Abstract
It has been shown that peripheral chemoreceptor sensitivity is enhanced in both clinical and experimental heart failure (HF) and that impairment of nitric oxide (NO) production contributes to this enhancement. In order to understand the cellular mechanisms associated with the alterations of chemoreceptor function and the actions of NO in the carotid body (CB), we compared the outward K+ currents (IK) of glomus cells in sham rabbits with that in HF rabbits and monitored the effects of NO on these currents. Ik was measured in glomus cells using conventional and perforated whole-cell configurations. IK was attenuated in glomus cells of HF rabbits, and their resting membrane potentials (−34.7 ± 1.0 mV) were depolarized as compared with those in sham rabbits (−47.2 ± 1.9 mV). The selective Ca2+-dependent K+ channel (KCa) blocker iberiotoxin (IbTx, 100 nm) reduced IK in glomus cells from sham rabbits, but had no effect on IK from HF rabbits. In perforated whole-cell mode, the NO donor SNAP (100 μm) increased IK in glomus cells from HF rabbits to a greater extent than that in sham rabbits (P < 0.01), and IbTx inhibited the effects of SNAP. However, in conventional whole-cell mode, SNAP had no effect. Nω-nitro-L-arginine (L-NNA, NO synthase inhibitor) decreased Ik in sham rabbits but not in HF rabbits. The guanylate cyclase inhibitor 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) inhibited the effect of SNAP on Ik. These results demonstrate that IK is reduced in CB glomus cells from HF rabbits. This effect is due mainly to the suppression of KCa channel activity caused by decreased availability of NO. In addition, intracellular cGMP is necessary for the KCa channel modulation by NO.
Activation of the sympathetic nervous system is one of the most profound and reproducible changes in chronic heart failure (HF) (Floras, 1993; Zucker et al. 1995; Esler et al. 1997). Previous studies have shown that peripheral chemoreceptor activation is an excitatory input that results in increased sympathetic outflow and blood pressure (Marshall, 1994). Studies from our laboratory have shown that in a rabbit model of pacing-induced HF, peripheral chemoreceptor activity is enhanced even under normoxic conditions. This enhanced sensitivity of the peripheral chemoreflex contributes, at least in part, to the sympathetic activation in this rabbit model of HF, because inhibition of peripheral chemoreceptor activity decreased renal sympathetic nerve activity (RSNA) in HF, but not in sham rabbits (Sun et al. 1999a). However, the cellular and molecular mechanisms involved in enhancing peripheral chemoreceptor sensitivity during HF still remain unclear.
The carotid body (CB) is the primary sensor site of the peripheral chemoreflex, and is composed of neurotransmitter-enriched glomus cells and glial-like sustentacular cells among other neuronal structures. Currently it is believed that glomus cells, which lie in synaptic apposition with the afferent nerve ending, are the initial sites of sensory transduction in the CB. The glomus cell membrane contains many voltage-gated ion channels. The suppression of outward K+ channels is proposed to contribute sequentially to the initial depolarization, activation of voltage-gated Ca2+ channels, release of neurotransmitter, and activation of discharge in the carotid sinus nerve (Gonzalez et al. 1994; Prabhakar, 1994).
NO is a gas molecule that functions as a transmitter in both the peripheral and central nervous systems. It is synthesized during the catalytic conversion of arginine to citrulline by the enzyme nitric oxide synthase (NOS) (Snyder, 1992). The extensive plexus of nerve fibres and vessels surrounding glomus cells contains neuronal NOS (nNOS) and endothelial NOS (eNOS) (Prabhakar et al. 1993; Wang et al. 1993), but NOS is not found in detectable levels in glomus and type II cells. However, these other neural and vascular cells may produce paracrine effects of their NO production on neighbouring glomus cells.
Our previous study has shown that a down-regulation of NO synthesis in the CB in HF rabbits contributes to the enhancement of CB chemoreceptor sensitivity (Sun et al. 1999b). We hypothesize that NO affects IK, which contributes to the altered peripheral chemoreceptor function in HF. To test this hypothesis, we compared IK of CB glomus cells in sham and pacing-induced HF rabbits and examined the effects of NO on IK. Our results show that IK was attenuated in CB glomus cells from HF rabbits and that down-regulation of NO attenuated IK via the inhibition of Ca2+-dependent K+ (KCa) channels. Furthermore, the ability of NO to activate IKCa in glomus cells appears to be mediated by a cGMP-dependent pathway. Preliminary results from this study have been published in an abstract form (Li & Schultz, 2003).
Methods
Pacemaker implant and production of HF
All experiments were carried out on male New Zealand White rabbits weighing 2.5–3.5 kg. Experiments were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health and the American Physiological Society's Guides for the Care and Use of Laboratory Animals.
Rabbits were anaesthetized with a cocktail consisting of 1.2 mg acepromazine, 5.9 mg xylazine and 58.8 mg ketamine, given as an i.m. injection. Using sterile technique, a left thoracotomy was performed as previously described (Sun et al. 1999a). The pericardium was opened and wire loop electrodes were attached to the left ventricle for pacing. Two sonomicrometer crystals (Sonometrics Corp., London, ON, Canada) were attached to opposing walls of the lateral left ventricle for measuring external diameter. All leads exited the chest between the 3rd and 4th ribs. The chest was closed in layers and evacuated. Rabbits were placed on an antibiotic regimen consisting of 5 mg kg−1 Baytril i.m. for 5 days. After the rabbits recovered from the thoracotomy (about 2 weeks), baseline left-ventricular end-systolic and end-diastolic external diameter (D), fractional shortening, and shortening velocity (dD/dtmax) were measured by sonomicrometry (Triton Technology Inc., San Diego, CA, USA). An arterial blood gas sample (0.25 ml) was taken by needle puncture of an ear artery periodically to monitor arterial blood gases. The pacing was started at 320 beats min−1, which was held for 7 days, and then the rate was gradually increased to 380 beats min−1, with an increment of 20 beats min−1 each week. The pacemaker was of our design, with its output usually being set at 4–5 V and 0.5 ms. Sonograms and blood gas (ABL5, Radiometer, Copenhagen) were done weekly, with the rabbits sitting quietly in a Plexiglas box and with the pacemaker turned off for at least 30 min before recordings were made. Sham-operated animals underwent a similar period of sonographic measurements with the pacemaker turned off. Rabbits with >40% reductions in dD/dtmax and shortening fraction were considered in HF (generally after 3–4 weeks). Any rabbit exhibiting abnormal arterial blood gases (Pa,O2 < 85 mmHg; 45 mmHg <Pa,CO2 < 30 mmHg) were excluded from study (Sun et al. 1999a).
