Abstract
Transient receptor potential (TRP) channels form cationic channels activated by diverse factors including mechanical stimuli, changes in osmolarity, pH and temperature, as well as the exogenous irritant, capsaicin. Metabotropic glutamate receptors have also recently been linked to TRP channel activation in neurones of the substantia nigra, hippocampus and cerebellum, suggesting a novel role for such channels in synaptic communication via endogenous neurotransmitters. We tested this for dopamine neurones in rat brain slices by characterizing the current–voltage relationship and pharmacology of EPSCs mediated by group I metabotropic glutamate receptor subtype 1 (mGluR1). Slow inward currents (273 ± 35 pA peak amplitude, 381 ± 25 ms latency, holding potential (Vh) =−73 mV) representing evoked mGluR1 EPSCs were isolated in the presence of antagonists of AMPA, NMDA, GABAA, GABAB, muscarinic and glycine receptors. CPCCOEt (100 μm), an mGluR1 antagonist, blocked the residual EPSC in all recordings. mGluR1-activated EPSCs reversed polarity near −10 mV, consistent with the involvement of a cationic channel. Extracellular application of the non-selective TRP channel blockers SKF 96365, flufenamic acid and ruthenium red caused reversible inhibition of mGluR1-activated EPSCs. These characteristics parallel those of mGluR1 activation with an agonist and indicate the involvement of a TRP-like channel in mGluR1-mediated EPSCs.
The canonical (or short) form of transient receptor potential (TRPC) channels are widely present in the brain (Strubing et al. 2001; Riccio et al. 2002; Sergeeva et al. 2003) and can be activated by Gq-coupled receptors and/or depletion of intracellular Ca2+ stores (Strubing et al. 2001; Clapham et al. 2002; Vennekens et al. 2002; Plant & Schaefer, 2003). Indeed, TRP-like channels can be activated by glutamate in cultured astrocytes (Pizzo et al. 2001) and by group 1 metabotropic glutamate receptors (mGluR-I) in CA3 neurones of the hippocampus (Gee et al. 2003) and dopamine neurones of the substantia nigra pars compacta (SNc) (Tozzi et al. 2003). During the preparation of this manuscript, Kim et al. (2003) published the first evidence that TRP channels can mediate synaptic transmission between neurones, namely the well-characterized mGluR-I subtype 1 (mGluR1)-mediated EPSC in Purkinje neurones of the cerebellum.
In dopamine neurones, mGluR-I activates either an inhibitory or an excitatory response depending on the recording conditions and the stimulus strength. Brief afferent stimulation (5 pulses) produces an IPSC mediated by mGluR-I and small conductance Ca2+-activated K+ channels (sK) while longer stimulation (10 pulses) evokes a complex response due to the summation of inhibitory and excitatory components (Shen & Johnson, 1997; Fiorillo & Williams, 1998; Iribe et al. 1999). Using a gluconate-based intracellular solution with moderate Ca2+ buffering which inhibits sK (Velumian et al. 1997), our group has studied isolated excitatory currents evoked by the agonist (S)-3,5-dihydroxyphenylglycine (DHPG, local application by pressure ejection through micropipettes) mediated selectively by mGluR1 in dopamine neurones of the SNc (Guatteo et al. 1999). DHPG activates a TRP-like cationic conductance with a reversal potential near 0 mV and sensitivity to several TRP channel blockers (Tozzi et al. 2003).
We wished to assess the involvement of TRP channels in mGluR1-mediated EPSCs in acute slice preparations of dopamine neurones. Thus we pharmacologically isolated a mGluR1-mediated synaptic current evoked by electrical stimulation and characterized its reversal potential and sensitivity to TRP channel blockers. This study demonstrates that TRP-like channels also mediate mGluR1-activated EPSCs in the SNc.
