Abstract
Short-term aldosterone coordinately regulates the cell-surface expression of luminal epithelial sodium channels (ENaC) and of basolateral Na+ pumps (Na+, K+-ATPase α1–β1) in aldosterone-sensitive distal nephron (ASDN) cells. To address the question of whether the subcellular localization of the Na+,K+-ATPase and its regulation by aldosterone depend on subunit isoform-specific structures, we expressed the cardiotonic steroid-sensitive human α isoforms 1–3 by retroviral transduction in mouse collecting duct mpkCCDc14 cells. Each of the three exogenous human isoforms could be detected by Western blotting. Immunofluorescence indicated that the exogenous α1 subunit to a large extent localizes to the basolateral membrane or close to it, whereas much of the α2 subunit remains intracellular. An ouabain-sensitive current carried by exogenous pumps could be detected in apically amphotericin B-permeabilized epithelia expressing human α1 and α2 subunits, but not the α3 subunit. This current displayed a higher apparent Na+ affinity in pumps containing human α2 subunits (10 mm) than in pumps containing human α1 (33.2 mm) or endogenous (cardiotonic steroid-resistant) mouse α1 subunits (mean: 16.3 mm). A very low mRNA level of the Na+,K+-ATPase γ subunit (FXYD2) in mpkCCDc14 cells suggested that this ancillary gene product is not responsible for the relatively low apparent Na+ affinity measured for a1 subunit-containing pumps. Aldosterone increased the pump current carried by endogenous pumps and by pumps containing the human α1 subunit. In contrast, the current carried by pumps with a human α2 subunit was not stimulated by the same treatment. In summary, quantitative basolateral localization of the Na+,K+-ATPase and its responsiveness to aldosterone require α1 subunit-specific sequences that differentiate this isoform from the α2 and α3 subunit isoforms.
The minimal functional unit of the Na+,K+-ATPase is a heterodimer of an alpha (α1–4) and a beta (β1–3) subunit (reviewed in Blanco & Mercer, 1998). The α subunit is the multi membrane-spanning catalytic subunit that performs ATP-driven extrusion of three Na+ in exchange for two K+. The β subunit is known to be required for structural and functional maturation of the α subunit and also slightly impacts on the K+ and Na+ activation kinetics of the mature Na+,K+-ATPase (Hasler et al. 1998). The small γ subunit or CHIF (FXYD protein family) is associated with the Na+,K+-ATPase in some kidney tubule segments and modulates pump kinetics (Crambert & Geering, 2003).
The Na+,K+-ATPase composed of α1 and β1 subunits appears to be expressed ubiquitously and fulfils housekeeping functions. The α1 subunit also appears to be the major or single α subunit to be expressed along the nephron (Lucking et al. 1996), although the Ki for ouabain and the apparent Na+ affinity display axial differences (Doucet & Barlet, 1986; Barlet-Bas et al. 1990). Recent functional and localization results obtained for the γ subunits and CHIF suggest that the localized expression of these small ancillary subunits along the tubule might explain these differences (Arystarkhova et al. 1999, 2002; Therien et al. 1999; Beguin et al. 2001; Crambert & Geering, 2003). The α2 subunit is expressed in brain, skeletal muscle and heart, the α3 subunit in brain and the α4 subunit exclusively in testis. Rat α1, 2 and 3 are 87% identical and their functional differences in terms of Na+ and K+ kinetics appear relatively small (Blanco & Mercer, 1998). Functional parameters might, however, be to some extent species specific and have been determined for human Na+,K+-ATPase only in non-mammalian expression systems, i.e. in Xenopus oocytes, insect SF9 cells and yeast (Yu et al. 1997; Crambert et al. 2000; Muller-Ehmsen et al. 2001).
The aldosterone-sensitive distal nephron (ASDN) is composed of the second half of the distal convoluted tubule (DCT2), the connecting tubule (CNT) and the collecting duct (CD) (Loffing et al. 2001). In this region, aldosterone action depends on transcription and translation (Summa et al. 2001) and it is the site where fine tuning of the final urinary Na+ excretion takes place (Verrey et al. 2000). The Na+ reabsorption machinery of the ASDN segment-specific epithelial cells is formed from the epithelial Na+ channel ENaC and, in DCT2, the Na+–Cl− cotransporter (NCC), which mediate luminal Na+ influx, and by the Na+,K+-ATPase that extrudes Na+ to the basolateral side and maintains the driving force for apical Na+ entry. Aldosterone controls Na+ reabsorption by regulating this transport in the short and long term (Verrey et al. 2000; Masilamani et al. 2002). Functionally, the short-term effect of aldosterone is already apparent 30 min after initiation of treatment (Verrey et al. 2000). We have recently shown in adrenalectomized rats that aldosterone increases the cell-surface expression of ENaC and of the Na+,K+-ATPase within 2 h (Loffing et al. 2001; Summa et al. 2001; Verrey et al. 2003). The effect of aldosterone on the cell-surface expression of Na+,K+-ATPase could be reproduced in vitro using the mouse cortical collecting duct-derived mpkCCDc14 cell line (Bens et al. 1999). In this model, the increase in cell-surface expression correlates with an increase in Na+ pump current. It is independent of apical Na+ influx during hormonal stimulation and depends on transcription and translation (Summa et al. 2001). Short-term stimulation of Na+,K+-ATPase by aldosterone has been previously described in Xenopus laevis A6 cells, another model for distal nephron Na+ reabsorption. However, unlike in mammalian cortical collecting duct (CCD) and mpkCCD cells, this stimulation was not mediated by an increase in the number of cell-surface pumps, meaning that the underlying mechanism is probably not the same.