Isolation of CB glomus cells
The carotid bifurcations on both sides were removed surgically from sham or HF rabbits anaesthetized with the cocktail described above. The rabbits were then killed with an intravenous injection of 150 mg kg−1 sodium pentobarbital. The removed tissue was placed in ice-cold Ca2+/Mg2+-free solution (mm: NaCl, 140; KCl, 5; Hepes 10; glucose 5; pH 7.2), and then minced with microscissors. The CBs were then subjected to a two-step enzymatic digestion protocol. Each step lasted for 30 min at 37°C. The enzymatic solution for the first step of digestion contained trypsin (type II, 2 mg ml−1) and collagenase (type IV, 2 mg ml−1); and that for the second step contained collagenase (4 mg ml−1) and bovine serum albumin (BSA, 5 mg ml−1). Other solutes in the solutions used for enzymatic digestion were the same as the above Ca2+/Mg2+-free solution. The digested tissue fragments were gently triturated for 1 min every 10 min during the process of digestion. CB cells were obtained after centrifugation of the digested tissue at 150 g for 5 min. The isolated cells were then resuspended in culture medium and plated onto culture wells. The culture medium consisted of a 50/50 mixture of Delbecco's modified Eagle's medium (DMEM) and Ham's F12 medium supplemented with antibiotics and 10% fetal bovine serum. The CB cells including glomus cells, other neural cells and vascular cells were cocultured at 37°C in a humidified atmosphere of 95% air–5% CO2 (Zhong et al. 1997) and studied within 24 h of dissociation.
Identification of glomus cells
Short-term cultured cells freshly isolated from CB in sham and HF rabbits contain several cell types, including glomus and type II cells. It has been shown that rabbit glomus cells are excitable cells and they exhibit Ca2+, K+ and Na+ currents, but type II cells are non-excitable and exhibit only small outward K+ currents. Therefore, at the beginning of each experiment, cells were superfused with an extracellular solution containing Na+. Those cells that exhibited Na+ current were considered to be glomus cells (Overholt & Prabhakar, 1997). Once the presence of Na+ current was confirmed, the extracellular solution was changed to solution containing 0.5 μm TTX to record IK (see below).
Recording of whole cell K+ currents and resting membrane potential
K+ currents were measured in the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981) using a Warner PC-501A patch-clamp amplifier (Warner Instrument Corp. Hamden, CT, USA), which allowed the same cell to be recorded in either the voltage-clamp or current-clamp mode. Patch pipettes had resistances of 4–6 MΩ when filled with (mm) 105 potassium aspartate, 20 KCl, 1 CaCl2, 10 EGTA, 5 Mg-ATP, 10 Hepes and 25 glucose, pH 7.2. In the perforated whole-cell configuration, nystatin was added to the patch-pipette solution at a final concentration of 300 μg ml−1 immediately before recording. The extracellular solution had the following composition (mm): 140 NaCl, 5.4 KCl, 2.5 CaCl2, 0.5 MgCl2, 5.5 Hepes, 11 glucose, 10 sucrose, pH 7.4. Na+ channels were blocked by TTX (0.5 μm).
Cell membrane capacitance (Cm) was determined by integrating the capacitive current evoked by a 5 mV voltage step and dividing the resulting charge by the voltage step. Mean series resistances, as determined from the decay of the capacitive transient, were 7.4 ± 0.5 MΩ (conventional whole-cell mode) and 13.2 ± 0.7 MΩ (perforated whole-cell mode), and were compensated electronically by 80–90%. Current traces were sampled at 10 kHz and filtered at 5 kHz. Holding potential was −80 mV. Current–voltage (I–V) relations were elicited by 400 ms test pulses from −80 mV to +80 mV applied in 10 mV increments (5 s between steps). Peak currents were measured for each test potential and were plotted against the corresponding test potential.
To study steady-state inactivation, we used two-step pulse protocols from a holding potential of −80 mV. First, a 10 s prepulse was applied at various potentials ranging from −110 to −10 mV (10 mV step) followed by a 400 ms test pulse to +20 mV. Inactivation curves were constructed from currents at the corresponding prepulse voltages and fit to the Boltzmann function:
where I is the peak current at each prepulse potential V; Imax is the maximum current recorded; V1/2 is the prepulse potential where I is half of the maximum; K1 is the slope factor at V1/2; and S is the non-inactivating component. To measure the resting membrane potentials, the amplifier was switched from voltage-clamp mode to current-clamp mode with zero current being delivered. pCLAMP 8.1 programs (Axon Instruments Inc., Union City, CA, USA) were used for data acquisition and analysis. All experiments were done at 22°C and the external solution equilibrated with air (PO2 of about 150 Torr). In order to measure the effect of each treatment, six to ten glomus cells from five to six rabbits were used in each group and the effects of one or two treatments were measured in one cell.
Test chemicals were all delivered to the recording bath through a fast-flow apparatus consisting of a linear array of borosilicate glass tubes (Overholt et al. 1995). Chemicals were added into syringe reservoirs, which contained extracellular solution and connected to the fast-flow apparatus. All chemicals were obtained from Sigma (St. Louis, MO, USA).
Data analysis
All data are presented as means ±s.e.m. Statistical significance was determined by Student's paired or unpaired t test for haemodynamic parameters, or a two-way ANOVA, with a Bonferroni procedure for post hoc comparisons of IK. Statistical significance was accepted when P < 0.05.
Results
Induction of heart failure
Rapid left ventricular pacing induced HF by the 3rd or 4th week of pacing. In paced rabbits, LV dD/dtmax and LV shortening fraction were reduced after 3 or 4 weeks of pacing, compared with prepaced baselines (P < 0.05, Table 1). There was no significant change in the LV dD/dtmax and LV shortening fraction between the baseline and the 3rd or 4th week in sham rabbits (Table 1).
Table 1.