Methods
Brain slice preparation
All experiments were carried out according to guidelines of the Ethics Committee of the Tor Vergata University for the use of animals in research. Albino Wistar rats of either sex (P22-28) were anaesthetized with 2-bromo-2-chloro-1,1,1-trifluoroethane (Sigma) inhalation and killed by decapitation. The brain was rapidly removed and horizontal slices (thickness 240 μm) were cut in 8–12°C artificial cerebrospinal fluid (ACSF) using a vibratome. ACSF contained (mm): NaCl, 126; KCl, 2.5; MgCl2, 1.2; NaH2PO4, 1.2; CaCl2, 2.4; glucose, 10; NaHCO3, 24; equilibrated with 95% O2 and 5% CO2. After incubation in a reservoir (1–4 h, 34°C), single slices were transferred into a recording chamber and completely submerged in continuously flowing ACSF (2.5 ml min−1, 33–34°C, pH 7.4). The chamber was mounted on the stage of an upright Axioskop microscope (Carl Zeiss, Oberkochen, Germany) equipped for infrared video microscopy (Hamamatsu, Hamamatsu City, Japan).
Electrophysiology
Whole cell patch-clamp recordings were obtained with an amplifier (Axopatch 1D, Axon Instruments) from visually and electrophysiologically identified dopamine neurones (Guatteo et al. 1999) of the SNc using patch pipettes (3–4 MΩ) made from borosilicate glass 1.5 mm (WPI, Sarasota, FL, USA) using a PP 83 Narishige puller (Tokyo, Japan). Membrane currents were digitized at 5 kHz through a Digidata 1322A A/D converter, acquired and analysed using pCLAMP software (Axon Instruments).
EPSCs were evoked using a pair of tungsten electrodes positioned on either side and within 2 mm of the patched cell. All EPSCs were recorded in the presence of CNQX (10 μm), AP5 (50 μm), picrotoxin (100 μm), CGP 55845 (0.5 μm), atropine (3 μm) and strychnine (1 μm) to block AMPA, NMDA, GABAA, GABAB, muscarinic and glycinergic receptors, respectively. In all cells, the identification of the residual EPSC was confirmed by blockade with the mGluR1 selective antagonist CPCCOEt (100 μm).
Recording pipettes were filled with a standard internal solution containing (mm): potassium gluconate 145; CaCl2, 0.1; MgCl2, 2; Hepes, 10; EGTA, 0.75; Mg2-ATP, 2; Na3GTP, 0.3; (pH 7.35 with KOH). For measurement of current–voltage relationships (I–Vs), potassium gluconate was replaced with caesium gluconate (pH 7.35 with CsOH). For EPSC I–V measurement, QX-314 (2 mm) was added to the internal solution to block Na+ currents and the h current (Ih) and ketamine 100 μm was added to the extracellular solution to ensure the blockade of NMDA receptor-mediated currents at depolarized potentials. For the measurement of DHPG I–Vs, the extracellular solution contained Na+, K+, Ih and GABAA channel blockers (containing in mm: NaCl, 106; TEA-Cl, 30; 4-aminopyridine (4AP), 5; KCl, 2.5; MgCl2, 1.2; CsCl, 2; CaCl2, 2.4; NaH2PO4, 1.2; NaHCO3, 24; glucose, 10; tetrodotoxin (TTX), 0.001; picrotoxin, 0.1). Extracellular channel blockers were applied for at least 10 min before measuring I–Vs and did not affect DHPG response amplitudes which measured 90 ± 8% of control responses measured in the same cell in standard ACSF (n= 6,Vh: −75 mV).
All results and figures have been corrected for calculated junction potential of 13–15 mV and should be noted when comparing these results with uncorrected data reported elsewhere.
Drugs
(S)-3,5-dihydroxyphenylglycine (DHPG), 1-[2-(4-Methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl-1H-imidazole hydrochloride (SKF 96365), 7(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate (CPCCOEt), picrotoxin, CGP 55845 and amino-5-phosphonoeptanoic acid (AP5) were obtained from Tocris Cookson (Bristol, UK); ruthenium red was from Fluka Chemie (Buchs, Switzerland); tetrodotoxin and N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium chloride (QX-314) were from Alomone Laboratories (Jerusalem, Israel); strychnine hydrochloride was from Roth (Karlsruhe, Germany); N-[3(trifluoromethyl)phenyl]anthranylic acid (flufenamic acid), atropine, ketamine, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and all other products were obtained from Sigma (Milan, Italy).
