Abstract
The effects of changing cytosolic [Mg2+] ([Mg2+]i) on l-type Ca2+ currents were investigated in rat cardiac ventricular myocytes voltage-clamped with patch pipettes containing salt solutions with defined [Mg2+] and [Ca2+]. To control [Mg2+]i and cytosolic [Ca2+] ([Ca2+]i), the pipette solution included 30 mm citrate and 10 mm ATP along with 5 mm EGTA (slow Ca2+ buffer) or 15 mm EGTA plus 5 mm BAPTA (fast Ca2+ buffer). With pipette [Ca2+] ([Ca2+]p) set at 100 nm using a slow Ca2+ buffer and pipette [Mg2+] ([Mg2+]p) set at 0.2 mm, peak l-type Ca2+ current density (ICa) was 17.0 ± 2.2 pA pF−1. Under the same conditions, but with [Mg2+]p set to 1.8 mm, ICa was 5.6 ± 1.0 pA pF−1, a 64 ± 2.8% decrease in amplitude. This decrease in ICa was accompanied by an acceleration and a –8 mV shift in the voltage dependence of current inactivation. The [Mg2+]p-dependent decrease in ICa was not significantly different when myocytes were preincubated with 10 μm forskolin and 300 μm 3-isobutyl-1-methylxanthine and voltage-clamped with pipettes containing 50 μm okadaic acid, to maximize Ca2+ channel phosphorylation. However, when myocytes were voltage-clamped with pipettes containing protein phosphatase 2A, to promote channel dephosphorylation, ICa decreased only 25 ± 3.4% on changing [Mg2+]p from 0.2 to 1.8 mm. In the presence of 0.2 mm[Mg2+]p, changing channel phosphorylation conditions altered ICa over a 4-fold range; however, with 1.8 mm[Mg2+]p, these same manoeuvres had a much smaller effect on ICa. These data suggest that [Mg2+]i can antagonize the effects of phosphorylation on channel gating kinetics. Setting [Ca2+]p to 1, 100 or 300 nm also showed that the [Mg2+]p-induced reduction of ICa was smaller at the lowest [Ca2+]p, irrespective of channel phosphorylation conditions. This interaction between [Ca2+]i and [Mg2+]i to modulate ICa was not significantly affected by ryanodine, fast Ca2+ buffers or inhibitors of calmodulin, calmodulin-dependent kinase and calcineurin. Thus, physiologically relevant [Mg2+]i modulates ICa by counteracting the effects of Ca2+ channel phosphorylation and by an unknown [Ca2+]i-dependent mechanism. The magnitude of these effects suggests that changes in [Mg2+]i could be critical in regulating l-type channel gating.
Cytosolic [Mg2+] ([Mg2+]i) in cardiac myocytes appears to be 0.6–1.3 mm (Buri & McGuigan, 1990; Hongo et al. 1994; Watanabe & Konishi, 2001) and is largely buffered in the cytosol by a variety of diffusible molecules (e.g. ATP) and proteins (Robertson et al. 1981; Fabiato, 1983; Konishi & Berlin, 1993). Changes in [Mg2+]i can have marked effects on fluxes through ion channels in cardiac myocytes (Agus et al. 1989; White & Hartzell, 1989; Agus & Agus, 2001). The first study of [Mg2+]i effects on l-type Ca2+ current (ICa) in frog myocytes showed that, under appropriate conditions (see below), increasing [Mg2+]i between 0.3 and 3.0 mm decreased ICa more than 50% (White & Hartzell, 1988). More recent studies in frog and guinea-pig myocytes have confirmed the marked inhibitory actions of increased [Mg2+]i on ICa (Yamaoka & Seyama, 1996a,b, 1998; Pelzer et al. 2001; Yamaoka et al. 2002); however, none have shown the large changes of current around physiologically relevant [Mg2+]i reported by White & Hartzell (1988). These recent results therefore raise the issue of whether [Mg2+]i is a physiologically important regulator of Ca2+ channel function.
Two general mechanisms could explain how Mg2+ regulates Ca2+ fluxes through l-type channels: alteration of ion permeation and modulation of channel gating properties. Cytosolic Mg2+ concentrations up to 10 mm do not decrease divalent cation conductance through single l-type Ca2+ channels (Kuo & Hess, 1993; Yamaoka & Seyama, 1998), so that it is unlikely that the reported effects of cytosolic Mg2+ on macroscopic ICa (White & Hartzell, 1988; Agus et al. 1989; Yamaoka & Seyama, 1996a,b, 1998; Pelzer et al. 2001) result from block of Ca2+ permeation through the channel pore. For this reason, we have focused on mechanisms by which [Mg2+]i could alter l-type channel gating properties.
l-type Ca2+ channel gating is regulated by at least three factors: membrane potential (Vm), cytosolic Ca2+ concentration ([Ca2+]i), and channel phosphorylation (McDonald et al. 1994). In this regard, a 10-fold increase in [Mg2+]i has been shown to produce a small negative shift in the Vm dependence for inactivation of Cd2+-sensitive Ba2+ current (Hartzell & White, 1989). Channel phosphorylation state also appears to be important in [Mg2+]i-dependent regulation of ICa. Earlier studies showed that increased [Mg2+]i inhibited ICa most prominently under conditions of high channel phosphorylation (White & Hartzell, 1988; Agus et al. 1989). Under basal, presumably low phosphorylation conditions, inhibitory actions of [Mg2+]i were less marked or not observed. Recent studies (Yamaoka & Seyama, 1998; Pelzer et al. 2001) suggest that this less pronounced reduction of ICa under basal conditions might reflect a shift in inhibitory [Mg2+] from a micromolar to millimolar range when the Ca2+ channel is phosphorylated and/or an inability of Mg2+ to modulate unphosphorylated channels. Finally, only one study examined how [Mg2+]i influences Ca2+-dependent regulation of Ca2+ channels (Yamaoka & Seyama, 1996a), and depending on [Mg2+]i, changes in [Ca2+]i increased or decreased ICa. The apparent complexity of these Mg2+ actions on ICa suggests that the mechanisms by which [Mg2+]i modulates l-type channel gating warrant further study.
The purpose of this study was therefore to determine whether physiologically relevant concentrations of cytosolic Mg2+ affect mechanisms, e.g. Vm, Ca2+, and channel phosphorylation, that regulate l-type channel gating. A whole-cell patch-clamp technique was used to measure l-type ICa density while dialysing cells with a pipette solution containing 40 mm Mg2+ buffers (30 mm citric acid and 10 mm ATP) to rapidly control [Mg2+]i levels. We found that increasing pipette [Mg2+] from 0.2 mm to 1.8 mm suppressed ICa density by ∼70%. This inhibitory effect was enhanced by [Ca2+]i but inhibited by channel dephosphorylation. However, neither Vm-dependent nor Ca2+/calmodulin-dependent mechanisms appeared to account for the marked reduction of ICa density by [Mg2+]i.
Portions of this work have appeared previously as a preliminary communication (Wang & Berlin, 2002).
Methods
Cell isolation
Adult rat ventricular myocytes were isolated enzymatically as previously described (Mitra & Morad, 1985) from male Sprague–Dawley rats (200–225 g). Animals received an intraperitoneal injection of sodium pentobarbitone (50–100 mg kg−1), and after full anaesthesia was achieved, a thoracotomy was performed to rapidly remove the heart, in accordance with the procedures approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey. Following isolation, myocytes were stored in a refrigerator and used within 1–8 h.
Measurement of membrane currents
Myocytes were placed in a chamber mounted on an inverted microscope (Nikon Inc., Japan) and superfused with a modified Tyrode solution (see Solutions below). Ca2+ current was measured in the whole-cell configuration with patch pipettes having resistances of 1.0–1.5 MΩ when filled with pipette solutions (see below). Outward K+ current was blocked by Cs+ and tetraethylammonium ions (TEA) in the pipette solution, while Na+ current was suppressed by addition of 30 μm tetrodotoxin (TTX) to the Tyrode solution and by depolarizing Vm to –40 mV with ramp pulses prior to test protocols (Nilius et al. 1985). All experiments were performed at room temperature. Experiments were conducted when whole-cell voltage clamps had time constants ranging from 100 to 300 μs without series resistance or capacitance compensation. Cell capacitance was estimated by integrating current elicited by 5 mV depolarizations from the holding potential of –70 mV
Experimental protocols
Cells were depolarized every 30 s from a holding potential of –70 mV to –40 mV with a 1 s ramp and then depolarized to a test potential of 0 mV for 200 ms. In some experiments, the I–V relationships were also obtained periodically by varying the test potential between –30 and +60 mV (in 10 mV increments) at 0.2 Hz. Displayed current records were obtained after 5 min in the whole-cell configuration to allow adequate intracellular dialysis (see Results), unless otherwise indicated. Data were analysed using pCLAMP software, version 8.0 (Axon Instruments, Union City, CA, USA), and ICa was calculated as 200 μm CdCl2-sensitive difference current. Displayed membrane currents are current recordings shown without linear leak subtraction, unless otherwise indicated.
