Abstract
Heart failure (HF) is a syndrome resulting from the inability of the cardiac pump to meet the energy requirements of the body. Despite intensive work, the pathogenesis of the cardiac intracellular abnormalities that result from HF remains incompletely understood. Factors that lead to abnormal contraction and relaxation in the failing heart include metabolic pathway abnormalities that result in decreased energy production, energy transfer and energy utilization. Heart failure also affects the periphery. Patients suffering from heart failure always complain of early muscular fatigue and exercise intolerance. This is linked in part to intrinsic alterations of skeletal muscle, among which decreases in the mitochondrial ATP production and in the transfer of energy through the phosphotransfer kinases play an important role. Alterations in energy metabolism that affect both cardiac and skeletal muscles argue for a generalized metabolic myopathy in heart failure. Recent evidence shows that decreased expression of mitochondrial transcription factors and mitochondrial proteins are involved in mechanisms causing the energy starvation in heart failure. This review will focus on energy metabolism alterations in long-term chronic heart failure with only a few references to compensated hypertrophy when necessary. It will briefly describe the energy metabolism of normal heart and skeletal muscles and their alterations in chronic heart failure. It is beyond the scope of this review to address the metabolic switches occurring in compensated hypertrophy; readers could refer to well-documented reviews on this subject.
Metabolic alterations in the heart
Specificity of cardiac metabolism
Myocardial function depends on a fine equilibrium between the work the heart has to perform to meet the requirements of the body and the energy that it is able to synthesize and transfer in the form of energy-rich phosphate bonds to sustain excitation–contraction coupling. Heart muscle is a highly oxidative tissue that produces more than 90% of its energy from mitochondrial respiration. Mitochondria occupy ≈30% of cardiomyocyte space and are well organized under the sarcolemma and in rows between myofilaments such that a constant diffusion distance exists between mitochondria and the core of myofilaments. During maximal exercise the heart uses more than 90% of its oxidative capacity, showing that there is no excess capacity of energy production over energy utilization (Mootha et al. 1997). There is a strict relationship in vitro and in vivo between oxygen consumption and cardiac work that occurs at constant global cellular ATP and phosphocreatine (PCr) concentrations. Therefore, a strong energy signalling pathways should exist to ensure a close matching between oxygen consumption and energy utilization. At present, the nature and function of such signals are still under debate. Oxygen availability, substrate limitation, ATP, ADP and PCr changes, inorganic phosphate, calcium, redox state and phosphotransfer systems have all been considered to play a role. Their relative contribution to energy metabolism homeostasis will depend on the mechanical load and the metabolic conditions the heart has to respond to. Among these factors, two of them have been extensively considered. One of the candidates for coupling aerobic metabolism and cardiac work is calcium as it regulates myosin and sarcoplasmic reticulum ATPases on the one hand, and the major mitochondrial dehydrogenases and F0/F1-ATPase on the other (Balaban, 2002). However, the assumption that respiration and contraction are simultaneously regulated by Ca2+ ions is not completely satisfactory, as parallel increases in cardiac work and oxygen consumption with increase in length (Frank-Starling mechanism) occurs at constant intracellular Ca2+ transients (Shimizu et al. 2002).
On the other hand, the muscle cell is not a well-mixed bag and the reactions involved in ATP generation and utilization are not governed by stochastic events, but are rather comprised within structural and functional entities, which are spatially and temporarily co-ordinated. Glycolytic enzymes are arranged in supramolecular complexes and bound to intracellular structures such as myofilaments and sarcoplasmic reticulum, where they participate in local energy production, more readily used by ion pumps and other membrane structures (Weiss & Hiltbrand, 1985). The presence of high-energy phosphotransfer systems is another essential feature of cardiac or striated muscle energy metabolism. Early in the seventies, Bessman identified the creatine kinase (CK) and adenylate kinase (AK) systems as energy shuttles (Bessman & Geiger, 1981). Since that time, considerable pieces of evidence have been accumulating to understand high-energy transfer in cardiac and muscle cells.
CK is present in variable amounts in heart and skeletal muscles and catalyses the reversible transfer of a phosphate moiety between ATP and creatine. Four different isoforms have been described and are expressed in a tissue-specific and developmentally regulated manner. CK exists as dimers composed of two subunits, M and B, giving three isoenzymes, MM, BB and MB. A fourth isoenzyme specifically found in the mitochondria (mi-CK) can form both octameric and dimeric structures (Wyss et al. 1992) and represents 20–40% of all CK activity in cardiac cells. CK isoenzymes are not evenly distributed and the CK system constitutes an example of a compartmentalized metabolic pathway. Myofibrillar MM-CK is a structural protein of the M-band and is functionally coupled to the myosin ATPase, thus providing enough energy to sustain maximal force and normal kinetics of contraction (Wallimann & Eppenberger, 1985; Ventura-Clapier et al. 1994). MM-CK is also strongly bound to the sarcoplasmic reticulum (SR) membranes where it is functionally coupled to the Ca2+-ATPase, and ensures efficient energy provision for calcium uptake (for review and further references see Ventura-Clapier et al. 1998). Another local functional coupling takes place in the intermembrane space of mitochondria, where mi-CK is found on the outer surface of the inner mitochondrial membrane, in the vicinity of the ATP–ADP translocator (ANT). During active oxidative phosphorylation, ATP generated in the matrix is exported by ANT in the intermembrane space where it is transphosphorylated by mi-CK to PCr and ADP. ADP is then immediately available for oxidative phosphorylation and further stimulates respiration (for reviews and further references see Wyss et al. 1992; Saks et al. 1994). These localized functional couplings between ATP-generating or -consuming enzymes and CK efficiently control local ATP/ADP ratios that thermodynamically and kinetically favour energy production in mitochondria (low ATP/ADP ratio) and energy consumption in cytosolic compartments (high ATP/ADP ratios). These sites are connected through the near-equilibrium CK reactions that take place in the cytosol, and that result in almost instantaneous transfer of phosphoryl groups to ATPases and of metabolic signal to mitochondria (Dzeja et al. 1998). Until recently, intracellular compartmentation of CK fluxes has been mostly neglected in the analysis of 31P NMR data, mainly because the cell has been considered so far as an homogeneous system. Recently, a new methodological approach allowing the quantification of unidirectional fluxes of localized CKs (Joubert et al. 2002b) and mathematical modelling (Aliev & Saks, 1997; Joubert et al. 2002a) provided strong evidence for the existence of localized adenine nucleotide pools interrelated through intracellular energy transfer by CK.
