Abstract
Inositol-1,4,5-trisphosphate (IP3)-dependent Ca2+ release represents the major Ca2+ mobilizing pathway responsible for diverse functions in non-excitable cells. In the heart, however, its role is largely unknown or controversial. In intact cat atrial myocytes, endothelin (ET-1) increased basal [Ca2+]i levels, enhanced action potential-evoked [Ca2+]i transients, caused [Ca2+]i transients with alternating amplitudes (Ca2+ alternans), and facilitated spontaneous Ca2+ release from the sarcoplasmic reticulum (SR) in the form of Ca2+ sparks and arrhythmogenic Ca2+ waves. These effects were prevented by the IP3 receptor (IP3R) blocker aminoethoxydiphenyl borate (2-APB), suggesting the involvement of IP3-dependent SR Ca2+ release. In saponin-permeabilized myocytes IP3 and the more potent IP3R agonist adenophostin increased basal [Ca2+]i and the frequency of spontaneous Ca2+ sparks. In the presence of tetracaine to eliminate Ca2+ release from ryanodine receptor (RyR) SR Ca2+ release channels, IP3 and adenophostin triggered unique elementary, non-propagating IP3R-dependent Ca2+ release events with amplitudes and kinetics that were distinctly different from classical RyR-dependent Ca2+ sparks. The effects of IP3 and adenophostin were prevented by heparin and 2-APB. The data suggest that IP3-dependent Ca2+ release increases [Ca2+]i in the vicinity of RyRs and thus facilitates Ca2+-induced Ca2+ release during excitation–contraction coupling. It is concluded that in the adult mammalian atrium IP3-dependent Ca2+ release enhances atrial Ca2+ signalling and exerts a positive inotropic effect. In addition, by facilitating Ca2+ release, IP3 may also be an important component in the development of Ca2+-mediated atrial arrhythmias.
During each heart beat an action potential depolarizes the cell membrane of cardiac myocytes to allow Ca2+ entry through voltage-gated Ca2+ channels. This relatively small amount of Ca2+ entry triggers a massive Ca2+-induced Ca2+ release (CICR) from intracellular SR Ca2+ stores by activating Ca2+-sensitive Ca2+ release channels (ryanodine receptors, RyRs) in the SR membrane. CICR represents the key step in excitation–contraction (E-C) coupling which provides the necessary amount of cytoplasmic Ca2+ to activate the contractile proteins resulting in contraction of the heart. Ca2+ release occurs from clusters of RyRs (Blatter et al. 1997) in the form of localized non-propagating elevations of [Ca2+]i, termed Ca2+ sparks. Here we use the term Ca2+ spark to refer strictly to elementary Ca2+ release events from RyRs. Ca2+ sparks are the building blocks of Ca2+ release and the spatio-temporal summation of these elementary Ca2+ release events forms the whole cell [Ca2+]i transient during E-C coupling.
IP3 is an important activator of a specific class of SR Ca2+ release channels, i.e. IP3 receptors (IP3Rs). IP3-dependent Ca2+ release represents the main avenue of intracellular Ca2+ release in electrically non-excitable cells (Berridge, 1997). In contrast, in cardiac tissue the main pathway of Ca2+ release occurs through RyRs, and IP3Rs are expressed at 1–2 orders of magnitude lower density than RyRs (Perez et al. 1997). Although IP3-dependent Ca2+ release in cardiac tissue was demonstrated early on (Hirata et al. 1984; Fabiato, 1986; Nosek et al. 1986), the role of IP3 in E-C coupling and cardiac function in the adult mammalian heart has remained highly controversial (Marks, 2000; Bers, 2001; Blatter et al. 2003). There is evidence that IP3-dependent signalling may be important during development (Rosemblit et al. 1999; Poindexter et al. 2001) and cardiac injury (Mouton et al. 1992; Jacobsen et al. 1996; Woodcock et al. 1997,1998; Harrison et al. 1998; Yamada et al. 2001), or may be relevant to the regulation of specific cellular functions such as propagation of electrical signals in Purkinje fibres, regulation of organellar and nuclear membrane permeability, Ca2+-dependent gene transcription, cardiac hypertrophy signalling and cell growth (e.g. Jaconi et al. 2000; for references see Bers, 2001). Atrial tissue expresses functional IP3-receptors at 6–10 times higher levels than ventricular myocytes and IP3Rs seem to colocalize with RyRs in the subsarcolemmal space (Lipp et al. 2000; Mackenzie et al. 2002). Although it has been proposed that IP3-dependent Ca2+ signalling plays a direct role in atrial E-C coupling under physiological as well as pathological conditions (see, e.g. Woodcock et al. 1998; Mackenzie et al. 2002), the spatio-temporal organization of IP3-dependent Ca2+ release and the specific mechanisms by which IP3 signalling modulates Ca2+ handling in atrial myocytes is not clear.