Picrotoxin, ruthenium red, flufenamic acid and strychnine were dissolved on the day of use. CPCCOEt and CGP 55845 were dissolved in DMSO, picrotoxin was dissolved in extracellular solution, QX-314 was dissolved in the intracellular solution and all other substances were dissolved in water. The final concentration of DMSO did not exceed 0.1% extracellularly which produced no independent effects on the neurones.
DHPG (100 μm) in extracellular solution was applied through a glass pipette whose open end (approx. 2 μm diameter) was positioned 30–50 μm from the cell body of the patched neurone. The DHPG pipette was connected to a pneumatic pico-pump (Picospritzer, WPI) set at 20 p.s.i., delivering a pressure-ejection pulse (1 s). Other drugs were bath-applied by switching the solution of the perfusion system using three-way taps with complete exchange of the solution in the recording chamber occurring in about 1 min.
All values in the text are expressed as mean ± standard error of the mean.
Results
Whole cell patch-clamp recordings were performed in dopamine neurones of the SNc in a well-defined region of tightly packed neurones adjacent to the medial terminal nucleus of the accessory optic tract. Dopamine neurones were identified by the presence of a large hyperpolarization-activated inward current, Ih (Lacey et al. 1989; Mercuri et al. 1995).
mGluR1 receptor-mediated EPSCs with a peak amplitude of 273 ± 35 pA (Vh=−73 mV, n= 15) were pharmacologically isolated (see Methods) from postsynaptic current responses to a 300 Hz stimulus train lasting 50 ms (i.e. 15 stimulations). This response peak occurred 381 ± 25 ms after the end of the stimulus train. Single exponential fits of mGluR1 EPSCs gave time constants of 0.199 ± 0.025 s for the increase in current amplitude and 0.809 ± 0.092 s for the decay.
Evoked EPSCs mediated by mGluR-I reverse near −10 mV
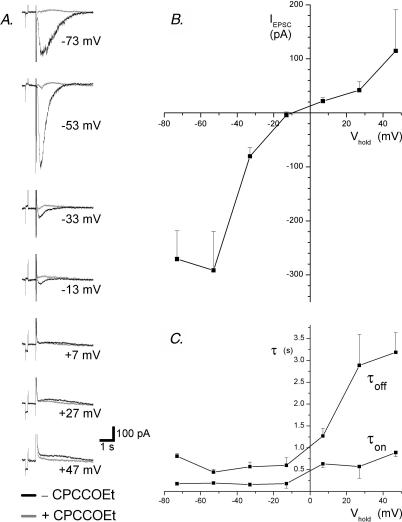
To investigate their I–V, mGluR1 EPSCs were recorded at holding potentials between −73 and +47 mV in seven cells. EPSCs were then re-recorded in the presence of the selective mGluR1 antagonist CPCCOEt (100 μm), which confirmed the absence of non-mGluR1-mediated components of the EPSC at all potentials in all cells (Fig. 1A). The EPSCs of three cells, including the example in Fig. 1A, were larger at −53 mV than at −73 mV, indicating a region of negative slope conductance in the I–V at hyperpolarized potentials. Another four cells, however, did not display this phenomenon with a positive slope conductance at all potentials. The EPSCs of all seven cells were inward at −33 mV and outward at +7 mV although EPSCs recorded at −13 mV showed both inward and outward components in some cells (not shown). An I–V of the mGluR1 EPSC was formed from the average EPSC amplitude of all cells and showed a reversal potential estimated to be −10 mV by extrapolation (Fig. 1B).
Figure 1. The I–V of mGluR1-mediated EPSCs.
A current traces show the EPSCs recorded from a midbrain dopamine neurone at the holding potentials indicated in the absence and presence of the mGluR1 antagonist CPCCOEt (100 μm). No averaging was performed. A 5 mV transient is included to control for the changes in access resistance and precedes the stimulus artefact (15 stimuli at 300 Hz). B, this I–V plot shows the peak difference between the EPSCs recorded in the presence and absence of CPCCOEt at each potential in 7 cells. C, this graph shows the time constants estimated by single exponential fits of the EPSC waveform during the increase (τon) and decrease (τoff) of the current amplitude at each potential for the same cells shown in B.
