Abstract
Recent observations have suggested that creatine supplementation might have a beneficial effect on glucoregulation in skeletal muscle. However, conclusive studies on the direct effects of creatine on glucose uptake and metabolism are lacking. The objective of this study was to investigate the effects of creatine supplementation on basal and insulin-stimulated glucose transporter (GLUT4) translocation, glucose uptake, glycogen content, glycogen synthesis, lactate production, glucose oxidation and AMP-activated protein kinase (AMPK) phosphorylation in L6 rat skeletal muscle cells. Four treatment groups were studied: control, insulin (100 nm), creatine (0.5 mm) and creatine + insulin. After 48 h of creatine supplementation the creatine and phosphocreatine contents of L6 myoblasts increased by ∼9.3- and ∼5.1-fold, respectively, but the ATP content of the cells was not affected. Insulin significantly increased 2-deoxyglucose uptake (∼1.9-fold), GLUT4 translocation (∼1.8-fold), the incorporation of D-[U-14C]glucose into glycogen (∼2.3-fold), lactate production (∼1.5-fold) and 14CO2 production (∼1.5-fold). Creatine neither altered the glycogen and GLUT4 contents of the cells nor the insulin-stimulated rates of 2-DG uptake, GLUT4 translocation, glycogen synthesis and glucose oxidation. However, creatine significantly reduced by ∼42% the basal rate of lactate production and increased by ∼40% the basal rate of 14CO2 production. This is in agreement with the ∼35% increase in citrate synthase activity and also with the ∼2-fold increase in the phosphorylation of both α-1 and α-2 isoforms of AMPK after creatine supplementation. We conclude that 48 h of creatine supplementation does not alter insulin-stimulated glucose uptake and glucose metabolism; however, it activates AMPK, shifts basal glucose metabolism towards oxidation and reduces lactate production in L6 rat skeletal muscle cells.
The physiological roles of creatine in the human body have been extensively investigated. Its main biochemical effect in skeletal muscle, usually described as the ‘energy shuttle’, is to transfer chemical energy from mitochondria, where ATP is produced, to the myofibrils (Wallimann et al. 1992; Ruggeri, 2000). In humans, approximately 95% of the total body creatine is found in skeletal muscle. More than 60% is in the form of phosphocreatine (PCr) and the remainder is stored in the non-phosphorylated form (Walker, 1979; Mesa et al. 2002). After Harris et al. (1992) demonstrated the efficacy of oral creatine intake for increasing skeletal muscle creatine content in humans, interest in the effects of oral creatine supplementation on skeletal muscle contractile performance and metabolism rapidly increased. It is now well established that the ingestion of a high dose (20–25 g per day) of oral creatine can rapidly (3–5 days) raise muscle total creatine content (Mesa et al. 2002). This elevation in muscle creatine storage has been associated with increased muscle power output during repeated short high-intensity exercise tasks (Greenhaff et al. 1993; Balsom et al. 1995) and enhanced effects of weight training on muscle volume and strength (Vandenberghe et al. 1997; American College of Sports Medicine, 2000). Additionally, It has been shown that the combination of creatine and carbohydrate supplements results in a greater postexercise muscle glycogen accumulation than carbohydrate alone (Robinson et al. 1999), suggesting that creatine could also exert an effect on peripheral glucose metabolism. Additional evidence suggesting that creatine supplementation might be effective in regulating peripheral glucose metabolism comes from a recent study on transgenic Huntington mice, which are hyperglycaemic. The addition of creatine to the diet of these mice significantly reduced hyperglycaemia, while improving the glucose response to intravenous glucose injection (Ferrante et al. 2000).
It has been suggested that the effects of creatine supplementation on glucose homeostasis may be due to an increase in insulin secretion (Gempel et al. 1996). Although some in vitro studies have indicated that creatine may increase insulin secretion modestly in the perfused rat pancreas (Alsever et al. 1970) and isolated mouse islets (Marco et al. 1976) or insulinoma cells (Gempel et al. 1996), evidence from in vivo human studies indicates that either one 5 g dose of creatine (Green et al. 1996a) or 3 days of creatine supplementation (Green et al. 1996b) does not alter insulin secretion. Therefore, we hypothesized that the glucoregulatory effect of creatine supplementation may be caused by a direct alteration in peripheral glucose metabolism independently of changes in insulin secretion.
Recently, it was reported that oral creatine supplementation increased by ∼40% the GLUT4 content in vastus lateralis muscle after rehabilitation training in subjects who had previously had one of their legs immobilized (Op't Eijnde et al. 2001a). It was also demonstrated that muscle glycogen and total creatine content were higher in creatine-supplemented subjects, but no data were presented regarding glucose uptake and other aspects of glucose metabolism in this study (Op't Eijnde et al. 2001a). In rats, it has also recently been reported that creatine supplementation reduces the PCr : TCr (total free creatine) ratio, suggesting an alteration in the energy state in muscle cells, but has no effect on glucose uptake in isolated plantaris muscles (Young & Young, 2002).
Energy and metabolic sensing in muscle cells have been attributed to AMP-activated protein kinase (AMPK) (Hardie et al. 1998; Winder, 2001), which has been shown to regulate glucose uptake and metabolism in skeletal muscle. An alteration of the energy state of the cell is likely to alter the activity of AMPK and the demand for glucose. Investigation of GLUT4 translocation and glucose uptake, as well as the fate of glucose via the pathways of glycogen synthesis, oxidation and lactate production, is crucial to characterizing the implications of altering the energy state of muscle cells by creatine supplementation. However, the possible effects of creatine supplementation on AMPK activation and the implications for glucose uptake and metabolism have not been fully investigated. This study was designed to enhance our understanding of the metabolic response of skeletal muscle with respect to glucose uptake and metabolism in response to creatine supplementation. Accordingly, we investigated the in vitro effects of short-term (48 h) creatine supplementation on glucose uptake, GLUT4 translocation, lactate production, glycogen synthesis, glucose oxidation, citrate synthase activity and AMPK phosphorylation in L6 rat skeletal muscle cells.
