Abstract
Non-noxious cooling stimuli were delivered to the shaved back of urethane-chloralose-anaesthetized, artificially ventilated rats using a plastic bag containing water at 24–40°C. Cooling of the skin by 2–6°C increased the rate of whole body oxygen consumption (V̇O2) and triggered electromyographic (EMG) activity recorded from the neck or femoral muscles. The cooling-induced V̇O2 responses did not depend on core (colonic) temperature and followed skin temperature in a graded manner. Pretreatment with the β-blocker propranolol (10 mg kg−1, i.v.) greatly attenuated the V̇O2 response but did not affect the EMG response. On the other hand, pretreatment with the muscle relaxant pancuronium bromide (2 mg kg−1, i.v.) affected the V̇O2 response very slightly but completely abolished the EMG activity. Accordingly, the cooling stimulus activated mainly non-shivering thermogenesis. Next, the contribution of the cerebral cortex to the cooling-induced thermogenesis was examined. Power spectral analysis of the electroencephalogram (EEG) showed that the cooling stimulus largely inhibited delta (0.5–3 Hz) waves, enhanced theta (3–8 Hz) waves, and slightly increased frequencies higher than 8 Hz. Pinching the hindpaw elicited changes in EEG similar to those elicited by skin cooling but did not increase the V̇O2. Therefore, there was no relationship between changes in the EEG and the magnitude of thermogenesis. Finally, skin cooling increased the V̇O2 of decorticated rats but did not increase that of decerebrated rats. The results suggest that the subcortical forebrain structure, but not cortical activation, is indispensable for non-shivering thermogenesis elicited by cooling stimulation of the skin.
Thermoafferent signals originating from cutaneous cold or warm receptors are not only involved in conscious temperature sensations but also play an important role in autonomic and behavioural responses of the organism to its thermal environment. Thermal information from the skin of the trunk is conveyed to the dorsal horn of the spinal cord. The thermoafferent pathway then projects to the lower brainstem structures and diverges further to sensory thalamic nuclei and regulatory hypothalamic areas (Hensel, 1981; Pierau, 1996). The hypothalamus, in particular the preoptic area (POA), contains neurones sensitive to temperatures of both local brain and skin, suggesting convergence and integration of peripheral and central thermal information (Boulant & Hardy, 1974). Because the POA exerts a diversity of thermoregulatory functions, including control of cutaneous blood flow for heat loss, muscle tone for shivering thermogenesis, and thermoregulatory behaviours, it is considered the primary controlling structure in thermoregulation (Boulant, 1980; Hensel, 1981; Simon et al. 1986; Kanosue et al. 1998).
However, changes in firing rate of POA neurones in response to thermal stimulation of the scrotum (Kanosue et al. 1984, 1985) or the trunk skin (Berner & Heller, 1998) were always found to be associated with desynchronizations of the electroencephalogram (EEG). These studies suggest that the apparent responses of POA neurones to thermal stimulation of the skin were not specific but reflected EEG activation caused by the thermal stimulation. Berner & Heller (1998) therefore raised a question as to whether or not peripheral temperature information is integrated in the POA and claimed the possibility that thermal information of the skin is integrated lower in the neuroaxis. Indeed, Blatteis & Banet (1986) showed that microknife separation of the POA from the rest of the brain had no significant effect on thermoregulatory functions in warm or cold environments and concluded that the POA is not essential for the integration of autonomic thermoregulatory responses.
A decrease in skin temperature elicits metabolic heat production, reflected by an increased oxygen consumption rate (V̇O2). Although unequivocal evidence exists on the hypothalamic control of thermogenesis (Boulant, 1980; Kanosue et al. 1998), it is still an open question as to whether thermoafferent signals that reach the POA play an essential role in cold-induced thermogenesis. Heat production can also be elicited by non-thermal stimulation such as systemic administration of capsaicin (Kobayashi et al. 1998) or an intraduodenal infusion of hypertonic solutions (Osaka et al. 2001). Interestingly, these thermogenic responses do not require the forebrain, because decerebrated rats exhibited clear heat production responses to capsaicin (Osaka et al. 2000) and duodenal osmotic stimulation (Osaka et al. 2002). Similarly, Kenney et al. (2000) demonstrated that whole-body heating in chloralose-anaesthetized, midbrain-transected rats increased renal and splenic nerve activity and concluded that forebrain neural structures were not required for mediating these responses.
The aim of the present study was to examine the involvement of the forebrain and activation of the cerebral cortex in cold-induced thermogenesis. For this purpose, anaesthetized rats were used because their baseline metabolic rate is stable and various surgical or pharmacological manipulations are possible. Although anaesthetized animals retain only limited ability to maintain body temperature, in particular, by metabolic heat production, acute body cooling increases activity of the sympathetic nerves innervating the interscapular brown adipose tissue (Talan et al. 1996; Morrison, 1999) and elicits non-shivering thermogenesis (Nour et al. 1984; Talan et al. 1996). Moreover, anaesthetized animals can exhibit shivering in response to lowering body temperature (Asami et al. 1988; Zhang et al. 1995; Tanaka et al. 2001). In this study, I examined the mechanism of thermogenic responses of urethane–chloralose-anaesthetized, artificially ventilated rats to mild cooling of the skin. However, because no systematic study has ever been reported on skin cooling-induced thermogenesis in anaesthetized animals, I evaluated firstly whether this response is an adequate model for the study of cold-induced thermogenesis.
