Abstract
Postischaemic acute renal failure (ARF) is influenced by sex. Na+,K+-ATPase (NKA) plays a crucial role in the pathogenesis of postischaemic ARF. We tested the impact of sex on mRNA, protein expression, cellular distribution and enzyme activity of NKA following renal ischaemia–reperfusion (I-R) injury. The left renal pedicle of uninephrectomized female (F) and male (M) Wistar rats was clamped for 55 min followed by 2 h (T2) and 16 h (T16) of reperfusion. Uninephrectomized, sham-operated F and M rats served as controls (n= 6 per group). Blood urea nitrogen, serum creatinine and renal histology were evaluated to detect the severity of postischaemic ARF. mRNA expression of NKA α1 and β1 subunits were detected by RT-PCR. The effect of I-R on cellular distribution was compared by Triton X-100 extraction. Cellular proteins were divided into Triton-insoluble and Triton-soluble fractions and assessed by Western blot. NKA enzyme activity was also determined. After the ischaemic insult blood urea nitrogen and serum creatinine were higher and renal histology showed more rapid progression in M versus F (P < 0.05). mRNA expression of the NKA α1 subunit decreased in I-R groups versus controls, but was higher in F versus M both in control and I-R groups (P < 0.05). However, protein levels of the NKA α1 subunit in total tissue homogenate did not differ in controls, but were higher in F versus M in I-R groups (P < 0.05). Triton X-100 extractability was lower in F versus M at T16 (P < 0.05). NKA enzyme activity was the same in controls, but was higher in F versus M in I-R groups (T2: 14.9 ± 2.3 versus 9.15 ± 2.21 U) (T16: 11.7 ± 4.1 versus 5.65 ± 2.3 U; P < 0.05). mRNA and protein expression of the NKA β1 subunit did not differ between F and M in any of the protocol. We concluded that NKA is more protected from the detrimental effects of postischaemic injury in females. Higher mRNA and protein expression of the NKA α1 subunit and higher enzyme activity might be additional contributing factors to the improved postischaemic renal function of female rats.
Ischaemia-induced acute renal failure (ARF) is characterized by impaired renal function, reduced glomerular filtration and tubular sodium reabsorption, which all have partly been related to the functional disorders of Na+,K+-ATPase (NKA) (Alejandro et al. 1995; Kwon et al. 2000).
NKA plays a pivotal role in the active transport of certain solutes and the maintenance of intracellular electrolyte homeostasis (Skou & Esmann, 1992). The physiological localization of NKA to the basolateral membrane is regulated by direct interactions with membrane-associated cytoskeletal proteins (Woroniecki et al. 2003). This polar distribution of NKA is essential for efficient enzyme function and sodium reabsorption by proximal tubular cells (Molitoris et al. 1991).
Renal ischaemia induces loss of tubular cell polarity (Brezis & Epstein, 1993), which is associated with a time-dependent translocation of NKA from the basolateral into the apical membrane domain of proximal tubular cells (Molitoris, 1992). For NKA to be translocated it must first dissociate from its actin-based cytoskeleton (Bidmon et al. 2000). Triton X-100 extraction fractionates the cellular pool of NKA into an insoluble pellet (cytoskeletal-associated fraction) and a soluble supernatant by simple differential centrifugation. This distribution has been used in several studies as a reproducible marker for postischaemic injury (Aufricht et al. 2002). Moreover, decreased NKA expression during I-R injury indicates that besides altered cellular distribution, impaired NKA synthesis could also play a role in the inefficient Na+ reabsorption and Na+ transport in postischaemic kidneys (Van Why et al. 1994).
Our previous study reported sexual dimorphism following I-R-induced ARF (Müller et al. 2002) in uninephrectomized rats. We demonstrated that female gender and female sex hormones improved survival and attenuated renal damage. The molecular and cellular bases for this sex difference need further investigation.
Regarding the male/female disparity in the susceptibility to renal ischaemic damage and the central importance of the NKA in renal physiology, we investigated the sexual differences in the postischaemic alterations of NKA. mRNA expression, protein level, cellular distribution of NKA α1 and β1 subunits and the enzyme activity were determined in a rat model of renal I-R injury-induced ARF.
Methods
Animal description and care
Experiments were performed on sexually mature, female (F) (weighing 240 ± 30 g) and male (M) (weighing 300 ± 35 g) Wistar rats. All experimental protocols were in compliance with the guidelines of the Committee on the Care and Use of Laboratory Animals of the Council on Animal Care at the Semmelweis University of Budapest, Hungary and University of Essen, Germany. Rats were fed a standard laboratory diet and water ad libitum.
Experimental protocol
General anaesthesia was induced by intraperitoneal administration of 50 mg kg−1 pentobarbital sodium (Nembutal, Abbott Laboratories, Budapest, Hungary). Body temperature was maintained at 37°C on a heating pad throughout the anaesthesia period. Renal ischaemia was accomplished by cross-clamping the left renal artery and vein for 55 min with an atraumatic vascular clamp. During the period of ischaemia the abdomen was temporarily closed to avoid excessive loss of fluids. Before the end of the period of ischaemia the clamp was withdrawn, the contralateral kidney was removed and the abdomen was closed.