Cardiac diameters and contractility in sham and HF rabbits
| Sham (n= 15) Baseline | 3–4 week | HF (n= 16) Baseline | 3–4 week | |
|---|---|---|---|---|
| ESD (% of control) | 100 ± 0.0 | 100.2 ± 1.4 | 100 ± 0.0 | 113.4 ± 2.4 |
| EDD (% of control) | 100 ± 0.0 | 100.4 ± 0.9 | 100 ± 0.0 | 110.3 ± 1.8 |
| dD/dtmax (mm s−1) | −11.2 ± 0.9 | −11.0 ± 1.1 | −10.9 ± 1.3 | −4.3 ± 0.8*# |
| % shortening | 10.3 ± 0.9 | 10.1 ± 0.8 | 10.1 ± 0.8 | 4.6 ± 0.5*# |
Data are means ±s.e.m. ESD, left ventricular end-systolic diameter; EDD, left ventricular end-diastolic diameter; dD/dtmax, 1st derivative of change in diameter; %shortening = (EDD – ESD)/EDD × 100%.
P < 0.05 versus baseline;
P < 0.05 versus sham rabbits at the same period.
Electrophysiological properties of CB glomus cells from sham and HF rabbits
The resting membrane potential of glomus cells in HF rabbits (−34.7 ± 1.0 mV, n= 21 cells from 20 rabbits) was depolarized (P < 0.05), compared with that in sham rabbits (−47.2 ± 1.9 mV, n= 18 cells from 18 rabbits). There was no significant difference in the whole-cell capacitance between sham (2.59 ± 0.18 pF) and HF rabbits (3.23 ± 0.35 pF). Thus, absolute currents measured in our recordings reflected current density in the cells and were not normalized.
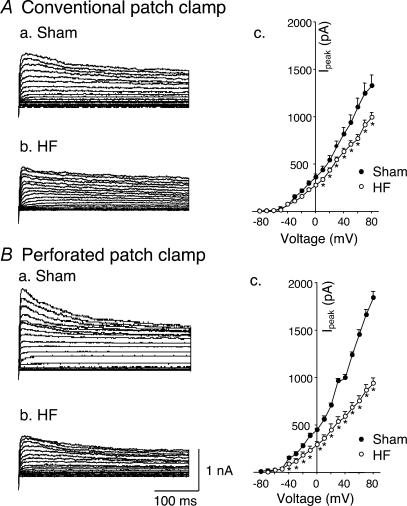
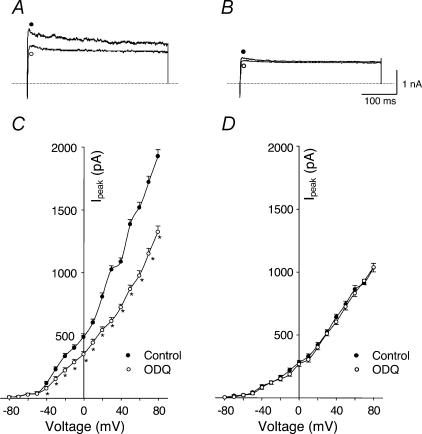
Figure 1Aa–c illustrates typical IK recordings and I–V curves obtained from glomus cells from sham and HF rabbits, with voltage steps from −80 mV under conventional whole-cell patch conditions. As illustrated in current traces in Fig. 1Aa and b, IK was attenuated in HF rabbits, compared with those in sham rabbits. Figure 1Ac shows the peak current–voltage relationships evoked by 400 ms depolarizing test pulses from −80 mV to +80 mV in 10 mV increments. From this curve, the attenuated IK in HF rabbits began near −30 mV and appeared to be voltage dependent.
Figure 1. Outward K+ currents of carotid body glomus cells from sham and HF rabbits.
A, outward K+ currents under conventional patch condition evoked in glomus cells from sham (Aa) and HF (Ab) rabbits by 400 ms depolarizing pulses from a holding potential of –80 mV to test pulses over the range of −80 to +80 mV in 10 mV increments. Ac, peak I–V relationships obtained in glomus cells from sham (n= 10 cells from 6 rabbits) and HF rabbits (n= 10 cells from 6 rabbits). B, outward K+ currents under perforated-patch condition evoked in glomus cells from sham (Ba) and HF (Bb) rabbits using the same pulse protocol above. Bc, peak I–V relationships obtained in glomus cells from sham (n= 8 cells from 6 rabbits) and HF rabbits (n= 9 cells from 6 rabbits). Data are means ±s.e.m. in each group. *P < 0.05versus sham. •, sham; ○, HF.
A potential limitation to the conventional whole-cell patch-recording technique used above is the possibility of the loss into the recording pipette of intracellular constituents that may influence K+ channel function. To assess this possibility we performed experiments using the perforated-patch technique, which restricts movement of intracellular contents into the pipette. From Fig. 1Ba–c it is evident that IK under perforated-patch conditions was larger than that under conventional patch conditions in glomus cells from sham rabbits, but IK was similarly decreased in glomus cells from HF rabbits with either recording technique. These results suggest that the activity of outward K+ channels in the glomus cells was influenced by an intracellular factor and this factor was absent in glomus cells from HF rabbits. We performed additional experiments described below to assess the role of cGMP as the candidate factor. Because IK was affected by the patch conditions, the remaining experiments were performed under perforated-patch conditions except as specifically mentioned.
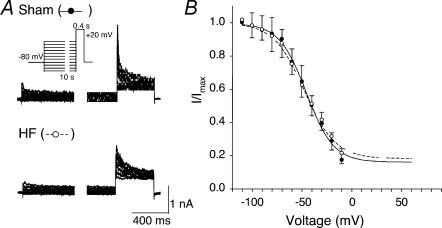
Steady-state inactivation was evaluated with a two-pulse protocol (Fig. 2A). The inactivating prepulse was 10 s in duration in order to reach a steady state of inactivation. The interpulse interval was 60 s to allow full recovery of inactivation. Figure 2B illustrates the mean inactivation curves obtained in eight cells from sham and HF rabbits. The mean inactivation curve exhibited a threshold at about −100 mV and failed to reach full inactivation at values greater than 0 mV. There was no difference in the inactivation curves between sham and HF rabbits (sham, V1/2=−45.6 mV, K1= 13.56, S= 0.16; HF, V1/2=−47.5 mV, K1=−15.44, S= 0.18).
Figure 2. Steady-state inactivation of outward K+ currents in carotid body glomus cells from sham and HF rabbits under perforated-patch conditions.