The aim of the present study was to test the hypothesis that the transcriptionally mediated effect of aldosterone on the cell-surface expression of Na+,K+-ATPase in mammalian CCD cells depends on isoform-specific structures of the α1 subunit. Therefore, we expressed the human Na+,K+-ATPase subunit isoforms α1, α2 and α3 in the mouse kidney cortical collecting duct cell line mpkCCDc14 and characterized their expression and their regulation by aldosterone. The results revealed an a isoform-specific distribution of the Na+,K+-ATPase and an α1 subunit-specific stimulation by aldosterone of the cell-surface Na+ pump function.
Methods
DNA constructs
The cDNAs encoding the human α1–3 subunits were obtained in pSD5 (a gift from Gilles Crambert and Käthi Geering) (Crambert et al. 2000). The original references and accession numbers are for hα1 (Kawakami et al. 1986) and D00099, for hα2 (Shull et al. 1989) and J05096, and for hα3 (Sverdlov et al. 1987) and X12910. In the case of hα1, we used a myc-tagged construct (gift from David Mordasini and Eric Féraille), because no species-specific antibody was available. cDNAs were subcloned into the retroviral vector pLPCX (Clonetech, Palo Alto, CA, USA), which contains a puromycin resistance gene. For control experiments, the enhanced green fluorescence protein (EGFP) was subcloned from EGFP.C1 into pLPCX.
Cell culture
The culture of mpkCCDcl4 cells (gift from Alain Vandewalle, Paris) (passages 18–43) was described by Bens et al. (1999). For electrophysiological measurements, mpkCCDcl4 cells were seeded on 12 mm diameter Transwell filters (Corning Costar, Bodenheim, Germany) and kept for 6–7 days in standard medium.
In the case of aldosterone stimulation, epithelia were transferred into serum- and hormone-deprived medium 12–20 h prior to the experiments. Aldosterone was used, because of the lack of sufficient mineralocorticoid receptor expression in these cells, at 10−6m to obtain near-maximal effects via the glucocorticoid receptor (Summa et al. 2001). The stimulatory effect on Na+ transport obtained via the glucocorticoid receptor is, in collecting duct cells, similar to that obtained via the mineralocorticoid receptor (Laplace et al. 1992). Na+ activation experiments were performed in standard medium. For immunofluorescence experiments, 24 mm diameter Transwell filters (Corning Costar) were used.
Phoenix ecotropic retrovirus producer cells, provided by the American Type Culture Collection (ATCC) with the permission of Dr G. Nolan (Stanford University), were cultured at 37°C and 5% CO2 in DMEM (Invitrogen, Basel, Switzerland, Cat. no. 41966-029) supplemented with 1 × 105 U ml−1 penicillin, 100 mg l−1 streptomycin, 2 mm l-glutamine and 10% fetal calf serum (FCS).
Retroviral transduction of mpkCCD cells
Stable cell lines of mpkCCDc14 cells expressing Na+,K+-ATPase α subunits were obtained according to Pear et al. (1997), using a slight modification of the protocol provided on the website http://www.stanford.edu/group/nolan/protocols/pro_helper_dep.html. Briefly, ecotropic pseudoretroviral producer cells (Phoenix) were plated 24 h prior to transfection at 50% density on 60 mm plates. The next day, 5 μg of the retroviral construct encoding either of the three Na+,K+-ATPase subunit α isoforms was applied using FuGENE6 (Roche, Basel, Switzerland) according to the manufacturer's recommendation. The medium was replaced after 24 h and the cells subsequently kept at 32°C. The pseudoretrovirus-containing supernatant was harvested after 24 h and filtered through a 45 μm sterile filter. After addition of Polybrene® to a final concentration of 4 μm, the entire supernatant (3 ml) was given to 5% confluent mpkCCDcl4 cells plated the day before on 6-well plates (35 mm diameter). The cells were centrifuged at 130 g and 32°C for 30 min and then kept for 6 h at 32°C (5% CO2) before the supernatant was replaced by usual medium. The procedure was repeated the next day using fresh retroviral supernatant harvested from the same Phoenix cells. A transduction efficiency of 70–100% was achieved when using EGFP (enhanced green fluorescent protein) as a marker. This protocol was repeated 2–5 times to achieve maximal expression. The resulting multiclonal cell lines were then kept under selective pressure, using first for one week 0.5 μg ml−1 and then 0.75–1 μg ml−1 puromycin.
Electrophysiological measurement of pump current (IP)
The potential difference and resistance across intact monolayers was measured with a Millicell (Millipore) device and the equivalent short-circuit current (Isc) was calculated according to Ohm's law. A 50% increase in equivalent Isc after 3 h aldosterone, compared with control cells, was set as a prerequisite for using the monolayer for pump current measurement (not reached in ∼5% of the cases). Na+ pump current (IP) was measured as previously described (Beron et al. 1995; Summa et al. 2001). Briefly, cells were washed and transferred to the chambers of a multichannel voltage-current clamp (Model VCC MC6 Revison B, Physiological Instruments, San Diego, CA, USA) and maintained under short-circuit conditions (clamped at 0 mV) in Na+-free buffer. Then, cells were permeabilized apically to monovalent ions using 35 μg ml−1 amphotericin B for 15 min at 37°C in Na+-free buffer containing (mm): 116 potassium gluconate, 1.8 CaCl2, 1.6 MgCl2, 0.8 KH2PO4, 2 d-glucose, 12 essential amino acids, 2 non-essential amino acids, 0.4 glutamine, 25 Hepes, 3 Ba(OH)2 and adjusted to pH 7.4 with Tris. Na+ was then added to the desired final concentration by mixing Na+ buffer with Na+-free buffer (Na+ replacement of K+) at both sides of the monolayer, and 3.5 min after Na+ addition, strophanthidin was added to the basolateral compartment to a final concentration of 50 μm to block exogenous cardiotonic steroid-sensitive pumps. One minute later, 1 mm ouabain was given to block the endogenous pumps.
