Abstract
We addressed the role of endothelial cells (ECs) in metabolic dilatation of skeletal muscle arterioles in anaesthetized mice in situ. Electrical field stimulation was used to contract the cremaster muscle for 15 s at 30 Hz. Diameter was observed using bright field microscopy. In controls, muscle contraction produced a 15.7 ± 1.5 μm dilatation from a baseline of 17.4 ± 1.6 μm. Endothelial denudation (−EC) via intraluminal perfusion of air abolished this response (1.6 ± 1.2 μm in −EC, P < 0.05), identifying endothelium as the primary vascular cell type initiating the dilatation. To investigate the role of EC Ca2+ in metabolic dilatation, arteriolar ECs were loaded with Fluo-4 AM or BAPTA AM by intraluminal perfusion, after which blood flow was re-established. Ca2+ activity of individual ECs was monitored as a function of change from baseline fluorescence using confocal microscopy. In ECs, whole cell Ca2+ increased (>10%, P < 0.05) during muscle contraction, and localized Ca2+ transients were increased (>20%, P < 0.05) during the first minute after contraction. Chelation of EC Ca2+ abolished the dilatations in response to muscle contraction (1.1 ± 0.7 μm, P < 0.05). Inhibition of P1 purinergic receptors (with xanthine amine congener) did not alter the rate of onset of the dilatation (P > 0.05) but decreased its magnitude immediately post stimulation (7.1 ± 0.9 μm, P < 0.05) and during recovery. These findings demonstrate obligatory roles for endothelium and EC Ca2+ during metabolic dilatation in intact arterioles. Furthermore, they suggest that at least two separate pathways mediate the local response, one of which involves stimulation of endothelial P1 purinergic receptors via endogenous adenosine produced during muscle activity.
A high degree of vasomotor tone maintains low levels of blood flow in resting skeletal muscle. During exercise, blood perfusion increases specifically in active muscle and in proportion to the intensity of the contractile activity. Signals originating at the site of the contracting fibres increase flow to the skeletal muscle (Gorczynski et al. 1978; Murrant & Sarelius, 2000; Segal, 2000) and regulate its distribution within the vascular network (Berg et al. 1997). In this way, local mechanisms play an important role in exquisitely coupling blood flow to regional metabolic demands.
Several local mechanisms are implicated in the dilator response to muscle contraction. For example, roles have been identified for release of vasoactive molecules (e.g. K+, adenosine (ADO), nitric oxide (NO)) from active skeletal muscle or surrounding vascular cells (Hnik et al. 1976; Proctor & Duling, 1982; Lau et al. 2000; Cohen & Sarelius, 2002; Murrant & Sarelius, 2002), alterations in PO2 (Gorczynski & Duling, 1978), and modulation of conducted dilator signals that are communicated along the length of the vessel to upstream sites (Berg et al. 1997; Cohen et al. 2000; Murrant & Sarelius, 2000). In fact, the integrated functional response is likely to be produced by a combination of several of these mechanisms acting in concert.
The exact dilator mechanisms recruited in specific scenarios of exercise are still being defined. One approach to understanding the integrated response to skeletal muscle contraction is to identify the primary vascular cell type and signalling intermediates that are required for the dilatation. Both endothelial (Saito et al. 1994; Berg et al. 1997; Segal & Jacobs, 2001) and smooth muscle (Laughlin & Korzick, 2001; Murrant & Sarelius, 2002) cell-dependent pathways are implicated in the metabolic dilator response. For example, contraction of muscle fibres underlying capillaries produces dilatations in upstream arterioles, directly substantiating the ability of ECs to initiate the functional response (Berg et al. 1997). On the other hand, of the two vascular cell types, smooth muscle cells (SMC) are in closer proximity to skeletal muscle. As a consequence, they will necessarily encounter the metabolic products of muscle contraction (e.g. ADO) or changes in environment of the active tissue (e.g. PO2) before ECs. This makes SMCs a likely candidate for independently mediating the response. The relative contribution of each vascular cell type to the dilatation in the exercise response has not been tested explicity at the arteriolar level. The first goal of this study was therefore to determine the extent of the EC-dependent component in the dilatation associated with skeletal muscle contraction.
The release and action of several key dilators (e.g. prostaglandins, NO) that originate from the endothelium during exercise (Hester et al. 1993; Nuttle et al. 1999; Lau et al. 2000) are associated with increases in EC Ca2+ (Falcone et al. 1993; Bolz et al. 1999; Tran et al. 2000), implying that a change in endothelial Ca2+ is likely to occur in response to muscle contraction. In earlier studies from our laboratory, we found that buffering EC Ca2+ eliminated the dilator response associated with 2 min of muscle contraction at 4 Hz, even though whole EC Ca2+ was unchanged from baseline immediately following the stimulation period (Murrant et al. 2004). This suggests that an increase in EC Ca2+ must be involved in the onset of the dilatation. Indeed, we have recently determined that increases in EC Ca2+ are required for dilatations that are initiated by stimulation of metabolically related pathways (Duza & Sarelius, 2003). Alternatively, it is possible that transitory, localized rather than (or in addition to) steady whole cell Ca2+ signals underlie the dilator response to muscle contraction. Thus, further goals of this study were to determine whether EC Ca2+ increases during skeletal muscle contraction, if there are associated changes in EC Ca2+ transients, and whether activation of EC Ca2+ dependent signals is required for the dilator response.
It is broadly accepted that ADO, a metabolite of muscle contraction and a potent dilator, is released into the extracellular space during exercise (Phair & Sparks, 1979; Honig & Frierson, 1980; Proctor & Duling, 1982). However, it is apparent that the role of ADO as a mediator of skeletal muscle blood flow changes during exercise is complex. For example, there is evidence for (Murrant & Sarelius, 2002) and against (Cohen & Sarelius, 2002) a role for ADO in metabolic vasodilatations in the same preparation. It is not clear whether such differentials within (Cohen & Sarelius, 2002; Murrant & Sarelius, 2002) and among (Phair & Sparks, 1979; Honig & Frierson, 1980; Proctor & Duling, 1982; Bockman & McKenzie, 1983; Poucher et al. 1990) skeletal muscles are due to variability in endogenous ADO availability and/or intrinsic differences in the ADO sensitivity of arterioles based on branch order and/or skeletal muscle source. We have shown recently that stimulation of P1 purinergic receptors targets ECs and requires an increase in EC Ca2+ for the associated vasodilatations (Duza & Sarelius, 2003). Furthermore, we have found in preliminary studies that ADO increases the frequency and synchronization of localized Ca2+ transients in ECs of skeletal muscle arterioles (authors' unpublished observations). Thus an additional goal of this work was to test if stimulation of P1 receptors subsequent to the release of endogenous ADO is involved in the dilator response (which we hypothesize is dependent on vascular endothelium and EC Ca2+) under the conditions of muscle contraction employed in the present study.
