Abstract
Purinergic receptors are a family of ubiquitous transmembrane receptors comprising two classes, P1 and P2 receptors, which are activated by adenosine and extracellular nucleotides (i.e. ATP, ADP, UTP and UDP), respectively. These receptors play a significant role in regulating ion transport in epithelial tissues through a variety of intracellular signalling pathways. Activation of these receptors is partially dependent on ATP (or UTP) release from cells and its subsequent metabolism, and this release can be triggered by a number of stimuli, often in the setting of cellular damage. The function of P2Y receptor stimulation is primarily via signalling through the Gq/PLC-β pathway and subsequent activation of Ca2+-dependent ion channels. P1 signalling is complex, with each of the four P1 receptors A1, A2A, A2B, and A3 having a unique role in different epithelial tissue types. In colonic epithelium the A2B receptor plays a prominent role in regulating Cl− and water secretion. In airway epithelium, A2B and A1 receptors are implicated in the control of Cl− and other currents. In the renal tubular epithelium, A1, A2A, and A3 receptors have all been identified as playing a role in controlling the ionic composition of the lumenal fluid. Here we discuss the intracellular signalling pathways for each of these receptors in various epithelial tissues and their roles in pathophysiological conditions such as cystic fibrosis.
Extracellular purines such as ATP and adenosine signal through membrane-associated purinergic receptors as autocrine and paracrine substances as well as neurotransmitters. The ubiquitous nature of these ligands as well as the abundance of expression of purinergic receptors account for purinergic control of diverse effects in many tissue types. Control of ion and fluid transport across epithelia is one such system in which purinergic regulation is highly significant, even considering the variety of hormones and other regulators that are well known to regulate epithelial transport. Purinergic receptors have been identified in most epithelial tissues and have been well characterized, specifically in the gastrointestinal, airway and kidney epithelia.
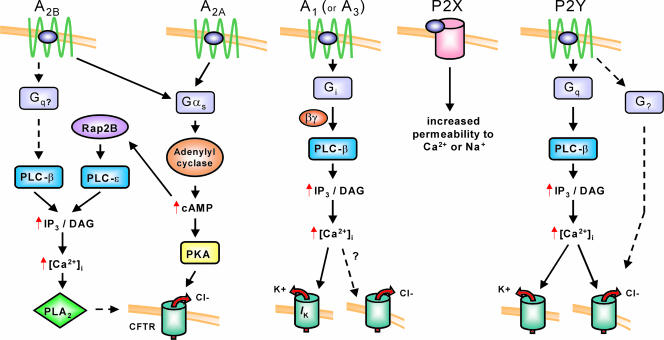
This large family of purinergic receptors has been subdivided into two major classes, P1 and P2, that have preferential affinity for adenosine and ATP, respectively. Figure 1 gives an overview of these receptors and their role in regulation of ion transport. The P1 receptors are a family of G protein-coupled receptors that signal through multiple intracellular effectors in response to nucleoside activation, primarily with adenosine. There are four known subtypes of P1 receptors, A1, A2A, A2B, and A3, each of which has been cloned and characterized biochemically and pharmacologically. The A1 receptor signals through the Gi/o family of G proteins, resulting most significantly in an inhibition of adenylyl cyclase but also, via βγ subunits, in the activation of phospholipase C-β (PLC-β), the activation of several K+ channels, and the inactivation of Ca2+ channels, among others. The A2A and A2B receptors classically signal primarily via Gs, resulting in activation of adenylyl cyclase, an increase in cAMP production, and subsequent activation of protein kinase A (PKA). In some cases activation of the A2B receptor has been documented to signal through other effectors, specifically mobilization of intracellular calcium, and this may result from interaction with Gq or other G proteins (Feoktistov & Biaggioni, 1997; Linden et al. 1999). It is also possible that in some cells cAMP may mobilize Ca2+ due to activation of phospho lipase C-ε (PLC-ε) via EPAC (exchange protein activated by cAMP) and RAP2B (Evellin et al. 2002). The A3 receptor signals primarily via Gi/o proteins. The primary downstream effects of A3 activation are inhibition of adenylyl cyclase function and, in some cases, activation of phospholipase C to provide an intracellular calcium signal. Inosine, a purine nucleoside metabolite of adenosine, has activity at the A3 receptor but has weak activity at the other P1 receptors (Jin et al. 1997; Ralevic & Burnstock, 1998; Fredholm et al. 2001).
Figure 1. Purinergic receptors important in regulating epithelial ion transport.
The P1 receptors A2A and A2B classically signal through Gs, resulting in an increase in cAMP and activation of PKA. A rise in intracellular Ca2+ noted in some cells in response to A2B receptor activation may result from receptor coupling to Gq and activation of PLC-β or from cAMP-activation of Rap2B that raises intracellular Ca2+ via PLC-ɛ. This rise in intracellular Ca2+ by either of these mechanisms likely accounts for the activation of PLA2 that contributes to the A2B activated Cl− current. The A1 receptor has been shown to be active in airway epithelial cell lines and evokes a Ca2+ response through the βγ subunits of Gi. This triggers basolateral K+ efflux and may trigger Cl− release. P2X receptors are ligand-gated ion channels and increase cell permeability to Na+ or Ca2+. P2Y receptors classically signal through Gq, resulting in an increase in intracellular Ca2+ and activation of Ca2+-activated Cl− and K+ channels, although a Ca2+-independent Cl− current resulting from P2Y activation has been identified.
The P2 family of receptors is further divided into metabotropic (G protein-coupled) P2Y receptors and ionotropic P2X receptors. There are currently eight known P2Y receptors (Y1, Y2, Y4, Y6, Y11, Y12, Y13 and Y14) and eight P2X receptors (P2X1-7 and P2XM). These receptors are classically activated by purine nucleotides, and the differing affinities for specific diphosphate or triphosphate nucleotides help to differentiate among similar receptor subtypes (Fredholm et al. 1994; Harden et al. 1997). The P2Y receptor preferences for naturally occurring ligands are summarized in Table 1 (King et al. 2001; Communi et al. 2001). A subset of these receptors can be activated by the pyrimidine nucleotides UTP and UDP, despite their original classification as ‘purinergic’ receptors. A receptor formally known as the UDP-glucose receptor has recently been added to the P2Y family and designated P2Y14 (Abbracchio et al. 2003). P2Y receptors classically signal through a Gq-dependent pathway, activating phospholipase C and mobilizing intracellular Ca2+, but some can modulate other effector pathways as well. P2X receptors are all activated by ATP, and, excepting P2X6, all are also activated by 2-MeSATP. BzATP is a selective agonist of P2X5 and P2X7 receptors (Khakh et al. 2001; Bo et al. 2003). P2X receptors are ligand-gated channels and their activation increases the permeability of the plasma membrane specifically to Na+ or Ca2+. In some cases antagonists have been developed which discriminate between P2X and P2Y receptor subtypes. These include the selective P2Y1 antagonist, MRS2279 (Boyer et al. 2002), and the selective P2Y12 antagonist AR-C69931 (Simon et al. 2002).
