Abstract
We examined relations between cellular currents activated near firing threshold and the initiation of action potentials by excitatory postsynaptic potentials (EPSPs) in CA1 pyramidal cells in vitro. Small voltage steps elicited sequences of inward–outward currents at hyperpolarized potentials, but evoked largely inward currents at near threshold potentials. Similarly small EPSP-like waveforms initiated largely inward currents while larger stimuli evoked sequences of inward followed by outward currents. Shorter rise times of EPSP-like waveforms accentuated a transient component of inward currents. Voltage clamp data were consistent with the voltage dependence of current clamp responses to injection of EPSP shaped waveforms. Small events were prolonged at subthreshold potentials and could elicit action potentials at long latencies while responses to larger EPSP waveforms showed less voltage dependence and tended to induce spikes at shorter, less variable latencies. The precision of action potentials initiated by white noise depended also on stimulus amplitude. High variance stimuli induced firing with high precision, while the timing of spikes induced by lower variance signals was more variable between trials. In voltage clamp records, high variance noise commands induced sequences of inward followed by outward currents, while lower variance versions of the same commands elicited purely inward currents. These data suggest that larger synaptic stimuli recruit outward as well as inward currents. The resulting inward–outward current sequences enhance the temporal precision of EPSP–spike coupling. Thus, CA1 pyramidal cells initiate action potentials with different temporal precision, depending on stimulus properties.
Neurones transmit information not only by their firing rate but also by the timing with which they discharge (Rieke et al. 1997). Precisely timed firing is crucial in both coding information and the synchronization of neuronal ensemble activity (König et al. 1996). Spike timing depends on processes intervening between the reception of an excitatory synaptic potential and the generation of an action potential. These processes include electrical propagation from dendritic sites towards the site of action potential initiation as well as the activation of intrinsic postsynaptic currents. The currents activated near threshold potential differ in distinct cell types and this variation affects the temporal precision of EPSP–spike coupling (Fricker & Miles, 2000).
The temporal precision of action potential generation in response to afferent excitation varies between different neurones. Spike timing tends to be most precise when postsynaptic cells receive biphasic excitatory–inhibitory signals. The inhibitory component may be extrinsic, such as an inhibitory synaptic potential (Pouille & Scanziani, 2001; Galarreta & Hestrin, 2001). Alternatively, an EPSP may activate intrinsic voltage-dependent K+ currents (Cassell & McLachlan, 1986; Brew & Forsythe, 1995; Martina et al. 1998; Ramakers & Storm, 2002) which by limiting synaptically induced depolarizations can constrain EPSP–spike coupling to be temporally precise (Csicsvari et al. 1998; Fricker & Miles, 2000).
In neocortical and hippocampal pyramidal cells, small EPSPs initiate voltage-dependent currents which are largely inward near threshold (Stuart & Sakmann, 1995; Fricker & Miles, 2000). These inward currents tend to prolong synaptic depolarizations so that action potentials may be initiated at long, variable latencies. On the other hand, noisy stimuli in vitro (Mainen & Sejnowski, 1995) or natural stimuli in vivo (Azouz & Gray, 2000) can induce temporally precise firing. These studies showed that action potentials were most precisely generated by depolarizing transients emerging from a hyperpolarized level, and suggested that the dynamic properties of voltage-dependent currents near threshold were responsible. Such precise coupling is important for theories on how pyramidal cells code information and generate synchrony (König et al. 1996) as well as on how synaptic plasticity depends on spike timing (Abbott & Nelson, 2000).
In this study, we attempt to reconcile the divergence in findings on the temporal precision of pyramidal cell firing by examining intrinsic currents activated by EPSP-like waveforms in CA1 pyramidal cells in vitro. First, we asked how the activation of intrinsic currents was related to temporal variability of spike firing in response to these waveforms. Then we compared the timing of action potentials and intrinsic currents elicited by noisy stimuli of variable frequency composition and amplitude. Low amplitude stimuli were found to induce largely inward currents in CA1 pyramidal cells and generate temporally imprecise firing. In contrast when stimulus amplitude was increased, intrinsic K+ currents were activated and the temporal precision of action potential generation was enhanced.
Methods
Slices
Experiments were performed according to local regulations, on hippocampal slices obtained from 9- to 14-day-old Sprague-Dawley rats. Animals were anaesthetized by intraperitoneal injection of a mixture of ketamine (200mgkg−1) and xylazine (800mgkg−1). After decapitation, the hippocampal formation was removed from the brain and cut into slices of 250μm thickness using a tissue slicer (Dosaka, Kyoto, Japan). Slices were maintained at room temperature in physiological saline bubbled with a mixture of 95% O2 and 5% CO2, before transfer into a heated chamber (32°C) for recording.
Solutions
Slices were prepared in and continuously superfused with a solution containing (mm): NaCl 130, KCl 3, CaCl2 2, MgCl2 2, NaH2PO4 1.3, NaHCO3 26, glucose 27.7, and saturated with 95% O2 and 5% CO2. Drugs were applied by bath application. Pipettes were filled with solutions containing (mm): potassium methylsulphate (KMeSO4) 150, Hepes 10, EGTA 0.1, ATP-Mg 4, GTP-Na 0.2, pH adjusted to 7.3 with KOH, osmolarity 290 mosmol l−1. Potassium methylsulphate was preferred to potassium gluconate to avoid the suppression of some K+ conductances, which is reported with gluconate as the major intracellular anion (Velumian et al. 1997). Na+ currents were measured in the presence of 500μm 4-aminopyridine (4-AP) in the extracellular solution. The addition of 100μm NiCl2, 1mm tetraethyl ammonium (TEA) and 2mm CsCl had no further effect on inward current amplitudes or kinetics. K+ currents were measured in the presence of 2μm tetrodotoxin (TTX) and 100μm nickel. In a few experiments 20μm 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzol[f]quinoxaline-7-sulphonamide (NBQX) was added to suppress synaptic currents mediated by AMPA receptors, but synaptic transmission was usually left intact. Blocking synaptic excitation and synaptic inhibition increases cellular input resistance, which might affect the integration of currents in different cellular compartments (Destexhe & Pare, 1999). Potassium methylsulphate was obtained from ICN (Orsay, France), NBQX from Tocris Cookson (Fisher Bioblock, Illkirch, France) and all other chemicals were purchased from Sigma (Lyon, France).
