Abstract
Hand–arm vibration syndrome is a vascular disease of occupational origin and a form of secondary Raynaud's phenomenon. Chronic exposure to hand-held vibrating tools may cause endothelial injury. This study investigates the biomechanical forces involved in the transduction of fluid vibration in the endothelium. Human endothelial cells were exposed to direct vibration and rapid low-volume fluid oscillation. Rapid low-volume fluid oscillation was used to simulate the effects of vibration by generating defined temporal gradients in fluid shear stress across an endothelial monolayer. Extracellular signal-regulated kinase (ERK1/2) phosphorylation and endothelin-1 (ET-1) release were monitored as specific biochemical markers for temporal gradients and endothelial response, respectively. Both vibrational methods were found to phosphorylate ERK1/2 in a similar pattern. At a fixed frequency of fluid oscillation where the duration of each pulse cycle remained constant, ERK1/2 phosphorylation increased with the increasing magnitude of the applied temporal gradient. However, when the frequency of flow oscillation was increased (thus decreasing the duration of each pulse cycle), ERK1/2 phosphorylation was attenuated across all temporal gradient flow profiles. Fluid oscillation significantly stimulated ET-1 release compared to steady flow, and endothelin-1 was also attenuated with the increase in oscillation frequency. Taken together, these results show that both the absolute magnitude of the temporal gradient and the frequency/duration of each pulse cycle play a role in the biomechanical transduction of fluid vibrational forces in endothelial cells. Furthermore, this study reports for the first time a link between the ERK1/2 signal transduction pathway and transmission of vibrational forces in the endothelium.
Hand–arm vibration syndrome (HAVS), also known as vibration-induced white finger, is a form of secondary Raynaud's phenomenon and is of occupational origin. HAVS is associated with prolonged exposure to vibration transmitted to the human hand and arm from hand-held power tools and vibrating machines. HAVS is both a vascular and neurological disorder. Acute inflammation in the hands and fingers can occur following heavy use of vibrating devices (Verdon, 1996; Lau et al. 1992; Liapina et al. 2002). With continued use of vibrating devices, chronic vascular symptoms are characterized by episodic vasospasm and blanching of the fingers on exposure to cold or emotional stress. As the condition progresses, vasospasms can occur even at room temperatures. Among workers using vibrating tools, the prevalence of vascular symptoms can be as high as 70% (Lie, 1998). To date, studies on the pathophysiological aspects of HAVS have focused primarily on the clinical manifestation and diagnosis of the syndrome (Ho & Belch, 1998), or the biodynamic response characteristics of whole hand–arm vibrational energy absorption (Dong et al. 2001; Rakheja et al. 2002). Little attention has been paid to the mechanochemical signal transduction of vibration in the vascular endothelium. Given the prevalence of the problem, it is increasingly important to identify and obtain a better understanding of the biomechanical forces involved in the pathophysiology of HAVS.
Vibration is characterized by rapidly changing compressive and expansive mechanical forces. In the fluid environment of the vasculature, such mechanical forces expose the endothelial monolayer not only to mechanical deformation, but also to rapid changes in fluid shear stress. Fluid shear stress is the frictional force that is generated parallel to the luminal cell surface as the mass of the cell is moved through its liquid environment about its equilibrium position. Whenever rapid periodic changes in fluid shear stress occur, two stimuli must be considered: the magnitude of the change in shear stress, and the temporal change in shear stress. Such temporal gradients in shear stress are defined as the localized change in shear stress over a small period of time at any given point. Temporal gradients in fluid shear stress have been shown to stimulate specific and distinct biochemical pathways in human endothelial monolayers (Bao et al. 1999, 2000, 2001). Large temporal gradients in fluid shear due to the change of shear direction have been linked to the pathogenesis of other endothelial and vascular disorders such as atherosclerosis (Ku et al. 1985; Ojha, 1994) and intimal hyperplasia (Keynton et al. 2001; Loth et al. 2003).
