Abstract
Stretching the stomach wall in young healthy subjects causes an increase in muscle sympathetic nerve activity and in blood pressure, the gastrovascular reflex. We compared healthy elderly subjects with healthy young subjects to find out whether the gastrovascular reflex attenuates in normal ageing and we studied whether there was a difference in autonomic function or gastric compliance that could explain this possible attenuation. Muscle sympathetic nerve activity, finger blood pressure and heart rate were continuously recorded during stepwise isobaric gastric distension using a barostat in eight healthy young (6 men and 2 women, 27 ± 3.2 years, mean ±s.e.m.) and eight healthy elderly subjects (7 men and 1 woman, 76 ± 1.5 years). Changes in cardiac output and total peripheral arterial resistance were calculated from the blood pressure signal. The baseline mean arterial pressure and muscle sympathetic nerve activity were higher in the elderly group (both P < 0.05) and muscle sympathetic nerve activity increase during the cold pressor test was lower in the elderly group (P = 0.005). During stepwise gastric distension, the elderly subjects showed an attenuated increase in muscle sympathetic nerve activity compared to the young subjects (P < 0.01). The older group tended to show a higher increase in mean arterial pressure (P = 0.08), heart rate (P = 0.06) and total peripheral arterial resistance (P = 0.09) The cardiac output rose slightly in both groups without significant difference between groups. The fundic compliance did not differ between groups. We conclude that stepwise gastric distension caused an increase in muscle sympathetic nerve activity in both groups, but the increase in the elderly was attenuated.
Digestion of food places a burden on the cardiovascular system. The blood flow in the superior mesenteric artery increases during a meal (Fujimura et al. 1997), indicating an increase in total splanchnic blood flow. To prevent a fall in systemic blood pressure during and after a meal, an increase in cardiac output and peripheral arterial resistance is needed.
In a normal condition, the systolic blood pressure does not fall after a meal in young healthy subjects (Mathias et al. 1989). This indicates that the cardiovascular system is activated in an early stage to prevent this fall in blood pressure. Directly after swallowing food, the stomach wall relaxes and is distended by the food bolus. This might be the trigger for the activation of the cardiovascular system.
Stepwise proximal gastric distension causes an increase in muscle sympathetic nerve activity (MSNA) and blood pressure in young healthy subjects. This phenomenon was named gastrovascular reflex (Rossi et al. 1998). The physiological relevance of this reflex may be to increase peripheral arterial resistance to compensate for the decrease of splanchnic arterial resistance that occurs during eating. The increase in blood pressure is possibly a consequence of baroreflex resetting caused by mechanoreceptor stimulation in the stomach wall (Rossi et al. 1998).
Healthy ageing is associated with changes in physiology with respect to food processing as well as to blood pressure regulation. Ageing is probably associated with impaired receptive relaxation and accommodation of the gastric fundus after a meal (MacIntosh et al. 2000; Rayner et al. 2000). In contrast to the effects of age on gastric mechanics, it seems that age has little, if any, effect on small intestinal or colonic motor function, and orocecal and whole-gut transit time are not affected in the healthy elderly (MacIntosh et al. 2000). The question remains whether ageing is associated with changes in the response to purely mechanical gastric distension without the effects of a meal mentioned above.
The aim of this study was to compare the influence of proximal gastric distension on sympathetic outflow, total peripheral arterial resistance, cardiac output, heart rate and blood pressure in young and in older healthy humans. Furthermore, we studied whether possible differences between the groups might be explained by a difference in fundic compliance or in autonomic status. Autonomic status was screened with the cold pressor test, Valsalva manoeuvre and spectral analysis of heart rate variability.
Methods
Population
Volunteers were recruited by advertisements in the local newspaper. All were free of overt cardiopulmonary disease as assessed from medical history taken by telephone. Older subjects were invited to the outpatient clinic to undergo a screening programme consisting of medical history and physical examination. Inclusion criteria for the elderly group were age > 70 and good health. The latter was based on a detailed health questionnaire and physical examination, in accordance with the modified exclusion criteria defining medically stable elderly subjects for exercise studies (Greig et al. 1994). To exclude postprandial hypotension in the elderly, older subjects underwent a standard ‘meal test’ (75 g glucose in 300 ml water) (Jansen et al. 1987) after an overnight fast on the day prior to the experiment. Postprandial hypotension was defined as a fall in systolic blood pressure of more than 20 mmHg within 2 h after a standard meal. The Ethics Committee of the Utrecht University Hospital approved the protocol and all experiments were carried out in accordance with the Declaration of Helsinki. Each subject gave written, informed consent prior to the commencement of the study.
Measurement instrumentation
Blood pressure was recorded continuously from the right hand with finger photoplethysmography (Finapres, Ohmeda, Englewood, CO, USA). Cardiac output (l min−1) and total peripheral resistance (medical units (MU); mmHg s ml−1) were obtained by pulse contour analysis (software: Beatscope 1.0; TNO TPD biomedical instrumentation, Academic Medical Centre, Amsterdam, the Netherlands). This method is accurate for measuring changes over time, but not for absolute values (Stok et al. 1993, 1999). For this reason we only report changes from baseline for cardiac output and total peripheral resistance. The electrocardiogram (ECG) was recorded using bipolar chest leads. Multifibre recordings of muscle sympathetic activity were made with a Tungsten microelectrode inserted in the peroneal nerve of the right leg (Vallbo et al. 1979; Wallin, 1999). A reference needle electrode was placed subcutaneously 2–3 cm away from the recording electrode. Small adjustments of the recording electrode were made until a site was found at which spontaneous sympathetic activity was recorded. Sympathetic bursts were identified by their characteristic morphology and relationship to R-waves (ECG). The correct position of the electrode was confirmed by the characteristic response to Valsalva manoeuvre (Ligtenberg et al. 1997).