Recordings
Patch pipettes were prepared (ESF electrode puller, Göttingen, Germany) from borosilicate glass of external diameter 1.5mm (Harvard Apparatus Ltd., Edenbridge, UK). Their resistance when filled with recording solution varied from 3 to 5MΩ. Recordings were made from pyramidal cells of the CA1 area of the hippocampus. Cells were identified visually using a Nikon microscope equipped with differential interference contrast (DIC) optics and a 40 × objective. Slices were illuminated with light passed through a high pass filter of cut-off 700 nm and images were obtained with a camera sensitive to infrared light (Hamamatsu C3077, Massy, France).
Whole-cell records were made with an Axopatch 200A amplifier operating in the fast current clamp or the voltage clamp mode (Axon Instruments, Union City, CA, USA). Stimulation and data acquisition were controlled by pCLAMP software (Axon Instruments). Signals were filtered at 5 kHz, digitized with a Digidata interface (Axon Instruments) at a sampling interval of 20μs, and stored on a computer. Leakage and capacitive currents were subtracted using a P/6 protocol with six correction pulses of amplitude one-sixth of the test pulse and opposite polarity. We noted no significant differences between a P/6 and a P/10 subtraction protocol. The holding potential was systematically used as the reference potential.
Whole-cell recordings were obtained by advancing a pipette through the slice under positive pressure until it was seen to distort the pyramidal cell body. Negative pressure was applied to form a high resistance seal and whole-cell access was established by applying a brief negative pressure pulse. Recordings were accepted when pyramidal cells had input resistances in the whole-cell configuration of 100–300MΩ and an access resistance of less than 10% of the input resistance. We waited for at least 5 min after whole-cell recording was established, to allow for perfusion of the cell with the pipette solution. Cells were excluded from analysis if either their input or access resistance, measured from responses to small hyperpolarizing pulses, changed by more than 20% during a recording.
Injections of simulated synaptic waveforms
Both single synaptic events and synaptic noise were simulated by somatic injection of waveforms created in the MATLAB programming environment (The Mathworks, Sèvres, France). The form of simulated synaptic potentials or currents follows the expression (1 – e−t/ton)e−t/toff with ton= 0.2ms and toff= 0.8ms. While these somewhat rapid kinetics were chosen to avoid inactivation of intrinsic cellular currents during the rising phase of injected events, we also systematically explored events with slower rising phases (Figs 2 and 5). Gaussian white noise was generated in the MATLAB programming environment at a frequency of 10 kHz and then low-pass filtered at 50 or 100 Hz with an analog filter (Frequency Devices, Haverhill, MA, USA). The frequency and amplitude composition of injected noise waveforms were close to those used by Mainen & Sejnowski (1995) and were chosen to mimic membrane potential fluctuations observed in recordings from the intact animal (Paréet al. 1998; Destexhe & Paré, 1999).
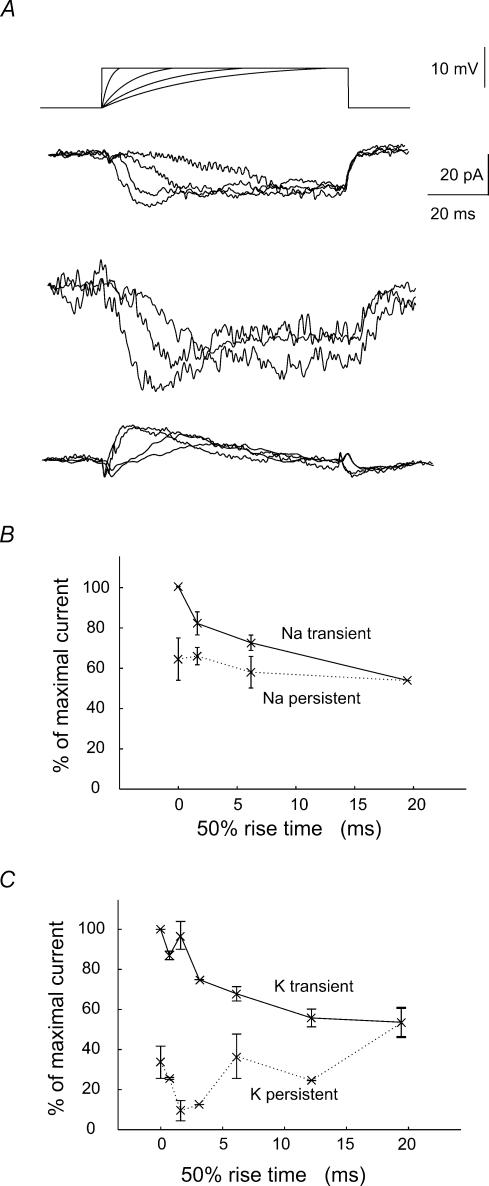
Figure 2. Na+ and K+ currents evoked by waveforms with rising phase of differing kinetics.
Pulse protocol: test waveforms consisted of an exponentially rising component of time constant 2–20ms which was then maintained at a potential depolarized by 10mV. The holding potential was −55mV and total duration of the waveform was 100ms. A shows from above the test waveforms and the net current, the pharmacologically isolated inward and the outward current. The summed inward and outward current was inward and was largely maintained during the pulse. Waveforms with the most rapid rising phase elicited a transient initial inward component. Inward current responses were recorded in the presence of 500μm 4-AP, 5mm TEA, 2mm Cs+ and 100μm Ni2+. Outward currents were recorded in the presence of TTX (1μm) and Ni2+ (100μm). B, increasing the kinetics of the rising phase of the pulse enhanced a transient component of inward currents (n= 4 cells). C, similarly, increasing the speed of the rising phase of the pulse enhanced the transient component of outward currents (n= 4 cells).
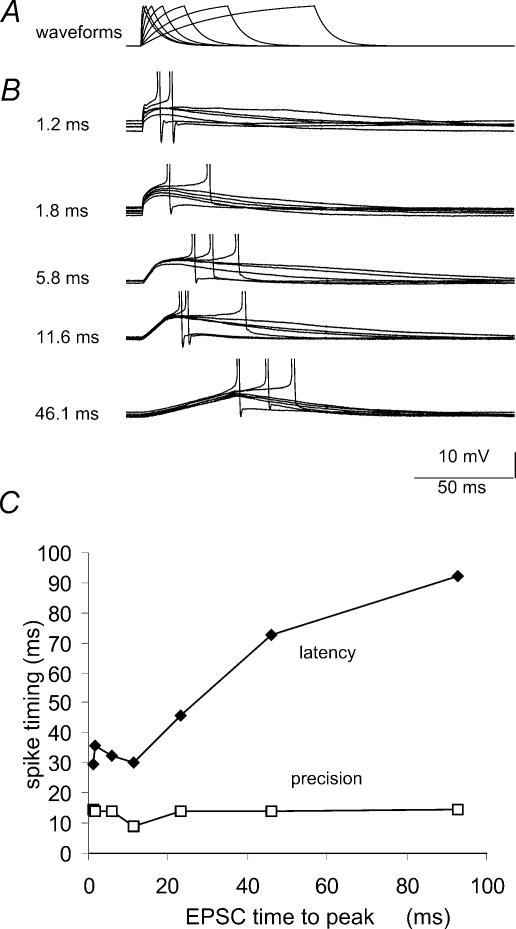
Figure 5. Dependence of the temporal precision of spike generation on the rise time of EPSP-like waveforms.