A, records obtained with the two-step pulse protocol (inset) in glomus cells. During the interpulse interval, the membrane was held at −80 mV for 60 s. B, the steady-state inactivation curves were plotted by normalizing the peak current evoked with the test pulse (+20 mV) against the potential of the prepulse. Data are means ±s.e.m., n= 8 cells from 6 rabbits in each group. Lines represent the best fit of the data to Boltzmann functions (see text). •, sham; ○, HF.
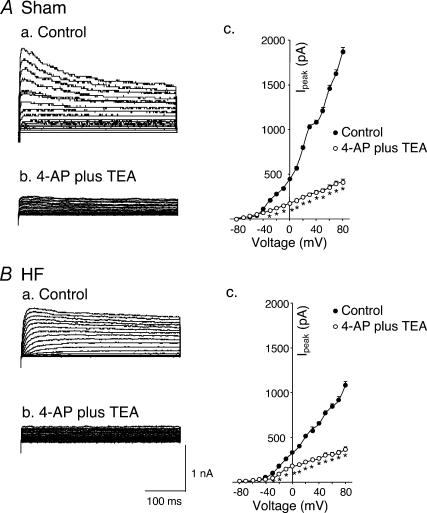
Pharmacological characterization of IK was carried out by the application of 1 mm 4-AP plus 1 mm TEA to the external bath. As shown in Fig. 3, 4-AP plus TEA markedly decreased IK to the same level in glomus cells from sham and HF rabbits (P < 0.05).
Figure 3. Effects of 4-AP plus TEA on outward K+ currents of carotid body glomus cells from sham and HF rabbits under perforated-patch conditions.
A, outward K+ currents of glomus cell from sham rabbit evoked by 400 ms depolarizing pulses from holding potential −80 mV to test pulses (−80 to +80 mV, 10 mV steps) in control conditions (Aa) and with 1 mm 4-AP plus 1 mm TEA (Ab). Ac, peak I–V relationships (n= 6 cells from 5 rabbits) obtained before and after administration of 1 mm 4-AP plus 1 mm TEA. B, outward K+ currents of glomus cell from HF rabbit evoked by same pulse protocol in control conditions (Ba) and with 1 mm 4-AP plus 1 mm TEA (Bb, n= 7 cells from 5 rabbits). Bc, peak I–V relationships obtained before and after administration of 1 mm 4-AP plus 1 mm TEA. *P < 0.05versus control. •, control; ○, 4-AP + TEA.
Effects of iberioxin (IbTx) on IK of CB glomus cells from sham and HF rabbits
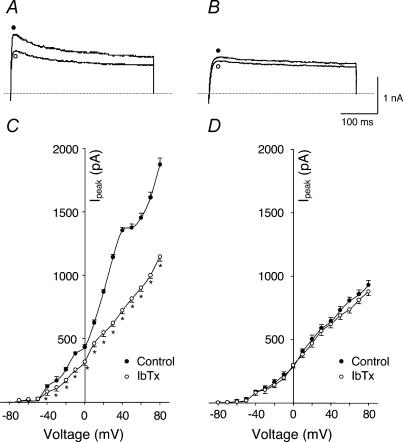
It has been shown that an IKCa component exists in rabbit carotid body glomus cells (Lopez-Lopez et al. 1993). Therefore, IKCa in glomus cells was further characterized by using the selective IKCa blocker, iberiotoxin (IbTx). In glomus cells from sham rabbits, 100 nm IbTx (saturated concentration) decreased IK (P < 0.05, Fig. 4A and C). However IbTx had no effect on IK in glomus cells from HF rabbits (Fig. 4B and D). These results suggested that the decrease of IK in glomus cells from HF rabbits was due to suppression of the KCa channel.
Figure 4. Effects of iberioxin (IbTx, Ca2+-dependent K+ channel blocker) on outward K+ currents of carotid body glomus cells from sham and HF rabbits under perforated-patch conditions.
A (sham rabbit) and B (HF rabbit), outward K+ currents elicited in the glomus cells by 400 ms test pulse from −80 to +70 mV before and after administration of 100 nm IbTx. C (sham rabbit, n= 7 cells from 6 rabbits) and D (HF rabbit, n= 8 cells from 6 rabbits), peak I–V relationships obtained from glomus cells before and after administration of IbTx. *P < 0.05versus control. •, control; ○, IbTx.
Effects of NO on IK of CB glomus cells from sham and HF rabbits
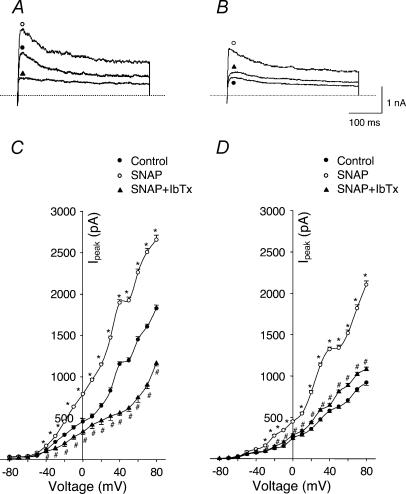
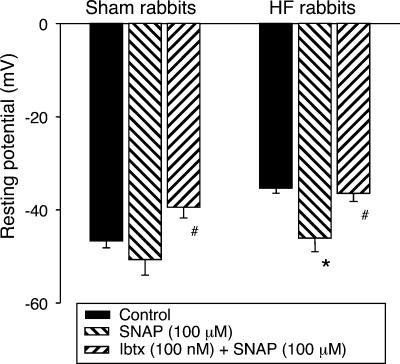
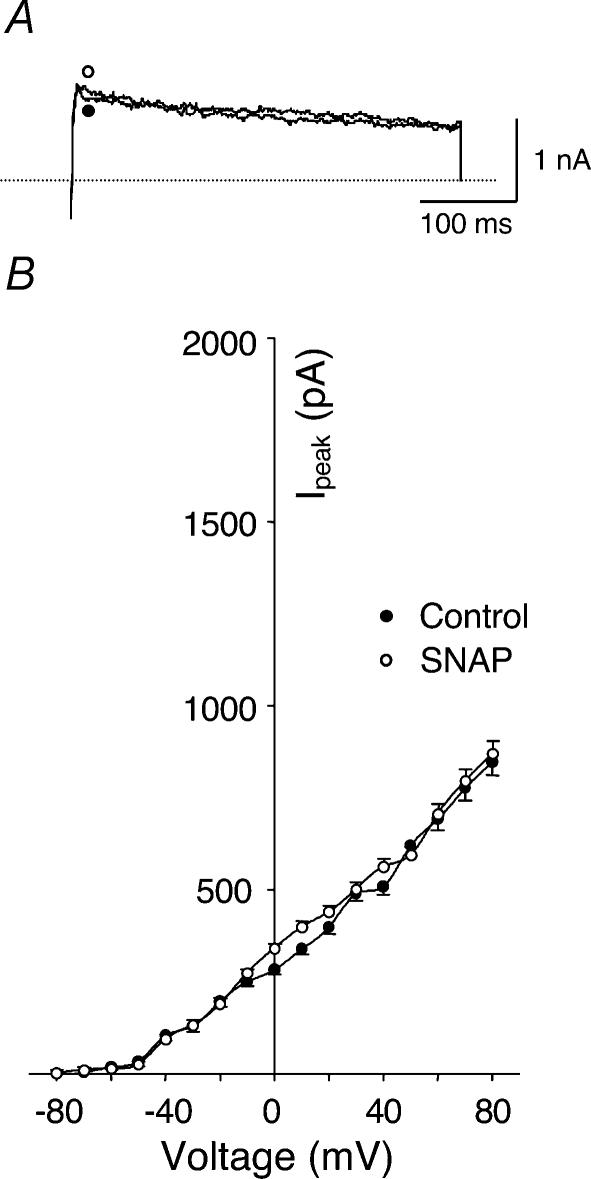