Methods
Male Wistar rats, weighing 350–500 g, were maintained at an ambient temperature of 24 ± 1°C with lighting between 07.00 h and 19.00 h for at least 1 week before the experiments. They had free access to water and laboratory food. The care of animals and all surgical procedures followed National Institute of Health and Nutrition guidelines.
After induction of anaesthesia with 2–3% isoflurane in air and cannulation of a femoral vein and the trachea, the rats were kept anaesthetized intravenously with urethane (600 mg kg−1) and α-chloralose (60 mg kg−1). An electromyogram (EMG) was recorded with a pair of Teflon-coated flexible stainless-steel wires inserted into the femoral muscles, filtered at 150 Hz–3 kHz, and monitored on an oscilloscope. In some experiments, the EMG was also recorded from the dorsal neck muscles. Occasionally, the signals were digitized at 4 kHz and stored on a hard disk. A mixture of urethane (70–80 mg kg−1 h−1) and chloralose (7–8 mg kg−1 h−1) was continuously administered with the aid of a syringe pump (KDS100, KD Scientific, MA, USA), started 90–120 min after the initial anaesthesia. This anaesthetic regimen was determined and slightly adjusted in each rat so that paw pinching evoked EMG activity recorded from the same limb but did not elicit withdrawal responses. Pinching the contralateral paw did not evoke EMG activity from the limb measured for the activity. Animals were killed by an overdose of anaesthetic at the end of experiment.
The back of each rat was shaved between the caudal end of the forelimbs and the rostral end of the hindlimbs, and the rats were positioned prone on an operating table heated to 32–33°C. Three thermocouples were glued to different sites on the shaved skin. The mean of these three readings was used as the measure of skin temperature (Ts). Colonic temperature (Tc) was measured with a fourth thermocouple inserted ∼50 mm into the anus. Tail skin temperature was occasionally measured with another thermocouple taped to the dorsal surface of the tail. The trunk and proximal part of the limbs were covered with a quilt to reduce heat dissipation. Respiration was maintained with an artificial respirator (Harvard pump 683). The intermittent expiratory gas from the respirator was introduced into a 30-ml reservoir, which was continuously ventilated with ambient air at a constant rate of 1 l min−1. The difference in O2 concentrations between reservoir and ambient air was measured with an O2 analyser (LC-700E, Toray, Japan). Values were corrected for metabolic body size (kg0.75). These signals were fed into a computer and recorded at 6-s intervals through a PowerLab system (ADInstruments, Australia) for on-line data display, storage, and off-line analysis. After the experiments, the data were averaged over 30-s intervals.
A pair of silver electrodes was placed on the somatosensory cortex for the bipolar recording of the EEG. A stainless-steel screw was placed in the skull over the cerebellum and used as an earthed reference. Electrodes were secured in place by covering with a dental acrylic resin. The signal was filtered (0.5–30 Hz), digitized at 100 Hz, and stored on a hard disk. The power spectrum was computed from a 1-min EEG record by using the PowerLab's spectrum software, which divided the EEG record into 50% overlapping successive 46–48 segments, each of 256 data points, applied the fast Fourier transformation (FFT) with a cosine-bell window, and calculated the average spectrum from these FFTs.
Thermal stimulation of the skin was delivered by placing a plastic bag containing 24–40°C water on to the shaved back for 2 min instead of the quilt that covered the rat. The bag was agitated every 30 s for 4–5 s to mix the water uniformly. Preliminary experiments showed that skin cooling for longer than 2 min or use of colder water resulted in a significant decrease in Tc; and therefore I did not analyse the effects of such stimulation. The quilt was replaced in its original position soon after the 2-min thermal stimulation. This procedure kept Ts within the non-noxious range of 28–40°C (Defrin et al. 2002). The water bag was made of a polyethylene sheet 70-μm thick and had a bottom surface area of 110 mm × 180 mm, and contained 340–360 ml of water. The bag covered not only the shaved back but also parts of the unshaved skin and the operating table on which the rat had been placed. The scrotum, tail, head and distal part of the limbs did not receive thermal stimulation. In most cases, the bag contained water at 28°C and lowered Ts by 4–5°C. The temperature of the operating table was increased by 1–2°C during repeated cooling episodes to compensate for the cooling-induced decrease in Tc. Preliminary experiments (n= 5) showed that the coefficient of variation of the decrease in Ts ranged between 1.2 and 3.8% when a rat received, at 30-min intervals, four consecutive applications of the bag containing water at the same temperature, thus indicating controlled and reproducible thermal stimulation in repeated trials.
The β-adrenoceptor blocker dl-propranolol hydrochloride was dissolved in physiological saline solution and i.v. administered at a dose of 10 mg kg−1. The neuromuscular blocking agent pancuronium bromide (Mioblock, Sankyo, Japan) was infused i.v. at a dose of 2 mg kg−1. A mixture of pancuronium (1 mg kg−1 h−1), urethane (70–80 mg kg−1 h−1), and chloralose (7–8 mg kg−1 h−1) was continuously infused to maintain the muscle relaxation and anaesthetic level. Skin cooling was applied before and 15–20 min after administration of the drugs. The effect of the drugs on the magnitude of cooling-induced thermogenesis was analysed using the paired t test.