A, current clamp stimuli followed an equation of the form (1 – e−t/ton)e−t/toff with ton= 12–90ms and toff= 0.8ms. In records from a given cell, the stimulus amplitude was adjusted to result in depolarizations of fixed amplitude close to 10mV. B, action potentials initiated by waveforms with different rise times. The holding potential was maintained at a level near −60mV, where about 50% of the stimuli initiated firing. C plots the mean latency and the standard deviation (‘precision’) of action potential latency against the time to peak of the waveforms that initiated firing (n= 6 cells).
Results
Multiple parameters influence the probability that firing is initiated when a postsynaptic cell recieves an excitatory synaptic event. They include the membrane potential of the cell as well as the amplitude and kinetics of the excitatory event. We examined the effect of these factors on both intrinsic currents of CA1 pyramidal cells and the temporal precision of evoked discharges. First we varied the amplitude and holding potential of stimuli with constant kinetics and then responses to stimuli with different kinetics were compared.
Na+ and K+ current responses to small steps at subthreshold voltages
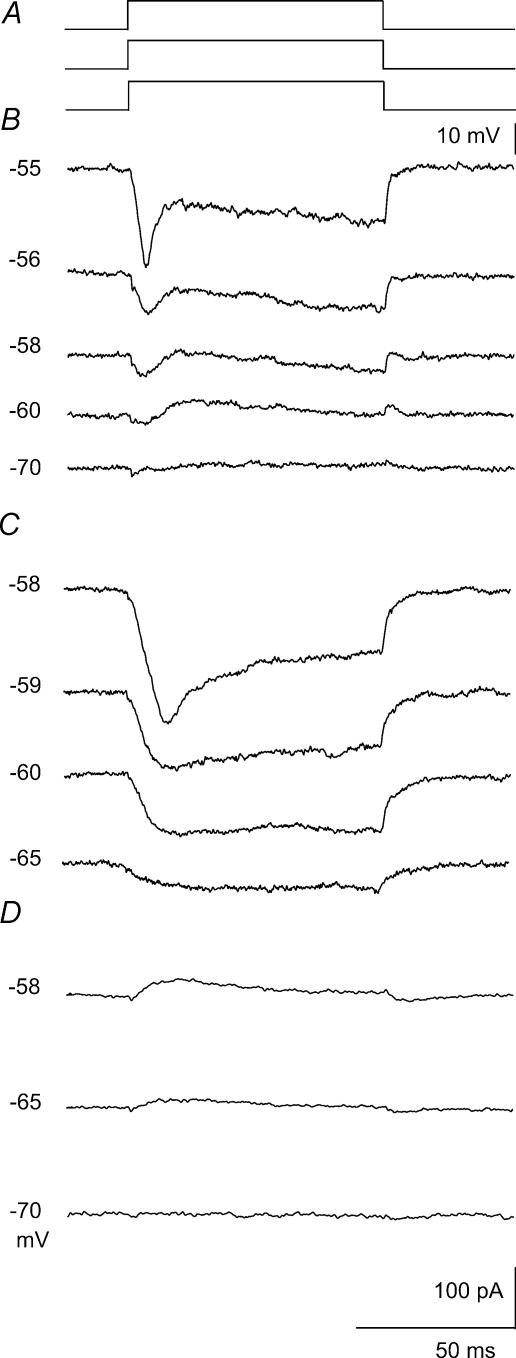
We first examined currents induced by stimuli of different amplitudes at varying holding potentials. Summed currents elicited by steps of 10mV (Fig. 1A) from holding potentials ranging between –80 and −50mV were recorded (Fig. 1B). These experiments revealed a voltage-dependent transition from a net outward current to a net inward current as the holding potential was shifted into the subthreshold range (Fig. 1B). At hyperpolarized potentials, small steps induced net outward currents, while at more depolarized potentials currents were largely inward (n= 5). The responses were dissected into inward (Fig. 1C) and outward components (Fig. 1D) by using either 4-AP (500μm, n= 4) or TTX (1μm, n= 4). The increase in peak amplitude of the Na+ current with depolarization was faster than that of K+ currents. Furthermore a significant maintained inward current (French & Gage, 1985) was evoked by steps from immediately subthreshold potentials (Fig. 1C). Its amplitude was usually larger than that of outward currents (Fig. 1D) resulting in a summed current that was entirely depolarizing at subthreshold potentials.
Figure 1. Voltage dependence of Na+ and K+ currents elicited by small current steps.
A, pulse protocol: holding potential varied over the range −70 to −55mV, just below action current threshold with a standard test pulse of +10mV and duration 100ms. B, net currents elicited at different holding potentials. Note the voltage dependent switch between largely outward currents at −70 and −60mV and predominantly inward currents in a narrow range of more depolarized subthreshold potentials. C, inward currents isolated in an external solution containing 4-AP (500μm), TEA (5mm), Cs+ (2mm) and Ni2+ (100μm). D, outward currents recorded in the presence of 1μm TTX and 100μm Ni2+ (different cell). The number of cells tested was 5 for net currents, 4 for inward currents and 4 for outward currents.
Only minor inactivation of Na+ and K+ currents during slow ramps
EPSPs vary in rise time as well as in amplitude. Rapidly rising synaptic depolarizations are suggested to initiate firing with high precision (Mainen & Sejnowski, 1995; Azouz & Gray, 2000). More slowly rising synaptic events may inactivate inward or outward currents (Martina & Jonas, 1997; Martina et al. 1998; Fricker et al. 1999) and so increase firing threshold and reduce the precision of EPSP to spike coupling. To investigate how EPSP rise time affects the activation of inward and outward currents governing the precision of firing, we examined currents induced by injecting pulses of rise times, measured at half height, in the range of 0.7–19.3ms (Fig. 2). The amplitude of pulses was 10mV and after reaching a peak potential was maintained to avoid the effects of incomplete activation. Summed current responses to these pulses injected from a holding potential of −55mV were inward throughout their time course. The contributions of inward (n= 4) and outward currents (n= 4) to summed responses were dissected in experiments with appropriate antagonists (4-AP, TEA, Cs+ and Ni2+ for Na+ currents and TTX and Ni2+ for K+ currents). The pulses with the fastest rise times initiated a rapid component of the Na+ current (Fig. 2B). For both Na+ and K+ currents, the peak current initiated by the most slowly rising ramp was about 60% of that initated by a square pulse (Fig. 2B and C). These data suggest that inactivation has relatively little effect on inward or outward currents during times corresponding to the kinetics of fast EPSPs.