Among phosphotransfer kinases, the CK system appears the most important, but others such as AK are also present and compartmentalized within the cell (Dzeja & Terzic, 2003). Moreover, it was recently shown that cell architecture is involved in energy regulation. Direct energy cross-talk between mitochondria and energy-consuming organelles (Kaasik et al. 2001) explains that locally produced ADP is more efficient than bulk ADP at stimulating mitochondrial respiration. In oxidative muscle cells, mitochondria with adjacent ADP-producing systems in myofibrils and in sarcoplasmic reticulum can be viewed as functional units representing the basic pattern of organization of muscle–cell energy metabolism (Saks et al. 2001). In CK-deficient muscles, phosphotransfer by other kinases, direct channelling with mitochondria, and glycolytic enzymes provide alternative routes for intracellular high-energy transfer (Dzeja et al. 1998; Boehm et al. 2000; Kaasik et al. 2001, 2003). This explains in part the preserved contractile function of CK–/– mice at moderate workloads (Saupe et al. 1998; Crozatier et al. 2002). These systems may not simply be redundant and more work is needed to understand their redundancy and/or specificity.
All these data show that maintaining energetic homeostasis despite fluctuating energy demand is an important prerequisite for contractile efficiency. This emphasizes the fact that cell architecture and metabolic networks are interrelated to build integrated phosphotransfer systems that improve cellular economy to tightly match cellular functions, and that alterations in this fine regulation can compromise cardiac function.
Heart failure and cardiac metabolism
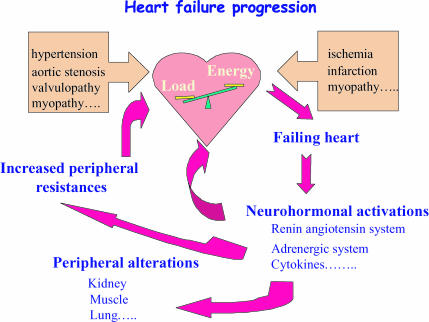
Mechanisms leading to cardiac pump failure can have multiple origins. This includes pressure overload, ischaemic heart disease resulting from altered coronary artery circulation or infarction, cardiomyopathies and defects in genes encoding proteins of a large panel of cellular functions, such as contractile apparatus, cytoskeleton, intercellular matrix and mitochondrial proteins. These defects result in (1) a mismatch between cardiac ability to eject blood and the needs of the body, and (2) a remodelling of cardiac structure initially to compensate for the impaired function (Fig. 1). In heart failure, the depression of contractile force is not matched by a concomitant depression of energy consumption, leading to mechanoenergetic uncoupling (Schipke, 1994; Saavedra et al. 2002).
Figure 1. Heart failure progression.
A mismatch between the load applied to the heart and the energy needed to meet the load may arise from mechanical and/or metabolic factors. The deleterious way into failure activates numerous pathways that increase peripheral resistance and induce compensatory as well as harmful skeletal muscle and cardiac remodelling. Increased peripheral resistances and adverse remodelling aggravate heart failure.
Optimal cellular bioenergetics rely on (1) adequate delivery of oxygen and substrates to the mitochondria, (2) the oxidative capacity of mitochondria, (3) adequate amounts of high-energy phosphate and the PCr/ATP ratio, (4) efficient energy transfer from mitochondria to sites of energy utilization, (5) adequate local regulation of ATP/ADP ratios near ATPases, and (6) efficient feedback signalling from utilization sites to maintain energetic homeostasis in the cell. Defects at these various steps of the cardiac energetic pathways have been found in cardiovascular diseases such as dilated and hypertrophic cardiomyopathies of various origins, cardiac conduction defects, and ischaemic heart diseases. Compromised energetics was recently proposed as a unifying mechanism to explain myocardium dysfunctions in hypertrophic cardiomyopathies (Ashrafian et al. 2003).
Substrates and oxygen availability. One important abnormality impairing high-energy phosphate synthesis in the failing heart is a decrease in coronary reserve that may limit nutrient and oxygen delivery to the cardiomyocytes at high workloads.
The heart is a metabolic omnivore able to meet its energy requirements from the oxidation of fatty acids, glucose, lactate and other oxidizable substrates. Despite a retrocontrol of fatty acid and glucose utilization, the heart functions best when it oxidizes both substrates simultaneously (Taegtmeyer, 2000). In HF, the chief myocardial energy substrates switch from fatty acids to glucose, with a down-regulation of the enzymes involved in fatty acid oxidation (Sack et al. 1996; Razeghi et al. 2001).
Glycolysis. It is usually accepted that during hypertrophy the chief myocardial energy source switches from fatty acid β-oxidation to glycolysis, a reversion to the fetal energy substrate preference pattern. Early switch from fatty acid to carbohydrate metabolism in hypertrophy results in improved efficiency of the heart as long as glucose can be oxidized (Taegmeyer, 2000). An increase in glycolysis and in glycolytic enzymes is observed in hypertrophy but rates of glucose oxidation are reduced and more lactate accumulates. As the process of remodelling progresses towards the uncompensated state, metabolic adaptation becomes insufficient with a lower capacity to oxidize glucose leading to decreased efficiency (Leong et al. 2003). In human heart failure, the glucose transporters GLUT-1, GLUT-4 and muscle glycogen synthase mRNA are down-regulated (Razeghi et al. 2001, 2002). Overexpression for the lactate transporter MCT1 in an experimental model of heart failure has been recently described, which could favour lactate transport (Johannsson et al. 2001). Heart failure is not accompanied by overexpression of glycolytic pathways and end-stage heart failure results in decreased glycolytic enzymes (De Sousa et al. 1999; Dzeja et al. 1999). This seems to be true for both hypertrophic and dilated cardiomyopathy (Kalsi et al. 1999).
GLUT-4 ablation induces hypertrophy (Abel et al. 1999), while GLUT-1 overexpression normalizes the PCr/ATP ratio and is protective against the development of heart failure induced by pressure overload (Liao et al. 2002). Although the exact mechanisms are not completely understood, this points towards a more important role of energy metabolism in the pathophysiology of heart failure than previously thought (Taegtmeyer, 2002).