Methods
Cell isolation
The procedure for cell isolation was approved by the Institutional Animal Care and Use Committee of Loyola University Chicago, Stritch School of Medicine. Adult mongrel cats of either sex (19 animals were used in this study) were anaesthetized with thiopental sodium (30 mg kg−1i.p.). Following thoracotomy hearts were quickly excised, mounted on a Langendorff apparatus, and retrogradely perfused with collagenase-containing solution at 37°C according to the method previously described (Kockskämper & Blatter, 2002; Sheehan & Blatter, 2003). All experiments were carried out at room temperature (22–24°C).
[Ca2+]i measurements
[Ca2+]i was measured in intact and permeabilized atrial myocytes with fluorescence laser scanning confocal microscopy. Intact atrial myocytes were loaded with the Ca2+ indicator fluo-4 by 20min incubation in Tyrode solution containing 20μm fluo-4 acetoxymethyl ester (fluo-4/AM; Molecular Probes, Eugene, OR, USA) at room temperature. Cells were superfused continuously (1 ml min−1) with normal Tyrode solution (composition in mm: NaCl 140; KCl 4; CaCl2 2; MgCl2 1; glucose 10; Hepes 10; pH 7.4 adjusted with NaOH). Fifteen to twenty minutes was allowed for de-esterification of the dye. [Ca2+]i measurements were performed with a laser scanning confocal microscope (Radiance 2000 MP, Bio-Rad, UK) equipped with a × 40 oil-immersion objective lens (N.A. = 1.3). Fluo-4 (and fluo-3 in permeabilized cells; see below) was excited with the 488nm line of an argon ion laser and fluorescence was measured at wavelengths >515nm. Images were acquired in the linescan mode (3 or 6ms per scan; pixel size 0.3μm). Whole-cell [Ca2+]i transients were obtained by averaging the entire cellular fluorescence signal from the line scanned. [Ca2+]i transients are presented as background-subtracted normalized fluorescence (F/F0) where F is the fluorescence intensity and F0 is resting fluorescence recorded under steady-state conditions at the beginning of an experiment. [Ca2+]i transients were evoked by electrical field stimulation (0.5 Hz). Ca2+ sparks were detected and quantified in terms of amplitude, spatial width and frequency using an automated detection algorithm (Cheng et al. 1999). Ca2+ spark frequencies are expressed as number of observed sparks per second and per 100μm of scanned distance in the confocal linescan mode (sparks s−1 (100μm)−1). Atrial myocytes were permeabilized with saponin (Zima et al. 2003). First, the cells were suspended in a solution containing (mm): potassium aspartate 100; KCl 20; EGTA 0.5; MgCl2 0.75; Hepes 10; pH 7.2 (KOH) and placed in the experimental chamber (final volume 50 μl) for 15 min. The cell surface membrane was permeabilized by adding 0.005% (w/v) saponin for 30 s. After 30 s the bath solution was exchanged for a saponin-free internal solution composed of (mm): potassium aspartate 100; KCl 15; KH2PO4 5; MgATP 5; EGTA 0.4; CaCl2 0.12; MgCl2 0.75; phosphocreatine 10; creatine phosphokinase 5 U ml−1; dextran (Mr: 40 000) 8%; Hepes 10; fluo-3 potassium salt 0.04; pH 7.2 (KOH). Free [Ca2+] and [Mg2+] of this solution were 100nm and 1mm, respectively (calculated using WinMAXC 2.05, Stanford University, CA, USA).
Drugs
IP3 and adenophostin were obtained from Calbiochem, and 2-aminoethoxydiphenyl borate (2-APB), ET-1, heparin (Mr 6000) and tetracaine were from Sigma.
Data analysis
Data are presented as the mean ±s.e.m. of n measurements. Statistical comparisons between groups were performed with Student's t test.