The stomach was distended by a non-compliant intragastric bag (maximal capacity: 700 ml) connected to a G & J Electronics Distender Series II™ barostat, a computer-controlled injection-aspiration air pump (Protocol Plus Data Scanner software; G & J Electronics, Toronto, Ontario, Canada). A similar approach was described in our previous report (Salet et al. 1998). In the current study the stomach was distended at fixed intragastric pressures (isobaric), using a staircase protocol (Whitehead & Delvaux, 1997). This method allows assessment of the function of tension receptors in the stomach wall. The barostat was connected to the intragastric bag by a single-lumen polyvinyl stomach tube (12 CH Levin stomach tube, Argyle, Sherwood Medical, Tullamore, Ireland). Pressure and volume transducers in the barostat allow real-time monitoring of gastric distension.
Gastric sensory function was assessed at the end of each distension step using visual analogue scales (VAS) (Sriwatanakul et al. 1983) for sensation of fullness, nausea and pain. Subjects were asked to quantify these three sensations from ‘no sensation’ to ‘unbearable fullness’ or ‘unbearable nausea, to the point of vomiting’ and ‘unbearable pain’.
ECG, respiration, finger arterial blood pressure and MSNA (mean rectified voltage neurograms) were recorded and monitored with an IBM-compatible PC, using POLY 5 (samples at 200 Hz; POLY 5, Physiocal Analysis Package, Inspector Research Systems B.V., Amsterdam, the Netherlands). Intragastric pressure and volume curves were monitored on-line with G & J Electronics Protocol Plus™ control software.
Experimental protocol
The study was performed after an overnight fast. All subjects were instructed to refrain from eating, drinking and smoking after 21.00 h the previous day. Just before the experiment, subjects were asked to empty their bladders. The experiment took place at 08.00 h in an air-conditioned room. The intragastric bag was introduced through the mouth into the proximal stomach. The throat was sprayed with a lidocaine solution (100 mg ml−1, 1 spray equals 10 μg), which resulted in a maximum anaesthetized period of 20 min (the actual measurement started about 45 min after the intragastric bag was introduced). The intragastric bag was insufflated with 200 ml of air to unfold the bag. After complete deflation of the bag, the catheter was connected to the barostat. Subjects lay on a bed at an angle of 30 deg in an anti-Trendelenburg supine position.
The hand that was used for the finger photoplethysmographic blood pressure recording (right hand) was kept warm with a cherrystone pillow to avoid vasoconstriction (Jagomagi et al. 2001) and was kept at a fixed vertical heart–hand distance to eliminate the influence of gravity on the observed blood pressure. The appropriate heart–hand height was determined by comparing the mean arterial blood pressure value of the Finapres with the mean arterial blood pressure value of at least two measurements with a sphygmomanometer.
After signals of good quality were obtained, baseline MSNA, blood pressure, heart rate and respiration rate were recorded for 5 min (first baseline period). During this period the subject was asked to sustain a comfortable breathing frequency of 15 breaths per minute assisted by a metronome. To assess baseline vagal and sympathetic responsiveness, the following provocation tests were subsequently performed: Valsalva manoeuvre (keeping a manometer inflated to 40 mmHg for 15 s) and a cold pressor test (CPT; the subject's left hand was immersed in ice water up to the wrist for 2 min). Each test was started only after signals had returned to baseline level.
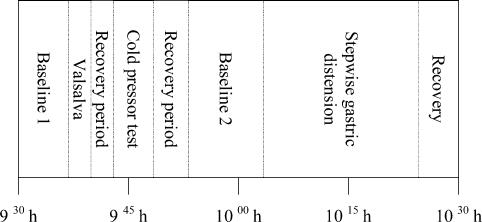
The provocation tests were followed by a second baseline (predistension) period for a duration of 10 min. During the second baseline period the minimal distension pressure (MDP; minimal pressure inside intragastric bag to overcome the intra-abdominal pressure; Mearin et al. 1991) was determined and sustained. One or two baseline blood pressure measurements with a sphygmomanometer were done to adjust the Finapres and to use for later analysis. Subsequently, intragastric pressure was increased in 3-min steps of 2 mmHg each, using a staircase protocol. In the last minute of each distension step, the subject was asked to quantify sensations of fullness, nausea, and pain on a VAS, ranging from 1 to 7. Gastric distension was ended after the seventh distension step (intragastric pressure = 14 mmHg) or after reaching maximal bag volume (700 ml) or maximum tolerable volume. Recording was continued after the gastric distension period for 15 min (recovery period) to ascertain normalization of the parameter values to baseline. Figure 1 shows the time line of the experiment protocol.
Figure 1. Time line of the protocol.
Not shown is the preparation period, which started at 08.00 h and was used for placing the intragastric bag and the finding of signals (MSNA, Finapres, ECG and pneumogram). During the first baseline period stable signals were obtained and used for comparison of baseline values. The recovery periods served to let all signals return to baseline levels. The values during the second baseline period served as baseline for the distension period. The heart rate variability during the second baseline period was used for spectral analysis.
Data analysis
Mean arterial blood pressure (MAP; mmHg) was calculated per heartbeat (time average MAP per beat) from the finger blood pressure curve. Heart rate (beats min−1) and beat length (interbeat interval (IBI); ms) were calculated beat to beat from the ECG using automatic R-wave detection. The stored integrated MSNA signal was analysed by software specially developed by our group. This software detected MSNA bursts based on their characteristic, more or less triangular shape using criteria on the slopes, duration and amplitude of the bursts. For instance, ‘EMG pulses’ were recognized from their steep front slope, and automatically rejected. Detected bursts were assigned to independently detected R-waves in the ECG, requiring the delay between the R-wave and the burst top to be within an a priori defined time window. Only one burst was assigned per R-wave. The results were shown graphically as stylised MSNA bursts together with the raw MSNA and ECG for visual inspection and, if necessary, correction.