Table 1.
Natural ligands for P2Y receptors
| Receptor | Preferred natural agonists |
|---|---|
| P2Y1 | ADP > ATP |
| P2Y2 | UTP = ATP |
| P2Y4 | UTP ≫ ATP |
| P2Y6 | UDP > UTP > ADP |
| P2Y11 | ATP |
| P2Y12 | ADP > ATP |
| P2Y13 | ADP > ATP |
| P2Y14 | UDP-glucose > UDP-galactose > UDP-N-acetylglucosamine |
In order to initiate signalling via purinergic receptors, nucleotides and their metabolites, specifically ATP, UTP, ADP, UDP, UDP-glucose or adenosine, must be present in the extracellular fluid at concentrations sufficient to activate receptors. Within cells, ATP levels are high and maintained within a narrow range. A variety of stimuli are capable of initiating ATP (or UTP) release from intact cells including mechanical stimuli, cell swelling, and inflammatory stimuli (Taylor et al. 1998; Roman & Fitz, 1999; Harden & Lazarowski, 1999). Once released, ATP at the luminal surface is able to activate P2 receptors or be readily converted to ADP and AMP via widely expressed ecto-nucleotidases present on the surface of cells (Zimmermann, 1996). AMP is converted to adenosine via the action of ecto-5′nucleotidase. Adenosine can be taken up into cells by nucleoside transporters and then be reconverted to AMP by adenosine kinase. Nucleoside transporters are thought to play a significant role in governing the effect of adenosine at the epithelial surface by controlling the extracellular concentration (Szkotak et al. 2001, 2003). Adenosine can be converted to inosine by the action of adenosine deaminase, found both inside and on the surface of cells. This ready release of nucleotides with a variety of insults and their subsequent metabolism have coevolved with multiple extracellular nucleotide signalling pathways that can utilize both ATP (or UTP) and adenosine and can efficiently work together to regulate ion transport across the epithelial surface. One commonly held belief is that, in many tissue types, this stimulation of ion transport and the flux of water that accompanies it are a natural defense system that functions to effectively wash away noxious stimuli in the setting of cellular damage or inflammation (Lazarowski & Boucher, 2001; Leipziger, 2003).
P2 receptors in epithelial transport
The role of P2 receptors in regulating epithelial transport has been recently reviewed by Leipziger (2003), and for that reason the majority of this review will focus instead on the role of P1 receptors in regulating secretion across the epithelium. It is worthwhile, however, discussing the basic signalling via P2 receptors in major tissue epithelia since extracellular adenosine is thought to arise from the breakdown of extracellular ATP, and therefore P1 and P2 signalling events are often triggered simultaneously by concurrent increases in extracellular ATP and adenosine.
P2 receptor signalling in gastrointestinal epithelia
P2 signalling is important in the gastrointestinal tract, where, in summary, P2 activation leads to activation of K+, HCO3−, and Cl− secretion and inhibition of Na+ reabsorption at various locations in the gallbladder, pancreas, small intestine, and colon (Roman & Fitz, 1999; Leipziger, 2003). There are a variety of P2 receptors implicated in regulating transport along the gastrointestinal tract, including some that have been well characterized: the P2Y6 receptor in the mouse gallbladder, the P2Y4 receptor in mouse jejunum, the P2Y1 and P2Y6 receptors in rat distal colon, the P2Y4 receptor in rat pancreas, and the P2Y2 receptor in guinea-pig pancreas (Leipziger et al. 1997; Cressman et al. 1999; Ishiguro et al. 1999; Hede et al. 1999; Kottgen et al. 2003). In gastrointestinal epithelial tissue, ATP does not directly activate Cl− secretion via P2 receptors since the cAMP-dependent cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel is thought to be the only chloride channel expressed, although there is longstanding debate on the existence of a Ca2+-dependent Cl− channel in the colonic epithelium (Anderson et al. 1992; Wagner et al. 1992; Rozmahel et al. 1996; Grubb & Gabriel, 1997; Barrett & Keely, 2000). Much of the controversy around this topic originates from the use of colonic epithelial cell lines such as T84 cells that express Ca2+-activated Cl− channels in their undifferentiated state, but down-regulate them upon forming differentiated monolayers (Anderson & Welsh, 1991; Morris et al. 1992). Despite the presumed absence of a Ca2+-activated Cl− channel in differentiated cell lines and in in vivo epithelial tissue, activation of P2 receptors can still lead to lumenal fluid accumulation via stimulation of K+ secretion and inhibition of Na+ reabsorption (Leipziger, 2003). Thus, the action of ATP in the colon is thought to stimulate water efflux as a protective response, as in other secretory epithelia. In the case of the colon, this would present clinically as secretory diarrhoea in response to an inflammatory stimulus.
P2 receptor signalling in respiratory epithelia
In bronchial epithelium, signalling via P2 receptors has received much attention because of the possible therapeutic potential in treating cystic fibrosis. The predominant P2 receptor in respiratory epithelium is the P2Y2 receptor that classically couples to Gq, increases intracellular Ca2+, and activates Ca2+-dependent Cl− channels, although other pathways for Cl− secretion in airways have been identified. Specifically, a Ca2+-independent and adenosine receptor independent Cl− current can be triggered by ATP or UTP in airway epithelia (Stutts et al. 1994; Inglis et al. 1999). The importance of the P2Y2 receptor in the mouse tracheal epithelium was confirmed in a knockout study in which mice deficient in this receptor lost 85–95% of the nucleotide-stimulated Cl− secretion in this tissue (Cressman et al. 1999). The net effect of P2Y2 activation is to increase Cl− secretion and K+ secretion and inhibit electrogenic Na+ absorption, all of which lead to water secretion (Mason et al. 1991; Clarke et al. 1997; Inglis et al. 1999; Leipziger, 2003). Because this pathway involves Ca2+-dependent Cl− channels rather than the cAMP-dependent cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel, activation of the P2Y2 receptor has been explored as a potential method of overcoming the Cl− secretion defect in cystic fibrosis.