In experiments that monitored indo-1 loading, fluorescence intensity at 410 nm (the isosbestic point of this indicator in our system) was measured during illumination with 360 nm light using methodologies previously described in Konishi & Berlin (1993).
Solutions
The pipette solution was composed of (mm): 100 caesium gluconate, 10 Pipes (caesium salt), 15 TEACl, 0.5 NaH2PO4, 0.1 Tris-GTP, 5 EGTA along with ATP, Mg-ATP, citric acid, magnesium citrate, MgCl2 and CaCl2 to produce free [Mg2+] of 0.2, 0.6 and 1.8 mm, pH 7.2, at specified free [Ca2+] of 1, 100 and 300 nm. Free [Mg2+] and [Ca2+] were calculated using a computer program (WinMAXC 2.40 obtained at http://stanford.edu/~cpatton/maxc.html). A second series of experiments was carried out using the same pipette solution with 5 mm BAPTA and 15 mm EGTA. In some experiments, indo-1 (K+ salt) was added to the pipette solution at a final concentration of 100 μm. The superfusion solution was a modified Tyrode solution containing (mm): 145 NaCl, 4 KCl, 2 CaCl2, 10 Hepes, 1 MgCl2, and 10 glucose, pH 7.4. When noted, CaCl2 in this solution was decreased to 0.5 mm.
Reagents
Unless specified, reagents were obtained from Sigma Chemical Corp. (St Louis, MO, USA). Autoinhibitory peptide (AIP; BioMol, Plymouth Meeting, PA, USA), calcineurin autoinhibitory peptide (CAP; CalBiochem, San Diego, CA, USA), okadaic acid (OA; CalBiochem), protein phosphatase 2A (PP2A; Upstate Biochemicals Inc., Lake Placid, NY, USA), and the potassium salt of indo-1 (Molecular Probes, Eugene, OR, USA) were added directly to pipette solutions. Several reagents purchased from CalBiochem (cyclosporine A, 3-isobutyl-1-methylxanthine (IBMX), KN-93, ryanodine, TTX and W7) and forskolin (BioMol) were prepared as concentrated stock solutions that were applied to bathing solutions 30 min prior to experiments. When DMSO or MeOH was used as the solvent for stock solutions, the final concentration in experimental solutions was less than or equal to 0.1%, and blank solutions containing 0.1% DMSO or MeOH were also prepared for control experiments.
Data analysis
Data are expressed as means ±s.e.m. for the number of cells indicated. Significance was determined using anova and Student's t test in commercial software (SigmaPlot, SPSS Inc., Chicago, IL, USA, and JMP IN, Duxbury, Pacific Grove, CA, USA). A P value less than 0.05 was considered statistically significant. The percentage change, confidence intervals (95%) and s.e.m. for current density ratios were calculated using Fieller's Theorem (Goldstein, 1964).
Results
Effect of [Mg2+]i on calcium current
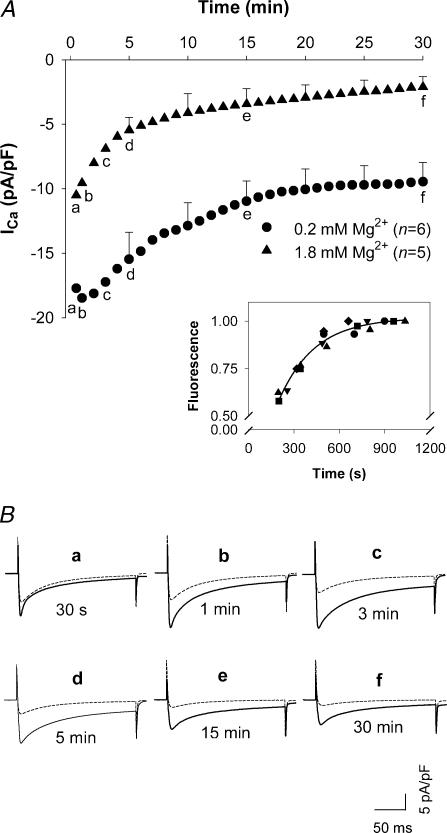
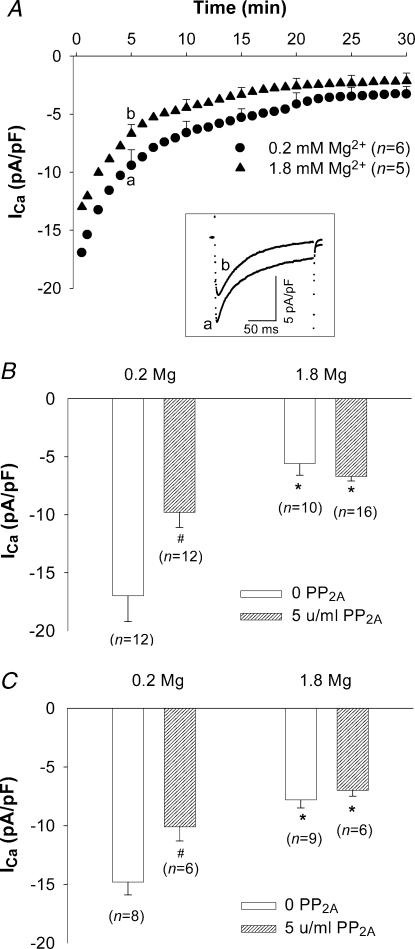
To assess the effects of [Mg2+]i on whole-cell ICa, time diaries of ICa were recorded for test depolarizations to 0 mV, elicited every 30 s, when cells were voltage-clamped with patch electrodes containing different concentrations of Mg2+ ([Mg2+]p) in the presence of 100 nm pipette [Ca2+] ([Ca2+]p). In the myocytes dialysed with 0.2 mm[Mg2+]p, ICa increased for 1–3 min after patch break-through followed by a long period of rundown before the current finally stabilized approximately 20 min into a 30 min observation period (Fig. 1A). The initial increase of ICa was probably caused by the relief from Mg2+ block of ICa due to the reduction of [Mg2+]i from a resting level of [Mg2+]i (∼1 mm) to 0.2 mm. The secondary rundown is thought to result from the washout of important cytoplasmic constituents (McDonald et al. 1994). In the myocytes dialysed with 1.8 mm[Mg2+]p, ICa at 30 s after patch break-through was much smaller and, with time, declined faster and to a much lower level than that with 0.2 mm[Mg2+]p. The time diaries show that ICa with 1.8 mm[Mg2+]p was 60%, 47%, 37%, 35%, 32%, 31% and 22% of ICa with 0.2 mm[Mg2+]p at 30 s, 1 min, 3 min, 5 min, 10 min, 15 min and 30 min after patch break-through, respectively. Representative currents at each of these time points are shown in Fig. 1B. Thus, differences in current density changed rapidly during the first 3–5 min after patch break-through and thereafter changed slowly.
Figure 1. L-type calcium current (ICa) recorded in rat ventricular myocytes dialysed with low and high [Mg2+]p.
A, time diaries of ICa in rat ventricular myocytes depolarized to 0 mV from a holding potential of –40 mV during dialysis with pipette solutions containing 0.2 mm and 1.8 mm[Mg2+]p. Time 0 coincides with patch break-through. Data are means with s.e.m. displayed for 5, 10, 15, 20, 25 and 30 min time points. The numbers of experiments are indicated in parentheses. Letters (a–f) in each time course correspond to the displayed currents. Inset: time course of indo-1 loading in patch-clamped myocytes. Fluorescence intensity was normalized to the maximal level measured in each of 5 cells (shown with different symbols). The continuous curve is the best exponential function with a time constant of 238 s. B, superimposed sample currents, continuous and dashed tracings were recorded with 0.2 mm and 1.8 mm[Mg2+]p, respectively.
The period of rapid change in relative current densities in cells voltage-clamped with 0.2 and 1.8 mm[Mg2+]p might be an indicator of dialysis from pipette solution to the cytosol. To test this assertion, the time course of indo-1 loading was examined in five cells that, when voltage-clamped, displayed an uncompensated time constant for decay of current during 5 mV depolarizations of 0.3 ms. Indo-1 loading was monitored by measuring fluorescence intensity at the isosbestic point for this Ca2+ indicator. These experiments showed that the time constant for increasing fluorescence intensity was 4 min (Fig. 1A, inset). This finding is consistent with our previous work (Berlin & Konishi, 1993). Given these data, in subsequent experiments, we waited for 5 min after patch break-through before ICa was measured. Within 5 min of establishing a whole-cell patch-clamp, cellular loading of small molecules, such as a fluorescent Ca2+ indicator, would be greater than 70% complete. Given the 40 mmol l−1 of Mg2+ buffers in our patch electrode solutions, this would imply that approximately 25 mmol l−1 of exogenous Mg2+ buffers, i.e. 20 mm citrate along with 70% of the difference between cytosolic and patch ATP concentrations, would have diffused into the cytosol within this time period. Such a large exogenous buffer concentration should be sufficient to overwhelm endogenous cytosolic Mg2+ buffering capacity and thereby gain control of [Mg2+]i.