We used electrical field stimulation of the mouse cremaster muscle as a model of skeletal muscle contraction during exercise. In situ observations of diameter and EC Ca2+ were made in the terminal vascular bed using intravital microscopy. We identified the primary cell type responsible for initiation of the dilatation produced by skeletal muscle contraction, and provided evidence for multiple roles of EC Ca2+ as an intermediate signalling molecule at different stages of the response. Finally, we demonstrated that P1 receptor-dependent signalling mechanisms contribute to the dilatation, and identified the associated time frame of the activity.
Methods
Animal preparation
All protocols were approved by the Animal Care and Use Committee of the University of Rochester and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, USA).
Male C57BL/6J mice (wt 24–28 g) were anaesthetized with pentobarbital sodium (75 mg kg−1i.p.) in saline. A tracheal cannula was placed to maintain a patent airway under anaesthesia. A jugular venous catheter was placed for administration of supplemental pentobarbital sodium as needed during surgery and throughout the experimental protocol: the depth of anaesthesia was assessed by monitoring the animal's respiration and reflex withdrawal to a tail pinch. Mouse body temperature was maintained at 37°C by convective heat. The right cremaster was exteriorized and prepared for in situ intravital microscopic observations as previously described (Lau et al. 2000). During surgery and experimental protocols the muscle preparation was continuously superfused (flow rate ∼5 ml min−1) with a bicarbonate-buffered physiological salt solution warmed to 36°C in a reservoir. The superfusion solution contained (mm): 131.9 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 30.0 NaHCO3, equilibrated with 5% CO2–95% N2 to maintain pH at 7.40 ± 0.05. Tubocurarine (4 μm) was added to the superfusion solution to eliminate neurally mediated twitching of skeletal muscles and associated tissue movement. At the completion of all experimental protocols, animals were given a lethal dose of pentobarbital sodium (i.v.).
After surgery, the preparation was allowed to stabilize for 30–45 min prior to data collection. Arterioles (maximum diameter ∼35 μm) in clear focus were chosen for study and visualized using an Olympus BX50WI microscope. The tissue was transilluminated with a tungsten arc lamp and imaged using a × 20 objective (numerical aperture 0.5). Images were displayed on a Sony monitor using a CCD camera (Dage MTI 72S) and recorded on videotape. Vessel diameter was measured offline using video calipers (Colorado Video Inc, model 321), calibrated with a videotaped stage micrometer. Diameter measurements were reproducible to ± 0.5 μm. Observations were made during a 1 min baseline period, the indicated stimulation period, and a 3 min recovery period (standard observation protocol) unless specified otherwise. A brief waiting time (∼5–10 min) was maintained between successive observations.
The vascular responsiveness of each preparation was evaluated at the end of all experimental protocols. Only data collected on preparations that displayed constriction in reponse to 19% O2 and dilatation in reponse to 10−4m acetlycholine (ACh) or 10−3m sodium nitroprusside (SNP) were kept for analysis (<5% of all preparations were discarded). Vessel diameter following at least 3 min of superfusion of the entire preparation with 10−4m ACh (Ca2+ experiments) or 10−3m SNP (all other experiments) was recorded for each observed arteriole and is reported as the maximum.
Muscle stimulation
A muscle stimulus protocol used elsewhere was used in the current study (Lau et al. 2000). Briefly, a two-pronged silver foil electrode (positive) was placed at the proximal end of the cremaster preparation, such that it made contact with the top and bottom muscle surfaces. A second silver foil electrode (negative) was placed on the support pedestal surrounding the distal end of the tissue preparation. Electrical field stimulation was used to contract the entire cremaster muscle at 30 Hz for 15 s (0.2 ms duration, 5–10 V).
Pharmacological interventions
Pressurized glass micropipettes placed at the vessel wall (within a distance of ∼10–20 μm) were used for localized application of agonists (10−4m ACh, ADO or noradrenaline (NA; norepinephrine) as previously described (Sarelius & Huxley, 1990). Flow out of the pipette (∼10 μm tip diameter) was achieved by a manometer system (30 cmH2O ejection pressure). FITC–dextran (100 μm) was added to the contents of the pipette and brief epi-illumination was used to confirm flow out of the pipette. It has been verified that the fluorescent tracer does not affect arteriolar responses (Frame & Sarelius, 1995). The entire preparation was exposed to 10−3m SNP or 10−5m xanthine amine congener (XAC, P1 antagonist) by adding each agent to the flowing superfusion solution.
Role of endothelium
To identify the primary vascular cell type (endothelium versus smooth muscle) responsible for producing the dilator response initiated by muscle contraction, observations were made before and after selective removal of ECs via air embolism. Responses were first studied in intact arterioles with local application of 10−4m ACh (for 2 min) and 30 Hz muscle contraction (control data). Then ECs were removed via air embolism as described elsewhere (Duza & Sarelius, 2003). Briefly, an arteriole was cannulated with a sharp micropipette containing air for perfusion of an air bubble through the microvascular network, after which blood flow was allowed to resume. A period of 20–25 min was allowed for vessel tone to re-establish prior to data collection. Arteriolar responses of the endothelium-denuded region to local application of ACh, muscle contraction and SNP (added to the superfusion solution) were then recorded. Lack of dilator response to ACh (an EC-dependent dilator), despite a vasomotor response to SNP (a SMC dependent dilator), was used as a criterion for identifying arteriolar regions with selective EC disruption. Generally, an arteriolar length of ∼200 μm was denuded of ECs using this approach.