F and M rats were re-anaesthetized (i.p., 50 mg kg−1 pentobarbital sodium). Blood samples were collected from the abdominal aorta and the kidney was removed at 2 h (T2), and 16 h (T16) of reperfusion (n= 6 per group). The animals were then exsanguinated. Uninephrectomized, sham-operated F and M rats served as controls (n= 6 per group). Kidney samples were immediately snap-frozen in liquid nitrogen or fixed in 4% buffered formalin (pH 7.4) for further investigations.
Measurements of blood urea nitrogen, serum creatinine, and serum sodium and potassium levels
All these parameters were determined photometrically with commercially available kits (Diagnosticum Ltd, Budapest, Hungary) on a Hitachi-712 automated spectrophotometer.
Renal histopathology
Paraffin sections of the excised kidney were stained with haematoxylin–eosin and periodic acid–Schiff reagent. Samples were coded and semiquantitatively evaluated in blind tests by light microscopy on a scale from 0 to 4 as follows: 0 none, 1 mild, 2 moderate, 3 massive, 4 severe. Leukocyte infiltration, nucleus atypia, vacuolization and hyalinization in the tubular cells, tubular dissolving, coming off and lack of the tubular cells were used for more detailed quantification as previously described (Heemann et al. 2000).
RT-PCR reaction
All reagents enzymes and isolation kits were purchased from Quiagen GmBH, Hilden, Germany.
Total RNA was extracted by RNeasy total RNA Isolation Kit as instructed by the manufacturer. First-strand cDNA was synthesized from 1 μg total RNA from each kidney sample, in master mix (5 × PCR buffer, 200 U SuperScript II RNase H− reverse transcriptase, 20 U RNaseOUT inhibitor and 3.75 pg μl−1 oligo dT12–18 primer).
PCR was performed in master mix (10 × PCR buffer, 2 mm MgCl2, 2 mm dNTPs, 1.5 U AmpliTaq DNA polymerase and 0.5 μm forward (F) and reverse (R) primers). The amplification profile consisted of denaturation at 94°C for 15 s, annealing 58°C (NKA α1 and β1) for 15 s, and extension 72°C for 30 s for 35 amplification cycles. Primer pairs for NKA α1 and β1 subunits were as follows. GenBank NKA α1 (NM 012504), sequence F: 5′-AGA TTT GAG CCG AGG CCT AAC ACC-3′; sequence R: 5′-TCC GCC CTT CAC CTC CAC CAG AT-3′ (product length: 418 bp). NKA β1 (NM 013113), sequence F: 5′-GCC CCG CCA GGA TTG ACA C-3′; sequence R: 5′-CTC ATC GCG CTT GCC AGT G-3′ (product length: 444 bp). PCR products were analysed on 2.5% agarose gel stained with ethidium bromide.
The RT samples were also used to generate glyceraldeyde-3-phosphate dehydrogenase (GAPDH) PCR products and their amount was considered as internal control. GAPDH forward and reverse primers were as follows: F: 5′-GGT GAA GGT CGG AGT CAA CG-3′; R: 5′-CTC ATC GCG CTT GCC AGT G-3′ (product length: 496 bp). The amplification profile was the same as for NKA, except that the annealing temperature was 55°C.
Tissue homogenization and cellular protein fractionation
One hundred milligrams of renal tissue was homogenized in chilled extraction buffer containing 60 mm Hepes, 1 mm EDTA, 1 mm EGTA, 100 mm NaCl, 0.5 mm phenylmethylsulphonyl fluoride, 0.75 mg l−1 leupeptine and 0.1 mm DTT, using a Potter Elvehejm homogeniser. The total tissue homogenate (TOT) was centrifuged at 680 g for 5 min at 4°C to pellet cell nuclei and large fragments.
Triton X-100 (0.1%) was added to the extraction buffer for cellular protein fractionation. One hundred milligrams of renal tissue was homogenized in this buffer and then centrifuged at 35000 g for 15 min at 4°C to separate the Triton-soluble supernatant fraction (SUP = non-cytoskeletal) from the Triton-insoluble pellet fraction (PEL = cytoskeletal). PEL was resuspended in extraction buffer, and all fractions (TOT, PEL and SUP) were saved at –80°C.
Western blot analysis
Protein determinations were performed in duplicate by Bradford analysis using bovine serum albumin as a standard. All reagents for PAGE and Western blot were purchased from Sigma Chemical Co. (MO, USA).
Samples were solubilized in a buffer of 12.5 mm Tris-HCl, pH 6.7, containing 4.0% SDS, 1 mm EDTA, 15% glycerol and 0.01% bromphenolblue. Samples (40 μg) were electrophoretically resolved on a 12.5% polyacrylamide gel and transferred to nitrocellulose membranes, which were blocked in 5% non-fat dry milk (NFDM) with 0.1% Tween-20 for 1 h at room temperature. The membranes were incubated with polyclonal antibodies of NKA α1 subunit (UBI, Lake Placid, NY, USA) diluted to 1 : 750 and β1 subunit (UBI) diluted to 1 : 1000 in 5% NFDM for 1 h at room temperature. The membranes were washed with Tween-phosphate-buffered saline, and incubated in peroxidase-conjugated goat anti-rabbit secondary antibody (DAKO, Glostrup, Denmark) diluted to 1 : 2000 in 5% NFDM for 30 min at room temperature.