P2 receptor signalling in renal epithelia
P2 signalling along the renal epithelium is similar to that found in the respiratory epithelium and gastrointestinal epithelium in that P2 receptor activation causes a rise in intracellular Ca2+ that initiates ion transport. The renal epithelium is complicated by the existence of multiple receptor subtypes and individual variations within each segment of the nephron. The role of each receptor subtype remains to be elucidated. In the nephron of both the rat and mouse, it is currently thought that P2Y1 and P2Y2 receptors play a primary role in epithelial tissues along the nephron with the P2Y6 receptor also recently implicated in the rat proximal collecting duct, thick ascending limb, and thin descending limb (Bailey et al. 2000; Schwiebert & Kishore, 2001). These receptors are of interest in this tissue primarily because of their role in regulating salt and water balance. Salt and water reabsorption are inhibited by activation of P2 receptors along the tubular lumen, making manipulation of these receptors of interest in hypertension (Kishore et al. 1995; McCoy et al. 1999; Leipziger, 2003). It has also been shown that P2 receptors play a significant role in regulating water balance independently of vasopressin. Hypervolaemia and ischaemic reperfusion injury are known to induce P2 expression along the nephron, while hypovolaemia down-regulates P2 expression, implicating these receptors in diuresis or water retention in certain pathophysiological states (Kishore et al. 1998, 2000; Schwiebert & Kishore, 2001).
P1 receptors in epithelial transport
Adenosine-mediated control of ion transport has been demonstrated in a variety of epithelial tissues and as a result of activation of all four P1 receptor subtypes. P1-receptor-mediated control of Cl− secretion via the cAMP-activated CFTR Cl− channel is among the most significant and most studied effects of adenosine on epithelial cells. Ca2+-activated currents stimulated primarily via the Gi/PLC-β pathway also play a major role in the diverse effects of P1 receptors in these tissues.
P1 receptor signalling in gastrointestinal epithelia
The best characterized tissue with regard to P1-mediated ion transport is the colonic epithelium. The interest in this tissue arises from the potent Cl− and water secretion stimulated by adenosine via the A2B receptor, which is clinically relevant as a contributing factor in secretory diarrhoea in the setting of inflammation and also as a possible target for treatment of cystic fibrosis. It is important to note that adenosine, unlike ATP acting via a P2 receptor, is known to have a direct effect on Cl− release in the colon via activation of CFTR. The A2B receptor has been identified on both the mucosal and basolateral aspects of colonic epithelial cells, and activation at either site results in Cl− secretion (Barrett et al. 1989; Strohmeier et al. 1995). A similar effect of A2B stimulation has been described in isolated rabbit ileum (Dobbins et al. 1984). A major source of adenosine in the bacteria-infected gut is neutrophils, which release adenine nucleotides that are rapidly converted to AMP and then to adenosine by 5′-nucleotidase, which is highly expressed on gut epithelium (Madara et al. 1993). The classical pathway for A2B-mediated Cl− release is via Gs activation of adenylyl cyclase, with a rise in cAMP that directly activates the CFTR Cl− channel. It has long been speculated, however, that another pathway may be involved based upon the saturation of Cl− secretion at levels of A2B stimulation that fail to saturate cAMP levels and the ability of adenosine to stimulate a Cl− efflux equal to that of forskolin at much lower cAMP levels (Barrett et al. 1989, 1990; Strohmeier et al. 1995; Clancy et al. 1999; Huang et al. 2001). This effect has been attributed to increased coupling of the A2B receptor to CFTR as well as to the existence of an alternative second messenger, and it now seems that both of these are likely to play a role in A2B-mediated Cl− secretion. With respect to coupling, A2B adenosine receptors are known to couple tightly to CFTR as well as adenylyl cyclase and PKA in airway epithelial cell models, as will be discussed later (Huang et al. 2001). With respect to an additional second messenger, a pathway that has recently been implicated in A2B-mediated Cl− secretion is the activation of phospholipase A2 (PLA2) in parallel with the activation of adenylyl cyclase (Barrett & Bigby, 1993; Bouritius et al. 1999; Cobb et al. 2002). It has been proposed that activation of PLA2 contributes to Cl− secretion via the effects of arachadonic acid (AA) or its metabolites, the precise mechanism of action of which is unknown but may be through effects on the electrochemical driving force for ion transport through CFTR (i.e. by activation of K+ channels), through direct effects of lipid species on CFTR or associated factors, or by locally increasing cAMP production in the vicinity of CFTR, an effect which may not be recognized with total cellular cAMP assays (Cobb et al. 2002).
P1 receptor signalling in airway epithelia
A2B activation of Cl− secretion at the lung epithelium is similar to that found in the colon, in which an increase in apical membrane Cl− conductance is produced through a Gs pathway activating adenylyl cyclase and raising cAMP (Lazarowski et al. 1992; Huang et al. 2001). This has been found in a transformed cell line from normal human airway epithelia (BEAS39), in a model for polarized serous cells thought to play a critical role in liquid secretion in cystic fibrosis (Calu-3), and in primary cultures of human nasal epithelium (Lazarowski et al. 1992; Huang et al. 2001). The stimulated secretory response was pharmacologically identified to be the result of A2B receptor activation and was lost in a cell line derived from a cystic fibrosis patient with a defect in ion transport at CFTR (CF/T43), implying that this ion channel is the one responsible for the A2B-mediated Cl− secretion (Lazarowski et al. 1992). It is worth noting that Calu-3 cells lack P2Y2 receptors, the primary P2 receptor controlling ion transport in lung epithelium, which further implicates adenosine, and not ATP, as an important player in ion secretion. When this A2B secretory response was compared to direct forskolin treatment, similar to what was found in the colonic epithelium, the A2B pathway produced the same level of Cl− secretion but a 9-fold lower level of cellular cAMP. This effect was attributed to tight colocalization of A2B, adenylyl cyclase, PKA and CFTR (Huang et al. 2001). Further characterization of this mechanism revealed that the A2B receptor is recruited to the plasma membrane upon stimulation and interacts with E3KARP (NHE3 kinase A regulatory protein) and ezrin (Sitaraman et al. 2002). Ezrin is a known PKA anchoring protein, and E3KARP associates with the COOH terminus of CFTR (Sun et al. 2000). These interactions may stabilize the receptor and downstream effector molecules in a signalling complex at the membrane. This compartmentalization of cAMP signalling challenges the idea of a readily diffusible second messenger and offers some understanding of how activation of pathways that utilize ubiquitous second messengers can function without global cellular activation.