The modulation of IK by NO was investigated by application of the NO donor S-nitroso-N-acetylpenicillamine (SNAP) and a NOS inhibitor, L-NNA, to the recording bath. As shown in Fig. 5, 100 μm SNAP significantly increased IK of glomus cells from HF rabbits and IbTx completely inhibited this effect (P < 0.05, Fig. 5B and D). Similarly, SNAP also enhanced the IK of glomus cells from sham rabbits (P < 0.05, Fig. 5A and C). At the same time, following the changes of IK induced by SNAP and IbTx, corresponding changes were induced in resting membrane potential. In glomus cells from both sham and HF rabbits, SNAP increased the resting membrane potential and IbTx inhibited this effect of SNAP (Fig. 6). However, in conventional whole-cell mode, SNAP did not change IK (Fig. 7) or membrane potential in either group (data not shown).
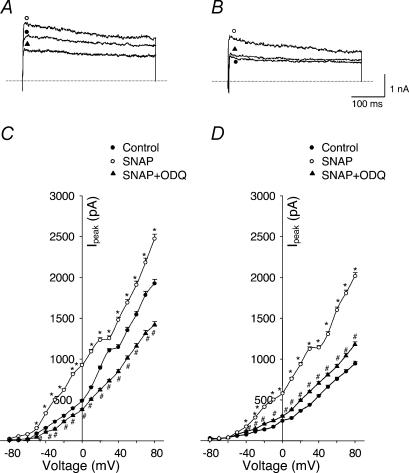
Figure 5. Effects of SNAP (NO donor) on outward K+ currents of carotid body glomus cells from sham and HF rabbits under perforated-patch conditions.
A (sham rabbit) and B (HF rabbit), outward K+ currents elicited in the glomus cells by 400 ms test pulse from −80 to +70 mV in control condition, with 100 μm SNAP alone and with 100 μm SNAP plus 100 nm IbTx. C (sham rabbit, n= 8 cells from 6 rabbits) and D (HF rabbit, n= 8 cells from 6 rabbits), peak I–V relationships obtained from glomus cells in control condition, with 100 μm SNAP alone and with 100 μm SNAP plus 100 nm IbTx. *P < 0.05 versus control; #P < 0.05versus SNAP. •, control; O, SNAP; ▴, SNAP + IbTx.
Figure 6. Effects of SNAP (NO donor) on resting membrane potential in carotid body glomus cells from sham and HF rabbits.
Data are means ±s.e.m., n= 8 cells from 6 rabbits in each group. *P < 0.05 versus control; #P < 0.05 versus SNAP.
Figure 7. Effects of SNAP (NO donor) on outward K+ currents of carotid body glomus cells from HF rabbits under conventional patch conditions.
A, outward K+ currents elicited in the glomus cells by 400 ms test pulse from −80 to +70 mV before and after administration of 100 μm SNAP. B, peak I–V relationships (n= 7 cells from 5 rabbits) obtained from glomus cells before and after administration of 100 μm SNAP. •, control; ○, SNAP.
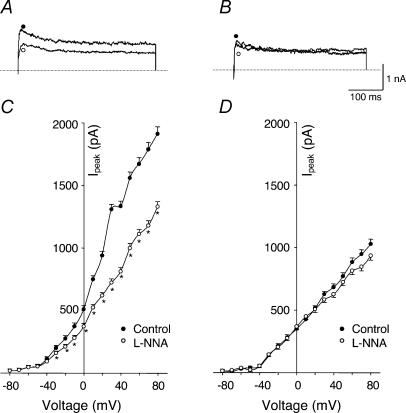
Since NOS inhibition by L-NNA can take several minutes, cells in the recording dish were preincubated for 30 min in normal extracellular solution containing 10 μm L-NNA. In sham rabbits, we examined the effect of L-NNA in 13 different glomus cells and its effect was clearly seen in 10 cells. As shown in Fig. 8A and C, L-NNA decreased IK (P < 0.05). However, L-NNA did not affect IK in glomus cells from HF rabbits (Fig. 8B and D). These results suggest that down-regulation of NOS activity resulted in the suppression of KCa channel activity in glomus cells from HF rabbits, and this effect contributed to the attenuated macroscopic IK in these cells. In addition, NO appeared to modulate the KCa channel via some intracellular factor(s) since SNAP was ineffective in conventional whole-cell recordings.
Figure 8. Effects of Nω-nitro-l-arginine (L-NNA, NOS inhibitor) on outward K+ currents of carotid body glomus cells from sham and HF rabbits under perforated-patch conditions.
