Abstract
In addition to action potential-evoked exocytotic release at neurohypophysial nerve terminals, the neurohormones arginine vasopressin (aVP) and oxytocin (OT) undergo Ca2+-dependent somatodendritic release within the supraoptic and paraventricular hypothalamic nuclei. However, the cellular and molecular mechanisms that underlie this release have not been elucidated. In the present study, the whole-cell patch-clamp technique was utilized in combination with high-time-resolved measurements of membrane capacitance (Cm) and microfluorometric measurements of cytosolic free Ca2+ concentration ([Ca2+]i) to examine the Ca2+ and stimulus dependence of exocytosis in the somata of magnocellular neurosecretory cells (MNCs) isolated from rat supraoptic nucleus (SON). Single depolarizing steps (≥20 ms) that evoked high-voltage-activated (HVA) Ca2+ currents (ICa) and elevations in intracellular Ca2+ concentration were accompanied by an increase in Cm in a majority (40/47) of SON neurones. The Cm responses were composed of an initial Ca2+-independent, transient component and a subsequent, sustained phase of increased Cm (termed ΔCm) mediated by an influx of Ca2+, and increased with corresponding prolongation of depolarizing step durations (20–200 ms). From this relationship we estimated the rate of vesicular release to be 1533 vesicles s−1. Delivery of neurone-derived action potential waveforms (APWs) as stimulus templates elicited ICa and also induced a ΔCm, provided APWs were applied in trains of greater than 13 Hz. A train of APWs modelled after the bursting pattern recorded from an OT-containing neurone during the milk ejection reflex was effective in supporting an exocytotic ΔCm in isolated MNCs, indicating that the somata of SON neurones respond to physiological patterns of neuronal activity with Ca2+-dependent exocytotic activity.
The process of Ca2+-dependent exocytotic fusion underlies the release of neurotransmitters for fast synaptic transmission and the secretion of neuropeptide hormones from presynaptic axon terminals. However, in addition to presynaptic sites of exocytosis, recent evidence indicates a strong likelihood for significant extrasynaptic vesicular release from somatodendritic regions of central and peripheral neurones, including those of the substantia nigra (Cheramy et al. 1981; Jaffe et al. 1998), dorsal root ganglia (Huang & Neher, 1996), and hypothalamic SON (Mason et al. 1984; Moos et al. 1984; Di Scala-Guenot et al. 1987). The voltage and ionic dependence of this activity points to the involvement of Ca2+-dependent secretory mechanisms similar to those described for more conventional presynaptic sites of activity-dependent vesicular exocytosis (Giovannucci & Stuenkel, 1997; Soldo et al. 1998; De Kock et al. 2003). Thus, it has been suggested that somatodendritic release may function to regulate the behaviour of specific classes of neurones at the level of the cell body in an autocrine or paracrine fashion via extrasynaptic mechanisms.
Magnocellular neurosecretory cells (MNCs) of the SON are responsible for the synthesis and vesicular packaging of the neurohormones aVP and OT, which are then released into the bloodstream from their neurosecretory terminals within the neurohypophysis. In addition, results from push–pull perfusion or microdialysis experiments conducted in vivo and radioimmunological detection of peptide release from SON tissue explants have demonstrated that depolarization of MNCs, whether induced experimentally or by physiological stimuli such as parturition and suckling, elicits significant intranuclear release of OT within the SON (Mason et al. 1984; Di Scala-Guenot et al. 1987; Moos et al. 1989; Neumann et al. 1993). Interestingly, this stimulated intranuclear secretion appeared to be decoupled from systemic release of the peptide, in that it continued after abatement of release of the neurohormone from the neurosecretory endings of the SON neurones (Neumann et al. 1993). It was further shown that exogenous application of OT to isolated SON in vitro induced Ca2+-dependent release of OT (Moos et al. 1984), and that this action correlated with the ability of OT to enhance the burst firing of OT-containing SON neurones (Lambert et al. 1993). On the basis of these data it has been posited that the functional role of intranuclear release of OT is to facilitate its continued secretion by providing positive feedback via postsynaptic receptors within the hypothalamic nuclei. By contrast, the activity of SON neurones was found to be reduced when OT acted at presynaptic receptors located on excitatory afferent inputs to the nucleus (Kombian et al. 1997). While the primary origin for the intranuclear release of OT remains unclear, the finding in anatomical studies that the rat SON contains few recurrent axonal collaterals (Mason et al. 1984) favours a major contribution from the dendrites and possibly somata of MNCs. Consistent with a somatodendritic origin of this intranuclear release are ultrastructural data that demonstrate the presence of large, dense-core secretory granules at somatic and dendritic sites in OT- and aVP-containing SON neurones and their clustering following high-potassium-induced depolarization (Pow & Morris, 1989; Wang et al. 1995). In addition, stimulus-induced somatic release is abolished following treatment of OT neurones with tetanus toxin, implicating exocytotic fusion in this process (De Kock et al. 2003).
A powerful and now widely adopted approach for monitoring secretory events in both endocrine cells and isolated nerve endings is to measure time-resolved changes in Cm (Neher & Marty, 1982; Joshi & Fernandez, 1988; Lindau & Neher, 1988). Changes in Cm (referred to here as ΔCm) that are elicited in response to depolarization reflect changes in cell surface area due to the addition (exocytosis) or retrieval (endocytosis) of vesicular membrane and provide an ‘on line’ measure of secretory activity at the single cell level. Only recently has this method been applied to investigate the exocytotic activity of MNC somata (De Kock et al. 2003). In the present study, we monitored ΔCm in combination with whole-cell patch recording of ICa and fura-2 microfluorometry to determine the characteristics of Ca2+-dependent vesicular release from the somata of supraoptic MNCs. Additionally, we sought to determine whether depolarization of neurones using physiologically relevant stimulus templates modelled on neurone-derived action potentials could activate exocytosis. We demonstrate that membrane depolarizations that elicited robust ICa and Ca2+ influx evoked substantial ΔCm responses. However, the exocytotic fusion of non-secretory organelles can be induced by high, non-physiological elevations in cytosolic Ca2+ (for example, that induced by photolysis of caged Ca2+ or intense stimulation by repetitive step depolarizations). Thus in addition in the current study we used physiologically relevant action potential based stimulation protocols to drive the fusion of secretory granules. Application of trains of action potential waveforms activated ΔCm in the somata of MNC in a frequency-dependent manner. These data indicate that the somata of SON neurones contain functional exocytotic machinery to perform Ca2+-dependent secretion.