While the A2B-stimulated Cl− secretory response was lost in the cystic fibrosis cell line CF/T43 specifically deficient in ion transport, manipulation of this receptor is still a consideration in treating cystic fibrosis patients with alternative CFTR mutations that preserve cAMP–PKA signalling and ion transport to some degree. For example, activation of the A2B receptor can augment impaired Cl− conductance at the R117H mutant CFTR, a channel which is known to localize to the cell surface and maintain normal PKA-dependent activation but which has reduced Cl− conductance (Clancy et al. 1999). A2B stimulation can also induce Cl− secretion through the ΔF508 CFTR, where the defect is in trafficking to the membrane and in which Cl− transport is preserved as long as the receptor spontaneously localizes to the cell surface a small percentage of the time or can be induced to localize by corrective molecules (Cobb et al. 2002). In Calu-3 cell monolayers, it was recently shown that phosphodiesterase inhibitors, specifically cilostazol, a phosphodiesterase 3 (PDE3) inhibitor, and papaverine, a non-specific PDE inhibitor, could induce a Cl− current that was additive when combined with adenosine. This effect is presumably obtained by the elevation of intracellular cAMP. The concentration of cilostazol that increased chloride current was below therapeutic plasma levels achieved during the treatment of peripheral vascular disease (Cobb et al. 2003). While this has yet to be studied in cell lines with mutated CFTR channels, it raises the possibility that PDE inhibitor therapy alone or in conjunction with pharmacological manipulation of A2B receptors could be of potential therapeutic benefit in cystic fibrosis.
A2B receptors are not the only P1 receptors implicated in controlling ion transport across airway epithelium. Some literature also implicates the A1 receptor, although the evidence for the role of this receptor has recently been called into question. RT-PCR studies have identified the A1 receptor in the airway epithelial cell line A549, a normal tracheal cell line (9HTEo-), and a CF submucosal cell line (2CFSMEo-), distinguishing these epithelial lines from the Calu-3 cell line in which the A1 receptor mRNA was not detected (McCoy et al. 1995; Szkotak et al. 2001, 2003). Antagonism at the A1 receptor is thought to stimulate Cl− efflux, as shown by the activity of the adenosine antagonist CPX in a variety of model systems (Schwiebert et al. 1992; McCoy et al. 1995; Haws et al. 1996). Importantly, the mechanism of action at this receptor seems to function even in the presence of a mutant CFTR gene, as shown by the ability of CPX to activate outward Cl− currents in primary nasal epithelial cells from wild-type as well as homozygous ΔF508 CF patients (Schwiebert et al. 1992). This was confirmed by showing that CPX could induce iodide efflux from recombinant cells expressing ΔF508 CFTR (Haws et al. 1996). To explain this effect in cells that are otherwise impaired in Cl− conductance, CPX must therefore work either to increase the Cl− conductance of mutant CFTR channels that manage to reach the plasma membrane or augment impaired membrane trafficking in these cells to increase accumulation of these channels at the cell membrane. It has recently been shown that, despite the common assumption that CPX is an A1 antagonist, this effect is probably due more to a direct interaction of CPX with CFTR than with the A1 receptor. CPX has been shown to bind directly to the first nucleotide-binding fold domain (NBF-1) of wild-type CFTR and with even higher affinity to the NBF-1 of the ΔF508 CFTR, and recent evidence shows that CPX can activate wild-type recombinant CFTR chloride channels in an isolated lipid bilayer system (Cohen et al. 1997; Arispe et al. 1998). This evidence supports the idea that, despite the long-standing belief that A1 antagonism activates cAMP-mediated Cl− efflux via CFTR, the more important signalling event may actually be via a direct interaction of the antagonist with CFTR. The validity of CPX for use in treating CF patients has been preliminarily explored, specifically in two phase I clinical trials that tested the safety of CPX administration, although the efficacy of this treatment has yet to be determined (Noone et al. 2001; McCarty et al. 2002).
Although the A1 receptor may not play a major role in regulating the cAMP-dependent CFTR chloride channel, it has been shown to regulate Ca2+-dependent processes in airway epithelial models. Specifically, in the wild-type human tracheal epithelial cell line 9HTEo-, the wild-type fetal trachea cell line 56FHTEo-, and the cystic fibrosis airway epithelial lines CFNPE9o- and CFPEo-, adenosine agonists have been shown to activate Ca2+-dependent K+ and Cl− currents, similar to the action of ATP at P2Y2 receptors in airway epithelia (Galietta et al. 1992; Rugolo et al. 1993). More recent work with the A549 airway epithelial cell line has confirmed the effect of A1 activation on Ca2+-activated K+ channels, although the effect on Cl− conductance has been questioned (McCoy et al. 1995; Szkotak et al. 2001).
P1 receptor signalling in renal epithelia
With regard to epithelial transport in the kidney, it has been known that adenosine stimulates sodium transport since Lang et al. (1985) first described it in Xenopus laevis renal epithelial A6 cells, a common model of the mammalian collecting duct. The net effect of adenosine stimulation is sodium transport from the lumenal to the serosal surface, making antagonists of these receptors possible candidates for diuretic therapy. Since this initial discovery, there has been much discussion about the specific adenosine receptor subtype involved in this response, with the A1, A2A and A3 receptors all being implicated. There is evidence that A1 stimulation, functioning through an increase in intracellular calcium and PKC activation, is largely responsible for apical stimulation of Na+ transport across the epithelial border (Macala & Hayslett, 2002). This is supported by the observed diuresis resulting from a decrease in sodium reabsorption thought to primarily occur in the proximal tubule following A1 receptor blockade (Wilcox et al. 1999). In direct opposition to this is the observation that intrarenal delivery of A1 agonists have been shown to induce natriuresis (Yagil, 1994). In addition, A1 activation has been shown to inhibit the activity of the NHE3 Na+–H+ exchanger when transfected into A6 cells (Di Sole et al. 1999), and this effect could decrease net Na+ reabsorption along the renal tubule. These conflicting results, implicating A1 agonism and antagonism in diuresis, highlight the ambiguity associated with P1 activation at the kidney epithelium. In addition to its effect on Na+ transport, stimulation of Cl− secretion following A1 receptor activation has been observed, specifically in the A6C1 subclone of the A6 cell line, and this secretory stimulation may account for some of the discrepancies in the observed effects of A1 activation and antagonism (Casavola et al. 1996; Banderali et al. 1999; Macala & Hayslett, 2002).
The effects of A2A receptor activation at the renal epithelium are equally debated. Basolateral A2A activation is thought to activate transepithelial Na+ transport via a cAMP–PKA-dependent pathway, although the role of this receptor has been debated. It has been proposed that this regulation of Na+ transport occurs through A2A-mediated control of intracellular pH via activation of the basolateral Na+–H+ exchanger and subsequent increase in intracellular pH (Casavola et al. 1997). The relationship between intracellular pH and Na+ transport is reciprocal such that the decrease in H+ concentration causes an increase in transepithelial Na+ transport. In contrast, A2A agonists have been shown to inhibit the Na+–H+ exchanger NHE3 in transfected A6 cells (Di Sole et al. 1999, 2002).