Blots were developed with enhanced chemiluminescence Western blotting detection (AP-Biotech, Buckinghamshire, UK). Computerized densitometry of the specific bands were analysed with Gel-Pro Analyser 3.1 software. The values were normalized to an internal standard and expressed as the relative optical density.
NKA activity
NKA activity was measured from the renal TOT used for Western blot analysis. Twenty micrograms of protein was added to the previously described reaction solution (Vásárhelyi et al. 1997). The final reaction volume was 225 μg. The linear rate of NADH oxidation was monitored for 150 s at 340 nm on a Hitachi-721 automated analyser. NKA activity was calculated from absorbance changes based on ouabain-sensitive ATP degradation. One unit of NKA represents 1 nmol of ATP degradation per milligram of protein per hour.
Statistical analysis
The data were analysed on STATISTICA.6 software (StatSoft Inc., USA) using ANOVA for multiple comparisons. If the criteria for parametric tests were not met, comparisons were made using Kruskal-Wallis ANOVA on ranks. Histological changes were analysed using the Kruskal-Wallis test followed by multiple pairwise comparisons according to Dunn's test. The criterion for significance was P < 0.05 in all experiments. The data are presented as means ±s.d.
Results
Blood urea nitrogen and serum creatinine, sodium and potassium levels after I-R injury
Blood urea nitrogen and serum creatinine levels did not differ between control F and M rats. At T2 and at T16 both blood urea nitrogen and serum creatinine were significantly lower in F versus M (P < 0.05) (Table 1). There were no differences between the sexes in serum Na and K levels in controls and at T2, but at T16 serum K levels were significantly higher in M versus F (P < 0.05).
Table 1.
Effect of I-R injury on blood urea nitrogen (BUN), and serum creatinine, sodium and potassium levels of F and M rats
| Group | Serum BUN (mm) | Serum creatinine (μm) | Serum Na (mm) | Serum K (mm) | |
|---|---|---|---|---|---|
| Control | F | 5.0 ± 0.4+§ | 53 ± 3+§ | 140 ± 4 § | 4.1 ± 0.4+§ |
| M | 6.2 ± 0.7+§ | 57 ± 7+§ | 144 ± 2 § | 4.7 ± 0.1+§ | |
| T2 | F | 7.5 ± 0.7*§ | 85 ± 5*§ | 138 ± 1 § | 5.1 ± 0.5 |
| M | 10.2 ± 1.0 § | 107 ± 7 § | 141 ± 3 § | 5.2 ± 0.6 § | |
| T16 | F | 27.2 ± 3.6* | 228 ± 7* | 133 ± 3 | 5.3 ± 0.4* |
| M | 37.2 ± 8.1 | 276 ± 7* | 134 ± 4 | 7.0 ± 0.9 |
BUN, serum creatinine, sodium and potassium levels were determined in serum blood of controls and at T2 and T16 hours of reperfusion following 55 min of renal ischaemia in F and M rats (n = 6 per group). Data are given as mean ±s.d..
P < 0.05 versus T2;
P < 0.05 versus T16.
P < 0.05 versus M;
Fifty-five minutes of warm renal ischaemia induced ARF as reflected by increased blood urea nitrogen, serum creatinine and serum potassium and by decreased sodium concentrations in both F and M (control versus T2 and T2 versus T16, P < 0.05).
Renal histopathological changes after I-R injury
To detect the severity of postischaemic ARF, kidney sections were examined for histopathological changes. Histological evaluation of kidneys from uninephrectomized rats revealed normal kidney structure in both males and females. At T2 there were no sex differences in these histological parameters (tubular necrosis: F, 1.0 (1–1) versus M, 1.0 (1–2); tubular dissolving: F, 1.0 (1–1) versus M, 1.0 (1–1); leukocyte infiltration: F, 1.0 (1–2) versus M, 1.0 (1–1)) (Fig. 1A and B). At T16 the extent of tubular necrosis and tubular dissolving was less severe in F versus M kidney samples (tubular necrosis: F, 2.5 (2–4) versus M, 4.0 (4–4); tubular dissolving: F, 3.0 (1–4) versus M, 4.0 (4–4), P < 0.05), while the extent of leucocyte infiltration did not differ significantly (F, 2.0 (1–2) versus M, 2.0 (2–3)) (Fig. 1C and D).
Figure 1. Histopathological changes in postischaemic kidneys of F and M rats.
Histopathological changes in postischaemic kidneys were determined and evaluated in PAS-stained kidneys of T2 female (A) and T2 male (B), and of T16 female (C) and T16 male (D) rats.
There was a continuous progression in the extent of tubular necrosis, tubular dissolving and peritubular leukocyte infiltration until T16 in both F and M (control versus T2, and T2 versus T16, P < 0.05).