Results
Endothelin effects on Ca2+ signals in intact atrial myocytes: involvement of IP3 signalling
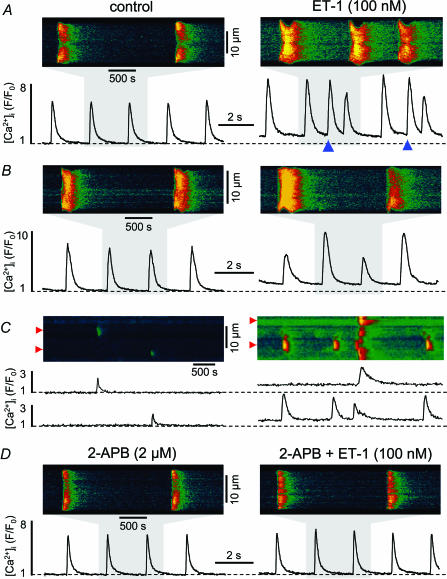
Neurohumoral stimuli (α-adrenergic agents, angiotensin II or endothelin) can cause an increase of [IP3] in atrial cells (Vogelsang et al. 1994). We used endothelin (ET-1), which binds to ET receptors (ETA receptor subtype), to study the effect of IP3-dependent Ca2+ signalling during E-C coupling in intact adult mammalian atrial myocytes. In electrically stimulated cells ET-1 (100nm; 10min exposure time) caused an increase in diastolic [Ca2+]i by 61 ± 11% (P < 0.05; observed in 93% of the cells tested; n= 14 cells). The amplitude (Fig. 1A) of electrically evoked action potential-dependent [Ca2+]i transients increased by 27 ± 8% (P < 0.05; 71% of the cells). This positive inotropic effect was typically seen after about 4min of exposure to ET-1, and the maximum effect of ET-1 on Ca2+ signalling was observed after 6–8 min. Furthermore, ET-1 caused spontaneous [Ca2+]i transients and Ca2+ waves (Fig. 1A and C; observed in 43% of the cells tested). A majority of cells developed Ca2+ alternans (Figs 1B; 64% of the cells). Ca2+ alternans occurred with a delay of 1–2min after the first signs of an ET-1-induced positive inotropic effect and was stable until the end of exposure to ET-1. In unstimulated cells ET-1 increased the frequency of spontaneous RyR-dependent Ca2+ sparks (Fig. 1C) from 0.96 ± 0.16 to 3.5 ± 1.1 sparks s−1 (100μm)−1 (P < 0.05; n= 6 cells). This effect of ET-1 was seen within the first minute of application of the agonist and was fully established after 6min of exposure to ET-1. The membrane permeant IP3R antagonist 2-APB (2–5μm) prevented or abolished all ET-1 effects on [Ca2+]i in atrial cells (Fig. 1D), suggesting that ET-1 effects were mediated by IP3, presumably through IP3-dependent Ca2+ release.
Figure 1. Effects of endothelin-1 (ET-1) on Ca2+ signalling in intact cat atrial myocytes.
Confocal linescan images and spatially averaged [Ca2+]i transients recorded from field-stimulated (0.5 Hz) atrial myocytes. A, ET-1 (100nm) increased diastolic [Ca2+]i and amplitude of electrically evoked [Ca2+]i transients. Spontaneous [Ca2+]i transients and Ca2+ waves (marked by the blue triangles in the right panel) occurred between triggered transients in the presence of ET-1. The traces represent [Ca2+]i expressed as normalized changes of fluo-4 fluorescence (F/F0). B, ET-1 elicited Ca2+ alternans. C, confocal linescan images of spontaneous Ca2+ sparks (top) and selected subcellular [Ca2+]i traces (F/F0, averaged over a distance of 1μm marked by the red triangles to the left of the images) under control conditions (left) and after application of ET-1 (right). ET-1 caused a significant increase in Ca2+ spark frequency and resting [Ca2+]i. D, the IP3R inhibitor 2-APB (2μm) prevented the effects of ET-1 on [Ca2+]i.