Mitochondria. Chronic heart failure is associated with morphological abnormalities of mitochondria such as increased number, reduced size and compromised structural integrity (Schaper et al. 1991). Mitochondrial injury is positively correlated with indices of heart failure severity such as plasma noradrenaline (norepinephrine), and left ventricle (LV) end-diastolic pressure and ejection fraction (Sabbah et al. 1992).
In human and experimental HF, decreases in the activity of complexes of the respiratory chain or Krebs cycle enzymes have been described. The reduced expression of mitochondrial proteins relates to limited ATP synthesis capacity and high-energy phosphate kinetic abnormalities in HF (Ning et al. 2000). Moreover, defective oxygen consumption rates and blunted mitochondrial regulation by the phosphate acceptors AMP, ADP and creatine are in favour of a lower myocardial energy production in HF via oxidative phosphorylation (Sanbe et al. 1995; Sharov et al. 1998, 2000; De Sousa et al. 1999). Due to the strict correlation between oxygen consumption and work, the decreased oxidative capacity of the failing myocardium will limit cardiac work at least for high workloads. However, even in basal conditions the cellular levels of ATP and PCr as well as the PCr/ATP ratio, all of which are controlled by oxidative phosphorylation, are altered in heart failure.
High-energy phosphates. The failing heart is unable to maintain its energetic reserve. Alterations in myocardial high-energy phosphates were identified in animal models and human hearts with LV hypertrophy or heart failure. A decrease in PCr/ATP ratio is consistently reported in failing human heart and experimental heart failure, even at moderate workloads. Creatine, creatine transporter, PCr and ATP are significantly reduced (Neubauer et al. 1999; Beer et al. 2002), and the decrease in the PCr/ATP ratio is a predictor of mortality in congenital heart failure (CHF) (Neubauer et al. 1997). However, the precise cellular mechanisms by which altered high-energy phosphate levels may compromise energy fluxes and contractility are not well understood. Of major importance, this is accompanied by an increase in ADP concentration and a resulting decrease in the phosphorylation potential that can affect ATPases involved in excitation–contraction coupling both thermodynamically and kinetically (Tian et al. 1997; De Sousa et al. 1999).
Energy transfer and feedback signalling. In addition to decreased energy production, HF also produces impairment in energy transfer and utilization. A generalized alteration of the creatine kinase system has long been observed. A decrease in total enzyme activity, alteration in the isoenzyme pattern and decreased CK fluxes are hallmarks of cardiac failure (Ingwall, 1993; Nascimben et al. 1996; Neubauer et al. 1997; De Sousa et al. 1999; Dzeja et al. 2000; Ye et al. 2001; Spindler et al. 2003). This includes a decrease in the cytosolic free or bound MM-CK and a dramatic drop in mi-CK protein and activity that is linearly correlated with the severity of the reduction of CK flux (Zhang, 2002). Decreased mi-CK coupling to oxidative phosphorylation has been consistently observed in animal models of cardiomyopathies of different origin and was suggested to be a marker of the transition between compensatory hypertrophy and failure (see Veksler & Ventura-Clapier, 1994 for review and further references), suggesting a generalized loss of integration between cytosolic signals and mitochondria, and energy signalling impairment. This is responsible for the altered energy fluxes and the lower PCr/ATP ratio, and for the incapacity of the failing myocardium to adapt its energy production to energy utilization as well as to mobilize its contractile reserve (Ingwall, 1993; Liao et al. 1996). Moreover, mi-CK can modulate mitochondrial permeability transition in the presence of creatine (Dolder et al. 2003). The drop in cardiac mi-CK could make the mitochondrial transition pore more prone to open, then favouring apoptotic cell death that may occur in heart failure.
Furthermore, the efficiency of the ATPases depends on an adequate energy supply and the effective withdrawal of the end products of ATP hydrolysis. Indeed, ATP and ADP exert a kinetic (through affinity and inhibition constants) as well as a thermodynamic (through free energy of ATP hydrolysis) control on energy transduction. In particular, a defect in the capacity of the SR to accumulate calcium is thought to participate in the pathophysiology of heart failure. Although there is evidence for a down-regulation of the sarco(endo)plasmic reticulum Ca2+α ATPase (SERCA) in CHF, the drop in MM-CK also compromises the ability of bound CK to stimulate SR calcium uptake (De Sousa et al. 1999). Due to a local lack of SR bound CK, the local ATP/ADP ratio will decrease, a mechanism that affects the kinetic and thermodynamic efficiency of SERCA (De Sousa et al. 1999).
Energy transfer can also be supported by adenylate kinase (AK) and glycolytic enzymes. Both pathways have been recognized as adaptive mechanisms supporting compromised muscle energetics in heart failure. However, the total compensatory potential of these systems is diminished, and the AK-mediated increase in respiration is blunted in heart failure (De Sousa et al. 1999; Dzeja et al. 2000). Moreover, it should be kept in mind that the increased flux through AK may contribute to the decrease in total ATP concentration because it stimulates adenine nucleotide degradation.
At present nothing is known concerning the possible fate of the direct energy cross-talk between mitochondria and intracellular energy-consuming organelles (Kaasik et al. 2001) in heart failure. Nevertheless these organelles are interconnected through the cytoskeleton network, which shows profound alterations in heart failure (Hein et al. 2000; Belmadani et al. 2002). This, together with the alterations in mitochondrial structure discussed above, suggest that the functional cross-talk between organelles will be also disrupted in heart failure, but this needs to be demonstrated. Heart failure is also accompanied by disturbances in ATP-sensing processes such as the cardioprotective KATP channel, gene expression and signalling systems (Dzeja et al. 2000).
In addition to decreased energy production, there is some evidence for energy wasting at the cellular level in cardiac dysfunction. The failing heart has a reduced mechanical efficiency that increases the energy cost of force production and the energy demands of the heart (Schipke, 1994; Ashrafian, 2002, 2003; Saavedra et al. 2002). The main, until now underestimated, consequence could be the thermodynamic limitation for Ca2+ handling that contributes to decreased contractile reserve in rat hearts (Tian et al. 1998; De Sousa et al. 1999). Altered calcium homeostasis is recognized as a key pathophysiological mechanism in heart failure, leading to altered contractile function and transcriptional activity. Calcium homeostasis depends on efficient energy-driven calcium and sodium pumps, while calcium concentration in turn determines energy expenditure through cellular ATPases and mitochondrial dehydrogenases. Disturbances in these finely controlled cellular processes make the myocyte enter a vicious cycle of energy mismatch and calcium dysregulation that may turn out to be highly detrimental, especially in periods of increased workload (De Sousa et al. 1999).