Methods
Neurone preparation
Acutely dissociated neurones of the hypothalamic SON were prepared from postnatal 17- to 25-day-old Sprague–Dawley rats as previously described (Soldo & Moises, 1998). Briefly, 400–500 μm slices containing the basal hypothalamic nucleus were collected. Segments of tissue containing the SON were dissected from regions adjacent to either side of the optic chiasm. The tissue fragments were enzymatically treated at 37°C with trypsin (Type I, 1.25 mg ml−1; Sigma, St Louis, MO, USA) and mechanically dissociated using several passages through a fire-polished Pasteur pipette. Dissociated neurones were plated onto poly-l-lysine-coated 35 mm culture dishes. Growth medium containing nerve growth factor was added approximately 1 h after plating. Neurones that had well-rounded cell bodies (14–38 μm) with no or only a few short extending processes were studied from 3 h to 1 day after plating. In a previous report, we had identified neurones of similar morphology as aVP- and OT-containing magnocellular SON neurones (Soldo & Moises, 1998).
All animals were handled in accordance with University of Michigan Institutional Animal Care Committee and NIH guidelines. Animals were deeply anaesthetized by CO2 gas and decapitated.
Electrophysiological recording of ICa and Cm
Voltage- and current-clamp recordings were obtained using the whole-cell variation of the patch clamp technique (Hamill et al. 1981). Patch electrodes, prepared from glass microhaematocrit capillary tubes (i.d. = 1.1–1.2 mm, o.d. = 1.3–1.4 mm; Fisher Scientific, Pittsburgh, PA, USA) and having resistances of 3–5 MΩ, were coated with Sylgard elastomer (Dow Corning, Midland, MI, USA). The voltage-clamp patch electrodes were filled with an internal recording solution of composition (mm): 102 caesium acetate, 11 CsCl, 1 MgCl2, 5 4-aminopyridine, 40 Hepes, 0.250 EGTA, 4 ATP, 0.1 GTP (pH 7.25–7.3; 290–300 mosmol (kg H2O)−1). In some experiments, the spatially averaged intrasomatic [Ca2+]i was determined by replacing 0.15 mm EGTA of the intracellular recording solution with the same concentration of the cell-impermeant Ca2+-sensitive fluorescent indicator fura-2 pentapotassium salt (Molecular Probes, Eugene, OR, USA). [Ca2+]i was monitored by dual-wavelength microspectrofluorometry in a manner that has been previously detailed (Giovannucci & Stuenkel, 1997). For current-clamp recordings of somatic action potentials, electrodes were filled with the following internal solution (mm): 130 potassium gluconate, 2 MgCl2, 10 Hepes, 1 EGTA, 0.5 CaCl2, 2 ATP, 0.1 GTP. Plated neurones were viewed through an inverted microscope and continuously superfused with a physiological saline of composition (mm): 140 NaCl, 1.2 MgCl2, 2.4 CaCl2, 10 Hepes, 11 glucose. For the recording of ICa, 140 mm NaCl in the extracellular solution was replaced with 100 mm tetraethylammonium chloride (TEA-Cl), 67 mm choline-Cl and 3.5 mm KCl. Both solutions were adjusted to pH 7.2–7.3 and 300–310 mosmol (kg H2O)−1. Current records were digitally corrected for leak currents by using an on-line P/4 subtraction protocol.
All recordings were made at room temperature using either an Axopatch 200A amplifier (Axon Instruments, Inc., Union City, CA, USA) or EPC9 amplifier (Heka, Lambrecht, Germany). Whole-cell capacitance (15–30 pF) and series resistance (6–10 MΩ) were corrected using compensation circuitry on the patch clamp amplifier. Series resistance compensation of 75–85% was typically obtained without inducing oscillations. High-resolution Cm measurements were used to monitor changes in membrane surface area (exocytotic activity) in response to large step depolarizations. The use of the whole-cell patch-clamp technique allows for the measurement of small, time-resolvable changes in Cm (ΔCm) by using a modified phase-tracking method (Joshi & Fernandez, 1988). ΔCm of isolated SON neurones was measured by monitoring the current response to a 30 mV r.m.s. sine wave at 1201 Hz applied to a holding potential of −90 mV. The current output of the voltage-clamp was monitored at two orthogonal phase angles, and the correct phase angle determined using a software-based phase-sensitive detector (Pulse Control 4.5, Richard J. Bookman, University of Miami, FL, USA). A 19.1 kHz sampling rate (16 samples per sinusoidal period) was used to compute one Cm point each 13.31 ms. Calibration pulses of 100 fF and 500 kΩ were generated at the beginning of each trace by the whole cell compensation circuit and by dithering of the phase-tracking resistor, respectively.
Depolarizing voltage step commands of varying duration (5–200 ms) were applied from a holding potential (Vh) of −90 mV to generate inward ICa and to evoke ΔCm for each neurone tested. In later experiments, an action potential was recorded under current clamp from a representative SON neurone and reintroduced as the stimulus waveform during voltage clamp recording. These action potential waveforms were applied at various frequencies to activate ICa and Cm responses. Bath solutions were applied by local superfusion of the individual neurone under study using a modified U-tube delivery system as previously described (Soldo & Moises, 1998). All data were analysed using an unpaired Student's t test, unless otherwise indicated, and expressed as means ±s.e.m. in the text.
Results
Features of depolarization-induced Cm responses in isolated SON neurones
To examine for depolarization-evoked exocytosis in isolated SON neurones, measurements of ICa, the associated changes in capacitance (ΔCm) and (in selected experiments) intracellular Ca2+ levels ([Ca2+]i) were performed under whole-cell patch clamp and with ionic conditions that isolated ICa. Baseline Cm was monitored for a period of 1 s after which the neurone was depolarized by a single 200 ms voltage step to +10 mV from a Vh of −90 mV. This voltage clamp protocol elicited maximum inward ICa responses in virtually all neurones examined under standard recording conditions and was therefore uniformly employed (unless otherwise indicated) to assess their capacity for Ca2+-dependent exocytosis. The ICa responses of SON consisted of both rapidly inactivating and more sustained components, as revealed by inspection of the traces from three neurones depicted in Fig. 1A (lower traces). These currents have been previously characterized in detail in terms of their biophysical and pharmacological properties (Fisher & Bourque, 1995; Foehring & Armstrong, 1996; Soldo & Moises, 1998).
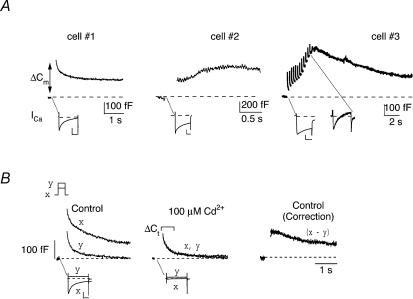
Figure 1. Depolarization-induced Cm responses recorded from MNC somata.