To better elucidate the biomechanical transduction of fluid vibrational forces in human endothelial cells, the present studies examined the effects of direct vibrational exposure and rapid low-volume fluid oscillation on primary human endothelial monolayers. Rapid low-volume fluid oscillation was used to simulate the effects of direct vibration by generating defined temporal gradients in fluid shear stress across the endothelial monolayer. This novel approach to the study of vibrational effects on the endothelium allows for the accurate quantification and manipulation of the magnitude and frequency of the temporal gradients applied to the endothelial monolayer. The rapid phosphorylation of the extracellular signal-regulated kinase ERK1/2 was used as a specific biochemical marker for the effects of vibration on the endothelium. ERK1/2 is a member the mitogen-activated protein kinase (MAPK) conserved cascade of kinases that stimulate the phosphorylation of transcription factors and other targets in response to extracellular signals such as growth factors, cytokines, and fluid shear stress (Widmann et al. 1999). Phosphorylation of the ERK1/2 protein was chosen as a marker of biomechanical transduction of vibration in human endothelial cells for two reasons. First, ERK1/2 is a known shear responsive protein and is rapidly and specifically phosphorylated within cells exposed to temporal gradients in fluid shear stress (Bao et al. 2000, 2001; Surapisitchat et al. 2001; Loth et al. 2003). Secondly, although the ERK pathway is thought to be primarily involved in the regulation of cell proliferation and differentiation (Karin & Hunter, 1995), phosphorylation of the ERK1/2 protein has also been shown to act as mediator of the inflammatory process (Suttles et al. 1999). Given the acute inflammation of the hands and fingers that often occurs following the heavy use of vibrating devices it is possible that the ERK1/2 pathway may be involved in this acute response to vibration.
To validate the physiological relevance of the rapid low-volume fluid oscillation model as an effective in vitro simulation of vibration, endothelial production and release of the potent vasoconstrictor endothelin-1 (ET-1) was also investigated. In patients diagnosed with HAVS, impaired endothelial release of nitric oxide and elevated levels of plasma ET-1 have been reported (Lau et al. 1995; Palmer & Mason, 1996; Ho & Belch, 1998). The rise in plasma ET-1 is thought to be a specific endothelial response to vibration, and not a simple marker of endothelial damage. Current research implicates an imbalance between ET-1 and localized deficiencies of calcitonin-gene-related peptide (a potent vasodilator that acts directly on the blood vessels in part by stimulating the release of NO from the endothelium (Goldsmith et al. 1996; Bull et al. 1996) as the main pathophysiological mechanism responsible for the vasospastic phenomenon in HAVS (Noël, 2000; Liapina et al. 2002).
Methods
Cell culture and treatment
Primary human umbilical vein endothelial cell (HUVEC) isolation was performed as previously described (Frangos et al. 1988). Umbilical cords were obtained from Sharp Memorial Hospital under the auspices of the Sharp Healthcare Institutional Review Board protocol no. 011081. Cells were seeded onto glass microscope slides and grown to confluence within 3 days in M199 media (Irvine Scientific) supplemented with 20% fetal bovine serum (FBS; Hyclone), 2 mml-glutamine, 0.5 U ml−1 penicillin and 0.05 mg ml−1 streptomycin. All cell cultures were maintained in a humidified 5% CO2–95% air incubator at 37°C. Prior to all experimental procedures, the HUVECs were serum-starved in ATP-free media containing 0.5% FBS for 4 h to establish quiescence in the monolayer. Dulbecco's modified Eagle's medium (DMEM; Irvine Scientific) containing 0.5% FBS, was used as the perfusing medium for all experimental procedures. All flow chambers and accompanying apparatus were maintained at 37°C throughout the experiment. Time-matched sham controls (slides mounted on flow chambers without flow) and static controls (undisturbed slides in Petri dishes) were performed for all experimental groups.
Platform vibration
Confluent HUVEC monolayers were mounted in conventional parallel-plate flow chambers (Frangos et al. 1988). The inflow and outflow ports of the chambers were sealed, and chambers were filled to maximum capacity with DMEM media. Chambers were secured to a vibrating platform and exposed to 10 min of vibration (30 Hz sinusoidal waveform at a relative acceleration of 1 or 2 g). HUVEC were quickly removed from the chamber, visually inspected to ensure an intact monolayer and normal morphology, harvested, and Western blot analysis performed on the cell lysates (see below).