The kinetics of outward mGluR1 EPSCs were much slower than those of inward EPSCs (Fig. 1C). Outward mGluR1 EPSCs recorded at +47 mV showed time constants of 0.89 ± 0.09 s for the increase in current amplitude and 3.18 ± 0.44 ms for the decay, representing an approximate fourfold increase in the respective time constant (τ) values measured at −75 mV.
Responses to pressure ejection of an mGluR1 agonist reverse near −17 mV
Local pressure-ejection application of DHPG selectively activates mGluR1 receptors in midbrain dopamine neurones (Guatteo et al. 1999). Responses to DHPG (100 μm, 1 s) showed an amplitude of h>340 ± 61 pA with time constants of 0.96 ± 0.09 s for the increase in current amplitude and 3.10 ± 0.34 s for the decay (Vh=−75 mV, n= 9). In comparison to synaptically mediated mGluR1 currents, DHPG-activated currents showed similar amplitudes (125% of mean EPSC amplitude, P= 0.42) but much slower kinetics (482 and 383% of mean EPSC τon and τoff, respectively, P < 0.0001 between-groups t tests).
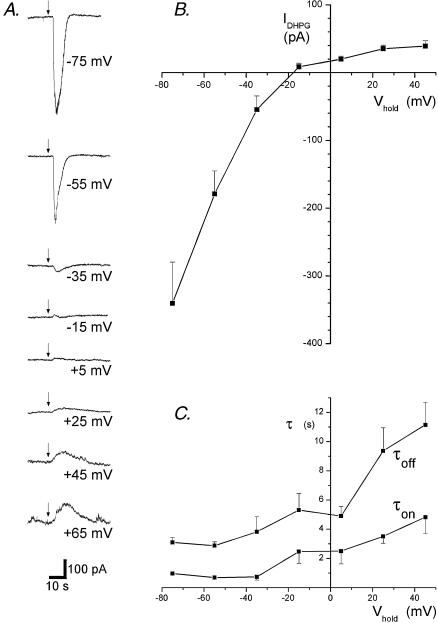
We have recently demonstrated the involvement of Na+- and K+-permeable TRP-like channels in the DHPG response of dopamine neurones, where a reversal potential of 0 mV was determined using ramp I–V protocols. To more directly compare mGluR1 EPSCs to agonist-evoked currents across a range of holding potentials, we measured responses to pressure ejection of DHPG at holding potentials between −75 and +45 mV in 20 mV intervals (Fig. 2A). In all nine cells, the DHPG current reversed polarity between −35 and +5 mV with a reversal potential of −17 mV estimated by extrapolation (Fig. 2B). As for mGluR1-mediated EPSCs measured near the reversal potential, the DHPG response at −15 mV showed inward followed by outward components in several cells (see example Fig. 2A).
Figure 2. The I–V of mGluR1 selective responses to DHPG.
A, traces show the current responses to pressure ejection of DHPG (100 μm, 1 s, arrows) at the holding potentials indicated. No averaging was performed. B, the histogram shows the I–V generated from measurements of the peak amplitude of the current evoked by DHPG at each holding potential in 7 cells. C, this graph shows the time constants estimated by single exponential fits of the EPSC waveform during the increase (τon) and decrease (τoff) of the current amplitude at each potential for the same cells shown in B.
The kinetics of DHPG activated currents, like those of mGluR1-mediated EPSCs, were much slower for outward than for inward currents (Fig. 2C). At holding potentials of +45 mV, the DHPG activated current had time constants of 4.82 ± 1.10 s for the rise and 11.15 ± 1.52 s for the decay, representing once again a roughly fourfold increase relative to the respective time constants of DHPG activated currents at holding potentials of −75 mV. Graphic analysis also revealed that these time constants, as for those of the mGluR1 EPSC, did not merely reflect the driving force of the current (i.e. the absolute difference between the reversal potential and the holding potential; Fig. 2C).
