Abstract
Before the preganglionic regulation of the adrenal medulla is established, hypoxia acts directly on the chromaffin cells to evoke the secretion of catecholamines. This direct action of hypoxia is suppressed by the gradual development of the preganglionic innervation and we have proposed that opioid peptides released from the adrenal splanchnic nerves may be responsible for this suppression. The effects of the specific opioid agonists DPDPE (δ-agonist), U-62066 (κ-agonist) and DALDA (μ-agonist) on the hypoxia-evoked response were investigated in both a whole-gland preparation and in isolated adrenal chromaffin cells using amperometry, whole-cell patch clamping and measurement of cytosolic [Ca2+]. The combined application of μ- and κ-type agonists abolished the hypoxia-evoked catecholamine secretion from whole perfused adrenal gland. In isolated chromaffin cells, μ- and κ-opioid agonists reduced the rise in [Ca2+]i that results from exposure to hypoxia. Both agonists decreased the voltage-dependent Ca2+ current in these cells. The μ-agonist increased the conductance through SK-type K+ channels and this action offset the decrease in K+ conductance produced by exposure to hypoxia. The κ-type agonist decreased the conductance through an action on BK-type K+ channels, a class of channels that are not involved in initiating the direct response to hypoxia. These data suggest that opioids, through their action on SK channels and voltage-dependent Ca2+ channels, may be responsible for the nerve-induced suppression of the hypoxic response of adrenal chromaffin cells and that these effects of endogenous opioids are mediated via μ- and κ-type receptors.
In a number of cells, including the glomus cells of the carotid body and the neuroepithelial bodies of the airways, oxygen-sensitive K+ channels play an important role in the responses to hypoxia (Lopez-Barneo et al. 2001). Hypoxia-induced closure of these K+ channels leads to depolarization of the cell membrane, resulting in activation of voltage-dependent Ca2+ channels and entry of Ca2+ from the extracellular compartment (Youngson et al. 1993; Prabhakar, 2000). Recently, we have shown that in sheep adrenal medullary chromaffin cells (AMCCs), two of the Ca2+-dependent K+ channels, the large conductance BK channel and the small conductance SK channel, are oxygen-sensitive (Keating et al. 2001). While sheep SK channels have not been cloned, the SK channels in sheep AMCCs have characteristics of human KCNN2 and KCNN3 (Gutman et al. 2003). Acute hypoxia results in closure of these SK channels, depolarization of the cell, and entry of Ca2+ into the cytosol (Keating et al. 2001). This direct response of AMCCs to hypoxia is seen in the mammalian fetus during early gestation, before the preganglionic regulation of the adrenal gland is established, and when hypoxaemia-evoked elevations in the concentration of circulating catecholamines are crucial for fetal survival (Cheung, 1990).
After the splanchnic innervation has developed, the direct, or non-neurogenic, response of the adrenal medulla to hypoxia is suppressed, and hypoxia-evoked catecholamine secretion occurs as a result of a neurogenic chemoreceptor reflex originating in the carotid body (Seidler & Slotkin, 1986; Cheung, 1990). Adrenal denervation restores the direct response of the AMCCs to hypoxia (Cheung, 1990), indicating that a factor released from the nerve terminals may be responsible for the suppression of the non-neurogenic hypoxic response. Nerve terminals in the adrenal medulla contain a number of biologically active agents including acetylcholine, vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), histamine and endogenous opioid peptides (Kobayashi et al. 1985; Holgert et al. 1998; Tornoe et al. 2000). It appears that the action of acetylcholine on nicotinic receptors is not responsible for the suppression of the non-neurogenic response of the AMCCs to hypoxia, as Cheung (1990) showed that pretreatment of sheep with the nicotinic antagonist hexamethonium does not restore the non-neurogenic response.
A review of the actions of the non-cholinergic neurotransmitters in the adrenal medulla indicates that those of the opioid peptides would best suit them for a role as suppressors of the direct hypoxic response. One reason for this is that acetylcholine (Montiel et al. 1995), VIP (Malhotra & Wakade, 1987), PACAP (Watanabe et al. 1995) and histamine (Donald et al. 2002) all promote catecholamine secretion from AMCCs.
Opioid peptides are present in both splanchnic nerve terminals (Kobayashi et al. 1985) and AMCCs (Livett et al. 1981; Wilson et al. 1982) and are cosecreted with catecholamines from AMCCs during nicotinic stimulation (Livett et al. 1981). Endogenous opioids inhibit the release of catecholamines from bovine and canine AMCCs (Saiani & Guidotti, 1982; Kimura et al. 1988), while the opioid receptor antagonist naloxone potentiates hypoxia-induced catecholamine secretion in fetal sheep in utero (Padbury et al. 1987; Martinez et al. 1988). The amounts of enkepahlin-containing opioids in rat preganglionic fibres increase with maturation in parallel with the suppression of the ‘direct’ hypoxic response (Holgert et al. 1994). All three major subclasses of opioid receptor, δ, κ, and μ, have been identified in the bovine adrenal medulla (Dumont & Lemaire, 1984; Bunn et al. 1988) and opioid agonists increase the current through BK channels (Twitchell & Rane, 1993) and decrease voltage-dependent Ca2+ currents (Kleppisch et al. 1992; Albillos et al. 1996). These facts support the hypothesis that opioids have a role in the developmental suppression of the hypoxic response of AMCCs.
These previously described effects of opioid receptor stimulation on BK channels and voltage-dependent Ca2+ currents would explain how opioid peptides released from the splanchnic nerve terminals diminish the release of catecholamines in response to a depolarizing stimulus such as hypoxia. They would not explain, however, the elimination of the hypoxic response. We have previously shown that in sheep AMCCs, closure of SK channels during episodes of hypoxia causes membrane depolarization and Ca2+ entry through voltage-dependent Ca2+ channels (Keating et al. 2001). Our hypothesis is that opioids mediate the suppression of hypoxia-evoked secretion of adrenal catecholamines by preventing the hypoxia-evoked depolarization produced by closure of the SK channels or by reducing Ca2+ current amplitude to such a degree as to prevent hypoxia-induced Ca2+ entry. The present study aimed to determine whether opioids prevent non-neurogenic hypoxia-evoked catecholamine secretion from the adrenal medulla, and if so, to determine the ion channels that are directly affected by the stimulation of opioid receptors. This information will allow us to evaluate whether opioid peptides can be considered as mediators of the neural suppression of the direct response of AMCCs to hypoxia.