There were no relevant differences between the sexes with regard to the other histological parameters investigated. None of the kidney samples presented glomerular histological changes, interstitial oedema or interstitial lymphocyte infiltration in any of the groups.
mRNA expression of NKA α1 and β1 subunits after I-R injury in kidney
Figure 2 shows mRNA expression of NKA α1 and β1 subunits and GAPDH using RT-PCR as detected in control, T2 and T16 kidneys of F and M animals. Significant differences between males and females were already obvious in control groups: mRNA expression of NKA α1 subunit was nearly twofold higher in F versus M (P < 0.05). At T2 the difference was even greater, since α1 mRNA expression was threefold higher in F versus M (P < 0.05). At T16 α1 mRNA expression was still significantly higher in F as compared to M (P < 0.05).
Figure 2. Effect of I-R injury on mRNA expression of Na+,K+-ATPase α1 and β1 subunits in F and M rat kidney.
mRNA expression of Na+,K+-ATPase α1 and β1 subunits were determined in kidney samples from controls, and at T2 and T16 hours of reperfusion following 55 min of renal ischaemia in F and M rats (n= 6 per group). Top panel, examples of RT-PCR analysis of Na+,K+-ATPase α1 and β1 subunits and GAPDH in kidney. A and B, results are given as the ratio of intensity of Na+,K+-ATPase α1 or β1 subunit mRNA to GAPDH mRNA. □,F; ▪,M. Data are given as mean ±s.d. *P < 0.05 versus M; +P < 0.05versus T2; §P < 0.05versus T16.
α1 mRNA expression temporarily decreased at T2 versus controls in both F and M; however, the decrement was twofold greater in M versus F (P < 0.05). At T16, α1 mRNA expression increased compared with T2 in both F and M (P < 0.05) (Fig. 2A).
Changes in mRNA expression of the NKA β1 subunit showed different dynamics to those of α1, since β1 mRNA decreased progressively to T16 in both F and M (control versus T2: P < 0.05; T2 versus T16: P < 0.05) without any sex differences (Fig. 2B).
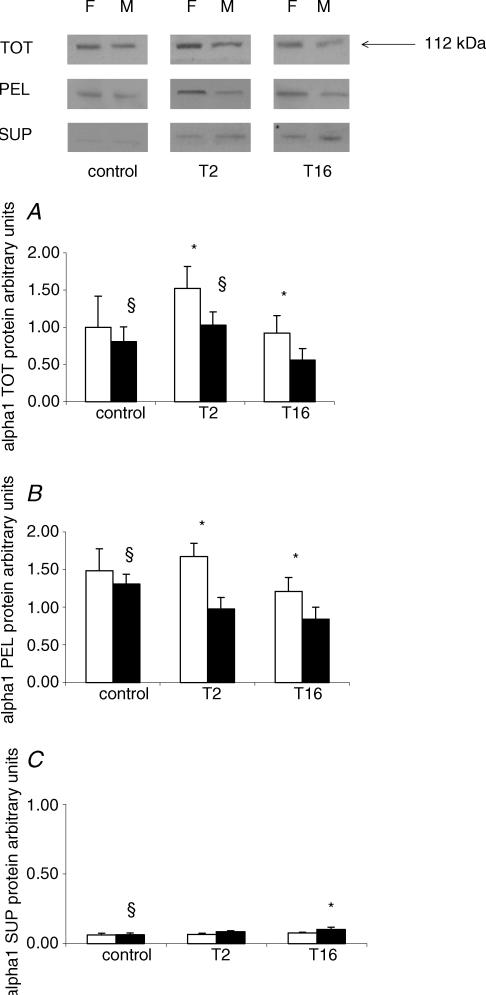
Protein expression and cellular distribution of NKA α1 and β1 subunits after I-R injury
To compare the effects of I-R injury on the synthesis of NKA in males and females, the protein levels of NKA α1 and β1 subunits were quantified using Western blot analysis in TOT tissue homogenate. To determine ischaemia-induced cellular redistribution of NKA, Triton X-100 extraction was used to divide TOT homogenate into Triton-soluble SUP and Triton-insoluble PEL fractions and protein levels of NKA α1 and β1 subunits were quantified.
α1 in TOT.In TOT homogenate of uninephrectomized, sham-operated control groups there was no difference between M and F in the protein levels of the α1 subunit. At T2 and at T16, the protein levels of NKA α1 were significantly higher in F versus M (P < 0.05). Changes of NKA α1 protein levels showed different dynamics in the TOT homogenates of F and M. While the α1 protein level did not change significantly during the investigated period in F, in M a marked decrease was detected at T16 versus control and T2 (P < 0.05) (Fig. 3A).
Figure 3. Effect of I-R injury on protein levels and cellular distribution of Na+,K+-ATPase α1 in F and M rat kidney.