A, representative traces of Cm (top) and corresponding ICa (bottom) responses recorded from three different neurones. Step depolarization to +10 mV for 200 ms from Vh=−90 mV evoked high-voltage-activated inward ICa in each neurone that differed in peak amplitude and rate of inactivation. In cells labelled 1 and 2, a jump-like increase in Cm was detected following delivery of a single depolarizing step. These Cm responses were of long duration, typically lasting several seconds and in the case of one neurone, included a slowly developing second phase of gradually increasing Cm (cell 2). In the third neurone, repetitive depolarization to +10 mV with a train of 12 pulses (50 ms in duration with 200 ms interpulse interval) evoked stepwise increases in Cm that summed to form a robust and prolonged phase of increased Cm, which decayed slowly back toward baseline over many seconds (cell 3). Note that significant Ca2+-dependent inactivation of ICa occurred upon delivery of each successive pulse within the train (currents evoked by the initial and last test pulse are shown for comparison, lower traces). B, records from another MNC illustrate ICa (lower) and Cm responses (upper) evoked by depolarizing steps to +10 mV (trace x) or +70 mV (trace y) from Vh=−90 mV in control media (left panel) and in the presence of the Ca2+ channel blocker, Cd2+ (100 μm) (middle panel). Stepping to +10 mV in control (left panel) evoked substantial inward ICa (trace x), accompanied by a large increase in Cm (trace x), consisting of a transient initial response and subsequent sustained phase with slower decay. By contrast, stepping to +70 mV in control (trace y, left panel) or to either test potential in the presence of Cd2+ (traces x and y, middle panel) evoked negligible ICa. Note that under these conditions where Ca2+ influx was negligible, only the transient component of the Cm increase was detected following depolarization to +10 mV (tracex in Cd2+) or +70 mV (tracey in control and Cd2+). To control for contamination of depolarization-evoked changes in Cm by this transient component (termed ΔCt in the text), controlΔCm responses were corrected as shown (x – y trace, right panel) by subtracting the Ca2+-independent ΔCt evoked by depolarization to +70 mV (trace y) from the total ΔCm evoked by a step to +10 mV (tracex). Verticalscale bars are 0.5 nA and 1 nA for current traces in A and B, respectively, and the time scale is 50 ms in all cases.
In response to a single depolarizing step to +10 mV, a large increase in Cm above baseline was detected in nearly all neurones examined (in 40/47 neurones; Fig. 1A upper traces). The depolarization-evoked Cm responses could be resolved into several kinetically distinct phases, beginning with an initial, rapidly activating transient component observed within 5–10 ms after termination of the voltage pulse, which we will refer to here as ΔCt. This response was followed by a slower and more prolonged phase of increased Cm that differed from neurone to neurone in its peak amplitude, duration and rate of decay (compare cells 1, 2 and 3, Fig. 1A). When isolated from ΔCt (see below), the latter response was represented in 37/40 neurones by an initial jump in Cm followed by a slower return of Cm to the resting level over a period of a few seconds (Fig. 1A, cell 1). In the three remaining neurones, the Cm responses were kinetically more complex and included a slowly increasing phase that developed with variable latencies after the initial jump in Cm (Fig. 1A, cell 2). Cm responses of this type were more reliably observed when neurones were stimulated repetitively with trains of step depolarizations (8–12 voltage pulses; Fig. 1A, cell 3). Based on their similarity to exocytotic ΔCm responses described in MNC nerve terminals (Lim et al. 1990; Hsu & Jackson, 1996; Giovannucci & Stuenkel, 1997), we interpret the initial jump and latent increases in Cm to reflect increases in membrane surface area that occur in response to depolarization-induced influx of Ca2+ and activation of Ca2+-dependent vesicular fusion. Conversely, the subsequent decay of ΔCm back to baseline has been shown to correspond to the net retrieval of vesicular membrane as the rate of endocytosis eventually exceeds that of the exocytotic response.
Correction of ΔCm for a Ca2+-independent Cm signal
The component referred to here as ΔCt closely resembled a transient, Ca2+-independent Cm response to membrane depolarization that has been observed both in the isolated nerve endings of SON neurones (Giovannucci & Stuenkel, 1997) and in bovine adrenal chromaffin cells (Horrigan & Bookman, 1994). These transient Cm signals were found to be unrelated to Ca2+-dependent exocytosis, but rather were attributed to a redistribution of voltage-sensitive gating charges within the membrane (Bezanilla et al. 1982; Horrigan & Bookman, 1994; Debus et al. 1995). The ΔCt detected in SON neurones was reliably evoked under conditions of Ca2+ channel blockade (100 μm Cd2+), as well as following depolarizations to potentials close to the estimated value of ECa (+70 mV under our recording conditions; Fig. 1B). Similar to that previously shown for nerve endings, the ΔCt amplitude was found to follow a Boltzmann-type relationship and saturated at steps above 0 mV (Giovannucci & Stuenkel, 1997). These results indicate that ΔCt was dissociated from voltage-dependent influx of Ca2+ and thus does not reflect Ca2+-dependent exo-endocytotic activity. As the presence of ΔCt interferes with measurement of the Ca2+-dependent Cm response, a correction was performed on all Cm measurements by doing a point by point subtraction of the ΔCt elicited by a +70 mV voltage step from the entire ΔCm response obtained upon depolarization to a test potential (routinely +10 mV) that elicited maximum inward ICa (Fig. 1B, correction trace). Henceforth, the term ΔCm will be used for the corrected step depolarization-evoked Cm responses recorded in MNC somata.
Properties of ΔCm in SON neurones
The amplitude of the depolarization-evoked ΔCm varied significantly between neurones (cf. traces from 3 neurones in Fig. 1A), despite the fact that the magnitude and duration of the depolarizing test pulse were adjusted to produce maximal inward ICa responses. On the other hand, successive ΔCm that were evoked in a given neurone remained relatively constant for approximately 20 min following patch rupture, after which rundown in the Cm response was common in all SON neurones tested. The variability in response among neurones, as well as the time-related changes in ΔCm can be attributed in part to differences in the magnitude of Ca2+ influx and secretory capacity of individual neurones. Presumably these differences correspond to the surface area of the respective somata (94–452 μm2), the number and subtype complement of Ca2+ channels, and/or in the respective number of proximal dendrites or axon stump length. The peak ΔCm recorded in 10 neurones in response to a single 200 ms depolarizing step that evoked maximal ICa ranged between 128 and 432 fF (mean ±s.e.m.; 248 ± 35 fF). These values correspond to the fusion of approximately 345 vesicles, assuming a dense-core vesicle diameter of 160 nm (Pow & Morris, 1989) and specific membrane capacitance of 9 fF μm−2 (Gentet et al. 2000). Nevertheless, this value should be considered a low estimate since exocytosis was likely to be initiated before the end of the voltage pulse. Moreover, as Cm measurements cannot distinguish between the fusion of large dense-core granules or microvesicles, nor distinguish between the temporally overlapping processes of exocytosis and endocytosis, these measurements may underestimate the secretory capacity of individual SON neurones.