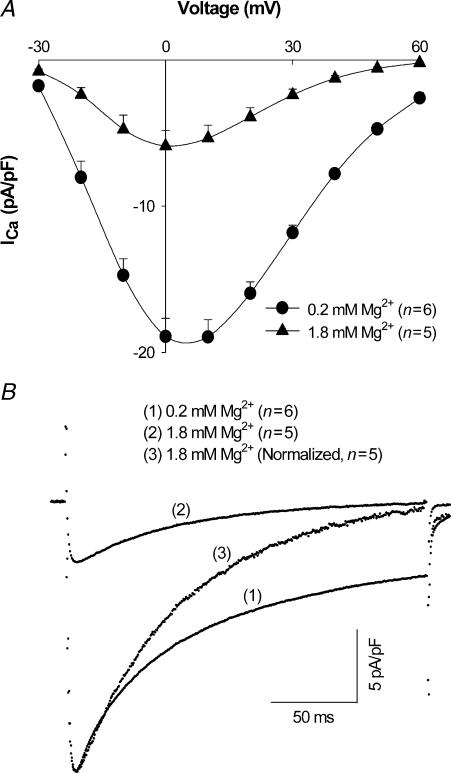
The Vm dependence of ICa was determined by a series of voltage pulses from –30 to +60 mV, as described in Methods. [Mg2+]p effects on the I–V relationship for ICa are illustrated in Fig. 2A. When [Mg2+]p was increased from 0.2 mm to 0.6 mm (not shown) and 1.8 mm, peak ICa amplitude was decreased by 56 ± 3.7%(n= 5) and 68 ± 3.5% (n= 5), respectively. Accounting for all experiments, including those where I–V relationships were not measured, increasing [Mg2+]p from 0.2 to 1.8 mm decreased peak ICa measured at 0 mV by 64 ± 2.8% (n= 10). Increasing [Mg2+]p also shifted the peak of the I–V relationship 5–10 mV in the negative direction and accelerated the rate of current inactivation, as shown by the normalization of current amplitudes in Fig. 2B.
Figure 2. Effect of [Mg2+]p on ICa.
A, current–voltage relationship of ICa in myocytes dialysed with 0.2 mm and 1.8 mm[Mg2+]p. Currents were recorded 5 min after establishing the whole-cell patch-clamp configuration. Data are means and s.e.m., with the number of experiments indicated in parentheses. B, average ICa tracings recorded at a test potential of 0 mV in rat ventricular myocytes dialysed with (1) 0.2 mm[Mg2+]p, (2) 1.8 mm[Mg2+]p and (3) 1.8 mm[Mg2+]p, normalized relative to that with 0.2 mm[Mg2+]p.
Effect of Mg2+ on the Vm dependence of calcium current
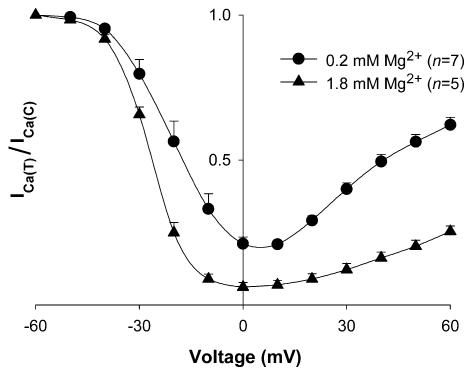
The observation that a decrease in ICa, when increasing [Mg2+]p, is accompanied by a shift of the I–V relationship towards negative Vm could be interpreted as an effect of [Mg2+]p on the Vm dependence of Ca2+ channel gating. Furthermore, the decrease in ICa that accompanies this leftward shift in the I–V relationship could imply that the Vm dependence of channel inactivation is also shifted to more negative potentials. To examine this possibility, the Vm dependence of channel inactivation was estimated with a two-pulse protocol consisting of a 400 ms prepulse (from –90 to +60 mV in 10 mV increments) followed, after a 3 ms interval at –40 mV, by a 200 ms test depolarization to 0 mV. Figure 3 shows the resulting inactivation curves at 0.2 mm and 1.8 mm[Mg2+]p. With 0.2 mm[Mg2+]p, the curve exhibited a characteristic ‘U’-shape, i.e. inactivation reached a maximum with prepulses to 0 mV but decreased with prepulses positive of 0 mV. However, at the higher [Mg2+]p, the degree of current inactivation was more complete at positive Vm. Since Vm-dependent inactivation of l-type channels requires a long period to reach a pseudo-steady state (Hadley & Lederer, 1991), this protocol was repeated using 3 s prepulses. Results similar to those with 400 ms prepulses were observed. With both protocols, leftward shifts in the inactivation curves were observed. With 400 ms prepulses, increasing [Mg2+]p from 0.2 to 1.8 mm shifted the Vm for half-maximal inactivation by −8.1 ± 0.7 mV (n= 5). The shift was −7.7 ± 1.0 mV (n= 5) with 3 s prepulses. These results suggested that [Mg2+]p can affect the Vm-dependence of channel gating. It should be pointed out that the degree of this shift is small enough that, at –40 mV, the change in Ca2+ channel availability is minor, and therefore, a shift in Vm-dependent inactivation is unlikely to account for the marked decrease of ICa observed with higher [Mg2+]p.
Figure 3. Effect of [Mg2+]p on inactivation curves for ICa.
The Vm dependence of channel inactivation was measured at 5 min after break-through into the whole-cell patch-clamp configuration with a 2-pulse protocol. ICa at 0 mV after a given prepulse (ICa(T)) is divided by ICa at 0 mV after a prepulse to –60 mV (ICa(C)). Data are means and s.e.m., with the number of experiments indicated in parentheses for each [Mg2+]p.
Ca2+ channel phosphorylation and Mg2+ effects on Ca2+ current
l-type Ca2+ channels are known to be regulated by channel phosphorylation (McDonald et al. 1994) and the phosphorylation state of the channel has been reported to be important in determining Mg2+ effects on ICa (White & Hartzell, 1988; Agus et al. 1989; Yamaoka & Seyama, 1998; Pelzer et al. 2001; Yamaoka et al. 2002). To investigate how the modulation of l-type Ca2+ channels by [Mg2+]p is affected by channel phosphorylation, we conducted a series of experiments measuring currents where l-type Ca2+ channels were likely to be in phosphorylated and dephosphorylated states, at low (0.2 mm) and high (1.8 mm) [Mg2+]p. To increase channel phosphorylation, cardiac myocytes were first preincubated for 30 min with 10 μm forskolin to activate adenylate cyclase and 300 μm 3-isobutyl-1-methylxanthine (IBMX) to inhibit cAMP and cGMP phosphodiesterases. l-type Ca2+ current was then measured 5 min after patch break-through with pipette solutions containing 50 μm okadaic acid (OA) to inhibit protein phosphatases that could dephosphorylate Ca2+ channels. This manoeuvre has been found to increase the ICa in frog and guinea-pig myocytes (Yamaoka & Seyama, 1998; Pelzer et al. 2001), presumably by cAMP-mediated phosphorylation of l-type Ca2+ channels.
Our experiments showed that in the presence of forskolin, IBMX and OA, ICa was dramatically increased to densities at 0 mV of 30–40 pA pF−1 with 0.2 mm[Mg2+]p (36.3 ± 2.1 pA pF−1, n= 3). To minimize voltage errors due to this high current density, extracellular Ca2+ concentration ([Ca2+]o) was therefore reduced from 2.0 to 0.5 mm. With 0.5 mm[Ca2+]o, ICa at 0 mV was 16.8 ± 1.4 pA pF−1 (n= 5) with 0.2 mm[Mg2+]p in cells exposed to forskolin, IBMX and OA. This current density was not significantly different from that observed under basal conditions with 2.0 mm[Ca2+]o.
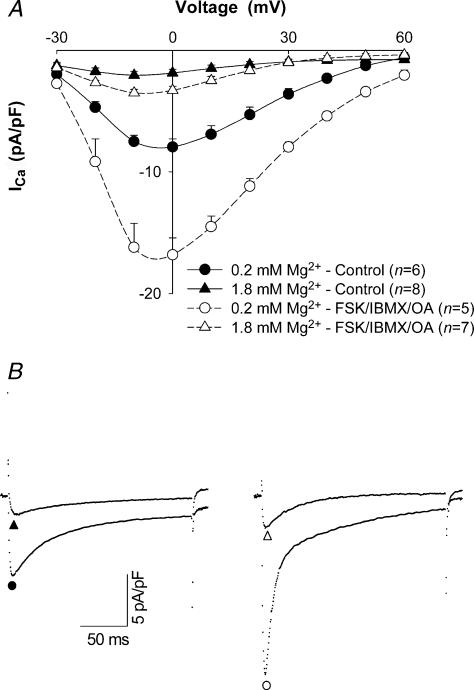
To evaluate the effect of [Mg2+]p on ICa, we first repeated experiments in Fig. 2A with 0.5 mm[Ca2+]o (Fig. 4A and B, left). Under these conditions, increasing [Mg2+]p from 0.2 mm to 1.8 mm decreased peak ICa amplitude by 75 ± 2.4% (n= 6) and shifted the peak of the I–V relationship 5–10 mV in the negative direction (Fig. 4A, continuous curves). This reduction of ICa was not statistically different from that observed with 2.0 mm[Ca2+]o, so changing [Ca2+]o appeared to have no effect on the decrease of ICa induced by increasing [Mg2+]p. In the presence of forskolin, IBMX and OA, peak ICa amplitude with 1.8 mm[Mg2+]p was 3.3 ± 0.4 pA pF−1 (n= 7). Thus, under these conditions, increasing [Mg2+]p from 0.2 mm to 1.8 mm produced a 79 ± 1.7% inhibition on peak ICa amplitude (Fig. 4A, dashed curves and Fig. 4B, right). Additionally, higher [Mg2+]p caused a –5 mV shift in the peak of the I–V relationship. Thus, increasing [Mg2+]i markedly reduced ICa under conditions promoting l-type Ca2+ channel phosphorylation.