Protein levels of the Na+,K+-ATPase α1 subunit were determined in total kidney tissue homogenate (TOT), the Triton-soluble supernatant (SUP) and the Triton-insoluble pellet (PEL) fraction of F and M rats in controls, and at T2 and T16 hours of reperfusion following 55 min of renal ischaemia (n= 6 per group). Top panel, examples of Western blot analysis of Na+,K+-ATPase α1 in TOT, PEL and SUP fractions. A–C, results for Na+,K+-ATPase α1 protein levels in TOT, PEL and SUP were normalized to an internal standard. □,F; ▪,M. Data are given as mean ±s.d.*P < 0.05versus M; §P < 0.05versus T16.
α1 in Triton-insoluble PEL. In the PEL fraction the changes were similar to those of the TOT homogenate. In control kidneys there were no differences between M and F in the protein level of the NKA α1 subunit. At T2 and T16, NKA α1 protein levels were higher in F versus M (P < 0.05). Similarly to TOT, in the PEL fraction NKA α1 protein levels did not change in F, while in M a significant decrease was detected at T16 as compared to controls (P < 0.05) (Fig. 3B).
α1 in Triton-soluble SUP. In the SUP fraction there was no difference between M and F in controls and T2. However, at T16 the protein level of the NKA α1 subunit was lower in F as compared to M (P < 0.05). While in M, the α1 protein level was higher in T16 versus controls, in F no significant changes were observed during the investigated period (P < 0.05) (Fig. 3C).
The expression of the NKA β1 subunit protein changed in a different manner to that of α1 in all cellular fractions (TOT, PEL and SUP).
β1 in TOT. At T2 the β1 protein level was reduced compared with controls in both F and M (P < 0.05). At T16, the protein levels of the NKA β1 subunit increased and returned to the control value (P < 0.05, T2 versus T16 in F and M). Differences between F and M were not observed at any investigated time point (Fig. 4A).
Figure 4. Effect of I-R injury on protein levels and cellular distribution of Na+,K+-ATPase β1 in F and M rat kidney.
Protein levels of the Na+,K+-ATPase β1 subunit were determined in total kidney tissue homogenate (TOT), Triton-soluble supernatant (SUP) and Triton-insoluble pellet (PEL) fractions of F and M rats in controls, and at T2 and T16 hours of reperfusion following 55 min of renal ischaemia (n= 6 per group). Top panel, examples of Western blot analysis of Na+,K+-ATPase β1 in TOT, PEL and SUP fractions. A–C, results for Na+,K+-ATPase β1 protein levels in TOT, PEL and SUP were normalized to an internal standard. □,F; ▪,M. Data are given as mean ±s.d.*P < 0.05versus M; †P < 0.05versus T2; §P < 0.05versus T16.
β1 in Triton-insoluble PEL and in Triton-soluble SUP. (The β1 protein level showed no significant changes during the investigated period; however, the tendency of change correlated with that of TOT. Differences between M and F were not detected at any time point (Fig. 4B and C).
NKA enzyme activity
The changes of enzyme activity followed those of NKA α1 protein levels in TOT and PEL. In controls the enzyme activity was the same in F and M. At T2 significantly higher NKA activity was detected in F compared to M (F versus M: 14.9 ± 2.3 versus 9.15 ± 2.21 U; P < 0.05). At T16 the difference between M and F still existed (F versus M: 11.7 ± 4.1 versus 5.65 ± 2.3 U; P < 0.05) (Fig. 5).
Figure 5. Effect of I-R injury on Na+,K+-ATPase enzyme activity in F and M rat kidney.
Na+,K+-ATPase activity was determined in kidney samples from controls, and at T2 and at T16 hours of reperfusion following 55 min of renal ischaemia in F (□) and M (▪) rats (n= 6 per group). Data are given as mean ±s.d.*P < 0.05versus M; +P < 0.05versus T2; §P < 0.05versus T16.
In M, NKA activity decreased progressively to T16 (control versus T2 versus T16: 15.89 ± 8.0 U versus 9.15 ± 2.21 U versus 5.65 ± 3.0 U; P < 0.05), while in F enzyme activity at T2 temporarily increased.
Discussion
Postischaemic renal injury is a leading cause of ARF and despite the recent progress in treatment the associated mortality and morbidity remain dismally high (Lameire & Vanholder, 2001). The pathogenesis of ARF is clearly multifactorial; however, altered function of NKA is considered to be a central factor of impaired cellular sodium and water homeostasis. Ischaemia was reported to induce a marked decrease in NKA activity by Van Why et al. (1994), Kim et al. (1995), Kwon et al. (2000) and Coux et al. (2001); however, all of these observations were made on male rats.
Female sex hormones are known, tissue-specific modulators of NKA (Dzurba et al. 1997; Labombarda et al. 2002); however, data about the kidney are limited and still controversial (Rafestin-Oblin et al. 1991; Mujais et al. 1993). Androgens have been studied even less. Male sex hormones have been shown either to stimulate NKA activity (Shima, 1992) or to have no effect (Kurihara et al. 1996; Sandhu et al. 1997).