Oscillatory flow experiments
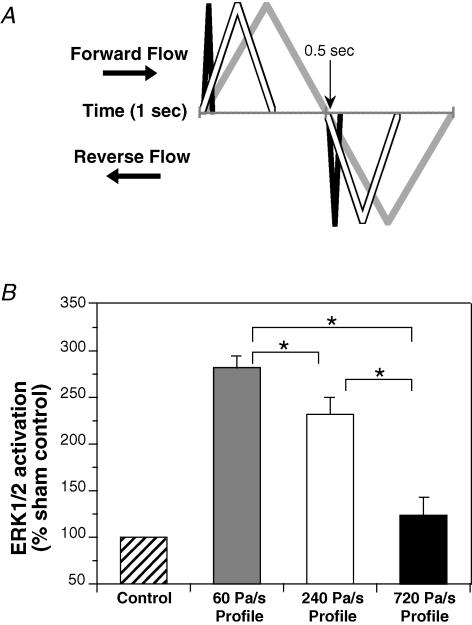
Confluent HUVEC monolayers were mounted in conventional parallel-plate flow chambers and subjected to one of the following flow profiles for 10 min. (1) Low temporal gradients: oscillating, approximately sinusoidal, flow with a frequency of 1, 4 and 8 Hz, where the amplitude was adjusted to yield a constant forward and reverse rate of acceleration of 70 Pa s−1. (2) High temporal gradients: oscillating, approximately sinusoidal, flow with a frequency of 4, 8 and 12 Hz, where the amplitude was adjusted to yield a constant rate of acceleration of 930 Pa s−1. (3) Matched frequency and peak amplitude: since the temporal gradient of shear stress depends on both the frequency and amplitude of the oscillation, the gradients were adjusted by introducing a pulsatile, non-sinusoidal, waveform. With a constant frequency of 1 Hz and peak flow rate of 0.6 ml s−1, flow profiles with low (60 Pa s−1), intermediate (240 Pa s−1) and high (720 Pa s−1) temporal gradients were generated (Fig. 4A). Immediately following exposure to oscillatory flow, HUVEC were quickly removed from the chamber, visually inspected to ensure an intact monolayer and normal morphology, harvested, and Western blot analysis performed on the cell lysates (see below).
Figure 4. Activation of ERK1/2 at a fixed frequency and matched peak amplitudes between three temporal gradient flow profiles.
A, diagrammatic representation comparing the low (grey line), intermediate (white line) and high (black line) temporal gradient flow profiles. At 1 Hz fluid flow (forward or reverse) is continuous throughout the low profile cycle. Small lag times between the forward and reverse components of flow are required in order to match both frequency and peak amplitude in the intermediate and high profiles. The magnitude of the peak amplitudes and pulse widths is not to scale. B, low temporal gradient flow profile (60 Pa s−1; n = 12), intermediate temporal gradient flow profile (240 Pa s−1; n = 10), and high temporal gradient flow profile (720 Pa s−1; n = 12). All values are means ±s.e.m. All values are significantly greater than sham control, except for 720 Pa s−1. *Significant difference between treatments.
Western blot analysis
Cells were collected immediately after fluid flow or vibrational stimulation. Cells were quickly washed with ice-cold PBS containing Na3VO4 (0.4 mm) and lysed in lysis buffer containing 63.5 mm Tris-HCl, 10% glycerol, 2% SDS, 1 mm Na3VO4, 1 mm phenylmethylsulphonyl fluoride (PMSF), 0.1 mm leupeptin, 5%β-mercaptoethanol, and 0.02% bromophenol blue (pH 6.8) (Houliston et al. 2001). Samples were resolved by 12% SDS-PAGE, and subsequently electroblotted onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P). Membranes were blocked with 5% milk in 10 mm Tris, 100 mm NaCl, and 0.1% Tween 20 (pH 7.4). Membranes were agitated in the same buffer with primary antibodies overnight at 4°C. Rabbit anti-ERK1/2 and antiphospho-ERK1/2 were purchased from Cell Signalling Technology Inc. (Beverly, MA, USA). Horseradish peroxidase-conjugated antirabbit was used as a secondary antibody (Cell Signalling). Immunodetection was carried out using enhanced chemiluminescence (Pierce). Protein band quantification was performed using a NIH image gel plotting macro. ERK1/2 activation was expressed as the ratio of phospho-ERK1/2 to total ERK1/2.
Endothelin-1 collection and assay
Confluent HUVEC monolayers were exposed to a 4, 8, or 12 Hz oscillating flow at the high temporal gradients flow profile (930 Pa s−1). After 10 min of exposure, chambers were rapidly purged of all perfusing media (2 ml). Perfusing media was collected and assayed for ET-1 content. HUVEC monolayers were also exposed to steady fluid shear stress at 1 Pa for 10 min in a low-volume (10 ml) recirculating flow loop (Frangos et al. 1988). The onset and cessation of flow was slowly ramped up and down over 30 s. Perfusing media was collected and assayed for ET-1 content. A commercially available endothelin enzyme-immunoassay kit (cat. no. BI-20092; Biomedica Gesellschaft mbH, Germany) was used to assay for ET-1 content.
Statistics
All experimental values are given as mean and standard error of the mean. All reported values of n refer to the number of separate and independent experiments from multiple primary HUVEC cultures. The normality of sample distributions was verified by the Kolmogorov-Smirnov test with tests for skewness and kurtosis. Differences between experimental groups were analysed by one-way ANOVA with frequency, temporal gradients, and steady flow as factors where appropriate. Dunnett's or Newman-Keuls post hoc tests were used to verify significant differences between treatments. Unless stated otherwise, P < 0.05 implies statistical significance.