Voltage and Ca2+ dependence of evoked ΔCm
Perfusion of SON neurones with the Ca2+ channel blocker, Cd2+ (100 μm), abolished the depolarization-evoked ICa and any associated ΔCm (Fig. 1B). This result indicated that Cm responses were largely dependent upon depolarization-induced influx of Ca2+ through voltage-dependent Ca2+ channels. Likewise, the ΔCm responses evoked by depolarizing pulses were abolished when the normal extracellular solution bathing the neurone was replaced with one containing no added Ca2+ (n= 3). To further assess the dependence of ΔCm on Ca2+ influx, we determined the amplitude–voltage relationship for ΔCm by evoking a series of ICa and ΔCm responses to single 200 ms depolarizing steps in increments of 10 mV from a Vh of −90 mV. In conjunction with these recordings, ratiometric monitoring was performed of the corresponding changes in [Ca2+]i in neurones that had been preloaded with the calcium-sensitive indicator, Fura-2. Figure 2A and B illustrates the results from a representative experiment. In this neurone, ΔCm was elicited at test potentials that evoked appreciable inward current and corresponding increases in [Ca2+]i and attained maximal amplitude at +10 mV, where ICa and [Ca2+]i also peaked. Conversely the evoked ΔCm decreased as the test potential approached the estimated value for ECa (∼+70 mV), consistent with the diminishment of Ca2+ influx. To test whether ΔCm might depend more directly on the total Ca2+ influx rather than peak ICa, we next determined the charge density of Ca2+ ion movement (QCa) that resulted from a 200 ms step to a particular test potential, calculated by integrating with respect to time the area under the corresponding ICa. Figure 2C provides a graphic comparison of the voltage-dependence of QCa (▪) and the amplitude–voltage relationship of the corresponding ΔCm response (▴), obtained by averaging measurements from six neurones. Although inducement of ΔCm occurred at a higher voltage threshold than Ca2+ influx (−10 and −20 mV, respectively), overall the voltage relationship of ΔCm closely followed the bell-shaped voltage profile for QCa. Moreover, simultaneous monitoring of changes in [Ca2+]i with Fura-2 showed that the depolarization-evoked increases in QCa resulted in corresponding changes in global [Ca2+]i. Increases in [Ca2+]i evoked by steps generated inward ICa that rose rapidly to peak values within 500 ms of the voltage pulse, after which [Ca2+]i gradually returned to resting levels over tens of seconds (Fig. 2B). The lag in inducement of ΔCm compared to QCa indicated that Ca2+ ions entering the cell must overcome some threshold value for activation of Ca2+-dependent secretion, which under our experimental conditions represented a mean influx of 37.6 pC. Because our [Ca2+]i measurements report global changes in intracellular Ca2+, we cannot deduce the nature of the Ca2+ signal that triggers exocytosis in SON neurones. Nonetheless, an additional observation offers at least some initial insight regarding its identity. In comparison to results obtained with standard internal solution (0.25 mm EGTA), our ability to evoke a ΔCm in response to a single 200 ms depolarizing pulse to +10 mV was markedly reduced (absent in 3 of 4 neurones tested) when recordings were obtained with high EGTA (10 mm) in the recording pipette (results not shown).
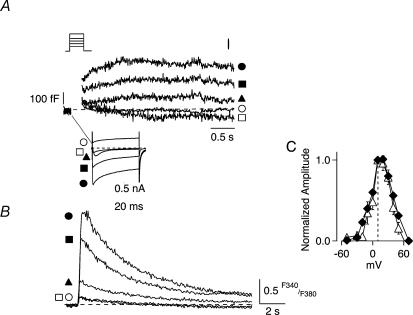
Figure 2. Voltage dependence of ΔCm parallels that for depolarization-evoked Ca2+ influx.
A, the relationships for ICa and ΔCm as a function of membrane potential were determined, as shown for a representative SON neurone, by delivering a series of 200 ms depolarizing steps to different test potentials in 10 mV increments from Vh=−90 mV. For purposes of clarity, only selected current (lower panel) and Cm traces (upper panel) from several test potentials are shown (□, −50; ▴, −30; ○, +10; ▪, +30; and ○, +70 mV). The amplitude of ICa (lower traces) increased in a voltage-dependent manner with increasing membrane depolarization up to a maximum obtained at +10 mV. A similar voltage-dependent profile was obtained for the corresponding increase in ΔCm (upper traces) measured at the termination of each depolarizing step. B, the increases in ICa and ΔCm recorded at a given test potential were accompanied by corresponding elevations in intracellular Ca2+ concentration ([Ca2+]i), as measured fluorometrically by inclusion of the Ca2+-sensitive dye fura-2 in the recording pipette. Note that stepping to test potentials that evoked either negligible or outward ICa (−50 and +70 mV, respectively), resulted in little change in [Ca2+]i and did not evoke ΔCm responses. C, direct comparison of the complete voltage-dependent relationship for the normalized amplitude of the depolarization-evoked ΔCm (Δ) and for the corresponding Ca2+ charge integral, QCa as a function of test potential (⋄) revealed that they were tightly correlated.
Estimations of the size of the releasable vesicle pool and rate of somatic secretion
The above results underscore the similarity of the Ca2+-dependent exocytotic ΔCm responses observed for both MNC somata and MNC nerve endings (Giovannucci & Stuenkel, 1997). To further investigate this similarity we estimated the initial rate of somatic release using a series of step depolarizations of increasing duration to deplete the soma of the readily available pool of granules, using a protocol employed previously for MNC terminals (Giovannucci & Stuenkel, 1997). The traces in Fig. 3A illustrate the results from a representative experiment in which we compared how the evoked ICa, corresponding elevation in [Ca2+]i and associated ΔCm changed as a function of test potential duration. An increase in Cm was first detected at a pulse duration of 20 ms, and the amplitude of ΔCm became larger as the pulse duration was lengthened up to 200 ms. Likewise, the magnitude of the induced elevation of [Ca2+]i also correlated with pulse duration. Figure 3B summarizes the results from 10 similar experiments, showing the mean ΔCm responses measured for each duration of the depolarizing pulse examined. The continuous line represents the best linear fit of the data, providing an exocytotic rate of 1110 fF s−1, or 1533 vesicles s−1. It should be appreciated, however, that these estimations might somewhat underestimate the rate of exocytosis owing to overlap of depolarizing pulses of long duration with the initial phase of the evoked ΔCm.