Methods
The Merino ewes used for these experiments, approved by the University of Adelaide Animal Ethics Committee, were killed with intravenous pentobarbitone (8.1 g). To obtain fetal adrenal glands, the fetus, anaesthetized from the maternal pentobarbitone injection, was removed through a laparotomy incision and decapitated.
Isolated perfused adrenal gland
Adrenals were collected from five fetal sheep at 136–145 days gestation and perfused following methods previously outlined by Adams et al. (1996). Adrenals were mounted in a perfusion chamber, which consisted of a glass funnel covered with a fine nylon mesh (2 × 2 mm) platform. Glands were perfused using a Minipuls 3 perfusion pump (Gilson Medical Electronics, France) at a rate of 1 ml min−1. The perfusion was carried out using Krebs bicarbonate solution containing (mm): NaCl, 120; KCl, 5; glucose, 10; CaCl2, 2.5; MgSO4, 1; NaHCO3, 25; NaH2PO4, 1; pH 7.4, maintained at 37°C and bubbled continuously with 95% O2+ 5% CO2(Po2= 587.2 ± 14.4 mmHg; n= 5); normoxic Krebs.
All adrenals were perfused for 90 min before being exposed to hypoxia. Adrenal hypoxia was generated by perfusion for 20 min with hypoxic Krebs bicarbonate solution (equilibrated with 1%O2+ 5% CO2+ 94% N2; Po2= 38.5 ± 2.7 mmHg; n= 5). Opioid agonists were included in the perfusate 20 min before glands were exposed to both opioids and hypoxia simultaneously. The Po2 of the perfusate was determined using an ABL 550 blood gas analyser (Radiometer, Denmark). The use of a three-way stopcock allowed for rapid switching between the normoxic and hypoxic Krebs buffers. For the measurement of secretion of catecholamines by the perfused gland, a pre-cut 10 μm diameter carbon fibre electrode (World Precision Instruments, USA), was positioned in the outflow of the gland. A holding voltage of +700 mV was applied between the carbon fibre tip and the reference electrode to allow oxidation of released catecholamine. Amperometric responses were monitored with a List EPC-7 amplifier (List, Germany), collected at 4 kHz, digitized with a TL-1 DMA acquisition system and monitored online with the Fetchex software from the pCLAMP 6.0 suite (Axon Instruments, USA).
Isolation of adrenal chromaffin cells
These experiments used isolated AMCCs from adult sheep. We have shown previously that these cells regain the ability to respond directly to hypoxia once the adrenal gland is denervated (Adams et al. 1996; Keating et al. 2001).
The maternal adrenal gland was removed and the medulla, dissected free of the cortex, was minced and incubated at 37°C for 45 min in a Ca2+-free Locke's solution consisting of (mm): NaCl, 154; KCl, 5.6; NaHCO3, 3.6; glucose, 5.6; Hepes, 5.0; pH 7.4, supplemented with collagenase (Type II, 0.2%; Worthington Biochemical Corporation, USA) and deoxyribonuclease (Type IV; 100 U ml−1; Sigma, USA). Repeated pipetting dispersed the tissue and the cells were washed twice in Ca2+-free Locke's solution. The cells were then resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco, USA) containing 10% charcoal-absorbed fetal bovine serum (Trace Scientific, Australia), 100 U ml−1 penicillin and 0.5 mg ml−1 streptomycin, plated on glass coverslips and maintained in culture.
Electrophysiology
Whole-cell recordings were conducted on cells 1–3 days after plating. Pipettes with a resistance between 2 and 4 MΩ were used and series resistance was 80–95% compensated. Currents were recorded with an EPC-9 amplifier (HEKA, Germany), data acquisition and analysis was performed on an IBM compatible computer using Pulse and Pulsefit software (version 8.11, HEKA). Corrections for liquid junction potential between the bath and electrode solutions were estimated by JPCalc (Barry, 1994). AMCCs, in a bath of approximately 500 μl, were superfused continuously at 2–3 ml min−1, always at room temperature. When recording whole-cell K+ currents with a constant [Ca2+]i, the extracellular solution contained (mm): NaCl, 140; KCl, 4; CaCl2, 2; MgCl2, 2; Hepes, 10; CdCl2, 0.2; glucose, 10; with pH adjusted to 7.4 with NaOH; and the internal solution contained (mm): KCl, 150; MgATP, 1; EGTA, 1; Hepes, 10; CaCl2, 0.89; adjusted to pH 7.2 with KOH. The free Ca2+ concentration in this internal solution was calculated to be 1 μm. When recording whole-cell K+ currents without blocking Ca2+ entry, the extracellular solution contained (mm): NaCl, 140; KCl, 4; CaCl2, 2; MgCl2, 2; Hepes, 10; glucose, 10; with pH adjusted to 7.4 with NaOH; and the internal solution contained (mm): KCl, 60; potassium glutamate, 75; MgCl2, 2; Na2ATP, 2; EGTA, 2; Hepes, 10; adjusted to pH 7.2 with KOH. The bath solution was equilibrated with either room air or 100% N2 using gas-impermeant tubing. Po2 was measured with an O2 microelectrode (World Precision Instruments, USA) placed near the recording site. With hypoxic superfusion, Po2 reached a level of 7–10 mmHg. Potassium conductance was estimated as the chord conductance between 10 and 60 mV using voltage ramps that increased linearly from −120 mV to 100 mV over 100 ms from a holding potential of −80 mV.