For Na+ activation experiments, IP was measured on each filter culture at a single Na+ concentration. Because the level of exogenous IP varied between cell lines and experiments, the absolute IP values were normalized to the mean IP measured at 30 mm Na+ during the same experiment and on the same cell type (for the endogenous or exogenous pumps IP), before values from different experiments were pooled. The Hill equation was used to fit curves with a non-linear regression analysis routine (Prism, GraphPad) to the pooled data points, using a Hill coefficient fixed at 2.3 (Pfeiffer et al. 1999). Experimental series in which any of the tested conditions (Na+ concentration or ± aldosterone) produced no exogenous IP were excluded (∼10% of the series).
RT-PCR
Total RNA was prepared using Rneasy Mini Kit (Qiagen Science, Germantown, MD, USA) from mpkCCD cells cultured on plastic and on filter supports and from mouse total kidney. A 100 ng sample of each RNA was used as template for cDNA synthesis in 25 μl reaction volume using random hexamer primers and the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA, USA). PCR was performed with Eurobiotaq polymerase (Eurobio, Les Ulis, France) using 1 μl of these cDNAs as a template and the following primers: γa sense, GAT CTG TCA GCG AAC AGT G; γb sense, CTA CCA TGG ACA GGT GGT A; γc sense, GAT TCT GGC TGG AAA CCT; antisense for γa, b, and c, GGA GTG GGG TGT TAG AGA A, CHIF sense, ATG GAG GAA ATC ACC TGT GC; CHIF antisense, GCA GGC TCT CCC AGT CAT AG. The following cycling parameters were used: denaturation, 94°C, 45 s; annealing, 60°C (10 cycles) and 57°C (20 cycles), 45 s; elongation, 72°C,45 s. The reaction products were run on agarose gels in the presence of ethidium bromide.
Immunoblotting
Cells from a 60 mm dish were washed 3 times with ice-cold PBS and lysed in 400 μl 2 × loading buffer (150 mm Tris (pH 6.8), 1.2% SDS, 30% glycerol, 0.0018% bromophenol blue, 2.5%β-mercaptoethanol). Lysates were incubated at 65°C for 5 min and equal amounts were loaded on the gel. After electrophoresis, proteins were transferred onto nitrocellulose (Protran, from Schleicher & Schuell, Dassel, Germany). Equal loading and transfer was verified qualitatively by Ponceau red staining (not shown). The membranes were then incubated first for 1 h at room temperature in blocking solution (5% milk powder, 0.4% IGEPAL (Sigma, Steinheim, Germany), 10 mm Tris-HCl pH 7.4, 0.9% NaCl, and then for 2 h with the primary antibody in blocking solution. Mouse monoclonal anti-myc was used at 1: 5000 (Invitrogen, Basel, Switzerland), polyclonal rabbit anti-α2 at 1: 4000 (gift from Alicia McDonough, Los Angeles, CA, USA) (Pressley, 1992) and mouse monoclonal IgG1 anti-α3 at 1: 1000 (Affinity BioReagents Inc., La-Roche, Switzerland). Incubation with the horseradish peroxidase (HRP)-coupled secondary antibody was for 1.5 h at room temperature using HRP-coupled sheep antimouse Ig at 1: 10 000 (Amersham, Life Science, Dübendorf, Switzerland) or HRP-coupled sheep antirabbit IgG at 1: 10 000 (Transduction Laboratories, San Diego, CA, USA). Signal was detected by chemiluminescence (SuperSignal® West Pico, Pierce, Rockford, IL, USA) and exposure to film (Kodak X-OMAT, Sigma).
Immunofluorescence
Washed filter cultures were fixed in a mixture of 3% paraformaldehyde and 0.2% Triton X-100 for 15 min at room temperature. Filters were then washed again three times, cut into squares and incubated overnight in 48-well plates at 4°C with primary antibodies diluted in 0.5% BSA–PBS solution (mouse anti-myc 1: 2000, goat anti-α2 (Sant Cruz Biotechnology, Nunningen, Switzerland) 1: 100). After three washes, filters were incubated for 4–6 h with the secondary antibody: anti-mouse, Cy3-conjugated, 1: 400 or anti-goat, Cy2-conjugated, 1: 100. After another round of washing, filters were mounted in DAKO-glycergel (DAKO, Glostrup, Denmark) containing 2.5% 1,4-diazabicyclo [2,2,2] octane (DABCO) as fading retardant. Confocal images were taken using a Leica laser scannig microscope (TCSSP, Wetzlar, Germany) equipped with a × 63 oil immersion objective. Filter cultures stained without primary and/or secondary antibody, served as controls.
Statistics
The results are expressed as means ±s.e.m. from n filter cultures (aldosterone stimulation, Na+ activation experiments) or independent experiments. Statistical analyses were done by unpaired, two-tailed Student's t test. A P value smaller than 0.05 was considered as significant.
Results
Exogenous human Na+,K+-ATPase α1 and α2 subunit isoforms form functional basolateral pumps in mpkCCDcl4 epithelia
mpkCCDc14 cells expressing the human α1, α2 and α3 subunits of the Na+,K+-ATPase were generated by repeated transduction and selected using puromycin. The exogenous subunits are expected to assemble with the endogenous β1 subunit and to form functional pumps as previously shown in other heterologous expression systems (Pfeiffer et al. 1999).