Na+ and K+ currents elicited by synaptic waveforms
These data show that depolarizing pulses of amplitudes up to 5–6mV, injected near threshold, always activate inward currents, and that outward currents are only evident when stimuli of larger amplitude (∼10mV) are injected from more hyperpolarized holding potentials. We asked whether similar behaviour was evident in responses to simulated EPSP waveforms (Fig. 3).
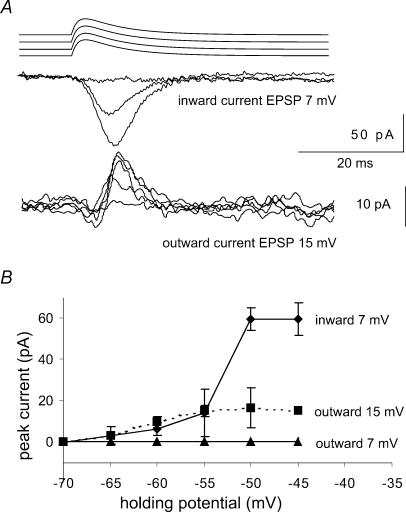
Figure 3. Na+ and K+ currents elicited by EPSP waveforms.
Pulse protocol: holding potential varied over the range −70 to −45mV, just below action current threshold. The test pulse was an EPSP waveform of the form (1 − e−t/ton)e−t/toff with ton= 0.2ms and toff= 0.8ms and of variable peak amplitude. Inward currents were isolated in the presence of 500μm 4-AP and outward currents were examined in the presence of 1μm TTX and 100μm Ni2+. EPSP waveforms of amplitude 7mV elicited purely inward currents while larger amplitude stimuli, here 15mV, elicited both inward and outward currents. B, plot of the mean and standard deviation of inward and outward currents for EPSP waveforms of amplitudes 7 and 15mV (inward currents, n= 3 cells; outward currents n= 7 cells).
EPSP-like waveforms of amplitude less than 8–10mV, activated currents that were exclusively inward over the range of holding potentials between −60 and −45mV, but did not elicit measurable K+ currents (Fig. 3). Larger voltage clamp waveforms activated sequences of inward followed by outward currents. Outward currents activated by larger EPSP-like waveforms increased as the holding potential was depolarized to −55mV. The amplitude of inward currents increased with depolarization until the action current threshold was reached (Fig. 3B; n= 4 for inward currents, n= 7 for outward currents). These data show that outward currents are activated only by larger EPSP waveforms, perhaps due to the steady state inactivation of K+ currents.
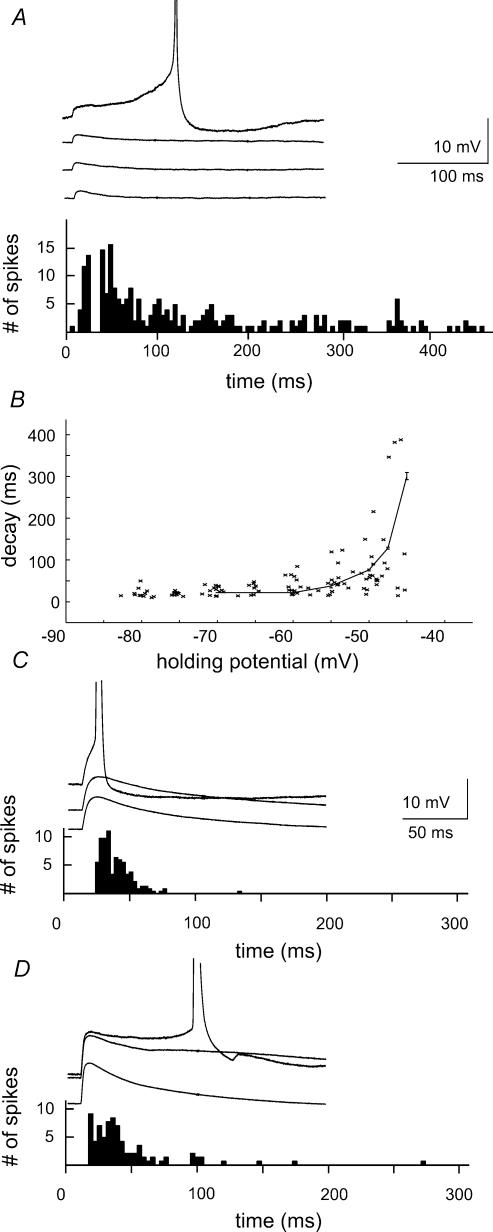
EPSPs are prolonged when outward currents are not activated
Current clamp records were used to explore the consequences for spike timing of this difference in synaptic activation of intrinsic currents. Synaptic waveforms of different amplitude were injected at different potentials. As shown in Fig. 4A, events induced by small amplitude currents were prolonged at subthreshold potentials (n= 9). Action potentials could be initiated from the rising phase of these events, but were also triggered at longer, variable latencies from a depolarizing plateau. The mean action potential latency measured from the onset of the stimulus was 125.4 ± 124.8ms. Figure 4B shows the voltage dependence of the decay of these responses, measured as the time from 90% to 10% of the peak. The latency distribution follows a continuous distribution with no evidence for two separate peaks. It resembles the voltage dependence of Na+ current activation, suggesting that the prolongation of synaptic waveforms may depend on activation of Na+ channels (Stuart & Sakmann, 1995; Fricker & Miles, 2000).
Figure 4. Voltage dependent amplification and firing induced by the injection of EPSP waveforms.
A, the time course of potentials induced by the injection of small EPSP waveforms (amplitude 3–5mV, form (1 – e−t/ton)e−t/toff with ton= 0.2ms and toff= 0.8ms) was prolonged on membrane depolarization. Action potential initiation was examined at a holding potential where approximately 50% of trials elicited a spike. Action potentials were initiated with a latency of 125 ± 124ms (mean ±s.d.). B, the voltage dependence of the decay time constant of potentials initiated by injection of EPSP waveforms. C, the time course of potentials induced by larger EPSP waveforms (amplitude 12–16mV) changed little with holding potential. Action potentials were initiated, at a holding potential where about 50% of trials elicited a spike, with latencies in the range 11–48ms (90% of spikes). D, in the same recording, EPSPs were prolonged in the presence of 500μm 4-AP. Ninety per cent of spikes were initiated with latencies in the range of 7–96ms.