Measurement of EC Ca2+
To test whether the arteriolar dilatation associated with muscle contraction involves EC Ca2+ as a signalling intermediate, we measured changes in EC Ca2+ and vessel diameter initiated by 30 Hz stimulation. The change in diameter initiated by muscle contraction was observed first using transillumination (as described above) in each preparation. To monitor EC Ca2+, arterioles were then perfused with 5 μm Fluo-4 AM indicator solution to load ECs with dye as described elesewhere for loading ECs with Fura (Duza & Sarelius, 2003). After 15 min of dye perfusion (total volume ∼10 μl) blood flow was allowed to resume in the test arteriole. A period of 10–15 min was allowed for intracellular de-esterification of the dye and re-establishment of vessel tone prior to data collection, after which EC Ca2+ responses were observed during contraction at 30 Hz.
An imaging system consisting of an Olympus BX50WI microscope fitted with a Nipkow disk scanning confocal head (Yokogawa Inc.) coupled to a GenIII+ ICCD (XR-Mega 10, Solamere Technology Group) was used to visualize Fluo-4-loaded cells. The depth of the confocal ‘slice’ in this system is ∼1 μm. Images were displayed on a Sony monitor and recorded on videotape during a 1 min baseline period, a 15 s contraction period and a 2 min recovery period. Images recorded on videotape were digitized at 5 Hz using a frame grabber (model CG7, Scion Image Inc) and analysed offline using NIH Image software. To monitor whole EC Ca2+ changes, a region of interest (ROI) encompassing the bulk of the cell body was identified on individual ECs that remained in focus during tetanic contraction (approximately half of all loaded cells). The average fluorescence intensity (grey scale range: 0−255; 0 = black, 255 = white) of the ROI was measured. Measurements were made for a 5 s baseline period, a 15 s contraction period, and a 30 s recovery period.
To quantify the frequency of localized EC Ca2+ transients, the mean fluorescence intensity was measured in a small ROI (∼15–25 μm2) positioned at the local site within an EC that was identified by visual inspection as being where the local Ca2+ increase originated. Fluoresence in this ROI was sampled at 5 Hz and background subtracted. Background was measured in an identical ROI positioned adjacent to the vessel in the avascular tissue space. We found that tissue movement during the 15 s period of muscle contraction precluded quantification of these localized Ca2+ transients because under these conditions it was not technically feasible to keep the small ROI both localized on the specified site within the EC and in focus. To qualify as the peak of a transient, we established a criterion from preliminary analyses (Duza, 2003) in which fluorescence intensity had to be greater than twice the maximum standard deviation of the signal (2–4%, attributed to noise) relative to the immediately preceding and following 0.6 or 0.8 s. Measurements were made for the 1 min baseline period and for each minute of the first 2 min of recovery after muscle contraction.
Vessel diameter and EC Ca2+ were measured in sequence, usually in the same arteriole (Fig. 1). Occasionally, if perfusion of the selected arteriole with Fluo-4 was not satisfactory, Ca2+ data were collected from a similar arteriole in the same tissue. Subsequent to collection of all muscle contraction data in each tissue, the entire preparation was exposed to 10−4m ACh (a concentration chosen to maximally increase EC Ca2+). There was a large elevation in Ca2+ throughout each EC body during exposure to ACh, which confirmed that the capacity to detect changes in Ca2+ was present in all ECs at the observed arteriolar site.
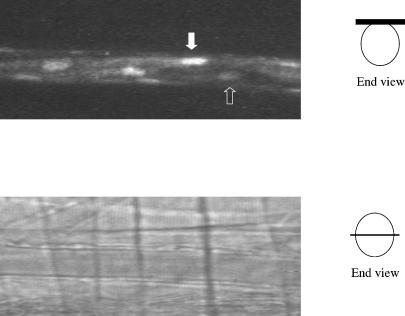
Figure 1. Examples of images used for analysis of EC Ca2+ and arteriolar diameter.
The upper panel shows a confocal image of a typical arteriole with Fluo-4-loaded endothelial cells. The confocal image plane is at the top of the arteriolar lumen (see schematic diagram at right, in which the black bar indicates the sampled confocal slice. Not to scale.). White arrow indicates nucleus of an EC with relatively bright fluorescence throughout; open arrow indicates nucleus of an EC with low fluorescence intensity in both nucleus and cytosol: ACh increased fluorescent intensity in all cells (not shown), indicating that all ECs were loaded with Fluo-4, but had different resting levels of Ca2+. Whole cell Ca2+ changes are measured in a region of interest (ROI) drawn to encompass one complete EC in a selected video image; Ca2+ oscillations are measured in a small ROI within the cell that is located at the same place in each EC image in a sampled series of video frames. The lower panel shows the same arteriole imaged by transillumination through the the same light path: diameter is measured with the plane of maximal radius in focus as shown in the schematic diagram on the right (not to scale).
Chelation of EC Ca2+
To determine if the change in EC Ca2+ associated with the dilatation initiated by muscle contraction is a required signalling intermediate, observations were made in the same preparations before and after chelation of EC Ca2+. Data were first collected in arterioles during local application of ACh (for 2 min) and muscle contraction (control). Then, to selectively buffer EC Ca2+, the microvascular network was intraluminally perfused with 5 μm BAPTA AM as described for Fluo-4 AM. After 10–15 min of BAPTA AM perfusion, blood flow was allowed to resume in the test arteriole and 20 min was allowed for intracellular de-esterification of the molecule. Responses of BAPTA-loaded, blood-perfused arterioles to local application of ACh and NA, muscle contraction, and SNP (added to the superfusion solution) were then recorded. The following functional responses were used to assess selective chelation of EC Ca2+: inhibition of the dilator response to ACh despite preservation of SMC-dependent contraction with NA (which requires an increase in Ca2+), and relaxation with SNP (which involves a change in Ca2+ sensitivity; Bolz et al. 1999).
Role of ADO
The roles of endogenous ADO and subequent P1 purinergic receptor-mediated signalling in the dilator response initiated by muscle contraction were examined. Observations were first made in arterioles during local application of ADO (for 2 min) and muscle contraction (control). Then the cremaster preparation was exposed to XAC. After 20 min of exposure to the antagonist, the same vessel's response to each stimulus was recorded again in the continued presence of the blocker.