Methods
Chemicals
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) was purchased from Toronto Research Chemicals, Inc. Amyloglucosidase, creatine hydrate, creatine kinase (CK), cytochalasin B, DTNB (5,5′dithiobis(2-nitrobenzoic acid)), glycogen, hexokinase (HK)/glucose-6-P dehydrogenase (G6P-DH)-conjugated enzymes, 3-phospho-glyceric acid, 3-phospho-glycerate kinase (PGK), glyceraldehydes-3-phosphate dehydogenase (GPDH), lactate dehydrogenase (LDH)/pyruvate kinase (PK)-conjugated enzymes, O-phenylenediamine di-hydrochloride (OPD), oxaloacetic acid, phenylethylamine, phosphoenolpyruvate and triethanolamine were purchased from Sigma (St Louis, MO, USA), acetyl-CoA, ADP, ATP, NADH and NADP were from Bioshop Canada Inc., Human insulin (Humulin®R) from Eli Lilly (Canada, Inc.) and d-[U-14C]glucose and 3H-2-deoxyglucose were from Amersham (Quebec, Canada). α-MEM and all other cell culture components were from Wisent (Quebec, Canada). Anti-myc antibody 9E10 was purchased from Santa Cruz (Santa Cruz, CA, USA). All other chemicals were of the highest grade available.
Cell culture conditions for L6Glut4-myc myoblasts
For all experiments L6 rat skeletal muscle cells were grown in minimum essential medium (α-MEM) supplemented with 10% (v/v) fetal bovine serum in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Cells were transfected to stably overexpress GLUT4 harbouring a myc epitope on the first exofacial loop of the transporter. This facilitated accurate quantitative analysis of GLUT4 translocation in intact cells. Myoblasts were grown to confluence and the following experimental conditions were assigned: control, cells cultivated with medium not supplemented with creatine and not subsequently stimulated with insulin; insulin, cells cultivated with medium not supplemented with creatine but subsequently stimulated with insulin; creatine, cells cultivated with medium supplemented with creatine but not subsequently stimulated with insulin; creatine + insulin, cells cultivated with medium supplemented with creatine and subsequently stimulated with insulin. The final creatine concentration in all media was 0.5 mm. For all experiments, creatine-supplemented medium was changed every 24 h and the cultivation, either with or without creatine, was maintained for 48 h. Cells were serum-deprived for 4 h (in the continued presence of creatine) prior to all experimental manipulations. The final concentration of insulin in all experiments was 100 nm.
Determination of glycogen, creatine, phosphocreatine and ATP contents
L6Glut4-myc myoblasts were seeded in 6-well plates and grown to confluence. Subsequently, the cells were incubated in the absence or presence of creatine (0.5 mm final concentration). After 0, 24 and 48 h of creatine treatment, the cells were washed 5 times with cold phosphate-buffered saline (PBS) and lysed in ice-cold perchloric acid. Subsequently, the cell lysates were neutralized with triethanolamine–K2CO3 solution, filtered, kept on ice and immediately used for assays (Wahlefeld & Siedel, 1985; Heinz & Weiber, 1985). Glycogen content was determined with amylo-glucosidase, as previously described (Passonneau & Lauderdale, 1974; Keppler & Decker, 1974). Total free creatine (TCr) and phosphocreatine (PCr) were determined by enzymatic assays using lactate dehydrogenase (LDH)–pyruvate kinase (PK)–creatine kinase (CK) and glucose-6-phosphate dehydrogenase (G6P-DH)–hexokinase (HK)–creatine kinase (CK), respectively, as previously described (Harris et al. 1974; Wahlefeld et al. 1985; Heinz et al. 1985). The PCr : TCr ratio was calculated by dividing the values obtained for phosphocreatine and free creatine, respectively. Creatine content in aliquots of the PBS used after the fifth wash was also determined to ensure that creatine was completely removed from the incubation medium before cell lysis. Adenosine-5′-triphosphate (ATP) was determined in the neutralized cell extract by an enzymatic assay with 3-phosphoglycerate kinase (Jaworek et al. 1974). Aliquots of the cell lysates were used to determine the protein content of the samples.
Glucose uptake
L6 myoblasts were cultivated in 12-well plates with or without creatine for 48 h and serum starved for 4 h before being incubated for 20 min with or without insulin. Subsequently, myoblasts were washed twice and glucose transport was assayed in Hepes-buffered saline solution (140 mm NaCl, 20 mm Hepes-Na, 2.5 mm MgSO4, 1 mm CaCl2, 5 mm KCl, pH 7.4) containing 10 μm 2-deoxy-d-glucose (0.5 μCi ml−1 2-deoxy-d-[3H]glucose) as previously described (Somwar et al. 2002). The incubation medium was aspirated, the cells were washed with ice-cold saline, and 1 ml of NaOH (0.05 m) was added to each well. Cell lysates were transferred to scintillation vials for radioactivity counting. Non-specific uptake was determined in the presence of cytochalasin B (10 μm) and was subtracted from all values.
Determination of cell surface GLUT4-myc
The L6Glut4-myc cells are stably transfected to express GLUT4 tagged on its first exofacial loop with a myc epitope (received as a kind gift from Dr Amira Klip, The Hospital for Sick Children, Toronto). The exofacial location of the myc epitope on GLUT4 in these cells allows the analysis of GLUT4 localization in intact cells. GLUT4-myc levels at the cell surface were measured by an antibody-coupled colorimetric assay as previously described (Somwar et al. 2002). Briefly, after 4 h of serum starvation, cells were incubated for 20 min in the presence or absence of insulin. Subsequently, cells were quickly washed in ice-cold PBS and incubated with anti-c-myc antibody (9E10, 1 : 200 dilution) for 60 min at 4°C. Cells were washed and fixed in 3% paraformaldehyde for 3 min on ice. The fixative was neutralized by incubation in 10 mm glycine in ice-cold PBS for 10 min. Cells were blocked in 10% goat serum for 10 min and then incubated with horseradish peroxidase-conjugated donkey anti-mouse IgG (1 : 1000 dilution, 4°C) for 60 min. Cells were washed 5 times with ice-cold PBS and incubated for 30 min at room temperature with 1 ml of OPD reagent (0.4 mg ml−1O-phenylenediamine di-hydrochloride and 0.4 mg ml−1 urea hydrogen peroxide in 0.05 m phosphate citrate buffer) per well. The reaction was stopped by adding 0.25 ml of 3 m HCl. The supernatant was collected and the absorbance was measured at 492 nm.