When Ca2+ currents were recorded, cells were bathed in a solution containing (mm): CaCl2, 10; MgCl2, 2; NaCl, 70; TEA Cl, 70; Hepes, 10; TTX, 3 × 10−4, 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), 0.1; and the internal solution consisted of (mm): TEA Cl, 60; caesium glutamate, 65; MgATP, 4; EGTA, 10; Hepes, 10; adjusted to pH 7.2 with TEA OH. The opioid agonists [d-Arg2, Lys4]-dermorphin-(1–4)-amide (DALDA), [d-Pen2,5]-enkephalin (DPDPE) and spiradoline mesylate (U-62066), all at 1 μm, were applied through the superfusion solution for 3–5 min
Calcium imaging
AMCCs were incubated with 20 μm fluo-3 AM and 0.0025% Pluronic acid in DMEM at 37°C for 30 min. During experiments, cells were superfused continuously at 2–3 ml min−1 with a solution containing (mm): NaCl, 140; KCl, 4; CaCl2, 2; MgCl2, 2; Hepes, 10; with pH adjusted to 7.4 with NaOH. Confocal microscopy with a water immersion lens was applied to single cells using an argon ion laser (Optiscan, Australia) scanning at a peak of 488 nm. Images were captured using confocal software (F900e, version 1.6, Optiscan, Australia) and analysed using Scion Image (Scion Corporation, USA). Laser intensity was reduced to 1% of maximum by use of neutral density filters and the number of scans per cell kept to a minimum to avoid photobleaching. Changes in intracellular Ca2+ levels were calculated as the ratio of the increase in the mean pixel value of the whole cell compared to that in the control period, where the pixel values are a grey scale ranging from zero to 255. All values of increased fluorescence were taken 60 s after the start of application of the hypoxic solution through the perfusion system. This ratio is calculated according to the equation:
where R= ratio of Ca2+ fluorescence change, Fmin= mean fluorescence intensity level during control period, F= mean fluorescence intensity level following 1 min of stimulation. This ratio was then multiplied by 100 to express any fluorescence changes as a percentage change from the resting fluorescence level.
Drugs
Apamin extracted from bee venom, NPPB and DMEM were obtained from Sigma Chemicals, USA, Fluo-3 AM and Pluronic F-127 from Molecular Probes, USA and the μ-agonist DALDA, δ-agonist DPDPE and κ-agonist U-62066 from RBI Chemicals, USA.
Statistics
Results are expressed as means ±s.e.m. The secretion of catecholamines by the perfused fetal adrenal gland in response to hypoxia in each experiment is expressed in relation to the mean level of catecholamine secretion in the 12 min baseline period preceding the onset of hypoxia. The catecholamine responses to hypoxia in the presence or absence of the opioid agonist were then compared using multifactorial ANOVA with experimental treatment (hypoxia alone or hypoxia + opioid) and sampling time as the specified variables. The Duncan's new multiple range test was used post ANOVA to identify significant differences between mean values. A probability level of 5%(P < 0.05) was taken as significant for all analyses. Student's paired t tests were used for two group comparisons in Ca2+ imaging experiments and comparisons of current amplitudes at specific voltages. An ANOVA with Tukey's post hoc test was used for multiple group comparisons when analysing chord conductance results. P < 0.05 was taken as the minimum level of significance.
Results
Opioid agonists and hypoxia-evoked secretion of catecholamines in the perfused adrenal gland
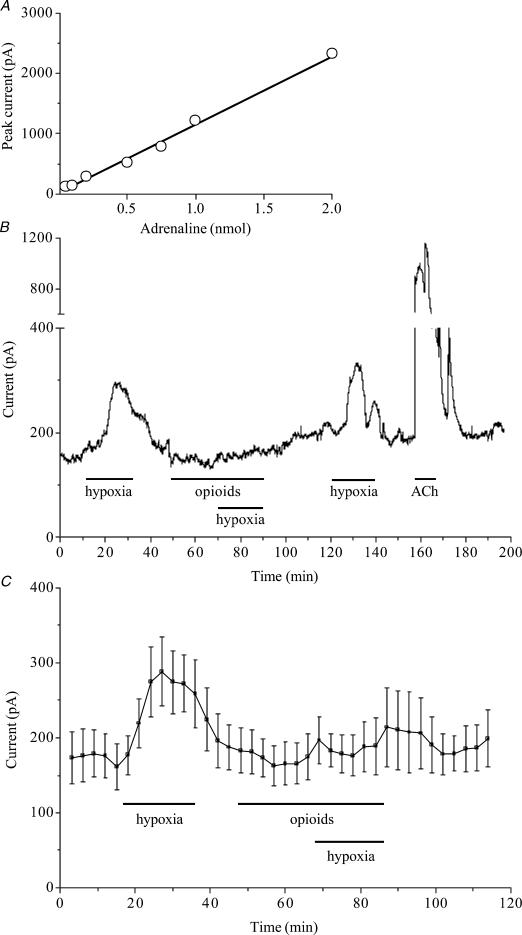
Allowing the effluent from the perfused adrenal gland from fetal sheep to flow over the carbon fibre electrode provided on-line measurement of catecholamine secretion. The electrode showed a linear response to increasing concentrations of catecholamines in the stream as demonstrated by the addition of adrenaline as a bolus to the perfusate (Fig. 1A). The isolated perfused adrenal gland allows repeated measurement of catecholamine secretion under controlled conditions (Fig. 1B). The response to hypoxia is repeatable, blocked reversibly by a combination of μ- and κ-opioid agonists, and smaller than the response to 100 μm acetylcholine (Fig. 1B). During the 12 min prior to exposure to hypoxia, the baseline current through the carbon fibre electrode was 176 ± 15 pA (n= 5; Fig. 1C). The adrenal glands were then perfused for 20 min with hypoxic Krebs solution which caused a significant increase in catecholamine secretion, with a peak current amplitude of 288 ± 46 pA(n= 5; P < 0.001) being reached after approximately 12 min exposure to hypoxia. This increase in secretion of catecholamines was fully reversed upon return to normoxia. The addition of the opioid agonists DALDA and U-62066 to the perfusate prevented the hypoxia-evoked secretion of catecholamine (Fig. 1C). When these agonists were added to the perfusate, the electrode current for the 12 min before exposure to hypoxia was 168 ± 13 pA (n= 5). In the presence of these opioid agonists, the peak electrode current during the hypoxic period was 197 ± 32 pA(n= 5) which was not significantly different from the current in the prehypoxic period.
Figure 1. Stimulation of μ- and κ-type opioid receptors diminishes hypoxia-evoked catecholamine secretion from whole perfused fetal adrenal glands.
A, response of the in-line carbon fibre electrode to the addition of adrenaline to the perfusion stream. B, raw current recording showing the release of catecholamines from an individual gland exposed to hypoxia in the presence or absence of μ- and κ-opioid agonists, and to 100 μm acetylcholine (ACh). C, pooled data from 5 adrenal glands exposed to hypoxia in the absence and then in the presence of both μ- and κ-type opioid agonists. For each gland the mean current in 3 min epochs was calculated and the data points are the mean ±s.e.m..