IP3-dependent Ca2+ signals in permeabilized cardiac myocytes
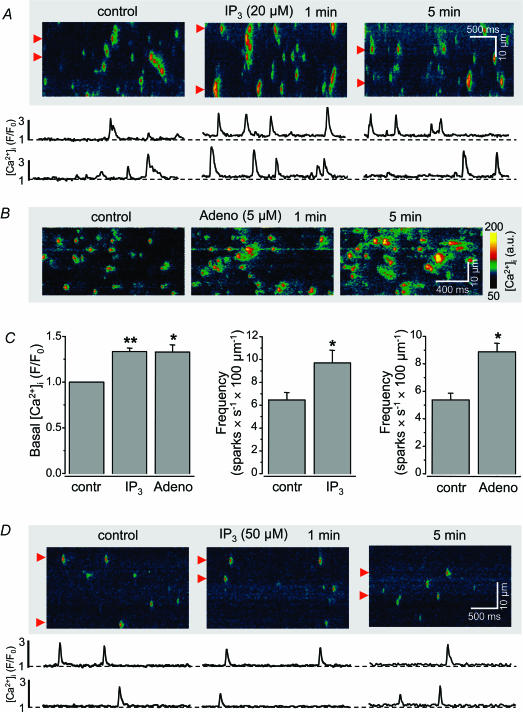
We tested the effect of the physiological agonist IP3 directly in saponin-permeabilized myocytes (Fig. 2A). IP3 (20μm) increased (Fig. 2C) basal [Ca2+]i by 34 ± 4% (P < 0.001; n= 12) and Ca2+ spark frequency from 6.4 ± 0.7 to 9.7 ± 1.1 sparks s−1 (100μm)−1 (P < 0.01; n= 12). However, IP3 did not alter Ca2+ spark amplitude or duration. The latter suggests that IP3 enhanced the probability of an SR Ca2+ release unit (cluster of RyRs) to liberate Ca2+ but did not change properties of the RyR cluster. Similar to IP3, adenophostin (5μm), a more potent IP3 agonist with two orders of magnitude higher affinity not subject to cellular enzymatic degradation, also increased (Fig. 2B and C) basal [Ca2+]i by 33 ± 7% (P < 0.01; n= 6) and raised the Ca2+ spark frequency from 5.4 ± 0.5 to 8.9 ± 0.6 sparks s−1 (100μm)−1 (P < 0.01; n= 6). The effects on basal [Ca2+]i and Ca2+ sparks were seen rapidly (<1 min) after exposure to the agonists, but required approximately 5min to develop fully. In contrast to atrial myocytes IP3 failed to change the frequency and properties of Ca2+ sparks in cat ventricular cells (n= 6 cells) indicating that the IP3 effect on Ca2+ signalling was specific to atrial myocytes (Fig. 2D).
Figure 2. Effects of IP3 and adenophostin on [Ca2+]i in saponin-permeabilized atrial and ventricular myocytes.
A, top, confocal linescan images (fluo-4 fluorescence images; a.u., arbitrary fluorescence intensity units) under control conditions and 1 and 5min after exposure to 20μm IP3. Bottom, local subcellular changes of [Ca2+]i (F/F0 averaged over 1μm indicated by the red triangles to the left of the images). B, effect of adenophostin (5μm; same experimental conditions as in A. C, average data of the effects of IP3 and adenophostin on basal [Ca2+]i and Ca2+ spark frequency. Statistically different at P < 0.01 (*) and P < 0.001 (**). A–C reflect data obtained from atrial myocytes. D, IP3 had no effect on basal [Ca2+]i and frequency and properties of Ca2+ sparks in permeabilized cat ventricular myocytes, suggesting that the IP3 effects were specific to atrial myocytes. [Ca2+]i was measured with fluo-3 pentapotassium salt (fluo-3 bath concentration was 40μm).
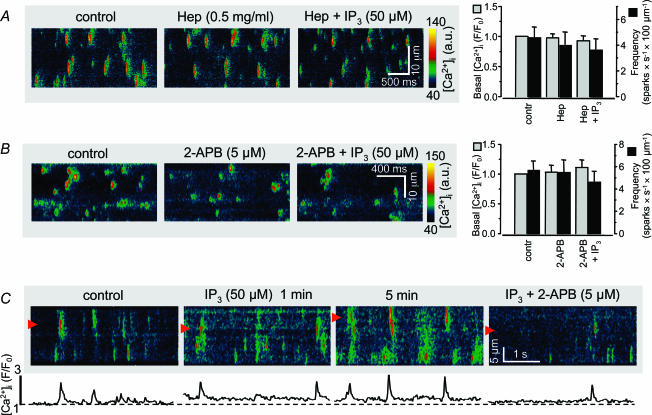
To assure that the observed effects of IP3 were indeed due to IP3-dependent Ca2+ release from the SR we applied the known IP3R antagonists heparin as well as 2-APB. In the presence of heparin (0.5 mg ml−1) IP3 (50μm) failed to increase basal [Ca2+]i and Ca2+ spark frequency (Fig. 3A). Similarly, the action of IP3 on basal [Ca2+]i and Ca2+ sparks was also prevented by 2-APB (5μm; Fig. 3B). In addition, inhibition of IP3Rs with 2-APB reversed the effects of IP3 on basal [Ca2+]i and Ca2+ spark frequency (Fig. 3C).