mGluR1 EPSCs are blocked by TRP channel blockers
mGluR1 receptor-mediated EPSCs were diminished by extracellular application of non-selective compounds known to block TRP channels and store-operated channels (SOCs). The imidazole SKF 96365 blocks SOCs and TRPC3, TRPC5, TRPC6 and TRPC7 channels at concentrations of 25–100 μm (Zhu et al. 1998; Halaszovich et al. 2000; Inoue et al. 2001; Clapham et al. 2002). SKF 96365 (100 μM) reversibly inhibited mGluR-mediated EPSCs to 3.9 ± 3.9% of control (n= 4; P < 0.05; Fig. 3A).
Figure 3. mGluR1-mediated EPSCs are inhibited by TRP channel blockers.
SKF 96365 (A) flufenamic acid (B) and ruthenium red (C) all reduce the mGluR1 EPSCs. Traces show the average of 3 EPSCs recorded in the conditions indicated. A 5 mV transient precedes the stimulus artefact (15 stimuli at 300 Hz). The histograms show the peak difference between the averaged EPSCs recorded in the presence and absence of CPCCOEt for each compound and normalized to the control EPSC in the same cell. Results came from 4 cells for each compound except for the washout of the effects of CPCCOEt, which was not achieved in all cells. Asterisks indicate statistical significance (P < 0.05, paired t tests). Rut r, ruthenium red; flufen, flufenamic acid; SKF, SKF 96365; CPCC, CPCCOEt.
Flufenamic acid is a non-steroidal anti-inflammatory drug known to increase TRPC6 currents but inhibits currents mediated by the TRPC3/7 subfamily and putative TRPC5 channels at 100 μm (Inoue et al. 2001; Tesfai et al. 2001; Lee et al. 2003). In midbrain dopamine neurones, 100 μm flufenamic acid caused a rapid and reversible inhibition of mGluR1-mediated EPSCs to 11 ± 3% of control(n= 4; P < 0.05; Fig. 3B).
Ruthenium red is a non-selective ryanodine receptor antagonist, which blocks TRPV-mediated responses (Zucchi & Ronca-Testoni, 1997), mitochondrial Ca2+ uptake (Rigoni & Deana, 1986) and voltage-operated Ca2+ channels (Cibulsky & Sather, 1999). Ruthenium red (20 μM) applied extracellularly rapidly and reversibly inhibited mGluR1-mediated EPSCs to 20 ± 7% of control (n= 4; P < 0.05; Fig. 3C).
After washout of the effects of SKF 96365, flufenamic acid or ruthenium red in all cells, CPCCOEt 100 μm was applied and shown to block the evoked EPSCs (5 ± 2% of control; n= 12; see Fig. 3). Thus the evoked EPSCs blocked by these TRP channel blockers were mediated by mGluR1.
Discussion
This short communication identifies the involvement of a TRP-like channel in evoked EPSCs mediated by metabotropic receptors, namely mGluR1, in native dopamine neurones in acute brain slices. The reversal potential and voltage-dependent kinetics of EPSCs, as well as their inhibition by three TRP channel blockers, matches that of DHPG-evoked currents, which are mediated by TRP-like channels (see Tozzi et al. 2003). The diffuse presence in the brain of mRNA for multiple TRP isoforms suggests the potential for this multifunctional channel superfamily to participate directly in neuronal transmission.
mGluR1 EPSC I–V is consistent with a cationic TRP channel
mGluR1 EPSCs reversed at a membrane potential of −10 mV, consistent with the involvement of a cationic channel and similar to the reversal potential of channels activated by DHPG, which we have previously shown to be permeable to K+ and Na+ (Guatteo et al. 1999; Tozzi et al. 2003). The reversal potential of the DHPG current measured at various holding potentials (−17 mV, current results) was more negative than estimates generated from ramp protocols (+1 mV, Tozzi et al. 2003). This may be due to a diminished contribution of the slow activating outward current components in rapid voltage ramp measurements, or the contribution of voltage-activated conductances in distal dendritic compartments where the voltage clamp may have been compromised.