Our voltage clamp data suggest that larger EPSP waveforms should activate both inward and outward currents. We tested this prediction by injecting current waveforms that induced voltage deflections of amplitude 10–15mV. The decay of these events was not prolonged at depolarized potentials (Fig. 4C, n= 7). Action potentials were consistently initiated on the rising phase or the peak of the depolarization with a mean latency of 25.6 ± 24.5ms, longer than that for the small EPSPs (P < 0.001, Mann-Whitney U test). The voltage clamp data suggest that outward currents act to curtail voltage-dependent prolongation of larger amplitude stimuli. Blocking K+ currents might then permit the emergence of plateau potentials and induce delayed firing. As shown in Fig. 4D, depolarizations resulting from synaptic waveforms were indeed prolonged by application of the antagonist 4-aminopyridine (4-AP, 500μm, n= 4) and the temporal precision of spike initiation was reduced. After 4-AP application the mean latency of action potentials increased to 38.5 ± 46.9ms (P < 0.05, Mann-Whitney U test). These results suggest that small stimuli activate purely inward currents in CA1 pyramidal cells. In contrast larger events, initiated from more hyperpolarized potentials, also activate outward currents which prevent voltage dependent EPSP amplification and do not permit late firing.
Dependence of spiking precision on EPSC rise time
Our voltage clamp data show that the activation of subthreshold inward and outward currents is relatively independent of stimulus rise time (Fig. 2). We explored the effect of EPSC rise time on spike initiation in current clamp experiments by examining responses to injections of synaptic waveforms with rise times between 1.2 and 90ms (Fig. 5A). The larger charge transfer associated with waveforms of the same peak current but slower kinetics resulted in significantly larger depolarizations and a more effective activation of voltage-dependent conductances. We therefore adjusted intensity so that currents with different rise times elicited voltage responses of similar amplitude from a holding potential of −60mV (Fig. 5B). The threshold for action potential discharge was independent of the EPSC rise time (correlation coefficient r2= 0.21). As expected, slower waveforms initiated action potentials at longer latencies (Fig. 5B and C). However, the precision of firing, measured as the standard deviation of action potential latency, did not depend on the rise time of the stimulus (Fig. 5C). Note that in Fig. 4 not only the mean latency of discharges, but also their variability depended on the amplitude of the injected synaptic waveform, resulting in a broader distribution of latencies for small synaptic waveforms. The data presented in Fig. 5 suggest that the rise time of synaptic waveforms has little influence on the temporal precision for action potential generation in CA1 pyramidal cells.
White noise with higher variance induces more precise spiking than noise with lower variance
These data establish relations between active currents initiated by isolated synaptic waveforms and the timing of action potential discharge. We next asked whether similar relations exist during more complex patterns of synaptic inputs (Destexhe & Pare, 1999). Temporally complex barrages of EPSCs, were mimicked using white noise stimuli resembling synaptic events (Mainen & Sejnowski, 1995). Noise stimuli were filtered with a low-pass cut-off at 50 or 100 Hz and noise waveforms (Fig. 6A) were scaled to provide stimuli of low, medium and high variance, with standard deviations in the range 5–20 pA.
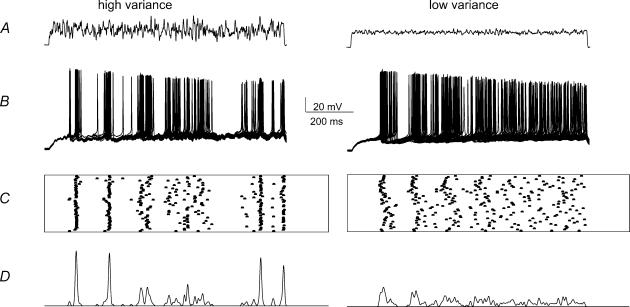
Figure 6. Spike timing in response to noise stimuli of different variance.
A, the noise stimuli of high and low variance. B, action potentials initiated by 50 trials using these waveforms. C, spike timing from successive trials is indicated in raster plots. D, plots of firing probability obtained by convolving the times of spike generation with a gaussian function with standard deviation equal to the duration of the trace (1 s) divided by the mean number of spikes. This procedure normalized for variations in the number of spikes.
High variance noise initiated action potentials more precisely than lower variance waveforms (n= 14 cells). Figure 6B shows superimposed spike trains initiated by sequential presentations of the same noise stimulus with high and low variance. The timing of action potentials initiated over 50 noise presentations is plotted in Fig. 6C, and these timing data are convolved with a gaussian waveform in Fig. 6D to show how the noise variance affected spike timing. The peaks in this representation of action potential timing reflect both the reliability and the temporal precision of spike initiation. As Fig. 6D shows, the high variance noise stimulus invariably resulted in larger peaks with less temporal dispersion than the low variance waveform.
Differential activation of inward and outward currents by noise with low and high variance
We attempted to dissect inward and outward currents initiated by noise waveforms in order to determine their influence on the timing of spike initiation. We examined voltage clamp responses to the same waveforms, rather than their time derivative, so that we could compare precisely currents in voltage clamp records with action potentials generated in current-clamp experiments. Figure 7 shows membrane current responses to voltage noise stimuli. We examined responses to noise waveforms of different levels of variance (with standard deviations 1.8, 3.7 and 6.9mV) at progressively more depolarized holding potentials (n= 9). We varied holding potentials over a range between hyperpolarized values at which no currents were induced, and potentials that were 1–2mV below action current threshold. We note that potentials corresponding to features in current responses (as in Fig. 7) were always comparable, since larger peak voltage stimuli were obtained in the high variance than in the medium or low variance waveforms.
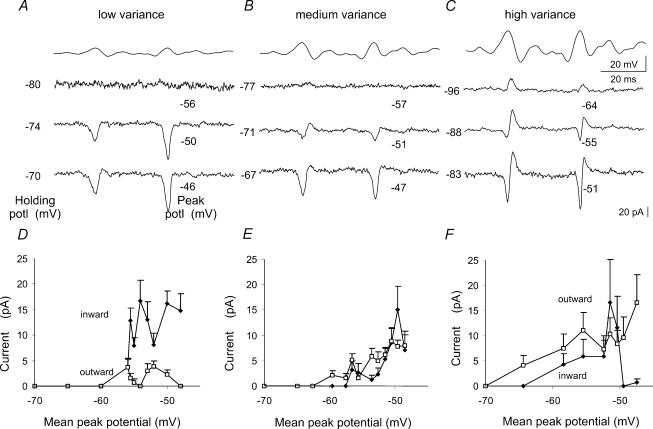
Figure 7. Voltage dependence of currents elicited by noise of different variances.