Results
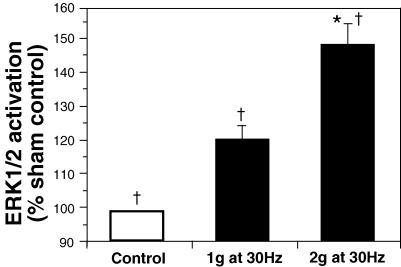
Direct platform vibration at 30 Hz sinusoidal waveform and a relative acceleration of 1 and 2g, resulted in a 120 ± 5.2% and 148 ± 6.7% increase in ERK1/2 phosphorylation, respectively, relative to sham control. Although there was a positive linear trend between sham, 1 g and 2 g (P <0.005), ERK1/2 phosphorylation was only significantly different from sham control at 2 g (Fig. 1).
Figure 1. Activation of ERK1/2 in HUVEC after 10 min of direct platform vibration.
For 1g at 30 Hz n = 5, and for 2g at 30 Hz n = 6. All values are means ±s.e.m.*Significant difference from sham control (P < 0.05). †Positive linear trend.
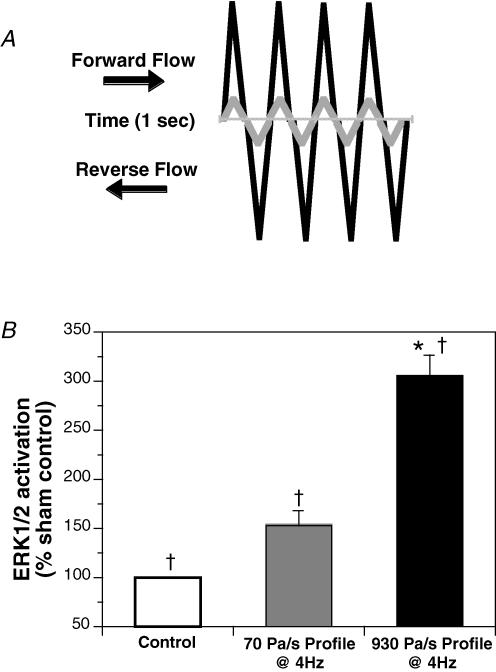
Similar to direct platform vibration, the activation of ERK1/2 by oscillating flow increased with the magnitude of the temporal gradient flow profile (Fig. 2). At a fixed oscillation frequency of 4 Hz, the low temporal gradient flow profile (70 Pa s−1) increased ERK1/2 activation by 153 ± 14% of sham control. The high temporal gradient flow profile (930 Pa s−1) increased ERK1/2 activation by 305 ± 22% of sham control. As with direct platform vibration, there was a positive linear trend with the increase in temporal gradient (P < 0.005), but ERK1/2 phosphorylation was only significantly different from sham control at the 930 Pa s−1 profile.
Figure 2. Activation of ERK1/2 as a function of the magnitude of the temporal gradient.
A, diagrammatic representation comparing the low (grey line) and high (black line) temporal gradient flow profiles. The magnitude of the peak amplitudes is not to scale. B, low temporal gradient flow profile (70 Pa s−1; n = 6), and high temporal gradient flow profile (930 Pa s−1; n = 8). All values are means ± s.e.m.*Significant difference from sham control (P < 0.05). †Positive linear trend.
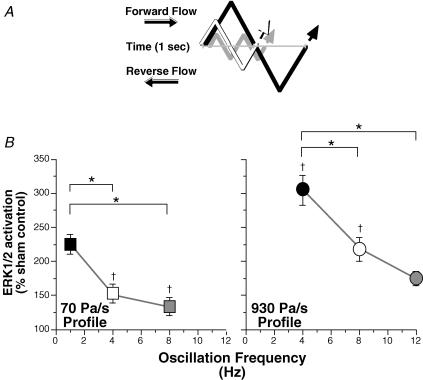
For both temporal gradient flow profiles, when the frequency of flow oscillation was increased, ERK1/2 activation decreased (Fig. 3). At 1, 4 and 8 Hz flow oscillation, the low temporal gradient flow profile (70 Pa s−1) activated ERK1/2 at 225 ± 14%, 153 ± 14% and 134 ± 13%, respectively, of sham control. All values of ERK1/2 activation were significantly greater than sham control, except for values at 8 Hz. There was no significant difference in ERK1/2 activation between 4 and 8 Hz. ERK1/2 activation at 1 Hz was significantly greater than at 4 and 8 Hz. A similar pattern was observed when increasing the frequency of flow oscillation for the high temporal gradient flow profile (930 Pa s−1). At 4, 8 and 12 Hz flow oscillation, ERK1/2 activation was increased 305 ± 22%, 218 ± 17% and 174 ± 10%, respectively, of sham control. All values were significantly greater than sham control. ERK1/2 activation was significantly greater at 4 Hz compared to 8 and 12 Hz, but there was no significant difference between 8 and 12 Hz. Consistent with Fig. 2, at identical oscillation frequencies, ERK1/2 activation was increased with the magnitude of the temporal gradient flow profile.