We have recently reported that sex and sex hormones influence the susceptibility to renal I-R injury, since female rats have better survival rates and improved renal recovery following ischaemic insult. We found that in male animals immaturity and castration significantly prolonged the survival. In contrast, in females ovariectomy, testosterone treatment and immaturity had no effect on postischaemic survival. Therefore we are tempted to speculate that it is not testosterone or oestrogen in itself, but the oestrogen/androgen ratio that plays a role in the different postischaemic susceptibility of male and female kidneys. (Müller et al. 2002). Considering the central role of NKA in the pathophysiology of ischaemia-induced ARF and the lack of data about its sex-specific changes, we investigated the differences between males and females in the alterations of NKA following I-R injury.
We are the first to show that the mRNA and protein expression, cellular distribution and enzyme activity of NKA differs in males and females after 55 min of warm ischaemia followed by reperfusion. We found that the mRNA expression of the NKA α1 subunit in females was already nearly twice that of males in sham-operated control animals and that this difference existed throughout the whole investigated period. At T2, α1 mRNA expression decreased in both sexes; however, the decrement was twofold greater in males compared to females. These results suggest that sex considerably influences the postischaemic mRNA expression of the NKA α1 subunit. This difference could be explained by decreased degradation, increased stability or transcription of mRNA in females. However, the stabilization of NKA α1 mRNA is more presumptive, since previous studies show that oestrogens might stabilize the mRNA of several genes after an ischaemic insult (Ruohola et al. 1999; Sasaki, 2003). Oestrogens were reported to prevent the access to endonucleases by binding to the target sites in the 3′ untranslated region (UTR) region of mRNA (Arao et al. 2002).
Protein levels correlated with mRNA expression after ischaemic insult with respect to the 8 h half-life time of the NKA α1 subunit. In the postischaemic reperfusion period a significant difference between males and females in α1 protein level was detected at each investigated time point. While in males α1 protein levels significantly decreased after the ischaemic insult, there was no remarkable change in females. This raises the possibility that impaired translation of NKA α1 protein occurs in males, but not in females. Transient ischaemia results in a long-lasting suppression of global protein synthesis. This is caused by the inhibition of translation initiation (Martin de la Vega et al. 2001) as a result of the phosphorylation of eukaryotic initiation factors; however, the exact molecular mechanism is very poorly understood. Akama & McEwen (2003) observed that oestrogen stimulates the rapid activation of specific signal transduction pathways, which initiates protein translation by alleviating the translational repression of initiation factor binding proteins. Thus it can be hypothesized that after the ischaemic insult protein synthesis is less impaired in females due to these mechanisms, while in males ischaemia induces a marked inhibition of protein synthesis.
On the other hand, it is also conceivable that the higher mRNA expression of α1 subunits in control females after ischaemic injury might serve as a pool for the translation of α1 subunit mRNA and potentiates an improvement in protein synthesis after I-R injury. Both of these hypotheses are conceivable, but further investigations are needed to understand the exact mechanism.
Besides inhibition of protein synthesis and NKA activity, transient relocation of NKA into the apical membrane domain is also a well-known feature of postischaemic ARF (Molitoris et al. 1991). Several clinical sequels such as renal sodium loss and reduced glomerular filtration rate can be explained by redistribution. For NKA to be translocated it must first be detached from its cytoskeletal anchorage (Molitoris, 1991), which has been showed by Triton X-100 extractibility method (Molitoris, 1992; Aufricht et al. 2002). In line with previous reports on male rats we also showed that the distribution of NKA changed to the supernatant fraction following the ischaemic insult in both males and females. However, the redistribution differed between males and females. While in males a marked translocation was observed into the apical membrane domain, in females the majority of NKA α1 subunit protein remained at the basal membrane. Moreover, we found that NKA α1 protein redistribution was less in postischaemic female kidneys than in males. This raises the possibility that the observed sexual disparity in the NKA α1 protein levels of total tissue homogenates could be attributed mostly to differences of pellet fraction.
We found a direct correlation between NKA α1 protein levels in the Triton-insoluble fractions and total tissue homogenates with enzyme activity. Simultaneously with higher α1 subunit protein levels, higher enzyme activity was detected in females than in males at each investigated time point following I-R injury. In line with the previous observations of Kajiwara et al. (1996) and Coux et al. (2002) obtained in males, we also detected a significant decrease in NKA activity in male rats, while in females enzyme activity did not change at all. Our results also indicate that the expressed α1 subunit is functionally active, since an increase of NKA α1 protein level parallels an elevation of NKA activity.
It is noteworthy that, in contrast to the α1 subunit, no difference between the sexes was detected either in mRNA or protein expression in the β1 subunit. Even the dynamics of alterations of the β1 subunit were different from those of α1, since β1 mRNA reached its lowest level at a later time point of reperfusion. Pressley (1988) and Bertorello et al. (1999) described the expression of both NKA α1 and β1 subunits as being independently regulated, which could explain their distinctive reactions to renal I-R injury.
One can speculate about the possible role of female sex in the alterations of the NKA α1 subunit following I-R injury. In accordance with our previous results, we confirmed that the structural damage of kidney tissue is less severe in female than in male rats. This raises the possibility that in females ischaemia leads to relatively milder ischaemic damage and therefore NKA is also less affected than in males. Due to this milder damage the redistribution of NKA is less pronounced, and therefore – as was seen in our model – more NKA remains functionally active in female than in male rats.