Finally, it has also been proposed that A3 receptors act at the apical surface to induce Cl− secretion. This effect is dependent on a rise in intracellular Ca2+ but is thought to be via a novel Gs–PKA stimulated increase in calcium entry rather than the traditional Gi–PLC-β-mediated increase in intracellular calcium (Reshkin et al. 2000).
Adenosine plays a role in controlling renal function that is distinct from the direct effect on ion transport in epithelial cells. It has recently been shown that adenosine is a key regulator of tubuloglomerular feedback via activation of A1 receptors on afferent arterioles (Schnermann & Levine, 2003). Accordingly, an A1 receptor knockout mouse shows a loss of macula densa control of renal vascular tone (Sun et al. 2001). Taken together with the significant effects of adenosine receptors on renal Na+ and Cl− transport, these data emphasize the importance of adenosine receptors in controlling the overall function of the kidney in both normal and pathogenic states, although further research is needed to resolve the conflicting reports of P1 function in the kidney.
P1 receptor signalling in other epithelial tissues
As previously discussed, the ubiquitous nature of purine nucleotide release by all cells in response to a variety of stimuli and the almost universal expression of purinergic receptors allows for P1- and P2-mediated control of ion transport in almost all epithelial subtypes explored. In accordance with this, P1 receptor control of ion secretion has been identified in such diverse tissue types as the non-pigmented ciliary epithelial cells of the retina, the vas deferens epithelium, and the middle ear epithelium.
The non-pigmented ciliary epithelial cells of the retina are a known location for A3 receptor-mediated control of epithelial transport. A3 activation causes an increase in aqueous humor production by increasing Cl− secretion, leading to an increase in intraocular pressure. This has been supported by the observed increase in intraocular pressure following in vivo administration of A3 receptor agonists as well as in the A3 receptor knockout mouse model in which the intraocular pressure was reduced (Mitchell et al. 1999; Carre et al. 2000; Avila et al. 2001, 2002). Adenosine stimulates Cl− channels of the ciliary epithelium, and this effect can be mimicked by administration of A3 agonists such as N(6)-(3-iodobenzyl)-5′-N-methylcarbamoyladenosine (IB-MECA) (Carre et al. 1997, 2000; Mitchell et al. 1999). Transcript of the A3 receptor has also been positively identified in human and rabbit ciliary epithelium by RT-PCR (Mitchell et al. 1999). It is also known that Cl− efflux can be induced by cell swelling, and this current is dependent on the same Cl− channels activated by A3 receptor activation. It was previously thought that these shared a common downstream signalling pathway, possibly by the volume-sensitive release of ATP (Carre et al. 2000). The evidence for this was from rabbit non-pigmented ciliary epithelial cells in which a volume-sensitive phosphatidylinositol 3-kinase Cl− efflux was dependent on activation of both PKC and PI3K, a common downstream pathway for Gi protein-coupled receptors such as the A3 receptor (Selbie & Hill, 1998; Shi et al. 2002). Recently, this concept of a shared common pathway for volume-sensitive and A3-induced Cl− currents has been questioned due to the discovery that the A3 receptor-induced Cl− current in human ciliary epithelial cells is independent of PI3K but dependent on mitogen-activated protein kinase (MAPK) activation through a Gβγ pathway (Shi et al. 2003). This leads to the question of whether there are signalling differences between rabbit and human ciliary epithelium or whether adenosine induces Cl− efflux signals through a separate pathway to that involved in volume-sensitive Cl− efflux, despite being dependent on the same effector Cl− channels (Shi et al. 2003).
With regard to the vas deferens epithelium, apical application of adenosine to primary human epithelial monolayers and freshly excised human vas deferens resulted in an increase in cAMP and subsequent anion (Cl− and HCO3−) release (Carlin et al. 2003). This recapitulates what has been shown in porcine vas deferens epithelia (Sedlacek et al. 2001). These results indicate that adenosine receptors modulate the lumenal environment of the deferent duct and may therefore play a role in male fertility. As in other tissue types, this has potential implications in treating cystic fibrosis, as over 97% of men with CF have reproductive dysfunction (Dean & Santis, 1994). In cultured gerbil middle ear epithelium, as in other tissues, adenosine has been shown to activate the cAMP–PKA system leading to Cl− secretion through the CFTR Cl− channels, and this effect is likely to be due to A2B receptor stimulation (Furukawa et al. 1998). In addition to these tissues in which adenosine receptors have been directly implicated in the stimulation of anion currents, cAMP-dependent currents have been identified in many epithelial tissue types including epithelia from mandibular and submandibular glands, rectal glands, epididymis, oviduct, and endometrium, and further research is likely to indicate that activation of adenosine receptors, particularly the A2B receptor, can stimulate a cAMP-mediated current in these tissues (Huang et al. 1993; Dinudom et al. 1995; Devor et al. 1995; Leung et al. 1995; Lee et al. 1999; Chan et al. 1999).
The role of P1 and P2 receptors in regulating ion transport at the epithelium is just one aspect of the diverse functions of these receptors. Further research needs to be initiated not only to further explore purinergic activity in epithelial tissues but also to determine the interactions between P1 and P2 receptors and between receptor functions in alternate tissue types. For example, the role of the A2B receptor in inducing water secretion in response to an inflammatory stimulus in the colon may combine with the A2A response on neutrophils recruited to the site to limit inflammation (Ohta & Sitkovsky, 2001). As more research on these receptors becomes available, it seems likely their manipulation will prove useful in clinical situations, especially in the treatment of cystic fibrosis and similar secretory defects.