The form of the mGluR1 EPSC I–V displayed double rectification with minimal outward current between −10 and +27 mV. Negative slope conductance at hyperpolarized potentials (< −53 mV) was also present in some cells. These properties also characterize TRPC channels in the TRPC1/4/5 subfamily studied in expression systems (Strubing et al. 2001; Plant & Schaefer, 2003). We have recently reported the presence of mRNA for individual or multiple combinations of these isoforms in dopamine cells. (Tozzi et al. 2003) Negative slope conductance at potentials more negative than −60 mV is revealed when multiple isoforms of this subfamily are coexpressed, but is lost during strong channel activation. The presence of negative slope conductance of the mGluR1 EPSC in some dopamine neurones may reflect the presence of multiple TRPC isoforms in these cells or differences in the degree of channel activation.
Voltage-dependent kinetics of mGluR1 responses
The kinetics of mGluR1-mediated EPSCs in dopamine neurones at hyperpolarized potentials was typical of the relatively slow synaptic currents mediated by metabotropic receptors including mGluR-I-mediated EPSCs in cerebellar Purkinje neurones (Reichelt & Knopfel, 2002; Kim et al. 2003) and dopamine neurones (Shen & Johnson, 1997). EPSC response latencies may also reflect diffusion delays introduced by the extra-synaptic location of mGluR1 receptors and/or the effector channels. Since time constants for EPSCs were roughly 4 times faster than those of currents evoked by localized pressure ejection of DHPG, mGluR1 receptors are probably located close to terminals (i.e. synaptic or perisynaptic).
The time course of both mGluR1-activated EPSCs and responses to brief activation of mGluR1 with an exogenous agonist were greatly retarded at depolarized potentials. The voltage dependence of mGluR1 response kinetics is unlikely to relate to the spatial relationship of receptors and effectors or second messengers but may reflect gating properties of the TRP-like channel involved. NMDA-mediated EPSCs (for example Anchisi et al. 2001) show a similar I–V shape and exhibit qualitatively similar voltage dependence in their kinetics.
Although mGluR1 EPSCs were much smaller at positive membrane potentials, they activated and deactivated/inactivated much more slowly. This may act to sustain mGluR1-activated excitatory currents following burst initiation when the membrane potential spends significant amounts of time at positive potentials.
Pharmacological identification of TRP channels mediating mGluR1 EPSCs
The sensitivity of mGluR1 EPSCs to TRP channel antagonists parallels that of current responses to DHPG pressure ejection. Although SKF 96365, flufenamic acid and ruthenium red have non-selective actions (see Results), we previously demonstrated the insensitivity of DHPG responses to blockers of voltage-activated Ca2+, Na+, K+ and h-current channels and that the inhibition of mGluR1 responses by ruthenium red was not mediated by its intracellular actions (Tozzi et al. 2003). Thus, we propose that mGluR1 EPSCs, like agonist-activated currents, are mediated by a TRP-like channel.
mGluR1-mediated EPSCs in cerebellar Purkinje neurones were also inhibited by SKF 96365 (Kim et al. 2003), despite previous evidence to the contrary (Tempia et al. 2001). TRPC1 channels, which mediate EPSCs in Purkinje neurones may also be involved in the mGluR1 EPSCs of dopamine neurones, most of which contain TRPC1 mRNA (Tozzi et al. 2003).
Physiological implications
Burst firing of afferent glutamatergic neurones is likely to activate mGluR1-mediated EPSCs in dopamine neurones. Glutamatergic neurones in the subthalamic nucleus project directly on to dopamine neurones in the substantia nigra. (Iribe et al. 1999). Subthalamic nucleus neurones display burst activity with 10–15 spikes per burst at frequencies of 100–400 Hz in vivo (Hollerman & Grace, 1992; Urbain et al. 2002). Such bursting activity in vivo is expected to cause mGluR1 EPSCs such as those recorded here using 15 stimuli at 300 Hz. Complex interactions between concurrent metabotropic and ionotropic glutamate receptor-mediated EPSCs and metabotropic IPSCs are likely, however.
Acknowledgments
This study was funded by two FIRB grants (codes: RBNE01WY7P-010 and RBNE017555-006).