Decerebration was achieved using a method previously described (Osaka et al. 1989). Briefly, the head was fixed in a stereotaxic apparatus, and a slit was made in the frontal plane of the skull at a position 4 mm rostral to the interaural line. An l-shaped small knife was bilaterally inserted into the brain through the slit with the aid of a micromanipulator until it made contact with the cranium floor. Care was taken not to damage the sagittal sinus. The level and completeness of the transection were later confirmed visually by examination of the cut on the ventral surface of the brain.
Decortication was performed using method similar to that described by Huston (1983). After an extensive craniotomy, the neocortex, dorsal hippocampus, and most of the striatum were bilaterally removed by aspiration. The thalamus, caudal part of striatum, preoptic area and its adjacent septal nuclei, ventral hippocampus and amygdala remained largely intact. The surface of the exposed brain was covered with a saline-soaked gelatine powder (Gelfoam powder, Pharmacia & Upjohn, Sweden) to prevent gradual bleeding. The incision in the skin was closed with a cyanoacrylate adhesive. The effects of skin cooling were not examined until 1 h after surgery.
Results
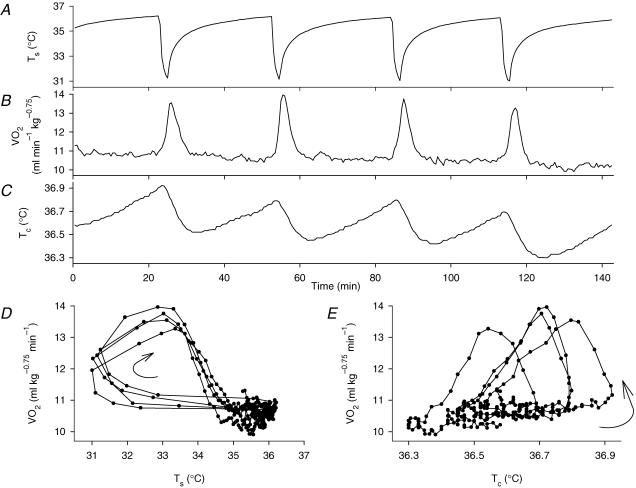
Skin cooling with the bag containing water at 26–28°C for 2 min lowered the Ts by 4–5°C from the resting warm temperature (35–38°C), and thereafter Ts recovered to its original level within 20–30 min (Fig. 1A). Baseline V̇O2 was 11.9 ± 0.2 ml kg−0.75 min−1 (mean ±s.e.m., n= 31). V̇O2 increased within 1 min after the onset of skin cooling and reached a peak increase by 2–3 ml kg−0.75 min−1 1–2 min after the removal of the water bag (Fig. 1B). The sequential plot of V̇O2 against Ts (Fig. 1D) showed a 60–90 s delay of the response to the drop in Ts, and this plot also demonstrated that the relationship between V̇O2 and Ts was reproducible in repeated skin cooling episodes. The magnitude of the thermogenic responses to repeated stimuli of the same temperature varied slightly: the coefficient of variation of the increase in V̇O2 was 12.6 ± 2.4% (mean ±s.e.m., n= 5) when a rat received four consecutive applications of cooling stimulation at the same temperature at 30-min intervals. This condition lasted approximately 3 h, and the amplitude of responses decreased gradually thereafter. The duration of each thermogenic response varied by 5–30 min among preparations, but was approximately the same in the same rat. A single cooling episode gradually decreased Tc by 0.2–0.4°C 6–10 min after removal of the water bag (Fig. 1C). V̇O2 increased before the fall in Tc, reached its peak value during the falling phase of Tc, and declined to the baseline level when Tc reached its minimal level. Although the increase in was always associated with the initial drop in Tc, the sequential plot of against Tc (Fig. 1E) shows that there was no constant relationship between V̇O2 and Tc. On the other hand, the temperature of the tail skin was 28–32°C and decreased by 0.1–0.5°C in parallel with Tc after skin cooling, suggesting a steady vasoconstrictive state and a lack of further vasoconstriction in response to cooling stimulation.
Figure 1. Thermogenic responses to skin but not core (colonic) temperature.
A representative record showing Ts (A), V̇O2 (B) and Tc (C) in response to repeated skin cooling episodes with a plastic bag containing water at 28°C. D and E, sequential plots of V̇O2 versusTs and Tc, respectively. Sequential points are joined by lines, and the direction of changes is shown by curved arrows. Although the V̇O2 response to skin cooling lagged behind Ts, resulting in hysteresis (D), the relationship between V̇O2 and Ts was constant in repeated cooling episodes. However, the relationship between V̇O2 and Tc varied during the experiment (E). These plots thus suggest that an input from the skin, but not from the core, determined the thermogenic response.