The exact functional consequences of the decreased CK fluxes in heart failure and whether they reflect adaptive or deleterious processes are difficult to assess. As very often observed in heart failure for other remodelling processes, it is highly possible that at first CK remodelling would serve as an adaptive mechanism during compensated hypertrophy. Such a mechanism has been proposed for the increased content of MB-CK in hypertrophy because the B isoenzyme has higher affinity for ADP (Ingwall, 1993). It can also be proposed that decreasing the CK shuttle in heart failure will slightly uncouple excitation–contraction coupling from mitochondrial energy production, and by this means preserve ATP production for other metabolic processes necessary for the survival of critically damaged cardiomyocytes, but at the expense of contractile activity (Ventura-Clapier et al. 1998).
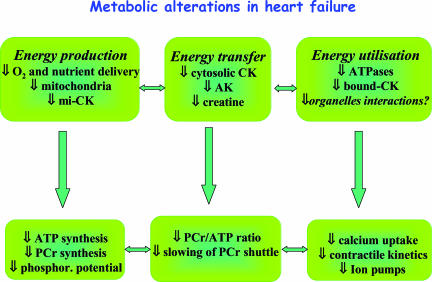
Thus, along with other cellular defects, the generalized drop in metabolic fluxes of the enzymatic systems involved in energy transfer provides a mechanism by which energy limitation may be an important factor underlying cardiac failure (Fig. 2). However, whether these metabolic alterations accompany or even precede the development of heart failure may depend on the aetiology of cardiac diseases.
Figure 2. Metabolic alterations in heart failure.
Defects in energy production, transfer and utilization have been described in both cardiac and skeletal muscle in HF. These defects lead to altered high-energy phosphate content and decreased phosphorylation potential that precipitate alterations in calcium homeostasis and contractility.
Metabolic alterations in skeletal muscle
Skeletal muscle metabolism
Skeletal muscle is a heterogeneous tissue composed of different fibre types that are characterized by the velocity of contraction and type of energy metabolism. Patterns and velocities of contraction depend on the myosin isoform and on energy production, transfer and utilization. Rapidly contracting muscles need a large amount of energy within a short period of time. Large PCr stores and a high CK activity can rephosphorylate ATP at a rate fast enough to cope with the high myosin-ATPase activity of these fibres. Once energy reserves are exhausted the amplitude and speed of contraction drop rapidly. Energy reserves are subsequently replenished by oxidative metabolism and glycolysis. This mode of contraction is described as ‘twitch now, pay later’ and is found mainly in locomotor muscles (Katz, 2001). Conversely, because postural muscles have to work for prolonged time periods they must permanently adjust their energy production to utilization. As this can only be achieved through mitochondrial respiration, these muscles have high oxidative capacities and efficient energy and signal transfers to fine-tune mitochondrial energy production (Zoll et al. 2002) on a ‘pay as you go’ basis. In addition, skeletal muscle cells also differ in mitochondrial regulation. Because mitochondrial permeability to ADP is restricted in oxidative muscles, mi-CK and AK control mitochondrial respiration. Such a restriction is absent in glycolytic muscles, thus demonstrating a tissue-specific regulation of mitochondrial function (Veksler et al. 1995).
Heart failure and skeletal muscle metabolism
The failing of the cardiac pump induces neurohormonal activation that increases the peripheral resistance and influences cardiac and skeletal muscle function and structure (Fig. 1). Reduced maximal exercise capacity of HF patients correlates poorly with central haemodynamics. This has led to the conclusion that peripheral factors are involved in the muscle weakness and increased fatiguability of the patients. Attention has therefore been focused on factors such as alterations in vascular function and intrinsic skeletal muscle abnormalities that may occur in this disease. They include fibre atrophy, cytoarchitectural remodelling, altered metabolism, and change in the fibre type composition. The main observation is a decreased proportion of type I (fatigue-resistant) fibres, compensated for by an increased proportion of fast type II (fatiguable) fibres and altered mitochondrial volume and enzyme activity (Drexler & Coats, 1996; Poole-Wilson & Ferrari, 1996).
Studies of skeletal muscle function in HF are relatively sparse. Impaired calcium homeostasis, reduced force and slower contractile kinetics were observed that are not related to intrinsic contractile properties and calcium sensitivity of contractile proteins (De Sousa et al. 2000; Lunde et al. 2001; Reiken et al. 2003). Thus, alterations in the contractile protein profile may not be the main determinant of exercise intolerance in HF.
On the other hand, large metabolic defects have been described in skeletal muscles from HF patients and animals. Skeletal muscles in HF exhibit a decreased mitochondrial volume that correlates with the aerobic capacities of the patients, suggesting a major contribution of altered oxidative metabolism to exercise intolerance in HF (Drexler et al. 1992). Decreased physical activity that takes part in muscle deconditioning cannot entirely explain these alterations (Simonini et al. 1996; Sullivan et al. 1997). In an animal model of heart failure, a marked fall in oxidative capacity and perturbations in the control of respiration by phosphate acceptors have been observed in oxidative (slow), glycolytic (fast) and even diaphragm muscles (De Sousa et al. 2000, 2001). Although the focus of fewer studies, the CK system is also clearly altered in skeletal muscle, with both the cytosolic and/or the mitochondrial isoforms of creatine kinase being affected (Hambrecht et al. 1999; De Sousa et al. 2000; Yamauchi et al. 2002). As for myocardium, the CK isoenzyme alterations could alter the functioning of mitochondria and sarcoplasmic reticulum, producing a mismatch between energy production and utilization, and altered calcium homeostasis.