Burst counts were calculated as burst frequency (number of sympathetic bursts per minute, NBM; bursts min−1) and as burst incidence (number of sympathetic bursts per 100 heartbeats, NBR). The burst area was calculated as the area under the curve (arbitrary units) per minute (ABM; a.u.c. min−1) and as the area per 100 heartbeats (ABR; a.u.c./100 heartbeats).
Autonomic status
Information on autonomic status of all subjects was obtained by the following tests
Spectral analysis of the heart rate variability
Five consecutive minutes of the baseline period were used for spectral analysis of the heart rate variability. Detection of R-waves in QRS complexes was used to obtain a list of interbeat intervals. The list of interbeat intervals was manually corrected for extra-systolic as well as missing heartbeats by interpolation. The linear trend was removed from the list and a cosine tip window was applied. The power spectrum was estimated as the squared amplitude of the result of a fast Fourier transform. From that, the power in the low frequency (LF: 0.04–0.15 Hz) and high frequency (HF: 0.15–0.40 Hz) ranges, and the LF/HF power ratio were calculated.
Valsalva
Baseline vagal cholinergic function was determined by calculating the IBImin/IBImax ratio (Valsalva ratio) from the minimum IBI in the ECG during elevated intrathoracic pressure (phase II) and the maximum IBI during the first 30 s after the release of intrathoracic pressure (phase IV) (Mathias & Bannister, 1999).
Cold pressor test
The effect of a cold pressor test was assessed by observing changes in MSNA, mean arterial pressure and heart rate. Two minutes of baseline were directly followed by 2 min of immersion of the right hand into ice water (cold pressor period) and 2 min of recovery. The cold pressor and recovery periods were divided into 30 s epochs. Mean absolute (mean arterial pressure, heart rate and NBM) or proportional (ABM) changes from baseline were calculated for each epoch. These delta values per epoch were used in a repeated measures anova with age group as the between-subject factor. The significance of changes over time and the difference between the groups were calculated.
Effect of gastric distension
Comparison of the baseline values of the young with that of the elderly was obtained for the following parameters: mean arterial pressure (sphygmomanometer), average heart rate and MSNA (NBM).
The effect of gastric distension was calculated by dividing this part of the measurement into separate periods. From each recording, an artefact-free 2-min period was selected from the second baseline period (barostat bag at MDP) and from the recovery period. Parameters (mean arterial pressure, heart rate, cardiac output, NBM, NBR and total peripheral resistance) were averaged over these 2-min periods to obtain the baseline and recovery values. For the seven barostat distension steps (+2 to +14 mmHg), the parameters were averaged over 3-min periods. All parameter step values were expressed relative to their baseline values (Δ; change from baseline). For the burst area parameters ABM and ABR, which were expressed in arbitrary units, proportional changes (%Δ) from baseline were calculated.
The Δ values of both groups showed a normal distribution. Repeated measures anova was performed for each parameter to compare changes from baseline and differences between groups during distension of the barostat bag. Data from the recovery period were not included. The (proportional) Δ values of the six distension steps were analysed with distension step as the within-subject factor and age group as the between-subject factor. Since the data did not fulfil the assumption of compound symmetry in most of the cases only multivariate results are shown (all subtests gave similar results).
Linear regression slopes
Most variables showed an approximately linear change during gastric distension. An estimation of this change was made using linear regression of the Δ values against the distension step number (independent variable). The slope of the regression line was calculated for each group (constant not in equation; baseline and recovery periods were not included).
Visual analogue perception scores
The association between the degree of gastric distension and the perception of pain, nausea and fullness, registered as VAS scores, was calculated using the Spearman correlation test. Changes over time and differences between groups in VAS scores were calculated with repeated measures anova.
Gastric wall compliance
An estimation of the compliance of the gastric wall (fundic compliance) was made by calculating the regression coefficient of the ‘intragastric pressure–volume’ (ΔV/ΔP) curves that were measured with the barostat. To minimize the influence of tension of the abdominal muscles, subjects were asked to relax those muscles as far as possible. Because distributions were normal, the group results were compared using Student's t test.
Missing data
All original signal curves were reviewed carefully off-line. Parts with missing data (for instance caused by turning off the Finapres for 30 s to avoid painful congestion of the finger) or with artefacts (for instance in the MSNA signal, caused by involuntary muscle tension) were marked and omitted in further analysis.
Missing data were considered in 1-min epochs, prior to calculating the 2- or 3-min averages mentioned above. A 1-min epoch was considered ‘missing’ if it contained significant artefacts. Inter- or extrapolation replaced these missing points using the group average trend. Of the numbers used, 5.6% consisted of replaced missing data, two-thirds of which occurred in the final (7th) distension step, due to measurements stopped early. For this reason, the 7th distension step was left out of all calculations.
General
Diferences were considered significant using a cut-off of P < 0.05. All statistics were performed with SPSS version 11. All results that showed normal distribution are expressed as the mean ± standard error of the mean (s.e.m.), other results as median and range.
Because the baseline heart rate did not differ between groups (Fig. 2) and the difference between results of burst counts (NBM and NBR) and burst size (ABM and ABR) were not significant, only results of NBM and ABM during CPT and gastric distension are shown.
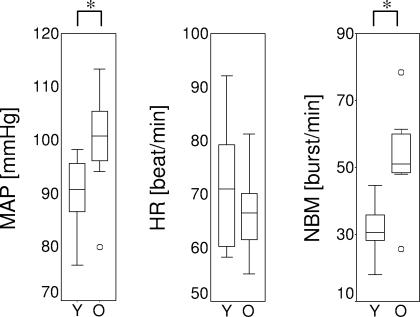
Figure 2. Baseline values.