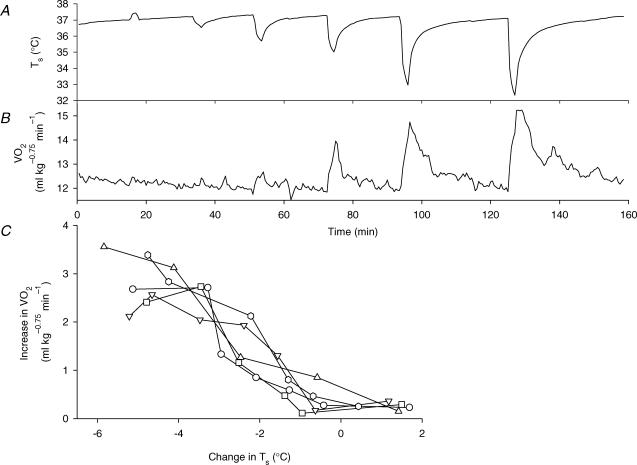
Figure 2A and B shows the thermogenic responses of a rat to various intensities of thermal stimulation of the skin, and Fig. 2C summarizes the responses of five rats. Skin cooling by more than 2°C elicited thermogenesis, the magnitude of which increased with the intensity of cooling. However, skin warming by 1–2°C had no effect on V̇O2, and skin cooling by 1°C also did not elicit a significant increase in V̇O2 above the level of baseline fluctuations.
Figure 2. Cooling intensity-dependent thermogenic responses.
Changes in Ts (A) and V̇O2 (B) of a rat in response to thermal stimulation of the skin delivered by a plastic bag containing water at 38, 36, 34, 32, 28, or 26°C. C, peak increase in V̇O2 plotted against change in Ts of five rats, showing that skin cooling of more than 1°C increased V̇O2 in an intensity-dependent manner. Each set of symbols and connecting lines shows data from the same rat.
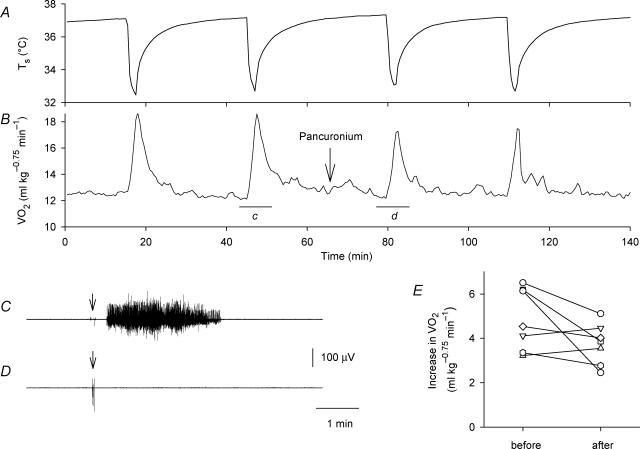
The rats usually had no significant background EMG activity, indicating a lack of spontaneous activity of the skeletal muscles. Skin cooling always elicited EMG activity recorded from the femoral or neck muscles (Fig. 3C; Fig. 4C and D). The EMG consisted of a series of continuous action potentials, and did not exhibit the rhythmic burst pattern that is typically observed during shivering. The rats also exhibited momentary shivering-like reactions around the neck 1–5 times during the 2-min cooling period, though they did not usually move the lower portions of their bodies. However, the skin cooling-induced EMG activity occurred continuously, and the activity lasted for 1–10 min even when the rats did not move their bodies, as judged by palpation and visual observation. On the other hand, skin warming did not elicit any body movement or EMG activity. These observations could suggest that shivering made a contribution to skin cooling-induced thermogenesis, though there was no definite relationship between the increase in and the occurrence of body movements. Therefore, the effects of the muscle relaxant pancuronium bromide were examined. Administration of pancuronium did not affect baseline V̇O2 but effectively blocked cooling-induced body movements and EMG activity (Figs 3C and D). However, cooling-induced thermogenesis largely persisted after administration of pancuronium (Fig. 3A and B). Figure 3E summaries the increase in V̇O2 of individual rats before and after administration of pancuronium. Although the average magnitude of cooling-induced thermogenesis decreased from 5.07 ± 0.50 ml kg−0.75 min−1 to 3.96 ± 0.37 ml kg−0.75 min−1 (mean ±s.e.m., n = 8) after administration of pancuronium, the difference was not statistically significant.
Figure 3. Effects of the muscle relaxant pancuronium bromide on skin cooling-induced thermogenesis and EMG responses.
Changes in Ts (A) and V̇O2 (B) of a rat in response to skin cooling before and after administration of pancuronium (2 mg kg−1, i.v., arrow). The horizontal bars ‘c’ and ‘d’ in B show the periods of the EMG records shown in C and D, respectively. The arrows in C and D show the onset of cooling stimulation. Administration of pancuronium completely blocked the cooling-induced EMG activity. E, cooling-induced increase in V̇O2 before and after administration of pancuronium in eight rats, showing the lack of a statistically significant change in the thermogenic response. Each set of symbols and connecting lines indicates an individual rat.
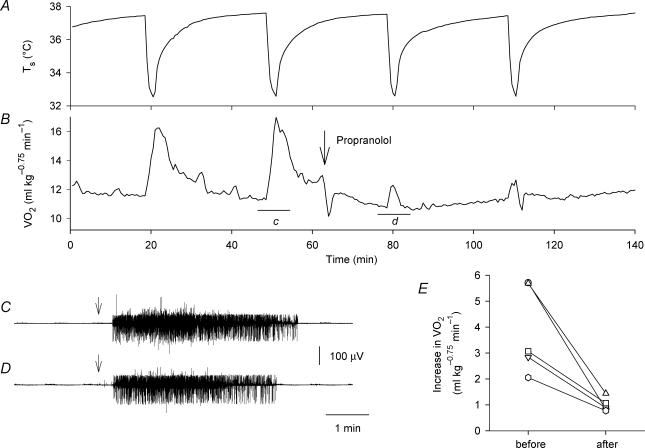
Figure 4. Contribution of β-adrenoceptors to skin cooling-induced thermogenesis.