Opioid agonists and hypoxia-evoked changes in Ca2+ entry into isolated adrenal chromaffin cells
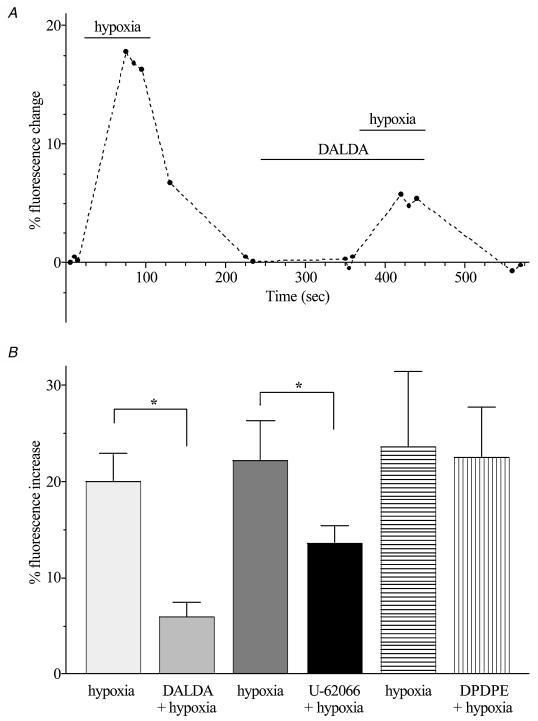
All single cell studies used AMCCs isolated from the adrenal medulla of adult sheep. After 1 min exposure to hypoxia, the [Ca2+]i increased as indicated by an increase of 20.0 ± 2.9% in fluorescence of fluo-3 loaded cells (P < 0.0001; Fig. 2). In the presence of the μ-type agonist DALDA (1 μm), while hypoxia evoked an increase in fluorescence (5.9 ± 1.5%; n= 7; P < 0.01), this was much smaller than that seen in the absence of this opioid agonist (P < 0.005; Fig. 2). Stimulation of κ-type opioid receptors also reduced the change in [Ca2+]i produced by hypoxia (Fig. 2). In the presence of U-62066 (1 μm), the change in fluorescence produced by hypoxia was reduced from 22.2 ± 4.1 to 13.5 ± 1.9% (n= 5; P < 0.05). Stimulation of δ-type opioid receptors with DPDPE did not alter the change in [Ca2+]i evoked by exposure to hypoxia. The resting [Ca2+]i in these cells was not altered by any of the three opioid agonists alone.
Figure 2. Hypoxia-evoked changes of intracellular [Ca2+] in the presence and absence of opioid receptor agonists.
A, fluorescence intensity of a single AMCC loaded with fluo-3 AM and exposed to hypoxia in the presence and absence of DALDA. The fluorescence reading at the beginning of the experiment was used as the baseline value. The lowest possible number of measurements are taken so as to reduce the effects of photobleaching. B, fluorescence of fluo-3 in each cell, measured 1 min after the application of various treatments, was compared to fluorescence in the control normoxic period. In each group of cells the [Ca2+]i during exposure to hypoxia alone was significantly greater than during normoxia. * Significant difference between responses in the absence and presence of the opioid agonist (P < 0.05); n= 7 (DALDA) or 5 (U-62066 and DPDPE).
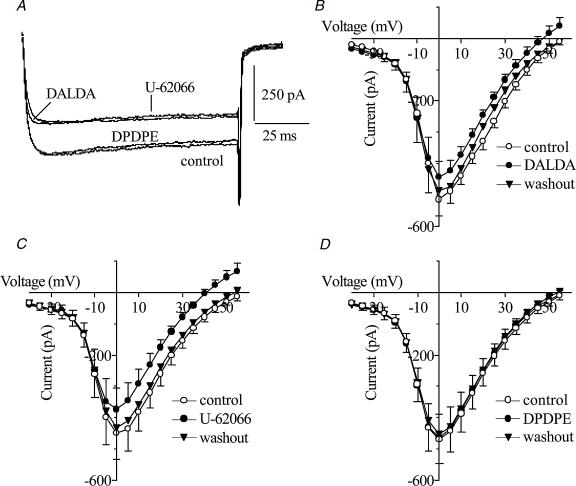
The effects of opioid agonists on voltage-dependent Ca2+ currents were determined by whole-cell patch-clamping (Fig. 3). Cells were treated with DALDA, DPDPE or U-62066 (1 μm) separately for approximately 3–5 min. The μ-agonist DALDA (Fig. 3B) and the κ-agonist U-62066 (Fig. 3C) decreased reversibly the Ca2+ current amplitude at 5 mV by approximately 17%(P < 0.05) and 20%(P < 0.01), respectively. Neither opioid agonist affected inward current at potentials more negative than −10 mV. DPDPE, a δ-agonist, had no effect on Ca2+ current amplitude at any voltage (Fig. 3D).
Figure 3. Effect of opioid agonists on voltage-dependent Ca2+ currents in sheep AMCCs.
A, Ca2+ currents evoked in one chromaffin cell by a 100 ms step pulse from −80 mV to 5 mV in control conditions and in the presence of 1 μm DALDA, U-62066 or DPDPE. B–D, the I–V relationship of Ca2+ current during control conditions (○), exposure to opioid receptor agonists (•), and upon washout with control solution (▾). The opioid agonists were DALDA (B, n= 4), U-62066 (C, n= 6) and DPDPE (D, n= 6). Cells were held at −80 mV and stepped to potentials ranging from −40 to 55 mV for 100 ms in increments of 5 mV.
Opioid agonists and K+ currents
Given the effect of μ- and κ-agonists on whole-cell Ca2+ currents and the presence of Ca2+-dependent K+ channels in sheep AMCCs, the direct effects of opioid agonists on K+ currents were measured with the cytosolic Ca2+ concentration clamped at 1 μm. This was achieved by the use of a Ca2+-buffered intracellular solution and a Ca2+ channel blocker, Cd2+ (200 μm), in the extracellular solution.