References
- Anchisi D, Scelfo B, Tempia F. Postsynaptic currents in deep cerebellar nuclei. J Neurophysiol. 2001;85:323–331. doi: 10.1152/jn.2001.85.1.323. [DOI] [PubMed] [Google Scholar]
- Cibulsky SM, Sather WA. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J Pharmacol Exp Ther. 1999;289:1447–1453. [PubMed] [Google Scholar]
- Clapham DE, Montell C, Schultz G, Julius D. The TRP ion channel family. IUPHAR Compendium, TRP Channels. 2002. pp. 1–40.
- Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Gerber U. Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J Physiol. 2003;546:655–664. doi: 10.1113/jphysiol.2002.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatteo E, Mercuri NB, Bernardi G, Knopfel T. Group I metabotropic glutamate receptors mediate an inward current in rat substantia nigra dopamine neurons that is independent from calcium mobilization. J Neurophysiol. 1999;82:1974–1981. doi: 10.1152/jn.1999.82.4.1974. [DOI] [PubMed] [Google Scholar]
- Halaszovich CR, Zitt C, Jungling E, Luckhoff A. Inhibition of TRP3 channels by lanthanides. Block from the cytosolic side of the plasma membrane. J Biol Chem. 2000;275:37423–37428. doi: 10.1074/jbc.M007010200. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and response to haloperidol. Brain Res. 1992;590:291–299. doi: 10.1016/0006-8993(92)91108-q. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Iribe Y, Moore K, Pang KC, Tepper JM. Subthalamic stimulation-induced synaptic responses in substantia nigra pars compacta dopaminergic neurons in vitro. J Neurophysiol. 1999;82:925–933. doi: 10.1152/jn.1999.82.2.925. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neuroscience. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. European J Neuroscience. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- Plant TD, Schaefer M. TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–450. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- Reichelt W, Knopfel T. Glutamate uptake controls expression of a slow postsynaptic current mediated by mGluRs in cerebellar Purkinje cells. J Neurophysiol. 2002;87:1974–1980. doi: 10.1152/jn.00704.2001. [DOI] [PubMed] [Google Scholar]
- Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- Rigoni F, Deana R. Ruthenium red inhibits the mitochondrial Ca2+ uptake in intact bovine spermatozoa and increases the cytosolic Ca2+ concentration. FEBS Lett. 1986;198:103–108. doi: 10.1016/0014-5793(86)81193-0. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Korotkova TM, Scherer A, Brown RE, Haas HL. Co-expression of non-selective cation channels of the transient receptor potential canonical family in central aminergic neurones. J Neurochem. 2003;85:1547–1552. doi: 10.1046/j.1471-4159.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. A slow excitatory postsynaptic current mediated by G-protein-coupled metabotropic glutamate receptors in rat ventral tegmental dopamine neurons. European J Neuroscience. 1997;9:48–54. doi: 10.1111/j.1460-9568.1997.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Tempia F, Alojado ME, Strata P, Knopfel T. Characterization of the mGluR(1)-mediated electrical and calcium signaling in Purkinje cells of mouse cerebellar slices. J Neurophysiol. 2001;86:1389–1397. doi: 10.1152/jn.2001.86.3.1389. [DOI] [PubMed] [Google Scholar]
- Tesfai Y, Brereton HM, Barritt GJ. A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein. Biochem J. 2001;358:717–726. doi: 10.1042/0264-6021:3580717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Urbain N, Rentero N, Gervasoni D, Renaud B, Chouvet G. The switch of subthalamic neurons from an irregular to a bursting pattern does not solely depend on their GABAergic inputs in the anesthetic-free rat. J Neurosci. 2002;22:8665–8675. doi: 10.1523/JNEUROSCI.22-19-08665.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velumian AA, Zhang L, Pennefather P, Carlen PL. Reversible inhibition of IK, IAHP, Ih and ICa currents by internally applied gluconate in rat hippocampal pyramidal neurones. Pflugers Arch. 1997;433:343–350. doi: 10.1007/s004240050286. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK) 293 cells. Evidence for a non-capacitative Ca2+ entry. J Biol Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]