Figure 4. Effect of [Mg2+]p on ICa in high phosphorylation conditions.
A, current–voltage relationships for ICa in myocytes dialysed with 0.2 mm and 1.8 mm[Mg2+]p in control (continuous curves) and high phosphorylation conditions (dashed curves) in the absence and presence of 10 μm forskolin (FSK), 300 μm IBMX and 50 μm OA, respectively, when [Ca2+]o was set at 0.5 mm. Currents were recorded at 5 min after break-through into the whole-cell patch-clamp configuration. Data are means and s.e.m., with the number of experiments indicated in parentheses. B, tracings of typical ICa records at a test potential of 0 mV in rat ventricular myocytes dialysed with 0.2 mm (•) and 1.8 mm[Mg2+]p (▴) in control conditions, and 0.2 mm (○) and 1.8 mm[Mg2+]p (▵) in high phosphorylation conditions.
To induce channel dephosphorylation, the catalytic subunit of protein phosphatase 2A (PP2A; 5 units ml−1) was included in the pipette solution. PP2A was chosen because l-type channels are complexed with stoichiometric amounts of PP2A (Davare et al. 2000) and PP2A is present in the heart (Herzig & Neumann, 2000). Furthermore, PP2A activity has no requirement for Mg2+ and Ca2+ (Herzig & Neumann, 2000; Rusnak & Mertz, 2000) so that this enzyme should be insensitive to the experimentally induced changes in cytosolic concentrations of these divalent cations.
The extent of cell dialysis with a protein, such as PP2A, is unknown in our experiments. For this reason, the time course of ICa was compared in the presence and absence of PP2A (Fig. 5A and Fig. 1, respectively). In cells superfused with 2 mm Ca2+-containing Tyrode solution and voltage-clamped with patch pipette solutions containing 0.2 mm[Mg2+]p and PP2A, the initial rate of current rundown was more rapid than in the absence of PP2A. After 5 min, average ICa density at 0 mV was 9.8 ± 1.3 pA pF−1 (n= 12), a level 63 ± 3.7% of that measured in the absence of PP2A (Fig. 5A). This decrease in ICa is statistically significant and is consistent with the results of duBell et al. (1996). Representative currents are shown in Fig. 5A, inset. Control experiments also showed that adding the enzyme carrier solution to the 0.2 mm Mg2+-containing pipette solution had no effect on ICa amplitude or kinetics. Thus, a 5-min period of cell dialysis appears to be sufficient for PP2A to produce significant effects on ICa.
Figure 5. Effect of [Mg2+]p on ICa in low phosphorylation conditions.
A, time diaries of ICa in rat ventricular myocytes depolarized to 0 mV from a holding potential of –40 mV during dialysis with 0.2 mm and 1.8 mm[Mg2+]p electrode solution containing 5 units ml−1 PP2A. Data are means with sample s.e.m. displayed for 5, 10, 15, 20, 25 and 30 min time points. The numbers of experiments are indicated in parentheses. Letters (a and b) in each time course correspond to the sample currents in the inset. Inset: superimposed currents recorded 5 min after patch break-through. B, ICa density in myocytes dialysed with 0.2 mm and 1.8 mm[Mg2+]p in control (0 PP2A) and low phosphorylation conditions (5 units ml−1 PP2A) in the presence of 100 nm[Ca2+]p. C, ICa density in the rat ventricular myocytes dialysed with 0.2 mm and 1.8 mm[Mg2+]p in control (0 PP2A) and low phosphorylation conditions (5 units ml−1 PP2A) in the presence of ∼1 nm[Ca2+]p. Currents were measured 5 min after break-through into the whole-cell patch-clamp configuration. Data are means and s.e.m., with the number of experiments indicated in parentheses. Significant changes of ICa, comparing low (0.2 mm) versus high [Mg2+]p (1.8 mm) and basal (0 PP2A) versus low phosphorylation conditions are indicated as * and #, respectively.
In the presence of PP2A, increasing [Mg2+]p from 0.2 mm to 1.8 mm suppressed peak current by 25 ± 3.4% (n= 10). The magnitude of this effect is statistically smaller than that observed under basal conditions (Fig. 5B). Interestingly, with 1.8 mm[Mg2+]p, ICa density at 0 mV was similar with or without PP2A added to the pipette solution (Fig. 5B). In addition, comparing the time diaries in Figs 1 and 5A shows that channel dephosphorylation had no significant effect on current magnitude in cells voltage-clamped with patch electrodes containing 1.8 mm[Mg2+]p. These results suggested that channel dephosphorylation reduced Mg2+ effects on ICa.
[Ca2+]i and Mg2+ effects on calcium current
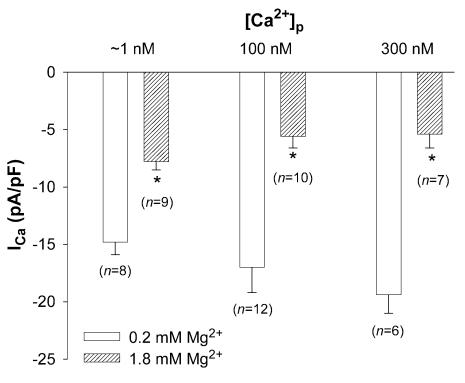
The experiments above were performed with [Ca2+]p set to 100 nm, similar to resting levels of [Ca2+]i in rat myocytes. To evaluate the possible influence of [Ca2+]i on [Mg2+]i-dependent modulation of current, ICa was measured at different [Mg2+]p in nominally Ca2+-free pipette solutions where free [Ca2+]p was approximately 1 nm. Under these conditions, peak ICa density at 0 mV in cells voltage-clamped with patch electrodes containing 0.2 mm Mg2+ was 14.8 ± 1.1 pA pF−1 (n= 8). Increasing [Mg2+]p from 0.2 mm to 1.8 mm altered I–V relationships and inactivation of ICa in a qualitatively similar manner to that with 100 nm[Ca2+]p. However, peak ICa amplitude was decreased by 45 ± 2.7% (Fig. 6), a significantly smaller reduction of ICa than observed with 100 nm[Ca2+]p.
Figure 6. Effect of [Mg2+]p on ICa with different [Ca2+]p.
Currents were measured at a test potential of 0 mV in myocytes dialysed with 0.2 mm and 1.8 mm[Mg2+]p at ∼1 nm, 100 nm and 300 nm[Ca2+]p. Data are means and s.e.m., with the number of experiments indicated in parentheses. Asterisks (*) indicate significant changes of ICa between low (0.2 mm) and high [Mg2+]p (1.8 mm).
When cells were voltage-clamped with patch electrodes containing 300 nm Ca2+ and 0.2 mm Mg2+, peak ICa density at 0 mV was 19.4 ± 1.6 pA pF−1 (n= 6). Increasing [Mg2+]p from 0.2 mm to 1.8 mm produced an inhibitory effect on ICa quantitatively similar to that observed with 100 nm[Ca2+]p (Fig. 6). These results, summarized in Table 1, suggested that [Ca2+]p did affect [Mg2+]p actions on ICa, particularly when [Ca2+]p was decreased to very low levels. The following experiments attempted to define how Ca2+ and Mg2+ might interact in the cell to modulate ICa.
Table 1.