References
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol. 1992;263:L1–L14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Welsh MJ. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991;88:6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Ma J, Jacobson KA, Pollard HB. Direct activation of cystic fibrosis transmembrane conductance regulator channels by 8-cyclopentyl-1,3-dipropylxanthine (CPX) and 1,3-diallyl-8-cyclohexylxanthine (DAX) J Biol Chem. 1998;273:5727–5734. doi: 10.1074/jbc.273.10.5727. [DOI] [PubMed] [Google Scholar]
- Avila MY, Stone RA, Civan MM. A(1)-, A(2A)- and A(3)-subtype adenosine receptors modulate intraocular pressure in the mouse. Br J Pharmacol. 2001;134:241–245. doi: 10.1038/sj.bjp.0704267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MY, Stone RA, Civan MM. Knockout of A3 adenosine receptors reduces mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002;43:3021–3026. [PubMed] [Google Scholar]
- Bailey MA, Hillman KA, Unwin RJ. P2 receptors in the kidney. J Auton Nerv Syst. 2000;81:264–270. doi: 10.1016/s0165-1838(00)00125-9. [DOI] [PubMed] [Google Scholar]
- Banderali U, Brochiero E, Lindenthal S, Raschi C, Bogliolo S, Ehrenfeld J. Control of apical membrane chloride permeability in the renal A6 cell line by nucleotides. J Physiol. 1999;519:737–751. doi: 10.1111/j.1469-7793.1999.0737n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KE, Bigby TD. Involvement of arachidonic acid in the chloride secretory response of intestinal epithelial cells. Am J Physiol. 1993;264:C446–C452. doi: 10.1152/ajpcell.1993.264.2.C446. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Cohn JA, Huott PA, Wasserman SI, Dharmsathaphorn K. Immune-related intestinal chloride secretion. II. Effect of adenosine on T84 cell line. Am J Physiol. 1990;258:C902–C912. doi: 10.1152/ajpcell.1990.258.5.C902. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Huott PA, Shah SS, Dharmsathaphorn K, Wasserman SI. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol. 1989;256:C197–C203. doi: 10.1152/ajpcell.1989.256.1.C197. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- Bo X, Jiang LH, Wilson HL, Kim M, Burnstock G, Surprenant A, North RA. Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol. 2003;63:1407–1416. doi: 10.1124/mol.63.6.1407. [DOI] [PubMed] [Google Scholar]
- Bouritius H, Bajnath RB, Groot JA. Microelectrode measurements of the effects of basolateral adenosine in polarized human intestinal epithelial cells in culture. Pflugers Arch. 1999;437:589–595. doi: 10.1007/s004240050821. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N(6)-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y(1) receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RW, Lee JH, Marcus DC, Schultz BD. Adenosine stimulates anion secretion across cultured and native adult human vas deferens epithelia. Biol Reprod. 2003;68:1027–1034. doi: 10.1095/biolreprod.102.009381. [DOI] [PubMed] [Google Scholar]
- Carre DA, Mitchell CH, Peterson-Yantorno K, Coca-Prados M, Civan MM. Adenosine stimulates Cl- channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1997;273:C1354–C1361. doi: 10.1152/ajpcell.1997.273.4.C1354. [DOI] [PubMed] [Google Scholar]
- Carre DA, Mitchell CH, Peterson-Yantorno K, Coca-Prados M, Civan MM. Similarity of A(3)-adenosine and swelling-activated Cl(-) channels in nonpigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2000;279:C440–C451. doi: 10.1152/ajpcell.2000.279.2.C440. [DOI] [PubMed] [Google Scholar]
- Casavola V, Guerra L, Reshkin SJ, Jacobson KA, Murer H. Polarization of adenosine effects on intracellular pH in A6 renal epithelial cells. Mol Pharmacol. 1997;51:516–523. [PMC free article] [PubMed] [Google Scholar]
- Casavola V, Guerra L, Reshkin SJ, Jacobson KA, Verrey F, Murer H. Effect of adenosine on Na+ and Cl- currents in A6 monolayers. Receptor localization and messenger involvement. J Membr Biol. 1996;151:237–245. doi: 10.1007/s002329900074. [DOI] [PubMed] [Google Scholar]
- Chan LN, Chung YW, Leung PS, Liu CQ, Chan HC. Activation of an adenosine 3′,5′-cyclic monophosphate-dependent Cl- conductance in response to neurohormonal stimuli in mouse endometrial epithelial cells: the role of cystic fibrosis transmembrane conductance regulator. Biol Reprod. 1999;60:374–380. doi: 10.1095/biolreprod60.2.374. [DOI] [PubMed] [Google Scholar]
- Clancy JP, Ruiz FE, Sorscher EJ. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am J Physiol. 1999;276:C361–C369. doi: 10.1152/ajpcell.1999.276.2.C361. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Chinet T, Boucher RC. Extracellular ATP stimulates K+ secretion across cultured human airway epithelium. Am J Physiol. 1997;272:L1084–L1091. doi: 10.1152/ajplung.1997.272.6.L1084. [DOI] [PubMed] [Google Scholar]
- Cobb BR, Fan LJ, Kovacs TE, Sorscher EJ, Clancy JP. Adenosine receptors and phosphodiesterase inhibitors stimulate Cl- secretion in Calu-3 cells. Am J Respir Cell Mol Biol. 2003;29:410–418. doi: 10.1165/rcmb.2002-0247OC. [DOI] [PubMed] [Google Scholar]
- Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. A(2) adenosine receptors regulate CFTR through PKA and PLA(2) Am J Physiol Lung Cell Mol Physiol. 2002;282:L12–L25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Lee G, Jacobson KA, Kim YC, Huang Z, Sorscher EJ, Pollard HB. 8-Cyclopentyl-1,3-dipropylxanthine and other xanthines differentially bind to the wild-type and delta F508 first nucleotide binding fold (NBF-1) domains of the cystic fibrosis transmembrane conductance regulator. Biochemistry. 1997;36:6455–6461. doi: 10.1021/bi970150v. [DOI] [PubMed] [Google Scholar]
- Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to G(i) J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(-) transport. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- Dean M, Santis G. Heterogeneity in the severity of cystic fibrosis and the role of CFTR gene mutations. Hum Genet. 1994;93:364–368. doi: 10.1007/BF00201659. [DOI] [PubMed] [Google Scholar]
- Devor DC, Forrest JN, Jr, Suggs WK, Frizzell RA. cAMP-activated Cl- channels in primary cultures of spiny dogfish (Squalus acanthias) rectal gland. Am J Physiol. 1995;268:C70–C79. doi: 10.1152/ajpcell.1995.268.1.C70. [DOI] [PubMed] [Google Scholar]