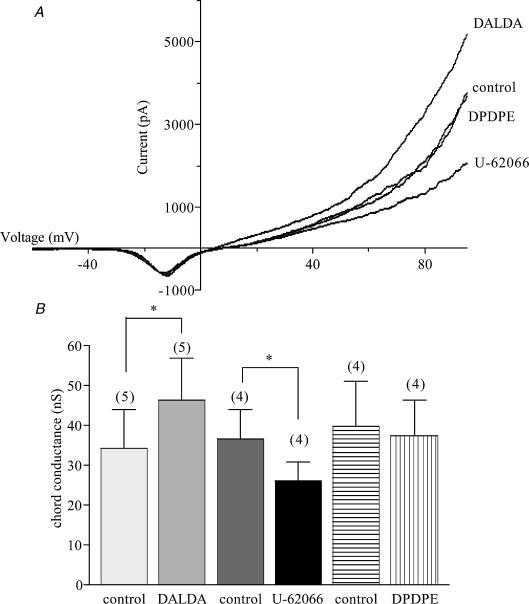
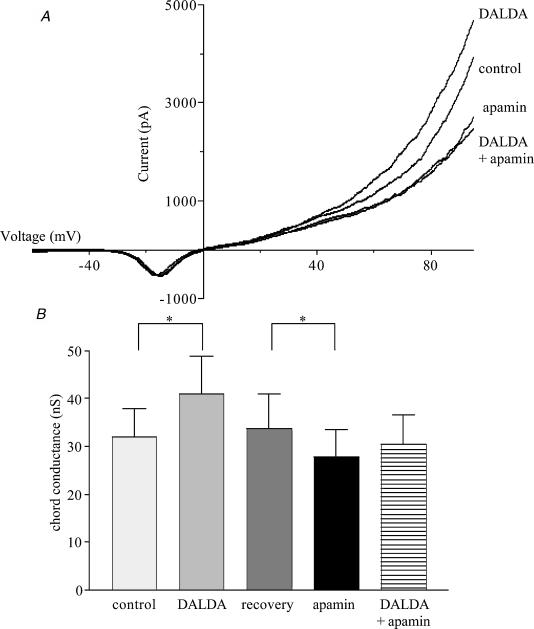
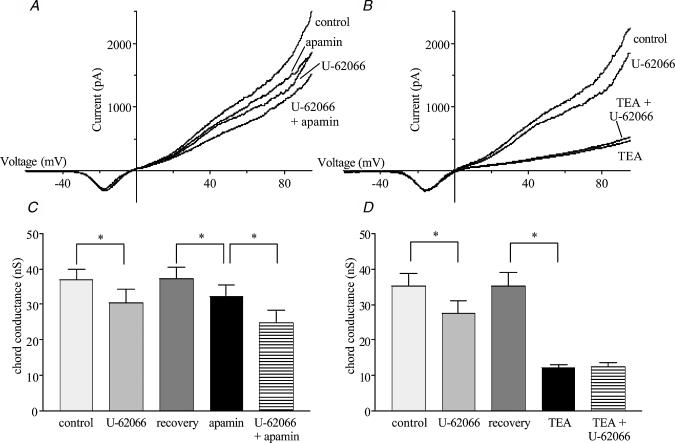
At positive membrane potentials, stimulation of either μ- or κ-type receptors caused significant changes in K+ conductance, with DALDA increasing the chord conductance (10–60 mV) by 35%, and U-62066 decreasing it by 28% (Fig. 4). DPDPE, the δ-agonist, had no effect on K+ conductance. Apamin (1 nm), a blocker of SK channels, and TEA (1 mm), a blocker of BK channels, were used to determine the contributions of these channels to the changes in membrane conductance produced by the μ- and κ-agonists. The increase in conductance produced by DALDA did not occur in the presence of apamin, indicating that the μ-agonist acted on SK channels (Fig. 5). In contrast, the decrease in conductance produced by U-62066 still occurred in the presence of apamin (Fig. 6A and fig.6B) but treatment of the cells with TEA prevented the κ-agonist from reducing conductance, indicating an action on BK channels (Fig. 6C and fig.6D).
Figure 4. Effect of opioid agonists on K+ conductance in AMCCs.
A, K+ currents recorded from a single cell held at −80 mV and ramped from −120 to 100 mV over 100 ms in the presence of μ-, κ-, and δ-opioid agonists. Each current trace is the average of 10 ramps. B, chord conductance measured in the range from 10 to 60 mV * Significant difference in the paired values (P < 0.05); n= 5 (DALDA) or 4 (U-62066 and DPDPE).
Figure 5. The contribution of SK channels to the μ-opioid-evoked increase in K+ conductance in sheep AMCCs.
A, the effect of DALDA (1 μm) and apamin (1 nm) on K+ currents recorded from a single cell held at −80 mV and ramped from −120 to 100 mV over 100 ms. Each current trace is the average of 10 ramps. B, chord conductance measured in the range from 10 to 60 mV * Significant difference between paired values (P < 0.05); n= 5 for all columns.
Figure 6. The contribution of SK and BK channels to the κ-opioid-evoked decrease in K+ conductance in AMCCs.
The effect of U-62066 on K+ currents recorded from single cells held at −80 mV and ramped from −120 to 100 mV over 100 ms in the presence and absence of apamin (A) and TEA (B). The effect of U-62066 on chord conductance measured in the range from 10 to 60 mV in the presence and absence of apamin (n= 4; C), and TEA (n= 5; D). * Significant difference between linked bars (P < 0.05)
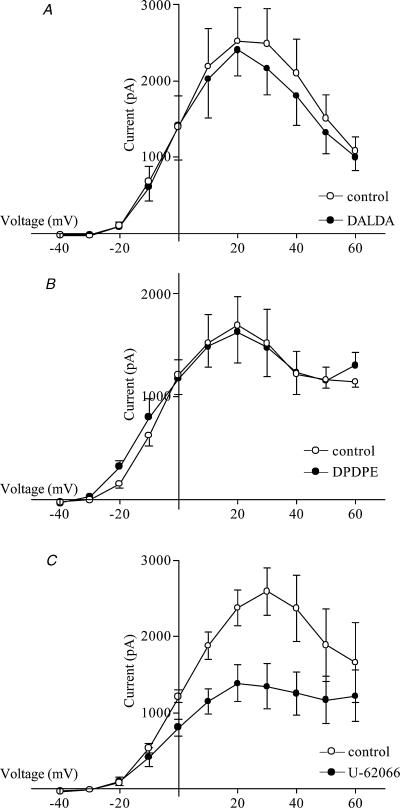
In physiological conditions, the effects of the μ- and κ-opioid agonists on Ca2+ currents and on Ca2+-dependent K+ currents will interact. In order to determine the balance between these effects, K+ currents were measured in cells in which the [Ca2+]i was not clamped during the depolarizing pulses. Under these conditions, the μ-agonist DALDA caused no significant change in outward current at any potential (n= 5; Fig. 7A). Stimulation of the κ-opioid receptors with U-62066 produced a significant reduction in outward current at membrane potentials from 0 to 50 mV (n= 5; P < 0.05; Fig. 7B). Stimulation with the δ-type opioid agonist DPDPE had no effect on outward current in these cells (Fig. 7C).