Lactate production
After the 48 h creatine supplementation period, cells were serum starved for 4 h, then incubated with or without insulin for 2 h, after which the media were collected for lactate determination. Total lactate released into the medium was measured by the lactate oxidase assay using a lactate kit (Sigma Diagnostics).
Glycogen synthesis
Glycogen synthesis was assessed by the incorporation of d-[U-14C]glucose into glycogen as previously described (Cuendet et al. 1976). Briefly, cells were cultivated in 6-well plates in the absence or presence of creatine. After 4 h of serum starvation, cells were incubated for 2 h with medium containing 0.15 μCi ml−1 of d-[U-14C]glucose with or without insulin. Cells were then quickly washed with ice-cold PBS and lysed in 0.5 ml of KOH (1 m). Cell lysates were used for overnight glycogen precipitation with ethanol. Precipitated glycogen was dissolved in water and transferred to scintillation vials for radioactivity counting.
Glucose oxidation
Glucose oxidation was measured by the production of 14CO2 from d-[U-14C]glucose as previously described (Ceddia et al. 1999) with a few modifications. Briefly, cells were incubated for 2 h in 60 × 15 mm Petri dishes with medium containing 0.15 μCi ml−1d-[U-14C]glucose with or without insulin. Each Petri dish was sealed with parafilm which had a piece of Whatman paper taped facing the inside of the Petri dish. The Whatman paper was wetted with 100 μl of phenylethylamine–methanol (1 : 1) to trap the CO2 produced during the incubation period. After 2 h of incubation, 200 μl of H2SO4 (4 m) was added to the cells, which were then incubated for 1 h at 37°C. Finally, the pieces of Whatman paper were removed and transferred to scintillation vials for radioactivity counting.
Citrate synthase (EC.4.1.3.7) maximum activity
L6Glut4-myc myoblasts were seeded in 12-well plates and grown to confluence. Subsequently, the cells were incubated either in the absence or presence of creatine (0.5 mm final concentration). After 0, 24 and 48 h, the cells were washed 3 times with ice-cold PBS (1 ml well−1) and subsequently lysed in 200 μl of ice-cold extraction buffer containing Tris-HCl (50 mm), MgCl2 (1 mm), KCl (100 mm), sucrose (250 mm) and 2-mercaptoethanol (30 mm). The suspension was kept on ice and centrifuged (9000 g, 15 min, 4°C). The supernatant was collected and the pellet of cell debris was discarded. For the measurement of citrate synthase activity, the assay buffer (950 μl final volume) contained tris-hydroxymethyl-aminomethane (100 mm), DTNB (0.2 mm), acetyl-CoA (0.1 mm) and Triton X-100 (0.05% v/v). The reaction was initiated by the addition of 20 μl of the enzyme extract and 30 μl of oxaloacetate (10 mm final concentration) as previously described (Srere et al. 1963; Alp et al. 1976; Ceddia et al. 2000). Absorbance at 412 nm (25°C) was then measured during 10 min. An aliquot of the enzyme extract was used to determine the protein content of each sample. The maximum activity of citrate synthase was expressed as nmoles of substrate converted to product per minute per mg of protein (nmol min−1 mg−1 of protein).
Western blot determination of GLUT4, P-AMPKα and P ACC
Cells were grown in 6-well plates and after 48 h in the presence or absence of creatine they were incubated for 30 min with or without insulin as indicated and then washed twice with ice-cold PBS. AICAR (1 mm, 30 min) was used as a positive control for acetyl-CoA carboxylase (ACC) phosphorylation. Subsequently, cells were lysed in 1 × SDS sample buffer (62.5 mm) Tris-HCl pH 6.8, 2% w/v SDS, 10% glycerol, 50 mm DTT, 0.01% w/v bromophenol blue, and protease and phosphatase inhibitors (1 μm Na3VO4, 1 μm leupeptin, 1 μm pepstatin, 1 μm okadaic acid and 1 μm PMSF), passed through a syringe several times and heated (65°C, 5 min). Aliquots (15 μl) of cell lysates were then subjected to SDS-PAGE (8% resolving gels), and then transferred to PVDF membranes (Bio-Rad Laboratories). The phosphorylation of AMPK and ACC was determined by using phospho-AMPK(Thr172)-specific (1 : 1000 dilution, Cell Signalling Technology) and phospho-ACC(S79)-specific (1 : 500 dilution, Upstate) antibodies, respectively. Equal loading of samples was confirmed by coomassie blue staining of the gels.
Determination of phosphorylation of the α-1 and α-2 subunits of AMPK
The phosphorylation of the α-1 and α-2 subunits of AMPK was determined after immunoprecipitation with specific antibodies (Santa Cruz Biotechnology, Inc.) against the α-1 and α-2 catalytic subunits as previously described (Stapleton et al. 1996) and protein A sepharose (Amersham Biosciences AB) beads. Immunoprecipitates were washed 5 times with lysis buffer (20 mm Tris pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mmβ-glycerophosphate, 1 mm Na3VO4, 1 μg ml−1 leupeptin, 1 mm PMSF) and the pellet was resuspended in 40 μl of SDS sample buffer and centrifuged for 30 s. Twenty microlitres of the supernatant was used for Western blot using phospho-AMPK(Thr172)-specific antibody (1 : 200 dilution, Cell Signalling Technology).
Statistical analysis
Data are presented as means ±s.e.m. Statistical analyses were performed by t test or one- or two-way analysis of variance (ANOVA) with Tukey-Kramer multiple comparison test or Bonferroni posthoc tests as indicated in the figure legends. The level of significance was set at P < 0.05.