Effects of experimental manoeuvres on [Mg2+]i-dependent modulation of ICa
| Experimental manoeuvres | Decrease in ICa density (%)† |
|---|---|
| Patch electrode Ca2+ ([Ca2+]p) | |
| ∼1 nm | 45 ± 2.7 (8)* |
| 100 nm | 64 ± 2.8 (10) |
| 300 nm | 71 ± 3.5 (6) |
| Change cytosolic Ca2+ buffering | |
| 10 μm Ryanodine | 71 ± 3.8 (5) |
| 5 mm BAPTA + 15 mm EGTA | 56 ± 3.5 (6) |
| Calmodulin blocker | |
| 50 μm W7 | 60 ± 4.9 (5) |
| Ca2+/calmodulin kinase II blockers | |
| 5 μm KN-93 | 59 ± 5.7 (6) |
| 100 μm AIP | 63 (3)‡ |
| Ca2+/calmodulin phosphatase | |
| 2B blockers | |
| 10 μm CsA | 56 ± 5.8 (6) |
| 10 μm CAP | 56 ± 2.5 (8) |
Values of n given in parentheses. †Percentage decrease of peak ICa at 0 mV in cells voltage-clamped with patch electrodes containing 1.8 mmversus 0.2 [Mg2+]p, calculated by Fieller's theorem (Goldstein, 1964). ‡The number of replicates is too small to calculate the mean and 95% Confidence Interval of the ratio, and therefore, the s.e.m. using Fieller's theorem. As a result, the ratio of the mean current density with 1.8 mm and 0.2 mm[Mg2+]i is listed. AIP, autoinhibitory peptide; CAP, calcineurin autoinhibitory peptide.
Significantly different from experiments with [Ca2+]p equal to 100 nm.
Effects of intracellular Ca2+ fluxes on Mg2+ modulation of calcium current
Ca2+ influx via l-type channels and sarcoplasmic reticulum (SR) Ca2+ release generate local increases in [Ca2+]i (Stern, 1992; Cheng et al. 1993). This local change in [Ca2+]i can significantly modulate both the amplitude and macroscopic inactivation kinetics of ICa in ventricular myocytes (Lacampagne et al. 1995; Sham et al. 1995; Qu & Campbell, 1998). To assess how [Ca2+]i participates in the regulation of ICa by [Mg2+]i, [Ca2+]i homeostasis was manipulated by two ways: (1) Ca2+ release from SR was blocked with 10 μm ryanodine and (2) Ca2+ buffering was increased by adding 5 mm BAPTA plus 15 mm EGTA to the pipette solution. In all experiments, [Ca2+]p was set to 100 nm.
In the presence of 10 μm ryanodine, peak ICa densities at 0 mV in cells voltage-clamped with [Mg2+]p of 0.2 mm and 1.8 mm were 16.7 ± 1.6 pA pF−1 (n= 7) and 4.5 ± 0.9 pA pF−1 (n= 5), respectively. This change was a 71 ± 3.8% decrease in peak ICa amplitude (Table 1), a value not significantly different from that observed without ryanodine. Thus, [Mg2+]p effects on ICa were not influenced by SR Ca2+ release.
To distinguish between local and global effects of Ca2+, slow (EGTA) and fast (BAPTA) Ca2+ buffering species were used. Since Ca2+ binding kinetics of BAPTA are about 100-fold faster than those of EGTA (Tsien, 1980), Ca2+ diffusion distances are quite short (< 100 nm) in the presence of millimolar BAPTA whereas Ca2+ diffusion distances can be considerably longer (∼1 μm) in the presence of millimolar EGTA (Allbritton et al. 1992). With 5 mm BAPTA and 15 mm EGTA included in patch pipette solutions, peak ICa densities at 0 mV in cells voltage-clamped with 0.2 mm and 1.8 mm[Mg2+]p were 18.9 ± 1.9 pA pF−1 (n= 6) and 7.8 ± 0.9 pA pF−1 (n= 6), respectively. These data show that increasing [Mg2+]p from 0.2 mm to 1.8 mm decreased peak ICa amplitude by 56 ± 3.5% (Table 1), a change not significantly different than the [Mg2+]p-induced decrease of ICa with 5 mm EGTA (64 ± 2.8%, n= 10). Thus, Ca2+ buffers with different kinetics did not affect Mg2+ actions on ICa.
Effects of Ca2+/CaM dependent signal-transduction pathways on Mg2+ modulation of Ca2+ current
The Ca2+-dependence of [Mg2+]i actions on ICa, and the inability of Ca2+ buffers to alter these actions, point to a possible role for a calmodulin-dependent process in the inhibition of ICa. To test this hypothesis, the [Mg2+]i dependence of ICa density was examined in the presence of various blockers of calmodulin (CaM) and CaM-dependent enzymes.
In cells voltage-clamped in the presence of the CaM inhibitor, W7 (50 μm), with pipette solutions containing 0.2 mm[Mg2+]p and 100 nm[Ca2+]p, ICa was 6.4 ± 0.8 pA pF−1 (n= 5), a 57 ± 12% decrease compared to ICa measured in the absence of W7. The size of this decrease is consistent with a previous report (Caulfield et al. 1991). Under the same conditions, except that myocytes were exposed to the CaM-dependent protein kinase (CaMKII) inhibitor, KN-93 (5 μm), in the superfusion solution, ICa was 9.8 ± 1.2 pA pF−1 (n= 5), a 37 ± 6% decrease compared to ICa measured in the absence of this inhibitor. This effect is similar to that reported by Yuan & Bers (1994) with a related CaMKII inhibitor. On the other hand, neither cyclosporin A (CsA, 10 μm in the superfusion solution) nor CAP (10 μm in the pipette solution), inhibitors of CaM-dependent protein phosphatase 2B (PP2B), produced any significant changes of ICa density in myocytes voltage-clamped with pipette solutions containing 0.2 mm[Mg2+]p and 100 nm[Ca2+]p.
Comparing ICa density in the presence of these various blockers with 0.2 mm and 1.8 mm[Mg2+]p suggested that a CaM-dependent process was not involved in the [Ca2+]i dependence of [Mg2+]i actions on ICa. For example, peak ICa at 0 mV in cells voltage-clamped with patch electrodes containing 1.8 mm[Mg2+]p was 3.6 ± 0.7 pA pF−1 (n= 6) and 2.2 ± 0.4 pA pF−1 (n= 5) in the presence of KN-93 and W7, respectively. These current densities represent a 59 ± 5.7% and 60 ± 4.9% decrease in ICa, respectively, when compared to cells voltage-clamped with electrodes containing 0.2 mm[Mg2+]p (see Table 1). This degree of current reduction was not significantly different from that observed in vehicle-control experiments (i.e. a 64% decrease in ICa).
Relationship between Ca2+ channel phosphorylation and [Ca2+]i on Mg2+-dependent reduction of ICa
The experiments above suggested that [Mg2+]i-dependent reduction of ICa was moderated by reducing channel phosphorylation and [Ca2+]i. The question therefore arises as to whether these two manoeuvres are acting via a common mechanism. To test this possibility, experiments in Fig. 5B were repeated except that pipette solutions were prepared without added Ca2+ (i.e. ∼1 nm free [Ca2+]p). Under these conditions and with PP2A (5 units ml−1) included in pipette solutions, increasing [Mg2+]p from 0.2 mm to 1.8 mm decreased peak ICa density from 10.1 ± 1.2 pA pF−1 (n= 6) to 7.0 ± 0.5 pA pF−1 (n= 6), a 23 ± 5.5% (n= 6) decrease (Fig. 5C). In the absence of PP2A, the degree of current reduction on increasing [Mg2+]p was 45 ± 2.7% (n= 8), significantly different from that observed in the presence of PP2A. Even so, by comparing Fig. 5B (100 nm[Ca2+]p) and Fig. 5C (∼1 nm[Ca2+]p), it was clear that PP2A had similar effects on ICa amplitude at both low and high [Mg2+]p, irrespective of [Ca2+]p. Thus, these results suggested that increased [Mg2+]i could block the effects of Ca2+ channel phosphorylation on ICa independently of [Ca2+]i at or below 100 nm.
Discussion
Experiments in this study demonstrated that increasing [Mg2+]p around the reported physiological concentration range, 0.6 and 1.3 mm (Buri & McGuigan, 1990; Hongo et al. 1994), produced a marked inhibitory modulation of l-type Ca2+ current, accelerated current inactivation and caused a negative shift in the Vm dependence of current inactivation. Furthermore, manipulating conditions to favour Ca2+ channel dephosphorylation, lessened the degree to which [Mg2+]i reduced Ca2+ current. This modulation was especially pronounced in the presence of [Ca2+]p (100–300 nm), similar to [Ca2+]i measured in cells at rest. Even so, the dependence of [Mg2+]p effects on channel phosphorylation conditions was largely unchanged over the range of [Ca2+]P tested.
Inhibition of L-type Ca2+ current by [Mg2+]i
During the course of whole-cell patch-clamp experiments, ICa declined faster and to a much lower level in the myocytes dialysed with 0.6 mm or 1.8 mm Mg2+-containing solutions than with solutions containing 0.2 mm Mg2+. At the time when current was routinely measured (5 min after break-through), the amplitude of peak ICa was 64% smaller in myocytes voltage-clamped with pipette solutions containing 1.8 mm Mg2+ than those voltage-clamped with 0.2 mm Mg2+, when [Ca2+]p was set to 100 nm with 5 mm EGTA in the patch electrode (Table 1). Previous studies have also shown that elevation of [Mg2+] in patch electrode solutions from as low as 1 μm up to 10 mm can dramatically suppress Ca2+ current in guinea-pig (Agus et al. 1989; Pelzer et al. 2001; Yamaoka et al. 2002) and frog cardiac myocytes (Yamaoka & Seyama, 1996a,b, 1998; Yamaoka et al. 2002). However, in these previous experiments, changing electrode solution Mg2+ concentration around a physiological range of [Mg2+]i produced considerably smaller changes in ICa than reported here. These results are, at least in a quantitative sense, different from the present data.