- Di Sole F, Casavola V, Mastroberardino L, Verrey F, Moe OW, Burckhardt G, Murer H, Helmle-Kolb C. Adenosine inhibits the transfected Na+–H+ exchanger NHE3 in Xenopus laevis renal epithelial cells (A6/C1) J Physiol. 1999;515:829–842. doi: 10.1111/j.1469-7793.1999.829ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sole F, Cerull R, Casavola V, Moe OW, Burckhardt G, Helmle-Kolb C. Molecular aspects of acute inhibition of Na+–H+ exchanger NHE3 by A2-adenosine receptor agonists. J Physiol. 2002;541:529–543. doi: 10.1113/jphysiol.2001.013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinudom A, Komwatana P, Young JA, Cook DI. A forskolin-activated Cl- current in mouse mandibular duct cells. Am J Physiol. 1995;268:G806–G812. doi: 10.1152/ajpgi.1995.268.5.G806. [DOI] [PubMed] [Google Scholar]
- Dobbins JW, Laurenson JP, Forrest JN., Jr Adenosine and adenosine analogues stimulate adenosine cyclic-3′,5′-monophosphate-dependent chloride secretion in the mammalian ileum. J Clin Invest. 1984;74:929–935. doi: 10.1172/JCI111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evellin S, Nolte J, vom Tysack KDF, Thiel M, Weernink PA, Jakobs KH, Webb EJ, Lomasney JW, Schmidt M. Stimulation of phospholipase C-epsilon by the M3 muscarinic acetylcholine receptor mediated by cyclic AMP and the GTPase Rap2B. J Biol Chem. 2002;277:16805–16813. doi: 10.1074/jbc.M112024200. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. VI. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Furukawa M, Ikeda K, Oshima T, Suzuki H, Yamaya M, Sasaki H, Takasaka T. A2 adenosine receptors in Mongolian gerbil middle ear epithelium and their regulation of Cl- secretion. Acta Physiol Scand. 1998;163:103–112. doi: 10.1046/j.1365-201x.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- Galietta LJ, Rasola A, Rugolo M, Zottini M, Mastrocola T, Gruenert DC, Romeo G. Extracellular 2-chloroadenosine and ATP stimulate volume-sensitive Cl- current and calcium mobilization in human tracheal 9HTEo-cells. FEBS Lett. 1992;304:61–65. doi: 10.1016/0014-5793(92)80589-9. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Gabriel SE. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am J Physiol. 1997;273:G258–G266. doi: 10.1152/ajpgi.1997.273.2.G258. [DOI] [PubMed] [Google Scholar]
- Harden TK, Lazarowski ER. Release of ATP and UTP from astrocytoma cells. Prog Brain Res. 1999;120:135–143. doi: 10.1016/s0079-6123(08)63551-7. [DOI] [PubMed] [Google Scholar]
- Harden TK, Lazarowski ER, Boucher RC. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- Haws CM, Nepomuceno IB, Krouse ME, Wakelee H, Law T, Xia Y, Nguyen H, Wine JJ. Delta F508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am J Physiol. 1996;270:C1544–C1555. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- Hede SE, Amstrup J, Christoffersen BC, Novak I. Purinoceptors evoke different electrophysiological responses in pancreatic ducts. P2Y inhibits K(+) conductance, and P2X stimulates cation conductance. J Biol Chem. 1999;274:31784–31791. doi: 10.1074/jbc.274.45.31784. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Fu WO, Chung YW, Zhou TS, Wong PY. Properties of cAMP-dependent and Ca(2+)-dependent whole cell Cl- conductances in rat epididymal cells. Am J Physiol. 1993;264:C794–C802. doi: 10.1152/ajpcell.1993.264.4.C794. [DOI] [PubMed] [Google Scholar]
- Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci U S A. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis SK, Collett A, McAlroy HL, Wilson SM, Olver RE. Effect of luminal nucleotides on Cl- secretion and Na+ absorption in distal bronchi. Pflugers Arch. 1999;438:621–627. doi: 10.1007/s004249900096. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Kitagawa M, Hayakawa T, Case RM, Steward MC. Luminal ATP stimulates fluid and HCO3− secretion in guinea-pig pancreatic duct. J Physiol. 1999;519:551–558. doi: 10.1111/j.1469-7793.1999.0551m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- King BF, Burnstock G, Boyer JL, Boeynaems JM, Weisman GA, Kennedy C, Jacobson KA, Humphries RG, Abbracchio MP, Gachet C, Miras-Portugal MT. The IUPHAR Compendium of Receptor Characterization and Classification. edn 2. IUPHAR. Media Publications; 2001. P2Y receptors; pp. 306–320. [Google Scholar]
- Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol. 1995;269:F863–F869. doi: 10.1152/ajprenal.1995.269.6.F863. [DOI] [PubMed] [Google Scholar]
- Kishore BK, Di Iulio D, Menon AG, Krane CM. Altered abundance and subcellular distribution of P2Y2-purinoceptor protein in inner medulla of thirsted and hydrated rats: possible basis for vasopressin-independent regulation of water transport (abstract) J Am Soc Nephrol. 2000;11:17A. [Google Scholar]
- Kishore BK, Wang Z, Rabb H, Haq M, Soleimani M. Upregulation of P2Y2 purinoceptor during ischemic reperfusion injury (IRI): possible relevance to diuresis of IRI (abstract) J Am Soc Nephrol. 1998;9:580A. [Google Scholar]
- Kottgen M, Loffler T, Jacobi C, Nitschke R, Pavenstadt H, Schreiber R, Frische S, Nielsen S, Leipziger J. P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP-mediated transport. J Clin Invest. 2003;111:371–379. doi: 10.1172/JCI16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang MA, Preston AS, Handler JS, Forrest JN., Jr Adenosine stimulates sodium transport in kidney A6 epithelia in culture. Am J Physiol. 1985;249:C330–C336. doi: 10.1152/ajpcell.1985.249.3.C330. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC. UTP as an extracellular signaling molecule. News Physiol Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Mason SJ, Clarke L, Harden TK, Boucher RC. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br J Pharmacol. 1992;106:774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl-/HCO3- exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- Leipziger J, Kerstan D, Nitschke R, Greger R. ATP increases [Ca2+]i and ion secretion via a basolateral P2Y-receptor in rat distal colonic mucosa. Pflugers Arch. 1997;434:77–83. doi: 10.1007/pl00008079. [DOI] [PubMed] [Google Scholar]
- Leung AY, Wong PY, Gabriel SE, Yankaskas JR, Boucher RC. cAMP- but not Ca(2+)-regulated Cl- conductance in the oviduct is defective in mouse model of cystic fibrosis. Am J Physiol. 1995;268:C708–C712. doi: 10.1152/ajpcell.1995.268.3.C708. [DOI] [PubMed] [Google Scholar]
- Linden J, Thai T, Figler H, Jin X, Robeva AS. Characterization of human A(2B) adenosine receptors: radioligand binding, western blotting, and coupling to G(q) in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol. 1999;56:705–713. [PubMed] [Google Scholar]
- Macala LJ, Hayslett JP. Basolateral and apical A1 adenosine receptors mediate sodium transport in cultured renal epithelial (A6) cells. Am J Physiol Renal Physiol. 2002;283:F1216–F1225. doi: 10.1152/ajprenal.00085.2002. [DOI] [PubMed] [Google Scholar]