Pulse protocol: noise command pulses of low, medium and high variance (standard deviations of 1.8, 3.7 and 6.9mV). Holding potentials were varied between −95 and −65mV and steps of 17 and 9mV were used for the low and medium variance waveform, respectively. Extracts of responses at a range of holding potentials, indicated to the left of the trace, are shown with the peak voltage reached during the trace indicated at the right. A, the low variance noise waveform elicited purely inward currents at all holding potentials. B, noise of medium variance induced inward currents which were sometimes succeeded by small outward currents. C, as the holding potential was depolarized, high variance noise commands induced first outward currents and then sequences of inward followed by outward currents. D to F, the mean amplitude of inward and outward currents measured from 15 points corresponding to the largest depolarizing transients during each noise trace plotted against the mean peak potential for noise of low (D), medium (E) and high (F) variance. Inward currents dominate in responses to the low variance waveform, while outward currents become larger than inward currents in responses to the high variance waveform.
At hyperpolarized holding potentials, low variance noise (Fig. 7A) evoked no currents. As the holding potential was depolarized, evoked currents were largely inward with small outward components. Similarly, noise stimuli of medium variance evoked no response at hyperpolarized potentials. As membrane holding potential was depolarized, sequences of inward followed by outward currents emerged, but with further depolarization current responses were largely inward (Fig. 7B). In contrast, when the holding potential was depolarized to levels at which currents were elicited, large variance noise stimuli first elicited purely outward currents (Fig. 7C). As the holding potential was further depolarized, mixed inward and outward currents were elicited. While inward currents predominated in responses to low variance waveforms, larger outward currents than inward currents were elicited by the high variance noise. These results are comparable to those obtained with isolated EPSP waveforms (Fig. 3). They show that while small synaptic events may elicit purely inward postsynaptic currents, larger amplitude stimuli initiate sequences of inward currents followed and curtailed by outward currents.
Outward currents contribute to precise spiking
Finally we compared the timing and properties of intrinsic currents activated by noise stimuli to the initiation and temporal precision of action potentials evoked by the same stimuli (Fig. 8A–C). Current and voltage clamp responses to the low, medium and high variance versions of the same waveforms were compared in nine cells. Action potentials were typically initiated at time points corresponding to inward current transients in companion voltage clamp responses. Low variance noise waveforms induced largely inward currents and the timing of action potential initiation was rather dispersed (Fig. 6). In contrast, high variance waveforms resulted in sequences of inward followed by outward currents. In current clamp experiments with the same waveforms, action potentials were generated with a reduced temporal dispersion. Figure 8D plots the temporal variability in action potential generation calculated as the standard deviation of spike occurrence at all points corresponding to the occurrence of inward currents in voltage clamp experiments with a given noise waveform. The mean amplitude of all inward and outward currents generated by low, medium and high variance waveforms (up to 15 currents per traces, n= 9 cells) at the most depolarized holding potential just below threshold is shown in Fig. 8E. Outward currents increased with the variance of the noise waveform, while the peak amplitude of inward currents was reduced in parallel as the waveform variance increased. This comparison of action potential timing and membrane currents suggests that outward currents which succeeded inward currents, prevented the delayed initiation of action potentials and so enhanced the precision of action potential generation.
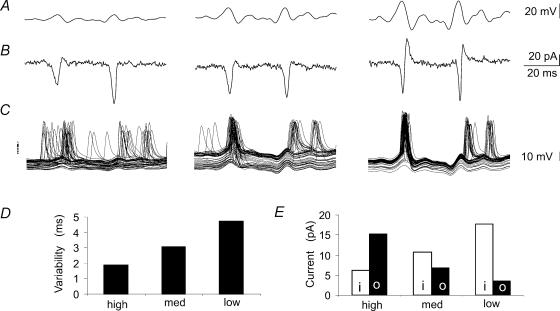
Figure 8. Membrane currents and action potentials initiated by noise stimuli.
A, portions of noise waveforms with small, medium and high variance. B, resulting cellular currents. C, the timing of action potentials induced by injecting the same waveforms in current clamp experiments. The apparent action potential ‘doublets’ result from superpositions of differently timed single action potentials in distinct sweeps. D, the variability in action potential timing calculated as the mean of the standard deviation in action potential timing near points at which currents were observed in voltage clamp. Data obtained at 9–14 time points for 50 stimulus trials in records from 9 cells. E, changes in the balance of inward and outward currents, measured from voltage clamp experiments as in B, corresponding to noise stimuli of low, medium and high variance. Inward, i, and outward, o, currents were measured as shown in Fig. 7 from the most depolarized holding potential. Peak inward and outward currents were measured from the same time windows (n= 14) used to calculate variability in spike timing (n= 9 cells). Outward currents increased with the variance of the noise waveform, while the apparent amplitude of inward currents was reduced as the temporally overlapping outward currents increased in amplitude.
Discussion
We have compared the timing of action potentials generated by CA1 pyramidal cells in response to the somatic injection of EPSP waveforms and noise stimuli. Small synaptic waveforms or noise with low variance generated largely inward cellular currents and initiated firing with poor temporal precision. Larger synaptic waveforms or noise of high variance activated outward currents and action potentials were initiated with a reduced temporal variability. We note that the amplitude of EPSP waveforms that generate sequences of inward followed by outward currents is rather larger than of a unitary synaptic event (Bolshakov & Siegelbaum, 1995; Isaac et al. 1996). Our data suggest that CA1 pyramidal cells will not discharge with high temporal precision in response to the firing of a single presynaptic cell. Instead precise firing seems likely to be induced only by synaptic depolarizations of amplitude greater than 8–10mV. Such depolarizations must correspond to the nearly synchronous firing of multiple presynatic cells (Paréet al. 1998; Destexhe & Paré, 1999).
We injected current pulses and synaptic waveforms via a somatic electrode and recorded responses with the same electrode. EPSPs impinge on dendritic membrane of CA1 pyramidal cells and action potentials are probably generated at axonal sites distant from the soma (Colbert & Johnston, 1996; Colbert & Pan, 2002). Our studies probably therefore underestimated contributions of conductances activated by EPSPs at dendritic sites and errors may have been introduced in measurements of the timing of action potential discharges at their initiation site. Whole cell studies on cells with an extended morphology probably suffer from an imperfect space clamp. However, since our study was limited to the effects of somatically generated waveforms on action potential generation, these errors were probably minimized. Both the density and biophysical properties of currents expressed by hippocampal neurones change during development (Spigelman et al. 1992; Falk et al. 2003). Our work was done on tissue from young animals, so results are strictly applicable to this age range. However, the relations between the properties of Na+ and K+ currents and timing of action potential generation probably apply more generally.