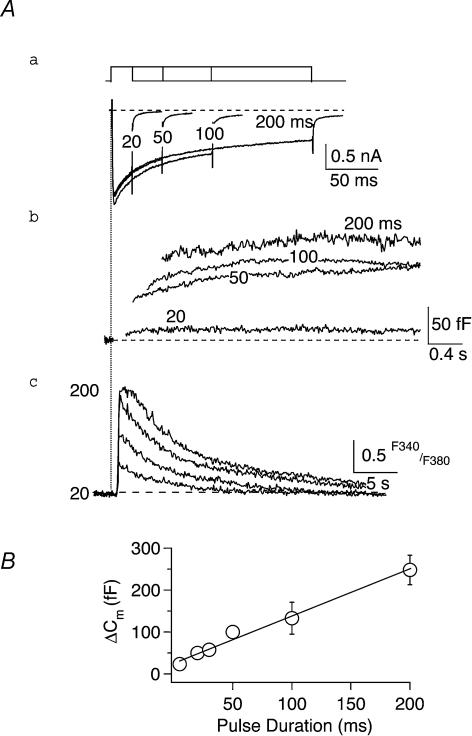
Figure 3. Determination of the rate of exocytosis.
A, records from a single SON neurone illustrate the changes in ICa (a), ΔCm (b) and [Ca2+]i (c) evoked in response to step depolarization to +10 mV from Vh=−90 mV, as a function of increasing the duration of the test pulse. Increasing the duration of the depolarizing step in roughly a 2-fold manner from 20 to 200 ms resulted in a concomitant lengthening in time course of the evoked ICa (Aa) and progressive increases in the peak amplitude of the corresponding ΔCm (Ab). Ac, the progressive increases in duration of the evoked Ca2+ influx and in peak ΔCm were paralleled by corresponding higher accumulations in [Ca2+]i. B, plotting of the averaged ΔCm evoked by depolarization +10 mV from Vh=−90 mV in 5 neurones, as a function of test pulse duration, yielded a linear relationship that could be best described by the following function (straight line): ΔCm= 1.11 × (pulse duration) + 27.2. The slope of this relationship indicates the initial rate of secretion was 1110 fF s−1, or 1533 vesicles s−1, under the present experimental conditions.
Properties of the delayed Cm increase in SON neurones
In 3 of 40 neurones stimulated with single depolarization steps and in 6 of 11 neurones tested with trains of stimulations, the Ca2+-dependent Cm response included an additional slowly increasing phase that emerged at variable latency after induction of the fast ΔCm and persisted following completion of the underlying ICa. As a result, the depolarization-evoked ΔCm in these neurones was at least biphasic, with one or more inflections in the rising phase of the Cm trace (see Fig. 1A, cells 2 and 3). The delayed Cm responses, which we term the secondary secretory phase (ΔCm,s), were only observed when neurones received single depolarizations 200 ms or longer in duration or after a repetitive series of eight to 12 pulses (50–200 ms) to test potentials that evoked maximal ICa. The latency to onset, rate of rise and amplitude of the ΔCm,s varied between neurones. Typically the ΔCm,s began to rise 0.5–1 s after the onset of depolarization and reached a mean maximum amplitude of 225 ± 92 fF.
It is worth noting that in MNC nerve endings, a similar, latent and slowly rising Ca2+-dependent Cm increase can be evoked in response to prolonged or repetitive step depolarizations (Seward et al. 1995; Giovannucci & Stuenkel, 1997). In this preparation, such responses required higher elevation in [Ca2+]i for activation, compared to the initial ΔCm and have been attributed, based on their additional properties (e.g. sensitivity to Ca2+ diffusion and exogenous buffering), to secretion from a distinct releasable granule pool (RRP) of larger capacity (Seward et al. 1995; Giovannucci & Stuenkel, 1997). The requirement of a larger Ca2+ influx to evoke the ΔCm,s was also found after normalizing the QCa for resting whole-cell Cm, arguing against the possibility that this might be related simply to differences in cell size. The protocols required to generate the ΔCm,s resulted in marked inter-trial variability and rapid rundown in the evoked ΔCm, precluding a more vigorous analysis of this latent and more sustained secretory response. Nevertheless, the above results strongly suggest that a ΔCm,s was only induced after the cumulative number of Ca2+ ions entering the cell overcame a threshold whose value exceeded that required for activation of secretion from an immediately releasable pool. In addition, they are consistent with the hypothesis that somatic secretion in SON neurones may involve the recruitment and Ca2+-dependent exocytosis of granules from more than one vesicular pool, recapitulating at least to some degree the events associated with depolarization-evoked release from their neurosecretory nerve endings.
Activation of ICa with action potential waveforms
The pulsatile stimuli used initially to examine for exocytotic activity in SON neurones generated Ca2+ currents unlikely to be encountered during normal spike behaviour. Therefore, an additional series of experiments was performed to ascertain whether somatic secretion could be supported by Ca2+ signals that more closely conform to those associated with physiological patterns of SON neuronal activity. For these experiments, we first recorded an action potential from a SON neurone under current clamp and reconstructed the action potential waveform (APW) for use as a voltage-clamp stimulus template to evoke action potential-generated currents. The APW depicted in Fig. 4A (upper trace) began from a baseline holding potential of −60 mV, and included an initial slow 2 ms voltage ramp to approximately −40 mV, followed by a rapid overshoot to approximately 20 mV and a subsequent rapid recovery back toward baseline, terminating with a slower afterhyperpolarization to −83 mV. The action potential properties depicted by this APW are similar to those previously characterized for SON neurone action potential firing recorded in vivo (Armstrong et al. 1994) and in slice preparations (Jourdain et al. 1998). A negative-directed APW (nAPW) of identical morphology, but opposite polarity, was also constructed and used as a voltage-clamp template to measure leak current (not shown). The nAPW-generated leak current traces were subtracted from uncorrected APW-generated current traces to obtain a measure of the APW-evoked inward currents. Under the same recording conditions used above to study step-evoked HVA Ca2+ currents, the APW-generated inward current response activated with peak depolarization of the APW, suggesting that this current required strongly depolarized potentials (>0 mV) for activation. The peak of the current response occurred during the falling phase of the APW (dotted line, Fig. 4). In addition, the current was reversibly blocked by Cd2+ treatment (200 μm; Fig. 4A, lower traces). This indicated that Ca2+ influx was through voltage-activated Ca2+ channels. We have previously shown that these APW-generated Ca2+ currents reflect the activation of L-, N-, R- and P/Q-type Ca2+ channels (Soldo et al. 1998).