Although decreases in mitochondrial volume and oxidative enzymes are a hallmark of muscle metabolic defects in HF, alterations in skeletal muscle mitochondrial function are not so clear in patients. On the one hand there is rapid phosphocreatine (PCr) depletion and increased lactate production during exercise, whilst PCr recovery is delayed at the end of exercise. Mitochondrial volume density and oxidative enzymes are decreased while glycolytic capacity is enhanced in the skeletal muscle of CHF patients (Drexler et al. 1992). On the other hand, the oxidative capacity of the vastus lateralis muscle of HF patients at the time of heart transplantation was found to be identical to that of sedentary individuals, showing that the mitochondrial oxidative phosphorylation pathway was preserved in these patients (Mettauer et al. 2001) and suggesting that energy limitation takes place upstream of mitochondria. This occurred despite lower CK and citrate synthase activities, but oxidative phosphorylation rather than the Krebs cycle are limiting mitochondrial respiration in cardiac and muscle cells (Rossignol et al. 1999). In humans, the low level of physical activity plays a role in the decreased oxidative capacity of the skeletal muscle. However, it should also be considered that new treatments of heart failure could have been protective for muscle energy metabolism especially in patients under angiotensin-converting enzyme (ACE) inhibitors therapy (Mettauer et al. 2001).
Origins of metabolic alterations
The general consensus is that heart failure markedly affects mitochondrial capacity and regulation, and phosphotransfer systems in both cardiac and skeletal muscles. The fact that heart, fast and slow muscles are all affected argues the case for a generalized metabolic myopathy in heart failure (Coats, 1996; Hardie & Pan, 2002). However, pathophysiological mechanisms underlying altered oxidative capacity and depressed phosphotransfer kinase systems still remain to be established.
These metabolic defects could arise from decreased transcription of mitochondrial proteins or from increased mitochondrial degradation. We recently addressed this question in an article published in this journal (Garnier et al. 2003).
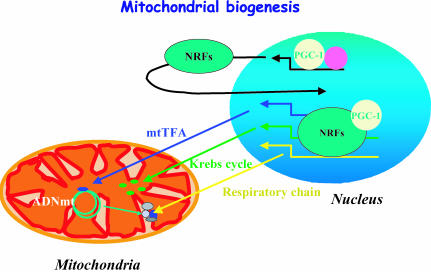
Mitochondrial biogenesis depends on the coordinated expression of the nuclear and mitochondrial genomes (Fig. 3). Mitochondria have their own DNA (mtDNA), encoding 13 subunits of the oxidative phosphorylation system (OXPHOS). The rest of the OXPHOS subunits as well as other mitochondrial proteins are encoded by the nucleus. Two transcription factors play a key role in the nucleo-mitochondrial communication. The nuclear respiratory factors (NRFs) bind and activate the promoters of various nuclear genes that encode for components of OXPHOS as well as for the mitochondrial transcription factor A (mtTFA) that regulate mtDNA replication and transcription (Scarpulla, 2002). Upstream of these transcription factors, a transcriptional coactivator of peroxisome proliferator-activated receptor gamma (PPARγ), known as PGC-1α, can coactivate mitochondrial biogenesis through its interaction with NRFs (Lehman & Kelly, 2002; Scarpulla, 2002). Moreover, PGC-1α also coactivates PPARα, the main ligand-dependent transcription factor of fatty acid oxidation (FAO) enzymes. Pressure overload-induced hypertrophy results in deactivation of PPARα and subsequent dysregulation of FAO enzyme gene expression, which sets the stage for abnormalities in cardiac lipid homeostasis and energy production (Barger & Kelly, 2000; Razeghi et al. 2002).
Figure 3. Mitochondrial biogenesis.
Mitochondrial biogenesis depends on the coordinated function of mitochondrial and nuclear genomes. The mitochondrial transcription factor (mtTFA) is encoded by the nuclear genome and activates transcription and replication of the mitochondrial DNA. mtTFA expression is controlled by nuclear respiratory factors (NRFs) that additionally stimulate the expression of numerous nuclear-encoded mitochondrial proteins. NRF expression and transcriptional activity are under the control of the transcriptional coactivator PGC-1α (transcriptional coactivator of peroxisome proliferator-activated receptor gamma). ADNmt, mitochondrial DNA.
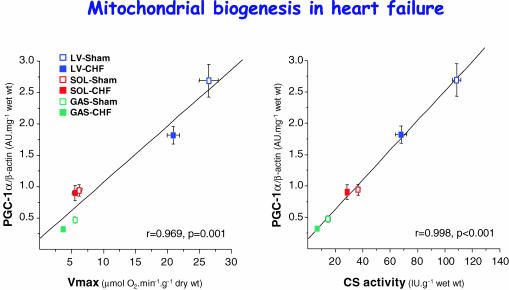
We have recently addressed this question in a model of HF in rats induced by aortic banding (Garnier et al. 2003). We showed that the decrease in oxidative capacity and mitochondrial enzyme activities in both cardiac and skeletal muscles is accompanied by a concurrent decrease in the expression of nuclear- and mitochondrial-encoded subunits of complex IV of the respiratory chain with no impairment of mitochondrial DNA replication. Moreover, alterations in the transcription of mitochondrial genes and in the respiratory chain function were accompanied by the down-regulation of PGC-1α, NRF-2α and mtTFA gene expression. PGC-1α mRNA level strongly correlated with expression of cytochrome oxidase (COX) subunits, COX I or COX IV and with mitochondrial markers (Fig. 4), suggesting that this factor, could set the tissue-specific oxidative capacity (Garnier et al. 2003). Depressed mitochondrial function in cardiac and skeletal muscles during HF is thus linked in part to a disturbed gene expression of mitochondrial proteins. In addition, increased reactive oxygen species and oxidative stress known to occur in heart failure may also mediate mitochondrial damage (Suematsu et al. 2003).
Figure 4. Mitochondrial biogenesis in heart failure.
The mRNA expression level of PGC-1α correlates with the enzymatic activity of citrate synthase (CS) or maximal respiration rate (Vmax) of fibres from left ventricle (LV), soleus (SOL) and gastrocnemius (GAS) of sham-operated and HF rats. r is the correlation coefficient and p is the statistical significance. This suggests that PGC-1α may set the oxidative capacity in different muscle types and in healthy and diseased muscles. (Adapted from Garnier et al. 2003.)
Again, this is not so clear in humans. Although a down-regulation of adult metabolic gene transcript profile has been observed in failing human heart (Razeghi et al. 2001), a recent study has excluded a generalized disturbance of mitochondrial gene expression (Scheubel et al. 2002). However, the fate of mitochondrial transcription factors has not been examined so far in the failing human heart. In line with maintained function, no evidence for altered mitochondrial biogenesis was found in skeletal muscle of HF patients (Garnier et al., unpublished results). Again, drug therapy may have been protective against these disturbances.