Materials
A 5 μl aliquot of 10−3m Fluo-4 AM (Molecular Probes, Eugene, OR, USA; dissolved in 100% DMSO) and 2 μl of 12.5 mg ml−1 Pluronic-127 (TEF Laboratories, Austin, TX, USA; made in 100% DMSO) stock solutions were mixed and diluted in 1 ml 0.9% NaCl for a final concentration of 5 μm Fluo-4 AM (indicator solution). A 50 μl aliquot of 10−3m BAPTA AM (Molecular Probes; dissolved in 100% DMSO) and 40 μl of 12.5 mg ml−1 Pluronic-127 (TEF Laboratories; made in 100% DMSO) stock solutions were mixed and diluted in 10 ml 0.9% NaCl (Ca2+ buffer solution). This resulted in a final concentration of 5 μm BAPTA AM. All other reagents were obtained from Sigma (St Louis, MO, USA). Solutions were freshly prepared daily in superfusion solution from stock solutions (10−2m ADO, ACh, NA and SNP in deionized H2O and 10−2m XAC in 0.1 N NaOH in saline).
Data analyis and statistics
All data are reported as means ±s.e.m. In the figures, time courses of responses are shown as the mean response at each time point for multiple arterioles. Elsewhere, we report the mean response at the first post-stimulation time point, or, where specified, the mean of the peak response for each vessel. The number of observations (n) refers to the number of arterioles (not less than 4 in each data set) and ECs (not less than 30) studied for diameter and Ca2+ measurements, respectively. For each experiment set, data were collected from a minimum of three animals: typically, only one arteriole was studied in each animal. Diameter data are expressed as an absolute diameter change (in μm) over 5 s intervals relative to baseline (averaged over 1 min). Whole cell Ca2+ data are normalized to baseline (averaged over 5 s) fluorescence intensity (for each cell) and reported at 1 s intervals. Local EC Ca2+ transients are expressed as average number of transients cell−1 min−1. Responses from multiple experiments were analysed by repeated-measures ANOVA with Dunnett's Multiple Comparision post hoc test, or Student's t test, as appropriate, to compare population differences. Differences were considered significant if P < 0.05.
Results
Contraction of the entire cremaster muscle for 15 s at 30 Hz induced a significant arteriolar dilatation of 15.7 ± 1.5 μm from a baseline of 17.4 ± 1.6 μm (n= 20, population pooled from all experiments). Mean maximum diameter for these vessels was 35.4 ± 2.1 μm. The time at which this peak response occurred varied slightly between vessels: on average it occurred 15 ± 5 s after termination of muscle contraction (i.e. 30 ± 5 s after initiation of electrical field stimulation). Though in some cases recovery was quicker, overall the arteriolar diameter had not returned to resting levels by the end of the 3 min recovery observation period.
Functional endothelium is vital for the dilatation
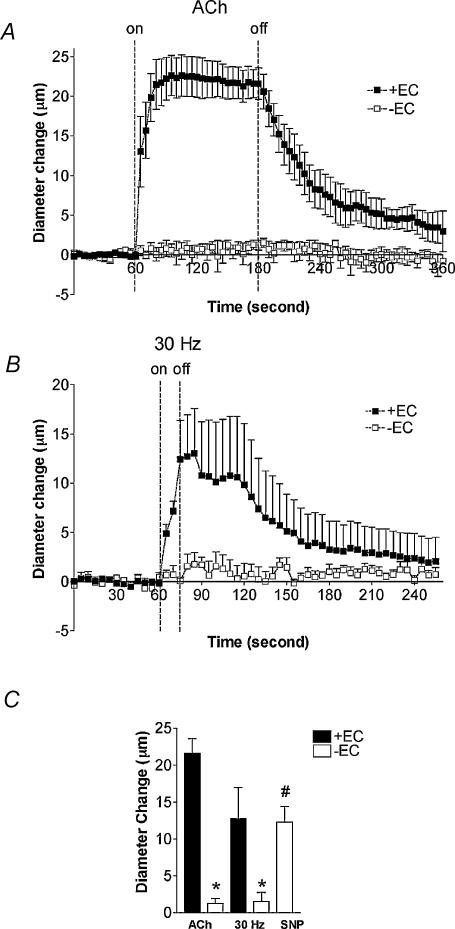
To determine whether ECs or SMCs are primarily responsible for the dilatation initiated by muscle contraction, observations were made in the same preparations before and after EC denudation via air embolism (n= 5). Air embolism resulted in a decrease in resting arteriolar diameter from 24.4 ± 1.7 to 17.6 ± 1.9 μm. EC denudation abolished Ach-induced dilatations (21.6 ± 2.0 versus 1.3 ± 0.6 μm, P < 0.05), establishing successful impairment of ECs with air treatment (Fig. 2A and C). Likewise, EC denudation abolished the dilatation (12.8 ± 4.2 versus 1.6 ± 1.2 μm, P < 0.05) produced by 30 Hz stimulation (Fig. 2B and C). As expected, endothelium-denuded vessels maintained their capacity to dilate in response to SNP and produced a peak dilatation of 12.3 ± 2.1 μm (P < 0.05 from baseline, Fig. 2C).
Figure 2. The role of endothelium in the arteriolar response to muscle contraction.
A, averaged time course of the response to 2 min local application of 10−4m ACh in the same vessels with (+EC) and without (−EC) endothelium. B, averaged time course of the response to 15 s of muscle contraction at 30 Hz in the same vessels ± EC. C, peak diameter changes to ACh and 30 Hz in controls (▪, + EC) and to ACh, 30 Hz and 10−3m SNP in endothelial denuded vessels (□, −EC). *Significantly different from +EC response (P < 0.05). #Significantly different from other −EC responses (P < 0.05). Values are means ±s.e.m. (n= 5).