In addition, sex hormones influence the expression of several cytokines and vasoregulatory factors, which are released following I-R injury. Some of them are reportedly implicated in the regulation of NKA (Husted et al. 2000; Qayyum et al. 2001). We have previously shown that male rats have higher prepro-endothelin levels than females after renal I-R injury (Müller et al. 2002). Endothelin was demonstrated to inhibit NKA in kidney (Greger, 2000), therefore it is tempting to speculate that higher endothelin levels in males might contribute to the relatively lower NKA activity. However, the role of several other agents and mechanisms, including different lipid peroxidation, membrane fluidity, etc. should also be taken into consideration.
In summary we demonstrated that NKA is more stable in females and it is protected from the detrimental effects of postischaemic injury. We reported that in female kidneys the mRNA and protein expression of α1 subunits and NKA activity is higher than in males following I-R injury.
These sex-specific alterations of NKA might be additional important characteristics that explain sex differences in postischaemic ARF. However, further investigations are needed to elucidate whether the different dynamics of alterations of NKA in females play a role in their improved adaptation to renal I-R injury.
Acknowledgments
This work was partly supported by OTKA-F042563-TO31986, ETT T-184-291/03, OMFB-TET CZ-4/02 and FKFP-0134/2001. The technical assistance of Edit Végh is gratefully acknowledged. V. Müller and A. Szabó are recipients of the Bolyai Scholarships.
References
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejandro VS, Nelson WJ, Huie P, Sibley RK, Dafoe D, Kuo P, Scandling JD, Jr, Myers BD. Postischemic injury, delayed function and Na+/K+-ATPase distribution in the transplanted kidney. Kidney Int. 1995;48:1308–1315. doi: 10.1038/ki.1995.415. [DOI] [PubMed] [Google Scholar]
- Arao Y, Kikuchi A, Ikeda K, Nomoto S, Horiguchi H, Kayama F. A+U-rich-element RNA-binding factor 1/heterogeneous nuclear ribonucleoprotein D gene expression is regulated by oestrogen in the rat uterus. Biochem J. 2002;361:125–132. doi: 10.1042/0264-6021:3610125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufricht C, Bidmon B, Ruffingshofer D, Regele H, Herkner K, Siegel NJ, Kashgarian M, Van Why S. Ischemic conditioning prevents Na,K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res. 2002;51:722–727. doi: 10.1203/00006450-200206000-00010. [DOI] [PubMed] [Google Scholar]
- Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol. 1999;276:L20–L27. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- Bidmonn B, Endemann M, Mueller T, Arbeiter K, Herkner K, Aufricht C. HSP-70 repairs tubule cell structure after renal ischemia. Kidney Int. 2000;58:2400–2407. doi: 10.1046/j.1523-1755.2000.00423.x. [DOI] [PubMed] [Google Scholar]
- Brezis M, Epstein FH. Cellular mechanisms of acute ischemic injury in the kidney. Annu Rev Med. 1993;44:27–37. doi: 10.1146/annurev.me.44.020193.000331. [DOI] [PubMed] [Google Scholar]
- Coux G, Trumper L, Elias MM. Cortical Na+,K+-ATPase activity, abundance, and distribution after in vivo renal ischemia without reperfusion in rats. Nephron. 2001;89:82–89. doi: 10.1159/000046048. [DOI] [PubMed] [Google Scholar]
- Coux G, Trumper L, Elias MM. Renal function and cortical Na++K+-ATPase activity, abundance and distribution after ischaemia-reperfusion in rats. Biochim Biophys Acta. 2002;1586:71–80. doi: 10.1016/s0925-4439(01)00087-4. [DOI] [PubMed] [Google Scholar]
- Dzurba A, Ziegelhoffer A, Vrbjar N, Styk J, Slezak J. Estradiol modulates the sodium pump in the heart sarcolemma. Mol Cell Biochem. 1997;176:113–118. [PubMed] [Google Scholar]
- Greger R. Physiology of renal sodium transport. Am J Med Sci. 2000;319:51–62. doi: 10.1097/00000441-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Heemann U, Szabo A, Hamar P, Muller V, Witzke O, Lutz J, Philipp T. Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury: possible connection to an interleukin-6-dependent pathway. Am J Pathol. 2000;156:287–293. doi: 10.1016/S0002-9440(10)64729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted RF, Sigmund RD, Stokes JB. Mechanisms of inactivation of the action of aldosterone on collecting duct by TGF-beta. Am J Physiol Renal Physiol. 2000;278:F425–F4733. doi: 10.1152/ajprenal.2000.278.3.F425. [DOI] [PubMed] [Google Scholar]