Effects of propranolol (10 mg kg−1, i.v., arrow in B) on skin cooling-induced changes in Ts (A) and V̇O2 (B). The horizontal bars ‘c’ and ‘d’ in B show the periods of the EMG records shown in C and D, respectively. The arrows in C and D show the onset of cooling stimulation. Administration of propranolol had no effect on the cooling-induced EMG activity. E, cooling-induced increase in V̇O2 before and after administration of propranolol in five rats, showing significant attenuation of thermogenesis. Each set of symbols and connecting lines indicates an individual rat.
Administration of propranolol alone (10 mg kg−1) decreased V̇O2 by 2–3 ml kg−0.75 min−1 for 3–5 min (n = 5), and thereafter the baseline level of V̇O2 was 0.5–1.5 ml kg−0.75 min−1 lower than the control level for 1–2 h. Figure 4A and B show that pretreatment with propranolol largely, but not completely, attenuated cooling-induced thermogenesis. All five rats examined showed a great decrease in their thermogenic response to skin cooling after propranolol administration (Fig. 4E). On the other hand, cooling-induced EMG activity was not affected by administration of propranolol (Fig. 4C and D).
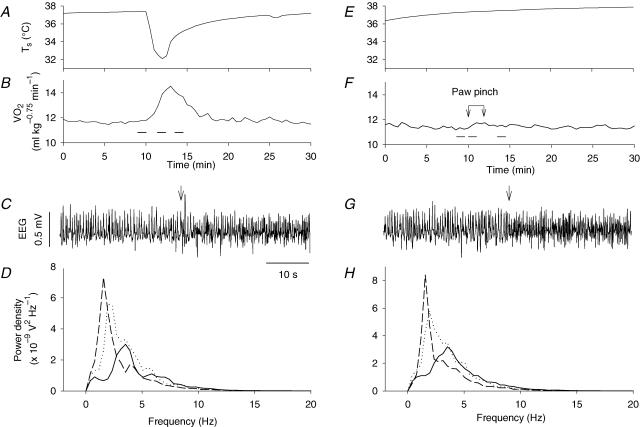
The spontaneous EEG consisted of mainly synchronized slow waves: power spectral analysis revealed that the proportions of delta (0.5–3 Hz), theta (3–8 Hz) and higher frequency (> 8 Hz) waves were 45–63%, 33–44% and 3–10% of the total power of the EEG, respectively (n = 6). Skin cooling abruptly changed the EEG to a theta-dominant state (Fig. 5A–D): the theta waves occupied 40–64% of the total power of the EEG during skin cooling, although the power of higher frequency waves also increased, to 7–14%. The EEG recovered to the delta wave-dominant state within 2 min after cooling stimulation, when V̇O2 was still higher than the baseline level. Noxious pinching of the hindpaw also changed the EEG to the theta wave-dominant state (Fig. 5E–H). The spectral composition of the EEG during paw pinching (Fig. 5H) was very similar to that during skin cooling (Fig. 5D). However, in contrast to skin cooling-induced thermogenesis, paw pinching increased V̇O2 only very slightly (Fig. 5F). Although in two to three tests in each of six rats examined paw pinching could increase V̇O2 to 1 ml kg−0.75 min−1, the magnitude of thermogenesis induced by pinching was always smaller than that elicited by skin cooling in the same rat.
Figure 5. Changes in EEG elicited by skin cooling and paw pinch.
Effects of skin cooling and paw pinching on Ts (A and E), V̇O2 (B and F), EEG (C and G), and the power spectrum of the EEG (D and H) recorded from a rat. The horizontal bars in B and F show the periods used to calculate power spectra of the EEG records shown in D and H, respectively. The arrows connected by a bar in F show the period of paw pinching. Dashed, continuous, and dotted lines in D and H show the respective power spectra obtained before, during, and after skin cooling (D) or paw pinching (H). The arrows in C and G show the onset of stimulation. Although the EEG was similarly affected by skin cooling and paw pinching, the thermogenic response was specific to skin cooling.