In these previous studies, electrode solutions contained no Mg2+ buffers (Agus et al. 1989) or weak Mg2+ buffering capacity at physiological [Mg2+]i, i.e. 4 mm ATP (Pelzer et al. 2001) or 3 mm ATP plus 5 mm EDTA (Yamaoka & Seyama, 1996a,b, 1998; Yamaoka et al. 2002). This issue is important because cytosolic Mg2+ is largely buffered by proteins and small molecules, such as ATP and phosphocreatine (Robertson et al. 1981; Fabiato, 1983; Konishi & Berlin, 1993) at concentrations that lead one to question the degree to which [Mg2+]i was controlled in previous studies, at least around physiological [Mg2+]i. In contrast, the original report investigating [Mg2+]i regulation of ICa in frog myocytes (White & Hartzell, 1988) showed that increasing pipette [Mg2+] in the range of 0.3–3.0 mm could significantly decrease ICa. In their experiments, pipette Mg2+ was buffered by 3 mm ATP and 5 mm phosphocreatine, which leads to a relatively higher Mg2+ buffering capacity at physiological concentrations. These results, consistent with the present data where 40 mm Mg2+ buffers were present in the patch electrode solution, suggest that changes in [Mg2+]i around a physiological set-point can have large effects in ICa.
Pipette solution compositions in this study were chosen to provide high Mg2+ buffering capacity at physiological [Mg2+]i, while maintaining MgATP in the millimolar range. Mg2+ buffering was provided by 30 mm citrate and 10 mm ATP. Since the dissociation constant of citrate for Mg2+ is 0.6 mm (calculated from binding constants in Martell & Smith, 1974), this compound should provide strong buffering through the range of [Mg2+]p used here. A previous report also showed that citrate (10 mm), applied intracellularly, had no effect on ICa (Hryshko & Bers, 1992). In any case, total citrate concentration in our pipette solutions was constant so that any citrate effects on ICa should have been systematic in this study.
Membrane potential and [Mg2+]i effects on ICa
In addition to markedly reducing ICa amplitude, increasing [Mg2+]p shifted the I–V relationship by 5–10 mV in the negative direction (Fig. 2A), a finding consistent with Hartzell & White (1989). Likewise, increasing [Mg2+]p from 0.2 to 1.8 mm shifted the Vm for half-maximal current inactivation by –8 mV (Fig. 3). This result, coupled with the acceleration of inactivation of ICa by high [Mg2+]p (Fig. 2B), suggests that increasing [Mg2+]p promotes Ca2+ channel inactivation. The mechanism responsible for the –8 mV shift in current inactivation was not explored in detail; however, two possibilities are obvious. Cytosolic Mg2+ could alter the kinetics of a Vm-dependent gating process and/or change surface charge shielding. Regardless of the particular mechanism, the effect of a –8 mV shift in steady state current inactivation would have only a minor effect on channel availability with a holding potential of –40 mV, as indicated in Fig. 3. Therefore, shifts in Vm-dependent channel gating are unlikely to explain the marked reduction of ICa produced by increasing [Mg2+]p.
Channel phosphorylation and [Mg2+]i effects on ICa
Channel phosphorylation state appears to exert a strong influence on Mg2+ modulation of ICa (White & Hartzell, 1988; Agus et al. 1989; Yamaoka & Seyama, 1998; Pelzer et al. 2001). Furthermore, the effects of increasing [Mg2+]i on ICa, i.e. decreased amplitude and accelerated inactivation, are consistent with a decrease in Ca2+ channel phosphorylation (Allen & Chapman, 1995; Mitarai et al. 2000). Therefore, effects of [Mg2+]i on ICa were investigated under conditions strongly favouring or antagonizing l-type channel phosphorylation.
The results of these experiments are quite clear. With 0.2 mm[Mg2+]p, manipulating phosphorylation conditions had a dramatic effect on ICa density, ranging from a level of 36 pA pF−1 in the presence of forskolin, IBMX and OA to less than 10 pA pF−1 in the presence of PP2A. Conversely, at 1.8 mm[Mg2+]p, these same manipulations had little effect on current density. These data, compiled or extrapolated from the experiments, are summarized in Table 2.
Table 2.
Effects of [Mg2+]i on phosphorylation-dependent modulation of ICa (pA pF−1)
| [Mg2+]p (mM) | ||
|---|---|---|
| Phosphorylation conditions† | 0.2 | 1.8 |
| Low (PP2A) | 9.8 ± 1.3 (12) | 6.7 ± 0.4 (16) |
| Basal | 17.0 ± 2.2 (12) | 5.6 ± 1.0 (10) |
| High (forskolin, IBMX, OA) | 36.3 ± 2.1 (3) | 7.6‡ |
Values of n given in parentheses.
[Ca2+]i set at 100 nm and [Ca2+]o to 2 mm in all experiments. Currents were measured at 0 mV.
This value is extrapolated by reducing the current density (36.3 pA pF−1) measured at 0.2 mm[Mg2+]i during superfusion with 2 mm[Ca2+]o by 79%, the reduction in current density measured upon increasing [Mg2+]i from 0.2 to 1.8 mm with 0.5 mm[Ca2+]o.
Viewed in another way, these data suggest that [Mg2+]p has a much greater modulatory role on ICa in high phosphorylation conditions (79% reduction) as compared to low phosphorylation conditions (25% reduction). White & Hartzell (1988) also showed that increasing [Mg2+]i ([Mg2+]p ranging from 0.3 to 3 mm) in frog ventricular myocytes had a much greater modulatory effect on ICa in conditions promoting Ca2+ channel phosphorylation, consistent with our results. Likewise, preincubation of guinea-pig myocytes with a non-specific kinase blocker, K252, to presumably decrease Ca2+ channel phosphorylation, abolished any effect of [Mg2+]i on ICa when cells were voltage-clamped with pipettes containing solutions in which [Mg2+] had been set from 1 μm to 5 mm (Pelzer et al. 2001). These results imply that channel phosphorylation is integral to Mg2+ actions on ICa.
Why cytosolic Mg2+ should produce a greater effect in conditions that promote channel phosphorylation is unclear. One consistent finding is that cytosolic Mg2+ inhibits ICa with high affinity under basal, presumably low phosphorylation, conditions (IC50= 4 μm; Yamaoka & Seyama, 1996b); however, under conditions promoting Ca2+ channel phosphorylation, the apparent affinity for Mg2+ inhibition of ICa shifts to well over 1 mm (Yamaoka & Seyama, 1998; Pelzer et al. 2001; Yamaoka et al. 2002). This finding might explain why we observe less pronounced effects of [Mg2+]i on ICa in the presence of PP2A, i.e. a [Mg2+]i of 0.2 mm would produce nearly maximal inhibition of ICa under dephosphorylating conditions so further increasing [Mg2+]i would have little additional effect on current. Alternatively, micromolar concentrations of GTP are reported to block [Mg2+]i-dependent effects on ICa (Yamaoka & Seyama, 1996b; Yamaoka et al. 2002), but channel phosphorylation is reported to overcome these effects of GTP (Yamaoka & Seyama, 1998). Since our pipette solutions contain 0.1 mm GTP, this second possibility seems quite plausible. In any case, our data establish that physiological [Mg2+]i is capable of regulating ICa in the presence of GTP to a degree which is dependent on the level of Ca2+ channel phosphorylation.
Two types of molecular mechanisms might explain how Mg2+ alters gating kinetics of phosphorylated l-type channels. First, the level of channel phosphorylation might be altered because the activity of several regulatory enzymes, such as adenylyl cyclases (Pieroni et al. 1995; Sunahara et al. 1996), phosphodiesterases (Sette & Conti, 1996; Percival et al. 1997) and phosphatases (Cohen et al. 1989; Herzig & Neumann, 2000), are affected by Mg2+ at concentrations up to the millimolar range. Second, l-type channel gating has been proposed to be modulated directly by both Mg2+ and GTP binding (Yamaoka & Seyama, 1998). Whether one or both of these mechanisms explain Mg2+ actions on ICa can only be determined by direct measurements of Mg2+ effects on Ca2+ channel phosphorylation.