A (sham rabbit) and B (HF rabbit), outward K+ currents elicited in the glomus cells by 400 ms test pulse from −80 to +70 mV, control and administration of 10 μm L-NNA. C (sham rabbit, n= 10 cells from 6 rabbits) and D (HF rabbit, n= 8 cells from 6 rabbits), peak I–V relationships obtained from glomus cells in control and administration of L-NNA. *P < 0.05versus control. •, control; ○, L-NNA.
Involvement of cGMP in the effect of NO on IK
To test whether enhancement of IK by NO is related to activation of guanylate cyclase, we examined the effects of SNAP on IK in the presence of 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ), a guanylate cyclase inhibitor. As shown in the current traces in Fig. 9A and B, and I–V curves in Fig. 9C and D, ODQ (1 μm) alone reduced IK in glomus cells from sham rabbits (P < 0.05) but not in those from HF rabbits. More importantly, ODQ inhibited the effects of SNAP on IK in cells from both sham and HF rabbits (P < 0.05, Fig. 10). We further inferred that if the effects of NO were mediated by cGMP, then 8-bromoguanosine-3′,5′-cyclic monophosphate (8-Br-cGMP), a cGMP analogue, should mimic the effects of SNAP on IK. Therefore we tested this possibility in seven additional cells from each group. Treatment with 8-Br-cGMP (1 mm) for 20 min increased IK in glomus cells from sham and HF rabbits by 132 ± 11% and 176 ± 19% of control, respectively (P < 0.01), at a test potential of +70 mV. These results support the idea that NO enhanced IK via a cGMP-dependent pathway.
Figure 9. Effects of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, guanylate cyclase inhibitor) on outward K+ currents of carotid body glomus cells from sham and HF rabbits under perforated-patch conditions.
A (sham rabbit) and B (HF rabbit), outward K+ currents elicited in the glomus cells by 400 ms test pulse from −80 to +70 mV, before and after administration of 1 μm ODQ. C (sham rabbit, n= 8 cells from 6 rabbits) and D (HF rabbit, n= 7 cells from 6 rabbits), peak I–V relationships obtained from glomus cells before and after administration of ODQ. *P < 0.05versus control. •, control; ○, ODQ.
Figure 10. Effects of SNAP on outward K+ currents through a cGMP-dependent mechanism.
A (sham rabbit) and B (HF rabbit), outward K+ currents elicited in the glomus cells by 400 ms test pulse from −80 to +70 mV in control condition, with 100 μm SNAP alone and with 100 μm SNAP plus 1 μm ODQ. C (sham rabbit, n= 6 cells from 6 rabbits) and D (HF rabbit, n= 6 cells from 6 rabbits), peak I–V relationships obtained from glomus cells in control condition, with 100 μm SNAP alone and with 100 μm SNAP plus 1 μm ODQ. *P < 0.05versus control; #P < 0.05versus SNAP. •, control; ○, SNAP; ▴, SNAP + ODQ.
Discussion
The results of the present study demonstrate that: (1) IK is attenuated in CB glomus cells from HF rabbits, and the resting membrane potential of glomus cells in HF rabbits is depolarized as compared with that in sham rabbits; (2) the selective KCa channel blocker IbTx decreased IK in glomus cells from sham rabbits, but had no effect on IK from HF rabbits; (3) the NO donor SNAP increased IK in glomus cells from sham and HF rabbits and IbTx inhibited the effect of SNAP; (4) the non-selective NOS inhibitor L-NNA reduced IK in sham rabbits but not in HF rabbits; (5) the guanylate cyclase inhibitor ODQ inhibited the effects of SNAP on IK. From these results, we conclude that: (1) the attenuated IK in glomus cells from HF rabbits is due to suppression of KCa channel activity; (2) NO activates KCa channels in rabbit glomus cells; (3) the decreased activity of KCa channels in glomus cells in HF rabbits is due to decreased NOS activity or bioavailability of NO; and (4) NO modulates the KCa channels via intracellular cGMP.
Functionally, K+ channels are classified into five families: (1) voltage-gated K+ (KV) channels, (2) KCa channels, which are intracellular Ca2+-sensitive and also voltage-sensitive, (3) ATP-sensitive K+ channels, (4) inward rectifier K+ channels, and (5) voltage-insensitive background K+ channels (Jan & Jan, 1997; Lesaga & Lazdunski, 2000). These channels are extraordinarily diverse and are expressed in many different cell types. In adult rabbits to the extent known, carotid body glomus cells express KV channels, KCa channels and HERG-like K+ channels (inward rectifier K+ channel; Lopez-Lopez et al. 1993, 1997; Overholt et al. 2000).
Of these K+ channels, which type is likely to account for attenuated IK in carotid body glomus cells from HF rabbits, and what regulates the change of resting membrane potential observed? The present study showed that IbTx inhibited IK in sham rabbits but had no effect in HF rabbits. These results suggest that KCa channel activity is markedly suppressed in glomus cells from HF rabbits, and the attenuated IK in HF rabbits is mainly due to decreased activity of these KCa channels. On the other hand, our results also do not imply that suppressed KCa channel activity exclusively caused the attenuated IK in HF rabbits. After administration of KV channel blockers, 4-AP and TEA, IK was reduced to the same level in both sham and HF rabbits (Fig. 3). However, in sham rabbits IbTx did not reduce the IK level to that seen in HF rabbits (Fig. 4). These results suggest that other KV channels are also involved in the attenuated IK in HF rabbits, but to a lesser extent.
Our results also suggest that the attenuated KCa activity contributed to the elevation in resting membrane potential observed in the HF glomus cells. SNAP lowered the resting potential to normal levels in HF cells, and this effect was reversed by IbTx. However, a HERG-like K+ channel has been reported in rabbit glomus cells which could also influence the resting membrane potential (Overholt et al. 2000). The contribution of HERG channels to the elevated membrane potential in the HF glomus cells remains to be determined.