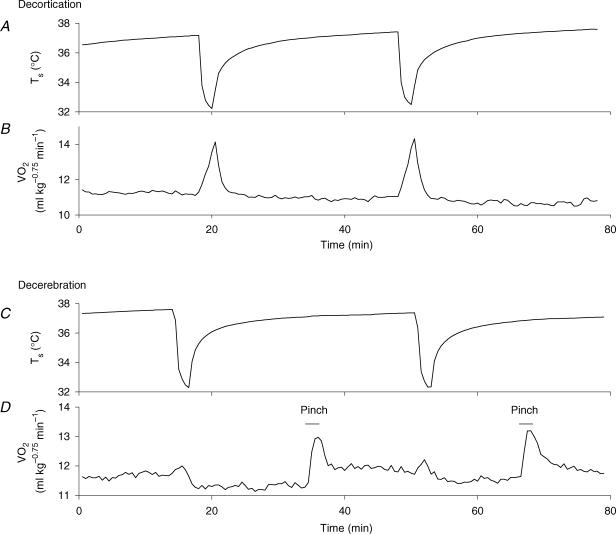
Two groups of four rats each were either decorticated or decerebrated. Their baseline V̇O2 value (11–12 ml kg−0.75 min−1) and occasional shivering-like reactions during the cooling episode were similar to those observed in intact rats. Whereas skin cooling elicited normal thermogenic responses in the decorticated rats (Fig. 6A and B), it decreased the V̇O2 of decerebrated rats by 0.3–1 ml kg−0.75 min−1 for 5–20 min. To confirm the lack of a thermogenic response in the decerebrated rats, I examined the effect of skin cooling ∼2 h after stopping the infusion of anaesthetics in two decerebrated rats. They exhibited a slight (at most 0.5 ml kg−0.75 min−1) increase in V̇O2 during the cooling episode, followed by a period of inhibition (Fig. 6C and D). However, pinching the hindpaw of these rats reproducibly increased V̇O2 by 1.2–1.7 ml kg−0.75 min−1 (Fig. 6D).
Figure 6. Involvement of the basal forebrain in skin cooling-induced thermogenesis.
Ts (A and C) and V̇O2 (B and D) of decorticated (A and B) or decerebrated (C and D) rats. Thermogenic responses of a decorticated rat to skin cooling were similar to those of intact rats. However, a decerebrated rat examined ∼2 h after stopping the infusion of urethane–chloralose solution exhibited a very small increase in V̇O2 followed by a period of inhibition, although paw pinching elicited significant thermogenic responses. These responses were reproducible in repeated tests. The bars in D show the period of paw pinching.
Discussion
A model of non-shivering thermogenesis: the skin cooling-induced V̇O2 response in anaesthetized rats
Mild non-noxious cooling of the skin elicited thermogenesis in anaesthetized rats. Deep anaesthesia generally blocks non-shivering thermogenesis (Albanese et al. 1994), whereas animals can exhibit thermogenic responses to cold under light anaesthesia (Nour et al. 1984). The anaesthetic regimen employed in the present study was based on the presence of EMG activity and the absence of withdrawal movements of the limb to paw pinching. There were, however, some variations in the magnitude of the V̇O2 response during repeated cooling episodes in a rat. The cause of this variation partly reflected the slight differences in baseline levels of Tc and V̇O2 in each cooling episode and might have been caused by minute changes in the depth of anaesthesia during the experiments. However, an average variation of 12.6% in the V̇O2 responses was considered sufficiently reproducible to allow an examination of the effects of the various treatments employed in the present study.
A plastic bag containing 340–360 g of water was used to deliver thermal stimulation. This procedure necessarily also involved mechanical stimulation due to the weight of the water. However, it is unlikely that thermogenic responses were elicited by mechanical stimulation, because skin warming by 1–2°C had no effect on V̇O2 and because skin cooling increased V̇O2 in a temperature-dependent manner. A water-perfused jacket has been used to study the effects of thermal stimulation of the trunk skin on single unit activity of medullary raphe neurones (Dickenson, 1977; Rathner et al. 2001) and on sympathetic fibres supplying the tail (Owens et al. 2002) in anaesthetized rats. However, the water bag method employed in the present study was simple and changes in Ts were of a similar magnitude and time course to those observed with the water jacket method, the main difference being that in the present study the stimulated area was limited to the dorsal skin of the trunk.
A decrease in either peripheral temperature or core temperature will produce an increase in metabolic heat production. Although skin cooling-induced thermogenesis was accompanied by a slight (0.2–0.4°C) decrease in Tc, a larger (1–7°C) decrease in Tc is generally employed to elicit body cooling-induced thermogenesis (Nour et al. 1984; Asami et al. 1988; Zhang et al. 1995; Talan et al. 1996; Tanaka et al. 2001). Moreover, in the present study, the increase in V̇O2 was well correlated with Ts but not with Tc. These results suggest that afferent signals originating from the skin, but not from the core, largely contributed to the thermogenesis observed. However, because the present experiments were not designed to measure the effect of core temperature in isolation, we cannot rule out a possible contribution from thermoreceptors in the spinal cord (Simon, 1974) or in the wall of the abdominal cavity (Riedel et al. 1973) to cold-induced thermogenesis.
There are two general types of heat production responses: shivering and non-shivering thermogenesis. The relative contributions of shivering versus non-shivering thermogenesis can be determined experimentally by giving muscle relaxants to block shivering or antagonists of the β-adrenoceptor to block non-shivering thermogenesis. Skin cooling occasionally evoked shivering-like body movements and always elicited EMG activity recorded from neck or femoral muscles. Since these observations suggested an involvement of shivering thermogenesis, the effects of the muscle relaxant pancuronium were examined. However, pretreatment with pancuronium did not significantly attenuate the skin cooling-induced thermogenesis, while it effectively blocked the body movements and EMG activity. Moreover, decerebrated rats did not exhibit thermogenesis but showed the muscular activity in response to skin cooling. Therefore, shivering contributed only a little amount, if at all, to the present thermogenic response. On the other hand, pretreatment with the β-blocker propranolol greatly attenuated the skin cooling-induced thermogenic response but did not affect the EMG response, indicating that non-shivering thermogenesis was mainly activated in the present experimental conditions. However, I cannot strictly exclude some involvement of shivering thermogenesis because skin cooling did elicit muscular activity and pretreatment with propranolol did not completely abolish the thermogenic response. Also, the threshold temperature of shivering thermogenesis is generally lower than that of non-shivering thermogenesis (Gautier, 2000). It is possible that in the present experimental conditions skin cooling activated both non-shivering and shivering thermogenesis, but the relative contribution of shivering to heat production was negligible.