Results
Creatine, phosphocreatine and ATP contents
The creatine and PCr contents of the cells increased from 6.75 ± 0.73 and 4.5 ± 0.36 μg (mg protein)−1 before supplementation to 46.39 ± 1.23 and 22.10 ± 1.48 μg (mg protein)−1 after 24 h and 62.36 ± 2.04 and 23.0 ± 0.78 μg (mg protein)−1 after 48 h of creatine supplementation (Fig. 1A), respectively. The expansion of the creatine and PCr contents, however, was not accompanied by an increase in the total ATP concentration in L6 muscle cells after creatine supplementation (5.40 ± 0.67 versus 5.07 ± 0.28 nmol (mg protein)−1 in the control and creatine supplemented cells, respectively) (Fig. 1B).
Figure 1. Creatine (Cr), phosphocreatine (PCr) (A) and ATP contents (B) of L6Glut4-myc myoblasts after incubation for 0, 24 and 48 h either in the absence (control) or presence of 0.5 mm creatine (creatine).
Data are representative of four independent experiments, with quadruplicate samples in each experiment. Data are expressed as means ±s.e.m.*P < 0.05versus PCr control at 0, 24 and 48 h; **P < 0.05versus Cr control at 0, 24 and 48 h; #P < 0.05versus Cr control at 0, 24 and 48 h and Cr at 24 h (two-way ANOVA).
Glucose uptake, GLUT4 translocation, glycogen synthesis, and GLUT4 and glycogen contents
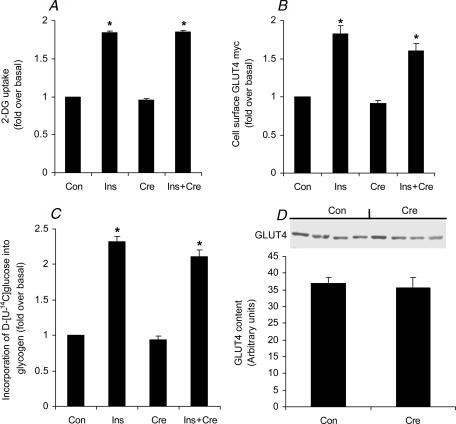
In the present study, glucose uptake (Fig. 2A), GLUT4 translocation (Fig. 2B) and the incorporation of d-[U-14C]glucose into glycogen (Fig. 2C) increased by ∼1.9-fold, ∼1.8-fold and 2.3-fold, respectively, after insulin stimulation. AICAR (1 mm, 30 min) was used as a positive control and it significantly increased glucose uptake by ∼1.7-fold (1.5 ± 0.19 and 2.53 ± 0.17 nmol (μg protein)−1 for the control and AICAR-treated cells, respectively) in L6 muscle cells. However, creatine supplementation neither altered the basal nor the insulin-stimulated rates of glucose uptake, GLUT4 translocation and glycogen synthesis. Furthermore, the GLUT4 protein content (Fig. 2D) was not altered by creatine supplementation in L6 rat skeletal muscle cells. The glycogen content of the cells increased from 3.38 ± 0.32 μmol (mg protein)−1 before supplementation to 4.36 ± 0.22 and 4.63 ± 0.55 μmol (mg protein)−1 after 24 and 48 h of creatine supplementation, respectively. However, there was no significant difference between the control and creatine-supplemented cells (Fig. 3).
Figure 2. Effects of creatine (Cre), insulin (Ins) and insulin plus creatine (Ins + Cre) on 2-deoxyglucose uptake (A), GLTU4 translocation (B) and glycogen synthesis (C).
D, representative blot of GLUT4 (55 kDa) in L6 rat skeletal muscle cells. Prior to insulin stimulation (20 min, 100 nm where indicated), cells were cultivated for 48 h either in the presence or absence of creatine. Data representative of 4 independent experiments with quadruplicate samples in each experiment (A, B and C). For GLUT4 content we performed 4 independent experiments with duplicates in each experiment (D). *P < 0.05versus control (Con) and creatine (one-way ANOVA).
Figure 3. Glycogen content of L6 rat skeletal muscle cells incubated for 0, 24 and 48 h either in the absence (control) or presence of creatine.
Data representative of 4 independent experiments with quadruplicate samples in each experiment. *P < 0.05versus control and creatine time 0 h (two-way ANOVA).
Lactate production, glucose oxidation and citrate synthase maximal activity
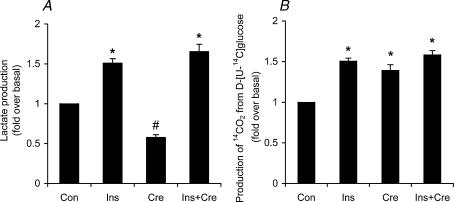
In the presence of insulin, the L6 myoblasts elicited a significant ∼1.5-fold increase in lactate production compared to control cells. Interestingly, creatine supplementation reduced the basal production of lactate by ∼42%, but did not alter the insulin-stimulated lactate production by the cells (Fig. 4A). Concomitantly, creatine supplementation significantly increased (1.4-fold) the basal production of 14CO2 from d-[U-14C]glucose but did not alter the effect of insulin on this variable (Fig. 4B). These results are supported by a significant increase (∼30%) in citrate synthase activity after creatine supplementation (Fig. 5). In fact, the maximal activity of citrate synthase increased from 21.22 ± 0.84 nmol min−1 (mg protein)−1 before supplementation to 23.40 ± 2.48 and 27.54 ± 1.4 nmol min−1 (mg protein)−1 after 24 and 48 h of creatine supplementation, respectively. Although there was a tendency towards an increase after 24 h, it only reached statistically significant values after 48 h of creatine supplementation (Fig. 5).
Figure 4. Effects of creatine (Cre), insulin (Ins) and insulin plus creatine (Ins + Cre) on the production of lactate (A) and 14CO2 (B) from d-[U-14C]glucose in L6 rat skeletal muscle cells.
Prior to insulin stimulation (2 h, 100 nm), cells were cultivated for 48 h either in the absence or presence of creatine (0.5 mm). Data representative of 4 independent experiments with quadruplicates in each experiment. *P < 0.05versus control (Con). #P < 0.05versus control, insulin and insulin + creatine (one-way ANOVA).