Baseline MAP (mean arterial pressure measured with sphygmomanometer), HR (heart rate) MSNA in burst frequency (NBM) per group (Y, Young; O, Old). Boxplots show median, range and interquartile range. ° Indication of excluding outliers; * indication of a significant difference between groups with Mann-Whitney U tests.
Results
Eleven healthy young subjects were recruited and they could all be included. The measurement failed in three subjects, due to practical problems (unable to swallow the barostat bag, MSNA signal could not be found or urge for micturition). Seven out of 16 recruited elderly could not be included due to hypertension, diabetes or postprandial hypotension and in one subject the MSNA signal could not be traced.
Experiments were completed on eight healthy young subjects (6 men and 2 women, mean age ±s.e.m.: 27 ± 3.2 years, range 21–40 years) and eight healthy elderly subjects (7 men and 1 woman, 76 ± 1.5 years, range 71–82). Body mass index was not different between young and older subjects (young, 23.23 ± 0.46 kg m−2versus older, 25.61 ± 1.37 kg m−2; P= 0.12). All subjects were normotensive (supine blood pressure (≤160/90 mmHg) and none took medication that could affect autonomic-circulatory function. Fourteen subjects (6 of the young and all of the elderly) tolerated seven steps of distension (MDP + 14 mmHg) and the remaining two subjects, six steps. For technical reasons, recordings were ended in one young subject before the end of the recovery period.
Effects of gastric distension (barostat)
Baseline parameters
Baseline mean arterial pressure (sphygmomanometer) and MSNA were higher in the elderly (Fig. 2; mean arterial pressure: P < 0.01; MSNA: P < 0.01). The heart rate did not differ significantly between the groups (P = 0.4). All parameters showed a trend to return to baseline level after the barostat distension was stopped (Fig. 3).
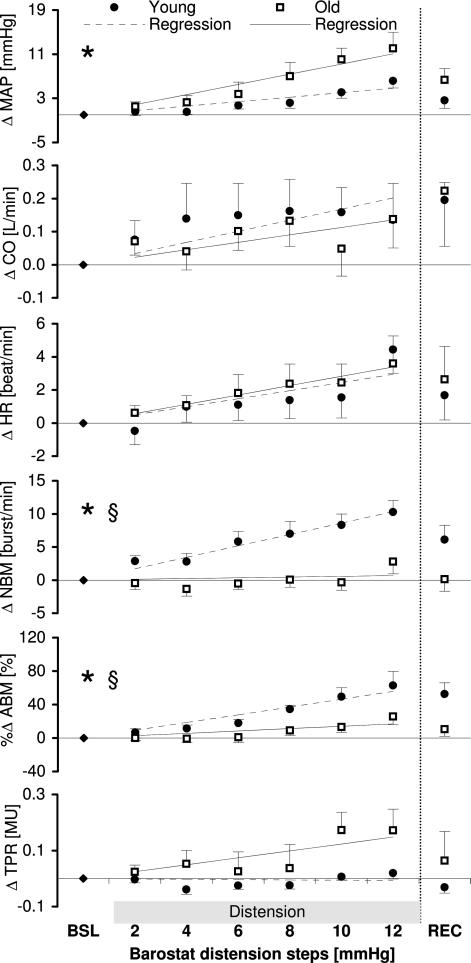
Figure 3. Course of parameters (Δ) during barostat gastric distension.
Changes in MAP (mean arterial pressure), HR (heart rate) and MSNA in NBM (burst frequency) and ABM (total area under the curve per minute) in reaction to stepwise stomach distension. * Indication of significant changes in time as calculated with repeated measures anova; § indication of a significant difference between the groups or a significant interaction. For details, see text.
Changes of parameters during stepwise barostat distension
The absolute values of variables are shown in Table 1 and the observed changes in mean arterial pressure, cardiac output, heart rate and MSNA (NBM and ABM) and total peripheral resistance during distension are illustrated in Fig. 3.
Table 1.
Course of parameters (absolute values) during barostat gastric distension
| Baseline | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | Step 6 | Recovery | |
|---|---|---|---|---|---|---|---|---|
| MAP young | 92 ± 3.8 | 93 ± 3.7 | 93 ± 3.9 | 94 ± 3.9 | 95 ± 3.7 | 97 ± 3.8 | 99 ± 3.7 | 95 ± 3.3 |
| MAP old | 107 ± 7.6 | 108 ± 7.3 | 109 ± 7.7 | 110 ± 8.0 | 114 ± 8.5 | 117 ± 8.4 | 119 ± 9.2 | 113 ± 8.2 |
| HR young | 72 ± 4.3 | 71 ± 4.4 | 72 ± 4.3 | 73 ± 4.4 | 73 ± 4.4 | 73 ± 4.8 | 76 ± 4.4 | 73 ± 4.6 |
| HR old | 67 ± 2.8 | 67 ± 2.9 | 67 ± 2.9 | 68 ± 2.7 | 69 ± 2.7 | 69 ± 2.5 | 70 ± 2.5 | 69 ± 3.4 |
| NBM young | 31.4 ± 2.8 | 34.3 ± 2.7 | 34.2 ± 2.1 | 37.3 ± 1.7 | 38.4 ± 2.1 | 39.8 ± 2.1 | 41.7 ± 2.3 | 37.5 ± 1.9 |
| NBM old | 52.9 ± 5.3 | 52.4 ± 5.1 | 51.5 ± 5.3 | 52.3 ± 4.8 | 52.9 ± 4.8 | 52.5 ± 5.0 | 55.7 ± 4.0 | 53.0 ± 4.4 |
Changes of mean arterial pressure (Finapres; MAP), heart rate (HR) and sympathetic burst frequency (NBM) in reaction to barostat distension steps. Values are mean+-standard error. Maximal mean difference from baseline is shown in bold characters. Significance of changes was calculated on the delta values, not on the absolute values. For statistical results, see text and Fig. 3.