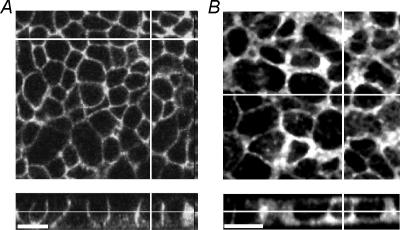
Western blots with isoform-specific antibodies showed that all three human α subunit isoforms were successfully expressed in mpkCCDcl4 cells (Fig. 1). Immunolocalization revealed that hα1myc is mostly expressed at or near the basolateral membrane (Fig. 2) as reported for the endogenous Na+,K+-ATPase (Bens et al. 1999). In contrast, hα2 was also expressed within the cells and possibly even to a small extent at the apical surface. It is not clear whether the presence of hα2 in the intracellular compartment is, to some extent, secondary to its overexpression. Although the immunoblotting had revealed a strong specific band for the ha3 subunit expressed in mpkCCDc14 cells, our attempts to immnunolocalize it did not reveal any specific labelling that was different from the background staining (not shown).
Figure 1. Western blot showing the expression of three human Na+,K+-ATPase α subunit isoforms in transduced mpkCCDcl4 cells.

Cell lysates from mpkCCDcl4 wild-type cells (lanes 2, 4 and 6) and mpkCCDcl4 cell lines expressing hα1myc (lane 1), hα2 (lane 3), and hα3 subunit (lane 5) were probed with anti-myc (lane 1 and 2), anti-α2 (lane 3 and 4) or anti-α3 (lane 5 and 6) antibody. Numbers on the left are kDa. The α subunits appear as ∼110 kDa bands. The band at ∼58 kDa in lanes 5 and 6 is unspecific.
Figure 2. Localization of human Na+,K+-ATPase α1 and α2 isoforms in transduced mpkCCDcl4 cells.
Immunofluorescence images of mpkCCDcl4 filter cultures expressing hα1myc and hα2 are shown in A and B, respectively. En face confocal images shown in the upper panels are taken at the level of the nucleus. The lower panels represent corresponding Z-Y reconstitutions. Bar: 15 μm; A, staining of the exogenous Na+,K+-ATPase hα1myc subunit in transduced mpkCCDcl4 cells reveals that a large portion of this protein is localized at or near the basolateral membrane. B, much of the staining of the exogenous hα2 Na+,K+-ATPase subunit is intracellular.
Functionally, the transduced mpkCCDc14 cell lines grown to confluence on permeable filter supports fulfilled the typical electrophysiological features of tight epithelia, displaying a high transepithelial electrical resistance ranging from 2.3 to 4.5 kΩ cm2. Similar values for transepithelial resistances were reported in the characterization study of mpkCCDc14 (Bens et al. 1999).
On transduced cells, the function of hybrid pumps containing a human α subunit associated with an endogenous mouse β1 subunit could be detected and distinguished from the endogenous pumps (mouse α1-β1) by the fact that they display a higher sensitivity towards cardiotonic steroids (Price & Lingrel, 1988). Thus, blocking the IP carried by the hybrid pumps was achieved by a low concentration of strophanthidin (50 μm) and the IP carried by endogenous pumps was subsequently blocked by a high concentration of the ∼10-fold more potent cardiotonic steroid ouabain (1 mm). Tracings from hα1myc- and hα2-transduced cells (Fig. 3) revealed the presence of a cardiotonic steroid-sensitive exogenous IP that generally amounted to 20–50% of the total IP (mean absolute values of endogenous and exogenous IP (30 mm Na+) are indicated in the legend of Fig 4. In contrast, no inhibition of exogenous current by strophanthidin was observed in hα3-transduced cells, which even often displayed a small increase in apical to basolateral current, the significance of which is unknown. Thus, hα3 did not form a measurable proportion of functional basolateral pumps.
Figure 3. Original recordings of endo- and exogenous Na+,K+-ATPase current (IP) in apically amphotericin-permeabilized mpkCCDcl4 cell epithelia.
The arrowheads indicate the addition of 30 mm Na+ into both apical and basolateral compartments. Strophanthidin (50 μm) blocked the Na+-activated current carried by the Na+ pumps containing an exogenous human α isoform, whereas 1 mm ouabain blocked the current carried by the Na+ pumps containing the ouabain-resistant endogenous mouse α1 subunit. In contrast to hα1myc- and hα2-transduced mpkCCDcl4 cells, hα3 subunit-transduced cells showed no exogenous, strophanthidin-sensitive IP.
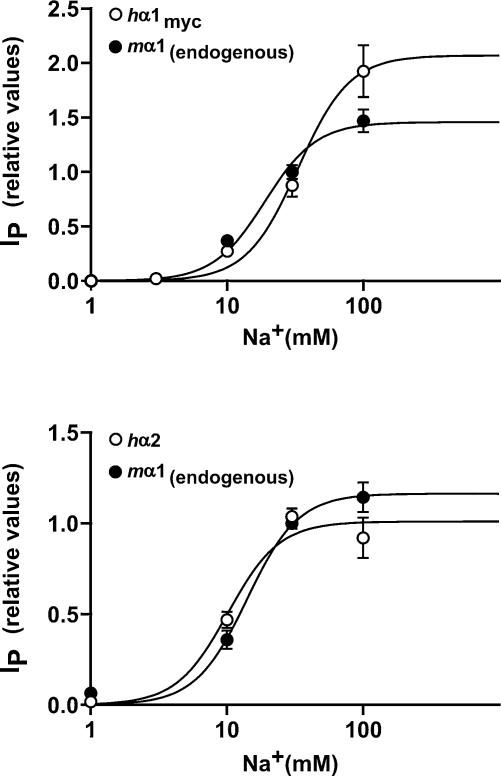
Figure 4. Na+-activation of endogenous Na+,K+-ATPase and of Na+,K+-ATPase containing an exogenous α subunit (hα1myc or hα2).