Figure 7. Modulation of whole-cell current by specific opioid agonists in adult sheep AMCCs.
I–V relationship in control conditions (○) and in the presence of an agonist (•). The agonists used, all at a concentration of 1 μm, were DALDA (n= 5; A), DPDPE (n= 4; B) and U-62066 (n= 5; C). Cells were held at −80 mV and stepped to potentials ranging from −40 to 60 mV for 200 ms.
Effect of μ-opioid agonist on the hypoxia-evoked reduction of K+ current
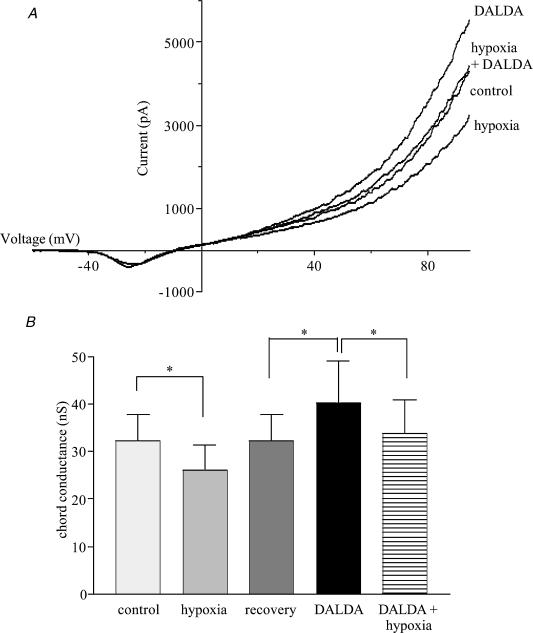
In cells in which [Ca2+]i was clamped, the ability of hypoxia to reduce overall membrane conductance was offset by the effects of DALDA (Fig. 8). Hypoxia decreased membrane conductance by 6.1 ± 1.2 nS from the value of 32.2 ± 5.2 nS seen in normoxic conditions. DALDA did not inhibit the ability of hypoxia to close K+ channels, as shown by the 6.6 ± 1.8 nS reduction in conductance produced by hypoxia in the presence of DALDA. The membrane conductance in the presence of both agents, 33.8 ± 6.4 nS, was, however, not different from that seen in control, normoxic cells.
Figure 8. The effects of μ-opioid receptor stimulation and hypoxia on K+ conductance.
A, example traces of the effect of hypoxia, 1 μm DALDA and both together on K+ currents in adult AMCCs held at −80 mV and ramped from −120 to 100 mV over 100 ms. Traces shown are the average of 10 ramps under each condition. B, chord conductance measured in the positive voltage range from 10 to 60 mV, n= 5 for all columns. * Significantly different from the bracketed value (P < 0.05).
Discussion
In this study we have shown that stimulation of μ- and κ-type opioid receptors suppresses the hypoxia-induced release of catecholamines in the fetal adrenal gland. Furthermore, μ-type receptor stimulation increased the current through the SK channels, thereby offsetting the decrease in K+ conductance produced by hypoxia. This, in conjunction with a reduction in Ca2+ current amplitude caused by stimulation of both μ- and κ-type receptors, abolishes the hypoxia-evoked increase in cytosolic [Ca2+]. We suggest that the developmental suppression of the hypoxic response of chromaffin cells in the adrenal gland is mediated via this mechanism and is induced by the increasing release of preganglionic opioids as splanchnic innervation matures.
The effect of opioid receptor stimulation on the direct hypoxic response was measured in both the isolated perfused fetal adrenal gland and single AMCCs of sheep. The concentrations of opioid peptides used in this study are likely to be in the physiological range, as estimates of opioid release from single AMCCs have suggested that the local opioid concentration is in the micromolar range (Castanas et al. 1985). Using an online analysis of catecholamine secretion with a carbon fibre electrode we were able to detect the small amounts of catecholamine secreted during hypoxic episodes as well as large amounts released by stimulation of the nicotinic cholinergic receptors due to the wide dynamic range of this system.
Combined stimulation of μ- and κ-type opioid receptors by their respective agonists, DALDA and U-62066, inhibited the hypoxia-evoked secretion of catecholamines by the isolated perfused adrenal gland of the fetal sheep. It is known that blockade of opioid receptors with naloxone increases catecholamine release in fetal sheep (Padbury et al. 1987; Martinez et al. 1988) and that, in rat, the amounts of enkephalin in the preganglionic fibres increase in parallel with the suppression of the direct hypoxic response (Holgert et al. 1994). Taken together with our current findings, the hypothesis that opioids have a role in the developmental suppression of the hypoxic response of chromaffin cells in the adrenal gland is strongly supported.
In order to identify the cellular mechanisms responsible for these actions of opioid agonists seen in the whole perfused adrenal gland, Ca2+ imaging and electrophysiology were performed on isolated adult AMCCs. We have shown previously that these cells respond to hypoxia in the same manner as fetal AMCCs (Keating et al. 2001). Stimulation of the μ- and κ-, but not δ-type, opioid receptors reduced the increase in [Ca2+]i that normally occurs during hypoxia. This could account for the ability of μ- and κ-type agonists to inhibit the hypoxia-induced secretion of catecholamines in the perfused glands. The lack of effect of the δ-type agonist would not be due to using a low concentration of this agonist, as we used 1 μm, which is over one hundred times greater than the KD for binding in rat brain membranes (Schlosser et al. 1995) and the IC50 for blocking the electrically stimulated mouse vas deferens (Haaseth et al. 1990).
The influx of Ca2+ produced by hypoxia in AMCCs occurs through voltage-dependent Ca2+ channels (Keating et al. 2001). The decrease in Ca2+ currents induced by the μ- and κ-opioid agonists that we have found in the sheep AMCCs supports earlier work in which μ-agonists decreased Ca2+ current in porcine AMCCs (Kitamura et al. 2002) and κ-agonists decreased Ca2+ currents in neurones of the guinea-pig myenteric plexus (Cherubini & North, 1985). Consistent with our experiments in which [Ca2+]i was measured, the δ-opioid agonist did not affect voltage-dependent Ca2+ currents. The reduction in Ca2+ currents caused by the μ- and κ-type agonists was, however, small, and would not appear to account completely for the diminished hypoxia-induced [Ca2+]i that was seen in the presence of these agonists.