Figure 3. Effect of inhibitors of IP3 receptors.
A, preincubation of permeabilized atrial myocytes with heparin prevented the effect of IP3 (50μm) on basal [Ca2+]i and Ca2+ sparks. B, same experiment as in A but with the IP3R blocker 2-APB (5μm). C, 2-APB reversed the increase of basal [Ca2+]i and Ca2+ spark frequency induced by preceding exposure to IP3.
Elementary IP3R-dependent Ca2+ release events in cardiac myocytes
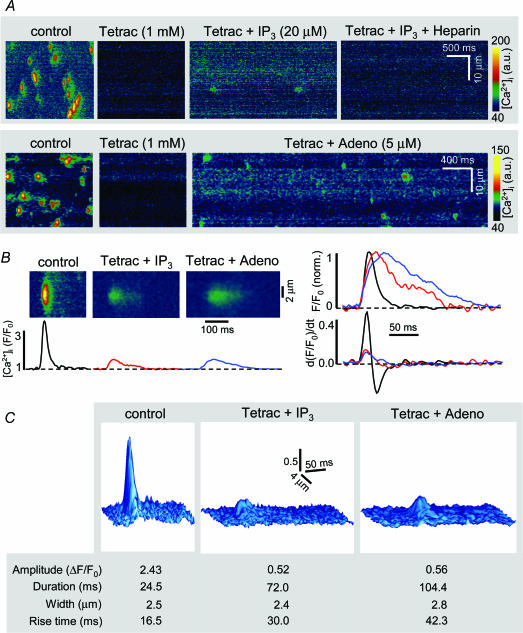
Elementary non-propagating IP3R-dependent Ca2+ release events, termed Ca2+ blips and puffs, have been observed in non-excitable cells such as oocytes (Parker & Yao, 1996), HeLa (Bootman et al. 1997) and vascular endothelial cells (Hüser & Blatter, 1997). They differ from RyR-mediated Ca2+ sparks in amplitude and kinetics, and have not been observed in cardiac myocytes, presumably because they are difficult to discern in the ‘Ca2+ noise’ from RyR-dominated Ca2+ release. We tested whether IP3-dependent puff-like events occurred in conditions where Ca2+ release via RyRs was blocked. For this purpose permeabilized atrial myocytes revealing spontaneous Ca2+ spark activity were treated with the RyR inhibitor tetracaine (Györke et al. 1997). Tetracaine instead of ryanodine was used to block the RyR, because ryanodine locks RyRs into a subconductance state which can lead to depletion of the SR. In control experiments we confirmed the inhibition of RyR activity by tetracaine. Tetracaine (1mm) reduced the open probability of the RyR channel on average by 98% (P < 0.01; n= 3) as measured with single channel recordings from RyRs incorporated into lipid bilayer (data not shown). Tetracaine also blocked spontaneous RyR-mediated Ca2+ sparks. Ca2+ spark frequency decreased from 6.0 ± 0.5 to 0.2 ± 0.1 sparks s−1 (100μm)−1 (P < 10−7; n= 12). After eliminating spontaneous Ca2+ sparks with tetracaine, permeabilized atrial myocytes were exposed to IP3 (20μm). Despite inhibition of RyRs, IP3 caused a significant increase of basal [Ca2+]i by 37 ± 7% (P < 0.001; n= 6). In addition, localized non-propagating [Ca2+] elevations appeared. Heparin completely abolished these Ca2+ release events. Adenophostin in the presence of tetracaine elicited the same type of elementary Ca2+ release events (Fig. 4A, bottom). Figure 4B shows averaged linescan images and [Ca2+]i profiles of RyR-mediated Ca2+ sparks (control) and IP3R-mediated elementary release events elicited with IP3 and adenophostin, respectively. In summary (Fig. 4C, bottom) the IP3R-dependent events had amplitudes which were 75–80% smaller than the average Ca2+ spark amplitude (see also surface plots of averaged RyR- and IP3R-mediated elementary release events, top panel of Fig. 4C). On average IP3R-mediated events were three to four times longer than Ca2+ sparks and the rise time was prolonged by approximately a factor of 2. The spatial spread of the two types of release events did not differ significantly. The differences in kinetics become evident when the average amplitudes were normalized (Fig. 4B, right). The first derivative of the [Ca2+]i transient (d(F/F0)/dt), which approximates the underlying Ca2+ flux (Sheehan & Blatter, 2003), revealed that the Ca2+ release flux of IP3-dependent events was clearly smaller. On rare occasions small non-propagating Ca2+ release events were observed in the presence of tetracaine alone. These events were approximately three times smaller in amplitude than Ca2+ sparks recorded under control conditions, however, they revealed otherwise the same spatial and temporal characteristics (average Ca2+ spark properties in tetracaine: amplitude ΔF/F0= 0.67; duration 26.6ms; width 2.0μm; rise time 17.2ms) as regular Ca2+ sparks (compare to tabulated values in Fig. 4C). Thus, these rare events were different from those observed after addition of IP3 and resulted from the opening of a smaller number of RyRs in a cluster of release channels. In summary, the IP3-dependent elementary Ca2+ release events we observed in permeabilized atrial myocytes were distinctly different from RyR Ca2+ sparks and were reminiscent of Ca2+ puffs typically observed in non-excitable tissue where IP3-dependent Ca2+ signalling is predominant (Parker & Yao, 1996; Berridge, 1997; Bootman et al. 1997; Hüser & Blatter, 1997).