The IP carried by endogenous pumps (•) and by pumps containing the exogenous subunit hα1myc (A) or hα2 (B) (○) were measured at various Na+ concentrations after permeabilization of the apical membrane to monovalent ions with amphotericin B. Shown are means of relative IP values ±s.e.m. that were each obtained from two cell lines expressing hα1myc or hα2. Because of the differential proportions of exogenous current, the values were normalized in each experiment to the IP measured at 30 mm Na+ (mean normalization values in hα1myc-expressing epithelia (4 experiments) were 4.95 ± 0.64 μA for endogenous pumps and 2.09 ± 0.62 μA for exogenous pumps, and in hα2-expressing epithelia (6 experiments) they were 3.07 + 0.80 μA for endogenous pumps and 2.21 ± 0.75 μA for exogenous pumps). Sigmoidal dose–response curves were fitted to the experimental points using a Hill coefficient fixed at 2.3 and forcing the curves through zero (Pfeiffer et al. 1999; Summa et al. 2001). A, the derived K0.5 for Na+ of the endogenous Na+,K+-ATPase is 19 mm (95% confidence interval 15.0–24.0 mm) and that of the ATPase containing the exogenous hα1myc is 33.2 mm (95% confidence interval 25.6–43.1 mm). B, the derived K0.5 values for Na+ in the hα2-expressing cell lines are 13.6 mm (95% confidence interval 11.5–16.0 mm) for the endogenous Na+,K+-ATPase and 10 mm (95% confidence interval 8.0–12.4 mm) for the hα2-containing Na+,K+-ATPase.
Na+ dependence of hα1myc- and hα2-containing Na+,K+-ATPase in mpkCCDcl4 cells
Measurements of the Na+ activation of cardiotonic steroid-sensitive pumps containing an exogenous α subunit indicated that pumps expressed in mpkCCDcl4 epithelia containing hα1myc have a lower apparent Na+ affinity (K0.5: 33.2 mm) than pumps containing hα2 (K0.5: 10 mm). The K0.5 measured for the endogenous Na+,K+-ATPase (mouse α1 subunit) was in between, namely 19 mm and 13.6 mm in cells expressing hα1myc and hα2 subunits, respectively (Fig. 4). The values obtained for hα1myc and hα2 were derived each from two independent cell lines.
To address the question of whether FXYD proteins might be expressed in mpkCCD cells and thus modulate the apparent Na+ affinity of the Na+,K+-ATPase, we used RT-PCR to test whether the mRNAs of the kidney-specific FXYD proteins, the Na+,K+-ATPase γ subunit isoforms a, b and c (FXYD2) and CHIF (FXYD4), were present in mpkCCD cells (data not shown) (Arystarkhova et al. 1999, 2002; Therien et al. 1999; Beguin et al. 2001; Crambert & Geering, 2003). The band corresponding to the CHIF cDNA fragment, amplified in 30 cycles from 4 ng RNA from mpkCCD filter cultures, was of similar intensity to that obtained from the same amount of mouse kidney RNA. In contrast, only the cDNA fragment of the γ subunit isoform b was amplified from mpkCCD RNA, and then only weakly, whereas using mouse kidney RNA as template, the fragments corresponding to γa and γb were strongly amplified and that corresponding to γc was weakly amplified. These results suggest that only CHIF, known to increase the apparent affinity of the Na+,K+-ATPase for Na+, is expressed at a substantial level in mpkCCD cells and thus may have impacted on the apparent Na+ affinity of the Na+,K+-ATPases containing the exogenously expressed human α1 (and α2) subunit.
Aldosterone stimulates pumps containing hα1myc subunits but not pumps containing hα2 subunits
Figure 5A and B shows that the Na+ transport measured as equivalent Isc was increased by aldosterone (3 h) in epithelia expressing hα1myc and hα2. Aldosterone also increased the total activity of the basolateral Na+,K+-ATPase in these cells, as indicated by an increase in total IP measured at 30 mm Na+ in amphotericin-permeabilized epithelia (Fig. 5C–F). In cell lines expressing hα1myc, the aldosterone-induced fractional change in endogenous IP was 1.53 ± 0.1 and the change in IP carried by hybrid pumps containing the hα1myc subunit was also increased (2.12 ± 0.42). In hα2-transduced cell lines, the endogenous IP was increased by a factor of 1.5 ± 0.16, whereas that carried by the Na+,K+-ATPase containing the exogenous hα2 subunit was at the level of the control (0.96 ± 0.07). In summary, aldosterone stimulated pumps containing the exogenous human α1 subunit (hα1myc) as much as endogenous pumps, whereas it did not stimulate pumps containing the hα2 subunit.
Figure 5. Stimulation by aldosterone (3 h) of IP (30 mmNa+) carried by pumps containing human Na+,K+-ATPase α isoforms in mpkCCDC14 cell epithelia.
A and B, the effect of aldosterone on transepithelial Na+ transport in intact epithelia (short-circuit current (Isc)) is shown for cells expressing hα1myc (A) and hα2 (B). C and D, the pump current IP was induced by the addition of 30 mm Na+ on apically amphotericin-permeabilized epithelia. The IP produced by the exogenous hybrid pumps was blocked first by the addition of 50 μm strophanthidin (dotted arrow) and subsequently the Ip carried by the endogeneous pumps was blocked by the addition of 1 mm ouabain (continuous line). Original tracings of epithelia expressing hα1myc (C) and hα2 (D) are shown. E shows the mean fractional change in IP induced by aldosterone (aldosterone (A) versus control (C)) in pumps containing the endogenous α1 and the exogenous hα1myc subunits (n= 4). F shows the effect of aldosterone on the IP carried by the pumps containing the endogenous α1 subunit and the lack of effect on those containing the exogenous hα2 subunit (n= 3). *P < 0.05; ***P < 0.01.