EC Ca2+ increases during muscle contraction
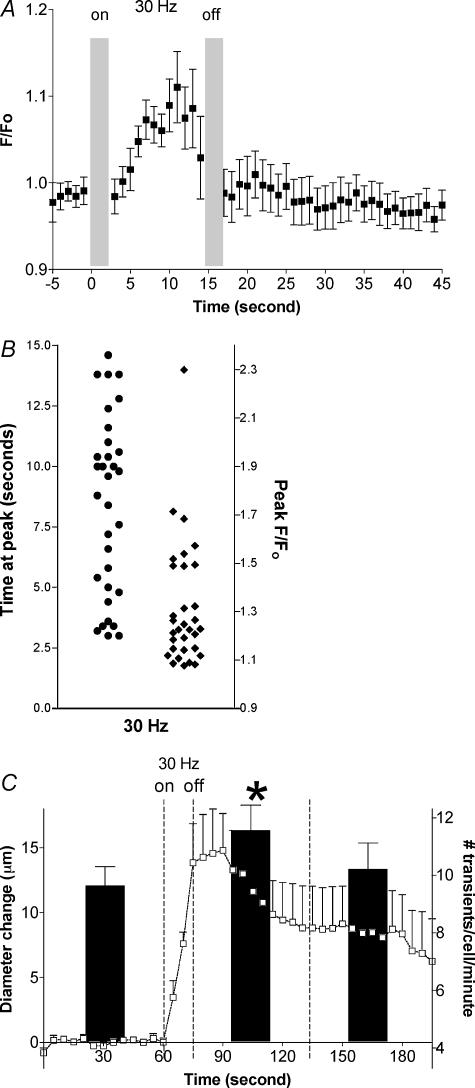
Dilatation in response to muscle contraction in this set of animals (n= 5) was 14.8 ± 2.8 μm from a baseline of 18.8 ± 2.7 μm. Muscle contraction increased whole EC Ca2+ from baseline during the stimulation period: this Ca2+ increase was less than that produced by 10−4m ACh. The averaged change in whole cell fluorescence at 1 s intervals in 32 ECs is shown in Fig. 3A. The mean increase in peak fluorescence attained during contraction (32 ± 5% from baseline, P < 0.05, n= 32) was reached at variable times during the contraction period (Fig. 3B), accounting for the lower apparent peak in fluorescence in the time-averaged data plotted in Fig. 3A. Significant lateral tissue movement associated with the initiation and termination of tetanic contraction precluded reliable measurement of whole cell Ca2+ for the first 0–3 s of stimulation and recovery. However, during the contraction the twitches are fused together and hence the tissue remains in focus for the bulk of the 15 s stimulation period, thereby allowing the measurement of whole cell Ca2+ in ECs during skeletal muscle contractile activity. The data in Fig. 3 show that most of the sampled ECs had reached their Ca2+ peak before the peak dilatation (shown in panel C of Fig. 3): Ca2+ was no longer different from baseline within seconds of cessation of the stimulation. Time courses of the change in whole cell Ca2+ (Fig. 3A) and metabolic dilatation (Fig. 3C) suggest that this increase in Ca2+ is more likely to be related to the activation of the vasodilator signal, while maintenance of the dilatation is achieved by other means.
Figure 3. Changes in endothelial cell Ca2+ and diameter in response to muscle contraction.
A, averaged time course of the change in whole cell Ca2+ during and after muscle contraction. EC Ca2+ response is expressed as relative change in the fluorescence emission intensity from normalized baseline (F/F0). Muscle contraction was from time = 0 s to time = 15 s: the grey bars indicate periods during which EC Ca2+ data were not obtainable due to tissue movement. (n= 32 ECs). B, scatter plots of time to peak fluorescence during the 30 Hz stimulation (•, left side) and peak fluorescence ratio (♦; right side) for the averaged data shown in A. Peak fluorescence in all ECs was always attained during the stimulation period. C, mean changes in localized EC Ca2+ transients (black bars, right y-axis, n= 62) during the 1 min period before and each of the 2 min periods after muscle contraction, shown in relation to the averaged time course of the diameter changes in the same preparations (□, left y-axis, n= 5). *Significantly different from frequency of localized transients before muscle contraction or in second minute post contraction (P < 0.05). Values are means ±s.e.m.
To test if muscle contraction alters local Ca2+ transients in ECs, measurements were made in the same cells (n= 62) over 1 min periods during baseline (control) and recovery from 30 Hz muscle contraction (Fig. 3C). Spontaneously occurring local transient increases in Ca2+ (9.6 ± 0.7 transients cell−1 min−1) were detected in arteriolar ECs during baseline. The frequency of this activity was increased significantly to 11.6 ± 0.9 transients cell−1 min−1 (P < 0.05, compared to control) during the first minute of recovery from contraction. Local Ca2+ transients had returned to control levels during the second minute of recovery (10.2 ± 0.9 transients cell−1 min−1, P > 0.05 compared to control, P > 0.05 compared to the first minute of post-stimulation recovery).
An increase in EC Ca2+ is required for the dilatation
To assess whether the increase in EC Ca2+ is obligatory for the dilatation associated with muscle contraction, observations were made in control vessels and following buffering of EC Ca2+ with BAPTA, in the same preparations (n= 4). BAPTA loading resulted in a decrease in resting arteriolar tone, though the vessels retained their capacity to dilate (control baseline diameter 19.9 ± 4.0 versus 23.2 ± 4.4 μm with BAPTA: maximum diameter with BAPTA was 31.5 ± 3.8 μm). Ach-induced dilatation was abolished following BAPTA loading (18.7 ± 4.1 versus 0.5 ± 1.0 μm, P < 0.05), establishing successful chelation of EC Ca2+ (Fig. 4A and C). Buffering EC Ca2+ also eliminated the response to muscle contraction (15.8 ± 2.7 versus 1.1 ± 0.7 μm, P < 0.05, Fig. 4B and C). BAPTA-loaded arterioles produced a peak dilatation of 9.9 ± 2.9 μm (P < 0.05 from baseline) in response to SNP, indicating that the treated vessels had the capacity to dilate (Fig. 4C). Additionally, they constricted significantly (P < 0.05) in response to locally applied NA, indicating that SMCs remained capable of increasing their intracellular Ca2+, and thus providing further confirmation that there had been selective buffering of EC Ca2+.