Much evidence indicates that NO produced within the CB is an inhibitory modulator of chemoreception (Prabhakar et al. 1993; Chugh et al. 1994; Wang et al. 1994; Alcayaga et al. 1997; Prabhakar, 1999; Sun et al. 1999b; Iturriaga et al. 2000a). The administration of the precursor l-arginine, NO donors (Prabhakar et al. 1993; Chugh et al. 1994; Wang et al. 1994; Iturriaga et al. 2000b), and NO gas (Iturriaga et al. 2000a) to the cat carotid body perfused in vitro reduces the chemosensory response to hypoxia. Non-specific NOS inhibitors, such as -nitro-l-arginine methyl ester (L-NAME), enhance the chemosensory response to hypoxia (Wang et al. 1994) or nicotine and NaCN (Valdes et al. 2003). In a previous study we demonstrated that the NO donor (SNAP) inhibited the baseline discharge of the carotid sinus nerve (CSN) and the chemosensitivity in sham and HF rabbits (Sun et al. 1999b). Our present results confirmed that SNAP enhanced IK and hyperpolarized the resting membrane potential in glomus cells from sham and HF rabbits, and IbTx completely abolished the effects of SNAP. We have shown that NO production and protein expression of NOS in CB from HF rabbits is lower than that from sham rabbits (Sun et al. 1999b; Zeng et al. 2002). From these data, we propose that decreased NO production contributed to the attenuated the IK in glomus cells from HF rabbits by preventing the activation of KCa channels.
In contrast to our study, Hatton & Peers (1996) found that SNAP did not affect IK recorded in CB glomus cells. One possibility for this discrepancy is that their study employed the conventional whole-cell patch-clamp technique. The present study also found that SNAP did not change the IK of glomus cells in the conventional whole-cell mode, but SNAP was effective in the perforated-patch mode. Taken together these results suggest that some intracellular factor(s) that is dialysed in conventional whole-cell recording is necessary for KCa channel modulation by SNAP. This notion is supported by Silva & Lewis's study (2002), in which KCa channel activity in rat CB glomus cells was enhanced by SNAP in cell-attached mode.
Our results indicate that this intracellular factor is cGMP. NO activates soluble guanylate cyclase, and many of its effects are mediated by stimulation of cGMP production (Snyder, 1992). cGMP is well known to activate K+ channels in vascular smooth muscle, and is the major mediator of NO-induced vasodilatation in many vascular beds (Vaandrager & de Jonge, 1996). The NO donor nitroglycerine has been shown to elevate cGMP levels in glomus cells (Wang et al. 1994). In rat CB glomus cells, NO enhances KCa channel activity through cGMP-dependent protein kinase G (Silva & Lewis, 2002). However, recently it has been shown that NO inhibits L-type Ca2+ channels in rabbit type I cells via a cGMP-independent mechanism (Summers et al. 1999). In rabbit aortic smooth muscle cells (Bolotina et al. 1994) and rat brain (Shin et al. 1997), NO activates KCa channels directly. The heterogeneity of these results could be due to the difference of species and tissues. Our results confirmed that the effect of NO on the IK in CB glomus cells was cGMP-dependent because ODQ inhibited the effects of SNAP on the IK and 8-Br-cGMP could mimic the effect of SNAP on the IK in both sham and HF rabbits.
It is known that NOS isoforms (eNOS, nNOS and iNOS) exist in the extensive plexus of nerve fibres and vessels surrounding glomus cells (Wang et al. 1993) but not in detectable levels in CB glomus cells and type II cells. Thus, a reasonable question is how NO production and NOS inhibitors can influence IK in isolated CB glomus cells. There may be at least two possible explanations for this phenomenon. First, NOS may be present in glomus cells at low levels that are difficult to detect (Prabhakar et al. 1993), but sufficient to influence K+ channel activity, or sequestered in mitochondria inaccessible to immunofluorescence antibodies (Alvarez et al. 2003). Secondly, even though glomus cells are dispersed in cell culture, they continue to coexist with other neural and vascular cells of the CB that possess substantial amounts of NOS. These cells may produce sufficient NO to exert a paracrine effect on the neighbouring glomus cells from which we recorded. This notion is supported by other electrophysiological studies in which paracrine effects can be observed from neighbouring cells in the recording media (Chapleau et al. 2001). Although we cannot ascertain the cellular origin of NOS activity in the present study, we observed that L-NNA reduced the IK in glomus cells from sham rabbits but not from HF rabbits, which demonstrated that lower activation of NOS was responsible for the attenuated IK in glomus cells from HF rabbits.
Our previous study has shown that the baseline discharge of CB chemoreceptor afferents in the normoxic state and their response to hypoxia are enhanced in pacing-induced HF rabbits as compared with sham rabbits (Sun et al. 1999b). Our present results indicate that IK is attenuated in CB glomus cells from HF rabbits in the normoxic state and the resting membrane potential of glomus cells from HF rabbits is depolarized as compared with that in sham rabbits. The depression in IK from the CB glomus cells in HF rabbits is consistent with the enhanced peripheral chemoreceptor function observed for CB afferent responses in HF rabbits. NO donors inhibited the baseline discharge of CSN (Sun et al. 1999b) and enhanced the IK in glomus cells in sham and HF rabbits. L-NNA increased the baseline discharge of CSN in sham rabbits but not HF rabbits (Sun et al. 1999b) and attenuated the IK in sham rabbits but not in HF rabbits. Based on these results, it is reasonable to assume that the attenuated IK in glomus cells induced by HF contributes to the enhancement of CB chemoreceptor function. Nevertheless, it is important to point out that other ion channels in glomus cells may be altered in HF due to changes in NO or other neuro-endocrine factors. These effects must be taken into consideration when fully assessing the functional significance of the results we describe here.
In conclusion, our results indicate that IK is attenuated in CB glomus cells from HF rabbits, and the attenuated IK is due mainly to the decreased activity of KCa channels. In the normal state, NO maintains this KCa channel activity via a cGMP-dependent signalling pathway, and during HF a decrease in NO induced by an attenuated NOS activity is responsible for the inactivation of the KCa channels.
Acknowledgments
The authors wish to thank Denise Arrick and Kaye Talbitzer for their technical assistance, and Dr Kurtis Cornish for his surgical assistance. This study was supported by a Program Project Grant from the Heart, Lung and Blood Institute of NIH (PO1-HL62222). Dr Sun was supported in part by a fellowship from the American Heart Association.