Figure 5. Maximum activity of citrate synthase in L6 rat skeletal muscle cells incubated for 0, 24 and 48 h either in the absence (control) or presence of creatine.
Data representative of 4 independent experiments with quadruplicates in each experiment. *P < 0.05versus control 0, 24 and 48 h (two-way ANOVA).
AMPK phosphorylation
Insulin did not alter AMPK phosphorylation in L6 skeletal muscle cells (Fig. 6A); however, AMPK phosphorylation was significantly increased (∼1.8-fold) under basal or insulin stimulated conditions after creatine supplementation (Fig. 6A). The increase in AMPK activity in our experiments was confirmed by the ∼2.2-fold increase in phosphorylation of a well-characterized substrate of AMPK, namely ACC (Fig. 6B), after creatine supplementation. Additionally, we used AICAR as a positive control for AMPK and ACC phosphorylation. The magnitude of ACC phosphorylation caused by creatine supplementation was similar to that obtained after the incubation of L6 muscle cells with AICAR (Fig. 6B). We also investigated if creatine regulated the phosphorylation of both α-1 and α-2 isoforms of AMPK. To do this, we immunoprecipitated α-1 and α-2 using isoform-specific antibody and then Western blotted the immunoprecipitates with phospho-AMPK(Thr172)-specific antibodies. Interestingly, phosphorylation of both isoforms was significantly increased by creatine supplementation. We detected a significant ∼2-fold increase in the phosphorylation state of both AMPK α-1 and α-2 isoforms, with no alteration in total AMPK α-1 and α-2 protein contents (Figs 6C and 6D).
Figure 6. Representative blots and their respective densitometric quantification of the effects of insulin (Ins) and creatine (Cre) on AMPK and ACC phosphorylation.
P-AMPKα (62 kDa, A), P-ACC (257 kDa, B), P-AMPK α-1 (62 kDa) and AMPK α-1 (62 kDa) (C), P-AMPK α-2 and AMPK α-2 (D) protein content. P-AMPK α-1 (C) and P-AMPK α-2 (D) are expressed as relative to AMPK α-1 and AMPK α-2 contents, respectively. Data representative of 4 independent experiments with duplicates in each experiment. AICAR (1 mm, 30 min) was used as a positive control for ACC phosphorylation. *P < 0.05versus control and insulin (A and B, ANOVA) and versus control (C and D, t test).
Discussion
Oral creatine supplementation in humans (Mesa et al. 2002) and rats (Young & Young, 2002) has been proven to increase the contents of creatine and phosphocreatine in skeletal muscle. In humans, creatine is usually administered as a dosage regimen consisting of a loading phase of 20 g day−1 (four times 5 g) for 5–7 days and a maintenance dosage of 3–5 g day−1 thereafter (Mesa et al. 2002). It has been reported that 1 h after a single oral dose (5 g) of creatine the serum concentration of this substance rises from 0.05 to 0.1 mmol l−1 (fasting serum values) to 0.6–0.8 mmol l−1 (Perski et al. 2003). In response to a 20 g oral dose, plasma creatine concentration reaches peak values of 2.17 mmol l−1 (50-fold increase) after 2.5 h of ingestion (Mesa et al. 2002). The uptake of creatine by muscle cells presents Michaelis-Menten kinetics, with a maximum rate of creatine uptake (Vmax) obtained at concentrations higher than 0.3–0.4 mmol l−1 (Sora et al. 1994). Based on these data, we decided to supplement the incubation medium with 0.5 mm l−1 of creatine, which corresponds to the concentration achieved in humans after a single 5 g oral dose of creatine. This value is also within the range that seems to elicit the maximal rate of muscle creatine uptake (Sora et al. 1994). We incubated the L6 muscle cells in the presence of 0.5 mm creatine for 48 h, since it has been established that the majority of muscle creatine accumulation is maximal in the first 2 days of oral supplementation (Kamber et al. 1999). The effectiveness of our supplementation regimen was confirmed by the ∼9.6- and ∼5.4-fold increases in creatine and PCr contents of the cells after 48 h of creatine supplementation, respectively. In fact, these increases were significantly higher than those reported in humans (15–20% increase in muscle total creatine content) after supplementation (Febbraio et al. 1995; Hultman et al. 1996; Casey et al. 1996; Robinson et al. 1999). Therefore, although we have attempted to recreate the physiological milieu as closely as possible, care must be taken when extrapolating our data regarding the effects of increased intracellular creatine and PCr on glucose uptake and metabolism to intact muscle tissue.
We did not find any significant alteration in GLUT4 content, basal or insulin-stimulated GLUT4 translocation or glucose uptake and glycogen content in these cells upon creatine supplementation. Additionally, contrary to what has been observed in humans (Robinson et al. 1999; Op't Eijnde et al. 2001a) and rats (Op't Eijnde et al. 2001b), we did not observe any alteration in either basal or insulin-stimulated glycogen synthesis after creatine supplementation. Interestingly, creatine supplementation has been reported to cause an increase in glycogen accumulation in humans only in muscles that are subject to exercise (Robinson et al. 1999; Op't Eijnde et al. 2001a). This suggests that some muscle contraction is necessary in order to observe any additive effect of creatine on glycogen synthesis in muscle. This could be one reason why creatine supplementation did not alter glycogen content and the basal or insulin-stimulated rates of glycogen synthesis in our in vitro model. In fact, our experiments were performed in non-contracting cells and the results obtained are in agreement with what has been reported in non-exercising rats in which creatine supplementation did not alter basal and insulin-stimulated glucose uptake (Young & Young, 2002), glycogen synthase activity (Op't Eijnde et al. 2001b; Rooney et al. 2002) and the incorporation of [14C]-glucose into glycogen (Op't Eijnde et al. 2001b) in skeletal muscle.