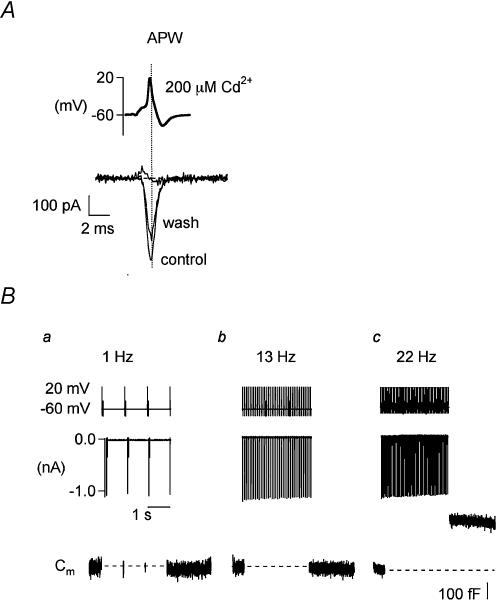
Figure 4. APWs generate frequency-dependent Cm responses in SON neurones.
A, the top trace depicts an APW voltage template (upper trace) that was constructed from a spontaneous action potential recorded under current clamp conditions in an acutely isolated SON neurone. Presentation of this APW as a stimulus template under voltage clamp evoked a large inward current in a representative SON neurone (lower traces, control). The evoked current was reversibly blocked by administration of the Ca2+ channel blocker Cd2+ (200 μm, asterick). B, ICa (middle traces) and ΔCm (lower traces) evoked by presentations of APW stimuli in another neurone are shown on a compressed time scale, as compared to that in A, such that APWs (top traces) and ICa (middle traces) appear as single lines above or below baseline, respectively. Baseline Cm measurements were obtained at Vh=−60 mV (lower traces) before delivery of a train of APWs at frequencies of 1 Hz (Ba), 13 Hz (Bb) and 22 Hz (Bc). The detection period for monitoring changes in capacitance in response APW presentations comprised a 15 ms window at the end of the second delivery of APW stimuli over a total 3 s stimulation time period, after which continual capacitance detection was performed for another 3 s (lower traces). Middle traces illustrate the evoked ICa responses corresponding to each presentation of APW at the indicated frequency.
APW-generated secretory responses
In the following experiments, individual SON neurones were clamped to Vh=−60 mV and baseline Cm was measured, after which APWs were delivered as the depolarizing stimulus. The APW-evoked current responses were recorded during a train of APWs. Cm measurements were captured in a 15 ms window and obtained at 1 Hz throughout the duration of the train (Fig. 4B). We applied APWs at physiologically relevant frequencies. Measurements of Cm (lower traces) obtained following presentation of each APW during a 3 s train of stimuli applied at a frequency of 1 Hz showed no change from baseline, as illustrated in Fig. 4Ba. Likewise, a 3 s train of APWs applied at frequencies up to 13 Hz (see Fig. 4Bb) was ineffective in eliciting an increase in Cm. On the other hand, trains of APWs delivered at these frequencies induced substantial ICa (Fig. 4B, middle traces). In contrast, a robust ΔCm was reliably evoked when APWs were presented at frequencies of 22 Hz or higher. For example, as shown in Fig. 4Bc, a robust ΔCm response was elicited within 2 s following application of a 22 Hz stimulus. Interestingly, unlike step depolarizations, APWs did not elicit the ΔCt. Neither nAPW nor trains of APW during Cd2+ blockade of Ca2+ influx induced a change in Cm. Thus no subtraction protocol for these records was performed.
Milk ejection-like burst pattern of APWs maintains secretion
OT- and aVP-containing SON neurones have been shown to exhibit specific patterns of action potential bursting that can be correlated with particular types of physiological stimuli, including suckling and parturition. For example, during the milk ejection reflex that occurs with suckling, OT neurones demonstrate a specific bursting pattern of discharge (ME burst) that is believed to sustain secretion of OT that, in turn, supports milk production. The ME burst pattern, characterized by in vivo recordings from rats (Jourdain et al. 1998), consists of a baseline discharge of low AP firing frequency (∼6 Hz) which, upon the burst, quickly reaches a peak frequency of firing of ∼33 Hz and then declines at a slower rate back to baseline (see Jourdain et al. 1998–Fig. 4B1). In a final series of experiments, we closely modelled this ME burst pattern with trains of APWs (Fig. 5A) and used this as a stimulus template to examine its ability to support exocytotic release from SON somata. When applied as the depolarizing stimulus, each APW in the ME burst template activated a brief inward current, as illustrated in Fig. 5B. The Ca2+ currents evoked by consecutive APWs of the burst stimulus are represented sequentially on the condensed time scale by the downward strokes below baseline, and these responses appeared similar in morphology and peak amplitude to those already described. The corresponding ΔCm response elicited by the ME burst of APWs is depicted in Fig. 5D (trace a). The initial 1 s presentation of APWs at the baseline rate of 6 Hz did not elicit a ΔCm. By contrast, the subsequent delivery of higher frequency trains of APWs, first at 33 Hz, followed by gradual changes in frequency to 11 Hz resulted in substantial increases in ΔCm. Measurements taken 1 s after initiation of the 33 Hz train of APWs revealed a robust ΔCm with incremental increases in Cm registered after delivery of each successive 1 s train of APWs throughout the duration of the ME burst pattern. Interestingly, the ΔCm continued to increase even as the frequency of APW presentation in the template declined below 22 Hz, a level estimated previously to represent the threshold for activation of a secretory response in these neurones (see Fig. 4B). In fact, upon cessation of the ME burst, the Cm trace displayed a slow, continual increase for a number of seconds thereafter. This latter result is entirely consistent with an increasing elevation in global [Ca2+]i to a level capable of sustaining secretory responses. Presentation of an ME burst of ‘negative’ APWs (nAWPs) resulted in small inward leak currents (Fig. 5C), but failed to elicit a Cm response in this neurone (Fig. 5D, traceb). Results similar to these were observed in three additional neurones examined in this manner.
Figure 5. ICa and corresponding ΔCm responses evoked by presentation of a physiologically relevant APW voltage template.
A, lower trace illustrates the composition of an APW voltage template modelled after an OT burst of action potentials during the milk ejection reflex (see Jourdain et al. 1998; Fig. 4B1). The OT-patterned APW burst was comprised of multiple 1 s segments, apportioned into successive trains of individual APW at frequencies of 6, 33, 33, 22, 13, 13, 11, 11, 11 and 6 Hz. B, continuous recording of individual ICa responses evoked in response to the OT-like train of APWs. Note the small decrement in peak amplitude of ICa evoked at the higher frequency APW trains (33 and 22 Hz), and restoration of peak amplitudes that occurs with delivery of the subsequent lower frequency trains. C, leak current responses evoked by deliverying a burst of negative APWs applied at the same frequencies as the OT-patterned burst of APWs. D, baseline capacitance measurements were obtained for several seconds before presentation of the OT-patterned APW burst. Subsequent to initiation of the burst, Cm measurements were obtained once every second within a 15 ms window (tracea). Cm measurements were also obtained during a similar train of nAPWs (trace b). Note that increases in ΔCm were first evoked during the 33 Hz APW trains, and then either continued to increase or reached a plateau with presentations of APW trains at lower frequency.