In excitable cells, Ca2+ entry through voltage-dependent Ca2+ channels is modulated by the activity of K+ channels, which are also known to be regulated by opioid agonists (Twitchell & Rane, 1993). Oxygen-sensitive SK and BK channels play a central role in the direct hypoxic response of the adrenal medulla (Keating et al. 2001) and are potential targets for opioid action. As the activity of SK and BK channels is regulated by [Ca2+]i, any effect of opioid agonists on them could either be directly on the channel or indirectly through alterations in [Ca2+]i. In order to determine any direct effect of the opioid agonists on the oxygen-sensitive K+ currents, measurements were made while [Ca2+]i was clamped at 1 μm. Under these conditions, stimulation with the μ-type opioid agonist DALDA increased the K+ conductance in the isolated AMCCs. Inhibition of the effect of DALDA on membrane conductance by 1 nm apamin indicates that the μ-agonist acts specifically on SK channels. Activation of SK channels by DALDA offset the action of hypoxia on these channels in that it prevented subsequent exposure to hypoxia from reducing the membrane conductance below that seen in normoxic cells in control conditions. The consequence of this, in cells that are not voltage-clamped, would be that DALDA, through its actions on SK channels, would inhibit the depolarization normally produced by hypoxia, suppressing the direct effect of hypoxia on catecholamine secretion by the adrenal medulla.
While activation of κ-type opioid receptors with U-62066 decreased the K+ conductance of AMCCs, this was not due to an action on SK channels because apamin did not alter the effect of U-62066. Rather, TEA, a blocker of BK channels, abolished the decrease in K+ current caused by U-62066. Previous work has demonstrated that it is SK channels, rather than BK channels, that are important for initiating the direct hypoxia-evoked secretion of catecholamines from sheep AMCCs (Keating et al. 2001). This would indicate that the suppression of K+ current by κ-type receptor stimulation does not interfere with the proposed effect of μ-type stimulation on resting membrane potential in these cells, a fact confirmed by our data in two different experiments. Firstly, κ-type stimulation does not cause secretion in vitro due to a lack of catecholamine release in the whole-gland preparations exposed to both μ- and κ-type agonists under normoxic conditions. It could be possible that no secretion occurs under these conditions because the depolarization caused by U-62066 is offset by a concomittant hyperpolarization produced by DALDA. However, our single cell Ca2+ imaging data refutes this possibility, as no increase in [Ca2+]i was seen in the presence of U-62066 alone. Also, a decrease in Ca2+ entry during hypoxia in the presence of our κ-type agonist, rather than any increase, was observed. This decrease in hypoxia-induced Ca2+ entry can most likely be attributed to the reduction in Ca2+ current seen upon κ-type stimulation.
In physiological conditions, the activity of the calcium-dependent K+ channels in these cells will be affected by the opioid-evoked changes in [Ca2+]i as well as the more direct effect of these agonists on BK and SK channels. The balance between these two effects was seen in the whole-cell currents measured in cells in which [Ca2+]i was not clamped. The whole-cell current appeared unchanged in the presence of the μ-agonist DALDA. The absence of any change caused by the μ-type agonist reflects the interaction between an increase in SK conductance and the reduction in SK and BK conductance resulting from the μ-agonist-induced decrease in Ca2+ currents. The decrease in the whole-cell outward current at positive potentials during κ-type receptor stimulation would reflect a combination of the reduction in BK conductance produced by the direct action of the κ-agonist on the channels and the indirect inhibition of BK and SK channels caused by the decrease in voltage-dependent Ca2+ currents.
With regard to the direct hypoxic response of sheep AMCCs, we have previously shown that closure of SK channels initiates the depolarization that leads to Ca2+ entry, possibly triggering catecholamine secretion. At resting membrane potential, the sole effect of the opioid agonists would be an increase in K+ conductance resulting from the direct action of the μ-receptor on SK channels. Voltage-dependent Ca2+ channels and BK channels are closed at these potentials and therefore would not be affected by opioid agonists.
In conclusion, our investigations have found a plausible explanation for the suppression of the non-neurogenic hypoxic response of the adrenal medulla upon innervation that involves the action of opioid peptides. It appears that both μ- and κ-type opioid receptors have roles to play in this mechanism, with stimulation of both receptor types causing a decrease in hypoxia-evoked Ca2+ entry via closure of voltage-gated Ca2+ channels, and μ-type activation increasing the K+ conductance through the action on SK channels.
Acknowledgments
This work was supported by the Australian Research Council and by the Channel 7 Children's Research Foundation of South Australia.