During distension, an approximately linear increase in all the variables was found. However, the standard errors indicate that the responses vary appreciably among the subjects. Mean arterial pressure increased during the barostat distension (Fig. 3). The slopes of the regression lines were 0.8 and 1.8 mmHg per distension steps for the young and elderly, respectively. Repeated measures analysis showed a significant change during distension (P = 0.003), but no significant interaction between distension step and group (P = 0.19) and no significant difference between subject effect for group (P = 0.08). This means that the increase in MAP during subsequent distension steps (as shown in Fig. 3) is significant, but both groups reacted in the same way statistically to the barostat distension steps.
Cardiac output rose slightly in both groups (maximal increase 0.14 l min−1 in both groups), but this increase was not significant (P = 0.6) and there was no significant difference between groups (P = 0.6).
The heart rate showed a trend (P = 0.06) towards increase in both groups (Fig. 3; slope in the young subjects 0.5 beats min−1 per distension step; in the elderly subjects 0.6 beats min−1 per distension step). There was no interaction between distension steps and group (P = 0.4) nor a between-subject effect for group (P = 0.7), indicating that there was no difference between the groups.
The change in number of sympathetic bursts (NBM) in reaction to the gastric distension was different in the two groups. The young subjects showed a steady increase, whereas the elderly did not seem to change at all (Fig. 3; slope in young 1.7 bursts min−1, in elderly 0.1 bursts min−1). Maximal increase in the young subjects was reached in the sixth step (mean 10 ± 1.7; range +2 to +16 bursts min−1). The increase of both groups together was significant (repeated measures analysis: P= 0.005). The interaction between distension steps and group was not significant (P = 0.05), but the between-subject effect (group) was (P = 0.002), indicating that the increase in the young was significantly higher than in the old group.
The difference between the groups in the area under the curve of the sympathetic bursts was less obvious compared to the number of burst measures of MSNA. Both groups showed an increase (Fig. 3; regression slope in young 9.3% per distension step, in the elderly 2.8% per step, both P < 0.01). In the elderly the increase started only after the fourth distension step. Maximal increase was reached in the sixth step in both groups. The interaction between distension steps and the group was not significant (P = 0.3), but the between-subject effect of the group was (P = 0.007). The observed increase in both groups was significant (P = 0.002) as well.
Total peripheral resistance did not increase in the young (0.02 mU increase in the 6th step), but the elderly did show an increase (0.17 mU in the 6th step). The difference between the groups was not significant (P = 0.09)
Comparable results were found when barostat volume and stomach tension (measure that combines pressure and volume inside the gastric barostat bag) were used as dependent instead of barostat pressure (because isobaric distension steps were used, there is no difference in using ‘steps’ or ‘pressure’).
Gastric sensory function
All subjects in the younger group and most subjects in the elderly group reported an increased feeling of fullness during distension (anova: P < 0.001; Spearman correlation coefficient: young, 0.85; elderly, 0.66). Most subjects did not feel nauseous, especially in the elderly group (anova: P= 0.2; Spearman correlation: young, 0.41; elderly, 0.35) or experience pain except at the higher distension steps (anova: P= 0.05; Spearman correlation: younger group, 0.68; elderly, 0.45). Only the VAS scores on nausea were significantly higher in the young (anova: fullness: P= 0.19; nausea: P= 0.03; pain: P= 0.9).
Fundic compliance
Fundic compliance was determined by calculating the linear regression coefficient of the pressure–volume curves (ΔV/ΔP) for each individual. Mean compliance of the young was 43.5 ± 2.4 ml mmHg−1, of the elderly 43.9 ± 5.1 ml mmHg−1. This difference was not statistically significant (P = 0.9; independent samples t test).
Autonomic function tests
Heart rate variability was calculated from IBIs in the baseline period. LF power, HF power did not differ significantly between the groups (all P≥ 0.4, data are not shown).
Valsalva ratios of all subjects were above 1.0, which is considered normal in our laboratory. The mean value of the younger group was slightly higher than that of the elderly, but this difference was not statistically significant (P = 0.4, data are not shown).
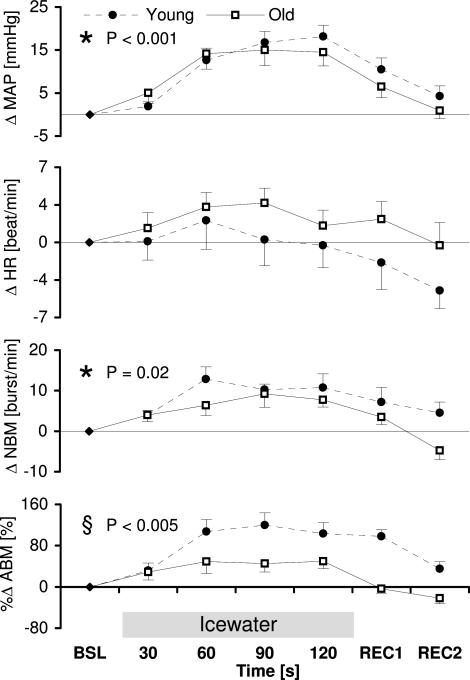
During the cold pressor test, NBM, ABM and MAP rose steadily, whereas the HR peaked at 1–1.5 min and declined slightly thereafter (Fig. 4). Repeated measures anova showed a significant change in time in mean arterial pressure and sympathetic burst frequency (NBM), a trend in sympathetic burst area (ABM: P= 0.05), but no significant change in time in heart rate (P = 0.13) (Fig. 4). The change in number of sympathetic bursts (NBM) in reaction to the CPT tended to be different in each group, the interaction between time and group was nearly significant (P = 0.05), but the between subject effect (group) was not significant (P = 0.10). The sympathetic burst area (ABM) showed a statistically different reaction between the groups. The young group showed a significantly higher increase compared to the elderly (P = 0.005).