Discussion
α subunit isoform-specific localization of Na+,K+-ATPase
The α1 subunit of the Na+,K+-ATPase is generally associated with the β1 subunit to form the ubiquitous Na+ pump that fulfils housekeeping functions in nearly every cell. This pump also plays a major role in driving specialized functions of cells and tissues, such as transepithelial transport of solutes and water in kidney tubules in which this isoform combination is (almost) the only one (Lucking et al. 1996). In cells of other tissues that express more than one α subunit isoform, their subcellular localization/distribution is differentiated, as shown for instance in heart muscle cells (Juhaszova & Blaustein, 1997; Slezak et al. 1997).
Using a retroviral expression system, we expressed the human α1–3 Na+,K+-ATPase subunit isoforms in mouse-derived kidney cortical collecting duct epithelial cells (mpkCCDc14 cell line). In the case of cells transduced with hα1 (NH2-terminal myc tag) and hα2 subunit, we observed a cardiotonic steroid-sensitive pump current that revealed the presence of functional hybrid pump units at the basolateral membrane. In contrast, hα3-containing hybrid pumps were not detected functionally in this membrane. Interestingly, the subcellular localization of hα1 and hα2 subunit isoforms was also not identical. Whereas the localization pattern observed by immunofluorescence for the α1 subunit was very similar to that observed for endogenous pumps (Bens et al. 1999), the α2 subunit was to a large extent also distributed intracellularly. The question of whether this intracellular localization is due to a lack of efficient signals for basolateral surface targeting/localization in the hα2 subunit and/or to its overexpression remains open.
α Subunit isoform-specific apparent Na+ affinity
The functional characteristics of α subunit isoforms have been studied mostly by expressing the naturally ouabain-resistant rat α1 subunit and/or the rat α2 and α3 subunits rendered ouabain-resistant by site-directed mutagenesis in cells that have a ouabain-sensitive α subunit or by overexpression of subunits in non-mammalian cells such as Xenopus laevis oocytes, insect SF9 cells or yeast. In terms of intracellular apparent Na+ affinity, isoform-specific differences have been noticed, some of which remain controversial. For instance, some studies suggest that α1 subunit-containing pumps have a higher apparent affinity for intracellular Na+ than those containing an α2 subunit (Munzer et al. 1994; Zahler et al. 1997; Crambert et al. 2000). In contrast, our earlier experiments performed in Xenopus kidney A6 cells and some other studies indicate that the apparent Na+ affinity of pumps containing a rat or Xenopus laevisα1 subunit is slightly lower than that of pumps with a rat α2 subunit (Jewell & Lingrel, 1991; Blanco et al. 1995; Pfeiffer et al. 1999; Horisberger & Kharoubi-Hess, 2002). In the present study, the apparent Na+ affinity measured for human α2 subunit -containing pumps is similar to the values obtained by others using Xenopus oocytes or the yeast expression system (Crambert et al. 2000; Muller-Ehmsen et al. 2001). However, the apparent Na+ affinity of pumps containing an α1 subunit (human or mouse) is, in the present study, lower than that of pumps with hα2 and values published for hα1 by others (Crambert et al. 2000; Muller-Ehmsen et al. 2001). The current values resemble those obtained previously for rat α1 subunits expressed in amphibian kidney A6 cells. Because we thought that in both cases the intrinsic apparent Na+ affinity of α1 subunit-containing pumps could have been decreased by the presence of an endogenous γ subunit in these cells of distal nephron origin, we tested the possible expression of such renal FXYD gene products in mpkCCD cells (Arystarkhova et al. 1999; Therien et al. 1999; Crambert & Geering, 2003). Surprisingly, the only mRNA for these FXYD proteins substantially expressed in mpkCCD cells was that of CHIF (FXYD4), a protein known to increase the apparent Na+ affinity of Na+,K+-ATPase. Thus, the relatively low apparent Na+ affinity of the pumps containing the human α1 subunit expressed in mpkCCD cells cannot apparently be explained by the expression of an endogenous Na+,K+-ATPase γ subunit (FXYD2).
α Subunit isoform-specific regulation of Na+,K+-ATPase by aldosterone
In mpkCCD cells, as in cortical collecting ducts, aldosterone induces the translocation of a substantial number of Na+,K+-ATPase units from an intracellular pool to the basolateral cell surface. Quantitatively, aldosterone increases, within 2–3 h, the amount of Na+,K+-ATPase accessible at the basolateral surface, by a factor of approximately 5 in tubules of adrenalectomized animal and by 20–50% in the mpkCCD cell culture system. This effect is independent of the apical influx of Na+ but requires ongoing transcription and translation, presumably because this effect is mediated by aldosterone-induced proteins (Summa et al. 2001). It is thought that the activation of Na+ pumps by aldosterone plays a parallel and supportive role to the activation of apical ENaC. Indeed, an increase in apical Na+ influx via ENaC needs to be compensated at the basolateral side by an increase in Na+ efflux via the Na+,K+-ATPase (Verrey et al. 2000). The aldosterone-induced increase in the number of functional pumps provides such a compensatory adaptation without requiring a rise in intracellular Na+ concentration. Without such a mechanism, the intracellular Na+ concentration would need to increase in order to kinetically activate the pumps. An increase in intracellular Na+ concentration would, by feedback inhibition, lead to a limitation of ENaC function and thus restrict the Na+ transport function (Verrey et al. 2000).