- McCarty NA, Standaert TA, Teresi M, Tuthill C, Launspach J, Kelley TJ, Milgram LJ, Hilliard KA, Regelmann WE, Weatherly MR, Aitken ML, Konstan MW, Ahrens RC. A phase I randomized, multicenter trial of CPX in adult subjects with mild cystic fibrosis. Pediatr Pulmonol. 2002;33:90–98. doi: 10.1002/ppul.10041. [DOI] [PubMed] [Google Scholar]
- McCoy DE, Schwiebert EM, Karlson KH, Spielman WS, Stanton BA. Identification and function of A1 adenosine receptors in normal and cystic fibrosis human airway epithelial cells. Am J Physiol. 1995;268:C1520–C1527. doi: 10.1152/ajpcell.1995.268.6.C1520. [DOI] [PubMed] [Google Scholar]
- McCoy DE, Taylor AL, Kudlow BA, Karlson K, Slattery MJ, Schwiebert LM, Schwiebert EM, Stanton BA. Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol. 1999;277:F552–F559. doi: 10.1152/ajprenal.1999.277.4.F552. [DOI] [PubMed] [Google Scholar]
- Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5′-Adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Peterson-Yantorno K, Carre DA, McGlinn AM, Coca-Prados M, Stone RA, Civan MM. A3 adenosine receptors regulate Cl- channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1999;276:C659–C666. doi: 10.1152/ajpcell.1999.276.3.C659. [DOI] [PubMed] [Google Scholar]
- Morris AP, Cunningham SA, Benos DJ, Frizzell RA. Cellular differentiation is required for cAMP but not Ca(2+) -dependent Cl- secretion in colonic epithelial cells expressing high levels of cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1992;267:5575–5583. [PubMed] [Google Scholar]
- Noone PG, Hamblett N, Accurso F, Aitken ML, Boyle M, Dovey M, Gibson R, Johnson C, Kellerman D, Konstan MW, Milgram L, Mundahl J, Retsch-Bogort G, Rodman D, Williams-Warren J, Wilmott RW, Zeitlin P, Ramsey B. Safety of aerosolized INS 365 in patients with mild to moderate cystic fibrosis: results of a phase I multi-center study. Pediatr Pulmonol. 2001;32:122–128. doi: 10.1002/ppul.1098. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Reshkin SJ, Guerra L, Bagorda A, Debellis L, Cardone R, Li AH, Jacobson KA, Casavola V. Activation of A(3) adenosine receptor induces calcium entry and chloride secretion in A(6) cells. J Membr Biol. 2000;178:103–113. doi: 10.1007/s002320010018. [DOI] [PubMed] [Google Scholar]
- Roman RM, Fitz JG. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology. 1999;116:964–979. doi: 10.1016/s0016-5085(99)70081-8. [DOI] [PubMed] [Google Scholar]
- Rozmahel R, Wilschanski M, Matin A, Plyte S, Oliver M, Auerbach W, Moore A, Forstner J, Durie P, Nadeau J, Bear C, Tsui LC. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat Genet. 1996;12:280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- Rugolo M, Mastrocola T, Whorle C, Rasola A, Gruenert DC, Romeo G, Galietta LJ. ATP and A1 adenosine receptor agonists mobilize intracellular calcium and activate K+ and Cl- currents in normal and cystic fibrosis airway epithelial cells. J Biol Chem. 1993;268:24779–24784. [PubMed] [Google Scholar]
- Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol. 2001;280:F945–F963. doi: 10.1152/ajprenal.2001.280.6.F945. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Kizer N, Gruenert DC, Stanton BA. GTP-binding proteins inhibit cAMP activation of chloride channels in cystic fibrosis airway epithelial cells. Proc Natl Acad Sci U S A. 1992;89:10623–10627. doi: 10.1073/pnas.89.22.10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlacek RL, Carlin RW, Singh AK, Schultz BD. Neurotransmitter-stimulated ion transport by cultured porcine vas deferens epithelium. Am J Physiol Renal Physiol. 2001;281:F557–F570. doi: 10.1152/ajprenal.2001.281.3.F557. [DOI] [PubMed] [Google Scholar]
- Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- Shi C, Barnes S, Coca-Prados M, Kelly ME. Protein tyrosine kinase and protein phosphatase signaling pathways regulate volume -sensitive chloride currents in a nonpigmented ciliary epithelial cell line. Invest Ophthalmol Vis Sci. 2002;43:1525–1532. [PubMed] [Google Scholar]
- Shi C, Szczesniak A, Mao L, Jollimore C, Coca-Prados M, Hung O, Kelly ME. A3 adenosine and CB1 receptors activate a PKC-sensitive Cl(-) current in human nonpigmented ciliary epithelial cells via a Gbetagamma-coupled MAPK signaling pathway. Br J Pharmacol. 2003;139:475–486. doi: 10.1038/sj.bjp.0705266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Filippov AK, Goransson S, Wong YH, Frelin C, Michel AD, Brown DA, Barnard EA. Characterization and channel coupling of the P2Y(12) nucleotide receptor of brain capillary endothelial cells. J Biol Chem. 2002;277:31390–31400. doi: 10.1074/jbc.M110714200. [DOI] [PubMed] [Google Scholar]
- Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem. 2002;277:33188–33195. doi: 10.1074/jbc.M202522200. [DOI] [PubMed] [Google Scholar]
- Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Fitz JG, Paradiso AM, Boucher RC. Multiple modes of regulation of airway epithelial chloride secretion by extracellular ATP. Am J Physiol. 1994;267:C1442–C1451. doi: 10.1152/ajpcell.1994.267.5.C1442. [DOI] [PubMed] [Google Scholar]
- Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem. 2000;275:29539–29546. doi: 10.1074/jbc.M004961200. [DOI] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkotak AJ, Ng AM, Man SF, Baldwin SA, Cass CE, Young JD, Duszyk M. Coupling of CFTR-mediated anion secretion to nucleoside transporters and adenosine homeostasis in Calu-3 cells. J Membr Biol. 2003;192:169–179. doi: 10.1007/s00232-002-1073-x. [DOI] [PubMed] [Google Scholar]
- Szkotak AJ, Ng AM, Sawicka J, Baldwin SA, Man SF, Cass CE, Young JD, Duszyk M. Regulation of K(+) current in human airway epithelial cells by exogenous and autocrine adenosine. Am J Physiol Cell Physiol. 2001;281:C1991–C2002. doi: 10.1152/ajpcell.2001.281.6.C1991. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. Am J Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Wagner JA, McDonald TV, Nghiem PT, Lowe AW, Schulman H, Gruenert DC, Stryer L, Gardner P. Antisense oligodeoxynucleotides to the cystic fibrosis transmembrane conductance regulator inhibit cAMP-activated but not calcium-activated chloride currents. Proc Natl Acad Sci U S A. 1992;89:6785–6789. doi: 10.1073/pnas.89.15.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CS, Welch WJ, Schreiner GF, Belardinelli L. Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J Am Soc Nephrol. 1999;10:714–720. doi: 10.1681/ASN.V104714. [DOI] [PubMed] [Google Scholar]
- Yagil Y. The effects of adenosine on water and sodium excretion. J Pharmacol Exp Ther. 1994;268:826–835. [PubMed] [Google Scholar]
- Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]