Figure 4. Elementary Ca2+ release events from IP3Rs in atrial myocytes.
A, confocal linescan images from permeabilized cat atrial myocytes. Top, tetracaine (1mm) abolished spontaneous Ca2+ spark activity. In the presence of tetracaine, IP3 (20μm) caused an increase in basal [Ca2+]i and the occurrence of non-propagating Ca2+ release events with significantly different amplitude and kinetics compared to Ca2+ sparks. Heparin abolished the elevation of basal [Ca2+] and Ca2+ release events. Bottom, localized Ca2+ release events elicited with adenophostin in the presence of tetracaine. B, left, average linescan images and F/F0 traces of Ca2+ sparks (black), and IP3- (red) and adenophostin- (blue) mediated Ca2+ release events. Right, normalized amplitudes of local [Ca2+]i transients (F/F0, norm.) and first derivatives (d(F/F0)/dt). d(F/F0)/dt serves as a measure of the underlying Ca2+ release flux. C, surface plot representation of averaged linescan images of Ca2+ sparks and IP3R-mediated Ca2+ release events from B. Bottom, table showing average values of Ca2+ release event amplitude (ΔF/F0), duration (measured at half maximum amplitude), spatial width (full width at half maximum amplitude) and rise time. ‘Control’ indicates RyR-mediated Ca2+ sparks recorded in the absence of tetracaine and IP3R agonists.
Discussion
Atrial myocytes reveal two classes of elementary Ca2+ release events
In this study we show that elementary Ca2+ release events from RyRs and IP3Rs coexist in atrial myocytes. Although available quantitative data on the spatio-temporal properties of Ca2+ sparks and Ca2+ puffs or blips vary considerably (see, e.g. summary in Bootman, 1996), distinct differences in amplitude and kinetics are described. IP3R-dependent elementary release events have slower rise and decline kinetics, last longer and have a smaller amplitude. The same observation was made in this study where the two types of release events could be compared directly. The IP3-dependent release events differed from RyR-dependent Ca2+ sparks in all of these parameters in the same manner. The duration of the IP3-dependent events observed in atrial myocytes compares fairly well with Ca2+ puffs observed in HeLa (Bootman et al. 1997) and vascular endothelial cells (Hüser & Blatter, 1997) which is in the range of 100–200ms, compared to the duration of a Ca2+ spark (<50ms; see, e.g. Bers 2001). Thus, the elementary IP3R-dependent events recorded from atrial myocytes are clearly reminiscent of Ca2+ puffs observed in non-excitable tissues.