Interestingly, we observed in the present study that pumps containing the human α1 subunit exogenously expressed in mpkCCD cells are also regulated by short-term aldosterone, whereas those containing the human α2 subunit were not affected. This contrasts with observations made in muscle cells stimulated with insulin in which both α1- and α2-containing Na+ pumps are translocated to the cell surface (Al-Khalili et al. 2003). This discrepancy highlights the fact that different cells are not equipped with identical regulated compartments and thus that seemingly similar mechanisms can involve different regulatory and/or target proteins.
In a former study using Xenopus laevis distal nephron A6 cells, we had observed that aldosterone regulates pumps containing α1 subunits and not pumps containing an α2 subunit, similar to the observation made now in mpkCCD cells. However, the mechanism of regulation observed in these amphibian cells did not concern the cell surface expression of the pumps (no Vmax effect) and was thus different from the present effect observed in mammalian collecting duct cells (Pfeiffer et al. 1999). The presently observed differential sensitivity towards aldosterone of pumps containing an α1 versus an α2 subunit, as well as their differential subcellular localization, suggests that the α1 subunit contains specific structures that direct the targeting and/or retention of pumps in specific membrane compartments and allow aldosterone to induce their translocation from an intracellular compartment to the basolateral surface. The differential response of pumps containing highly similar human α subunit isoforms (α1 versusα2) opens the possibility of identifying the structures within the α1 subunit that are required for this regulatory action.
Acknowledgments
The authors thank Gilles Crambert and Käthi Geering (Lausanne) for the human α subunit cDNAs, Alicia McDonough (Los Angeles) for the polyclonal anti-α2 antibody, David Mordasini and Eric Féraille (Geneva) for the hα1myc cDNA, Alain Vandewalle (Paris) for the mpkCCDcl4 cells and Christian Gasser for the artwork. This study was supported by the Swiss NSF grant 31-59141.99 to François Verrey.
References
- Al-Khalili L, Yu M, Chibalin AV. Na+,K+-ATPase trafficking in skeletal muscle: insulin stimulates translocation of both alpha 1- and alpha 2-subunit isoforms. FEBS Lett. 2003;536:198–202. doi: 10.1016/s0014-5793(03)00047-4. [DOI] [PubMed] [Google Scholar]
- Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit modulates Na+ and K+ affinity of the renal Na,K- ATPase. J Biol Chem. 1999;274:33183–33185. doi: 10.1074/jbc.274.47.33183. [DOI] [PubMed] [Google Scholar]
- Arystarkhova E, Wetzel RK, Sweadner KJ. Distribution and oligomeric association of splice forms of Na+-K+-ATPase regulatory gamma-subunit in rat kidney. Am J Physiol Renal Physiol. 2002;282:F393–F407. doi: 10.1152/ajprenal.00146.2001. [DOI] [PubMed] [Google Scholar]
- Barlet-Bas C, Cheval L, Khadouri C, Marsy S, Doucet A. Difference in the Na affinity of Na+-K+-ATPase along the rabbit nephron: modulation by K. Am J Physiol. 1990;259:F246–F250. doi: 10.1152/ajprenal.1990.259.2.F246. [DOI] [PubMed] [Google Scholar]
- Beguin P, Crambert G, Guennoun S, Garty H, Horisberger JD, Geering K. CHIF, a member of the FXYD protein family, is a regulator of Na,K-ATPase distinct from the gamma-subunit. EMBO J. 2001;20:3993–4002. doi: 10.1093/emboj/20.15.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol. 1999;10:923–934. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]
- Beron J, Mastroberardino L, Spillmann A, Verrey F. Aldosterone modulates sodium kinetics of Na,K-ATPase containing an alpha 1 subunit in A6 kidney cell epithelia. Mol Biol Cell. 1995;6:261–271. doi: 10.1091/mbc.6.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Blanco G, Sanchez G, Mercer RW. Comparison of the enzymatic properties of the Na,K-ATPase alpha 3 beta 1 and alpha 3 beta 2 isozymes. Biochemistry. 1995;34:9897–9903. doi: 10.1021/bi00031a011. [DOI] [PubMed] [Google Scholar]
- Crambert G, Geering K. FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase. Sci STKE. 2003;2003:RE1. doi: 10.1126/stke.2003.166.re1. [DOI] [PubMed] [Google Scholar]
- Crambert G, Hasler U, Beggah ATYuC, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na, K- ATPase isozymes. J Biol Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- Doucet A, Barlet C. Evidence for differences in the sensitivity to ouabain of NaK-ATPase along the nephrons of rabbit kidney. J Biol Chem. 1986;261:993–995. [PubMed] [Google Scholar]
- Hasler U, Wang X, Crambert G, Beguin P, Jaisser F, Horisberger JD, Geering K. Role of beta-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J Biol Chem. 1998;273:30826–30835. doi: 10.1074/jbc.273.46.30826. [DOI] [PubMed] [Google Scholar]
- Horisberger JD, Kharoubi-Hess S. Functional differences between alpha subunit isoforms of the rat Na,K-ATPase expressed in Xenopus oocytes. J Physiol. 2002;539:669–680. doi: 10.1113/jphysiol.2001.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell EA, Lingrel JB. Comparison of the substrate dependence properties of the rat Na,K-ATPase alpha 1, alpha 2, and alpha 3 isoforms expressed in HeLa cells. J Biol Chem. 1991;266:16925–16930. [PubMed] [Google Scholar]