The V̇O2 response to skin cooling always lagged behind Ts, resulting in hysteresis. The time lag probably reflected various processes of thermogenesis. These include, for example, (1) neural processing, such as the temporal summation of impulses in the afferent/efferent pathways, (2) the biochemical processes in the cells where thermogenesis occurs, and (3) the lag between the O2 consumption in the cells and the decrease in pulmonary O2 concentration. In addition, the delayed participation of the core thermoreceptors could also contribute to the time lag.
Involvement of the basal forebrain and lack of the contribution of the cerebral cortex in skin cooling-induced thermogenesis
Both skin cooling and paw pinching similarly attenuated the delta waves and enhanced the theta waves in the EEG, suggesting that the cerebral cortex was affected non-specifically by these stimuli. However, this non-specific influence could not account for the skin cooling-induced thermogenesis, because skin cooling elicited significantly larger thermogenic responses than did paw pinching. Although thermal stimulation of the scrotum or the trunk skin desynchronized the EEG in rats anaesthetized with 1–1.5 g kg−1 urethane (Kanosue et al. 1984, 1985; Berner & Heller, 1998), the changes in the EEG that occurred in the present study were largely limited to the frequency range below 8 Hz, indicating the absence of arousal patterns. Similarly, Rathner et al. (2001) showed that skin cooling excited medullary raphé neurones without accompanying changes in the EEG of rats anaesthetized with isoflurane. Thus, cortical activation is not indispensable for the full expression of skin cooling-induced thermogenesis.
Decorticated rats still exhibited thermogenic responses to skin cooling, which result also demonstrated that any effect of cortical arousal could not account for the skin cooling-induced thermogenesis. However, decerebrated rats did not increase V̇O2 in response to thermal stimulation. Because the cerebral transection was made between the hypothalamus and midbrain, the subcortical forebrain structure is responsible for this response. It is likely that the POA in the hypothalamus is critically involved in this response, because bilateral microinjections of bicuculline, a GABAA receptor antagonist, into the POA blocked skin cooling-induced thermogenesis (author's unpublished observation). On the other hand, paw pinching elicited small but significant thermogenic responses in decerebrated rats when supplemental anaesthetics were not administered. Thus, the lower brainstem or spinal cord could mediate thermogenesis elicited by nociceptive stimulation.
The present study demonstrated that cutaneous thermoafferents reaching the basal forebrain, possibly the POA of the hypothalamus, are indispensable to non-shivering thermogenesis. Thermoregulation is achieved by several hierarchically arranged subsystems, including the spinal cord, lower brainstem, and hypothalamus (Satinoff, 1978). The lack of thermoregulatory deficits in chronic rats subjected to microknife separation of the POA from the rest of the brain (Blatteis & Banet, 1986) could be attributable to the compensatory function of the intact subsystems. Similarly, many classic studies have shown the recovery of thermoregulatory abilities in animals or patients after partial or complete transections of the spinal cord, although these responses were much reduced in comparison with those in intact animals or patients and were not sufficient to maintain body temperature. To understand the organization and compensatory function of the thermoregulatory system, it is critical to define neural pathways and transmitters among thermoregulatory subsystems. In particular, our knowledge on thermoafferent pathways is still quite fragmentary. The preparation employed in the present study will be useful for elucidating the organization of thermoafferents and the mechanisms underlying non-shivering thermogenesis.
Acknowledgments
This study was supported by grants from the Ministry of Education, Science and Culture of Japan for Scientific Research (No. 15590218) and from the Japan Health Sciences Foundation (KH51059).