Figure 3. Activation of ERK1/2 as a function of the frequency of flow oscillation.
A, diagrammatic representation of three oscillation frequencies for a given temporal gradient flow profile. For a given flow profile, the black line represents the lowest frequency, the white line represents the middle frequency, and the grey line represents the highest frequency. Absolute frequency and magnitude of the peak amplitudes are not to scale. B, low temporal gradient flow profile (70 Pa s−1; n = 6; left panel), and high temporal gradient flow profile (930 Pas−1; n = 8; right panel). Sham control = 100%. All values are means ±s.e.m. All values are significantly greater than sham control, except for 70 Pa s−1 at 8 Hz. *Significant difference between frequencies within the same profile. †Significant difference between profiles at the same frequency.
When the frequency of flow oscillation was fixed at 1 Hz and the peak amplitudes of the forward and reverse rates of fluid acceleration were held equipoise between flow profiles, activation of ERK1/2 was greatest with the low (60 Pa s−1) temporal gradient flow profile (Fig. 4). For oscillating fluid flow at a frequency of 1 Hz and a temporal gradient of 60 Pa s−1, ERK1/2 activation was 282 ± 12% of sham control. At a frequency of 1 Hz, ERK1/2 activation for the intermediate (240 Pa s−1) and high (720 Pa s−1) temporal gradient flow profiles was 231 ± 19% and 123 ± 18% of sham control, respectively. Activation of ERK1/2 was significantly greater than sham control for both the 60 and 240 Pa s−1 profiles. ERK1/2 activation by the 720 Pa s−1 profile was not significantly different to the sham control.
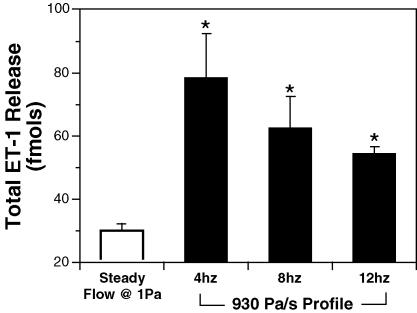
Endothelial production and release of ET-1 was also stimulated by flow oscillation (Fig. 5). After 10 min of steady fluid shear stress (devoid of temporal gradients), the total amount of ET-1 produced was 30 ± 2 fmol. The total ET-1 production when exposed to the high temporal gradient flow profile (930 Pa s−1) for 10 min at 4, 8 and 12 Hz was 78 ± 14, 62 ± 10 and 54 ± 2 fmol, respectively. Compared to ET-1 production under steady fluid shear stress, exposure to flow oscillation significantly increased ET-1 production at all frequencies. Although the mean total production of ET-1 was reduced with increasing oscillation frequency, the trend was not significant.
Figure 5. Endothelial production of ET-1 is enhanced by flow oscillation.
Steady fluid shear stress was devoid of temporal gradients (n = 7). For 4 and 8 Hz n = 7, for 12 Hz n = 10. All values are means ±s.e.m.*Significant difference from steady fluid shear stress.
Discussion
In the present studies, rapid low-volume fluid oscillation was used to simulate the effects of direct vibration by generating defined temporal gradients in fluid shear stress across an endothelial monolayer. By cultivating HUVEC monolayers used in this study on a rigid glass substrate, the cyclic deformation component of vibration is effectively minimized. When the effects of direct platform vibration (Fig. 1) were compared to the effects of rapid low-volume fluid oscillation (Fig. 2), both methods were found to increase ERK1/2 activation in response to increases in oscillational magnitude. Although the mechanical stimulation in both methods is derived primarily from the frictional force of the cell's rapid movement through its liquid environment, the frictional force generated by direct platform vibration is a function of the relative gravitational acceleration of the platform, of which inertia is a large component. The quantification and manipulation of the vibrational forces generated by platform vibration is complicated and is dependent upon the mass and orientation of the flow chamber(s) mounted on the vibrating platform. The novel utilization of rapid low-volume fluid oscillation to simulate the effects of direct vibration on the endothelium allows for the accurate quantification and manipulation of the magnitude and frequency of the vibrational forces applied to the endothelial monolayer.