- Kajiwara I, Kawamura K, Hiratsuka Y, Takebayashi S. The influence of oxygen free radical scavengers on the reduction of membrane-bound Na+-K+-ATPase activity induced by ischemia/reperfusion injury in the canine kidney. Nephron. 1996;72:637–643. doi: 10.1159/000188952. [DOI] [PubMed] [Google Scholar]
- Kim YK, Woo JS, Kim YH, Jung JS, Kim BS, Lee SH. Effect of renal ischemia on organic compound transport in rabbit kidney proximal tubule. Pharmacol Toxicol. 1995;77:121–129. doi: 10.1111/j.1600-0773.1995.tb01000.x. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Frokiaer J, Han JS, Knepper MA, Nielsen S. Decreased abundance of major Na+ transporters in kidneys of rats with ischemia-induced acute renal failure. Am J Physiol Renal Physiol. 2000;278:F925–F939. doi: 10.1152/ajprenal.2000.278.6.F925. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Gonzalez SL, Gonzalez DM, Guennoun R, Schumacher M, de Nicola AF. Cellular basis for progesterone neuroprotection in the injured spinal cord. J Neurotrauma. 2002;19:343–355. doi: 10.1089/089771502753594918. [DOI] [PubMed] [Google Scholar]
- Lameire N, Vanholder R. Pathophysiologic features and prevention of human and experimental acute tubular necrosis. J Am Soc Nephrol. 2001;12:S20–S32. [PubMed] [Google Scholar]
- Martin de la Vega C, Burda J, Nemethova M, Quevedo C, Alcazar A, Martin ME, Danielisova V, Fando JL, Salinas M. Possible mechanisms involved in the down-regulation of translation during transient global ischaemia in the rat brain. Biochem J. 2001;357:819–826. doi: 10.1042/0264-6021:3570819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA. The potential role of ischemia in renal disease progression. Kidney Int. 1992;36:S21–S25. [PubMed] [Google Scholar]
- Molitoris BA, Geerdes A, McIntosh JR. Dissociation and redistribution of Na+,K+-ATPase from its surface membrane actin cytoskeletal complex during cellular ATP depletion. J Clin Invest. 1991;88:462–469. doi: 10.1172/JCI115326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujais SK, Nora NA, Chen Y. Regulation of the renal Na:K pump: role of progesterone. J Am Soc Nephrol. 1993;3:1488–1495. doi: 10.1681/ASN.V381488. [DOI] [PubMed] [Google Scholar]
- Müller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G, Tulassay T, Szabo AJ. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 2002;62:1364–1371. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- Pressley TA. Ion concentration-dependent regulation of Na,K-pump abundance. J Membr Biol. 1988;105:187–195. doi: 10.1007/BF01870996. [DOI] [PubMed] [Google Scholar]
- Qayyum I, Zubrow AB, Ashraf QM, Kubin J, Delivoria-Papadopoulos M, Mishra OP. Nitration as a mechanism of Na+,K+-ATPase modification during hypoxia in the cerebral cortex of the guinea pig fetus. Neurochem Res. 2001;26:1163–1169. doi: 10.1023/a:1012331108641. [DOI] [PubMed] [Google Scholar]
- Rafestin-Oblin ME, Couette B, Barlet-Bas C, Cheval L, Viger A, Doucet A. Renal action of progesterone and 18-substituted derivatives. Am J Physiol. 1991;260:F828–F832. doi: 10.1152/ajprenal.1991.260.6.F828. [DOI] [PubMed] [Google Scholar]
- Ruohola JK, Valve EM, Karkkainen MJ, Joukov V, Alitalo K, Harkonen PL. Vascular endothelial growth factors are differentially regulated by steroid hormones and antiestrogens in breast cancer cells. Mol Cell Endocrinol. 1999;149:29–40. doi: 10.1016/s0303-7207(99)00003-9. [DOI] [PubMed] [Google Scholar]
- Sasaki R. Pleiotropic functions of erythropoietin. Intern Med. 2003;42:142–149. doi: 10.2169/internalmedicine.42.142. [DOI] [PubMed] [Google Scholar]
- Shima S. Effects of androgen treatment on adenylate cyclase system in rat hepatic membranes. Pharmacol Toxicol. 1992;70:429–433. doi: 10.1111/j.1600-0773.1992.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Skou JC, Esmann M. The Na,K-ATPase. J Bioenerg Biomembr. 1992;24:249–261. doi: 10.1007/BF00768846. [DOI] [PubMed] [Google Scholar]
- Van Why SK, Mann AS, Ardito T, Siegel NJ, Kashgarian M. Expression and molecular regulation of Na+-K+-ATPase after renal ischemia. Am J Physiol. 1994;267:F75–F85. doi: 10.1152/ajprenal.1994.267.1.F75. [DOI] [PubMed] [Google Scholar]
- Vásárhelyi B, Ver Á, Szabo T, Tulassay T. Measurement of Na+/K+-ATPase activity with an automated analyzer. Clin Chem. 1997;43:1986–1987. [PubMed] [Google Scholar]
- Woroniecki R, Ferdinand JR, Morrow JS, Devarajan P. Dissociation of spectrin-ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia. Am J Physiol Renal Physiol. 2003;284:F358–F364. doi: 10.1152/ajprenal.00100.2002. [DOI] [PubMed] [Google Scholar]