Figure 4. Course of parameters (Δ) during cold pressor test.
Changes in MAP (mean arterial pressure), HR (heart rate) and MSNA in burst frequency (NBM) and burst area (ABM) in reaction to a cold pressor test. BSL, baseline; REC, recovery. * Indication of significant changes in time as calculated with repeated measures anova; § indication of a significant difference between the groups or a significant interaction.
Discussion
The main finding of the present study is that the elderly subjects showed reduced reactivity of MSNA during stepwise gastric distension. This reduced reactivity to gastric distension could be part of a more generalized difference in autonomic function between young and old, as the reactivity of sympathetic burst area (ABM) in reaction to a cold pressor test was reduced in the elderly as well. Fundic compliance did not differ between the groups. Therefore, it is unlikely that reduced reactivity of MSNA in reaction to gastric distension is related to fundic compliance.
Although the effect of proximal gastric distension induced by food intake is different from that of stretching the stomach wall by means of barostat in this study, both result in an elongation of the gastric wall and activation of stretch receptors (Scheffer et al. 2002).
The higher baseline MSNA, which was found in the older group (Fig. 2), has been described by others as well, and is considered an age-related physiological change (Sundlof & Wallin, 1978; Iwase et al. 1991). Ageing also reduces the MSNA responses to Valsalva (Matsukawa et al. 1998), to gravitational stress (Iwase et al. 1991) and to vestibular stimuli (Ray & Monahan, 2002). In contrast with our result, Ray & Monahan (2002) found that the increase in total activity of MSNA in response to a cold pressor test in the elderly was not attenuated, although the change in burst frequency in older subjects was less than that in young subjects. It should be noted that they did not examine subjects above the age of 70. Regarding higher baseline MSNA in the elderly, it is our experience that the difference in baseline MSNA between young and older subjects is the most manifest in the 70+ age group. This is also the reason why the elderly group in this study consisted of subjects who were over 70.
Superior mesenteric artery flow and diameter increased in response to a meal. (Fujimura et al. 1997) These phenomena can be interpreted as an effect of a decrease in splanchnic arterial resistance, possibly by the release of vasoactive intestinal hormones. The gastrovascular reflex (increase in MSNA and blood pressure in reaction to distension of the stomach) prevents a fall in blood pressure by increasing local arterial resistance in muscles. With regard to the physiological mechanism of the gastrovascular reflex, it was hypothesized in our previous study that the sympathetic response is a direct (neural) effect of stomach distension mediated by tension receptors in the stomach wall through vagal afferent fibres, comparable to a spinal reflex (Rossi et al. 1998). This hypothesis is supported by the findings from another study (O'Donovan et al. 2002) and an ongoing experiment (van Orshoven et al. 2002) in which intraduodenal glucose infusion is applied as a trigger. In these experiments the MSNA and blood pressure started to change only after 10 min, whereas in the present study the increase of MSNA already occurred within 1 min. We hypothesize that stretching the stomach wall increases MSNA through a direct, fast-acting neural pathway and that intraduodenal glucose infusion increases MSNA through a slow, indirect pathway by the release of vasoactive intestinal peptides. This might cause splanchnic blood pooling and subsequently a fall in venous return of blood to the heart and a lower cardiac output, which activates the baroreflex and thus increases MSNA.
The slope of the regression line of MSNA during the stepwise gastric distension was less steep in the elderly. On the other hand, total peripheral resistance and blood pressure tended to increase more in the elderly than in the young. These findings seem to be in contradiction with each other: a stronger increase in MSNA in the young group would be expected to lead to a stronger increase in total peripheral arterial resistance and subsequently more increase in blood pressure. A likely explanation is that in older subjects the decrease in splanchnic arterial resistance induced by gastric distension is less pronounced than in young subjects. This may be caused by a reduced compliance of the splanchnic arteries in the elderly. This assumption is in line with the well-known fact that ageing causes reduction in central arterial compliance (Monahan et al. 2001). In non-compliant arteries, the ability to show vasodilatation is reduced. More pronounced vasodilatation in the splanchnic system of the young group might explain why total arterial resistance and blood pressure did not increase that much. Although this explanation seems reasonable, the current study does not give direct evidence for it. Further experiments with a direct measurement of splanchnic flow, for instance by the non-invasive duplex ultrasound method, are desirable.
Furthermore, a small increase of sympathetic activity has a larger effect on blood pressure in non-compliant arteries than in compliant arteries. This may explain why the blood pressure in the elderly increased strongly despite their attenuated MSNA response. Reduced vascular compliance may also explain the finding that mean arterial pressure has a significant positive correlation to an increase in MSNA in elderly, but not in young, subjects (Watanabe et al. 1993).
It could be argued that the attenuated increase in MSNA in the elderly during distension relates to their higher baseline. MSNA consists of rhythmic spontaneous discharges that are synchronous to the heartbeat, and NBM cannot exceed the heart rate (Sundlof & Wallin, 1978). Our results of the cold pressor test, also found by others (Ray & Monahan, 2002), strongly suggest that the ‘ceiling effect’ is unlikely, since the elderly showed a similar NBM to the young.
It could be argued that the higher increase of MSNA in young subjects is due to an indirect effect (discomfort) of gastric distension since the VAS scores in nausea during gastric distension is a significant difference between the young and the elderly group. However, pain and other stress reactions are characterized by a prompt and immediate increase in heart rate (Freyschuss et al. 1990) and in the present study the minor increase in heart rate was not significantly different between the groups. We consider the difference in the results of the gastric sensory test as an expression of decreased perception of gastric distension in the elderly, which was also found by others (Rayner et al. 2000).