The major observation of this study was that fluid vibrations lead to phosphorylation of the ERK1/2 protein, and the release of the potent vasoconstrictor ET-1. Although the involvement of ET-1 in the pathogenesis of HAVS has been reported by others (Lau et al. 1995; Palmer & Mason, 1996), this is the first report linking the activation of the ERK1/2 pathway with the transduction of vibrational forces in endothelial cells. This finding may explain recent reports of localized ERK1/2 activation within the vascular smooth muscle of arteriovenous grafts where patterns of disturbed fluid flow result in increased vein wall vibration (Loth et al. 2003). Given that ERK1/2 can act as a pro-inflammatory pathway (Suttles et al. 1999), this may also account for the acute in vivo inflammation often observed in the hands and fingers of workers following heavy use of vibrating devices (Verdon, 1996; Lau et al. 1992; Liapina et al. 2002) This study does not suggest that ERK1/2 activation directly leads to ET-1 release. ERK1/2 and ET-1 were chosen as known markers of temporal gradients and HAVS, respectively. However, the release of ET-1 does validate the rapid low-volume fluid oscillation model as an effective in vitro simulation of the in vivo physiological response to vibration. This finding also supports the findings of Ziegler et al. (1998), who reported that the expression of ET-1 mRNA in cultured endothelial cells was specifically upregulated when exposed to oscillatory mechanical forces.
Biomechanically, another important observation of this study was the apparent counter effect of temporal gradients in fluid shear stress, and the attenuation effects of fluid oscillation frequency on the biomechanical transduction of vibrational forces in human endothelial cells. At a fixed oscillation frequency, ERK1/2 activation increased with the absolute magnitude of the temporal gradient in shear stress (Fig. 2). However, when the oscillation frequency of fluid flow was increased and the absolute magnitude of the temporal gradient was held constant, ERK1/2 activation was attenuated with the increase in oscillation frequency (Fig. 3). The attenuation of ERK1/2 activation may be explained by the effects of inertia at the membrane–fluid interface of the cell. The elasticity of the cell membrane may attenuate vibration in a manner similar to a rubber damper that attenuates motor vibrations. Given that ERK1/2 activation at identical frequencies was greater at 930 Pa s−1 compared to 70 Pa s−1, it would suggest that not only the absolute magnitude of the temporal gradient was important, but the duration of each pulse cycle (pulse width) may also be important. The reduction in pulse width with increasing oscillation frequency is illustrated in Fig. 3A. To corroborate this model, varying temporal gradients were produced while keeping both magnitude and frequency constant with a non-sinusoidal waveform (Fig. 4). Sharp, spike-like waveforms with narrow pulse widths contain a high part of their spectral energy in high-frequency components, as opposed to triangular and sinusoidal waveforms with larger pulse widths, where the base spectral component dominates. Consistently, spike-like waveforms show reduced ERK1/2 activation in spite of higher temporal gradients. This may be due to the high-order spectral components of the spike-like waveform being absorbed and attenuated by the elastic fluid-membrane system. Consistent with this finding, ET-1 release was also stimulated by rapid low-volume fluid oscillation as compared to steady shear stress (Fig. 5). As with the attenuation of ERK1/2 activation in response to increasing frequencies of low-volume fluid oscillation (Fig. 3), ET-1 release was also attenuated with the increase in oscillation frequency.
Furthermore, these studies would also suggest that vibrating hand tools operating at high frequencies may be less damaging to the endothelium than those operating at low frequencies. The observed reduction of ERK1/2 activation with increasing frequency indicates that the elastic properties of the cell monolayer itself attenuate high-frequency vibration. This is consistent with the mechanical mode of energy transfer into tissue. Vibrational energy depends primarily on acceleration, which increases both with vibrational amplitude and frequency. Any viscoelastic material, including tissue, strongly attenuates the vibrational amplitude at high frequencies. The frequency-weighted acceleration concept assumes that the harmful effects of acceleration are independent of frequency between 6.3 and 16 Hz, but progressively decrease with higher frequencies (Burström et al. 1998). Even in a relatively non-compliant system such as the one used in this study, almost all of the vibrational amplitude at frequencies above 15 Hz is completely eliminated (data not shown). As a result, all assessments of vibrational damage should be based upon the component with the largest acceleration magnitude (International Standard ISO 8041, 1985). Although heavy-duty pneumatic tools can operate at frequencies in excess of 200 Hz (Tranmax Machinery Co. Ltd., Tai-Ping City, Taiwan), the vibrational amplitude of these tools is rather low. Tools such as jack hammers and rock drills, which typically operate in the 3–15 Hz range (Swastic Driling Co. Ltd., New Delhi, India), have a much greater vibrational amplitude. Therefore the frequency ranges used in this study were appropriate to further elucidate the biomechanical and molecular mechanisms that underlie the pathophysiology of HAVS.