[Ca2+]i and [Mg2+]i effects on ICa
In the presence of 100 nm[Ca2+]p, increasing [Mg2+]i from 0.2 to 1.8 mm[Mg2+]p suppressed the amplitude of ICa by 64%. However, when [Ca2+]p was nominally zero (∼1 nm), increasing [Mg2+]p from 0.2 to 1.8 mm decreased the amplitude of ICa by only 45%, a statistically smaller effect. Conversely, when [Ca2+]p was increased to 300 nm, the [Mg2+]i-induced reduction of ICa was not different (71%) from that observed with 100 nm[Ca2+]p (Table 1 and Fig. 6). These results suggest that the [Mg2+]p effect is greater in the presence of 100 nm and 300 nm[Ca2+]p than that with ∼1 nm[Ca2+]p and that, to some degree, the effects of [Mg2+]p on ICa are achieved in a Ca2+-dependent manner, i.e. [Mg2+]i and [Ca2+]i interact to regulate ICa.
[Mg2+]i effects on [Ca2+]i regulation of ICa
The question then is how [Mg2+]i affects the [Ca2+]i regulation of ICa. Our data (Fig. 6) show that increasing [Ca2+]p (1–300 nm) tended to increase ICa at low [Mg2+]p (0.2 mm) whereas increasing [Ca2+]p tended to decrease ICa at high [Mg2+]p (1.8 mm). These trends in the data suggest that [Mg2+]i might determine the pattern of [Ca2+]i-dependent regulation of ICa, e.g. positive or negative regulation of ICa.
This latter point was not pursued because, given cell-to-cell variability, a demonstration of statistically significant changes in ICa as a function of [Ca2+]i and [Mg2+]i would have required many more experiments. Nonetheless, these results are interesting because the reported effects of increasing [Ca2+]i on ICa have varied widely in previous studies. Yamaoka & Seyama (1996a) have reported that increasing [Ca2+]i from 10 nm to 1 μm facilitates ICa in frog myocytes voltage-clamped with electrodes containing either 0.1 or 1 mm Mg2+. At the higher electrode [Mg2+], their results appear opposite of those reported here. No other papers have directly investigated if an interaction between [Mg2+]i and [Ca2+]i might regulate ICa. However, many papers have reported that increasing [Ca2+]i can either facilitate (Bates & Gurney, 1993; Gurney et al. 1989; Hirano & Hiraoka, 1994) or inhibit ICa and single Ca2+ channels (Morad et al. 1988; Hadley & Lederer, 1991; Hirano & Hiraoka, 1994; You et al. 1994). Reviewing these papers does not provide a clear picture about the role of [Mg2+]i in these changes of ICa; however, the present results do suggest that changing [Mg2+]i can affect the manner in which [Ca2+]i, around resting levels, might modulate ICa.
Possible mechanisms underlying [Ca2+]i modulation of [Mg2+]i effects on ICa
To investigate how [Ca2+]i is involved in the modulation of ICa by [Mg2+]i, we manipulated [Ca2+]i in two ways: blocking SR Ca2+ release with ryanodine, and buffering [Ca2+]i with the fast Ca2+ chelator, BAPTA. With 100 nm[Ca2+]p, increasing [Mg2+]p from 0.2 to 1.8 mm decreased ICa amplitude to a similar degree in the presence and absence of 10 μm ryanodine, an indication that SR Ca2+ release was not involved in [Mg2+]i effects on ICa. Furthermore, the [Mg2+]i-induced decrease of ICa was similar whether fast (5 mm BAPTA + 15 mm EGTA, 100 nm[Ca2+]p) or slow Ca2+ buffer systems (5 mm EGTA, 100 nm[Ca2+]p) were included in the pipette solution. These results indicate that buffering increases of [Ca2+]i, irrespective of the kinetics and capacity of the Ca2+ chelator, does not affect Mg2+ actions on ICa, consistent with our observation that increasing [Ca2+]i with 300 nm[Ca2+]p also does not significantly change [Mg2+]i-dependent modulation of ICa. Instead, our data show that [Ca2+]i must be decreased below 100 nm for the interaction between Mg2+ and Ca2+ to be observed.
Many potential sites exist on or near the l-type Ca2+ channel where Ca2+ binding could regulate ICa (Hering et al. 2000; Herzig & Neumann, 2000). Each of these sites is also likely to be a potential site for Mg2+ binding. Even so, our experiments focused on a possible role of calmodulin (CaM) for several reasons. First, CaM can bind both Mg2+ and Ca2+ (Haiech et al. 1981) and Mg2+ binding to CaM interferes with Ca2+-dependent regulation of enzyme function (Ohki et al. 1997). Second, the Ca2+ affinity of CaM when it is bound to IQ motif peptides is approximately 50 nm (Black et al. 2002), in the same range of Ca2+ concentrations used in our pipette solutions. The IQ motif of the l-type channel is located at the C-terminal tail and CaM interaction near this site is thought to participate in channel inactivation and facilitation (Zühlke et al. 1999; DeMarla et al. 2001). Thus, a reasonable expectation is that Mg2+ might modulate this Ca2+-dependent mechanism of channel gating or vice versa. To test this hypothesis, the effects of CaMKII inhibitors (KN-93 and AIP), calcineurin inhibitors (CsA and CAP) and the CaM inhibitor W7 were tested on [Mg2+]i-dependent modulation of ICa. None of these agents significantly altered the effects of [Mg2+]i on ICa. Therefore, our data indicate that a CaM-dependent mechanism does not explain the interaction of [Ca2+]i and [Mg2+]i to modulate ICa. Nevertheless, considering that W7, probably like other calmodulin blockers, may not produce a specific blockade of calmodulin (Klockner & Isenberg, 1987) and Ca2+/CaM-dependent inactivation (Imredy & Yue, 1994; Victor et al. 1997), we cannot entirely rule out the involvement of a Ca2+/CaM-dependent facilitation/inactivation mechanism in the regulation of ICa by [Mg2+]i.
Participation of [Ca2+]i in the phosphorylation-dependent regulation of ICa by [Mg2+]i
Since our results show that [Mg2+]i effects on ICa amplitude are dependent on channel phosphorylation, we looked at whether this phosphorylation-dependent regulation of Mg2+ effects on ICa is related in some manner to [Ca2+]i dependence of Mg2+ actions. The effect of increasing [Mg2+]p was examined under low and basal phosphorylation conditions with ∼1 nm and 100 nm[Ca2+]p. With both [Ca2+]p, Mg2+ effects were comparable, i.e. under basal phosphorylation conditions, increasing [Mg2+]p produced a greater decrease in ICa than in the dephosphorylated channel. Most clearly, high [Mg2+]p minimized the effect of channel phosphorylation on ICa with both [Ca2+]p. We interpret these data as suggesting that Mg2+ can affect two mechanisms, one phosphorylation-dependent and the other Ca2+-dependent, that modulate l-type Ca2+ channel gating properties.
In summary, the present data show that changes of [Mg2+]p between 0.2 mm and 1.8 mm strongly suppress cardiac ICa. These data suggest that cytosolic Mg2+ is a potential regulator of ICa at physiological concentrations. This modulation of ICa by [Mg2+]i is larger in the presence of resting levels of [Ca2+]i, an indication of an interaction between [Mg2+]i and [Ca2+]i. However, Ca2+/CaM-dependent signal pathways do not appear to be involved in this modulatory action of [Mg2+]i. Shifts in Vm-dependent gating are also unlikely to be responsible for Mg2+ actions. Instead, our results suggest that the channel phosphorylation state plays a predominant role in [Mg2+]i-induced modulation of ICa.
Acknowledgments
The authors wish to thank Ms Renee Green for excellent technical assistance, Drs Masato Konishi and Roman Shirokov for helpful discussions about the data. This work is supported by the National Institutes of Health (HL 69020).