It has been suggested that decreased fundic compliance could be a cause of postprandial hypotension (Morley, 2001) by attenuating the incentive of the gastrovascular reflex. The results of the present study, as well as of another study (Rayner et al. 2000) showed no difference in fundic compliance between the young and the elderly. These results cannot reject the hypothesis, since we only studied healthy elderly subjects and not patients suffering from postprandial hypotension. However, we think this explanation is less likely, because we would then expect to find at least a minor difference between young and old.
In the present study, normal results for both young and older groups on Valsalva manoeuvre and similar results of the spectral analysis of the heart rate variability suggest normal autonomic function. The findings that the area under the curve of the MSNA of the elderly group showed an increase during proximal gastric distension and during CPT and that the variables decreased toward initial values after the end of provocations indicate an intact baroreflex control of MSNA.
In normal subjects superior mesenteric artery flow increases postprandially, which causes an increase in splanchnic-mesenteric blood volume. The systemic blood pressure is maintained due to compensation by baroreflex-mediated decrease in the volume of the muscle and probably the skin resistance bed. In patients with autonomic failure, the superior mesenteric artery flow increase response to feeding is mostly preserved, but the auto-regulatory response is reduced or absent, and therefore systemic blood pressure passively falls as a larger blood volume is transferred into the splanchnic bed (Fujimura et al. 1997). We would emphasize that MSNA is dominated by vasoconstrictor impulses destined for muscle blood vessels and the main control of MSNA is exerted by arterial baroreceptors (Wallin & Elam, 1997). An increase in MSNA is associated with intramuscular vasoconstriction. The increase in MSNA and blood pressure during stretching of the stomach wall in both the young and the elderly found in the present study might be considered as a reflex preventing a fall in systemic blood pressure during the ingestion of a meal.
Limitation of the present study
The performance of the experiments was technically demanding, which limited the number of subjects studied and thereby the power of the statistical tests. As an illustration, observed powers were calculated for some repeated measures analyses of the gastric distension effect (for P= 0.05, using SPSS 11.0). For mean arterial pressure, the observed power was 0.4 for both the between-groups effect and the interaction between the distension step and the group. For HR, the observed powers were 0.6 for the effect of the step, and smaller than 0.3 for the interaction and group effect. Low powers indicate a low chance of finding a significant difference in the available sample, assuming a stated difference exists in the population.
Although we do not know how reproducible the results of this study are, it is a well-known fact that intraindividual variations in MSNA are small (Wallin & Elam, 1997). In our previous study we also found a high reproducibility of MSNA in healthy subjects and chronic renal disease patients, both at rest as well as in reaction to provocations (Ligtenberg et al. 1999).
The measurement of cardiac output and total peripheral resistance by means of pulse contour analysis has its limitation for assessing the absolute values (Remmen et al. 2002). Therefore we used this method only to express the relative changes of cardiac output and total peripheral resistance during gastric distension. This has proven to be a valuable non-invasive method, particularly when the body position has not changed during the measurement (Stok et al. 1999).
To summarize, the present study shows that stepwise gastric distension causes an increase in MSNA in young and older subjects. However, the increase in MSNA is attenuated in the elderly group. Further studies are needed to determine whether the gastrovascular reflex is altered in patients with postprandial hypotension, a disorder which frequently occurs in the elderly.
References
- Freyschuss U, Fagius J, Wallin BG, Bahlin G, Perski A, Hjemdahl P. Cardiovascular and sympathoadrenal response to mental stress: a study of sensory intake and rejection reactions. Acta Physiol Scand. 1990;139:173–183. doi: 10.1111/j.1748-1716.1990.tb08910.x. [DOI] [PubMed] [Google Scholar]
- Fujimura J, Camilleri M, Low PA, Novak V, Novak P, Opfer-Gehrking TL. Effect of perturbations and a meal on superior mesenteric artery flow in patients with orthostatic hypotension. J Auton Nerv Syst. 1997;67:15–23. doi: 10.1016/s0165-1838(97)00087-8. [DOI] [PubMed] [Google Scholar]
- Greig CA, Young A, Skelton DA, Pippet E, Butler FM, Mahmud SM. Exercise studies with elderly volunteers. Age Ageing. 1994;23:185–189. doi: 10.1093/ageing/23.3.185. [DOI] [PubMed] [Google Scholar]
- Iwase S, Mano T, Watanabe T, Saito M, Kobayashi F. Age-related changes of sympathetic outflow to muscles in humans. J Gerontol. 1991;46:M1–M5. doi: 10.1093/geronj/46.1.m1. [DOI] [PubMed] [Google Scholar]
- Jagomagi K, Raamat R, Talts J. Effect of altering vasoactivity on the measurement of finger blood pressure. Blood Press Monit. 2001;6:33–40. doi: 10.1097/00126097-200102000-00006. [DOI] [PubMed] [Google Scholar]
- Jansen RW, Penterman BJ, Van Lier HJ, Hoefnagels WH. Blood pressure reduction after oral glucose loading and its relation to age, blood pressure and insulin. Am J Cardiol. 1987;60:1087–1091. doi: 10.1016/0002-9149(87)90358-4. [DOI] [PubMed] [Google Scholar]