Nothing is known at present of the signalling pathways leading to a down-regulation of PGC-1α and decreased energy metabolism in HF. In the progression from compensated hypertrophy to failure, there is a generalized hyperactivation of hormones (renin–angiotensin–aldosterone system, endothelin-1, adrenaline (epinephrine)), neuromediators (noradrenaline) and cytokines (interleukins, tumour necrosis factors (TNFs)), but many of the systems activated in hypertrophy have been shown to activate rather than inhibit the expression of PGC-1α. On the other hand, signalling pathways that are not activated during the compensated phase of hypertrophy are stimulated after the transition to the decompensated stage, one example being the Akt/PKB pathway (Haq et al. 2001). Cardiac-specific expression of a constitutively active mutant of Akt mediates a nearly threefold down-regulation of PGC-1α mRNA expression (Cook et al. 2002). TNFα, angiotensin II and endothelin-1, which are dramatically increased in heart failure, may potentially activate the Akt pathway (Molkentin & Dorn, 2001). This provides a possible link between neurohumoral activation, decreased PGC-1α expression, and altered cardiac and skeletal muscle mitochondrial function in HF, but needs further investigation.
Alterations in the expression of creatine kinase isoenzymes are a hallmark of heart failure but signalling pathway and transcription factors involved in creatine kinase alterations remain to be established.
Recently, observations that spontaneously occurring genetic mutations in the γ regulatory subunit of the AMP-activated protein kinase (AMPK) give rise to a skeletal and cardiac muscle disease associated with the Wolff-Parkinson-White syndrome, including ventricular pre-excitation, progressive conduction system disease and cardiac hypertrophy, emphasizes the potential importance of this signalling pathway in heart failure (Ashrafian et al. 2003). Indeed, AMPK is activated in cardiac hypertrophy (Tian et al. 2001); however, the potential role of this energy sensor in heart failure also remains to be established.
Conclusions
Despite the diversity of origin and of clinical manifestation of heart failure, defects in energy metabolism are increasingly considered as a important determinant in the progression of the disease (Lopaschuk et al. 2002; Ashrafian et al. 2003; Watkins, 2003). Heart failure is a multiorgan syndrome, affecting different cell types and producing multiple neuro-hormonal activations. Within muscle cells, this pathology affects most intracellular organelles and pathways. In myocytes, calcium and energy homeostasis are intrinsically linked so that affecting one will automatically be reflected in the other. The decreased efficiency of mechanotransduction and inadequate calcium uptake and release result in a mismatch between energy production and utilization, and may influence calcium homeostasis and contractility. It is thus not surprising that ameliorating calcium homeostasis results in improved cardiac energetics (del Monte et al. 2001) and that in turn improving myocardial energetics normalizes calcium cycling (Hasenfuss et al. 2002; Liao et al. 2002). Gradual accumulation of defects in various steps in myocardial energetic signalling, along with compromised compensatory mechanisms, precipitates the failure of the whole cardiac energetic system, ultimately contributing to myocardial dysfunction. Improving the understanding of myocardial bioenergetics will provide new perspectives for heart failure therapy.
Although improving symptoms, long-term inotropic therapy has proven somewhat deleterious due to the fact that it simultaneously increases energy demand, thereby exaggerating the mismatch between energy yield and use. Rather, drugs that ultimately result in decreased energy demand and/or increased energy yield such as angiotensin-converting enzymes, β-adrenergic receptor antagonists and diuretics are the only ones to increase survival in CHF. It emerges that therapy aimed at modulating energy metabolism could be a new alternative approach to the improvement of a failing myocardium. Drugs that shift energy metabolism away from fatty acids to carbohydrates have been developed and are currently under trial (Lopaschuk et al. 2002). Pyruvate, an oxidative substrate, can improve calcium cycling and contractile force (Hasenfuss et al. 2002). Administration of l-carnitine was recently shown to reduce apoptosis in skeletal muscle of CHF rats (Vescovo et al. 2002). Such therapeutic strategy will cope with the proposal that it is worthwhile to ‘feed a tired energy-starved horse’ rather than ‘whip the horse’ with inotropic agents (Katz, 2000). Similarly, the transcriptional cascade and PGC-1α could be new targets for increasing the energetic efficiency of the heart and skeletal muscles. As it is a determining factor in the progression of heart failure and could affect cell metabolism, normalizing neuroendocrine activation may represent a promising therapeutic target for improved energetics.
Acknowledgments
We thank R. Fischmeister for continuous support and Dr B. Mettauer for critical reading of the manuscript. R. V.-C. is supported by CNRS.