A voltage-dependent switch in net currents near firing threshold
Our data show that a voltage-dependent switch occurs in the net current elicited by small pulses as membrane potential is depolarized towards firing threshold (Fig. 1). Inward currents always preceded outward currents. However, while net currents were largely outward at hyperpolarized potentials, summed currents were completely inward near threshold potentials. This behaviour seems to result from the combination of an increased maintained component of Na+ current together with a voltage-dependent increase in the steady state inactivation of K+ currents.
The voltage dependence of intrinsic currents induced by EPSP-shaped waveforms (Fig. 3) was similar to that of step commands, and may explain the firing precision observed with simulated synaptic currents of different kinetics (Fig. 4). Small stimuli elicited largely inward currents, which were blocked by tetrodotoxin and so were carried by voltage-dependent Na+ channels. These Na+ currents showed relatively little inactivation (Fig. 2). Similar behaviour in this potential range in a number of cell types has been attributed to a persistent Na+ current (French et al. 1990; Fleidervish & Gutnick, 1996; Magistretti & Alonso, 1999), although recent work (Taddese & Bean, 2002) suggests that it may not be necessary to invoke a separate current. Na+ channel availability is significantly reduced at near threshold potentials (Fricker et al. 1999; Taddese & Bean, 2002). In conditions of low availability, the stochastic behaviour of single channels becomes important (Schneidman et al. 1998) and this seems likely to be one factor underlying the variability in timing of action potentials (Fig. 4A).
Larger stimuli delivered from more hyperpolarized potentials activated in addition an outward current which was suppressed by 4-aminopyridine and so corresponds to inactivating voltage-dependent potassium channel. A role for transient K+ currents in accelerating EPSP decay was first noted at synapses made with sympathetic ganglion cells (Cassell & McLachlan, 1986). These K+ currents may ensure that only a short time window is available for action potential discharge (Brew & Forsythe, 1995; Fricker & Miles, 2000; Ramakers & Storm, 2002). At other synapses, however, inactivating K+ currents may have opposite effects so that EPSPs initiate firing at long, and variable, latencies. For instance, dendro-dendritic EPSPs impinging on mitral cells of the olfactory bulb activate a transient potassium current with similar kinetics to the AMPA component of the EPSP. Mitral cell discharge is thus initiated at long-latency by the NMDA component of the synaptic event (Schoppa & Westbrook, 1999). For inactivating K+ currents to ensure delayed firing, depolarizing influences, such as a long-lasting NMDA receptor mediated synaptic excitation or a substantial Na+ current must be maintained. Delayed firing cannot occur, if the excitation decays more quickly than the outward current inactivates, as in the present experiments.
EPSC rise time and the precision of CA1 pyramidal cell firing
In neocortical pyramidal cells, the rate of membrane depolarization affects the probability of action potential generation (Nowak et al. 1997) and, by some measures, their threshold (Azouz & Gray, 2000). This effect is suggested to depend on the rate of transition of Na+ channels into their inactivated state. Slower synaptic events of rise times similar to the kinetics of Na+ channel inactivation might then initiate action potentials at more depolarized potentials, and with longer, more variable latencies (Harsch & Robinson, 2000). However, the time constants of inactivation for Na+ currents are close to 20ms at subthreshold potentials (authors' unpublished observations; Martina & Jonas, 1997; Fricker et al. 1999). Furthermore inward currents induced by step depolarizations to subthreshold potentials had substantial persistent components (Fig. 1). AMPA receptor-mediated EPSPs recorded from CA1 pyramidal cells have a time to peak of 4–8ms and so Na+ channel inactivation should be far from complete. However, an alternative explanation for the enhanced efficacy with which rapid depolarizations initiate firing may lie in our observation (Fig. 2) that waveforms with rapid kinetics initiate a transient inward current which is absent from responses to more slowly rising EPSP waveforms. Our data suggest that depolarizations with rise times slower than about 10ms induce largely maintained inward currents (cf. Fleidervish & Gutnick, 1996; Magistretti & Alonso, 1999).
EPSPs with slower rise times often have a slow decay. In our experiments we used ramps rather than EPSP-like waveforms to explore the influence of EPSP kinetics in an attempt to avoid effects due to deactivation and incomplete activation. The data shown in Fig. 2A suggest that fast EPSPs only partially activate Na+ and K+ currents.
Comparison of responses to noisy stimuli and to single synaptic waveforms
Our comparison of the variability in spike timing with intrinsic currents elicited in response to the same waveforms seems to resolve a paradox. Noisy current stimuli injected into layer V cortical pyramidal cells evoke precise spike timing (Mainen & Sejnowski, 1995). In contrast, EPSP waveforms injected into the same cells, activate intrinsic Na+ currents, which prolong synaptic events and initiate action potentials at long, variable latencies (Stuart & Sakmann, 1995). In CA1 pyramidal cells, our data suggest that action potentials may be initiated either precisely or with poor temporal precision according to the amplitude of the stimulus. Small stimuli activate currents that are largely inward and induce firing with variable timing. In contrast, larger stimuli induce firing more precisely since they evoke biphasic sequences of inward–outward currents.
Our comparison of the relation between the mode of cellular firing and intrinsic currents generated by noise stimuli of different variances complements previous data suggesting that action potentials are most precisely initiated by rapid depolarizations from hyperpolarized potentials (Mainen & Sejnowski, 1995; Azouz & Gray, 2000). Our voltage clamp data suggest that rapidly rising waveforms are more likely to initiate a transient component of inward current. Furthermore, in our current clamp experiments increasing the variance of the test waveform tended to augment hyperpolarizing as well as depolarizing transients. Hyperpolarizing transients should tend to remove K+ current inactivation and thus enhance the probability that a succeeding depolarizing current transient would initiate a biphasic inward–outward sequence of intrinsic currents.