In conclusion, this study was designed to investigate the biomechanical transduction of fluid vibrational forces in human endothelial cells. The role of fluid vibration in direct mechanical vibration is a widely unexplored field. These studies are the first to suggest that vibrational energy is transduced into biochemical signals within the endothelium at least in part, via the ERK1/2 pathway. This work has pathophysiological implications beyond the vasculature. For example, HAVS is both a vascular and neurological disorder. Neurological symptoms of pain and paresthesia may be explained by injury to peripheral structures and reduced sensory nerve conduction velocity of the distal radial nerve (Hirata et al. 2002), but could also be due to changes in cortical somatotopic mapping of the hand in the brain (Lundborg et al. 2002). The transmission of vibrational energy to this region of the brain would undoubtedly involve fluid oscillations similar to those used in this study. Furthermore, vibration is an excitatory stimulus for both vestibular and proprioceptive afferents. Vibration applied either to the skull or to the neck muscles of subjects after unilateral vestibular deafferentation induces nystagmus and a shift of the subjective visual horizontal (Karlberg et al. 2003). On even more of a basic cellular level, direct mechanical vibration has also been shown to disrupt chromosomes during metaphase and prevent cellular proliferation (Naruse, 2002). We feel that the findings of the current study can be applied as effectively to these diverse physiological effects of vibration as it can be applied to the endothelium alone.
Acknowledgments
The authors wish to thank Susan Toyama, Kelly Bell, and Nathan McKnight for their valuable assistance. This study was supported by NASA grant: NAG8-1589 and NHLBI grant: HL-40696.
References
- Bao X, Clark CB, Frangos JA. Temporal gradient in shear-induced signaling pathway – involvement of MAP kinase, c-fos and connexin-43. Am J Physiol Heart Circ Physiol. 2000;278:H1598–H1605. doi: 10.1152/ajpheart.2000.278.5.H1598. [DOI] [PubMed] [Google Scholar]
- Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NF kappa B, and egr-1. Arterioscler Thromb Vasc Bio. 1999;19:996–1003. doi: 10.1161/01.atv.19.4.996. [DOI] [PubMed] [Google Scholar]
- Bao X, Lu C, Frangos JA. Mechanism of temporal gradients in shear-induced ERK1/2 activation and proliferation in endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281:H22–H29. doi: 10.1152/ajpheart.2001.281.1.H22. [DOI] [PubMed] [Google Scholar]
- Bull HA, Hothersall J, Chowdhury N, Cohen J, Dowd PM. Neuropeptides induce release of nitric oxide from human dermal microvascular endothelial cells. J Invest Dermatol. 1996;106:655–660. doi: 10.1111/1523-1747.ep12345471. [DOI] [PubMed] [Google Scholar]
- Burström L, Lundström R, Hagberg M, Nilsson T. Comparison of different measures for hand-arm vibration exposure. Safety Sci. 1998;28:3–14. [Google Scholar]
- Dong RG, Rakheja S, Schopper AW, Han B, Smutz WP. Hand-transmitted vibration and biodynamic response of the human hand-arm: a critical review. Crit Rev Biomed Eng. 2001;29:393–439. doi: 10.1615/critrevbiomedeng.v29.i4.20. [DOI] [PubMed] [Google Scholar]
- Frangos JA, McIntire LY, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- Goldsmith PC, Leslie TA, Hayes NA, Levell NJ, Dowd PM, Foreman JC. Inhibitors of nitric oxide synthase in human skin. J Invest Dermatol. 1996;106:113–118. doi: 10.1111/1523-1747.ep12328204. [DOI] [PubMed] [Google Scholar]
- Hirata M, Sakakibara H, Abe M. Reduced sensory nerve conduction velocity of the distal part of the radial nerve among patients with vibration syndrome. Electromyogr Clin Neurophysiol. 2002;42:113–118. [PubMed] [Google Scholar]
- Ho M, Belch JJ. Raynaud's phenomenon: state of the art. Scand J Rheumatol. 1998;27:319–322. doi: 10.1080/03009749850154311. [DOI] [PubMed] [Google Scholar]