- Ligtenberg G, Blankestijn PJ, Oey PL, Klein IHH, Dijkhorst-Oei LT, Boomsma F, Wieneke GH, Van Huffelen AC, Koomans HA. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999;340:1321–1328. doi: 10.1056/NEJM199904293401704. [DOI] [PubMed] [Google Scholar]
- Ligtenberg G, Blankestijn PJ, Oey PL, Wieneke GH, van Huffelen AC, Koomans HA. Cold stress provokes sympathoinhibitory presyncope in healthy subjects and hemodialysis patients with low cardiac output. Circulation. 1997;95:2271–2276. doi: 10.1161/01.cir.95.9.2271. [DOI] [PubMed] [Google Scholar]
- MacIntosh C, Morley JE, Chapman IM. The anorexia of aging. Nutrition. 2000;16:983–995. doi: 10.1016/s0899-9007(00)00405-6. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Bannister R. Investigation of autonomic disorders. In: Mathias CJ, Bannister R, editors. Autonomic Failure, a Textbook of Clinical Disorders of the Autonomic Nervous System. 4. Oxford: Oxford University Press; 1999. pp. 169–195. [Google Scholar]
- Mathias CJ, da Costa DF, Fosbraey P, Bannister R, Wood SM, Bloom SR, Christensen NJ. Cardiovascular, biochemical and hormonal changes during food-induced hypotension in chronic autonomic failure. J Neurol Sci. 1989;94:255–269. doi: 10.1016/0022-510x(89)90235-9. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Baroreflex control of muscle sympathetic nerve activity is attenuated in the elderly. J Auton Nerv Syst. 1998;73:182–185. doi: 10.1016/s0165-1838(98)00128-3. [DOI] [PubMed] [Google Scholar]
- Mearin F, Cucala M, Azpiroz F, Malagelada JR. The origin of symptoms on the brain-gut axis in functional dyspepsia. Gastroenterology. 1991;101:999–1006. doi: 10.1016/0016-5085(91)90726-2. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281:H284–H289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- Morley JE. Editorial: Postprandial hypotension – the ultimate Big Mac attack. J Gerontol A Biol Sci Med Sci. 2001;56:M741–M743. doi: 10.1093/gerona/56.12.m741. [DOI] [PubMed] [Google Scholar]
- O'Donovan D, Feinle C, Tonkin A, Horowitz M, Jones KL. Postprandial hypotension in response to duodenal glucose delivery in healthy older subjects. J Physiol. 2002;540:673–679. doi: 10.1113/jphysiol.2001.013442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Orshoven NP, van Schelven LJ, Jansen PA, Horowitz M, Akkermans LM, van Huffelen AC, Oey PL. The effect of intra-duodenal glucose on muscle sympathetic nerve activity (MSNA) is attenuated in older subjects (Abstract) Clin Auton Res. 2002;12:297. doi: 10.1007/s10286-008-0452-5. [DOI] [PubMed] [Google Scholar]
- Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation. 2002;105:956–961. doi: 10.1161/hc0802.104289. [DOI] [PubMed] [Google Scholar]
- Rayner CK, MacIntosh CG, Chapman IM, Morley JE, Horowitz M. Effects of age on proximal gastric motor and sensory function. Scand J Gastroenterol. 2000;35:1041–1047. doi: 10.1080/003655200451153. [DOI] [PubMed] [Google Scholar]
- Remmen JJ, Aengevaeren WR, Verheugt FW, van ver Werf T, Luijten HE, Bos A, Jansen RW. Finapres arterial pulse wave analysis with Modelflow is not a reliable non-invasive method for assessment of cardiac output. Clin Sci (Lond) 2002;103:143–149. doi: 10.1042/cs1030143. [DOI] [PubMed] [Google Scholar]
- Rossi P, Andriesse GI, Oey PL, Wieneke GH, Roelofs JM, Akkermans LM. Stomach distension increases efferent muscle sympathetic nerve activity and blood pressure in healthy humans. J Neurol Sci. 1998;161:148–155. doi: 10.1016/s0022-510x(98)00276-7. [DOI] [PubMed] [Google Scholar]
- Salet GA, Samsom M, Roelofs JM, Berge Henegouwen GP, Smout AJ, Akkermans LM. Responses to gastric distension in functional dyspepsia. Gut. 1998;42:823–829. doi: 10.1136/gut.42.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer RC, Akkermans LM, Bais JE, Roelofs JM, Smout AJ, Gooszen HG. Elicitation of transient lower oesophageal sphincter relaxations in response to gastric distension and meal ingestion. Neurogastroenterol Motil. 2002;14:647–655. doi: 10.1046/j.1365-2982.2002.00366.x. [DOI] [PubMed] [Google Scholar]
- Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther. 1983;34:234–239. doi: 10.1038/clpt.1983.159. [DOI] [PubMed] [Google Scholar]
- Stok WJ, Baisch F, Hillebrecht A, Schulz H, Meyer M, Karemaker JM. Noninvasive cardiac output measurement by arterial pulse analysis compared with inert gas rebreathing. J Appl Physiol. 1993;74:2687–2693. doi: 10.1152/jappl.1993.74.6.2687. [DOI] [PubMed] [Google Scholar]
- Stok WJ, Stringer RC, Karemaker JM. Noninvasive cardiac output measurement in orthostasis: pulse contour analysis compared with acetylene rebreathing. J Appl Physiol. 1999;87:2266–2273. doi: 10.1152/jappl.1999.87.6.2266. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wallin BG. Intraneural recordings of normal and abnormal sympathetic activity in humans. In: Mathias CJ, Bannister R, editors. Autonomic Failure. A Textbook of Clinical Disorders of the Autonomic Nervous System. 4. Oxford: Oxford University Press; 1999. pp. 223–225. [Google Scholar]
- Wallin BG, Elam M. Microneurography and autonomic dysfunction. In: Low PA, editor. Clinical Autonomic Disorders. 2. Philadelphia: Lippincott-Raven; 1997. pp. 233–243. [Google Scholar]
- Watanabe T, Kobayashi F, Furui H, Tanaka T, Horibe H, Takeshima N, Iwase S, Mano T. Assessment of sympathetic nerve activity controlling blood pressure in the elderly using head-up tilt. Environ Res. 1993;62:251–255. doi: 10.1006/enrs.1993.1110. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo-Wellcome Research, UK. Dig Dis Sci. 1997;42:223–241. doi: 10.1023/a:1018885028501. [DOI] [PubMed] [Google Scholar]