Figure 4. Role of EC Ca2+ chelation in the arteriolar response to muscle contraction.
A, averaged time course of the response to 2 min local application of 10−4m ACh in controls, and in the same arterioles after ECs had been loaded with BAPTA to chelate Ca2+. B, averaged time course of the response to 15 s of muscle contraction at 30 Hz in the same arterioles as in A. C, peak diameter changes produced by ACh and 30 Hz in controls (filled columns) and ACh, 30 Hz and 10−3m SNP in BAPTA-loaded vessels (open columns). *Significantly different from response in controls (P < 0.05). #Significantly different from other responses in BAPTA-loaded vessels (P < 0.05). Values are means ±s.e.m. (n= 4).
ADO participates in sustaining the dilatation
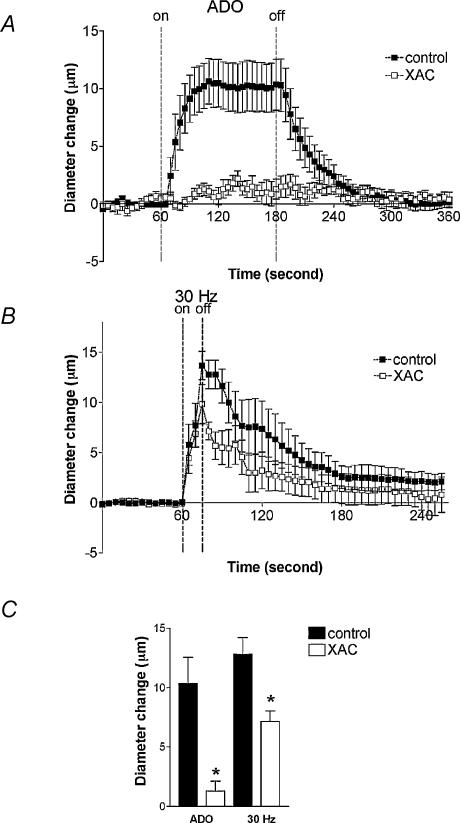
To investigate whether the dilator response initiated by muscle contraction involves stimulation of P1 receptors subsequent to production of endogenous ADO, observations were made in the same arterioles before (control) and during exposure to XAC (n= 6). Baseline and maximal diameters in these vessels were 15.6 ± 1.6 and 30.5 ± 1.2 μm, respectively. Treatment with XAC abolished dilatations in response to ADO applied locally for 2 min (10.3 ± 2.2 versus 1.3 ± 0.9 μm, P < 0.05), indicating successful inhibition of P1 receptors (Fig. 5A and C). With XAC treatment, the rate of onset of dilatation in response to muscle contraction was unchanged but the magnitude of the dilatation immediately post stimulation (12.8 ± 1.4 versus 7.1 ± 0.9 μm, P < 0.05) and during recovery was decreased (Fig. 5B and C). Collectively, these findings indicate that ADO does not play an essential role in initiating the dilatation in response to 30 Hz muscle contraction, but is involved in maintenance of the dilatation, suggesting that at least two separate mechanisms must mediate the response.
Figure 5. Arteriolar response to muscle contraction in the absence or presence of XAC, a P1 purinergic receptor antagonist.
A, averaged time course of the response to 2 min local application of 10−4m ADO in controls, and in the same arterioles in the presence of 10−5m XAC. B, averaged time course of the response to 15 s of muscle contraction at 30 Hz in the same arterioles as in A. C, peak diameter changes to ADO and 30 Hz in controls (filled columns) and in the presence of XAC (open columns). *Significantly different from response in controls (P < 0.05). Values are means ±s.e.m. (n= 6).
Discussion
This study demonstrates that in intact blood-perfused arterioles, manifestation of the dilatation initiated by skeletal muscle contraction is dependent on the presence of a functional endothelium. We established that the ability to change EC Ca2+ is a required element in the response to this physiological stimulus. We found that whole cell Ca2+ increases only during stimulation, and, importantly, its peak occurs prior to the peak change in vessel diameter. The activity of localized EC Ca2+ transients on the other hand, is increased relative to baseline immediately post contraction, and like whole cell Ca2+, returns to resting levels sooner than vessel diameter. Furthermore, our findings indicate that at least two endothelial pathways mediate the dilatation. We conclude this because the P1 purinergic receptor-dependent pathway is not involved in the onset of the dilatation, whereas P1 receptor-mediated effects of endogenous ADO are partially responsible for the maintenance of the dilatation during later phases of the response. We speculate that a separate mechanism must mediate an early component of the dilatation, perhaps, for example, K+ released during contraction.
While both ECs and SMCs can independently mediate changes in diameter, contraction or relaxation of SMCs is ultimately what constricts or dilates arterioles. Accordingly, it is reasonable to expect that SMCs will directly mediate a component of exercise hyperaemia. However, an important finding of this study is that the dilator response to muscle contraction is abolished in the absence of intact endothelium, indicating that in fact ECs are the primary vascular cell type reponsible for the initiation of metabolic dilatation in these arterioles. We denuded the EC layer by air embolism, an approach that is frequently used in isolated blood vessels and has recently been applied to small intact arterioles in situ (Duza & Sarelius, 2003). EC-denuded arterioles spontaneously displayed vessel tone and responded to the SMC-dependent dilator SNP, confirming that the lack of dilator response to muscle contraction was related specifically to impairment of EC function and not to a general loss of vessel integrity from the denudation procedure. Our finding that ECs are essential for the exercise hyperaemia response in these arterioles is consistent with earlier studies from our laboratory showing that metabolic dilatation in small arterioles can be initiated remotely by capillaries, and therefore by implication, ECs (Berg et al. 1997; Cohen et al. 2000). A key contribution of the current study is that it identifies an essential role for ECs in arterioles as well, where signalling pathways involving either vascular cell type would be available.