The GLUT4 and glycogen contents of the cells were not altered by creatine supplementation, and although we did not observe any effect of creatine on basal and insulin-stimulated GLUT4 translocation and glucose uptake, L6 muscle cells supplemented with creatine significantly increased (∼40%) their basal production of 14CO2 from d-[U-14C]glucose. This suggests that creatine supplementation shifted basal glucose metabolism towards oxidation, which is compatible with the significant reduction (∼42%) in basal lactate production observed in the creatine-supplemented cells. These observations are also in agreement with data obtained from mitochondria isolated from muscles of creatine-supplemented human subjects (Walsh et al. 2001) and from mice cardiac skinned muscle fibres incubated with creatine (Saks et al. 2000). In both models, oxygraphic measurements point towards an increase in mitochondrial respiration in the presence of creatine (Saks et al. 2000; Walsh et al. 2001). It has been demonstrated that in the presence of creatine the mitochondrial isoform of creatine kinase (mi-CK) uses ATP to produce ADP, which is channelled directly to adenine nucleotide translocase for regulation of respiration (Saks et al. 2000). This means that creatine, by activating mi-CK, changes the energy state of the cell and directly controls the mitochondrial energy production (Saks et al. 2000). The increase in CO2 production that we observed in our creatine-supplemented L6 muscle cells could have resulted from the elevation of creatine concentration in the cell, leading to higher mi-CK activity and increased oxidative phosphorylation. It has been repeatedly reported that creatine supplementation increases total creatine concentration in muscle in both humans (Greenhaff et al. 1994; Green et al. 1996a,b; Hultman et al. 1996; Steenge et al. 1998; Robinson et al. 1999) and rats (Brannon et al. 1997; McMillen et al. 2001; Young & Young, 2002). Interestingly, the expansion of the total creatine (TCr) pool in muscle is due to an increase in both the free and phosphorylated (PCr) forms of creatine. However, PCr does not seem to increase in the same proportion as free creatine in the muscle cell, and hence the PCr : TCr ratio actually falls after supplementation (Greenhaff et al. 1994; Green et al. 1996a,b; Hultman et al. 1996; Steenge et al. 1998). This was also the case in our experiments, since the PCr : TCr ratios were 0.67 and 0.37 for control and 48 h creatine-supplemented cells, respectively. Considering that the PCr : TCr ratio reflects cellular energetics (Connet, 1988; Op't Eijnde et al. 2001a,b), a decrease in PCr : TCr might have been interpreted as an altered energy state in our resting muscle cells, which in turn might have increased their basal glucose oxidation rate. This is compatible with our data showing increases (∼30%) in both glucose oxidation and maximal activity of citrate synthase after 48 h of creatine supplementation.
The AMP-activated protein kinase (AMPK) has been established as a protein that monitors the metabolic and energetic states of the muscle cells (Hardie et al. 1998; Winder, 2001). Additionally, it has been shown that AMPK activity is modulated by the PCr : TCr ratio (Pontikos et al. 1998). A fall in the PCr : TCr ratio within the cell would be expected therefore to cause activation of AMPK as a result of the release of inhibition exerted by PCr (Pontikos et al. 1998). Interestingly, we found an ∼1.8-fold increase in AMPK phosphorylation. Previous studies have reported specific induction of AMPK α-2 isoform activity in skeletal muscle by exercise (Wojtaszewski et al. 2000; Fuji et al. 2000; Stephens et al. 2002), suggesting that this isoform may be involved in the metabolic responses observed in contracting skeletal muscles. A more detailed analysis of changes in activity of the catalytic AMPK isoforms in our study revealed an ∼2-fold increase in phosphorylation of both α-1 and α-2 isoforms in creatine-supplemented muscle cells. Additionally, we demonstrated that similar amounts of the α-1 and α-2 AMPK isoforms were present in L6 muscle cells. Therefore, the metabolic effects we observed in the present study with non-contracting L6 muscle cells are likely to be due to the activation of both α-1 and α-2 AMPK isoforms. This is again different from exercise conditions, which lead to the degradation of intracellular glycogen content (Fuji et al. 2000) and other energy substrates and may cause isoform-specific activation of the catalytic subunits of AMPK. In fact, the exercise-induced increase in the α-2-specific activity of AMPK has been reported to be intensity dependent (Wojtaszewski et al. 2000; Fuji et al. 2000) and inversely correlated with glycogen depletion in skeletal muscle (Fuji et al. 2000; Stephens et al. 2002), suggesting that the glycogen content of muscle cells may indeed play an important role in triggering the multiple cellular effects of AMPK.
AMPK activation has been suggested to play a key role in increasing glucose uptake in contracting skeletal muscle (Zierath, 2002). Yet we do not observe an increase in glucose uptake following creatine supplementation, despite activation of AMPK. In order to demonstrate that L6 muscle cells have the ability to react to increased AMPK activity by elevating glucose transport, we treated cells with AICAR and observed a significant increase (∼1.7-fold) in glucose uptake. However, it has been reported that AMPK activity and glucose uptake may be completely dissociated in contracting perfused slow-twitch rat muscle (Derave et al. 2000). It seems that the major factor that dissociates AMPK activation from an increase in glucose uptake in skeletal muscle is the glycogen content of the cells (Derave et al. 2000). In our experiments the glycogen content was similar in the control and creatine-supplemented conditions and this may be the reason why the increased phosphorylation of AMPK in our creatine-supplemented cells was not followed by an increment in glucose uptake. Furthermore, there are many instances where activation of a given mediator of glucose uptake does not lead to glucose uptake, e.g. PI3-kinase (Jiang et al. 1998). It may also be that creatine activates AMPK in a compartment-specific manner such that only a subset of AMPK not involved in stimulating glucose uptake is activated.
One intriguing point in our results is the fact that creatine increased the basal glucose oxidation rate and this may have resulted from AMPK activation; however, when the glycolytic flux was acutely increased by insulin in the creatine-supplemented cells the rate of glucose oxidation was not affected at all by creatine, despite a similar increase in AMPK phosphorylation. At the present time we do not have a precise explanation for this finding. However, it is possible that the increase in glucose oxidation observed under basal conditions, which was compensated for by a proportional reduction in lactate production, was already sufficient to meet the new energetic demands of the cells in order to adjust for the reduction in the PCr : TCr ratio caused by creatine supplementation. Therefore, no additional increases in glucose oxidation were necessary, even though the glycolytic flux in the cells was acutely increased by insulin stimulation.