Cross-talk between IP3R- and RyR-dependent Ca2+ release: modulation of CICR by IP3
In atrial myocytes Ca2+ release from the SR during E-C coupling occurs primarily through RyRs. We have shown that in cat atrial myocytes inhibition of RyRs reduces the [Ca2+]i-transient amplitude by ∼90% (Kockskämper et al. 2001; Sheehan & Blatter, 2003). Nonetheless, in atrial tissue IP3-dependent Ca2+ release exerts an important modulatory role for Ca2+ signalling during E-C coupling by facilitating Ca2+ release via RyRs. IP3-dependent Ca2+ release makes also a small direct contribution to the [Ca2+]i transient although in quantitative terms this effect is likely to be small based on the small number of IP3Rs and the magnitude of IP3-dependent Ca2+ release events. Ca2+ affects the behaviour of both types of channels. Ca2+ is the primary activator of RyRs, and cytoplasmic as well as lumenal Ca2+ change the sensitivity of the release channel to CICR. The open probability (Po) of the IP3R type-2 (cardiac) shows a steep Ca2+ dependence in the range of 10–100nm, but is rather Ca2+-independent at [Ca2+] > 100nm (Ramos-Franco et al. 1998), i.e. at [Ca2+] encountered in cardiac cells at rest as well as during activation. Type-2 IP3R has the highest sensitivity to IP3, suggesting, together with its Ca2+-independence at [Ca2+] > 100nm, that the cardiac IP3R functions as a pure IP3 sensor. This suggests that IP3Rs are rather unaffected by Ca2+ release from RyR. In contrast, release of Ca2+ from IP3Rs can impose critical changes of [Ca2+]i in the microenvironment of the RyRs which facilitates CICR from neighbouring RyR Ca2+ release sites. In the present study we found IP3-dependent release events in both, in subsarcolemmal as well as in deeper regions of the cell, suggesting that IP3-dependent Ca2+ release may affect CICR from both junctional and non-junctional SR of atrial myocytes (Blatter et al. 2003; Sheehan & Blatter, 2003). In summary, IP3 can exert a positive inotropic effect by enhancing Ca2+ release from the SR and contraction in a beat-to-beat fashion. This may indeed represent one of the mechanisms through which neurohumoral agents such as α-adrenergic agonists, angiotensin II or endothelin modulate cardiac Ca2+ signalling and contractility.
IP3-dependent Ca2+ signalling and atrial arrhythmias
IP3-dependent Ca2+ signalling has been implied in cardiac arrhythmias due to ischaemia and reperfusion injury, inflammatory processes and developing cardiac failure (see, e.g. Woodcock et al. 1998; Mackenzie et al. 2002). Our data lend direct support to the notion that IP3-dependent Ca2+ release plays a causal role in the genesis of atrial arrhythmias. IP3 caused spontaneous [Ca2+]i transients and Ca2+ waves as well as Ca2+ alternans (Fig. 1), all disturbances in Ca2+ signalling related to cardiac arrhythmias (see Kockskämper & Blatter, 2002). Our observation that IP3-dependent Ca2+ signalling is pivotal for atrial E-C coupling and is responsible for the pro-arrhythmogenic disturbances of cellular Ca2+ homeostasis suggests that therapeutic agents which target the IP3R and the IP3/Ca2+ signalling cascade may prove beneficial for the prevention and treatment of cardiac arrhythmias (Woodcock et al. 1998).
Acknowledgments
Supported by NIH grant HL-62231 (LAB). We wish to thank Drs S. L. Lipsius and G. A. Mignery for helpful discussions. The technical assistance of A. Pezalla is gratefully acknowledged.
References
- Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd edn. Dordrecht, Netherlands: Kluwer; 2001. [Google Scholar]
- Blatter LA, Hüser J, Rios E. Sarcoplasmic reticulum Ca2+ release flux underlying Ca2+ sparks in cardiac muscle. Proc Natl Acad Sci U S A. 1997;94:4176–4181. doi: 10.1073/pnas.94.8.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter LA, Kockskämper J, Sheehan KA, Zima AV, Hüser J, Lipsius SL. Local calcium gradients during excitation–contraction coupling and alternans in atrial myocytes. J Physiol. 2003;546:19–31. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD. Hormone-evoked subcellular Ca2+ signals in HeLa cells. Cell Calcium. 1996;20:97–104. doi: 10.1016/s0143-4160(96)90099-8. [DOI] [PubMed] [Google Scholar]
- Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol. 1997;499:307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, et al. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Inositol (1,4,5)-trisphosphate induced release of Ca2+ from the sarcoplasmic reticulum of skinned cardiac cell. Biophys J. 1986;49:190a. [Google Scholar]