References
- Albanese CT, Nour BM, Rowe MI. Anesthesia blocks nonshivering thermogenesis in the neonatal rabbit. J Pediatr Surg. 1994;29:983–986. doi: 10.1016/0022-3468(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Asami T, Hori T, Kiyohara T, Nakashima T. Convergence of thermal signals on the reticulospinal neurons in the midbrain, pons and medulla oblongata. Brain Res Bull. 1988;20:581–596. doi: 10.1016/0361-9230(88)90217-1. [DOI] [PubMed] [Google Scholar]
- Berner NJ, Heller HC. Does the preoptic anterior hypothalamus receive thermoafferent information. Am J Physiol. 1998;274:R9–R18. doi: 10.1152/ajpregu.1998.274.1.R9. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Banet M. Autonomic thermoregulation after separation of the preoptic area from the hypothalamus in rats. Pflugers Arch. 1986;406:480–484. doi: 10.1007/BF00583370. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic control of thermoregulation. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus. Vol. 3. New York: Marcel Dekker, Inc.; 1980. pp. 1–82. (part A, Behavioral Studies of the Hypothalamus). [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain. 2002;125:501–510. doi: 10.1093/brain/awf055. [DOI] [PubMed] [Google Scholar]
- Dickenson AH. Specific responses of rat raphé neurones to skin temperature. J Physiol. 1977;273:277–293. doi: 10.1113/jphysiol.1977.sp012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H. Body temperature regulation in the rat. J Therm Biol. 2000;25:273–279. doi: 10.1016/s0306-4565(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Hensel H. Thermoreception and Temperature Regulation. London: Academic Press; 1981. [PubMed] [Google Scholar]
- Huston JP. Ablation and stimulation of the brain. In: Bure J, Bureová O, Huston JP, editors. Techniques and Basic Experiments for the Study of Brain and Behavior. 2. Amsterdam: Elsevier; 1983. pp. 217–263. [Google Scholar]
- Kanosue K, Hosono T, Zhang Y-H, Chen XM. Neuronal networks controlling thermoregulatory effectors. In: Sharma HS, Westman J, editors. Brain Function in Hot Environment. Vol. 115. Amsterdam: Elsevier; 1998. pp. 49–62. Progress in Brain Research. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Nakayama A, Ishikawa Y, Hosono T. Threshold temperatures of diencephalic neurons responding to scrotal warming. Pflugers Arch. 1984;400:418–423. doi: 10.1007/BF00587543. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Nakayama A, Ishikawa Y, Hosono T, Kaminaga T, Shosaku A. Responses of thalamic and hypothalamic neurons to scrotal warming in rats: non-specific responses. Brain Res. 1985;328:207–213. doi: 10.1016/0006-8993(85)91031-5. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Pickar JG, Weiss ML, Saindon CS, Fels RJ. Effects of midbrain and spinal cord transections on sympathetic nerve responses to heating. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1329–R1338. doi: 10.1152/ajpregu.2000.278.5.R1329. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T, Namba Y, Inoue S, Lee TH, Kimura S. Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am J Physiol. 1998;275:R92–R98. doi: 10.1152/ajpregu.1998.275.1.R92. [DOI] [PubMed] [Google Scholar]
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol. 1999;276:R962–R973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- Nour BM, Boudreaux JP, Rowe MI. An experimental model to study thermogenesis in the neonatal surgical patient. J Pediatr Surg. 1984;19:764–770. doi: 10.1016/s0022-3468(84)80365-6. [DOI] [PubMed] [Google Scholar]
- Osaka T, Kobayashi A, Inoue S. Thermogenesis induced by osmotic stimulation of the intestines in the rat. J Physiol. 2001;532:261–269. doi: 10.1111/j.1469-7793.2001.0261g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T, Kobayashi A, Inoue S. Vago-sympathoadrenal reflex in thermogenesis induced by osmotic stimulation of the intestines in the rat. J Physiol. 2002;540:665–671. doi: 10.1113/jphysiol.2001.013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T, Kobayashi A, Lee TH, Namba Y, Inoue S, Kimura S. Lack of integrative control of heat production and heat loss after capsaicin administration. Pflugers Arch. 2000;440:440–445. doi: 10.1007/s004240000313. [DOI] [PubMed] [Google Scholar]
- Osaka T, Yoshimatsu H, Kannan H, Yamashita H. Activity of hypothalamic neurons in conscious rats decreased by hyperbaric environment. Brain Res Bull. 1989;22:549–555. doi: 10.1016/0361-9230(89)90110-x. [DOI] [PubMed] [Google Scholar]
- Owens NC, Ootsuka Y, Kanosue K, McAllen RM. Thermoregulatory control of sympathetic fibres supplying the rat's tail. J Physiol. 2002;543:849–858. doi: 10.1113/jphysiol.2002.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierau FK. Peripheral thermosensors. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology Environmental Physiology. Vol. 1. New York: Oxford University Press; 1996. pp. 85–104. section 4. [Google Scholar]
- Rathner JA, Owens NC, McAllen RM. Cold-activated raphé-spinal neurons in rats. J Physiol. 2001;535:841–854. doi: 10.1111/j.1469-7793.2001.t01-1-00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel W, Siaplauras G, Simon E. Intra-abdominal thermosensitivity in the rabbit as compared with spinal thermosensitivity. Pflugers Arch. 1973;3540:59–70. doi: 10.1007/BF00592197. [DOI] [PubMed] [Google Scholar]
- Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201:16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- Simon E. Temperature regulation: the spinal cord as a site of extrahypothalmic themoregulatory funcitons. Rev Physiol Biochem Pharmacol. 1974;71:1–76. doi: 10.1007/BFb0027660. [DOI] [PubMed] [Google Scholar]
- Simon E, Pierau F-K, Taylor DCM. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986;66:235–300. doi: 10.1152/physrev.1986.66.2.235. [DOI] [PubMed] [Google Scholar]
- Talan MI, Kirov SA, Kosheleva NA. Nonshivering thermogenesis in adult and aged C57BL/6J mice housed at 22°C and at 29°C. Exp Gerontol. 1996;31:687–698. doi: 10.1016/s0531-5565(96)00095-2. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tonouchi M, Hosono T, Nagashima K, Yanase-Fujiwara M, Kanosue K. Hypothalamic region facilitating shivering in rats. Jpn J Physiol. 2001;51:625–629. doi: 10.2170/jjphysiol.51.625. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats. J Physiol. 1995;485:195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]