References
- Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev MK, Saks VA. Compartmentalized energy transfer in cardiomyocytes: use of mathematical modelling for analysis of in vivo regulation of respiration. Biophys J. 1997;73:428–445. doi: 10.1016/S0006-3495(97)78082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H. Cardiac energetics in congestive heart failure. Circulation. 2002;105:e44–e45. [PubMed] [Google Scholar]
- Ashrafian H, Redwood C, Blair E, Watkins H. Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet. 2003;19:263–268. doi: 10.1016/S0168-9525(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–1274. doi: 10.1016/s0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- Belmadani S, Pous C, Ventura-Clapier R, Fischmeister R, Mery PF. Post-translational modifications of cardiac tubulin during chronic heart failure in the rat. Mol Cell Biochem. 2002;237:39–46. doi: 10.1023/a:1016554104209. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Boehm E, Ventura-Clapier R, Mateo P, Lechene P, Veksler V. Glycolysis supports calcium uptake by the sarcoplasmic reticulum in skinned ventricular fibres of mice deficient in mitochondrial and cytosolic creatine kinase. J Mol Cell Cardiol. 2000;32:891–902. doi: 10.1006/jmcc.2000.1130. [DOI] [PubMed] [Google Scholar]
- Coats AJS. The ‘muscle hypothesis’ of chronic heart failure. J Mol Cell Cardiol. 1996;28:2255–2262. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- Crozatier B, Badoual T, Boehm E, Ennezat PV, Guenoun T, Su J, Veksler V, Hittinger L, Ventura-Clapier R. Role of creatine kinase in cardiac excitation-contraction coupling: studies in creatine kinase-deficient mice. FASEB J. 2002;16:653–660. doi: 10.1096/fj.01-0652com. [DOI] [PubMed] [Google Scholar]
- del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+− ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Bigard X, Mateo P, Serrurier B, Ventura-Clapier R. Dual influence of disease and increased load on diaphragm muscle in heart failure. J Mol Cell Cardiol. 2001;33:699–710. doi: 10.1006/jmcc.2000.1336. [DOI] [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Bigard X, Mateo P, Ventura-Clapier R. Heart failure affects mitochondrial but not myofibrillar intrinsic properties of skeletal muscle. Circulation. 2000;102:1847–1853. doi: 10.1161/01.cir.102.15.1847. [DOI] [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterations – Implications in heart failure. Circ Res. 1999;85:68–76. doi: 10.1161/01.res.85.1.68. [DOI] [PubMed] [Google Scholar]
- Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- Drexler H, Coats AJ. Explaining fatigue in congestive heart failure. Annu Rev Med. 1996;47:241–256. doi: 10.1146/annurev.med.47.1.241. [DOI] [PubMed] [Google Scholar]
- Drexler H, Riede U, Munzel T, Konig H, Funke E, Jusu H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Pucar D, Redfield MM, Burnett JC, Terzic A. Reduced activity of enzymes coupling ATP-generating with ATP-consuming processes in the failing myocardium. Mol Cell Biochem. 1999;201:33–40. doi: 10.1023/a:1007016703229. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Redfield MM, Burnett JC, Terzic A. Failing energetics in failing hearts. Curr Cardiol Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Zeleznikar RJ, Goldberg ND. Adenylate kinase: Kinetic behavior in intact cells indicates it is integral to multiple cellular processes. Mol Cell Biochem. 1998;184:169–182. [PubMed] [Google Scholar]
- Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Gielen S, Linke A, Möbius-Winkler S, Yu J, Niebauer J, Jiang H, Fiehn E, Schuler G. Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J Am Coll Cardiol. 1999;33:174–179. doi: 10.1016/s0735-1097(98)00531-2. [DOI] [PubMed] [Google Scholar]
- Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Maier LS, Hermann HP, Luers C, Hunlich M, Zeitz O, Janssen PM, Pieske B. Influence of pyruvate on contractile performance and Ca2+ cycling in isolated failing human myocardium. Circulation. 2002;105:194–199. doi: 10.1161/hc0202.102238. [DOI] [PubMed] [Google Scholar]
- Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45:273–278. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- Ingwall JS. Is cardiac failure a consequence of decreased energy reserve? Circulation. 1993;87:VII58–VII62. [Google Scholar]
- Johannsson E, Lunde PK, Heddle C, Sjaastad I, Thomas MJ, Bergersen L, Halestrap AP, Blackstad TW, Ottersen OP, Sejersted OM. Upregulation of the cardiac monocarboxylate transporter MCT1 in a rat model of congestive heart failure. Circulation. 2001;104:729–734. doi: 10.1161/hc3201.092286. [DOI] [PubMed] [Google Scholar]
- Joubert F, Hoerter JA, Mazet JL. Modeling the energy transfer pathways. Creatine kinase activities and heterogeneous distribution of ADP in the perfused heart. Mol Biol Rep. 2002a;29:177–182. doi: 10.1023/a:1020321711771. [DOI] [PubMed] [Google Scholar]
- Joubert F, Mazet JL, Mateo P, Hoerter JA. 31P NMR detection of subcellular creatine kinase fluxes in the perfused rat heart: contractility modifies energy transfer pathways. J Biol Chem. 2002b;24:18469–18476. doi: 10.1074/jbc.M200792200. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Ventura-Clapier R. From energy store to energy channeling: a study in creatine kinase deficient fast skeletal muscle. FASEB J. 2003;17:708–710. doi: 10.1096/fj.02-0684fje. [DOI] [PubMed] [Google Scholar]
- Kalsi KK, Smolenski RT, Pritchard RD, Khaghani A, Seymour AML, Yacoub MH. Energetics and function of the failing human heart with dilated or hypertrophic cardiomyopathy. Eur J Clin Invest. 1999;29:469–477. doi: 10.1046/j.1365-2362.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- Katz AM. Heart Failure. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- Katz AM. Physiology of the Heart. 3. New York: Lippincott Williams & Wilkins, Raven Press; 2001. [Google Scholar]
- Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- Leong HS, Brownsey RW, Kulpa JE, Allard MF. Glycolysis and pyruvate oxidation in cardiac hypertrophy – why so unbalanced. Comp Biochem Physiol Mol Integr Physiol. 2003;135:499–513. doi: 10.1016/s1095-6433(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- Liao RL, Nascimben L, Friedrich J, Gwathmey JK, Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy – Relationship to contractile performance. Circ Res. 1996;78:893–902. doi: 10.1161/01.res.78.5.893. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Rebeyka IM, Allard MF. Metabolic modulation: a means to mend a broken heart. Circulation. 2002;105:140–142. [PubMed] [Google Scholar]
- Lunde PK, Dahlstedt AJ, Bruton JD, Lannergren J, Thoren P, Sejersted OM, Westerblad H. Contraction and intracellular Ca2+ handling in isolated skeletal muscle of rats with congestive heart failure. Circ Res. 2001;88:1299–1305. doi: 10.1161/hh1201.092041. [DOI] [PubMed] [Google Scholar]
- Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol. 2001;38:947–954. doi: 10.1016/s0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Dorn GW., II Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Arai AE, Balaban RS. Maximum oxidative phosphorylation capacity of the mammalian heart. Am J Physiol. 1997;41:H769–H775. doi: 10.1152/ajpheart.1997.272.2.H769. [DOI] [PubMed] [Google Scholar]
- Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and non failing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Remkes H, Spindler M, Horn M, Wiesmann F, Prestle J, Walzel B, Ertl G, Hasenfuss G, Wallimann T. Downregulation of the Na+-creatine cotranporter in failing human myocardium and in experimental heart failure. Circulation. 1999;100:1847–1850. doi: 10.1161/01.cir.100.18.1847. [DOI] [PubMed] [Google Scholar]
- Ning XH, Zhang JY, Liu JB, Ye Y, Chen SH, From AHL, Bache RJ, Portman MA. Signaling and expression for mitochondrial membrane proteins during left ventricular remodeling and contractile failure after myocardial infarction. J Am Coll Cardiol. 2000;36:282–287. doi: 10.1016/s0735-1097(00)00689-6. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson PA, Ferrari R. Role of skeletal muscle in the syndrome of chronic heart failure. J Mol Cell Cardiol. 1996;28:2275–2285. doi: 10.1006/jmcc.1996.0220. [DOI] [PubMed] [Google Scholar]
- Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106:407–411. doi: 10.1161/01.cir.0000026392.80723.dc. [DOI] [PubMed] [Google Scholar]
- Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, Warren MS, He KL, Yi GH, Wang J, Burkhoff D, Vassort G, Marks AR. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol. 2003;160:919–928. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol R, Malgat M, Mazat JP, Letellier T. Threshold effect and tissue specificity – Implication for mitochondrial cytopathies. J Biol Chem. 1999;274:33426–33432. doi: 10.1074/jbc.274.47.33426. [DOI] [PubMed] [Google Scholar]
- Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- Sack MN, Rader TA, Park SH, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, Fleck E, Seppet E. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks VA, Khuchua ZA, Vasilyeva EV, Belikova OY, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells – Role of coupled creatine kinases in in vivo regulation of cellular respiration – A synthesis. Mol Cell Biochem. 1994;133:155–192. doi: 10.1007/BF01267954. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Tanonaka K, Kobayasi R, Takeo S. Effects of long-term therapy with ACE inhibitors, captopril, enalapril and trandolapril, on myocardial energy metabolism in rats with heart failure following myocardial infarction. J Mol Cell Cardiol. 1995;27:2209–2222. doi: 10.1016/s0022-2828(95)91551-6. [DOI] [PubMed] [Google Scholar]
- Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Schaper J, Froede R, Hein St Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- Scheubel RJ, Tostlebe M, Simm A, Rohrbach S, Prondzinsky R, Gellerich FN, Silber RE, Holtz J. Dysfunction of mitochondrial respiratory chain complex I in human failing myocardium is not due to disturbed mitochondrial gene expression. J Am Coll Cardiol. 2002;40:2174–2181. doi: 10.1016/s0735-1097(02)02600-1. [DOI] [PubMed] [Google Scholar]
- Schipke JD. Cardiac efficiency. Basic Res Cardiol. 1994;89:207–240. doi: 10.1007/BF00795615. [DOI] [PubMed] [Google Scholar]
- Sharov VG, Goussev A, Lesch M, Goldstein S, Sabbah HN. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1998;30:1757–1762. doi: 10.1006/jmcc.1998.0739. [DOI] [PubMed] [Google Scholar]
- Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Todaka K, Burkhoff D. Load dependence of ventricular performance explained by model of calcium–myofilament interactions. Am J Physiol Heart Circ Physiol. 2002;282:H1081–H1091. doi: 10.1152/ajpheart.00498.2001. [DOI] [PubMed] [Google Scholar]
- Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circ Res. 1996;79:128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- Spindler M, Engelhardt S, Niebler R, Wagner H, Hein L, Lohse MJ, Neubauer S. Alterations in the myocardial creatine kinase system precede the development of contractile dysfunction in beta(1)-adrenergic receptor transgenic mice. J Mol Cell Cardiol. 2003;35:389–397. doi: 10.1016/s0022-2828(03)00015-4. [DOI] [PubMed] [Google Scholar]
- Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb FR, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exercise. 1997;29:860–866. doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H. Metabolism – The lost child of cardiology. J Am Coll Cardiol. 2000;36:1386–1388. doi: 10.1016/s0735-1097(00)00870-6. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H. Switching metabolic genes to build a better heart. Circulation. 2002;106:2043–2045. doi: 10.1161/01.cir.0000036760.42319.3f. [DOI] [PubMed] [Google Scholar]
- Tian R, Halow JM, Meyer M, Dillmann WH, Figueredo VM, Ingwall JS, Camacho SA. Thermodynamic limitation for Ca2+ handling contributes to decreased contractile reserve in rat hearts. Am J Physiol. 1998;275:H2064–H2071. doi: 10.1152/ajpheart.1998.275.6.H2064. [DOI] [PubMed] [Google Scholar]
- Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- Tian R, Nascimben L, Ingwall JS, Lorell BH. Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation. 1997;96:1313–1319. doi: 10.1161/01.cir.96.4.1313. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Anflous K, Mateo P, van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice. 2. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- Veksler V, Ventura-Clapier R. In situ study of myofibrils, mitochondria and bound creatine kinases in experimental cardiomyopathies. Mol Cell Biochem. 1994;133:287–298. doi: 10.1007/978-1-4615-2612-4_19. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Kuznetsov A, Veksler V, Boehm E, Anflous K. Functional coupling of creatine kinases in muscles: Species and tissue specificity. Mol Cell Biochem. 1998;184:231–247. [PubMed] [Google Scholar]
- Ventura-Clapier R, Veksler V, Hoerter JA. Myofibrillar creatine kinase and cardiac contraction. Mol Cell Biochem. 1994;133:125–144. doi: 10.1007/BF01267952. [DOI] [PubMed] [Google Scholar]
- Vescovo G, Ravara B, Gobbo V, Sandri M, Angelini A, Della Barbera M, Dona M, Peluso G, Calvani M, Mosconi L, Dalla Libera L. L-Carnitine: a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am J Physiol Cell Physiol. 2002;283:C802–810. doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Eppenberger HM. Localization and function of M-line-bound creatine kinase M-band model and creatine phosphate shuttle. Cell Muscle Motility. 1985;6:239–285. doi: 10.1007/978-1-4757-4723-2_8. [DOI] [PubMed] [Google Scholar]
- Watkins H. Genetic clues to disease pathways in hypertrophic and dilated cardiomyopathies. Circulation. 2003;107:1344–1346. doi: 10.1161/01.cir.0000057860.52586.9c. [DOI] [PubMed] [Google Scholar]
- Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. 1985;75:436–447. doi: 10.1172/JCI111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M, Smeitink J, Wevers RA, Wallimann T. Mitochondrial creatine kinase – A key enzyme of aerobic energy metabolism. Biochim Biophys Acta. 1992;1102:119–166. doi: 10.1016/0005-2728(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–1576. doi: 10.1161/01.cir.103.11.1570. [DOI] [PubMed] [Google Scholar]
- Zhang J. Myocardial energetics in cardiac hypertrophy. Clin Exp Pharmacol Physiol. 2002;29:351–359. doi: 10.1046/j.1440-1681.2002.03657.x. [DOI] [PubMed] [Google Scholar]
- Zoll J, Sanchez H, N'Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R, Mettauer B. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2002;543:191–200. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]