Our study also shows that changes in EC Ca2+ are essential for metabolic dilatation. In both this and earlier studies (Murrant et al. 2004), we observed that whole cell Ca2+ does not remain elevated in ECs following the cessation of muscle contraction, which suggests that this Ca2+-dependent signalling pathway operates in an early phase of the integrated metabolic response. We were unable to quantify localized Ca2+ transients in ECs during muscle contraction, but were able to show that the frequency of these localized oscillations was increased immediately post stimulation. We also showed that inhibition of the ability of ECs to alter Ca2+ abolished the metabolic vasodilatation. Although we cannot eliminate the possibility that all EC function was abolished by BAPTA loading, our data are most simply interpreted as supporting an obligatory role for EC Ca2+ changes in the response. It is established that intracellular Ca2+ has the remarkable capacity to fuel a variety of outcomes based on the frequency, amplitude and spatial cues of its signal (Berridge, 1997; Rottingen & Iversen, 2000). Our data support a model where distinct EC Ca2+ signalling patterns activated in response to skeletal muscle contraction play different roles in the orchestration of the integrated dilator response. In particular, an increase in whole cell Ca2+ in ECs is an essential early signalling event in metabolic vasodilatation, while transitory localized EC Ca2+ events might be involved in the sustained component of the response. The dilatation persisted through the 3 min period during which recovery was monitored, whereas Ca2+ transient events had returned to control levels by the second minute post stimulation. Our data clearly indicate therefore that maintenance of the dilatation and the subsequent recovery to control diameter are achieved by means that are independent of increases in the amplitude of the whole cell Ca2+ increase, but that could be dependent on the Ca2+ transients. For example, in isolated endothelial cell systems, focal elevation of subplasmalemmal Ca2+ has been implicated in triggering eNOS activation and subsequent NO synthesis (Graier et al. 1998; Teubl et al. 1999; Schneider et al. 2002).
As discussed earlier, a role for ADO, albeit complex, has consistently been identified in exercise hyperaemia. We recently showed that in small arterioles, endothelial purinergic (P1) receptors both mediate the dilator response to extracellular purines via a Ca2+-dependent pathway (Duza & Sarelius, 2003) and also modulate EC Ca2+ transients (authors' unpublished observations); this provided the rationale for examining the role of P1 receptor-dependent pathways in metabolic dilatation in the present study. Our present result extends our previous work (Cohen & Sarelius, 2002; Murrant & Sarelius, 2002) by showing that ADO is not responsible for the initial onset of dilatation. The earlier studies also implicated ATP-dependent K+ (KATP) channels in metabolic vasodilatation; however, glibenclamide did not affect the metabolic vasodilatation produced with the stimulation parameters used in the present study (authors' unpublished observations). This supports our conclusion from the earlier work that ADO does not act via KATP channels in this metabolic response. Our current study shows that ADO clearly contributes to the sustained component of the response, perhaps via modulation of localized Ca2+ transients in ECs. Measurements of changes in the amplitude of localized Ca2+ transients was beyond the capacity of our measurement system. We (authors' unpublished observations) and others (Burdyga et al. 2003) have observed that ECs in situ display significant oscillations in local Ca2+ activity, and we have observed in preliminary studies (authors' unpublished observations) that ADO is capable of modifying this activity. Clearly, this result and earlier studies (Honig & Frierson, 1980; Proctor & Duling, 1982) serve to emphasize that the contribution of ADO to metabolic dilatation is only one component of a multifaceted response. The novel contribution of the present work is the insight that it provides regarding the cell type that is the likely target for this purinergic pathway, as collectively, our data from this and earlier work (Duza & Sarelius, 2003) provide indirect evidence that the stimulated P1 receptors are on ECs rather than SMCs. We note that endothelial P1 receptor-dependent pathways are also essential in mediation of the metabolic vasodilatation initiated by hypoxia (Marshall, 2000).
During skeletal muscle contraction, not only is total blood volume supplied to the exercising tissue increased, but flow is redistributed such that active regions receive more flow (Gorczynski et al. 1978; Berg et al. 1997; Murrant & Sarelius, 2000). An array of signalling mechanisms must work in concert to permit this precisely graded response that matches the vascular dilatation to metabolic activity. The most significant contribution of the present study is that it identifies endothelium as primarily responsible for mediation of metabolic coupling in the terminal vasculature. We also report that this response depends on EC Ca2+ changes; there is both an increase in whole cell Ca2+ during muscle contraction, and increased frequency of localized EC Ca2+ transients immediately post stimulation. An increase in EC Ca2+ is essential for this metabolic response to occur. Finally, the study shows that P1 purinergic receptor-mediated signalling contributes to the sustained part of the dilatation, but not its initiation. Collectively, these findings expand the current understanding of how signals originating from contracting muscle fibres influence arteriolar diameter, which ultimately couples blood flow to metabolism in exercising tissue.
Acknowledgments
We thank Dr C. L. Murrant for critical discussion, and Ms. P. A. Titus for superb technical assistance. The work was supported by NIH HL56574.