References
- Agus MSD, Agus ZS. Cardiovascular actions of magnesium. Crit Care Clin. 2001;17:175–186. doi: 10.1016/s0749-0704(05)70158-5. [DOI] [PubMed] [Google Scholar]
- Agus ZS, Kelepouris E, Dukes I, Morad M. Cytosolic magnesium modulates calcium channel activity in mammalian ventricular cells. Am J Physiol. 1989;256:C452–C455. doi: 10.1152/ajpcell.1989.256.2.C452. [DOI] [PubMed] [Google Scholar]
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Chapman RA. The effect of a chemical phosphatase on single calcium channels and the inactivation of whole-cell calcium current from isolated guinea-pig ventricular myocytes. Pflugers Arch. 1995;430:68–80. doi: 10.1007/BF00373841. [DOI] [PubMed] [Google Scholar]
- Bates SE, Gurney AM. Ca2+-dependent block and potentiation of L-type calcium current in guinea-pig ventricular myocytes. J Physiol. 1993;466:345–365. [PMC free article] [PubMed] [Google Scholar]
- duBell WH, Lederer WJ, Rogers TB. Dynamic modulation of excitation–contraction coupling by protein phosphatases in rat ventricular myocytes. J Physiol. 1996;493:793–800. doi: 10.1113/jphysiol.1996.sp021423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin JR, Konishi M. Ca2+ transients in cardiac myocytes measured with high and low affinity Ca2+ indicators. Biophys J. 1993;65:1632–1647. doi: 10.1016/S0006-3495(93)81211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DJ, Halling DB, Pate P, Mandich DV, Hamilton SL, Altschuld RA. Effect of Ca2+ channel IQ peptides on Ca2+ binding to calmodulin. Biophys J. 2002;82:106a. [Google Scholar]
- Buri A, McGuigan JA. Intracellular free magnesium and its regulation, studied in isolated ferret ventricular muscle with ion-selective microelectrodes. Exp Physiol. 1990;75:751–761. doi: 10.1113/expphysiol.1990.sp003457. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Robbins J, Sim JA, Brown DA, MacNeil S, Blackburn GM. The naphthalenesulphonamide calmodulin antagonist W7 and its 5-iodo-1-C8 analogue inhibit potassium and calcium currents in NG108-15 neuroblastoma x glioma cells in a manner possibly unrelated to their antagonism of calmodulin. Neuroscience Lett. 1991;125:57–61. doi: 10.1016/0304-3940(91)90130-l. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying extraction-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cohen P, Klumpp S, Schelling DL. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989;250:596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Davare MA, Horne MC, Hell JW. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2000;275:39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- DeMarla CD, Soong TW, Alselkhan BA, Alvanla RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Biostatistics: An Introductory Text. New York: MacMillan; 1964. pp. 187–189. [Google Scholar]
- Gurney AM, Charnet P, Pye JM, Jargeot J. Augmentation of cardiac calcium current by flash photolysis of intracellular caged-Ca2+ molecules. Nature. 1989;341:65–68. doi: 10.1038/341065a0. [DOI] [PubMed] [Google Scholar]
- Hadley RW, Lederer WJ. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiech J, Klee CB, Demaille JG. Effects of cations on affinity of calmodulin for calcium: ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Biochemistry. 1981;20:3890–3897. doi: 10.1021/bi00516a035. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, White RE. Effects of magnesium on inactivation of the voltage-gated calcium current in cardiac myocytes. J General Physiol. 1989;94:745–767. doi: 10.1085/jgp.94.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering S, Berjukow S, Sokolov S, Marksteiner R, Weiß RG, Kraus R, Timin EN. Molecular determinants of inactivation in voltage-gated Ca2+ channels. J Physiol. 2000;528:237–249. doi: 10.1111/j.1469-7793.2000.t01-1-00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Hiraoka M. Dual modulation of unitary L-type Ca2+ channel currents by [Ca2+]i in fura-2-loaded guinea-pig ventricular myocytes. J Physiol. 1994;480:449–463. doi: 10.1113/jphysiol.1994.sp020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo K, Konishi M, Kurihara S. Cytoplasmic free Mg2+ in rat ventricular myocytes studied with the fluorescent indicator furaptra. Jap J Physiol. 1994;44:357–378. doi: 10.2170/jjphysiol.44.357. [DOI] [PubMed] [Google Scholar]
- Hryshko LV, Bers DM. Citrate alters Ca channel gating and selectivity in rabbit ventricular myocytes. Am J Physiol. 1992;262:C191–C198. doi: 10.1152/ajpcell.1992.262.1.C191. [DOI] [PubMed] [Google Scholar]
- Imredy JP, Yue DT. Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Klockner U, Isenberg G. Calmodulin antagonists depress calcium and potassium currents in ventricular and vascular myocytes. Am J Physiol. 1987;253:H1601–H1611. doi: 10.1152/ajpheart.1987.253.6.H1601. [DOI] [PubMed] [Google Scholar]
- Konishi, Berlin JR. Ca2+ transients in cardiac myocytes measured with low affinity fluorescent indicator, furaptra. Biophys J. 1993;64:1331–1343. doi: 10.1016/S0006-3495(93)81494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C, Hess P. Block of the L-type Ca2+ channel pore by external and internal Mg2+ in rat phaeochromocytoma cells. J Physiol. 1993;466:683–706. [PMC free article] [PubMed] [Google Scholar]
- Lacampagne A, Brette F, Le Guennec JY. Presence of a hump during the inactivation phase of the L-type calcium current of guinea-pig ventricular cardiomyocytes. Biophys J. 1995;70:A271. [Google Scholar]
- Martell AE, Smith RM. Critical Stability Constants. Vol. 6. New York: Plenum Press; 1974. [Google Scholar]
- McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Mitarai S, Kaibara M, Yano K, Taniyama K. Two distinct inactivation processes related to phosphorylation in cardiac L-type Ca2+ channel currents. Am J Physiol. 2000;279:C603–C610. doi: 10.1152/ajpcell.2000.279.3.C603. [DOI] [PubMed] [Google Scholar]
- Mitra R, Morad M. A uniform enzymatic method of dissociation of myocytes from heart and stomachs of vertebrates. Am J Physiol. 1985;249:H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Morad M, Davies NW, Kaplan JH, Lux HD. Inactivation and block of calcium channels by photo-released Ca2+ in dorsal root ganglion neurons. Science. 1988;241:842–844. doi: 10.1126/science.2457253. [DOI] [PubMed] [Google Scholar]
- Nilius B, Hess P, Lansman JB, Tsien RW. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985;316:443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Ohki SY, Ikura M, Zhang MJ. Identification of Mg2+-binding sites and the role of Mg2+ on target recognition by calmodulin. Biochemistry. 1997;36:4309–4316. doi: 10.1021/bi962759m. [DOI] [PubMed] [Google Scholar]
- Pelzer S, La CC, Pelzer KL. Phosphorylation-dependent modulation of cardiac calcium current by intracellular free magnesium. Am J Physiol. 2001;281:H1532–H1544. doi: 10.1152/ajpheart.2001.281.4.H1532. [DOI] [PubMed] [Google Scholar]
- Percival MD, Yeh B, Falgueyret JP. Zinc dependent activation of cAMP-specific phosphodiesterases (PDE4A) Biochem Biophys Res Commun. 1997;241:175–180. doi: 10.1006/bbrc.1997.7542. [DOI] [PubMed] [Google Scholar]
- Pieroni JP, Harry A, Chen J, Jacobowitz O, Magnusson RP, Iyengar R. Distinct characteristics of the basal activities of adenylyl cyclases 2 and 6. J Biol Chem. 1995;270:21368–21373. doi: 10.1074/jbc.270.36.21368. [DOI] [PubMed] [Google Scholar]
- Qu Y, Campbell DL. Modulation of L-type calcium current kinetics by sarcoplasmic reticulum calcium release in ferret isolated right ventricular myocytes. Can J Cardiol. 1998;14:263–272. [PubMed] [Google Scholar]
- Robertson SP, Johnson JD, Potter JD. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+ Biophys J. 1981;34:559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: Form and function. Physiol Rev. 2000;80:1483–1521. [Google Scholar]
- Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- Sham JSK, Cleeman L, Morad M. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci U S A. 1995;92:121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Victor RG, Rusnak F, Sikkink R, Marban E, O'Rourke B. Mechanism of Ca2+-dependent inactivation of L-type Ca2+ channels in GH3 cells: direct evidence against dephosphorylation by calcineurin. J Membr Biol. 1997;156:53–61. doi: 10.1007/s002329900187. [DOI] [PubMed] [Google Scholar]
- Wang M, Berlin JR. Regulation of L-type Ca current in cardiac myocytes by an interaction between cytosolic Ca2+ and Mg2+ Biophys J. 2002;82:104a. [Google Scholar]
- Watanabe M, Konishi M. Intracellular calibration of the fluorescent Mg2+ indicator furaptra in rat ventricular myocytes. Pflugers Arch. 2001;442:35–40. doi: 10.1007/s004240000499. [DOI] [PubMed] [Google Scholar]
- White RE, Hartzell HC. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988;239:778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- White RE, Hartzell HC. Magnesium ions in cardiac function: regulator of ion channels and second messengers. Biochem Pharmacol. 1989;38:859–867. doi: 10.1016/0006-2952(89)90272-4. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Seyama I. Regulation of Ca2+ channel by intracellular Ca2+ and Mg2+ in frog ventricular cells. Pflugers Arch. 1996a;431:305–317. doi: 10.1007/BF02207267. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Seyama I. Modulation of Ca2+ channels by intracellular Mg2+ ions and GTP in frog ventricular myocytes. Pflugers Arch. 1996b;432:433–438. doi: 10.1007/s004240050155. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Seyama I. Phosphorylation modulates L-type Ca channels in frog ventricular myocytes by changes in sensitivity to Mg2+ block. Pflugers Arch. 1998;435:329–337. doi: 10.1007/s004240050519. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Yuki T, Kawase K, Munemori M, Seyama I. Temperature-sensitive intracellular Mg2+ block of L-type Ca2+ channels in cardiac myocytes. Am J Physiol. 2002;282:H1092–H1101. doi: 10.1152/ajpheart.00585.2001. [DOI] [PubMed] [Google Scholar]
- You Y, Pelzer DJ, Pelzer S. Modulation of calcium current density by intracellular calcium in isolated guinea pig ventricular cardiomyocytes. Biochem Biophys Res Comm. 1994;204:732–740. doi: 10.1006/bbrc.1994.2520. [DOI] [PubMed] [Google Scholar]
- Yuan WL, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994;267:H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- Zühlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]