- Houliston RA, Pearson JD, Wheeler-Jones CPD. Agonist-specific cross talk between ERKs and p38 MAPK regulates PGI2 synthesis in endothelium. Am J Physiol Cell Physiol. 2001;281:C1266–C1276. doi: 10.1152/ajpcell.2001.281.4.C1266. [DOI] [PubMed] [Google Scholar]
- International Standard ISO 8041. Geneva: International Organization for Standardization; 1985. Human response to vibration – measuring instrumentation. [Google Scholar]
- Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- Karlberg M, Aw ST, Black RA, Todd MJ, MacDougall HG, Halmagyi GM. Vibration-induced ocular torsion and nystagmus after unilateral vestibular deafferentation. Brain. 2003;126:956–964. doi: 10.1093/brain/awg091. [DOI] [PubMed] [Google Scholar]
- Keynton RS, Evancho MM, Sims RL, Rodway NV, Gobin A, Rittgers SE. Intimal hyperplasia and wall shear in arterial bypass graft distal anastomoses: an in vivo model study. J Biomech Eng. 2001;123:464–473. doi: 10.1115/1.1389461. [DOI] [PubMed] [Google Scholar]
- Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillation shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Lau CS, Khan F, Brown R, McCallum P, Belch JJ. Digital blood flow response to body warming, cooling, and rewarming in patients with Raynaud's phenomenon. Angiology. 1995;46:1–10. doi: 10.1177/000331979504600101. [DOI] [PubMed] [Google Scholar]
- Lau CS, O'Dowd A, Belch JJ. White blood cell activation in Raynaud's phenomenon of systemic sclerosis and vibration induced white finger syndrome. Ann Rheum Dis. 1992;51:249–252. doi: 10.1136/ard.51.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liapina M, Tzvetkov D, Vodenitcharov E. Pathophysiology of vibration-induced white fingers; current opinion: a review. Cent Eur J Public Health. 2002;10:16–20. [PubMed] [Google Scholar]
- Lie JT. Visceral intestinal Buerger's disease. Int J Cardiol. 1998;66(Suppl. 1):S249–S256. doi: 10.1016/s0167-5273(98)00176-4. [DOI] [PubMed] [Google Scholar]
- Loth F, Fischer PF, Arslan N, Bertram CD, Lee SE, Royston TJ, Shaalan WE, Bassiouny HS. Transitional flow at the venous anastomosis of an arteriovenous graft: potential activation of the ERK1/2 mechanotransduction pathway. J Biomech Eng. 2003;125:49–61. doi: 10.1115/1.1537737. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Rosen B, Knutsson L, Holtas S, Stahlberg F, Larsson EM. Hand-arm-vibration syndrome (HAVS): is there a central nervous component? An fMRI study. J Hand Surg. 2002;27:514–519. doi: 10.1054/jhsb.2002.0813. [DOI] [PubMed] [Google Scholar]
- Naruse Y. Mechanical vibration model for chromosomes in metaphase of mitosis and possible application to the interruption of cell division. Biosystems. 2002;66:55–63. doi: 10.1016/s0303-2647(02)00033-3. [DOI] [PubMed] [Google Scholar]
- Noël B. Pathophysiology and classification of the vibration white finger. Int Arch Occup Environ Health. 2000;73:150–155. doi: 10.1007/s004200050021. [DOI] [PubMed] [Google Scholar]
- Ojha M. Wall shear stress temporal gradient and anastomotic intimal hyperplasia. Cir Res. 1994;74:1227–1231. doi: 10.1161/01.res.74.6.1227. [DOI] [PubMed] [Google Scholar]
- Palmer KT, Mason H. Serum endothelin concentrations in workers exposed to vibration. Occup Environ Med. 1996;53:118–124. doi: 10.1136/oem.53.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakheja S, Wu JZ, Dong RG, Schopper AW, Boileau PE. A comparison of biodynamic models of the human hand–arm system for applications to hand-held power tools. J Sound and Vibration. 2002;249:55–82. [Google Scholar]
- Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci U S A. 2001;98:6476–6481. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttles J, Milhorn DM, Miller RW, Poe JC, Wahl LM, Stout RD. CD40 signaling of monocyte inflammatory cytokine synthesis through an ERK1/2-dependent pathway. A target of interleukin (IL-4 and IL-10 anti-inflammatory action. J Biol Chem. 1999;274:5835–5842. doi: 10.1074/jbc.274.9.5835. [DOI] [PubMed] [Google Scholar]
- Verdon ME. Overuse syndromes of the hand and wrist. Prim Care. 1996;23:305–319. doi: 10.1016/s0095-4543(05)70278-5. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]