References
- Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol. 1997;272:H2693–H2700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Exp Biol. 1997;200:315–319. doi: 10.1242/jeb.200.2.315. [DOI] [PubMed] [Google Scholar]
- Bockman EL, McKenzie JE. Tissue adenosine content in active soleus and gracilis muscles of cats. Am J Physiol. 1983;244:H552–H559. doi: 10.1152/ajpheart.1983.244.4.H552. [DOI] [PubMed] [Google Scholar]
- Bolz SS, de Wit C, Pohl U. Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels. Br J Pharmacol. 1999;128:124–134. doi: 10.1038/sj.bjp.0702775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga T, Shmygol A, Eisner DA, Wray S. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium. 2003;34:27–33. doi: 10.1016/s0143-4160(03)00019-8. [DOI] [PubMed] [Google Scholar]
- Cohen KD, Berg BR, Sarelius IH. Remote arteriolar dilations in response to muscle contraction under capillaries. Am J Physiol Heart Circ Physiol. 2000;278:H1916–H1923. doi: 10.1152/ajpheart.2000.278.6.H1916. [DOI] [PubMed] [Google Scholar]
- Cohen KD, Sarelius IH. Muscle contraction under capillaries in hamster muscle induces arteriolar dilation via KATP channels and nitric oxide. J Physiol. 2002;539:547–555. doi: 10.1113/jphysiol.2001.013388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duza T. PhD thesis. Rochester NY USA: University of Rochester; 2003. Roles of endothelial cell calcium and purinergic receptors in local metabolic coupling and vascular communication in arterioles in situ. [Google Scholar]
- Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+ Am J Physiol Heart Circ Physiol. 2003;285:H26–H37. doi: 10.1152/ajpheart.00788.2002. [DOI] [PubMed] [Google Scholar]
- Falcone JC, Kuo L, Meininger GA. Endothelial cell calcium increases during flow-induced dilation in isolated arterioles. Am J Physiol. 1993;264:H653–H659. doi: 10.1152/ajpheart.1993.264.2.H653. [DOI] [PubMed] [Google Scholar]
- Frame MD, Sarelius IH. L-arginine-induced conducted signals alter upstream arteriolar responsivity to 1-arginine. Circ Res. 1995;77:695–701. doi: 10.1161/01.res.77.4.695. [DOI] [PubMed] [Google Scholar]
- Gorczynski RJ, Duling BR. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol. 1978;235:H505–H515. doi: 10.1152/ajpheart.1978.235.5.H505. [DOI] [PubMed] [Google Scholar]
- Gorczynski RJ, Klitzman B, Duling BR. Interrelations between contracting striated muscle and precapillary microvessels. Am J Physiol. 1978;235:H494–H504. doi: 10.1152/ajpheart.1978.235.5.H494. [DOI] [PubMed] [Google Scholar]
- Graier WF, Paltauf-Doburzynska J, Hill BJ, Fleischhacker E, Hoebel BG, Kostner GM, Sturek M. Submaximal stimulation of porcine endothelial cells causes focal Ca2+ elevation beneath the cell membrane. J Physiol. 1998;506:109–125. doi: 10.1111/j.1469-7793.1998.109bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RL, Eraslan A, Saito Y. Differences in EDNO contribution to arteriolar diameters at rest and during functional dilation in striated muscle. Am J Physiol. 1993;265:H146–H151. doi: 10.1152/ajpheart.1993.265.1.H146. [DOI] [PubMed] [Google Scholar]
- Hnik P, Holas M, Krekule I, Kuriz N, Mejsnar J, Smiesko V, Ujec E, Vyskocil F. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflugers Arch. 1976;362:85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Honig CR, Frierson JL. Role of adenosine in exercise vasodilation in dog gracilis muscle. Am J Physiol. 1980;238:H703–H715. doi: 10.1152/ajpheart.1980.238.5.H703. [DOI] [PubMed] [Google Scholar]
- Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Korzick DH. Vascular smooth muscle: integrator of vasoactive signals during exercise hyperemia. Med Sci Sports Exerc. 2001;33:81–91. doi: 10.1097/00005768-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Adenosine and muscle vasodilatation in acute systemic hypoxia. Acta Physiol Scand. 2000;168:561–573. doi: 10.1046/j.1365-201x.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Duza T, Kim MB, Cohen KC, Sarelius IH. Arteriolar dilations induced by contraction of hamster cremaster muscle are dependent on changes in endothelial cell calcium. Acta Physiol Scand. 2004 doi: 10.1046/j.0001-6772.2003.01241.x. in press. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Local and remote arteriolar dilations initiated by skeletal muscle contraction. Am J Physiol Heart Circ Physiol. 2000;279:H2285–H2294. doi: 10.1152/ajpheart.2000.279.5.H2285. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Reg Integrat Physiol. 2002;282:R969–R978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- Nuttle LC, Ligon AL, Farrell KR, Hester RL. Inhibition of phospholipase A2 attenuates functional hyperemia in the hamster cremaster muscle. Am J Physiol. 1999;276:H1289–H1294. doi: 10.1152/ajpheart.1999.276.4.H1289. [DOI] [PubMed] [Google Scholar]
- Phair RD, Sparks HV. Adenosine content of skeletal muscle during active hyperemia and ischemic contraction. Am J Physiol. 1979;237:H1–H9. doi: 10.1152/ajpheart.1979.237.1.H1. [DOI] [PubMed] [Google Scholar]
- Poucher SM, Nowell CG, Collis MG. The role of adenosine in exercise hyperaemia of the gracilis muscle in anaesthetized cats. J Physiol. 1990;427:19–29. doi: 10.1113/jphysiol.1990.sp018158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor KG, Duling BR. Adenosine and free-flow functional hyperemia in striated muscle. Am J Physiol. 1982;242:H688–H697. doi: 10.1152/ajpheart.1982.242.4.H688. [DOI] [PubMed] [Google Scholar]
- Rottingen J, Iversen JG. Ruled by waves? Intracellular and intercellular calcium signalling. Acta Physiol Scand. 2000;169:203–219. doi: 10.1046/j.1365-201x.2000.00732.x. [DOI] [PubMed] [Google Scholar]
- Saito Y, Eraslan A, Hester RL. Role of endotheliumderived relaxing factors in arteriolar dilation during muscle contraction elicited by electrical field stimulation. Microcirculation. 1994;1:195–201. doi: 10.3109/10739689409148274. [DOI] [PubMed] [Google Scholar]
- Sarelius IH, Huxley VH. A direct effect of atrial peptide on arterioles of the terminal microvasculature. Am J Physiol. 1990;258:R1224–R1229. doi: 10.1152/ajpregu.1990.258.5.R1224. [DOI] [PubMed] [Google Scholar]
- Schneider JC, El Kebir D, Chereau C, Mercier JC, Dall'Ava-Santucci J, Dinh-Xuan AT. Involvement of Na(+)/Ca(2+) exchanger in endothelial NO production and endothelium-dependent relaxation. Am J Physiol Heart Circ Physiol. 2002;283:H837–H844. doi: 10.1152/ajpheart.00789.2001. [DOI] [PubMed] [Google Scholar]
- Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand. 2000;168:511–518. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol (Lond) 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teubl M, Groschner K, Kohlwein SD, Mayer B, Schmidt K. Na(+)/Ca(2+) exchange facilitates Ca(2+)-dependent activation of endothelial nitric-oxide synthase. J Biol Chem. 1999;274:29529–29535. doi: 10.1074/jbc.274.41.29529. [DOI] [PubMed] [Google Scholar]
- Tran QK, Ohashi K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000;48:13–22. doi: 10.1016/s0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]