A, Heinemann U, Ballanyi K. Ca2+- and metabolism-related changes of mitochondrial potential in voltage-clamped CA1 pyramidal neurons in situ. J Neurophysiol. 2000;83:1710–1721. doi: 10.1152/jn.2000.83.3.1710. [DOI] [PubMed] [Google Scholar]
- Soto-Blanco B, Marioka PC, Gorniak SL. Effects of long-term low-dose cyanide administration to rats. Ecotoxicol Environ Saf. 2002;53:37–41. doi: 10.1006/eesa.2002.2189. [DOI] [PubMed] [Google Scholar]
- Taylor JR. An Introduction to Error Analysis. University Science Books,Oxford University Press,Oxford: 1982. [Google Scholar]
- Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol. 1999;82:2163–2170. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- Tylleskar T, Banea M, Bikangi N, Cooke RD, Poulter NH, Rosling H. Cassava cyanogens and konzo, an upper motoneuron disease found in Africa. Lancet. 1992;339:208–211. doi: 10.1016/0140-6736(92)90006-o. [DOI] [PubMed] [Google Scholar]
- Vergun O, Sobolevsky AI, Yelshansky MV, Keelan J, Khodorov BI, Duchen MR. Exploration of the role of reactive oxygen species in glutamate neurotoxicity in rat hippocampal neurones in culture. J Physiol. 2001;531:147–163. doi: 10.1111/j.1469-7793.2001.0147j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J Neurochem. 2002;80:616–625. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]