References
- Adams MB, Simonetta G, McMillen IC. The non-neurogenic catecholamine response of the fetal adrenal to hypoxia is dependent on activation of voltage sensitive Ca2+ channels. Brain Res Dev Brain Res. 1996;94:182–189. doi: 10.1016/0165-3806(96)00054-5. [DOI] [PubMed] [Google Scholar]
- Albillos A, Carbone E, Gandia L, Garcia AG, Pollo A. Opioid inhibition of Ca2+ channel subtypes in bovine chromaffin cells: selectivity of action and voltage-dependence. Eur J Neurosci. 1996;8:1561–1570. doi: 10.1111/j.1460-9568.1996.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Meth. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bunn SJ, Marley PD, Livett BG. The distribution of opioid binding subtypes in the bovine adrenal medulla. Neuroscience. 1988;27:1081–1094. doi: 10.1016/0306-4522(88)90212-6. [DOI] [PubMed] [Google Scholar]
- Castanas E, Bourhim N, Giraud P, Boudouresque F, Cantau P, Oliver C. Interaction of opiates with opioid binding sites in the bovine adrenal medulla. I. Interaction with δ and μ sites. J Neurochem. 1985;45:677–687. doi: 10.1111/j.1471-4159.1985.tb04046.x. [DOI] [PubMed] [Google Scholar]
- Cherubini E, North RA. μ and k opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci U S A. 1985;82:1860–1863. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY. Fetal adrenal medulla catecholamine response to hypoxia – direct and neural components. Am J Physiol. 1990;258:R1340–R1346. doi: 10.1152/ajpregu.1990.258.6.R1340. [DOI] [PubMed] [Google Scholar]
- Donald AN, Wallace DJ, McKenzie S, Marley PD. Phospholipase C-mediated signalling is not required for histamine-induced catecholamine secretion from bovine chromaffin cells. J Neurochem. 2002;81:1116–1129. doi: 10.1046/j.1471-4159.2002.00915.x. [DOI] [PubMed] [Google Scholar]
- Dumont M, Lemaire S. Opioid receptors in bovine adrenal medulla. Can J Physiol Pharmacol. 1984;62:1284–1291. doi: 10.1139/y84-215. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels. Pharmacol Rev. 2003;55:583–586. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- Haaseth RC, Sobczyk-Kojiro K, Medzihradsky F, Smith CB, Mosberg HI. Single residue modifications of the delta opioid receptor selective peptide, [D-Pen2,D-Pen5]-enkephalin (DPDPE). Correlation of pharmacological effects with structural and conformational features. Int J Pept Protein Res. 1990;36:139–146. doi: 10.1111/j.1399-3011.1990.tb00957.x. [DOI] [PubMed] [Google Scholar]
- Holgert H, Dagerlind A, Hokfelt T. Immunohistochemical characterization of the peptidergic innervation of the rat adrenal gland. Horm Metab Res. 1998;30:315–322. doi: 10.1055/s-2007-978891. [DOI] [PubMed] [Google Scholar]
- Holgert H, Dagerlind A, Hokfelt T, Lagercrantz H. Neuronal markers, peptides and enzymes in nerves and chromaffin cells in the rat adrenal medulla during postnatal development. Brain Res Dev Brain Res. 1994;83:35–52. doi: 10.1016/0165-3806(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Keating DJ, Rychkov GY, Roberts ML. Oxygen sensitivity in the sheep adrenal medulla: role of SK channels. Am J Physiol Cell Physiol. 2001;281:C1434–C1441. doi: 10.1152/ajpcell.2001.281.5.C1434. [DOI] [PubMed] [Google Scholar]
- Kimura T, Katoh M, Satoh S. Inhibition by opioid agonists and enhancement by antagonists of the release of catecholamines from the dog adrenal gland in response to splanchnic nerve stimulation: evidence for the functional role of opioid receptors. J Pharmacol Exp Ther. 1988;244:1098–1102. [PubMed] [Google Scholar]
- Kitamura G, Ohta T, Kai T, Kon Y, Ito S. Inhibitory effects of opioids on voltage-dependent Ca2+ channels and catecholamine secretion in cultured porcine adrenal chromaffin cells. Brain Res. 2002;942:11–22. doi: 10.1016/s0006-8993(02)02648-3. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Ahnert-Hilger G, Gollasch M, Spicher K, Hescheler J, Schultz G, Rosenthal W. Inhibition of voltage-dependent Ca2+ channels via α2-adrenergic and opioid receptors in cultured bovine adrenal chromaffin cells. Pflugers Arch. 1992;421:131–137. doi: 10.1007/BF00374819. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Miyabayashi T, Uchida T, Yanaihara N. Met-enkephalin-Arg6-Gly7-Leu8 in large-cored vesicles of splanchnic nerve terminals innervating guinea pig adrenal chromaffin cells. Neurosci Lett. 1985;53:247–252. doi: 10.1016/0304-3940(85)90545-2. [DOI] [PubMed] [Google Scholar]
- Livett BG, Dean DM, Whelan LG, Udenfriend S, Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981;289:317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Malhotra RK, Wakade AR. Vasoactive intestinal polypeptide stimulates the secretion of catecholamines from the rat adrenal gland. J Physiol. 1987;388:285–294. doi: 10.1113/jphysiol.1987.sp016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Padbury J, Shames L, Evans C, Humme J. Naloxone potentiates epinephrine release during hypoxia in fetal sheep: dose–response and cardiovascular effects. Pediatr Res. 1988;23:343–347. doi: 10.1203/00006450-198804000-00001. [DOI] [PubMed] [Google Scholar]
- Montiel C, Lopez MG, Sanchez-Garcia P, Maroto R, Zapater P, Garcia AG. Contribution of SK and BK channels in the control of catecholamine release by electrical stimulation of the cat adrenal gland. J Physiol. 1995;486:427–437. doi: 10.1113/jphysiol.1995.sp020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padbury JF, Agata Y, Polk DH, Wang DL, Callegari CC. Neonatal adaptation: naloxone increases the catecholamine surge at birth. Pediatr Res. 1987;21:590–593. doi: 10.1203/00006450-198706000-00017. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Saiani L, Guidotti A. Opiate receptor-mediated inhibition of catecholamine release in primary cultures of bovine adrenal chromaffin cells. J Neurochem. 1982;39:1669–1676. doi: 10.1111/j.1471-4159.1982.tb08001.x. [DOI] [PubMed] [Google Scholar]
- Schlosser B, Kudernatsch MB, Sutor B, ten Bruggencate G. δ, μ and κ opioid receptor agonists inhibit dopamine overflow in rat neostriatal slices. Neurosci Lett. 1995;191:126–130. doi: 10.1016/0304-3940(94)11552-3. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Ontogeny of adrenomedullary responses to hypoxia and hypoglycemia: role of splanchnic innervation. Brain Res Bull. 1986;16:11–14. doi: 10.1016/0361-9230(86)90005-5. [DOI] [PubMed] [Google Scholar]
- Tornoe K, Hannibal J, Jensen TB, Georg B, Rickelt LF, Andreasen MB, Fahrenkrug J, Holst JJ. PACAP-(1–38) as neurotransmitter in the porcine adrenal glands. Am J Physiol Endocrin Metab. 2000;279:E1413–E1425. doi: 10.1152/ajpendo.2000.279.6.E1413. [DOI] [PubMed] [Google Scholar]
- Twitchell WA, Rane SG. Opioid peptide modulation of Ca2+-dependent K+ and voltage-activated Ca2+ currents in bovine adrenal chromaffin cells. Neuron. 1993;10:701–709. doi: 10.1016/0896-6273(93)90171-m. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Shimamoto N, Takahashi A, Fujino M. PACAP stimulates catecholamine release from adrenal medulla: a novel noncholinergic secretagogue. Am J Physiol. 1995;269:E903–E909. doi: 10.1152/ajpendo.1995.269.5.E903. [DOI] [PubMed] [Google Scholar]
- Wilson SP, Chang KJ, Viveros OH. Proportional secretion of opioid peptides and catecholamines from adrenal chromaffin cells in culture. J Neurosci. 1982;2:1150–1156. doi: 10.1523/JNEUROSCI.02-08-01150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]