- Juhaszova M, Blaustein MP. Distinct distribution of different Na+ pump alpha subunit isoforms in plasmalemma. Physiological implications. Ann N Y Acad Sci. 1997;834:524–536. doi: 10.1111/j.1749-6632.1997.tb52310.x. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Ohta T, Nojima H, Nagano K. Primary structure of the alpha-subunit of human Na,K-ATPase deduced from cDNA sequence. J Biochem (Tokyo) 1986;100:389–397. doi: 10.1093/oxfordjournals.jbchem.a121726. [DOI] [PubMed] [Google Scholar]
- Laplace JR, Husted RF, Stokes JB. Cellular responses to steroids in the enhancement of Na+ transport by rat collecting duct cells in culture. Differences between glucocorticoid and mineralocorticoid hormones. J Clin Invest. 1992;90:1370–1378. doi: 10.1172/JCI116003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol. 2001;280:F675–F682. doi: 10.1152/ajprenal.2001.280.4.F675. [DOI] [PubMed] [Google Scholar]
- Lucking K, Nielsen JM, Pedersen PA, Jorgensen PL. Na-K-ATPase isoform (alpha 3, alpha 2, alpha 1) abundance in rat kidney estimated by competitive RT-PCR and ouabain binding. Am J Physiol. 1996;271:F253–F260. doi: 10.1152/ajprenal.1996.271.2.F253. [DOI] [PubMed] [Google Scholar]
- Masilamani S, Wang X, Kim GH, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol. 2002;283:F648–F657. doi: 10.1152/ajprenal.00016.2002. [DOI] [PubMed] [Google Scholar]
- Muller-Ehmsen J, Juvvadi P, Thompson CB, Tumyan L, Croyle M, Lingrel JB, Schwinger RH, McDonough AA, Farley RA. Ouabain and substrate affinities of human Na+-K+-ATPase alpha1beta1, alpha2beta1, and alpha3beta1 when expressed separately in yeast cells. Am J Physiol Cell Physiol. 2001;281:C1355–C1364. doi: 10.1152/ajpcell.2001.281.4.C1355. [DOI] [PubMed] [Google Scholar]
- Munzer JS, Daly SE, Jewell-Motz EA, Lingrel JB, Blostein R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J Biol Chem. 1994;269:16668–16676. [PubMed] [Google Scholar]
- Pear W, Scott M, Nolan GP. Generation of High Titre, Helper-Free Retroviruses by Transient Transfection. Totowa, NJ, USA: Humana Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R, Beron J, Verrey F. Regulation of Na+ pump function by aldosterone is alpha-subunit isoform specific. J Physiol. 1999;516:647–655. doi: 10.1111/j.1469-7793.1999.0647u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressley TA. Phylogenetic conservation of isoform-specific regions within alpha-subunit of Na+-K+-ATPase. Am J Physiol. 1992;262:C743–C751. doi: 10.1152/ajpcell.1992.262.3.C743. [DOI] [PubMed] [Google Scholar]
- Price EM, Lingrel JB. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988;27:8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- Shull MM, Pugh DG, Lingrel JB. Characterization of the human Na,K-ATPase alpha 2 gene and identification of intragenic restriction fragment length polymorphisms. J Biol Chem. 1989;264:17532–17543. [PubMed] [Google Scholar]
- Slezak J, Schulze W, Okruhlicova L, Tribulova N, Singal PK. Cytochemical and immunocytochemical localization of Na,K-ATPase alpha subunit isoenzymes in the rat heart. Mol Cell Biochem. 1997;176:107–112. [PubMed] [Google Scholar]
- Summa V, Mordasini D, Roger F, Bens M, Martin PY, Vandewalle A, Verrey F, Feraille E. Short term effect of aldosterone on Na,K-ATPase cell surface expression in kidney collecting duct cells. J Biol Chem. 2001;276:47087–47093. doi: 10.1074/jbc.M107165200. [DOI] [PubMed] [Google Scholar]
- Sverdlov ED, Monastyrskaya GS, Broude NE, Ushkarev YA, Melkov AM, Smirnov YV, Malyshev IV, Allikmets RL, Kostina MB, Dulubova IE, Kiyatkin NI, Grishin AV, Modyanov NN, Ovchinnikov YA. Family of human Na+, K+-ATPase genes. Structure of the gene of isoform alpha-III. Dokl Biochem. 1987;297:426–431. [Google Scholar]
- Therien AG, Karlish SJ, Blostein R. Expression and functional role of the gamma subunit of the Na, K-ATPase in mammalian cells. J Biol Chem. 1999;274:12252–12256. doi: 10.1074/jbc.274.18.12252. [DOI] [PubMed] [Google Scholar]
- Verrey F, Hummler E, Schild L, Rossier BC. Control of Na+ transport by aldosterone. In: Seldin DW, Giebiesch G, editors. The Kidney, Physiology and Pathophysiology. 3. Philadelphia: Lippincott. Williams & Wilkins; 2000. pp. 1441–1471. [Google Scholar]
- Verrey F, Summa V, Heitzmann D, Mordasini D, Vandewalle A, Féraille E, Zecevic M. Jorgensen P, editor. Short-term aldosterone action on Na,K-ATPase surface expression: role of aldosterone-induce SGK1. Ann N Y Acad Sci. 2003;986:570–278. doi: 10.1111/j.1749-6632.2003.tb07253.x. In 10th International Conference on Na,K-Atpase and Related Cation Pumps. [DOI] [PubMed] [Google Scholar]
- Yu C, Xie Z, Askari A, Modyanov NN. Enzymatic properties of human Na,K-ATPase alpha1beta3 isozyme. Arch Biochem Biophys. 1997;345:143–149. doi: 10.1006/abbi.1997.0255. [DOI] [PubMed] [Google Scholar]
- Zahler R, Zhang ZT, Manor M, Boron WF. Sodium kinetics of Na,K-ATPase alpha isoforms in intact transfected HeLa cells. J General Physiol. 1997;110:201–213. doi: 10.1085/jgp.110.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]