In vivo, action potentials are induced by membrane fluctuations resulting from the summation of multiple synaptic events. We attempted to analyse this process using first simple stimuli, then waveforms similar to synaptic events of variable kinetics and amplitude and finally with noise stimuli. Summed synaptic events in vivo may be characterized according to amplitude, frequency content and membrane potential. Our use of white noise stimuli let us avoid assumptions on the exact nature of these fluctuations Identical noise waveforms were injected in current and voltage clamp experiments, so that the precision of spike generation could be compared directly to cellular currents elicited by the same waveform. It might be argued that a better comparison would be made by injecting in current clamp the differential of a given waveform. However, in our experiments, values for the membrane time constant were significantly shorter than the frequency of our injected noise signal chosen to fit the frequency range of natural synaptic events (Mainen & Sejnowski, 1995). We found that the potential fluctuations resulting from injections of current noise at hyperpolarized potentials where intrinsic currents were little activated were better correlated with the injected waveform than with its integral (data not shown). In other conditions, with injected noise signals of higher frequency components, or with neurones of long time constant, injection of the integral of the current clamp waveform as the test stimulus in voltage clamp might provide a better comparison.
Functional significance of the temporal precision in EPSP–spike coupling
Our results suggest that CA1 pyramidal cells discharge precisely when large afferent stimuli arrive at hyperpolarized potentials. It seems that excitatory synaptic inputs which induce precise firing must consist of multiple, synchronous EPSPs (Stevens & Zador, 1998; Harsch & Robinson, 2000). As shown by Pouille & Scanziani (2001), the sequential occurrence of an EPSP followed at short latency by a feedforward inhibition is another way by which CA1 pyramidal cell firing can be restricted to a short time window. However, small EPSPs arriving in isolation at depolarized potentials should induce firing at variable and often long latencies. Such long latency firing seems likely to underly the maintenance of reverberating neuronal population activities (Burns, 1954), which may be important in several types of neuronal operations (Aksay et al. 2001; Wang, 2002).
Acknowledgments
We would like to thank Alain Destexhe, Andreas Draguhn, Andreas Herz, Desdemona Fricker, Uwe Heinemann and Dietmar Schmitz for helpful advice and comments on the manuscript. INSERM, the NIH (MH054671), the FRM, the FRC, the FFRE, the DFG GK238 and the Ministère de la Recherche provided financial support.
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl.):1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci. 2001;4:184–193. doi: 10.1038/84023. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci U S A. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. J Neurosci. 1995;15:8011–8022. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns BD. The mechanism of after-bursts in cerebral cortex. J Physiol. 1954;127:168–188. doi: 10.1113/jphysiol.1955.sp005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell JF, McLachlan EM. The effect of a transient outward current (IA) on synaptic potentials in sympathetic ganglion cells of the guinea-pig. J Physiol. 1986;374:273–288. doi: 10.1113/jphysiol.1986.sp016079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci. 1996;16:6676–6686. doi: 10.1523/JNEUROSCI.16-21-06676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–538. doi: 10.1038/nn0602-857. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Pare D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol. 1999;81:1531–1547. doi: 10.1152/jn.1999.81.4.1531. [DOI] [PubMed] [Google Scholar]
- Falk T, Kilani RK, Strazdas LA, Borders RS, Steidl JV, Yool AJ, Sherman SJ. Developmental regulation of the A-type potassium-channel current in hippocampal neurons: role of the Kvbeta 1.1 subunit. Neuroscience. 2003;120:387–404. doi: 10.1016/s0306-4522(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Gutnick MJ. Kinetics of slow inactivation of persistent sodium current in layer V neurons of mouse neocortical slices. J Neurophysiol. 1996;76:2125–2130. doi: 10.1152/jn.1996.76.3.2125. [DOI] [PubMed] [Google Scholar]
- French CR, Gage PW. A threshold sodium current in pyramidal cells in rat hippocampus. Neurosci Lett. 1985;56:289–293. doi: 10.1016/0304-3940(85)90257-5. [DOI] [PubMed] [Google Scholar]
- French CR, Sah P, Buckett KJ, Gage PW. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J General Physiol. 1990;95:1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker D, Miles R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron. 2000;28:559–569. doi: 10.1016/s0896-6273(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Fricker D, Verheugen JA, Miles R. Cell-attached measurements of the firing threshold of rat hippocampal neurones. J Physiol. 1999;517:791–804. doi: 10.1111/j.1469-7793.1999.0791s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- Harsch A, Robinson HP. Postsynaptic variability of firing in rat cortical neurons: the roles of input synchronization and synaptic NMDA receptor conductance. J Neurosci. 2000;20:6181–6192. doi: 10.1523/JNEUROSCI.20-16-06181.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Hjelmstad GO, Nicoll RA, Malenka RC. Long-term potentiation at single fiber inputs to hippocampal CA1 pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:8710–8715. doi: 10.1073/pnas.93.16.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- Magistretti J, Alonso A. Biophysical properties and slow voltage-dependent inactivation of a sustained sodium current in entorhinal cortex layer II principal neurons – a whole cell and single channel study. J General Physiol. 1999;114:491–509. doi: 10.1085/jgp.114.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- Martina M, Jonas P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J Physiol. 1997;505:593–603. doi: 10.1111/j.1469-7793.1997.593ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Sanchez-Vives MV, McCormick DA. Influence of low and high frequency inputs on spike timing in visual cortical neurons. Cereb Cortex. 1997;7:487–501. doi: 10.1093/cercor/7.6.487. [DOI] [PubMed] [Google Scholar]
- Paré D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons in vivo. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Ramakers GM, Storm JF. A postsynaptic transient K+ current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 2002;99:10144–10149. doi: 10.1073/pnas.152620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Warland D, de Ruyter van Steveninck R, Bialek W. Spikes: Exploring the Neural Code. Cambridge, MA, USA: MIT Press; 1997. [Google Scholar]
- Schneidman E, Freedman B, Segev I. Ion channel stochasticity may be critical in determining the reliability and precision of spike timing. Neural Comput. 1998;10:1679–1703. doi: 10.1162/089976698300017089. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Nat Neurosci. 1999;2:1106–1113. doi: 10.1038/16033. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Zhang L, Carlen PL. Patch-clamp study of postnatal development of CA1 neurons in rat hippocampal slices: membrane excitability and K+ currents. J Neurophysiol. 1992;68:55–69. doi: 10.1152/jn.1992.68.1.55. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Zador AM. Input synchrony and the irregular firing of cortical neurons. Nat Neurosci. 1998;1:210–217. doi: 10.1038/659. [DOI] [PubMed] [Google Scholar]
- Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Velumian AA, Zhang L, Pennefather P, Carlen PL. Reversible inhibition of IK, IAHP, Ih and ICa currents by internally applied gluconate in rat hippocampal pyramidal neurones. Pflügers Arch. 1997;433:343–350. doi: 10.1007/s004240050286. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Probabilistic decision making by slow reverberation in cortical circuits. Neuron. 2002;36:955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]