- Györke S, Lukyanenko V, Györke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J Physiol. 1997;500:297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SN, Autelitano DJ, Wang BH, Milano C, Du XJ, Woodcock EA. Reduced reperfusion-induced Ins(1,4,5)P3 generation and arrhythmias in hearts expressing constitutively active α1B-adrenergic receptors. Circ Res. 1998;83:1232–1240. doi: 10.1161/01.res.83.12.1232. [DOI] [PubMed] [Google Scholar]
- Hirata M, Suematsu E, Hashimoto T, Hamachi T, Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984;223:229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüser J, Blatter LA. Elementary events of agonist-induced Ca2+ release in vascular endothelial cells. Am J Physiol. 1997;273:C1775–C1782. doi: 10.1152/ajpcell.1997.273.5.C1775. [DOI] [PubMed] [Google Scholar]
- Jacobsen AN, Du XJ, Lambert KA, Dart AM, Woodcock EA. Arrhythmogenic action of thrombin during myocardial reperfusion via release of inositol 1,4,5-triphosphate. Circulation. 1996;93:23–26. doi: 10.1161/01.cir.93.1.23. [DOI] [PubMed] [Google Scholar]
- Jaconi M, Bony C, Richards SM, Terzic A, Arnaudeau S, Vassort G, et al. Inositol 1,4,5-trisphosphate directs Ca2+ flow between mitochondria and the endoplasmic/sarcoplasmic reticulum: a role in regulating cardiac autonomic Ca2+ spiking. Mol Biol Cell. 2000;11:1845–1858. doi: 10.1091/mbc.11.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockskämper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol. 2002;545:65–79. doi: 10.1113/jphysiol.2002.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockskämper J, Sheehan KA, Bare DJ, Lipsius SL, Mignery GA, Blatter LA. Activation and propagation of Ca2+ release during excitation–contraction coupling in atrial myocytes. Biophys J. 2001;81:2590–2605. doi: 10.1016/S0006-3495(01)75903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, et al. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–342. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, et al. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR. Cardiac intracellular calcium release channels: role in heart failure. Circ Res. 2000;87:8–11. doi: 10.1161/01.res.87.1.8. [DOI] [PubMed] [Google Scholar]
- Mouton R, Genade S, Huisamen B, Malan M, Lochner A. The effect of ischaemia-reperfusion on [3H]inositol phosphates and ins (1,4,5) P3 levels in cardiac atria and ventricles – a comparative study. Mol Cell Biochem. 1992;115:195–202. doi: 10.1007/BF00230331. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Williams MF, Zeigler ST, Godt RE. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol. 1986;250:C807–C811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- Parker I, Yao Y. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J Physiol. 1996;491:663–668. doi: 10.1113/jphysiol.1996.sp021247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez PJ, Ramos-Franco J, Fill M, Mignery GA. Identification and functional reconstitution of the Type-2 InsP3 receptor from ventricular cardiac myocytes. J Biol Chem. 1997;272:23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- Poindexter BJ, Smith JR, Buja LM, Bick RJ. Calcium signaling mechanisms in dedifferentiated cardiac myocytes: comparison with neonatal and adult cardiomyocytes. Cell Calcium. 2001;30:373–382. doi: 10.1054/ceca.2001.0249. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemblit N, Moschella MC, Ondriasa E, Gutstein DE, Ondrias K, Marks AR. Intracellular calcium release channel expression during embryogenesis. Dev Biol. 1999;206:163–177. doi: 10.1006/dbio.1998.9120. [DOI] [PubMed] [Google Scholar]
- Sheehan KA, Blatter LA. Regulation of junctional and non-junctional sarcoplasmic reticulum calcium release in excitation–contraction coupling in cat atrial myocytes. J Physiol. 2003;546:119–135. doi: 10.1113/jphysiol.2002.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsang M, Broede-Sitz A, Schafer E, Zerkowski HR, Brodde OE. Endothelin ETA-receptors couple to inositol phosphate formation and inhibition of adenylate cyclase in human right atrium. J Cardiovasc Pharmacol. 1994;23:344–347. [PubMed] [Google Scholar]
- Woodcock EA, Lambert KA, Phan T, Jacobsen AN. Inositol phosphate metabolism during myocardial ischemia. J Mol Cell Cardiol. 1997;29:449–460. doi: 10.1006/jmcc.1996.0287. [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Matkovich SJ, Binah O. Ins(1,4,5)P3 and cardiac dysfunction. Cardiovasc Res. 1998;40:251–256. doi: 10.1016/s0008-6363(98)00187-4. [DOI] [PubMed] [Google Scholar]
- Yamada J, Ohkusa T, Nao T, Ueyama T, Yano M, Kobayashi S, et al. Up-regulation of inositol 1,4,5 trisphosphate receptor expression in atrial tissue in patients with chronic atrial fibrillation. J Am Coll Cardiol. 2001;37:1111–1119. doi: 10.1016/s0735-1097(01)01144-5. [DOI] [PubMed] [Google Scholar]
- Zima AV, Kockskämper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol. 2003;550:765–783. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]