In summary, our study demonstrates that 2-day treatment of L6 rat skeletal muscle cells with creatine increased the proportion of glucose being oxidized and caused a corresponding reduction in lactate production (Fig. 7) in L6 rat skeletal muscle cells. The ability of creatine to phosphorylate and activate AMPK may be responsible for this effect. Although creatine supplementation caused an increase in AMPK activity and GLUT4 and glycogen contents, the ability of insulin to stimulate GLUT4 translocation, glucose uptake and glycogen synthesis was not affected in these cells.
Figure 7. Schematic representation of the effects of creatine on glucose uptake and metabolism in L6 rat skeletal muscle cells.
Creatine supplementation did not alter either basal or insulin-stimulated GLUT4 translocation (1), glucose uptake (2), or glycogen synthesis (3). The GLUT4 and glycogen contents of the cells were not altered by creatine supplementation either. However, the basal production of lactate was reduced while the maximum activity of citrate synthase and the production of CO2 were increased after creatine supplementation. ⇑= increase; ⇓= reduction; ⇔= no effect; PM = plasma membrane; CT = creatine transporter.
Acknowledgments
We thank the Canadian Diabetes Association for funding this research (G.S. is the recipient of a scholarship in honor of the late Mary A. Bodington and R.B.C. is the recipient of a postdoctoral fellowship in honor of the late Norman J. Newell). Thanks are due to Dr Amira Klip for the kind gift of L6Glut4-myc cells and GLUT4 antibody.
References
- Alp PR, Newsholme EA, Zamit VA. Activities of citrate synthase and NAD+-linked isocitrate dehydrogenase in muscles from vertebrates and invertebrates. Biochem J. 1976;154:689–700. doi: 10.1042/bj1540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsever RN, Georg RH, Sussman KE. Stimulation of insulin secretion by guanidinoacetic acid and other guanidinine derivatives. Endocrinology. 1970;86:332–336. doi: 10.1210/endo-86-2-332. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. [DOI] [PubMed] [Google Scholar]
- Balsom PD, Soderlund K, Sjodin B, Ekblom B. Skeletal muscle metabolism during short duration high-intensity exercise: influence of creatine supplementation. Acta Physiol Scand. 1995;154:303–310. doi: 10.1111/j.1748-1716.1995.tb09914.x. [DOI] [PubMed] [Google Scholar]
- Brannon TA, Adams GR, Connif CL, Baldwin KM. Effects of creatine loading and training on running performance and biochemical properties of rat skeletal muscle. Med Sci Sports Exerc. 1997;29:489–495. doi: 10.1097/00005768-199704000-00010. [DOI] [PubMed] [Google Scholar]
- Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am J Physiol. 1996;271:E31–E37. doi: 10.1152/ajpendo.1996.271.1.E31. [DOI] [PubMed] [Google Scholar]
- Ceddia RB, William WN, Jr, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and metabolism. Int J Obes. 1999;23:75–82. doi: 10.1038/sj.ijo.0800762. [DOI] [PubMed] [Google Scholar]
- Ceddia RB, William WN, Jr, Lima FB, Flandin P, Curi R, Giacobino JP. Leptin stimulates uncoupling protein-2 mRNA expression and Krebs cycle activity and inhibits lipid synthesis in isolated rat white adipocytes. Eur J Biochem. 2000;267:5952–5958. doi: 10.1046/j.1432-1327.2000.01664.x. [DOI] [PubMed] [Google Scholar]
- Connet RJ. Analysis of metabolic control: New insights using a scale creatine kinase model. Am J Physiol. 1988;254:R949–R959. doi: 10.1152/ajpregu.1988.254.6.R949. [DOI] [PubMed] [Google Scholar]
- Cuendet GS, Loten GE, Jeanrenaud B, Renold E. Decreased basal, non-insulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J Clin Invest. 1976;58:1078–1088. doi: 10.1172/JCI108559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihleman J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Flanagan TR, Snow RJ, Zhao S, Carey MF. Effect of creatine supplementation on intramuscular TCr, metabolism and performance during intermittent, supramaximal exercise in humans. Acta Physiol Scand. 1995;155:387–395. doi: 10.1111/j.1748-1716.1995.tb09988.x. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci. 2000;15:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaiser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorel A, Goodyear LJ. Exercise induces isoforms-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Comm. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Gempel K, Brdizyczka D, Kaddurah-Daouk R, Wallimann T, Kaufhold P, Gerbitz KD. The creatine analog cyclocreatine increases insulin secretion in INS-1 cells via K+ channel independent mechanism. Diabetologia. 1996;39(suppl.):A31. [Google Scholar]
- Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL. Carbohydrate feeding augments skeletal muscle creatine accumulation during creatine supplementation in man. Am J Physiol. 1996a;271:E821–E826. doi: 10.1152/ajpendo.1996.271.5.E821. [DOI] [PubMed] [Google Scholar]
- Green AL, Simpson EJ, Littlewood JJ, Macdonald IA, Greenhaff PL. Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiol Scand. 1996b;158:195–202. doi: 10.1046/j.1365-201X.1996.528300000.x. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Bodin K, Soderlund K, Hultman E. The effect of oral creatine supplementation on muscle phosphocreatine resynthesis. Am J Physiol. 1994;266:E725–E730. doi: 10.1152/ajpendo.1994.266.5.E725. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Casey A, Short AH, Harris R, Soderlund K, Hultman E. Influence of oral creatine supplementation on muscle torque during repeated bouts of maximal voluntary exercise in man. Clin Sci. 1993;84:565–571. doi: 10.1042/cs0840565. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the Eukaryotic Cell. Ann Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Laboratory Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci. 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Heinz F, Weiber H. Creatine phosphate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 3. Weinheim: VCH-Verlagsgesellschaft; 1985. pp. 507–514. [Google Scholar]
- Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in man. J Appl Physiol. 1996;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- Jaworek D, Gruber W, Bergmeyer HU. Adenosine-5′-triphosphate. Determination with 3-phosphoglycerate kinase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2. New York: Academic Press Inc.; 1974. pp. 2097–2101. [Google Scholar]
- Jiang T, Sweeney G, Rudolf MT, Klip A, Traynor-Kaplan A, Tsien RY. Membrane-permeant esters of phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:11017–11024. doi: 10.1074/jbc.273.18.11017. [DOI] [PubMed] [Google Scholar]
- Kamber M, Koster M, Kreis R, Walker G, Boesch C, Hoppeler H. Creatine supplementation – part I: performance, clinical chemistry, and muscle volume. Med Sci Sports Exerc. 1999;31:1763–1769. doi: 10.1097/00005768-199912000-00011. [DOI] [PubMed] [Google Scholar]
- Keppler D, Decker K. Glycogen. determination with amyloglucosidase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2. New York: Academic Press Inc.; 1974. pp. 1196–1201. [Google Scholar]
- McMillen J, Donovan CM, Messer JI, Willis W. Energetic driving forces are maintained in resting rat skeletal muscle after dietary creatine supplementation. J Appl Physiol. 2001;90:62–66. doi: 10.1152/jappl.2001.90.1.62. [DOI] [PubMed] [Google Scholar]
- Marco J, Calle C, Hedo JA, Villanueva ML. Glucagon-releasing activity of guanidine compounds in mouse pancreatic islets. FEBS Lett. 1976;64:52–54. doi: 10.1016/0014-5793(76)80246-3. [DOI] [PubMed] [Google Scholar]
- Mesa JLM, Ruiz JR, Gonzalez-Gross MM, Sainz AG, Garzon MJC. Oral creatine supplementation and skeletal muscle metabolism in physical exercise. Sports Med. 2002;32:903–944. doi: 10.2165/00007256-200232140-00003. [DOI] [PubMed] [Google Scholar]
- Op't Eijnde B, Richter EA, Henquin JC, Kiens B, Hespel P. Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiol Scand. 2001b;171:169–176. doi: 10.1046/j.1365-201x.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- Op't Eijnde B, Urso B, Richter EA, Greenhaff PL, Hespel P. Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes. 2001a;50:18–23. doi: 10.2337/diabetes.50.1.18. [DOI] [PubMed] [Google Scholar]
- Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974;60:405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- Perski AM, Muller M, Derendorf H, Grant M, Brazeau GA, Hochhaus G. Single- and multiple-dose pharmacokinetics of oral creatine. J Clin Pharmacol. 2003;43:29–37. doi: 10.1177/0091270002239703. [DOI] [PubMed] [Google Scholar]
- Pontikos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J. 1998;17:1688–1699. doi: 10.1093/emboj/17.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TM, Sewell DA, Hultman E, Greenhaff PL. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J Appl Physiol. 1999;87:598–604. doi: 10.1152/jappl.1999.87.2.598. [DOI] [PubMed] [Google Scholar]
- Rooney K, Bryson J, Phuyal J, Denyer G, Caterson I, Thompson C. Creatine supplementation alters insulin secretion and glucose homeostasis in vivo. Metabolism. 2002;51:518–522. doi: 10.1053/meta.2002.31330. [DOI] [PubMed] [Google Scholar]
- Ruggeri P. Factors modifying creatine accumulation in human skeletal muscle. In: Paoletti R, Poli A, Jackson AS, editors. Creatine: From Basic Science to Clinical Applications. London: Kluwer Academic Publishers; 2000. pp. 59–63. [Google Scholar]
- Saks VA, Kongas O, Vendelin M, Kay L. Role of the creatine/phosphocreatine system in the regulation of mitochondrial respiration. Acta Physiol Scand. 2000;168:635–641. doi: 10.1046/j.1365-201x.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- Somwar R, Koterski S, Sweeney G, Sciotti R, Djuric S, Berg C, Trevillian J, Scherer PE, Rondinone CM, Klip A. A dominant-negative p38 MAPK mutant an novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J Biol Chem. 2002;277:50386–50395. doi: 10.1074/jbc.M205277200. [DOI] [PubMed] [Google Scholar]
- Sora I, Richman J, Santoro G, Wei HB, Wang Y, Vanderah T, Horvath R, Nguyen M, Waite S, Roeske WR, Yamamura HI. The cloning and expression of a human creatine transporter. Biochem Biophys Res Commun. 1994;204:419–427. doi: 10.1006/bbrc.1994.2475. [DOI] [PubMed] [Google Scholar]
- Srere PA, Brazil H, Gonen L. The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem Scand. 1963;17:S129–S134. [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michel BJ, Teh T, House CM, Fernadez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Steenge GR, Lambourne J, Casey A, Macdonald IA, Greenhaff PL. The stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am J Physiol. 1998;275:E974–E979. doi: 10.1152/ajpendo.1998.275.6.E974. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, Canny BJ, Michel BJ, Kemo BE, McConell GK. Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Am J Physiol. 2002;282:E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- Vandenberghe K, Goris M, Van Heckle P, Van Leemputte M, Vangerven L, Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training. J Appl Physiol. 1997;83:2055–2063. doi: 10.1152/jappl.1997.83.6.2055. [DOI] [PubMed] [Google Scholar]
- Wahlefeld AW, Siedel J. Creatine and creatinine. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 3. Weinheim: VCH-Verlagsgesellschaft; 1985. pp. 488–507. [Google Scholar]
- Walker JB. Creatine: Biosynthesis, regulation and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Tonkonogy M, Soderland K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978. doi: 10.1111/j.1469-7793.2001.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91:1017–1028. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Young RE. The effect of creatine supplementation on glucose uptake in rat skeletal muscle. Life Sci. 2002;71:1731–1737. doi: 10.1016/s0024-3205(02)01941-0. [DOI] [PubMed] [Google Scholar]
- Zierath JR. Exercise effects of muscle insulin signaling and action. Invited review: Exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol. 2002;93:773–781. doi: 10.1152/japplphysiol.00126.2002. [DOI] [PubMed] [Google Scholar]