Although we did not consistently identify OT neurones in this study, in some experiments we identified single soma-type postrecording using aVP- or OT-specific antisera (dot blot method previously described in Soldo & Moises, 1998). In 16 cases we were able to conclusively identify neurone type (roughly 50 : 50 distribution, one neurone expressing both aVP and OT). No consistent difference in the Cm responses between the two cell types was observed. This indicates that the Ca2+-dependent Cm increases can be induced in both OT and aVP soma and that stimulus pattern or frequency may be a major determinant of electrical activity-induced release characteristics.
Discussion
There is a growing consensus that the neuropeptide OT regulates rhythmic electrical activity and development of MNCs at the level of the SON. It has been proposed that MNCs may independently regulate the pulsatile release of OT via positive retrograde signalling (Moos et al. 1984; Jourdain et al. 1998) and the inhibition of excitatory afferents (Hirasawa et al. 2001; Kombian et al. 2002). In addition, OT signalling has been proposed to induce morphological and developmental changes in MNCs (Chevaleyre et al. 2000, 2001).
In this paper we used time-resolved membrane capacitance measurements to directly demonstrate that isolated MNC somata undergo Ca2+-dependent secretory activity. Importantly, our work reveals that the neuronal somata exhibit multiple phases of Cm, indicating that there are functionally distinct secretory granule pools, similar to that observed in their isolated nerve terminals (Lim et al. 1990; Seward et al. 1995; Hsu & Jackson, 1996; Giovannucci & Stuenkel, 1997; Nowycky et al. 1998). The Ca2+ and activity dependence of these phases indicate that functional exocytotic machinery is also present in the somata and that Ca2+-dependent exocytotic activity underlies somatodendritic peptidergic secretion. Thus, modulation of the sizes of these granule pools might provide a basis for plasticity of the secretory response to meet specific physiological demands.
Cm detection in conjunction with whole-cell voltage clamp revealed that exocytotic events were preferentially evoked with depolarizing voltage steps or with high frequency action potential waveform stimulations that evoked substantial Ca2+ influx via activation of voltage-gated Ca2+ channels. Voltage-dependent capacitance responses consisted of at least three phases: (1) an initial Ca2+-independent transient component that obfuscated the first 10–15 ms of the Ca2+-dependent Cm components; (2) a residual Ca2+-dependent ΔCm that could be measured following subtraction of the Ca2+-independent transient and cessation of the depolarizing voltage pulse; and (3) a more persistent component that induced ΔCm with varying delays. Accordingly, we have termed the two Ca2+-dependent Cm components as indicative of rapidly releasable and slowly releasable secretory granule pools, respectively.
The Ca2+ dependence of the ΔCm attributed to the rapidly releasable pool was established by abolition of the evoked ΔCm during Ca2+ channel blockade, by showing a correlation between the voltage–response relationship of Ca2+ channel activation and ΔCm, and by demonstrating linearity between the flux of Ca2+ across the membrane and peak ΔCm amplitudes. It has been previously shown in neurohypophysial nerve endings that Ca2+-dependent increases in Cm eventually saturate and that this property may reflect the release from, and the eventual depletion of, a rapidly releasable pool of vesicles that are primed and docked at the cellular membrane (Giovannucci & Stuenkel, 1997). The Cm increase detected immediately following a brief depolarization was interpreted to reflect the exocytosis of a subpopulation of vesicles that are immediately available to fuse with the membrane. Likewise, stepwise changes in Cm were evoked in MNC somata in response to brief depolarizations, and these changes eventually saturated when challenged by repetitive stimulations. The diminution of the ΔCm occurred despite evidence that [Ca2+]i continued to increase beyond this point. This observation is consistent with the depletion of an immediately releasable pool of granules that is sensitive to localized domains of high Ca2+ influx. In several neurones we detected a delayed and persistent ΔCm that was initiated during repetitive pulses or that appeared to activate several seconds beyond termination of long-duration pulses. Because of the variability of the delayed ΔCm, and the small number of cells that exhibited this profile, we did not investigate these events further. However, similar responses have been described in neurohypophysial nerve endings and chromaffin cells and postulated to reflect the recruitment of a slowly releasable pool of vesicles.
Although we clearly demonstrated that step depolarizations applied either repetitively or with long duration could evoke secretory activity, we also tested whether such activity could be induced using stimulations and experimental conditions that were more physiologically relevant. An additional set of experiments demonstrated that action potential waveforms, capable of activating short duration inward ICa, could evoke ΔCm in SON neurones if applied in trains of greater than 22 Hz. The Ca2+ efficacy of trains of high-frequency APW stimulations to evoke exocytotic responses may be greater than that of depolarizing pulses due to a relationship between Ca2+ entry and the interpulse interval between stimulations (Engisch et al. 1997). That intermittent stimulations can maintain increases in ΔCm suggests that the secretory machinery is capable of summating small quantities of Ca2+ entry per APW stimulation. Moreover, the repetitive stimulus protocol used here spans several seconds, which provides sufficient time for activation of Ca2+-dependent signalling systems. In addition, recent evidence suggests that Ca2+-induced Ca2+ release or OT or AVP evoked Ca2+ release from internal Ca2+ stores might play a role in modulating the release of peptide from the cell bodies of MNCs.
Our results support previous findings that have indicated exocytotic activity at extrasynaptic sites. Ultrastructural data have provided indirect evidence that is consistent with the exocytosis of vesicles at both synaptic and non-synaptic somatodendritic sites (sympathetic ganglia: Zaidi & Matthews, 1997; supraoptic nucleus: Pow & Morris, 1989; Wang et al. 1995). Direct measurements obtained using amperometry and single-cell immunoblots have provided strong evidence for Ca2+-dependent secretion of monoamines and neuropeptides from cell bodies in central and spinal cord neurones (substantia nigra: (Cheramy et al. 1981; Jaffe et al. 1998; dorsal root ganglia: Huang & Neher, 1996).