References
- Alcayaga J, Iturriaga R, Ramirez J, Readi R, Quezada C, Salinas S. Cat carotid body chemosensory response to non-hypoxia stimuli is inhibited by sodium nitroprusside both in situ and in vitro. Brain Res. 1997;767:384–387. doi: 10.1016/s0006-8993(97)00805-6. [DOI] [PubMed] [Google Scholar]
- Alvarez S, Valdez LB, Zaobornyj T, Boveris A. Oxygen dependence of mitochondrial nitric oxide synthase activity. Biochem Biophys Res Commun. 2003;305:771–775. doi: 10.1016/s0006-291x(03)00818-0. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Li Z, Meyrelles SS, Ma XY, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann NY Acad Sci. 2001;940:1–19. doi: 10.1111/j.1749-6632.2001.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Chugh DK, Katayama M, Mokashi A, Debout DE, Ray DK, Lahiri S. Nitric oxide-related inhibition of carotid chemosensory nerve activity in the cat. Respir Physiol. 1994;97:147–156. doi: 10.1016/0034-5687(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Esler M, Kaye D, Lambert G, Esler D, Jennings G. Adrenergic nervous system in heart failure. Am J Cardiol. 1997;80:7L–14L. doi: 10.1016/s0002-9149(97)00844-8. [DOI] [PubMed] [Google Scholar]
- Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am Coll Cardiol. 1993;22:72A–84A. doi: 10.1016/0735-1097(93)90466-e. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Peers C. Hypoxic inhibition of K+ currents in isolated rat type I carotid body cells: evidence against the involvement of cyclic nucleotides. Pflugers Arch. 1996;433:129–135. doi: 10.1007/s004240050258. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Mosqueira M, Villanueva S. Effects of nitric oxide gas on carotid body chemosensory response to hypoxia. Brain Res. 2000a;855:282–286. doi: 10.1016/s0006-8993(99)02369-0. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Villanueva S, Mosqueira M. Dual effects of nitric oxide on cat carotid body chemoreception. J Appl Physiol. 2000b;89:1005–1012. doi: 10.1152/jappl.2000.89.3.1005. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan TN. Cloned potassium channels from eukaryotes and prokaryotes. Annu Rev Neurosci. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- Lesaga F, Lazdunski M. Molecular and functional properties of two-pore domain potassium channels. Am J Physiol. 2000;279:R793–R801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Li YL, Schultz HD. Attenuated Ca2+-dependent potassium current in carotid body glomus cells of heart failure rabbit: Involvement of nitric oxide. FASEB J. 2003;17:A401. doi: 10.1113/jphysiol.2003.057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, DeLuis DA, Gonzalez C. Properties of a transient K+ current in chemoreceptor cells of rabbit carotid body. J Physiol. 1993;460:15–32. doi: 10.1113/jphysiol.1993.sp019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Gonzalez C, Perez-Garcia MT. Properties of ionic currents from isolated adult rat carotid body chemoreceptor cells: effect of hypoxia. J Physiol. 1997;499:429–441. doi: 10.1113/jphysiol.1997.sp021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Overholt JL, Ficker E, Yang T, Shams H, Bright GR, Prabhakar NR. HERG-like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body. J Neurophysiol. 2000;83:1150–1157. doi: 10.1152/jn.2000.83.3.1150. [DOI] [PubMed] [Google Scholar]
- Overholt JL, Prabhakar NR. Ca2+ current in rabbit carotid body glomus cells is conducted by multiple types of high voltage-activated Ca2+ channels. J Neurophysiol. 1997;78:2467–2474. doi: 10.1152/jn.1997.78.5.2467. [DOI] [PubMed] [Google Scholar]
- Overholt JL, Saulino A, Drumm ML, Harvey RD. Rectification of whole cell cystic fibrosis transmembrane conductance regulator chloride current. Am J Physiol. 1995;268:C636–C646. doi: 10.1152/ajpcell.1995.268.3.C636. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Neurotransmitters in the carotid body. Adv Exp Med Biol. 1994;360:57–69. doi: 10.1007/978-1-4615-2572-1_6. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Respir Physiol. 1999;115:161–168. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Chang CH, Agani FH, Haxhiu MA. Nitric oxide in the sensory function of the carotid body. Brain Res. 1993;625:16–22. doi: 10.1016/0006-8993(93)90132-7. [DOI] [PubMed] [Google Scholar]
- Shin JH, Chung S, Park EJ, Uhm DY, Suh CK. Nitric oxide directly activates calcium-activated potassium channels from rat brain reconstituted into planar lipid bilayer. FEBS Lett. 1997;415:299–302. doi: 10.1016/s0014-5793(97)01144-7. [DOI] [PubMed] [Google Scholar]
- Silva JM, Lewis DL. Nitric oxide enhances Ca2+-dependent K+ channel activity in rat carotid body cells. Pflugers Arch. 2002;443:671–675. doi: 10.1007/s00424-001-0745-1. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Nitric oxide: first in a new class of neurotransmitters. Science. 1992;257:494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR. Nitric oxide inhibits L-type Ca2+ current in glomus cells of the rabbit carotid body via a cGMP-independent mechanism. J Neurophysiol. 1999;81:1449–1457. doi: 10.1152/jn.1999.81.4.1449. [DOI] [PubMed] [Google Scholar]
- Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol. 1999a;86:1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol. 1999b;86:1273–1282. doi: 10.1152/jappl.1999.86.4.1273. [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, de Jonge HR. Signaling by cGMP-dependent protein kinases. Mol Cell Biochem. 1996;157:23–30. doi: 10.1007/BF00227877. [DOI] [PubMed] [Google Scholar]
- Valdes V, Mosqueira M, Rey S, Rio RD, Iturriaga R. Inhibitory effects of NO on carotid body: contribution of neural and endothelial nitric oxide synthase isoforms. Am J Physiol. 2003;284:L57–L68. doi: 10.1152/ajplung.00494.2001. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Bredt DS, Fidone SJ, Stensaas LJ. Neurons synthesizing nitric oxide innervate the mammalian carotid body. J Comp Neurol. 1993;336:419–432. doi: 10.1002/cne.903360308. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Stensaas LJ, Bredt DS, Dinger B, Fidone SJ. Localization and actions of nitric oxide in the cat carotid body. Neuroscience. 1994;60:275–286. doi: 10.1016/0306-4522(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Zeng YC, Sun SY, Xia XH, Reddy P, Li Y, Schultz HD. Effect of exercise and heart failure on NOS isoforms in the carotid body of rabbits. FASEB J. 2002;16:A829. [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J Physiol. 1997;503:599, 612. doi: 10.1111/j.1469-7793.1997.599bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–341. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]