The Cm measurements of suproptic neurones described in the current study and one previously reported (Soldo et al. 1998) indicate that electrical activity at the cell soma can induce Ca2+-dependent exocytotic activity. These data largely agree with Cm measurements of supraoptic neurones recently reported by de Kock et al. (2003). However, whereas de Kock et al. observed Cm changes induced by application of a single AP waveform, a major observation of our study was that repetitive application of AP waveforms was required to evoke Cm increases. Application of a single AP waveform under our conditions induced only a Ca2+-independent Cm transient, consistent with our previous demonstration that a short-duration depolarization (<5 ms) in isolated neurohypophysial nerve terminals was ineffective at consistently evoking an exocytotic response. The observation that robust, prolonged electrical activity that induced substantial rises in [Ca2+]i was required to induce somatic Cm increases indicates that dendritic release of peptide may be highly regulated. Thus, although our work and the work of others (Kombian et al. 1997; De Kock et al. 2003) clearly indicates that Ca2+ influx through voltage-dependent Ca2+ channels can induce the release of neuropeptide, it does not suggest that influx is necessarily the preferred route by which OT is released from dendrites. In fact work of Ludwig et al. (2002) demonstrated that increases in dendritic [Ca2+]i activates and enhances OT release, whereas back propagation of AP from the neural stalk was ineffective. As such, the apparent differences in the control of peptide release reported in these studies may indicate modulation of size of the releasable pool of secretory vesicles and the plastic nature of somatodendritic secretion.
Thus, our work supports and extends the concept of somatodendritic release as an important regulator of hypothalamic rhythmicity and development, and indicates that exocytotic release of peptide from somata or dendrites participates in a bidirectional pathway for communication between pre- and postsynaptic elements within the SON.
Acknowledgments
We thank Dr Xiaoming Hou and Bi Yu for providing technical assistance, and Charles Pluto for comments. Various portions of this work were supported by an American Heart Association Scientist Development Grant 0130231N to D.R.G., National Institute on Drug Abuse Grants DA 03365 to H.C.M and DA 07268 (postdoctoral training fellowship) to B.L.S, and NIH Grant NS 39914 to E.L.S.
References
- Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol. 1994;475:115–128. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Taylor RE, Fernandez JM. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J General Physiol. 1982;79:21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Dayanithi G, Moos FC, Desarmenien MG. Developmental regulation of a local positive autocontrol of supraoptic neurons. J Neurosci. 2000;20:5813–5819. doi: 10.1523/JNEUROSCI.20-15-05813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Moos FC, Desarmenien MG. Correlation between electrophysiological and morphological characteristics during maturation of rat supraoptic neurons. Eur J Neurosci. 2001;13:1136–1146. doi: 10.1046/j.0953-816x.2001.01489.x. [DOI] [PubMed] [Google Scholar]
- De Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus K, Hartmann J, Kilic G, Lindau M. Influence of conductance changes on patch clamp capacitance measurements using a lock-in amplifier and limitations of the phase tracking technique. Biophys J. 1995;69:2808–2822. doi: 10.1016/S0006-3495(95)80154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scala-Guenot D, Strosser MT, Richard P. Electrical stimulations of perifused magnocellular nuclei in vitro elicit Ca2+-dependent, tetrodotoxin-insensitive release of oxytocin and vasopressin. Neurosci Lett. 1987;76:209–214. doi: 10.1016/0304-3940(87)90717-8. [DOI] [PubMed] [Google Scholar]
- Engisch KL, Chernevskaya NI, Nowycky MC. Short-term changes in the Ca2+ -exocytosis relationship during repetitive pulse protocols in bovine adrenal chromaffin cells. J Neurosci. 1997;17:9010–9025. doi: 10.1523/JNEUROSCI.17-23-09010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1995;486:571–580. doi: 10.1113/jphysiol.1995.sp020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring RC, Armstrong WE. Pharmacological dissection of high-voltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. J Neurophysiol. 1996;76:977–983. doi: 10.1152/jn.1996.76.2.977. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys J. 2000;79:314–320. doi: 10.1016/S0006-3495(00)76293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci DR, Stuenkel EL. Regulation of secretory granule recruitment and exocytosis at rat neurohypophysial nerve endings. J Physiol. 1997;498:735–751. doi: 10.1113/jphysiol.1997.sp021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Kombian SB, Pittman QJ. Oxytocin retrogradely inhibits evoked, but not miniature, EPSCs in the rat supraoptic nucleus: role of N- and P/Q-type calcium channels. J Physiol. 2001;532:595–607. doi: 10.1111/j.1469-7793.2001.0595e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Hsu SF, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. J Physiol. 1996;494:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LY, Neher E. Ca2+ -dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C, Fernandez JM. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988;53:885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Israel JM, Dupouy B, Oliet SH, Allard M, Vitiello S, Theodosis DT, Poulain DA. Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro. J Neurosci. 1998;18:6641–6649. doi: 10.1523/JNEUROSCI.18-17-06641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Pittman QJ. Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus. Prog Brain Res. 2002;139:235–246. doi: 10.1016/s0079-6123(02)39020-4. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Moos FC, Richard P. Action of endogenous oxytocin within the paraventricular or supraoptic nuclei: a powerful link in the regulation of the bursting pattern of oxytocin neurons during the milk-ejection reflex in rats. Neuroscience. 1993;57:1027–1038. doi: 10.1016/0306-4522(93)90046-i. [DOI] [PubMed] [Google Scholar]
- Lim NF, Nowycky MC, Bookman RJ. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990;344:449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Mason WT, Ho YW, Hatton GI. Axon collaterals of supraoptic neurones: anatomical and electrophysiological evidence for their existence in the lateral hypothalamus. Neuroscience. 1984;11:169–182. doi: 10.1016/0306-4522(84)90221-5. [DOI] [PubMed] [Google Scholar]
- Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Seward EP, Chernevskaya NI. Excitation-secretion coupling in mammalian neurohypophysial nerve terminals. Cell Mol Neurobiol. 1998;18:65–80. doi: 10.1023/a:1022575126738. [DOI] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Seward EP, Chernevskaya NI, Nowycky MC. Exocytosis in peptidergic nerve terminals exhibits two calcium-sensitive phases during pulsatile calcium entry. J Neurosci. 1995;15:3390–3399. doi: 10.1523/JNEUROSCI.15-05-03390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo BL, Giovannucci DR, Stuenkel EL, Moises HC. Opioids modulate Ca2+ currents evoked by action potential waveforms in rat supraoptic nucleus neurons. J Neurosci. 1998;24:1081. [Google Scholar]
- Soldo BL, Moises HC. μ-Opioid receptor activation inhibits N- and P-type Ca2+ channel currents in magnocellular neurones of the rat supraoptic nucleus. J Physiol. 1998;513:787–804. doi: 10.1111/j.1469-7793.1998.787ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ward AR, Morris JF. Oestradiol acutely stimulates exocytosis of oxytocin and vasopressin from dendrites and somata of hypothalamic magnocellular neurons. Neuroscience. 1995;68:1179–1188. doi: 10.1016/0306-4522(95)00186-m. [DOI] [PubMed] [Google Scholar]
- Zaidi ZF, Matthews MR. Exocytotic release from neuronal cell bodies, dendrites and nerve terminals in sympathetic ganglia of the rat, and its differential regulation. Neuroscience. 1997;80:861–891. doi: 10.1016/s0306-4522(96)00664-1. [DOI] [PubMed] [Google Scholar]